Abstract
Objectives
No copper intrauterine device (IUD) type is known to better suit young nulliparous women who tend to experience higher rates of IUD discontinuation compared with their older parous counterparts. A systematic review to determine which IUDs have higher continuation rates in young nulliparous women was undertaken.
Design
Systematic review and meta-analyses of available evidence based on IUD type.
Data sources
AMED, BNI, CINAHL, DARE, EMBASE, EMCARE, HMIC, MEDLINE, PsycINFO, PubMed, TRIP, and the Cochrane Library electronic databases were searched from inception to 11 May 2022; as well as the Bandolier, Medicines and Healthcare products Regulatory Agency, Faculty of Sexual and Reproductive Healthcare, Royal College of Obstetricians and Gynaecologists, Department of Health, National Institute for Health and Care Excellence, Scottish Intercollegiate Guidelines, WHO and Google Scholar websites.
Eligibility criteria
All studies on IUDs currently available in the UK or comparable (same design and size) to those available in the UK, involving nulliparous women of any age including those aged under 30.
Data extraction and synthesis
Independently extracted data were assessed as low risk of bias using the Mixed Methods Appraisal Tool. Random effects meta-analyses of proportions were performed where data, including subgroups, were amenable to quantitative synthesis. Heterogeneity was reported using tau2 and I2 statistics, and sensitivity analyses were also performed.
Results
Nineteen studies involving 13 045 nulliparous women were included but the heterogeneity of participant ages, parity and IUD types made quantitative synthesis of outcome data in totality inappropriate. The highest continuation rate obtained was 91.02% (95% CI 88.01% to 93.64%) for the smaller TCu 380A at 12 months post insertion.
Conclusions
Evidence for IUD use in young nulliparous women based on IUD type remains limited. Smaller sized IUD types appear better suited to this group of IUD users, however, more research is needed.
PROSPERO registration number
CRD42019120969.
Keywords: reproductive medicine, community gynaecology, public health
STRENGTHS AND LIMITATIONS OF THE STUDY.
The first reported systematic review exploring intrauterine device (IUD) types in young nulliparous women.
A wide range of data sources, unrestricted to randomised controlled trials, was reviewed —an approach more representative of the real world.
Articles for inclusion were limited to publications in the English language.
Some data were obtained by calculation and measurements of graphs or figures where these data were not numerically specified in reports.
Most studies did not differentiate between nulligravid and nulliparous participants.
Introduction
The highest rates of unintended pregnancy and terminations of pregnancy, which contribute to poor sexual health, are in women aged 20–24 followed by those aged 25–29.1 Increasing uptake of long-acting reversible contraceptives (LARCs), such as copper intrauterine contraception, in these women is yet to yield a proportional reduction in pregnancy terminations. This is attributable to their higher LARC discontinuation rates.2
Copper intrauterine contraception is the LARC with the greatest number of brands, with 21 copper intrauterine devices (IUDs) available in the UK.3 IUDs are of various shapes, sizes, total copper surface area and copper distribution on the IUD frame. They have changed little over the last 40 years. No IUD type has been shown to be associated with better outcomes regarding unwanted effects that lead to early IUD discontinuation. This early IUD discontinuation excludes discontinuation due to IUD user choice alone or the wish to conceive. IUD continuation rates tend to be surrogate for IUD satisfaction and/or acceptability. Studies have shown IUD discontinuation rates to be higher in adolescents and women in their 20s compared with their older counterparts, as well as in nulliparous compared with parous women.4–8
Previous systematic reviews and guidance suggest that IUD size and shape may be a factor in discontinuation, and have recommended future research investigate which IUD types are associated with less pain, bleeding and discontinuation.7 9–11 The identification and use of IUDs with higher continuation rates and fewer unwanted effects could improve outcomes including IUD satisfaction for young nulliparous women. A systematic review and meta-analysis were therefore undertaken to investigate continuation rates and reasons for discontinuation of IUDs, currently available, or comparable to those currently in use in the UK, based on IUD type involving women aged under 30.
Objectives
This study aimed to determine which currently available IUDs have higher continuation rates, in nulliparous women aged under 30, by systematically reviewing published studies. Discontinuation rates and reasons for discontinuation were secondary outcomes.
