Abstract
Bladder cancer is defined as a urinary tract malignancy that threatens men’s and women’s health. Due to the side effects of common chemotherapies, novel therapeutic strategies are necessary to overcome the issues concerning bladder cancer treatments. Nanotechnology has been suggested as a means to develop the next-generation objectives of cancer diagnosis and treatment among various novel therapies. Owing to the special characteristics that they can offer, silver nanoparticles (AgNPs) were investigated in this study to evaluate their apoptotic impact on bladder cancer 5637 cells. In this study, an MTT assay was conducted and appropriate concentrations of AgNPs were selected. Moreover, reactive oxygen species (ROS) production and apoptosis levels were determined using fluorimetric and Annexin/PI flow cytometry assays, respectively. Moreover, the activity of caspase 3,7, mRNA expression of Bax (Bcl-2-associated X) and Bcl-2 (B-cell lymphoma 2) were assessed based on colorimetric and qRT-PCR methods, respectively. The results indicated that AgNPs can significantly reduce the viability of 5637 cells in a dose-dependent mode as well as having the ability to elevate ROS production. Flow cytometry data showed that AgNPs lead to a remarkable increase in the apoptosis rate as compared with the control. Consistent with this, the induction of apoptosis was revealed by the overexpression of Bax, accompanied by a reduction in Bcl-2 expression compared to the control. Furthermore, AgNPs remarkably stimulated caspase 3,7 activation. In summary, AgNPs can mediate apoptosis in 5637 cells via excessive ROS formation, up-regulating Bax/Bcl-2 expression, and caspase 3,7 activation.
Keywords: Apoptosis, Silver, Metal Nanoparticles, Urinary Bladder Neoplasms
INTRODUCTION
Cancer has a serious impact on human health today and is known for its high morbidity and mortality worldwide.1 Bladder cancer (BC) is the most frequent urinary malignant tumor with a high incidence in males. In general, two different types of BC are often reported under the titles of non-muscle invasive BC (NMIBC) and muscle-invasive or metastatic BC. Various types of treatments are employed to combat BC such as transurethral resection (TUR), intravesical chemotherapy, and radical cystectomy. However, due to the side effects and unsuccessful outcomes of the conventional therapies, novel therapeutic strategies are essential to treat a reasonable percentage of patients with BC.2,3 Among the different novel treatments, nanotechnology has recently been introduced to address the next-generation objectives of cancer research and treatment.4,5,6 In comparison to small molecule agents, nanoparticles (NPs) exhibit unique physicochemical features, including a high surface area to volume ratio that allows them to easily permeate live cells.7 Metallic NPs are considered among the most demanding research areas in modern materials in light of their unique optical properties. They are also frequently employed in biological applications such as tumor eradication in combination with immunotherapy.8,9 Silver NPs (AgNPs) are one of the most common metallic NPs and have distinctive yet special physicochemical and biological characteristics making them a promising agent in various fields such as medicine, agriculture, industry, and pharmaceuticals.10,11,12 Recently, AgNPs have been proposed for cancer therapy, mostly because of their antitumor effects on cancer cells which to a great extent are similar to chemotropic agents’ mechanisms which both lead to cancer cell death.13,14,15 Apoptosis may be promoted as a highly controlled process of planned cell death that is governed by intra- and extracellular signaling pathways. It is widely reported that NPs may induce apoptosis or necrosis.16 The antitumor potential of several Food and Drug Administration (FDA)-approved agents is built upon inhibition of the cell survival and proliferation pathways and their effects on apoptosis signaling pathway’s alterations in cancer cells.17 Thus, measuring B-cell lymphoma protein 2 (Bcl-2), Bcl-2 associated X (Bax), cytochrome complex (Cyt-c), cysteine-aspartic protease-9 (caspase-9) and caspase-3 in a great number of research works have revealed that several types of NPs, including AgNPs,18 copper NPs19 and AuNPs20,21 can induce apoptosis in a variety of cell lines. In addition, many reports indicated that reactive oxygen species (ROS) generation results in the regulation of Bax and Bcl-2 expression level. These two proteins have indeed a vital role in apoptosis.22 Moreover, over-expression of Bax and downregulation of Bcl-2 may be a consequence of NPs-induced mitochondrial damage which leads to the formation of mitochondrial permeability transition pores. The aforementioned process results in the release of Cyt-c into the cytosol and subsequently, the formation of apoptotic protease activating factor-1 (Apaf-1). Then, pro-caspase-9 is proteolytically cleaved and a complex such as an apoptosome is assembled leading to the activation of caspase-3 and -7 and thus induce apoptosis.23,24 Understanding apoptotic signaling pathways will help clinical researchers properly exploit anticancer agents. This motivated us to investigate the apoptotic effects of AgNPs on bladder cancer 5637 cells.
