Abstract
The aim was to compare the effectiveness of Brandt-Daroff, Semont and Epley maneuver in BPPV resolution. A Single Blind RCT in a Secondary Care Center was performed. Inclusion criteria were: patients with unilateral rotatory nystagmus on Dix-Hallpike Maneuver (DHM). Exclusion criteria: other causes of peripheral or central vertigo. Patients were randomized into 4 groups: Brandt-Daroff, “sham”, Semont and Epley. Patients underwent allocation, 1st visit (at 1 week with reprise of original maneuver if persistent nystagmus) and 2nd visit (2 to 4 weeks) with repetitions of both DHM and DHI. Main Outcome Measures: Absence of nystagmus on DHM at 1st and 2nd visit evaluations and DHI score. Resolution was defined as the abscence of nystagmus. We included 34 patients (25 females, 9 males). Patients were randomized to Brandt-Daroff (n = 9), “sham” (n = 7), Semont (n = 9) and Epley (n = 9) group. Overall mean age was 59.85 years (SD ± 13.10). A total of 47.06% patients (n = 16) had negative DHM at 1st visit. Resolution for Brandt-Daroff was 22.22%, “sham” 28.57%, Semont 44.44% and Epley 88.88% (p = 0.024); at 2nd visit follow up, Epley achieved 100% resolution (other maneuvers: 42.86%, 16.67%, 44.44%, respectively. P = 0.006). The DHI improvement at 2nd visit for Brandt-Daroff was 21.17 points, “sham” 8.05, Semont 14.67 and Epley 61.78 (p = 0.001). Epley maneuver was superior to Brandt Daroff, “sham” and Semont maneuvers on nystagmus resolution and DHI improvement in patients with BPPV.
Keywords: Benign Paroxysmal Positional Vertigo, Epley maneuver, Semont maneuver, Brandt-Daroff exercises, Placebo, Randomized controlled trial
Introduction
Benign Paroxysmal Positional Vertigo (BPPV) is one of the most common causes of vertigo in patients with vestibular disorders [1] and also, the most common cause of peripheral vertigo [2]. BPPV was first described by Barany (1923) [2] but it was until 1952 when Margaret Dix and Charles Hallpike created the diagnostic test named after them [3]. Dix-Hallpike Maneuver (DHM) is nowadays considered the gold standard for diagnosis. The incidence of BPPV estimated is 64/100,000 per year, with an annual prevalence of 2.4% [4]. BPPV is characterized by rotatory dizziness symptoms according to postural changes of the head, such as side-lying or turning head to left or right [1]. The etiology of BPPV is explained by two theories: cupulolithiasis, in which the adhesion of otoliths to the cupula in any of the three semicircular canals makes it denser than the endolymphatic flow and, as a result, more susceptible to gravity effects. Canalithiasis on the other hand is the free-floating otoconia in the semicircular canals. These otoconia, stimulate the ampullary crests, establishing a rotatory feeling dependent on the postural head movement related to gravity. Among the three semicircular canals, the posterior semicircular canal is the most affected (80%), and the superior canal the less frequently implicated [5–7].
Positional nystagmus is the most important finding for BPPV diagnosis. Through the characteristics of nystagmus it is possible to identify the semicircular canal involved, allowing also to distinguish between cupulolithiasis or canalithiasis and thus selecting the most appropriate treatment [7]. Posterior semicircular canal nystagmus has a torsional component (clockwise or counter-clockwise) associated with a superior vertical element that persists for less than one minute [8].
Classic eye movements within Dix-Hallpike Maneuver, suggestive clinical history and the absence of another related pathology integrate the diagnosis of BPPV [9]. If the patient has no presence of nystagmus on Dix-Hallpike Maneuver, it is recommended to perform the Roll-Test maneuver in search for lateral canal BPPV.
Diverse maneuvers based on cupulolithiasis and canalithiasis theories have been proposed as treatment for BPPV, the more relevant are Brandt-Daroff, Semont and Epley, the last one being the most widely used [8]. The purpose of these maneuvers is to return otoconia particles from the involved canal back to the utricular macula with symptom resolution [10]. Nevertheless, there are still numerous publications in search for the best maneuver. The ideal maneuver would have a prompt BPPV resolution and less BPPV recurrences.