Methods
An appraisal of previous systematic reviews, including publications by the Cochrane Collaboration Fertility Regulation Group, Faculty of Sexual and Reproductive Healthcare (FSRH) and National Institute for Health and Care Excellence (NICE), was performed. A search strategy was developed in conjunction with an Electronic Services Librarian. These informed the design of this systematic review and its protocol.
This study is reported as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (see online supplemental material 1). Its protocol was registered on the International Prospective Register of Systematic Reviews database (see online supplemental material 2).12 The protocol included other studies besides randomised controlled trials (RCTs) reporting on IUD continuation, in case the RCTs determined eligible for inclusion in the systematic review were too few to address the review question.
bmjopen-2021-060606supp001.pdf (61.6KB, pdf)
bmjopen-2021-060606supp002.pdf (48.3KB, pdf)
Selection criteria
Inclusion criteria
Inclusion criteria are as follows: articles published in English, on studies in women who are nulliparous and aged under 30, that involved IUDs available or of the same design and size, to those available in the UK.
Exclusion criteria
Exclusion criteria are as follows: articles not published in English, studies solely in parous women aged 30 or over 30, that involved IUDs not available, or not of the same design and size to those available in the UK.
Where studies on IUDs currently available in the UK were lacking, studies with IUDs comparable in shape, size, total copper surface area or distribution on the IUD frame to those currently available in the UK were included. Where studies involving only nulliparous women aged under 30 were lacking, studies with nulliparous women of all ages (incorporating those aged under 30) were also included in the review.
Search strategy
Nine electronic databases—the Allied and Complementary Medicine (AMED), British Nursing Index (BNI), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Excerpta Medica Database (EMBASE), Nursing and Allied Health Professionals Database (EMCARE), Health Management Information Consortium (HMIC), General Medical Database (MEDLINE), Psychology and Allied Fields (PsycINFO) and PubMed—were searched. The search terms were (copper intrauterine).ti, ab OR (copper intrauterine device).ti, ab OR (copper coil).ti, ab OR (copper IUD).ti, ab OR (copper T).ti, ab from database inception to 7 February 2021 (updated to 11 May 2022). The following additional sources were searched using the term ‘copper intrauterine’: the Cochrane Library, Database of Abstracts and Reviews of Effects (DARE), Turning Research into Practice (TRIP) database, National Electronic Library of Health (merged with MEDLINE), Bandolier, Medicines and Healthcare products Regulatory Agency, FSRH, Royal College of Obstetricians and Gynaecologists, Department of Health, NICE, Scottish Intercollegiate Guidelines and WHO websites. A Google Scholar search was also undertaken using the term ‘copper intrauterine device young nulliparous’. The full search strategy is provided as a supplementary file (online supplemental material 3).
bmjopen-2021-060606supp003.pdf (51.7KB, pdf)
Relevant articles published in English were identified by two authors and these were exported into an Endnote library on completion of all the searches. Following deduplication, the relevant articles obtained from the searches were exported to Rayyan, a web app for systematic reviews (rayyan.ai). In Rayyan, further deduplication yielded unique entries of which abstracts, and then full texts, were screened independently by two authors to assess eligibility for inclusion in the systematic review based on the inclusion/exclusion criteria. Additional citation screening of reference lists of both included and excluded studies was performed. Screening was initially done in batches of 20, then later increased to 50. Agreements were obtained between the first two authors and did not require a third review. Selected articles were RCTs and observational studies published in English, involving IUDs available or comparable to those in the UK, and involving nulliparous women aged under 30.
Quality assessment and data summary
All articles selected for inclusion in the systematic review underwent a quality assessment using the Mixed Methods Appraisal Tool (MMAT), v.2018.13 The MMAT risk of bias tool was chosen because it was applicable to all the study types selected for inclusion. The highest total MMAT score conforming with best quality was seven, while the lowest possible score equating with poorest quality was zero. Included articles were initially quality assessed by the two authors separately and then agreement was reached.