MATERIALS AND METHODS
1. Chemicals
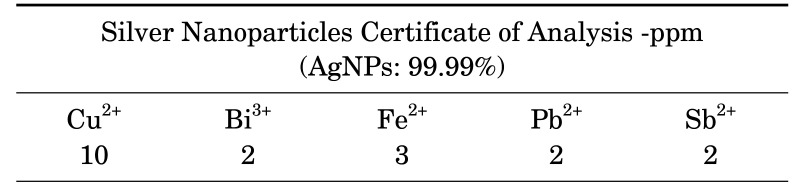
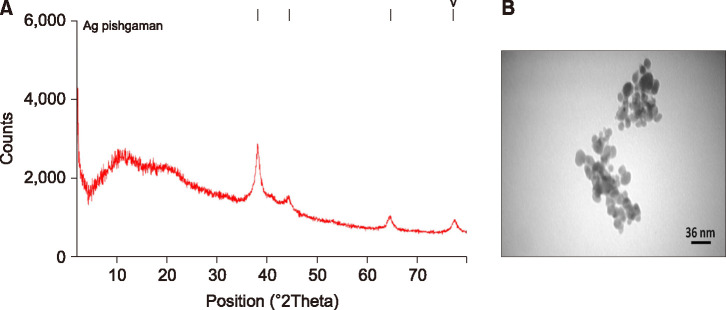
Spherical AgNPs with the size of 30-50 nm, and coated with Poly Vinyl Pyrrolidone, was procured from Iranian Nanomaterials Pioneers Company, NANOSANY (Mashhad, Iran). Table 1 shows the AgNPs certificate of analysis as the purity of NPs was 99.99%. Moreover, X-Ray Diffraction (XRD) patterns and Transmission electron microscopy (TEM) of these nanoparticles are presented in Fig 1A and B, respectively. XRD is reported to identify the crystalline phases and thereby reveal their chemical composition information.
TABLE 1. Silver nanoparticles (AgNPs) Certificate of Analysis-ppm.
Certificate is adopted from Nanosany company. The relative concentration of some of the materials in the AgNPs is reported in parts per million (ppm) and the purity of AgNPs is 99.99%.
FIG. 1. (A) X-Ray Diffraction (XRD) patterns and (B) Transmission electron microscopy (TEM) of Poly Vinyl Pyrrolidone-coated silver nanoparticles (PVP-AgNPs) are provided from Iranian Nanomaterials Pioneers Company, NANOSANY. XRD is reported to identify crystalline phases and thereby reveal chemical composition information.
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) powder was obtained from Sigma-Aldrich.
2. Cell culture and treatment
The 5637-tumor cell line of the bladder was purchased from Pasteur Institute cell collection, (Tehran, Iran). RPMI-1640 medium (KRPM500) supplemented with 10% fetal bovine serum (FBS) (KFBS100) and 1% penicillin-streptomycin (BI-1203) was used to culture 5637 cells. Subculture (passage) of cells was performed using Trypsin-EDTA 0.25% solution (KRT100).
The NPs were diluted in RPMI-1640 medium in order to obtain the desired concentrations, and then, the 5637 cells were exposed to different concentrations of AgNPs.
3. Determination of cell viability via MTT assay
In brief, the 5637 cells were suspended in medium and then seeded in a 96-well plate at a concentration of 2×104 cells per well. After that, the plate was incubated upon the cells reaching the appropriate confluency. Next, diluted AgNPs with different concentrations (0, 3.9, 7.8, 15.6, 31.25, 62.5 and 125 µg/mL)25 were transferred into the wells. After 24 h incubation, the MTT solution was added and incubated for 4h in an incubator. Subsequently, we removed the content of each well and added dimethyl sulfoxide (DMSO) (DNA biotech, Iran) in order to dissolve the formazan crystals. The absorbance of the control and experimental samples were recorded at 570 nm with an ELISA plate reader (RT-2100C Microplate Reader, China). Finally, the viability of 5637 cells was determined using the formula as presented below.
| Percentage of viability=Absorption (treated-well) / Absorption (control-well)×100. |
4. Determination of intracellular ROS production
The effect of AgNPs on ROS production was assessed via the microplate-fluorimetric method according to the manufacturer’s protocol (Kiazist, Iran).
In a nutshell, a concentration of 25×103 5637 cells per well were grown in a black 96-well plate. After incubating overnight, the cells were exposed to selected doses of AgNPs (0, 30, 50, 60 µg/mL) adopted from the MTT assay. After 24 h, the content of the wells was aspirated and 100 µl DCFDA (2’, 7’-dichlorofluorescin diacetate) solution was added to the experimental wells and then incubated for 45 minutes at 37℃. Lastly, the fluorescence intensity was recorded with excitation and emission wavelengths of Ex/Em=485/528 nm using a fluorescence microplate reader (Bio-Tek Instruments, Winooski-USA).26 The data were reported as fold change in comparison to the untreated cells (control).
5. Apoptosis detection by flow cytometry
Apoptosis level in 5637 cells was determined using Annexin-V Apoptosis Detection Kit (MabTag) following the manufacturer’s manual and descriptions according to Baghbani-Arani et al.27
First, the 5637 cells were cultured in T25 culture flasks and then were treated with desired concentrations of AgNPs for 24 h. Then, the cells were detached from each T25 culture flask. The pellet of control and treated cells were resuspended in 90 µL of diluted (1x) Annexin-V binding buffer. Next, 5 µL of Annexin-V conjugate and 5 µL of propidium iodide (PI) solution were transferred into the tubes including the cell suspension and then placed in a dark room for 20 minutes. Subsequently, 400 µL of Annexin-V binding buffer was included. After centrifuging at 400×g for 5 minutes, Annexin-V binding buffer (1X) was added and apoptosis cell death was assessed with a flow cytometer system (Life Technologies Attune NxT). Eventually, flow cytometry data were analyzed using FlowJo V10 CL software.