Because of the subjective symptomatology and the intricacy to quantify the improvement after the implemented therapy on patients with vertigo, Jacobson and Newman in 1990 [11] developed a tool named Dizziness Handicap Inventory (DHI) with 25 questions divided into three domains: emotional (9 items), functional (9 items) and physical (7 items). The minimum score is 0 and the maximum score is 100. Since the creation of this tool, it has been widely accepted, used on multiple vertigo and dizziness related studies and validated in more than 17 languages [12].
The purpose of this article was to measure the efficacy of the different Particle Repositioning Maneuvers (PRM) as the treatment for posterior canal BPPV, assessed through resolution of nystagmus on Dix-Hallpike Maneuver as well as improvement on the DHI score.
Methods
Type of study
Single Blind, Randomized Controlled Clinical Trial.
Subjects
Patients with BPPV diagnosis performed through Dix-Hallpike Maneuver, with no previous treatment, who attended the Otorhinolaryngology and Head and Neck Surgery service from March 2013 to February 2014.
Ethical Approval
The protocol was approved by the Ethics Committee of this institution with registry number of 0126, and was in accordance to the Helsinki Declaration. Each participant provided individual informed consent.
Inclusion Criteria
There was no age or gender restriction. The principal requirement for inclusion was diagnosis of posterior canal BPPV, with positive nystagmus (up beating and torsional) on the DHM.
Exclusion Criteria
Patients were excluded if central vertigo or other causes of peripheral vertigo were suspected (for example: Meniere disease, vestibular neuritis). Patients with previous brain or cervical injury, recent medical treatment (vestibular suppressants) or refusal to sign the informed consent.
Elimination Criteria
Failure to attend to the first visit follow up after the allocation visit.
Study Procedure
Patients were randomized into 4 groups through a computerized system and were assigned the therapeutic maneuver. With randomization complete, demographic and clinical data were obtained. Only one maneuver was performed for each visit.
In the Brandt-Daroff group, exercises were performed as originally described [13] with 30 min sessions each day. “Sham maneuver”represented the placebo group, the clinician performed a Semont maneuver but to the non affected contralateral side.
There were two visits after allocation: first visit (1 week) and second visit (from two to four weeks). After 2nd visit and end of study, patients were treated according to their clinician best decision.
Outcome Measurements
Absence of nystagmus was assessed during the 1st and 2nd visit. The primary outcome measurement was the presence or abscence of nystagmus, evaluated on the Dix-Hallpike Maneuver (negative or positive). Resolution was defined as the abscence of nystagmus. Recurrence was defined as negative nystagmus on the 1st visit and positive nystagmus on the 2nd visit.
Study Instrument
The Dizziness Handicap Inventory (DHI) [14] was applied for subjective evaluation of vertigo in the allocation evaluation and the 2nd visit. We considered reduction of symptoms if these categories were improved (with a decrement in score) from allocation to 2nd visit.
Blinding
This study was blinded to the participants only. Patients in the placebo group received a sham maneuver to the contralateral side, therefore, patients were not aware of not receiving any effective treatment for their BPPV. Nevertheless, they knew the possibilities of the different maneuvers in this study and the probability of not receiving any effective maneuver at all.
Statistical Analysis
Descriptive statistic with central tendency measures and data dispersion through mean and standard deviation were used in case of continuous variables and frequency and proportions in case of categorical variables. The comparison between groups was performed with one-way analysis of variance (ANOVA) for continuous variables and X2 and Fisher’s exact test for categorical variables. A p ≤ 0.05 was considered as statistically significant.
The collected data was introduced in the Statistical Package for the Social Sciences (SPSS) version 21 (IBM Corp., Armonk, NY, USA) for its statistical analysis.
Results
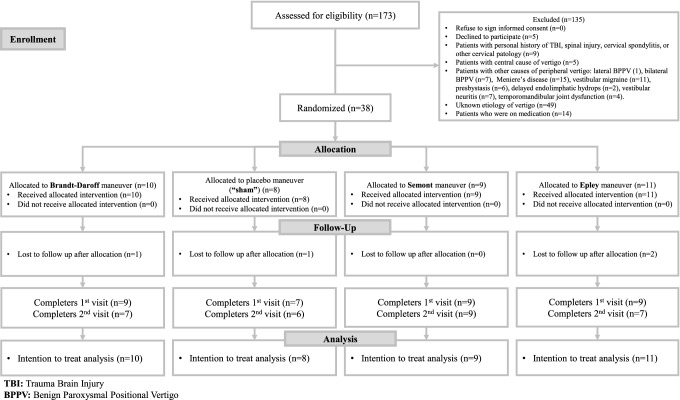
One hundred and seventy-three patients were assessed for eligibility, but only 38 were randomized. In order to see the enrollment process see Fig. 1. Participants were randomized into four groups: Brandt-Daroff (n = 10), “sham” (n = 8), Semont (n = 9) and Epley (n = 11). Four patients were lost to follow up after allocation and were eliminated. After applying elimination criteria, the groups were as follow: Brandt-Daroff (n = 9), “sham” (n = 7), Semont (n = 9) and Epley (n = 9). All patients were considered for the intention to treat analysis.
