Abstract
Background
Early-life respiratory tract infections might affect chronic obstructive respiratory diseases, but conclusive studies from general populations are lacking. Our objective was to examine if children with early-life respiratory tract infections had increased risks of lower lung function and asthma at school age.
Methods
We used individual participant data of 150 090 children primarily from the EU Child Cohort Network to examine the associations of upper and lower respiratory tract infections from age 6 months to 5 years with forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), FEV1/FVC, forced expiratory flow at 75% of FVC (FEF75%) and asthma at a median (range) age of 7 (4–15) years.
Results
Children with early-life lower, not upper, respiratory tract infections had a lower school-age FEV1, FEV1/FVC and FEF75% (z-score range: −0.09 (95% CI −0.14– −0.04) to −0.30 (95% CI −0.36– −0.24)). Children with early-life lower respiratory tract infections had a higher increased risk of school-age asthma than those with upper respiratory tract infections (OR range: 2.10 (95% CI 1.98–2.22) to 6.30 (95% CI 5.64–7.04) and 1.25 (95% CI 1.18–1.32) to 1.55 (95% CI 1.47–1.65), respectively). Adjustment for preceding respiratory tract infections slightly decreased the strength of the effects. Observed associations were similar for those with and without early-life wheezing as a proxy for early-life asthma.
Conclusions
Our findings suggest that early-life respiratory tract infections affect development of chronic obstructive respiratory diseases in later life, with the strongest effects for lower respiratory tract infections.
Short abstract
This meta-analysis of 150 000 children suggests that mostly lower respiratory tract infections are associated with an increased risk of asthma and lower lung function. This is independent from preceding respiratory tract infections or early-life asthma. https://bit.ly/3weE62I
Introduction
Respiratory tract infections are common in early life [1, 2]. An accumulating body of evidence suggests that early-life respiratory tract infections have short-term consequences, but also affect the development of both the respiratory and immune systems [3–6]. Thus, early-life respiratory infections may predispose individuals to chronic respiratory diseases such as asthma in later life.
Previous individual observational studies have shown inconsistent findings on the associations of respiratory tract infections in early life with the risk of wheezing or asthma in later life, which ranges from a 1.5- to 10-fold increased risk [7–13]. Relatively few observational studies focused on lung function as an outcome, which showed that early-life respiratory tract infections were associated with a lower lung function in childhood or adulthood [14–18]. Most studies considered only severe respiratory infections, e.g. requiring hospitalisation, or specific pathogens found in nasal lavage fluids or other biological samples. This, however, might reflect a subset of infections only, which is not representative of mostly less severe upper and lower respiratory tract infections in the general population. Studying the associations of early-life upper and lower respiratory tract infections separately with lung function and asthma using individual participant data from the general European population allows better harmonisation of the data, usage of the same set of confounders and more powerful analyses compared with these separate studies with different definitions of respiratory tract infections and respiratory outcomes, measured at different ages and often with limited power. We hypothesised that mostly lower respiratory tract infections in early life would be associated with lower lung function and an increased risk of asthma.
Therefore, we conducted an individual participant data meta-analysis among 150 090 children from 38 European birth cohorts to examine the associations of early-life upper and lower respiratory tract infections with lung function and asthma at school age.
Methods
General design
We identified 53 European pregnancy and birth cohorts from the EU Child Cohort Network (www.lifecycle-project.eu) and a birth cohort registry (www.birthcohorts.net) [19]. Inclusion criteria were cohorts that had included children born between 1989 and 2013, had available data on early-life respiratory tract infections and childhood lung function and/or asthma, had approval for the study from local institutional review boards, and gave written informed consent for using their data and the possibility to exchange original data. Of the invited cohorts, some did not respond (n=3), were unable to participate due to lack of data (n=10) or had other reasons for nonparticipation (n=2), leading to a total of 38 cohorts (24 from the EU Child Cohort Network) with 150 090 mother–child pairs for the current analyses (supplementary figure S1). Cohorts shared original data, and data harmonisation and analysis was performed within the lead institute (Generation R Study Group, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands).
Early-life respiratory tract infections
Information on respiratory tract infections was obtained at the ages of 6 months, 1, 2, 3, 4 and 5 years, and reflected any upper or lower respiratory tract infection in the last 6 or 12 months. For most cohorts (74% (n=110 067)), data on respiratory tract infections were obtained by questionnaires (supplementary table S1). Other methods to obtain information on respiratory tract infections included the use of registry data or interviews. Upper respiratory tract infections included croup, whooping cough, ear infection, throat infection, rhinitis and cold. Lower respiratory tract infections included bronchitis, bronchiolitis, pneumonia and chest infections. Infections were preferably doctor-diagnosed in order to limit the possibility that symptoms of asthma were misdiagnosed as infections or due to allergy. Early-life respiratory tract infections were categorised into upper respiratory tract infections (no/yes) and lower respiratory tract infections (no/yes).
School-age lung function and asthma
The main respiratory outcomes used were lung function and asthma (median (range) age 7 (4–15) years). Lung function was measured by spirometry, and comprised forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), FEV1/FVC and forced expiratory flow at 75% of FVC (FEF75%). All cohorts performed spirometry according to American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines. Cohorts provided absolute values of all lung function measurements, and these were subsequently converted into sex-, age-, height- and ethnicity-adjusted z-scores based on the Global Lungs Initiative reference values by the primary data analyst [20]. Asthma was defined as ever doctor diagnosis of asthma (no/yes) diagnosed at or after age 5 years, which was preferably obtained by questionnaire (40% (n=60 036)) through questions adapted from the International Study on Asthma and Allergy in Childhood (ISAAC) [21]. Other methods to obtain information on asthma were healthcare registry data, interviews and symptom diary or report. If cohorts had data on lung function or asthma measured at multiple time-points, we only used data from the age closest to the median age of all cohorts (7 years) in the full meta-analysis. If cohorts had both lung function and asthma data available (16% (n=23 955)), we used data obtained at concomitant ages.
Covariates
Information on socioeconomic, lifestyle and growth-related factors was mostly obtained by questionnaire, with diaries or registry data as other methods of data ascertainment (supplementary table S1). Covariates were selected from the literature and were visualised by means of a directed acyclic graph. The final set of confounders included maternal age, education, ethnicity, parity, smoking during pregnancy, history of asthma or atopy and pet keeping, and child's sex, gestational age at birth, birthweight, season of birth, breastfeeding and daycare attendance. We obtained information on early-life wheezing by questions adapted from the ISAAC on wheezing in the past 12 months at the ages of 1, 2, 3 and 4 years [21]. As asthma is difficult to diagnose at young ages and early-life wheezing is a strong predictor of later asthma development, we used wheezing as a proxy for early-life asthma to assess whether the associations between early-life respiratory tract infections and school-age lung function and asthma differed between those with and without early-life wheezing.
Statistical analyses
We conducted a one-stage random effects meta-analysis to study the associations of any upper and lower respiratory tract infections in early life with lung function and asthma at school age. For this analysis, individual participant data from all cohorts were combined in one analysis and were modelled simultaneously taking into account the clustering of participants within studies by using a random intercept at cohort level. With this, potential differences in cohorts and geographical regions were taken into account. First, we studied any upper and lower respiratory tract infections at all different ages separately, using linear regression models for lung function and logistic regression models for asthma as the outcome. Our first model was unadjusted, our second model was adjusted for socioeconomic, lifestyle and growth-related factors based on their known associations with lung function and asthma from literature, and a third model was additionally adjusted for preceding upper or lower respiratory tract infections, as appropriate, to minimise bias due to vulnerability to these infections. We considered the second model (confounder model) as our main model.