Data extracted from articles included IUD type, study location(s) and year of publication, age of women, gravidity/parity of women, IUD continuation and discontinuation rates and reasons for IUD discontinuation. Where a rate was not specified but could be reliably calculated, this was done to one decimal place. If a continuation rate was not specified, this was obtained by subtracting the discontinuation rate from 100, or adding all stated rates for reasons for discontinuation (where these were mutually exclusive) and subtracting from 100, if the report suggested such a calculation to be valid. If a discontinuation rate was not specified, this was obtained by subtracting a stated continuation rate from 100, or by adding all stated rates for reasons for discontinuation (where these were mutually exclusive), if the report suggested such a calculation was valid. Gross rates (obtained after excluding participants lost to follow-up or removals to conceive) were used, except where only net cumulative rates were reported. Measurements were performed to obtain data from published graphs or figures where rates had been reported in this format but not numerically specified.
An Excel data collection form was developed, piloted with three articles selected for inclusion by one author, then revised and amended by the second author before proceeding to data extraction. Data from the 19 selected articles included in the review were extracted by one author into the Excel spreadsheet and checked by the second author.
Data analysis
Where available, data were amenable to quantitative synthesis, random effects meta-analyses of proportions were performed using the metaprop suite of commands on STATA 16. Variances were stabilised using the Freeman-Tukey double arcsine transformation. This approach provides better approximation and leads to results between 0% and 100% when synthesising proportions from small samples and multiple studies in meta-analyses.14 Where possible, subgroup analysis was performed to examine differences between nulliparous women aged ≤30 years and nulliparous women of any age. Statistical heterogeneity was reported using I2 and tau2 statistics, since random effects meta-analyses were being performed. The I2 value describes the percentage of the variability in effect estimates that is due to statistical heterogeneity (reflecting methodological diversity among the included studies) as opposed to chance. Conventionally, while an I2 value <40% may not be significant, a value >50% may represent substantial heterogeneity and a value >75% may indicate considerable heterogeneity.15 The tau2 statistic measure of ‘between-study variance’, unlike the I2 statistic, is not affected by size of included studies in a meta-analysis and hence may be considered more appropriate for estimating heterogeneity.16 The effect of removing individual studies on the overall effect size (ES) was explored in sensitivity analyses (online supplemental material 4). Publication bias was examined by producing Doi plots and generating LFK index values, being considered a more appropriate measure of publication bias than funnel plots/Egger’s test when performing meta-analyses of proportions.17
bmjopen-2021-060606supp004.pdf (70.6KB, pdf)
Patient and public involvement
The FSRH is the UK organisation committed to meeting the highest SRH standards, ensuring improvements in population SRH and supporting SRH professionals. The FSRH’s Contraceptive Priority Setting Partnership in liaison with the James Lind Alliance yielded over 700 responses from patients, practitioners and the public that identified: ‘Which interventions increase uptake and continuation of effective contraception including long-acting methods…?’ as the top SRH research priority.18 This influenced the research aims. IUD users attending a sexual health clinic over a 4-week period were consulted about improving access to and use of intrauterine contraception. Their suggestions, which included studying women’s experiences with IUDs, were used in developing the research question, aim and study design. The Consumer Panel of the North East Research Design Service was also consulted and the proposed research presented to them. The research plan was modified in line with their feedback.
Results
Only one study, a prospective (non-RCT) cohort study, provided information on an IUD available in the UK, solely involving nulliparous users aged under 30.19 This was inadequate to address the review question. As per the systematic review protocol, other studies on IUDs currently available in the UK or IUDs comparable to those available in the UK (table 1) involving nulliparous women of all ages (so not limited to those aged under 30) were also screened. An IUD was considered comparable if at least two out of its four characteristics (copper surface area, shape/design, width and arms flexibility) equated with IUDs currently used in the UK. So, for example, the Nova T200 was comparable because it has the same shape/design as a Nova T380, the same width as a Nova T380/Cu T380A/TCu 380A and TT380 slimline, and the same flexible arms as a Nova T380 (table 1).
Table 1.