Fig. 1.
Consort flow diagram
From these 34 participants, 73.50% (n = 25) were females, 26.50% (n = 9). Overall mean age was 59.85 years (SD ± 13.10), range from 34 to 83 years. The comorbidities found in this study were: diabetes mellitus 14.70% (n = 5), hypertension 38.20% (n = 13), dyslipidemia 11.80% (n = 4), hypothyroidism 5.90% (n = 2) and prolonged rest in 17.60% (n = 6). There was no clinical history of hyperthyroidism in our population. None of these clinical variables were statistically significant between groups. Demographic and clinical characteristics of the population are depicted on Table 1.
Table 1.
Demographic and clinical characteristics of the population
| Total | Brandt-Daroff group (n = 9) | “Sham” group (n = 7) | Semont group (n = 9) | Epley group (n = 9) | p Value | |
|---|---|---|---|---|---|---|
|
Age (mean, SD, years) |
59.85 ± 13.10 | 59.66 | 55.28 | 64.66 | 58.77 | 0.600 |
| Gender |
M = 9 F = 25 |
M = 3 (33.33%) F = 6 (66.66%) |
M = 1 (14.28%) F = 6 (85.71%) |
M = 1 (11.11%) F = 8 (88.88%) |
M = 4 (44.44%) F = 5 (55.55%) |
0.343 0.898 |
| Diabetes mellitus | 5 | 1 (11.11%) | 1 (14.28%) | 2 (22.22%) | 1 (11.11%) | 0.898 |
| Hypertension | 13 | 4 (44.44%) | 3 (42.85%) | 4 (44.44%) | 2 (22.22%) | 0.721 |
| Dyslipidemia | 4 | 0 (0%) | 0 (0%) | 3 (33.33%) | 1 (11.11%) | 0.104 |
| Hypothyroidism | 2 | 2 (22.22%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.116 |
| Prolonged rest | 6 | 2 (22.22%) | 1 (14.28%) | 0 (0%) | 3 (33.33%) | 0.303 |
|
Affected ear-side |
R = 24 L = 10 |
R = 7 (77.77%) L = 2 (22.22%) |
R = 6 (85.71%) L = 1 (11.11%) |
R = 6 (66.66%) L = 3 (33.33%) |
R = 5 (55.55%) L = 4 (44.44%) |
0.564 |
|
Nystagmus latency (mean, SD, seconds) |
5.41 s ± 5.81 s | 4.55 s | 3.14 s | 8.44 s | 5 s | 0.204 |
|
Nystagmus duration (mean, SD, seconds) |
10.5 s ± 3.75 s | 8.88 s | 11.14 s | 11.11 s | 11 s | 0.715 |
M: Males, F: Females, R: Right, L: Left, CW: Clockwise, CCW: Counter-clockwise, s: Seconds
The DHM for right posterior canal was positive in 70.60% (n = 24) and left posterior canal in 29.40% (n = 10). Mean latency duration was of 5.41 ± 5.81 s (range 1 to 35 s). Nystagmus mean duration was 10.5 ± 3.75 s (range 5 to 20 s).
Resolution Rates
Global 1st visit resolution rate (absence of nystagmus) in our population was 47.06% (n = 16). Resolution rate of Epley maneuver (88.8%) was superior when compared to Brandt-Daroff (22.2%), “sham” (28.5%) and Semont maneuver (44.4%) (p = 0.024), as displayed on Table 2.
Table 2.