As a sensitivity analysis, we conducted a two-stage random effects meta-analysis to study the associations of early-life respiratory tract infections with the main lung function outcome FEV1/FVC and asthma (no/yes). For this analysis, we used linear and logistic regression models per cohort, after which pooled regression coefficients (β-values) from the per-cohort effect estimates were calculated. We tested for heterogeneity between effect estimates by using I2-values [22].
We performed additional analyses on the main models of our one-stage random effects meta-analysis. We additionally stratified for early-life wheezing to examine whether associations of early-life respiratory tract infections with lung function and asthma were different among children with and without symptoms of early-life wheezing. Also, to assess differences in results related to trajectories of post-natal lung growth, we repeated our analyses in strata of children aged <9 and ≥9 years at the time of outcome assessment. This cut-off was based on both data availability and age of change in FEV1/FVC trajectories [23]. We performed sensitivity analyses by applying a complete case analysis to explore any differences between complete and noncomplete case analyses, excluding cohorts that used parental report of asthma not according to the ISAAC, excluding cohorts that used other methods to assess respiratory tract infections rather than questionnaire of parental report, or that comprised a large number of participants (>5% of the total), and two cohorts that assessed lung function at age 4 years because reliable and valid measurements of lung function below the age of 4 years in population-based cohorts is difficult. For all analyses, missing values in covariates were used as an additional group in the categorical variables to prevent exclusion of noncomplete cases. Measures of association were z-score differences or odds ratios presented with their 95% confidence intervals. Analyses were performed with SPSS version 25.0 for Windows (IBM, Armonk, NY, USA) and RevMan version 5.3 (Nordic Cochrane Centre, Copenhagen, Denmark).
Results
Participant characteristics
The characteristics of asthma, lung function and respiratory tract infections are shown in tables 1 and 2, and supplementary table S2. The prevalence of upper and lower respiratory tract infections was highest at the age of 1 year (mean 62.9% and 23.0%, respectively) and thereafter decreased until the age of 5 years (42.6% and 15.0%, respectively) (table 2). The mean prevalence of asthma across all cohorts was 12.3%. Characteristics of covariates can be found in supplementary table S3.
TABLE 1.
Characteristics of asthma and lung function in participating cohorts
| Cohort name (country) | Age at outcome, years | Participants, n | Asthma, % (n) | FEV1, z-score | FVC, z-score | FEV1/FVC, z-score | FEF75%, z-score |
| ABIS (Sweden) | 5 | 12 618 | 4.6 (578) | NA | NA | NA | NA |
| ALSPAC (UK) | 8 | 8376 | 21.7 (1605) | −0.34±1.01 | −0.50±1.02 | 0.42±1.07 | NA |
| BAMSE (Sweden) | 8 | 3402 | 12.4 (420) | 0.46±0.95 | 0.65±0.93 | −0.36±0.89 | NA |
| BiB (UK) | 5 | 2674 | 8.3 (223) | NA | NA | NA | NA |
| BILD (Switzerland) | 6 | 254 | 5.6 (14) | −0.00±0.95 | −0.19±0.97 | 0.41±0.97 | NA |
| CoNER (Italy) | 8 | 214 | 6.1 (13) | −1.02±0.87 | 1.73±0.80 | 1.80±0.50 | NA |
| COPSAC 2000 (Denmark) | 7 | 290 | 19.7 (57) | −0.26±1.09 | −0.58±1.06 | 0.78±1.17 | 2.01±1.14 |
| COPSAC 2010 (Denmark) | 5 | 550 | 22.4 (123) | −0.11±1.00 | −0.18±1.00 | 0.17±0.98 | 1.53±0.92 |
| DNBC (Denmark) | 7 | 34 437 | 15.2 (5250) | NA | NA | NA | NA |
| EDEN (France) | 6 | 900 | 18.6 (167) | −1.3±1.65 | −1.63±1.65 | 0.87±1.12 | 1.33±1.93 |
| FLEHS (Belgium) | 10 | 110 | 7.3 (8) | NA | NA | NA | NA |
| GASPII (Italy) | 9 | 464 | 13.1 (61) | −0.01±0.88 | 0.05±0.76 | −0.15±0.97 | NA |
| Generation R (Netherlands) | 10 | 5441 | 9.3 (436) | 0.15±0.98 | 0.19±0.93 | −0.11±0.96 | 0.02±0.92 |
| Generation XXI (Portugal) | 7 | 5485 | 6.1 (331) | 0.56±0.96 | 0.38±0.94 | 0.29±0.89 | 1.39±1.93 |
| GINI (Germany) | 15 | 1965 | 12.9 (217) | −0.58±0.92 | −0.53±0.90 | −0.11±1.00 | −0.13±0.95 |
| HUMIS (Norway) | 9 | 2384 | 5.3 (127) | NA | NA | NA | NA |
| INMA Gipuzkoa (Spain) | 4 | 277 | NA | −0.60±1.15 | −0.54±1.15 | −0.05±0.91 | −0.16±1.00 |
| INMA Menorca (Spain) | 12 | 422 | 6.4 (27) | −0.16±1.07 | 0.01±1.13 | −0.24±1.19 | −0.06±1.13 |
| INMA Sabadell (Spain) | 4 | 406 | NA | −0.57±1.30 | −0.48±1.37 | −0.08±1.03 | −0.25±1.13 |
| INMA Valencia (Spain) | 8 | 455 | NA | 0.30±1.08 | 0.30±1.10 | −0.04±0.95 | 0.04±0.90 |
| Isle of Wight (UK) | 10 | 1327 | 19.9 (264) | NA | NA | NA | NA |
| KOALA (Netherlands) | 7 | 1875 | 7.6 (141) | −0.13±0.95 | 0.16±0.94 | −0.55±0.84 | NA |
| LRC (UK) | 12 | 3978 | 20.3 (809) | −0.11±1.17 | −0.16±1.09 | 0.23±1.05 | 0.20±0.98 |
| Lifeways Cross-Generation Cohort Study (Ireland) | 9 | 138 | 6.5 (9) | NA | NA | NA | NA |
| LISA (Germany) | 15 | 941 | 9.7 (77) | −0.50±0.93 | −0.44±0.97 | −0.12±0.98 | −0.12±0.90 |
| LucKi (Netherlands) | 6 | 337 | 15.4 (52) | NA | NA | NA | NA |
| LUKAS (Finland) | 6 | 374 | 9.9 (37) | −0.08±1.09 | 0.30±1.00 | −0.73±0.84 | −0.48±1.01 |
| MAS-90 (Germany) | 7 | 826 | 6.6 (44) | 0.28±1.09 | 0.06±0.91 | 0.41±1.00 | NA |
| Millennium Cohort Study (UK) | 11 | 14 917 | 15.3 (2284) | NA | NA | NA | NA |
| MoBa (Norway) | 7 | 34 542 | 10.6 (3677) | NA | NA | NA | NA |
| NINFEA (Italy) | 7 | 1072 | 3.0 (32) | NA | NA | NA | NA |
| PELAGIE (France) | 6 | 941 | 11.3 (106) | NA | NA | NA | NA |
| PIAMA (Netherlands) | 11 | 2810 | 11.3 (299) | 0.52±0.92 | 0.37±0.87 | 0.21±1.01 | NA |
| REPRO_PL (Poland) | 7 | 106 | 2.1 (2) | 0.33±1.20 | 0.23±1.16 | 0.18±1.15 | 2.22±1.05 |
| Rhea (Greece) | 7 | 596 | 9.3 (55) | −0.01±1.16 | 0.18±1.18 | −0.33±1.03 | −0.22±1.06 |
| STEPS (Finland) | 5 | 713 | 8.3 (59) | NA | NA | NA | NA |
| SWS (UK) | 6 | 2033 | 14.1 (287) | 0.02±0.96 | −0.12±1.03 | −0.14±1.08 | NA |
| WHISTLER (Netherlands) | 5 | 1438 | 8.1 (116) | 0.43±1.06 | −0.38±1.00 | 1.71±0.87 | 1.99±0.79 |
| Total | Median 7 | 150 090 | 12.3 (18 007) | −0.02±1.10 | −0.03±1.11 | 0.03±1.07 | 0.35±1.37 |
z-scores for lung function measurements are presented as mean±sd. NA: not available.