6. RNA extraction and quantitative reverse transcription-PCR (qRT-PCR)
The whole RNA was extracted from the control and AgNPs-treated 5637 cells using Kiazol reagent according to Kiazist kit instruction. The quantity of RNA was measured via determining the absorbance at 260/280 nm by NanoDrop One UV-Vis Spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and also the quality was checked by %1 gel agarose electrophoresis. The RNA samples converted to cDNA by reverse transcriptase enzyme using BioFact™ RT Series cDNA synthesis kit and qRT-PCR was done on a LightCycler®96 instrument (Roche, Germany). The primer sequences of Bax, Bcl-2 and β actin, used for the analysis, were designed by primer3 software and verified by Blast search. The primers sequences are displayed in Table 2. Furthermore, the specificity of primers was checked according to the melting-curve analysis. the relative expression level was determined based on 2−ΔΔCt method28 in which the target gene expression was normalized with the expression level of β actin as an internal control.
TABLE 2. Primer sequences used in qRT-PCR reaction.
The primer sequences were designed by primer3 software and checked by Blast search.
7. Determination of caspase 3,7 activity
A cell lysate of 5637 cells was prepared after 24-h incubation with AgNPs following the Kiazist Kit manual (Hamadan, Iran).
Briefly, 55.5 µL of the working solution containing caspase buffer, DTT and caspase substrate and also 50 µL of the cell lysate were transferred to a 96-well plate and then maintained for 1.5 h at 37℃. An ELISA plate reader was used to record the absorption at 405 nm. It is important to note that, p-nitroaniline (pNA) was regarded as standard and the activity of caspase 3,7 was estimated based on the standard curve. Finally, the results were expressed as mU/mg protein. In addition, a Bradford assay29 was carried out to normalize the results.
8. Statistical analysis
All experimental data were reported as mean±standard deviation (SD) using GraphPad prism 9 software. Triplicate determinations were carried out to determine the SD. One-way ANOVA following Tukey’s test was used to analyze the data along with specifying the statistically significant differences among the studied groups at a significance level of p<0.05.
RESULTS
1. Effects of AgNPs on 5637 cell viability
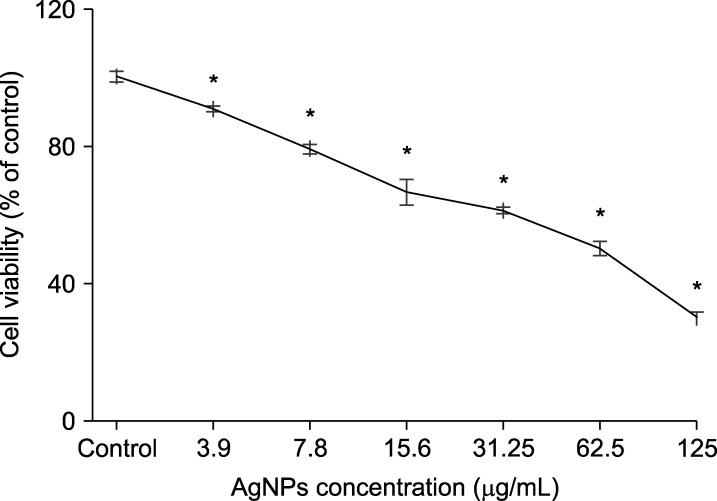
As it is shown in Fig. 2, AgNPs could reduce the 5637 cells viability in a dose-dependent mode in which the IC50 (half-maximal inhibitory concentration) value was estimated to be 51.12 µg/mL after 24 h incubation. The viability of 5637 cells was remarkably decreased in cells exposed to AgNPs in doses ranging from 3.9-125 µg/mL compared to the control untreated cells (100% cell viability). According to IC50 concentration, we selected the 30, 50 and 60 µg/mL doses of AgNPs for subsequent experiments.
FIG. 2. Effects of silver nanoparticles (Ag NPs) on the viability of 5637 bladder cancer cells after 24-h incubation are adopted from MTT assay. The viability of treated-cells are effectively reduced in a dose dependent manner in which the IC50 value is calculated to be 51.12 µg/mL. Results are reported as mean±SD. *p<0.05 compared to control (untreated cells).
2. Intracellular ROS production in the presence of AgNPs
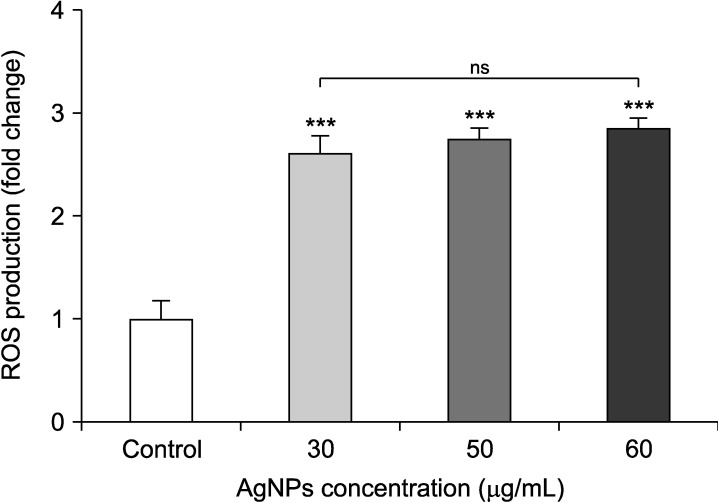
Results revealed that ROS production was significantly increased in AgNPs-treated cells with respect to the control (p<0.001), while there were no noticeable differences among different concentrations (p>0.05). According to Fig. 3, ROS generation was augmented more than two-fold in cells exposed to AgNPs compared to the control cells (p<0.001).