Success rate of original maneuver assigned
| Brandt-Daroff group (n = 9) | “Sham” group (n = 7) | Semont group (n = 9) | Epley group (n = 9) | p Valuea | |
|---|---|---|---|---|---|
| 1st visit follow up | 0.024 | ||||
| Positive nystagmus (n, %) | 7 (77.77%) | 5 (71.42%) | 5 (55.55%) | 1 (11.11%) | |
| Negative nystagmus (n, %) | 2 (22.22%) | 2 (28.57%) | 4 (44.44%) | 8 (88.88%) | |
| Recurrence (n, %) | − | − | − | − | |
| Lost to follow-up (n) | − | − | − | − | |
| Total (n) | 9 | 7 | 9 | 9 | |
| 2nd visit follow up | 0.006 | ||||
| Positive nystagmus (n, %) | 4 (57.14%) | 5 (83.33%) | 5 (55.56%) | 0 (0%) | |
| Negative nystagmus (n, %) | 3 (42.86%) | 1 (16.67%) | 4 (44.44%) | 7 (100%) | |
| Recurrence (n, %) | 0 (0%) | 0 (0%) | 1 (11.11%) | 0 (0%) | |
| Lost to follow-up (n) | 2 | 1 | 0 | 2 | |
| Total (n) | 9 | 7 | 9 | 9 |
aFisher test
Data analyzed with X2 test at 1st visit follow up confirmed Epley superiority against Brandt-Daroff exercises (p = 0.004), “sham” (p = 0.013) and Semont maneuver (p = 0.046).
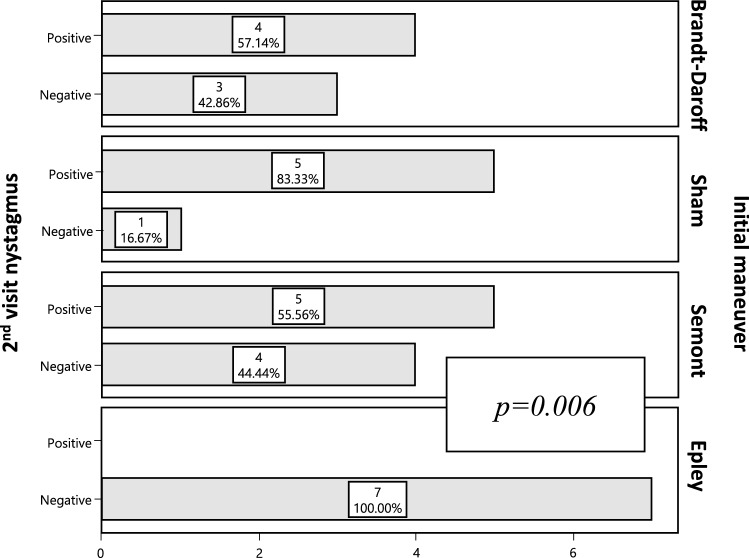
Twenty-nine patients received a 2nd visit evaluation. The resolution rate of BPPV was 51.72% (n = 15) with the original maneuver assigned. Epley maneuver was superior on the resolution of nystagmus compared to Brandt-Daroff exercises (100% vs 42.86%), “sham” (100% vs 16.67%) and Semont maneuver (100% vs 44.4%) (p = 0.006). See Fig. 2.
Fig. 2.
Nystagmus resolution on dix-hallpike maneuver: 2nd visit follow up
When comparing Epley to each of the other maneuvers, these outcomes remained significant at the 2nd visit follow up, with Epley being superior in the resolution rate (Brandt-Daroff p = 0.018, “sham” p = 0.002, Semont p = 0.017).
Intention to Treat Analysis
Additionally, two intention to treat analysis were performed considering the four patients lost after allocation and the five patients lost after 1st visit. First scenario: all nine patients (four patients lost after allocation and five patients lost after 1st visit) were considered to have positive nystagmus at 2nd visit. In this scenario Epley was superior only against “sham” [Brandt-Daroff (70% vs 30%; p = 0.074), “sham” (70% vs 12%; p = 0.015), Semont (70% vs 40%; p = 0.178)]. Second scenario: all nine patients were considered to have negative nystagmus at 2nd visit, in this situation Epley was statistically significant superior compared to all other maneuvers [Brandt-Daroff (100% vs 60%; p = 0.025), “sham” (100% vs 37%; p = 0.003), Semont (100% vs 50%; p = 0.010).
A third scenario of intention to treat analysis was calculated considering only the five patients lost after 1st visit. In this scenario the last evaluation at 1st week (DHM, nystagmus present or absent) was assumed to persist at the second visit. In this manner, Epley was superior to all other maneuvers [Brandt-Daroff (100% vs 33%; p = 0.009), “sham” (100% vs 28%; p = 0.005), Semont (100% vs 44%; p = 0.029)].