TABLE 2.
Prevalence of upper and lower respiratory tract infections among children
| Prevalence, % (n) | |
| Upper respiratory tract infections | |
| 6 months | 41.2 (36 564) |
| 1 year | 62.9 (58 949) |
| 2 years | 46.0 (27 119) |
| 3 years | 47.7 (35 641) |
| 4 years | 42.8 (11 159) |
| 5 years | 42.6 (19 424) |
| Lower respiratory tract infections | |
| 6 months | 6.7 (3587) |
| 1 year | 23.0 (13 297) |
| 2 years | 16.0 (9045) |
| 3 years | 16.0 (11 117) |
| 4 years | 11.8 (2354) |
| 5 years | 15.0 (5783) |
Respiratory tract infections and lung function
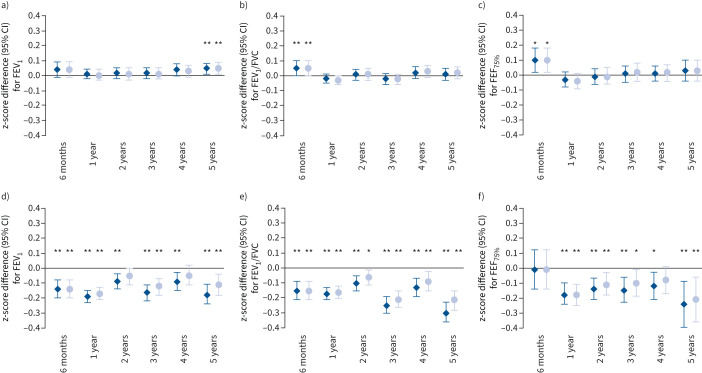
Unadjusted associations of upper and lower respiratory tract infections with lung function are provided in supplementary table S4. After adjustment for socioeconomic, lifestyle and growth-related factors, only upper respiratory tract infections at the age of 6 months were associated with a higher FEV1/FVC and FEF75% (z-score difference: 0.05 (95% CI 0.00–0.10) and 0.10 (95% CI 0.03–0.19), respectively), and upper respiratory tract infections at the age of 5 years with a higher FEV1 (z-score difference: 0.04 (95% CI 0.00–0.08)) (figure 1 and supplementary table S5). After additional adjustment for preceding upper respiratory tract infections, the direction and size of the effect estimates remained similar (figure 1 and supplementary table S6). Lower respiratory tract infections at all ages were associated with a lower FEV1 and FEV1/FVC (z-score difference range: −0.09 (95% CI −0.14– −0.04) to −0.30 (95% CI −0.36– −0.23)) (figure 1 and supplementary table S5). Only lower respiratory tract infections at age 1 year were associated with a lower FVC (z-score difference: −0.08 (95% CI −0.12– −0.04)). Additionally, lower respiratory tract infections at all ages, except at the age of 6 months, were associated with a lower FEF75% (z-score difference range: −0.12 (95% CI −0.21– −0.03) to −0.24 (95% CI −0.39– −0.09)). After additional adjustment for preceding lower respiratory tract infections, the direction of the effect estimates remained, but the sizes attenuated (z-score difference range: −0.08 (95% CI −0.12– −0.04) to −0.21 (95% CI −0.36– −0.06)) (figure 1 and supplementary table S6).
FIGURE 1.
Associations of early-life a–c) upper and d–f) lower respiratory tract infections with school age: a, d) forced expiratory volume in 1 s (FEV1), b, e) FEV1/forced vital capacity (FVC) and c, f) forced expiratory flow at 75% of FVC (FEF75%). Data are presented as change in z-score (95% confidence interval), derived from multilevel linear regression models. The dark blue diamonds represent models adjusted for maternal history of asthma and atopy, ethnicity, education level, smoking during pregnancy, parity and pet keeping, and child's sex, gestational age at birth, birthweight, season of birth, breastfeeding, and daycare attendance. The light blue circles represent models additionally adjusted for preceding a–c) upper or d–f) lower respiratory tract infections. *: p<0.05; **: p<0.01.
Respiratory tract infection and asthma
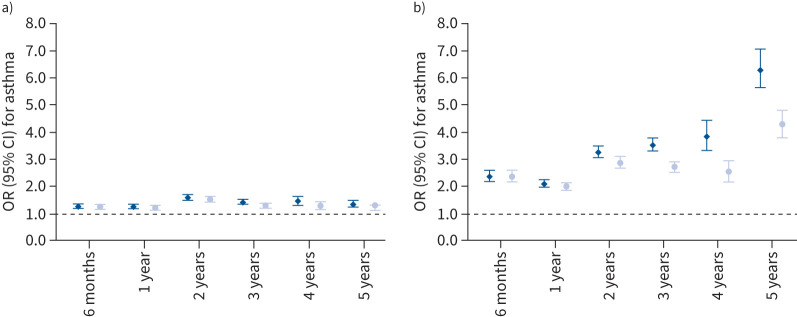
Unadjusted associations of upper and lower respiratory tract infections with asthma are provided in supplementary table S4. Upper respiratory tract infections at all ages were associated with an increased risk of asthma (OR range: 1.25 (95% CI 1.18–1.32) to 1.57 (95% CI 1.48–1.67)) (figure 2 and supplementary table S5). Also, lower respiratory tract infections at all ages were associated with an increased risk of asthma (OR range: 2.10 (95% CI 1.98–2.22) to 6.30 (95% CI 5.64–7.04)). After additional adjustment for preceding upper or lower respiratory tract infections (as appropriate), the effect estimates slightly attenuated and this decreasing effect was stronger with increasing age (figure 2 and supplementary table S6).
FIGURE 2.
Associations of early-life a) upper and b) lower respiratory tract infections with school-age asthma. Data are presented as odds ratio (95% confidence interval), derived from multilevel logistic regression models. The dark blue diamonds represent models adjusted for maternal history of asthma and atopy, ethnicity, education level, smoking during pregnancy, parity and pet keeping, and child's sex, gestational age at birth, birthweight, season of birth, breastfeeding and daycare attendance. The light blue circles represent models additionally adjusted for preceding a) upper or b) lower respiratory tract infections. p-values all <0.01.