Characteristics of IUDs in the included studies
| IUD brand/name | Copper (mm2) | Shape/design | Width (mm) | Arms’ flexibility |
| Currently available in the UK | ||||
| Cu T380A/TCu 380 A/TT380 Slimline | 380 | T with arm bands | >30 | No |
| TCu 380A Nul/Mini TT380 slimline | 380 | T with arm bands | 23.2 | No |
| Multiload Cu 375 | 375 | Ω | 16–20.5 | Yes, flex down |
| Nova T380 | 380 | T without arm bands | >30 | Yes, flex up |
| Comparable to those available in the UK | ||||
| Nova T200 | 200 | T without arm bands | ≥30 | Yes, flex up |
| TCu 300 | 300 | T without arm bands | >30 | No |
| Cu T200/TCu 200 | 200 | T without arm bands | >30 | No |
| TCu 220C | 220 | T without arm bands | >30 | No |
IUD, intrauterine device.
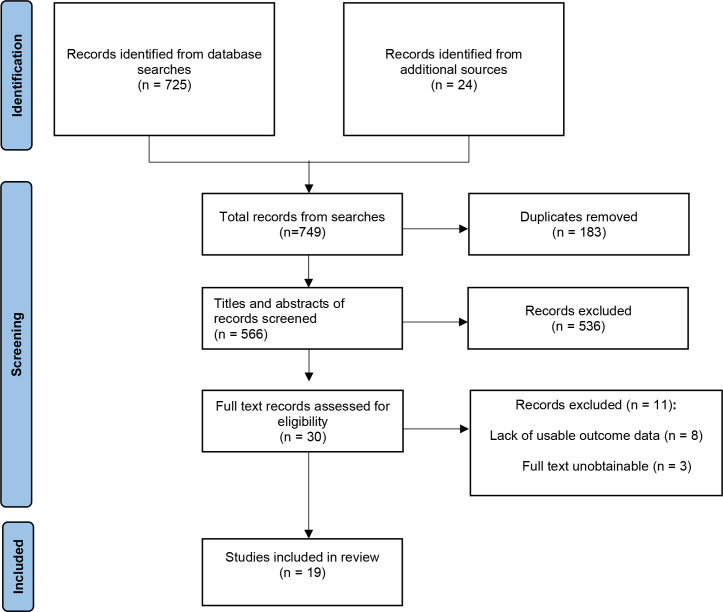
Thirty records were obtained and their full texts assessed where possible. Eleven records were excluded, either for lack of usable outcome data (n=85 20–26) or because their full texts were unobtainable (n=327–29) (see online supplemental material 5). A total of 19 studies on IUDs available or comparable to those available in the UK, involving 13 045 nulliparous women, were included in the systematic review (table 2).19 30–47 Figure 1 depicts a PRISMA flow diagram detailing the search and selection process.48
Table 2.
Characteristics of the included studies
| Study/authors | Year | Country | Study design | Study objectives | IUDs in study | Quality (MMAT score) |
| Abraham et al19 | 2015 | USA | Prospective cohort | Relationship among young age, nulliparity and continuation of long-acting reversible contraceptives | Copper T380A | Good (7) |
| Akintomide et al30 | 2019 | UK | Retrospective records review | Discontinuation rates and reasons for discontinuation at 1 year of the small-sized Mini TT380 Slimline IUD compared with the standard-sized TT380 Slimline | Mini TT380 slimline TT380 slimline |
Good (6) |
| Allonen et al31 | 1980 | Denmark, Finland Sweden |
RCT—double blind | Continuation rates and reasons for discontinuation at 2 years of the Nova T200 and Copper T200 | Nova T200 Copper T200 |
Good (6) |
| Elkhateeb et al32 | 2020 | Egypt | Prospective cohort | Acceptability of IUD use in nulliparous women by both women and healthcare providers | Copper T380A | Good (7) |
| Fugere33 | 1990 | Canada | Prospective cohort | Clinical performance of the Nova T200 IUD over 5 years | Nova T200 | Good (7) |
| Hall and Kutler34 | 2016 | USA | Prospective cohort | Experience and satisfaction of nulliparous intrauterine contraception users at 1, 6, 12 and 18 months | Copper T380A | Good (7) |
| Kaislasuo et al35 | 2015 | Finland | Prospective cohort | Menstrual characteristics and ultrasonographic uterine cavity measurements predict bleeding and pain in nulligravid women using intrauterine contraception | Nova T380 | Good (7) |
| Larsen et al36 | 1981 | Denmark | RCT—patient blind | Comparison of clinical performances of Progestasert and Copper T200 at 12 months | Copper T200 | Good (5) |
| Lewit37 | 1973 | USA | Prospective cohort | Two years’ experience of the Copper T200 | Copper T200 | Good (7) |
| Liedholm and Sjöberg 38 | 1974 | Sweden | Prospective cohort | Two years’ experience with the Copper T200 and comparison between nulliparous and parous women | Copper T200 | Good (7) |