The five patients lost to follow up after 1st visit patients were contacted by a phone call and 3 patients declared improvement (1 patient from “sham” group and 2 from Epley group). One patient moved to another city (1 from Brandt-Daroff group) and the fifth subject declined to continue in the study (1 from Brandt-Daroff group).
Dizziness Handicap Inventory
Total basal DHI score was 52.82 (SD ± 25.47) points. At 2nd visit evaluation only 28 patients completed the DHI, and the total 2nd visit mean score was 26.85 (SD ± 29.43). DHI improvement at 2nd visit was 21.17 points for Brandt-Daroff, 8.05 for “sham”, 14.67 for Semont and 61.78 for Epley (p < 0.001) as evidenced on Table 3.
Table 3.
Dizzines handicap inventory (Dhi) score improvement At Basal and 2nd visit follow up
| Brandt-Daroff group (n = 9) | “Sham” group (n = 7) | Semont group (n = 9) | Epley group (n = 9) | p Value& | |
|---|---|---|---|---|---|
|
Basal DHI (n = 34) |
56.88 | 41.71 | 47.11 | 63.11 | 0.334 |
| 2nd visit DHI (n = 28) | 35.71 | 33.66 | 32.44 | 1.33 | 0.116 |
|
Improvement (difference between 2nd visit and basal DHI score, %) |
− 21.17, 37.21% | − 8.05, 19.29% | − 14.67, 31.13% | − 61.78, 97.89% | < 0.001 |
&ANOVA Analysis (Analysis of variance). DHI: Dizziness Handicap Inventory
ANOVA analysis was non significant on total score of second week DHI (p = 0.116) among the different treatment groups.
Recurrences
Only 1 patient of the Semont group had recurrent nystagmus at 2nd visit follow up, the patient was treated successfully with repetition of original Semont maneuver.
Discussion
We report a randomized controlled clinical trial that proves the effectiveness of the Epley maneuver. This was evident on the patients that completed the study (first and second visit follow up) and also, on the intention to treat analysis.
Fortunately, the majority of BPPV patients are resolved with otoconia particle repositioning maneuvers. Nevertheless, there are on the literature controversial results on the effectivity of these diverse maneuvers. Questions such as which maneuver has the least recurrences, or which has the most rapid improvement, both objectively and subjectively, are not adequately answered, there is still a need for RCTs. Our study adds on this scientific background and answers these vital questions.
Hilton and Pinder [15], on a Cochrane systematic review, described the effectiveness of the Epley maneuver. This last study included 11 different studies with a total of 745 patients and concluded that Epley maneuver was more effective for BPPV resolution compared to the control group, “sham” maneuver and Brandt-Daroff exercises, but additionally described a similar effectivity to Semont and Gans maneuvers.
Our present study shows superior effectivity of the Epley maneuver in vertigo control and nystagmus resolution on the 1st visit if compared to Brandt-Daroff group (88.88% vs 22.22%, p = 0.004), “sham” group (88.88% vs 28.57%, p = 0.013) and the Semont group (88.88% vs 44.44%, p = 0.046). On the 2nd visit of our study, we achieved a 100% nystagmus resolution (n = 7) in patients assigned to Epley maneuver, which was statistical significant compared with the rest of the maneuvers (Brandt-Daroff p = 0.018, “sham” p = 0.002, Semont p = 0.017).
Interestingly, a multicentric double-blind study described improvement of the Epley maneuver observed within the first 20 min (63.9%) of the maneuver, in contrast with Semont (37.5%) and placebo (38.7%), and this effect persisted at one week follow up (Epley 94.4%, Semont 71.9% and placebo 71.0%, p = 0.023) [16].
Gupta [17], compared effectivity with similar PRMs as our study. This author described improvement in 90% (n = 27) of patients treated with the Epley maneuver immediately after the therapeutic maneuver, in contrast to 73.33% (n = 22) of Semont group and 50% (n = 15) of Brandt-Daroff group. In our study, the absence of nystagmus on the 1st visit in the Semont group and “sham” group were even lower (44.44% and 28.57%, respectively). Our outcomes in nystagmus resolution are similar to Sen [18], who obtained a resolution of 87% on the Epley group (n = 26) and 57% (n = 17) on the Semont group, both groups at first week follow up.