Additional and sensitivity analyses
The two-stage random effect meta-analyses using combined effects showed similar magnitude and strength of effects as the one-stage random effects meta-analysis, with low to moderate heterogeneity (I2 range: 0–72%) (supplementary table S8). The associations of upper and lower respiratory tract infections with lung function and asthma did not materially differ for those without and with early-life wheezing at the same age as the respiratory tract infection or for children aged <9 and ≥9 years (supplementary table S7, table 3 and supplementary table S9, respectively). Results did not materially change when we restricted our analyses to cohorts that used ISAAC-based questionnaires of asthma, that used parental report of respiratory tract infections with questionnaire, complete cases (supplementary table S9), when leaving out one cohort at a time with a large number of participants (supplementary table S10) or when leaving out the two cohorts that assessed lung function at age 4 years (data not shown).
TABLE 3.
Associations of any early-life upper and lower respiratory tract infections with school-age asthma, stratified for early-life wheezing
| Asthma, no early-life wheezing OR (95% CI) | Asthma, early-life wheezing OR (95% CI) | |
| Upper respiratory tract infections | ||
| 6 months | 1.11 (1.03–1.21)** | 1.03 (0.87–1.22) |
| 1 year | 1.19 (1.08–1.32)** | 1.22 (1.06–1.41)** |
| 2 years | 1.20 (1.04–1.37)* | 1.14 (0.95–1.37) |
| 3 years | 1.17 (1.06–1.30)** | 1.00 (0.86–1.16) |
| 4 years | 1.19 (1.01–1.41)* | 1.01 (0.86–1.19) |
| Lower respiratory tract infections | ||
| 6 months | 2.09 (1.45–3.01)** | 1.40 (1.18–1.66)** |
| 1 year | 2.28 (1.97–2.66)** | 1.87 (1.63–2.13)** |
| 2 years | 2.25 (1.89–2.68)** | 1.87 (1.59–2.20)** |
| 3 years | 2.67 (2.12–3.35)** | 1.43 (1.21–1.69)** |
| 4 years | 2.54 (1.98–3.28)** | 1.45 (1.17–1.80)** |
Data are presented as odds ratio with 95% confidence interval, derived from multilevel logistic regression models. Models are adjusted for maternal history of asthma and atopy, ethnicity, education level, smoking during pregnancy, parity and pet keeping, and child's sex, gestational age at birth, birthweight, season of birth, breastfeeding and daycare attendance. Early-life wheezing reflects wheezing at the same age as upper or lower respiratory tract infections. *: p<0.05; **: p<0.01.
Discussion
Our results from an individual participant meta-analysis among 150 090 participants from 38 cohorts across Europe demonstrate that 1) early-life upper respiratory tract infections were associated with an increased risk of school-age asthma, not lung function, and 2) early-life lower respiratory tract infections were associated with increased risks of both school-age lower lung function (FEV1, FEV1/FVC and FEF75%) and asthma. The effect sizes for the associations of lower respiratory tract infections with asthma were much larger than those for the association of upper respiratory tract infections with asthma. The strength of the effects slightly decreased when adjusting for preceding respiratory tract infections. Results were not modified by wheezing in early-life, suggesting that these associations could in part be present irrespective of possible early-life susceptibility to asthma.
Comparison with previous studies
We showed that mostly early-life lower respiratory tract infections were associated with increased risks of school-age lower lung function and asthma, both before and after age 9 years. The results are in line with a meta-analysis of 15 studies demonstrating that rhinovirus wheezing illness in the first 3 years of life is associated with a 2-fold increased risk of asthma or wheezing at older childhood ages [24]. These findings were present both before and after the childhood age of 10 years. The large majority of studies have assessed specific pathogens of the respiratory infections, mostly rhinovirus or respiratory syncytial virus in relation to later-life chronic respiratory diseases. Relatively few cohort studies focused on respiratory infections such as pneumonia or bronchiolitis. A birth cohort showed that lower respiratory tract infections were associated with an increased risk of asthma at age 7 years, while repeated upper respiratory tract infections in the first year of life were associated with a decreased risk [25]. One study demonstrated that pneumonia in childhood was associated with a lower FEV1/FVC at age 7 years, but only in those with current asthma [26]. Another study demonstrated that severe bronchiolitis during infancy was associated with a 2.5-fold increased risk of asthma at age 5 years [13]. Studies assessing the association of early-life respiratory tract infections with lung function in later life are scarce. A systematic review showed that respiratory infections until age 3 years are associated with a lower FEV1 % pred at the age of 7.5–20 years [27]. The novelty of our study is that it adds to these findings by demonstrating that in the general European population, early-life lower respiratory tract infections, including bronchitis, bronchiolitis, pneumonia and chest infection, are associated with not only lower FEV1 but also lower FEV1/FVC and FEF75%, and an increased risk of asthma, which could have persistent and profound effects on later-life respiratory function and health. The use of harmonised data and the same set of confounders, and diagnoses of respiratory tract infections in the general population as opposed to specific pathogens in hospital-based populations, leads to better generalisability of the results.
Possible mechanisms
In this study, we found that both upper and lower respiratory tract infections are associated with an increased risk of asthma, while only lower respiratory tract infections are associated with lower lung function. The effect sizes for the associations of upper respiratory tract infections with asthma were smaller than the effect sizes for the association of lower respiratory tract infections with asthma; upper respiratory tract infections were not associated with lower lung function. Although the effect sizes for the associations of upper respiratory tract infections with asthma remained when additionally adjusted for concomitant lower respiratory tract infections (data not shown), we cannot fully rule out that this observed association is due to misclassifications of infections or concomitant infections. We consider the observed associations of upper respiratory tract infections at age 6 months with higher FEV1/FVC and FEF75% most likely as chance findings rather than biologically true observations. Both the immune and respiratory systems are still developing in the first years of life, and any disturbance in this development could be associated with adverse respiratory health in later life [28–31]. It is likely that both upper and lower respiratory tract infections have an effect on the immune system through adapted T-helper 2 and regulatory T-cell responses, which could subsequently lead to an increased risk of asthma [32]. Additionally, lower respiratory tract infections might have a more direct effect on the lungs through disruption of normal lung development and growth, specifically in the smaller airways. This could in turn lead to a lower lung function, predominantly airway obstruction and airflow limitation. This is in line with the findings that lower respiratory tract infections have an adverse effect on FEV1, FEV1/FVC and FEF75%, but not FVC. Some have suggested that the association of early-life respiratory tract infections with lung function and asthma might be explained by a pre-existing underlying predisposition [27, 33]. We demonstrated that the association of respiratory tract infections with lung function and asthma does not differ between those with and without concomitant wheezing. This suggests that asthma susceptibility does not modify these associations, although we cannot fully rule out overlap of respiratory symptoms due to respiratory tract infections and asthma if both are present. This is supported by a cohort study demonstrating that lower respiratory tract infections in infancy are associated with a lower lung function at age 1 year, irrespective of lung function at age 6 weeks [16]. In line with the Developmental Origins of Health and Disease hypothesis, studies have suggested that the effect of respiratory tract infections in early life on respiratory health carries on until adulthood [34–36]. Additionally, lung function trajectories, either obstructive or restrictive phenotypes, are shown to persist into adolescence and adulthood [37]. Whether early-life risk factors, altered lung function and diagnosis of asthma in childhood either separately or combined lead to adverse respiratory health such as asthma or chronic obstructive pulmonary disease in adulthood needs to be carefully elucidated. Last, our results could potentially be explained by reverse causation. This suggests that those with lower lung function or asthma in early life have an increased risk of respiratory infections in later life. To minimise this reversed effect, we additionally adjusted for preceding respiratory tract infections, but lacked appropriate statistical methods to fully rule this out on a meta-analysis-based level.