| Luukkainen et al39 | 1979 | Denmark, Finland Sweden |
RCT—double blind | Experience and clinical performance of the Nova T200 and Copper T200 at 12 months | Nova T200 Copper T200 |
Good (6) |
| Luukkainen et al40 | 1987 | Denmark, Finland, Hungary, Norway, Sweden | RCT—no blinding | Use-effectiveness and clinical performance of levonorgestrel-releasing and copper-releasing intrauterine devices at 12 months | Nova T200 | Good (6) |
| Mishell et al41 | 1973 | USA | Prospective cohort | Continuation and clinical performance of TCu 200 in nulliparous women | Copper T200 | Good (7) |
| Nygren et al42 | 1981 | Denmark, Finland Sweden |
RCT—double blind | Continuation rates and reasons for discontinuation at 3 years of the Nova T200 and Copper T200 | Nova T200 Copper T200 |
Good (7) |
| Ostergard and Gunning43 | 1979 | USA | RCT—blinding not stated | Continuation and clinical performances of Copper T200 and Dalkon Shield in nulligravid women at 12 months | Copper T200 | Good (5) |
| Otero-Flores et al44 | 2003 | Mexico | RCT—single (patient) blind | Comparison of clinical performance of three different IUDs in nulliparous women | Copper T380A Copper T380A Nul Multiload 375 sl |
Good (6) |
| Roy et al45 | 1974 | USA | Prospective cohort | Experience with three different IUD models in nulliparous women at 1 year | Copper T380A Copper T300 Copper T200 |
Good (7) |
| Sivin and Stern46 | 1979 | USA | RCT—double blind | Experience of three different IUDs in nulliparous and parous women | Copper T380A Copper T220C Copper T200 |
Good (5) |
| Timonen et al47 | 1974 | Finland | Prospective, single (patient) blind | Use-effectiveness of Copper T300 at 1 year | Copper T300 | Good (7) |
IUD, intrauterine device; MMAT, Mixed Methods Appraisal Tool; RCT, randomised controlled trial.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
bmjopen-2021-060606supp005.pdf (98.8KB, pdf)
All included studies were generally of good quality (mean 6.42 [5-7]; see online supplemental material 6 for quality and risk of bias assessments). The lowest MMAT score of five obtained was awarded to three RCTs published in 1979 and 1981 and may relate to inadequate reporting.36 43 46 Their reports did not confirm that randomisation had been appropriately performed,36 46 randomised groups were comparable at baseline,43 46 nor that outcome assessors were blinded to the intervention provided.36 43
bmjopen-2021-060606supp006.pdf (99.2KB, pdf)
Although the outcome data obtained were considered homogeneous, studies’ designs, participant ages and parity, and IUD types were not; making a quantitative synthesis of the outcome data in totality inappropriate. Results were therefore grouped into three to include studies involving: (1) IUD types currently available in the UK and only nulliparous women aged ≤30; (2) IUD types currently available in the UK and nulliparous women of all ages; (3) IUD types comparable to those available in the UK and nulliparous women of all ages (table 3). The estimated continuation rates at 12 months by IUD type, obtained from the included studies with data amenable to synthesis, is reported in table 4. Tau2 values for heterogeneity of the included studies are provided separately (see online supplemental material 7).
Table 3.