In an article that included two hundred patients by Ajayan [19], an improvement of 84% (n = 84) with the Epley maneuver and 81% (n = 81) for the Semont maneuver was achieved on the first week. At one month-follow up a resolution of 96% and 93% was obtained and in the 3 months-follow up it was 95% and 94% for Epley and Semont maneuvers, respectively. No statistical significance was reached and both maneuvers were considered equally effective. On the other hand, the Semont maneuver required more maneuvers to achieved success (p = 0.0529) compared to the Epley. Unfortunately, our study did not evaluate BPPV resolution in the long term and this needs to be considered as part of our limitations.
In a meta-analysis by Zhang [20], Semont maneuver was superior against the untreated group (p = < 0.01) and sham maneuver group (p = < 0.01) but not superior to Epley maneuver (RR = 0.83, 95% CI 0.68–1.00, p = 0.05) or Brandt-Daroff exercises (RR = 1.32, 95% CI 1.00–1.75, p = 0.05). Zhang also described that Semont maneuver recurrence rates were not statistically significant except when compared with the group with no intervention.
According to Benito [21] the effectivity of a unique Epley maneuver up to 7 days was 82% (compared with the 88% obtained in our study). The previously cited author refers a better outcome after 3 months in patients treated with Epley maneuver than patients treated with Semont maneuver.
There is controversy in the literature, between effectiveness of the Epley and Semont maneuvers, furthermore, some authors have reported they are equally effective [6, 15, 19, 22, 23]. Nevertheless, other authors have concluded that the Epley maneuver is superior and requires less maneuver repetitions [16–18, 24, 25].
The main strength of this study is the intention to treat analysis we performed considering various outcomes. Epley was not superior to Brandt-Daroff exercises in the first intention to treat analysis. Nonetheless, Epley superior effectiveness against Semont maneuver was evident on the second scenario (nine patients at 2nd visit as negative nystagmus, p = 0.010) and on the third scenario (1st visit nystagmus status assumed to persist in the second visit, p = 0.029). Additionally, Epley was statistical significant superior to Semont among the 29 patients that attended the second visit follow up (p = 0.006).
The definition of resolution of BPPV in most studies is considered as the absence of nystagmus. However, the impact in quality of life through DHI is rarely evaluated. Our study applied the DHI in an effort to understand the patient subjective symptoms. We have described an improvement on DHI values after treatment of all groups. We only considered total DHI score, according to Van De Wyngaerde, et al. [26] total DHI score is warranty of general dizziness evaluation. Rodrigues, et al. [27] described both subscale and total DHI scores, with the latter outcomes similar to ours. Rodrigues also found that recurrence rates were lower in any PRM performed if concomitant vestibular exercises were associated (p = 0.023).
It is important to consider that other authors, such as Sreenivas [28], have found association between diabetes mellitus and BPPV recurrence (p = 0.015). Additionally, other authors [29–31] have also demonstrated the association of osteoporosis and BPPV presence.
This single blinded randomized clinical control trial shows a short term follow up superiority of the Epley maneuver against all other maneuvers (Brandt Daroff, Semont and Placebo—“sham”). Furthermore, this study adds on the use of DHI as a form to evaluate the subjective symptoms of BPPV patients. Based on our results we recommend the performance of the Epley maneuver as a primary maneuver on a BPPV patient.
Conclusions
Epley maneuver was superior to Brandt Daroff, “sham” and Semont maneuvers on nystagmus resolution and Dizziness Handicap Inventory improvement in the treatment of patients with BPPV.
Acknowledgements
We would like to thank the department of Otolaryngology of CIDOCS and Autonomous University of Sinaloa.
Author's Contributions
All authors contributed to the manuscript and are accountable of all aspects of the manuscript.
Funding
Part of this study’s data was a poster presentation at the American Academy of Otolaryngology, 2015.
Data Availability
Available upon request.
Code Availability
SPSS was used.
Declarations
Conflict of interest
The authors declares that they have no conflict of interests.
Ethics Approval
Research and ethical approval was obtained in CIDOCS, number 0126.
Consent to Participate
All subjects signed informed consent.