FIG. 3. Effects of silver nanoparticles (Ag NPs) on ROS production in bladder cancer 5637 cells. The results are reported as fold change in comparison to the untreated cells (control) and expressed as mean±SD. AgNPs could obviously increase the ROS production. ***p<0.001 compared to the control (untreated cells). ns: non-significant differences between experimental groups.
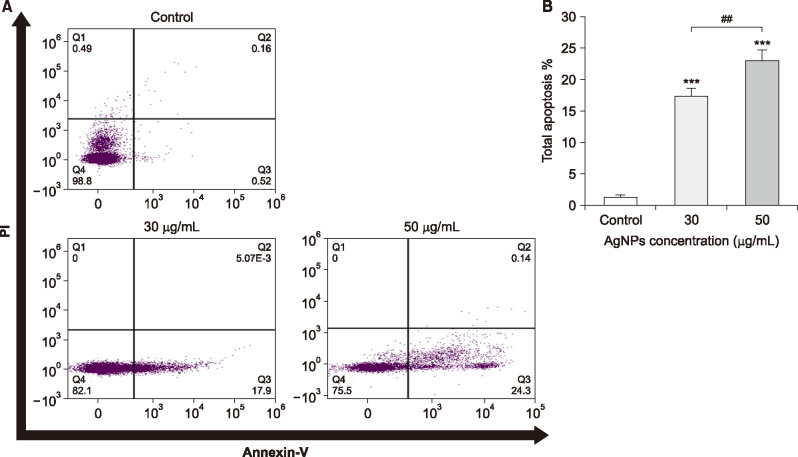
3. Induction of apoptosis by AgNPs (Annexin V/PI)
The percentage of cell apoptosis were quantitatively detected by the Annexin V–FITC/PI double staining assay. As it is depicted in Fig. 4A, exposure to AgNPs could meaningfully increase the rate of apoptosis in AgNPs-treated 5637 cells compared to the untreated control cells in which the main apoptotic cells were in the early apoptotic phase. Furthermore, necrotic or PI-positive cells were not observed in experimental groups (Fig. 4A). Statistical analysis of flow cytometry data indicated that AgNPs could remarkably enhance the total apoptosis in comparison to the control cells (Fig. 4B) (p<0.05).
FIG. 4. (A) Silver nanoparticles (AgNPs) induce apoptosis in bladder cancer 5637 cells. The 5637 cells were treated with 30 and 50 µg/mL of AgNPs for 24-h, and then apoptosis was evaluated by Annexin V–FITC/PI flow cytometry assay compared to the untreated control cells. The four quadrants of flow cytometry graph represent Necrotic (Q1), Late apoptotic (Q2), Early apoptotic (Q3) and Viable (Q4) cells. (B) Total apoptosis level (early and late apoptotic cells) in 30 and 50 µg/ml-treated cells compared to the control. The results are reported as mean±SD (n=3). ***p<0.001 compared to the control and ##p<0.01 shows significant differences between groups.
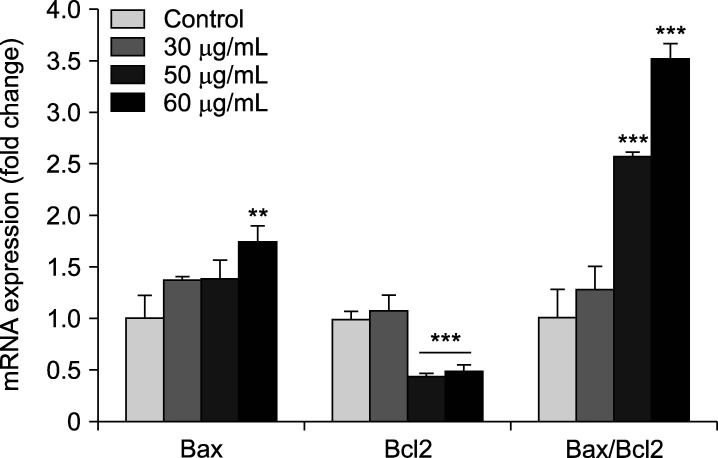
4. Apoptotic gene expression changes in the presence of AgNPs
As it is demonstrated in Fig. 5, AgNPs could meaningfully alter Bax and Bcl-2 genes expression in 5637 cells. qRT-PCR results showed an over-expression of Bax (pro-apoptotic gene) in cells exposed to 60 µg/mL of AgNPs (p<0.01). Meanwhile, the expression level of Bcl-2 (anti-apoptotic gene) was remarkably down-regulated in 50 and 60 µg/mL-treated cells compared to the control (p<0.001). Moreover, the elevation of Bax/Bcl-2 ratio was obtained from data as emphasize that the apoptosis was induced in AgNPs-treated cells at the concentrations of 50 and 60 µg/mL compared with the control (p<0.001). However, the Bax/Bcl-2 ratio was not statistically significant in cells incubated with 30 µg/mL dose of AgNPs (p>0.05).