Strengths and limitations
The main strengths of this study include the use of a large dataset with individual participant data from across Europe, with harmonised data and the same set of confounders. The large majority of cohorts used ISAAC-based questionnaires commonly used in epidemiological studies for asthma diagnosis rather than providing medication, with potential side-effects for measuring lung function reversibility for relatively healthy subjects of population-based cohorts, and ATS/ERS criteria for spirometry, leading to homogeneity of data ascertainment. Last, we used various statistical methods and sensitivity analyses to test the robustness of the results. However, some limitations do apply. First, lung function measurements were available in ∼17% of the cohorts, and therefore we were not able to reliably assess mediation of lung function in the association between respiratory tract infections and asthma. Second, we did not have information on lung function in early life and therefore were not able to assess change in lung function due to respiratory tract infections. Further studies should also focus on forced expiratory flow at 25–75% of FVC (FEF25–75%) as a lung function outcome as this measure might be the first declining lung function parameter as a result of small airway impairment obtained in early life. We also did not have information on bronchodilator reversibility, which might have biased the diagnosis of asthma. Additionally, even though we used individual participant data to allow harmonisation of the data, there is heterogeneity both in terms of assessment and prevalence of respiratory tract infections across the cohorts. This could in part reflect true differences in prevalence between different countries, but it is also likely that this is due to differences in data collection, including ascertainment of the diagnoses. Due to nonconsistent data availability we were not able to study a possible mediating effect of antibiotic use. However, in a previous study we found no mediating effect of antibiotic use in the association of respiratory tract infections with lung function and asthma [17].
Conclusions
In conclusion, early-life upper respiratory tract infections are associated with an increased risk of school-age asthma. Early-life lower respiratory tract infections are associated with lower lung function at school age, indicative of airway obstruction and airflow limitation, and even stronger increased risk of asthma. These results suggest that predominantly lower respiratory tract infections could have a direct effect on lung development and subsequent chronic respiratory diseases.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02395-2021.Supplement (654.1KB, pdf)
Shareable PDF
Acknowledgements
ABIS: ABIS has been supported by the Swedish Research Council (K2005-72X-11242-11A, K2008-69X-20826-01-4) and the Swedish Child Diabetes Foundation (Barndiabetesfonden), JDRF Wallenberg Foundation (K 98-99D-12813-01A), Medical Research Council of Southeast Sweden (FORSS) and the Swedish Council for Working Life and Social Research (FAS2004–1775), and Östgöta Brandstodsbolag. ALSPAC: We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. The UK Medical Research Council and Wellcome (217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors, and E.R. van Meel and L. Duijts will serve as guarantors for the contents of this paper. A comprehensive list of grant funding is available on the ALSPAC website (www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf). BAMSE: BAMSE was funded by the Swedish Research Council, the Swedish Heart Lung Foundation, ALF Region Stockholm and SFO Epidemiology Karolinska Institutet. E. Mélen is supported by a European Research Council grant (TRIBAL, 757919). BiB (Born in Bradford): BiB is only possible because of the enthusiasm and commitment of the children and parents in BiB. We are grateful to all the participants, practitioners and researchers who have made BiB happen. The BiB study presents independent research commissioned by the National Institute for Health Research Collaboration for Applied Health Research and Care (NIHR CLAHRC) and the Programme Grants for Applied Research funding scheme (RP-PG-0407-10044). Core support for BiB is also provided by the Wellcome Trust (WT101597MA). BILD: This study was funded by the Swiss National Science Foundation (320030_163311). CoNER: Funds were obtained from the special programme (Programmi speciali – Art.12 bis, comma 6 D.lgs.229/99 Sanitaria e della Vigilanza sugli Enti) funded by the Italian Ministry of Health. Approval for the study was obtained from the Ethics Committee of the S. Orsola-Malpighi Teaching Hospital in April 2004 (52/2004/U/Tess). COPSAC 2000 and COPSAC 2010: All funding received by COPSAC is listed on www.copsac.com. The Lundbeck Foundation (R16-A1694), Ministry of Health (903516), Danish Council for Strategic Research (0603-00280B) and Capital Region Research Foundation have provided core support to the COPSAC research centre. We express our deepest gratitude to the children and families of the COPSAC 2000 and COPSAC 2010 cohort studies for all their support and commitment. We acknowledge and appreciate the unique efforts of the COPSAC research team. DNBC (Danish National Birth Cohort): The authors would like to thank the participants, the first Principal Investigator of DNBC, Jørn Olsen, the scientific managerial team and DNBC secretariat for being, establishing, developing and consolidating the DNBC. The DNBC was established with a significant grant from the Danish National Research Foundation. Additional support was obtained from the Danish Regional Committees, Pharmacy Foundation, Egmont Foundation, March of Dimes Birth Defects Foundation, Health Foundation and other minor grants. The DNBC Biobank has been supported by the Novo Nordisk Foundation and Lundbeck Foundation. Follow-up of mothers and children has been supported by the Danish Medical Research Council (SSVF 0646, 271-08-0839/06-066023, O602-01042B, 0602-02738B), Lundbeck Foundation (195/04, R100-A9193), Innovation Fund Denmark 0603-00294B (09-067124), Nordea Foundation (02-2013-2014), Aarhus Ideas (AU R9-A959-13-S804), University of Copenhagen Strategic Grant (IFSV 2012) and Danish Council for Independent Research (DFF-4183-00594, DFF-4183-00152). A. Pinot de Moira is funded by a Lundbeck Foundation grant (R264-2017-3099). EDEN: We thank the EDEN mother–child cohort study group (I. Annesi-Maesano, J.Y. Bernard, J. Botton, M.A. Charles, P. Dargent-Molina, B. de Lauzon-Guillain, P. Ducimetière, M. de Agostini, B. Foliguet, A. Forhan, X. Fritel, A. Germa, V. Goua, R. Hankard, B. Heude, M. Kaminski, B. Larroque†, N. Lelong, J. Lepeule, G. Magnin, L. Marchand, C. Nabet, F. Pierre, R. Slama, M.J. Saurel-Cubizolles, M. Schweitzer and O. Thiebaugeorges). We thank all funding sources for the EDEN study (not allocated for the present study but for the cohort): Foundation for Medical Research (FRM), National Agency for Research (ANR), National Institute for Research in Public health (IRESP: TGIR cohorte santé 2008 programme), French Ministry of Health (DGS), French Ministry of Research, INSERM Bone and Joint Diseases National Research (PRO-A) and Human Nutrition National Research Programs, Paris-Sud University, Nestlé, French National Institute for Population Health Surveillance (InVS), French National Institute for Health Education (INPES), the European Union FP7 programmes (FP7/2007-2013, HELIX, ESCAPE, ENRIECO, MeDALL projects), Diabetes National Research Program (in collaboration with the French Association of Diabetic Patients (AFD)), French Agency for Environmental Health