Consent for Publication
this study is not considered for publication in any other journal. The submitted work is original and the authors consent to be considered for publication in the indian journal of otolaryngology.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Imai T, Takeda N, Ikezono T, Shigeno K, Asai M, Watanabe Y, et al. Classification, diagnostic criteria and management of benign paroxysmal positional vertigo. Auris Nasus Larynx. 2017;44:1–6. doi: 10.1016/j.anl.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Bárány R (1923) Disease of the Otolith Apparatus. J Nerv Ment Dis 57(1): 70. https://journals.lww.com/jonmd/Citation/1923/01000/Disease_of_the_Otolith_Apparatus.74.aspx
- 3.Dix M, Hallpike C. The pathology, symptomatology and diagnosis of certain common disorders of the vestibular system. Proc R Soc Med. 1952;45:341–354. [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Wang W, Zhang A, Bai X, Zhang S. Epley and Semont Maneuvers for posterior canal benign paroxysmal positional vertigo: a network meta-analysis. Laryngoscope. 2016;126:951–955. doi: 10.1002/lary.25688. [DOI] [PubMed] [Google Scholar]
- 5.Patangay KK, Ansari R. Benign paroxysmal positional vertigo: our experience. Indian J Otolaryngol Head Neck Surg. 2016;68:39–41. doi: 10.1007/s12070-014-0818-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh SY, Kim JS, Choi KD, Park JY, Jeong SH, Lee SH, et al. Switch to Semont maneuver is no better than repetition of Epley maneuver in treating refractory BPPV. J Neurol. 2017;264:1892–1998. doi: 10.1007/s00415-017-8580-2. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira AK, Suzuki FA, Boari L. Is it important to repeat the positioning maneuver after the treatment for benign paroxysmal positional vertigo? Braz J Otorhinolaryngol. 2015;81:197–201. doi: 10.1016/j.bjorl.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez-Vázquez P, Franco-Gutiérrez V, Soto-Varela A, Amor-Dorado JC, Martín-Sanz E, Oliva-Domínguez M, et al. Practice guidelines for the diagnosis and management of benign paroxysmal positional vertigo otoneurology committee of spanish otorhinolaryngology and head and neck surgery consensus document [In Spanish] Acta Otorrinolaringol Esp. 2017;69:345–366. doi: 10.1016/j.otorri.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Von Brevern M, Bertholon P, Brandt T, Fife T, Imai T, Nuti D, et al. Benign paroxysmal positional vertigo: diagnostic criteria. Consensus document of the committee for the classification of vestibular disorders of the bárány society [In Spanish] Acta Otorrinolaringol Esp. 2017;68:349–360. doi: 10.1016/j.otorri.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Cetin YS, Ozmen OA, Demir UL, Kasapoglu F, Basut O, Coskun H. Comparison of the effectiveness of Brandt-Daroff Vestibular training and Epley Canalith repositioning maneuver in benign Paroxysmal positional vertigo long term result: A randomized prospective clinical trial. Pak J Med Sci. 2018;34:558–563. doi: 10.12669/pjms.343.14786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson GP, Newman CW. The development of the dizziness handicap inventory. Arch Otolaryngol Head Neck Surg. 1990;116:424–427. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- 12.Ceballos LR, Vargas AM. Application and utility of the Dizziness Handicap Inventory in patients with vertigo from the Otolaryngology Service of the Specialties Hospital of the National Medical Center XXI Century [In Spanish] An Med Asoc Med Hosp ABC. 2004;49:176–183. [Google Scholar]
- 13.Brandt T, Daroff RB. Physical therapy for benign paroxysmal positional vertigo. Arch Otolaryngol. 1980;106:484–485. doi: 10.1001/archotol.1980.00790320036009. [DOI] [PubMed] [Google Scholar]
- 14.Yorker A, Ward I, Vora S, Combs S, Keller-Johnson T. Measurement characteristics and clinical utility of the dizziness handicap inventory among individuals with vestibular disorders. Arch Phys Med Rehab. 2013;94:2313–2314. doi: 10.1016/j.apmr.2013.07.007. [DOI] [Google Scholar]
- 15.Hilton MP, Pinder MK. The Epley (canalith repositioning) maneuver for benign paroxysmal positional vertigo (Review). Cochrane Database Syst Rev 2014; 12: CD003162. [DOI] [PMC free article] [PubMed]