FIG. 5. Exposure to silver nanoparticles (AgNPs) leads to apoptosis in bladder cancer 5637 cells. Alterations of pro-(Bax) and anti-apoptotic (Bcl-2) genes expression in 5637 cells are adopted from qRT-PCR assay. The ratio of Bax to Bcl-2 shows the rate of apoptosis. Fold change is calculated using 2−ΔΔCt method and the β-actin gene expression was regarded as an internal control. The results are reported as mean±SD (n=3). **p<0.01 and ***p<0.001 compared to untreated control cells.
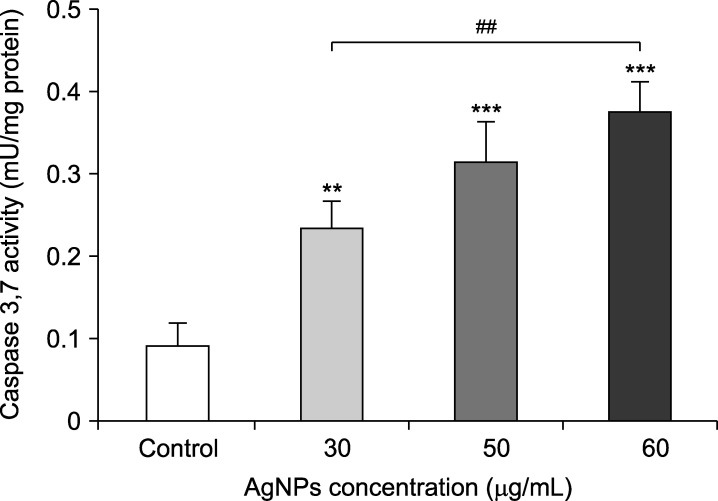
5. AgNPs induce apoptosis through caspase 3,7 activation
The effects of AgNPs on caspase 3,7 activity are demonstrated in Fig. 6. A noticeable increase in caspase 3,7 activity was observed when the bladder cancer 5637 cell line were treated with different concentrations of AgNPs compared to the control cells (p<0.01). Furthermore, the activity level was significantly higher in 60 µg/mL-treated cells compared to those exposed to 30 µg/mL of AgNPs (p<0.01).
FIG. 6. Effects of silver nanoparticles (Ag NPs) on caspase 3,7 activity in bladder cancer 5637 cells. AgNPs could significantly stimulate the activity of caspase 3,7. Results are reported as mean±SD. **p<0.01, ***p<0.001 compared to the control (untreated cells). ##p<0.01 shows significant differences between groups.
DISCUSSION
Typically, cells prevent excessive growth and tumorigenesis by regulating vital processes such as cell growth and proliferation; however, the disruption of cellular processes might lead to cancer development.30 BC is currently known as one of the major causes of morbidity and death, especially in men.3
Since nanotechnology has shown favorable and promising results in cancer therapy, we aimed to explore the anticancer action of AgNPs in bladder cancer 5637 cell line, which was derived from a grade II tumor of bladder. To reach this goal, the apoptotic pathway was evaluated via ROS production, flow cytometry assay, Bax/Bcl-2 genes expression and the activity of caspase 3,7.
The MTT method is usually carried out to estimate the viability of cells in the presence of anticancer agents. The results of the MTT viability assay clearly showed that the rate of viability declined in the 5637 cancer cells exposed to AgNPs which is in full compliance with some other related studies.31,32,33 For instance, Acharya et al.31 reported that eco-friendly AgNPs have anticancer effects on colorectal cancer cells (HCT-116) in which the dose-dependent inhibitory effects were determined by MTT assay with a 50% cytotoxic concentration (CC50) of 48.84±0.78 µg/mL after 24-h. More importantly, Elhawary et al.34 synthesized spherical AgNPs with average size of 9.22 nm using extract of Jasminum ficinale L. and subsequently examined their anticancer potential on 5637 bladder cancer cells. The IC50 of 9.22 nm AgNPs was reported to be 13.09 µg/mL in 5637 cells. It seems that the larger size of AgNPs have less cytotoxicity in 5637 cells as the IC50 was calculated to be 51.12 µg/mL after being exposed to 30 nm AgNPs. In this regard, it is reported that the size of AgNPs plays an important role in their cytotoxic effects. Besides, several studies have reported that smaller AgNPs exert more toxicity within cells compared to larger ones in equivalent concentrations.35
In the current study, the ability of AgNPs to stimulate ROS production in 5637 cells was demonstrated by fluorimetric assay. Consistent with this, Tian et al.33 reported that ROS generation was stimulated in AgNPs-treated A549 lung cancer cells. In fact, ROS formation is a strong hallmark of cancer cells linked with proliferation action. Mechanistically, cells can take up AgNPs through phagocytosis, endocytosis or diffusion and then translocated into the mitochondria, nucleus, and redox-regulating organelles. This process may result in ROS production, known as a principal mechanism of many anticancer drugs. On the other hand, excessive ROS formation leads to mitochondrial dysfunction, membrane destruction, and oxidative stress-induced DNA damage and eventually triggers cell death via apoptosis in many cancerous and non-cancerous cell lines.36,37,38,39