Safety (now ANSES), Mutuelle Générale de l'Education Nationale complementary health insurance (MGEN), French national agency for food security, and French speaking association for the study of diabetes and metabolism (ALFEDIAM). The funding source had no involvement in the conception of the present study. FLEHS: This study was conducted within the framework of the Flemish Centre of Expertise on Environment and Health, funded by the Dept of the Environment of the Flemish Government, Flemish Agency of Care and Health, and Flemish Dept of Economy, Science and Innovation. GASPII: The GASPII cohort was funded by the Italian Ministry of Health (2001), the research leading to these results has received funding from the European Community's Seventh Framework Program under grant agreement 261357 (MeDALL). Generation R: This study was funded by Erasmus MC Rotterdam, Erasmus University Rotterdam and the Netherlands Organisation for Health Research and Development. V.W.V. Jaddoe received a grant from the European Research Council (ERC-2014-CoG-648916). L. Duijts received funding from cofunded ERA-Net on Biomarkers for Nutrition and Health (ERA HDHL), Horizon 2020 (696295; 2017), the Netherlands Organisation for Health Research and Development (ZonMw; 529051014; 2017), Science Foundation Ireland (SFI/16/ERA-HDHL/3360), and European Union (ALPHABET project). The project received funding from the European Union's Horizon 2020 research and innovation programme (LIFECYCLE, 733206, 2016; EUCAN-Connect 824989; ATHLETE, 874583). The researchers are independent from the funders. The study sponsors had no role in the study design, data analysis, interpretation of data or writing of this report. Generation XXI: Generation XXI was supported by the European Regional Development Fund (ERDF) through the Operational Programme Competitiveness and Internationalization and national funding from the Foundation for Science and Technology (FCT), Portuguese Ministry of Science, Technology and Higher Education, and by the Unidade de Investigação em Epidemiologia – Instituto de Saúde Pública da Universidade do Porto (EPIUnit) (UIDB/04750/2020), Administração Regional de Saúde Norte (Regional Dept of Ministry of Health) and Fundação Calouste Gulbenkian. A.C. Santos is founded by FCT Investigator contracts IF/01060/2015. GINI: The GINIplus study was mainly supported for the first 3 years by the Federal Ministry for Education, Science, Research and Technology (interventional arm) and Helmholtz Zentrum München (former GSF) (observational arm). The 4- and 6-year follow-up examinations of the GINIplus study were covered from the respective budgets of the five study centres (Helmholtz Zentrum München (former GSF), Research Institute at Marien-Hospital, Wesel, LMU Munich, TU Munich and from 6 years onwards also from IUF – Leibniz Research Institute for Environmental Medicine at the University of Düsseldorf). HUMIS: We thank all mothers for participating in the HUMIS study. HUMIS was funded by a grant from the Norwegian Research Council (226402). The HUMIS study was approved by the Norwegian Data Inspectorate (2002/1398) and by the Regional Ethics Committee for Medical Research in Norway (S-02122), and the specific use in the current study was approved by the Ethics Committee as well (2010/1259/REK sør-øst). INMA: Gipuzkoa: This study was funded by grants from Instituto de Salud Carlos III (FIS-PI09/00090, FIS-PI18/01142 including FEDER funds), CIBERESP, Dept of Health of the Basque Government (2013111089) and annual agreements with the municipalities of the study area (Zumarraga, Urretxu, Legazpi, Azkoitia y Azpeitia and Beasain). Menorca: This study was funded by grants from Instituto de Salud Carlos III (Red INMA G03/176; CB06/02/0041; 97/0588; 00/0021-2, PI061756; PS0901958, PI14/00677 including FEDER funds), CIBERESP, Beca de la IV convocatoria de Ayudas a la Investigación en Enfemerdades Neurodegeneratives de La Caixa, and EC contract QLK4-CT-200-00263. Sabadell: This study was funded by grants from Instituto de Salud Carlos III (Red INMA G03/176; CB06/02/0041; PI041436; PI081151 including FEDER funds), Generalitat de Catalunya-CIRIT 1999SGR 00241 and Fundació La marató de TV3 (090430). ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya. M. Casas holds a Miguel Servet fellowship (CP16/00128) funded by Instituto de Salud Carlos III and cofunded by the European Social Fund “Investing in your future”. Valencia: This study was funded by grants from the European Union (FP7-ENV-2011 cod 282957 and HEALTH.2010.2.4.5-1), Spain: Instituto de Salud Carlos III (Red INMA G03/176, CB06/02/0041; FIS-FEDER: PI03/1615, PI04/1509, PI04/1112, PI04/1931, PI05/1079, PI05/1052, PI06/1213, PI07/0314, PI09/02647, PI11/01007, PI11/02591, PI11/02038, PI13/1944, PI13/2032, PI14/00891, PI14/01687, PI16/1288, PI17/00663; Miguel Servet-FEDER CP11/00178, CP15/00025, CPII16/00051), Generalitat Valenciana: FISABIO (UGP 15-230, UGP-15-244, UGP-15-249), and Alicia Koplowitz Foundation 2017. Isle of Wight: This study was funded by grants from the National Institutes of Health USA (R01HL082925), Asthma UK (364), Isle of Wight NHS Trust and the British Medical Association. KOALA: The collection of data relevant for this study was funded by grants from the Netherlands Organisation for Health Research and Development (ZonMw; 2100.0090) and the Netherlands Asthma Foundation (3.2.03.48, 3.2.07.022). The researchers are independent from the funders. The funders had no role in the study design, data analysis, interpretation of data or writing of this report. We thank the children and parents for their participation in the KOALA study. LRC (Leicestershire Respiratory Cohorts): This study was funded by grants from the Swiss National Science Foundation (SNF: 320030-182628, 320030-162820, 3233-069348, 3200-069349) and Asthma UK 07/048. Lifeways Cross-Generation Cohort Study: This study was funded by the Health Research Board, Ireland, and the Irish Dept of Health and Children's Health Promotion Policy Unit. LISA: The LISA study was mainly supported by grants from the Federal Ministry for Education, Science, Research and Technology and in addition from Helmholtz Zentrum München (former GSF), Helmholtz Centre for Environmental Research – UFZ, Leipzig, Research Institute at Marien-Hospital Bad Honnef for the first 2 years. The 4-, 6-, 10- and 15-year follow-up examinations of the LISA study were covered from the respective budgets of the involved partners (Helmholtz Zentrum München (former GSF), Helmholtz Centre for Environmental Research – UFZ, Leipzig, Research Institute at Marien-Hospital Wesel, Pediatric Practice, Bad Honnef, IUF – Leibniz Research Institute for Environmental Medicine at the University of Düsseldorf) and in addition by a grant from the Federal Ministry for Environment (IUF Düsseldorf, FKZ 20462296). Further, the 15-year follow-up examination of the LISA study was supported by the Commission of the European Communities, the Seventh Framework Program: MeDALL project. This project has received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (949906). LucKi: LucKi is supported by Child and Youth Health Care Zuyderland, Public Health Service South Limburg and Maastricht University. We thank all parents and children for their participation in LucKi. LUKAS: This study was funded by research grants from the Academy of Finland (139021, 287675, 296814, 296817, 308254); Juho Vainio Foundation; EVO/VTR funding; Päivikki and Sakari Sohlberg Foundation; Farmers’ Social Insurance Institution (Mela); Finnish Cultural Foundation; Foundation for Pediatric Research; European Union QLK4-CT-2001-00250; and Finnish Institute for Health and