- 16.Lee JD, Shim DB, Park HJ, Song CI, Kim MB, Kim CH, et al. A multicenter randomized double-blind study: comparison of the epley, semont, and “sham” maneuvers for the treatment of posterior canal benign paroxysmal positional vertigo. Audiol Neurootol. 2014;19:336–341. doi: 10.1159/000365438. [DOI] [PubMed] [Google Scholar]
- 17.Gupta AK, Sharman KG, Sharma P. Effect of epley, semont maneuvers and brandt-daroff exercise on quality of life in patients with posterior semicircular canal benign paroxysmal positional vertigo (PSCBPPV) Indian J Otolaryngol Head Neck Surg. 2018;71:99–103. doi: 10.1007/s12070-018-1322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sen K, Sarkar A, Raghavan A. Comparative efficacy of Epley and Semont maneuver in benign paroxysmal positional vertigo: A prospective randomized double-blind study. Astrocyte. 2016;3:96–99. doi: 10.4103/2349-0977.197214. [DOI] [Google Scholar]
- 19.Ajayan PV, Aleena PF, Jacobo AM. Epley’s maneuver versus Semont’s maneuver in treatment of posterior canal benign positional paroxysmal vertigo. Int J Res Med Sci. 2017;5:2854–2860. doi: 10.18203/2320-6012.ijrms20172574. [DOI] [Google Scholar]
- 20.Zhang X, Qian X, Lu L, Chen J, Liu J, Lin C, et al. Effects of Semont maneuver on benign paroxysmal positional vertigo: a meta-analysis. Acta Otolaryngol. 2016;137:63–70. doi: 10.1080/00016489.2016.1212265. [DOI] [PubMed] [Google Scholar]
- 21.Benito-Orejas JI, Poncela-Blanco M, Díez-González L, Álvarez-Otero R, Aguilera-Aguilera G, Intraprendente-Martini JF, et al. Practice guideline for benign paroxysmal positional vertigo [In Spanish] Revista ORL. 2017;8:157–196. doi: 10.14201/orl201783.15655. [DOI] [Google Scholar]
- 22.Anagnostou E, Stamboulis E, Kararizou E. Canal conversion after repositioning procedures: comparison of Semont and Epley maneuver. J Neuro. 2014;261:866–869. doi: 10.1007/s00415-014-7290-2. [DOI] [PubMed] [Google Scholar]
- 23.Kinne BL, Perla MJ, Weber DT. Semont maneuver versuss Epley maneuver for canalithiasis of the posterior semicircular canal: a systematic review. Phys Ther Rev. 2016;21(2):102–108. doi: 10.1080/10833196.2016.1228511. [DOI] [Google Scholar]
- 24.Radtke A, von Brevern M, Tiel-Wilck K, Mainz-Perchalla A, Neuhauser H, Lempert T. Self-treatment of benign paroxysmal positional vertigo Semont maneuver vs Epley procedure. Neurology. 2004;63:150–152. doi: 10.1212/01.WNL.0000130250.62842.C9. [DOI] [PubMed] [Google Scholar]
- 25.Emad MA, Radwa MY. Effect of Epley maneuver versus Semont maneuver on vertigo in post-menopausal women. Int J Rehabil Res. 2017;6:1–4. [Google Scholar]
- 26.Van De Wyngaerde KM, Lee MK, Jacobson GP, Pasupathy K, Romero-Brufau S, McCaslin DL. The component structure of the dizziness handicap inventory (DHI) Otol Neurotol. 2019;40:1217–1223. doi: 10.1097/MAO.0000000000002365. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues DL, de Oliveira CAP, Pires de Oliveira CA, Bahmad F., Jr Effect of vestibular exercises associated with repositioning maneuvers in patients with benign paroxysmal positional vertigo: a randomized controlled clinical trial. Otol Neurotol. 2019;40:824–829. doi: 10.1097/MAO.0000000000002324. [DOI] [PubMed] [Google Scholar]
- 28.Sreenivas V, Natashya S, Sumy P. The Role of Comorbidities in Benign Paroxysmal Positional Vertigo. Ear Nose Throat J 2019. [DOI] [PubMed]
- 29.Talaat HS, Abuhadied G, Talaat AS, Abdelaal MS. Low bone mineral density and vitamin D deficiency in patients with benign positional paroxysmal vertigo. Eur Arch Otorhinolaryngol. 2014;272:2249–2253. doi: 10.1007/s00405-014-3175-3. [DOI] [PubMed] [Google Scholar]
- 30.Byun H, Chung JH, Lee SH, Park CW, Kim EM, Kim I. Increased risk of benign paroxysmal positional vertigo in osteoporosis: a nationwide population-based cohort study. Sci Rep. 2019;9:3469. doi: 10.1038/s41598-019-39830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He LL, Li XY, Ho MM, Li XQ. Association between bone mineral density and benign paroxysmal positional vertigo: a meta-analysis. Eur Arch Otorhinolaryngol. 2019;276:1561–1571. doi: 10.1007/s00405-019-05345-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available upon request.
SPSS was used.