Summary of findings
| Study | IUD types (N*) | Age at insertion (years) | Study period | Continuation rates % (n) |
Discontinuation rates % (n) |
Removal for bleeding/pain % (n) | Expulsion % (n) | Pregnancy % (n) |
| Studies of IUD types currently available in the UK only involving nulliparous women aged ≤30 | ||||||||
| RCT | ||||||||
| Otero-Flores et al 44*† | TCu 380A (375) TCu 380A Nul (367) ML Cu 375 sl (374) |
23.2±6.8 22.4±6.6 22.6±6.4 |
12 months | 30.7 (115) 91.3 (335) 89.0 (333) |
69.3 (260) 8.7 (32) 11.0 (41) |
61.6 (231) 3.81 (14) 6.68 (25) |
3.47 (13) 1.91 (7) 1.87 (7) |
1.07 (4) 0.54 (2) 0.00 (0) |
| Non-RCT | ||||||||
| Abraham et al19 | Cu T380A (201) Cu T380A (44) Cu T380A (201) Cu T380A (44) |
20–25 <20 20–25 <20 |
12 months 24 months |
82 [95% CI 76-87] 79 [95% CI 64-89] 73 [95% CI 66-79] 64 [95% CI 48-77] |
ns ns |
ns ns |
ns ns |
ns ns |
| Hall and Kutler34 | Cu T 380A (21) | 18–30 | 12 months | 73.7 (14) | 26.3 (5) | 10.5 (2) | 10.5 (2) | 5.26 (1) |
| Studies of IUD types currently available in the UK involving nulliparous women of all ages | ||||||||
| RCTs | ||||||||
| Sivin and Stern46ठ| TCu 380A (2254) TCu 220C (1301) TCu 200 (4215) |
<20–35+ <20–35+ <20–35+ |
2 years | 55.7 57.8 54.2 |
44.3 42.2 45.8 |
21.9 19.5 16.8 |
7.8 9.8 9.8 |
0.8 1.6 5.1 |
| Non-RCTs | ||||||||
| Akintomide et al30 | TT380 Slimline (27) Mini TT380 Slimline (53) |
15–37 16–37 |
1 year | 66.7 (18) 86.8 (46) |
33.3 (9) 13.2 (7) |
ns ns |
3.7 (1) 3.77 (2) |
0 (0) 0 (0) |
| Elkhateeb et al32 | TCu 380A (90) | 16–>30 | 6 months | 94.4 (85) | 5.6 (5) | ns | 0 (0) | ns |
| Kaislasuo et al35† | Nova T380 (42) | 18–43 | 1 year | 83.3 (35) | 16.7 (7) | ns | 4.76 (2) | ns |
| Roy et al45 | TCu 380A (785) TCu 300 (347) TCu 200 (472) |
<14–>33 15–>33 <14–>33 |
12 months | 81.9 80.7 74.2 |
18.1 19.3 25.8 |
9.1 9.2 10.7 |
3.8 6.1 5.4 |
0.2 0.6 1.7 |
| Studies of IUD types comparable to those available in the UK involving nulliparous women of all ages | ||||||||
| RCTs | ||||||||
| Luukkainen et al39§¶ | Nova T200 (ns) Cu T200 (ns) |
≤19–≥35 ≤19–≥35 |
12 months | ns ns |
ns ns |
15.3 23.4 |
6 10.8 |
0.53 2.3 |
| Allonen et al31§¶ | Nova T200 (ns) Cu T200 (ns) |
≤19 -≥35 ≤19 -≥35 |
24 months | ns ns |
ns ns |
23.5 24 |
6.5 14 |
1.14 5.28 |
| Nygren et al42§ | Nova T200 (ns) Cu T200 (ns) |
<20 ->35 | 36 months | 36.9 31.0 |
ns ns | 28.3 (74) 28.2 (68) |
10.3 (27) 10.7 (26) |
1.5 (4) 6.5 (15) |
| Larsen et al36§ | Cu T200 (99) | 15–44 | 12 months | 73 | 27** | 16 | 5 | 1 |
| Luukkainen et al40 | Nova T200 (77) | 17–40 | 12 months | 73.1 | 26.9** | 10.4 | 9.2 | 0 |
| Ostergard and Gunning43 | TCu 200 (117) TCu 200 (115) |
18–34 | 6 months 12 months |
88.9 (104) 73.0 (84) |
11.1 (13) 27.0 (31) |
6.0 (7) 12.2 (14) |
3.41 (4) 6.09 (7) |
0 (0) 0 (0) |
| Non-RCTs | ||||||||
| Fugere33 | Nova T200 (54) | 17–42 | 24 months | ns | ns | 17.2 | 1.9 | 0 |