Welfare, Finland. MAS-90: This study was funded by grants from the German Federal Ministry of Education and Research (MBMF; 07015633m 07ALE27, 01EE9405/5, 01EE9406) and the German Research Foundation (DFG; KE1462/2-1). Millennium Cohort Study: This study was funded by the Economic and Social Research Council and a consortium of UK government funders. We are grateful to the participating families and the Centre for Longitudinal Studies (CLS), UCL Institute of Education, for the use of these data and to the UK Data Service for making them available. However, neither CLS nor the UK Data Service bear any responsibility for the analysis or interpretation of these data. This work was supported by the Welcome Trust (187389/B/08/Z). MoBa: The Norwegian Mother, Father and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and Ministry of Education and Research. We are grateful to all the participating families in Norway who take part in this ongoing cohort study. This research was supported by the Research Council of Norway through its Centres of Excellence funding scheme (262700). NINFEA: The authors are grateful to all the participants of the NINFEA cohort. The NINFEA study was partially funded by the Compagnia San Paolo Foundation. This research was partially funded by the European Union's Horizon 2020 research and innovation programme (LIFECYCLE, 733206). PELAGIE: We are grateful to the families who participated and continue to participate in the study. The cohort is supported by INSERM and received funding from the French National Research Agency, Fondation de France, French Agency for Food, Environmental and Occupational Health & Safety, National Institute for Public Health Surveillance (InVS), French Ministry of Labour, and French Ministry of Ecology. PIAMA: This study was funded by the Netherlands Organisation of Health Research and Development, Netherlands Organisation for Scientific Research, Netherlands Asthma Fund, Netherlands Ministry of Spatial Planning, Housing and the Environment, and Netherlands Ministry of Health, Welfare and Sport. REPRO_PL: This study was funded by the National Science Center Poland (DEC-2014/15/B/N27/00998). Rhea: This study was funded by the European Union Social Fund and the Hellenic Ministry of Health (“Program of prevention and early diagnosis of obesity and neurodevelopment disorders in preschool age children in the prefecture of Heraklion, Crete, Greece”; MIS 349580, NSRF 2007–2013). Additional funding from the National Institute of Environmental Health Sciences (NIEHS) supported L. Chatzi (R01ES030691, R01ES029944, R01ES030364, R21ES029681, R21ES028903, P30ES007048). STEPS: This study was funded by the University of Turku, Abo Akademi University, Turku University Hospital, Academy of Finland (123571, 140251, 277535) and Foundation for Pediatric Research Finland. SWS: This study was funded by the Medical Research Council, British Heart Foundation, Arthritis Research UK, Food Standards Agency, NIHR Southampton Biomedical Research Centre and the European Union's Seventh Framework Programme (FP7/2007–2013), project EarlyNutrition (289346), and the European Union's Horizon 2020 research and innovation programme (LIFECYCLE, 733206). WHISTLER: The WHISTLER birth cohort was supported with a grant from the Netherlands Organisation for Health Research and Development (2001-1-1322) and by an unrestricted grant from GlaxoSmithKline Netherlands. GlaxoSmithKline had no role in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the report for publication. WHISTLER-Cardio was supported with an unrestricted strategic grant from the University Medical Center Utrecht (UMCU).
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.01214-2022
Data sharing: Individual participant data will not be available for sharing.
Author contributions: E.R. van Meel, S.M. Mensink-Bout, H.T. den Dekker, J.C. de Jongste, V.W.V. Jaddoe and L. Duijts contributed to the study design, data analysis plan, data collection, data analysis, data interpretation, writing, reviewing the manuscript critically and gave consent for submission. All other authors contributed equally to study design, data analysis plan, data collection, reviewing the manuscript critically and gave consent for submission.
Conflict of interest: T.S. Ahluwalia received funding for the current manuscript from the Novo Nordisk Foundation (NNF180C0052457). I. Annesi-Maesano is member of the ATS Environment Health Policy Committee, the ERS Ethics and Integrity Committee, and the French IRD Ethics Committee. S.H. Arshad received funding for the manuscript from Asthma UK (364) and National Institutes of Health USA (R01HL082925). H. Bisgaard received funding for the current manuscript from the Lundbeck Foundation (R16-A1694), Ministry of Health (903516), Danish Council for Strategic Research (0603-00280B) and Capital Region Research Foundation. M. Eggesbø received paid honorarium for making small videos relating to allergy and asthma, by the Norwegian LHL organisation. U. Frey received funding for the manuscript from the Swiss National Science Foundation (320030_204717/1), and is chair of the National Steering Board, Swiss Personalized Health Network (SPHN). H. Inskip received funding for the manuscript from the UK Medical Research Council and the European Union, and was President for the Society for Social Medicine and Population Health. J. Jerzynska received funding for the current manuscript from the National Science Centre, Poland (DEC-2014/15/B/N27/00998). A.M. Karvonen received funding for the present manuscript from the Academy of Finland (139021, 287675, 296814, 296817, 308254), Juho Vainio Foundation, EVO/VTR funding, Pavivikki and Sakari Sohlberg Foundation, Farmers’ Social Insurance Institution (Mela), Finnish Cultural Foundation, Foundation for Pediatric Research, and the European Union (QLK4-CT-2001-0250). M. Mommers received grants from the Research Council of Norway (262700) and European Research Council (947684). A. Pinot de Moira received a Lundbeck Foundation fellowship (R264-2017-3099). V. Peltola received funding for the present manuscript from the Academy of Finland and Foundation for Pediatric Research Finland. K.C. Pike received consulting fees from Novartis and Spiriva, payment or honoraria for lectures from Novartis, and is participating on a data safety monitoring board or advisory board for Adherium. K. Polanska received funding for the current manuscript from the National Science Centre, Poland (DEC-2014/15/B/N27/00998), grant PRNF-218-AI-1/07 from Norway through the Norwegian Financial Mechanisms within the Polish–Norwegian Research Fund, and the Ministry of Science and Higher Education, Poland (PBZ-MEiN-/8/2//2006). G. Roberts is president of the BSACI. A.C. Santos received funding for the current manuscript from FCT Investigators contracts (IF/01060/2015). J. Sunyer received a grant from the European Research Council (Prenatal exposure to urban AIR pollution and pre- and postNatal Brain development (AIR-NB), 785994). J. Usemann received grants from the Palatin Foundation, University of Basel Switzerland, Swiss Cancer League and Swiss Lung Foundation, and payments or honoraria for lectures from Vertex and Zurich Lung Foundation. V.W.V. Jaddoe received a grant from the European Research Council (ERC-2014-CoG-648916). L. Duijts received funding from cofunded ERA-Net on Biomarkers for Nutrition and Health (ERA HDHL), Horizon 2020 (696295; 2017), the Netherlands Organisation for Health Research and Development (ZonMw; 529051014; 2017), Science Foundation Ireland (SFI/16/ERA-HDHL/3360), and the European Union (ALPHABET project). All other authors declare no conflict of interest.