According to the flow cytometry results, AgNPs considerably induce apoptosis in 5637 cells. In related work, Joseph et al.40 observed that AgNPs increase the percentage of apoptotic cells in MCF-7 cell line using Annexin V-FITC/PI apoptosis method and flow cytometry. Furthermore, their results showed that AgNP-treated MCF-7 cells are shifted into the early apoptotic phase after 72 h which is approximately in accordance with the present study results in 24 h. To elucidate the Annexin V-FITC/PI assay in more details Annexin V can basically bind to phosphatidylserine which is located in the outer membrane of apoptotic cells whereas PI usually enters the cells with destroyed membrane integrity.41 Thus, PI and Annexin V-positive cells are regarded as necrotic and apoptotic cells, respectively. Besides, apoptosis could have occurred due to the alterations of apoptotic-related genes’ expression. As such, we evaluated the Bax and Bcl-2 genes expression that effectively contribute to the apoptosis pathway. In connection with apoptosis effects of AgNPs, some reports42,43,44,45 have shown promising anticancer potentials of AgNPs leading to an upregulation of Bax and a reduction in the Bcl-2 gene expression. However, according to our results, it seems that the induced apoptosis in 30 µg/mL-treated cells may not be related to Bax/Bcl-2 alteration. In this regard, we can also hypothesize that some other apoptotic pathways could be involved in AgNPs-mediated cell death. For instance, it was reported that AgNPs induce apoptosis in HCT116 cells via upregulation of Bcl-2 homologous antagonist/killer (Bak) and P53 along with downregulation of Poly [ADP-ribose] polymerase 1 (PARP1) and P21 genes expression.46
In fact, one of the important mechanisms for the resistance of tumor cells against anticancer agents may be related to their inclination to avoid apoptosis. Therefore, there is a substantial demand to propose novel drugs that potentially target apoptosis pathways in cancer cells.17 What is more, it has been well demonstrated that overexpression of Bax and Bak, as two components of the Bcl-2 family, sensitizes cells to apoptosis by permeabilization of outer mitochondrial membrane, resulting in release of proteins into cytosol. Subsequently, caspases enzymes are activated and initiate cell death.47
Our results showed that the AgNPs effectively enhance the activity of caspase 3,7 in 5637 cells.
Moreover, Ullah et al.48 have stated that AgNPs induce apoptosis in MCF-7 breast cancer cells through activation of caspase 3 and 9. In this direction, it was found that caspase family is involved in the mediation of apoptosis in which executive caspases (caspases 3, 6, and 7) can be activated by initiator caspases (caspases 2, 8, 9, and 10) and subsequently promote apoptosis cascade.17,48 All in all, according to the aforementioned arguments, it seems that AgNPs can be suggested as a great candidate for further investigations to eradicate bladder cancer cells.
In conclusion, the viability of bladder cancer 5637 cells was reduced after 24-h incubation with AgNPs. Besides, AgNPs could promote apoptosis via excessive ROS production, caspase 3,7 activation, overexpression of Bax and a remarkable reduction in Bcl-2 expression. Moreover, flow cytometry data verified the capability of AgNPs as an apoptosis inducer in 5637 cells. Despite the considerable efforts of this study to discover the anticancer activity of AgNPs on bladder cancer cells, further research might still be required to realize the mitochondrial-mediated apoptosis pathways.
ACKNOWLEDGEMENTS
The current work has been extracted from a research project at Hamadan University of Medical Sciences, Iran. The present research work was financially supported by Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences (No. 9903271752). Hamadan University of Medical Sciences approved this study by IR.UMSHA.REC.1399.203
Footnotes
CONFLICT OF INTEREST STATEMENT: None declared.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Carradori S, Cristini C, Secci D, Gulia C, Gentile V, Di Pierro GB. Current and emerging strategies in bladder cancer. Anticancer Agents Med Chem. 2012;12:589–603. doi: 10.2174/187152012800617768. [DOI] [PubMed] [Google Scholar]
- 3.Piergentili R, Carradori S, Gulia C, De Monte C, Cristini C, Grande P, et al. Bladder cancer: innovative approaches beyond the diagnosis. Curr Med Chem. 2014;21:2219–2236. doi: 10.2174/0929867321666140304110231. [DOI] [PubMed] [Google Scholar]
- 4.Navya PN, Kaphle A, Srinivas SP, Bhargava SK, Rotello VM, Daima HK. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019;6:23. doi: 10.1186/s40580-019-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg MS. Improving cancer immunotherapy through nanotechnology. Nat Rev Cancer. 2019;19:587–602. doi: 10.1038/s41568-019-0186-9. [DOI] [PubMed] [Google Scholar]
- 6.Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018;9:1410. doi: 10.1038/s41467-018-03705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XH. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano. 2007;1:133–143. doi: 10.1021/nn700048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid G. Nanoparticles: from theory to application. 2nd ed. Hoboken: John Wiley & Sons; 2011. [Google Scholar]