References
- 1.Chonmaitree T, Revai K, Grady JJ, et al. . Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis 2008; 46: 815–823. doi: 10.1086/528685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudan I, Boschi-Pinto C, Biloglav Z, et al. . Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 2008; 86: 408–416. doi: 10.2471/BLT.07.048769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Openshaw PJ, Tregoning JS. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev 2005; 18: 541–555. doi: 10.1128/CMR.18.3.541-555.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holt PG. Programming for responsiveness to environmental antigens that trigger allergic respiratory disease in adulthood is initiated during the perinatal period. Environ Health Perspect 1998; 106: Suppl. 3, 795–800. doi: 10.1289/ehp.98106795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daley D. The evolution of the hygiene hypothesis: the role of early-life exposures to viruses and microbes and their relationship to asthma and allergic diseases. Curr Opin Allergy Clin Immunol 2014; 14: 390–396. doi: 10.1097/ACI.0000000000000101 [DOI] [PubMed] [Google Scholar]
- 6.Wark PAB, Ramsahai JM, Pathinayake P, et al. . Respiratory viruses and asthma. Semin Respir Crit Care Med 2018; 39: 45–55. doi: 10.1055/s-0037-1617412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson DJ, Gangnon RE, Evans MD, et al. . Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 2008; 178: 667–672. doi: 10.1164/rccm.200802-309OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnelykke K, Vissing NH, Sevelsted A, et al. . Association between respiratory infections in early life and later asthma is independent of virus type. J Allergy Clin Immunol 2015; 136: 81–86. doi: 10.1016/j.jaci.2015.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusel MM, de Klerk NH, Kebadze T, et al. . Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol 2007; 119: 1105–1110. doi: 10.1016/j.jaci.2006.12.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montgomery S, Bahmanyar S, Brus O, et al. . Respiratory infections in preterm infants and subsequent asthma: a cohort study. BMJ Open 2013; 3: e004034. doi: 10.1136/bmjopen-2013-004034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James KM, Gebretsadik T, Escobar GJ, et al. . Risk of childhood asthma following infant bronchiolitis during the respiratory syncytial virus season. J Allergy Clin Immunol 2013; 132: 227–229. doi: 10.1016/j.jaci.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigurs N, Aljassim F, Kjellman B, et al. . Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010; 65: 1045–1052. doi: 10.1136/thx.2009.121582 [DOI] [PubMed] [Google Scholar]
- 13.Balekian DS, Linnemann RW, Hasegawa K, et al. . Cohort study of severe bronchiolitis during infancy and risk of asthma by age 5 years. J Allergy Clin Immunol Pract 2017; 5: 92–96. doi: 10.1016/j.jaip.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 14.Chan JY, Stern DA, Guerra S, et al. . Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics 2015; 135: 607–616. doi: 10.1542/peds.2014-3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guilbert TW, Singh AM, Danov Z, et al. . Decreased lung function after preschool wheezing rhinovirus illnesses in children at risk to develop asthma. J Allergy Clin Immunol 2011; 128: 532–538. doi: 10.1016/j.jaci.2011.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray DM, Turkovic L, Willemse L, et al. . Lung function in African infants in the Drakenstein child health study. Impact of lower respiratory tract illness. Am J Respir Crit Care Med 2017; 195: 212–220. doi: 10.1164/rccm.201601-0188OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Meel ER, den Dekker HT, Elbert NJ, et al. . A population-based prospective cohort study examining the influence of early-life respiratory tract infections on school-age lung function and asthma. Thorax 2018; 73: 167–173. doi: 10.1136/thoraxjnl-2017-210149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bui DS, Lodge CJ, Burgess JA, et al. . Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med 2018; 6: 535–544. doi: 10.1016/S2213-2600(18)30100-0 [DOI] [PubMed] [Google Scholar]
- 19.Jaddoe VWV, Felix JF, Andersen AN, et al. . The LifeCycle Project-EU Child Cohort Network: a federated analysis infrastructure and harmonized data of more than 250,000 children and parents. Eur J Epidemiol 2020; 35: 709–724. doi: 10.1007/s10654-020-00662-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quanjer PH, Stanojevic S, Cole TJ, et al. . Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asher MI, Keil U, Anderson HR, et al. . International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995; 8: 483–491. doi: 10.1183/09031936.95.08030483 [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 23.Quanjer PH, Stanojevic S, Stocks J, et al. . Changes in the FEV1/FVC ratio during childhood and adolescence: an intercontinental study. Eur Respir J 2010; 36: 1391–1399. doi: 10.1183/09031936.00164109 [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Pan Y, Zhu Y, et al. . Association between rhinovirus wheezing illness and the development of childhood asthma: a meta-analysis. BMJ Open 2017; 7: e013034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Illi S, von Mutius E, Lau S, et al. . Early childhood infectious diseases and the development of asthma up to school age: a birth cohort study. BMJ 2001; 322: 390–395. doi: 10.1136/bmj.322.7283.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perret JL, Lodge CJ, Lowe AJ, et al. . Childhood pneumonia, pleurisy and lung function: a cohort study from the first to sixth decade of life. Thorax 2020; 75: 28–37. doi: 10.1136/thoraxjnl-2019-213389 [DOI] [PubMed] [Google Scholar]
- 27.Kouzouna A, Gilchrist FJ, Ball V, et al. . A systematic review of early life factors which adversely affect subsequent lung function. Paediatr Respir Rev 2016; 20: 67–75. doi: 10.1016/j.prrv.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 28.de Vries E, de Groot R, de Bruin-Versteeg S, et al. . Analysing the developing lymphocyte system of neonates and infants. Eur J Pediatr 1999; 158: 611–617. doi: 10.1007/s004310051162 [DOI] [PubMed] [Google Scholar]
- 29.Dunnill MS. Postnatal growth of the lung. Thorax 1962; 17: 329–333. doi: 10.1136/thx.17.4.329 [DOI] [Google Scholar]
- 30.Kajekar R. Environmental factors and developmental outcomes in the lung. Pharmacol Ther 2007; 114: 129–145. doi: 10.1016/j.pharmthera.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 31.Larsen JM, Brix S, Thysen AH, et al. . Children with asthma by school age display aberrant immune responses to pathogenic airway bacteria as infants. J Allergy Clin Immunol 2014; 133: 1008–1013. doi: 10.1016/j.jaci.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 32.Heinonen S, Rodriguez-Fernandez R, Diaz A, et al. . Infant immune response to respiratory viral infections. Immunol Allergy Clin North Am 2019; 39: 361–376. doi: 10.1016/j.iac.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramette A, Spycher BD, Wang J, et al. . Longitudinal associations between respiratory infections and asthma in young children. Am J Epidemiol 2018; 187: 1714–1720. doi: 10.1093/aje/kwy053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986; 1: 1077–1081. doi: 10.1016/S0140-6736(86)91340-1 [DOI] [PubMed] [Google Scholar]
- 35.Barker DJ, Godfrey KM, Fall C, et al. . Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ 1991; 303: 671–675. doi: 10.1136/bmj.303.6804.671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Backman K, Ollikainen H, Piippo-Savolainen E, et al. . Asthma and lung function in adulthood after a viral wheezing episode in early childhood. Clin Exp Allergy 2018; 48: 138–146. doi: 10.1111/cea.13062 [DOI] [PubMed] [Google Scholar]
- 37.Wang G, Hallberg J, Charalampopoulos D, et al. . Spirometric phenotypes from early childhood to young adulthood: a Chronic Airway Disease Early Stratification study. ERJ Open Res 2021; 7: 00457-2021. doi: 10.1183/23120541.00457-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02395-2021.Supplement (654.1KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02395-2021.Shareable (997.2KB, pdf)