- 9.Evans ER, Bugga P, Asthana V, Drezek R. Metallic nanoparticles for cancer immunotherapy. Mater Today (Kidlington) 2018;21:673–685. doi: 10.1016/j.mattod.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnaraj C, Muthukumaran P, Ramachandran R, Balakumaran MD, Kalaichelvan PT. Acalypha indica Linn: biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol Rep (Amst) 2014;4:42–49. doi: 10.1016/j.btre.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamida RS, Albasher G, Bin-Meferij MM. Oxidative stress and apoptotic responses elicited by Nostoc-synthesized silver nanoparticles against different cancer cell lines. Cancers (Basel) 2020;12:2099. doi: 10.3390/cancers12082099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Zhang B, Chang X, Gan J, Li W, Niu S, et al. Silver nanoparticles modulate mitochondrial dynamics and biogenesis in HepG2 cells. Environ Pollut. 2020;256:113430. doi: 10.1016/j.envpol.2019.113430. [DOI] [PubMed] [Google Scholar]
- 13.Egorova EM, Kaba SI, Kubatiev AA. In: Nanobiomaterials in Cancer Therapy: Applications of Nanobiomaterials. Grumezescu AM, editor. Oxford: Elsevier; 2016. Toxicity of silver nanoparticles obtained by bioreduction as studied on malignant cells: is it possible to create a new generation of anticancer remedies? pp. 505–542. [Google Scholar]
- 14.El-Deeb NM, El-Sherbiny IM, El-Aassara MR, Hafez EE. Novel trend in colon cancer therapy using silver nanoparticles synthesized by honey bee. J Nanomed Nanotechnol. 2015;6:265 [Google Scholar]
- 15.Lim HK, Gurung RL, Hande MP. DNA-dependent protein kinase modulates the anti-cancer properties of silver nanoparticles in human cancer cells. Mutat Res Genet Toxicol Environ Mutagen. 2017;824:32–41. doi: 10.1016/j.mrgentox.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Wu LY, Yang WX. Nanoparticles induce apoptosis via mediating diverse cellular pathways. Nanomedicine (Lond) 2018;13:2939–2955. doi: 10.2217/nnm-2018-0167. [DOI] [PubMed] [Google Scholar]
- 17.Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17:395–417. doi: 10.1038/s41571-020-0341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YS, Kim DW, Lee YH, Oh JH, Yoon S, Choi MS, et al. Silver nanoparticles induce apoptosis and G2/M arrest via PKCζ-dependent signaling in A549 lung cells. Arch Toxicol. 2011;85:1529–1540. doi: 10.1007/s00204-011-0714-1. [DOI] [PubMed] [Google Scholar]
- 19.Sarkar A, Das J, Manna P, Sil PC. Nano-copper induces oxidative stress and apoptosis in kidney via both extrinsic and intrinsic pathways. Toxicology. 2011;290:208–217. doi: 10.1016/j.tox.2011.09.086. [DOI] [PubMed] [Google Scholar]
- 20.Pérez-Hernández M, Del Pino P, Mitchell SG, Moros M, Stepien G, Pelaz B, et al. Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold nanoprisms. ACS Nano. 2015;9:52–61. doi: 10.1021/nn505468v. [DOI] [PubMed] [Google Scholar]
- 21.Daei S, Ziamajidi N, Abbasalipourkabir R, Khanaki K, Bahreini F. Anticancer effects of gold nanoparticles by inducing apoptosis in bladder cancer 5637 cells. Biol Trace Elem Res. 2022;200:2673–2683. doi: 10.1007/s12011-021-02895-9. [DOI] [PubMed] [Google Scholar]
- 22.Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch Toxicol. 2015;89:289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- 23.Sharma V, Anderson D, Dhawan A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2) Apoptosis. 2012;17:852–870. doi: 10.1007/s10495-012-0705-6. [DOI] [PubMed] [Google Scholar]
- 24.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmadian E, Dizaj SM, Rahimpour E, Hasanzadeh A, Eftekhari A, Hosain Zadegan H, et al. Effect of silver nanoparticles in the induction of apoptosis on human hepatocellular carcinoma (HepG2) cell line. Mater Sci Eng C Mater Biol Appl. 2018;93:465–471. doi: 10.1016/j.msec.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Wu T, Duan X, Hu C, Wu C, Chen X, Huang J, et al. Synthesis and characterization of gold nanoparticles from Abies spectabilis extract and its anticancer activity on bladder cancer T24 cells. Artif Cells Nanomed Biotechnol. 2019;47:512–523. doi: 10.1080/21691401.2018.1560305. [DOI] [PubMed] [Google Scholar]
- 27.Baghbani-Arani F, Movagharnia R, Sharifian A, Salehi S, Shandiz SAS. Photo-catalytic, anti-bacterial, and anti-cancer properties of phyto-mediated synthesis of silver nanoparticles from Artemisia tournefortiana Rchb extract. J Photochem Photobiol B. 2017;173:640–649. doi: 10.1016/j.jphotobiol.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Yezhelyev MV, Gao X, Xing Y, Al-Hajj A, Nie S, O’Regan RM. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 2006;7:657–667. doi: 10.1016/S1470-2045(06)70793-8. [DOI] [PubMed] [Google Scholar]
- 31.Acharya D, Satapathy S, Somu P, Parida UK, Mishra G. Apoptotic effect and anticancer activity of biosynthesized silver nanoparticles from marine algae Chaetomorpha linum extract against human colon cancer cell HCT-116. Biol Trace Elem Res. 2021;199:1812–1822. doi: 10.1007/s12011-020-02304-7. [DOI] [PubMed] [Google Scholar]
- 32.Dou Y, Tu F, Wu Y, Wang X, Lu G, Zhao L. Facile preparation of Kaolin supported silver nanoparticles mediated by Thymbra spicata extract and investigation of the anti-human lung cancer properties. J Saudi Chem Soc. 2021;25:101303 [Google Scholar]
- 33.Tian S, Saravanan K, Mothana RA, Ramachandran G, Rajivgandhi G, Manoharan N. Anti-cancer activity of biosynthesized silver nanoparticles using Avicennia marina against A549 lung cancer cells through ROS/mitochondrial damages. Saudi J Biol Sci. 2020;27:3018–3024. doi: 10.1016/j.sjbs.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elhawary S, El-Hefnawy H, Mokhtar FA, Sobeh M, Mostafa E, Osman S, et al. Green synthesis of silver nanoparticles using extract of Jasminum officinal L. leaves and evaluation of cytotoxic activity towards bladder (5637) and breast cancer (MCF-7) cell lines. Int J Nanomedicine. 2020;15:9771–9781. doi: 10.2147/IJN.S269880. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Skalska J, Strużyńska L. Toxic effects of silver nanoparticles in mammals--does a risk of neurotoxicity exist? Folia Neuropathol. 2015;53:281–300. doi: 10.5114/fn.2015.56543. [DOI] [PubMed] [Google Scholar]
- 36.Arora S, Jain J, Rajwade JM, Paknikar KM. Cellular responses induced by silver nanoparticles: In vitro studies. Toxicol Lett. 2008;179:93–100. doi: 10.1016/j.toxlet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Piao MJ, Kang KA, Lee IK, Kim HS, Kim S, Choi JY, et al. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol Lett. 2011;201:92–100. doi: 10.1016/j.toxlet.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Foldbjerg R, Dang DA, Autrup H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol. 2011;85:743–750. doi: 10.1007/s00204-010-0545-5. [DOI] [PubMed] [Google Scholar]
- 39.Chairuangkitti P, Lawanprasert S, Roytrakul S, Aueviriyavit S, Phummiratch D, Kulthong K, et al. Silver nanoparticles induce toxicity in A549 cells via ROS-dependent and ROS-independent pathways. Toxicol In Vitro. 2013;27:330–338. doi: 10.1016/j.tiv.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 40.Joseph J, Khor KZ, Moses EJ, Lim V, Aziz MY, Abdul Samad N. In vitro anticancer effects of Vernonia amygdalina leaf extract and green-synthesised silver nanoparticles. Int J Nanomedicine. 2021;16:3599–3612. doi: 10.2147/IJN.S303921. Erratum in: Int J Nanomedicine 2021;16:6263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yedjou CG, Izevbigie EB, Tchounwou PB. Vernonia amygdalina-induced growth arrest and apoptosis of breast cancer (MCF-7) cells. Pharmacol Pharm. 2013;4:10.4236/pp.2013.41013. doi: 10.4236/pp.2013.41013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Nuairi AG, Mosa KA, Mohammad MG, El-Keblawy A, Soliman S, Alawadhi H. Biosynthesis, characterization, and evaluation of the cytotoxic effects of biologically synthesized silver nanoparticles from Cyperus conglomeratus root extracts on breast cancer cell line MCF-7. Biol Trace Elem Res. 2020;194:560–569. doi: 10.1007/s12011-019-01791-7. [DOI] [PubMed] [Google Scholar]
- 43.Mousavi B, Tafvizi F, Zaker Bostanabad S. Green synthesis of silver nanoparticles using Artemisia turcomanica leaf extract and the study of anti-cancer effect and apoptosis induction on gastric cancer cell line (AGS) Artif Cells Nanomed Biotechnol. 2018;46(1):499–510. doi: 10.1080/21691401.2018.1430697. [DOI] [PubMed] [Google Scholar]
- 44.Aslany S, Tafvizi F, Naseh V. Characterization and evaluation of cytotoxic and apoptotic effects of green synthesis of silver nanoparticles using Artemisia Ciniformis on human gastric adenocarcinoma. Mater Today Commun. 2020;24:101011 [Google Scholar]
- 45.Ramar M, Manikandan B, Marimuthu PN, Raman T, Mahalingam A, Subramanian P, et al. Synthesis of silver nanoparticles using Solanum trilobatum fruits extract and its antibacterial, cytotoxic activity against human breast cancer cell line MCF 7. Spectrochim Acta A Mol Biomol Spectrosc. 2015;140:223–228. doi: 10.1016/j.saa.2014.12.060. [DOI] [PubMed] [Google Scholar]
- 46.Narasimha VR, Latha TS, Pallu R, Panati K, Narala VR. Anticancer activities of biogenic silver nanoparticles targeting apoptosis and inflammatory pathways in colon cancer cells. J Clust Sci. 2022;33:2215–2231. [Google Scholar]
- 47.Edlich F. BCL-2 proteins and apoptosis: recent insights and unknowns. Biochem Biophys Res Commun. 2018;500:26–34. doi: 10.1016/j.bbrc.2017.06.190. [DOI] [PubMed] [Google Scholar]
- 48.Ullah I, Khalil AT, Ali M, Iqbal J, Ali W, Alarifi S, et al. Green-synthesized silver nanoparticles induced apoptotic cell death in MCF-7 breast cancer cells by generating reactive oxygen species and activating caspase 3 and 9 enzyme activities. Oxid Med Cell Longev. 2020;2020:1215395. doi: 10.1155/2020/1215395. [DOI] [PMC free article] [PubMed] [Google Scholar]