| Lewit37 | TCu-200 (2099) Nulligravid subgroup: TCu-200 (1585)† Age subgroups: TCu-200 (1130) TCu-200 (2468) TCu-200 (1513) TCu-200 (683) TCu-200 (449) |
15–49 15–49 15–19 20–24 25–29 30–34 35–49 |
1 year 1 year 1 year 1 year 1 year 1 year 1 year |
73.3 75.9 67.3 73.8 77.6 81.7 85.2 |
26.7 24.1 32.7 26.2 22.4 18.3 14.8 |
9.4 9.6 7 8.3 5.8 7.9 6.8 |
10.7 8.7 15 8.5 8.7 6 3.1 |
1.3 0.8 2.3 2.8 1.5 0.4 0.3 |
| Liedholm and Sjöberg38 | T-Cu 200 (208) | 14–40 | 12 months 24 months |
70.2 60.3 |
29.8 39.7 |
18.1 28 |
0.5 0.5 |
2.9 (6) 2.9 (6) |
| Mishell et al41§ | TCu 200 (471) | 14–33 | 3 months 6 months 12 months |
92.6 84.5 74.2 |
7.4 15.5 25.8 |
2.8 5.8 10.7 |
2.6 4.7 5.4 |
0.2 0.4 1.7 |
| Timonen et al47 | T Cu-300 (138) | <25–40+ | 12 months | 84.7 | 15.3 | 7.2 | 1.6 | 1.6 |
*Sample size or participants excluding those lost to follow-up or removals to plan pregnancy.
†Nulligravid women only.
‡A combination of double blind studies.
§Net cumulative rates.
¶Data obtained from graphs or figures.
**Not stated; obtained by subtraction of continuation rate from 100.
IUD, intrauterine device; ns, not stated; RCT, randomised controlled trial.
Table 4.
Estimated continuation rates at 12 months of IUD types from the included studies
| IUD type | Continuation rates with numbers of patients (n) and statistical heterogeneity (tau2 and I2) values of studies included in subgroup | ||
| Nulliparous women aged <30 | Nulliparous women of any age | Overall effect size (all studies) | |
| TCu 380A* | 81.60% (95% CI 76.52% to 86.21%)† (n=264; tau2=0.0; I2=0.0%, p=0.69)19 34 |
80.97% (95% CI 76.04% to 85.48%) (n=971; tau2=0.005; I2=27.6%, p=0.25)19 30 45 |
81.93% (95% CI 79.66% to 84.09%) (n=1235; tau2=0.0; I2=0.0%, p=0.62)19 30 34 45 |
| Smaller TCu 380A‡ |
Not applicable—only one study group | 91.02% (95% CI 88.01% to 93.64%) (n=420; tau2=0.0; I2=0.0%, p=0.51)30 44 |
91.02% (95% CI 88.01% to 93.64%) (n=420; tau2=0.0; I2=0.0%, p=0.51)30 44 |
| TCu 300 | Not applicable—no study | 81.92% (95% CI 78.35% to 85.24%) (n=485; tau2=0.0; I2=17.3%, p=0.27)45 47 |
81.92% (95% CI 78.35% to 85.24%) (n=485; tau2=0.0; I2=17.3%, p=0.27)45 47 |
| TCu 200 | 73.03% (95% CI 67.63% to 78.10%) (n=5111; tau2=0.010; I2=94.2%, p=<0.01)37 |
76.51% (95% CI 72.67% to 80.14%) (n=3277; tau2=0.012; I2=84.0%, p=<0.01)37–39 41 43 45 |
75.44% (95% CI 72.32% to 78.43%) (n=8388; tau2=0.012; I2=89.9%, p=<0.01)37–39 41 43 45 |
| Nova T200 | Not applicable—no study | 73.21% (95% CI 70.10% to 76.22%) (n=818; tau2=0.0; I2=0.0%, p=0.94)39 40 |
73.21% (95% CI 70.10% to 76.22%) (n=818; tau2=0.0; I2=0.0%, p=0.94)39 40 |
*Excludes Otero-Flores et al’s study data.
†Includes women aged 30 from Hall and Kutler’s study data.
‡TCu 380A Nul/Mini TT380 Slimline IUDs.
IUD, intrauterine device.
bmjopen-2021-060606supp007.pdf (72KB, pdf)
Studies of IUD types currently available in the UK only involving nulliparous women aged ≤30
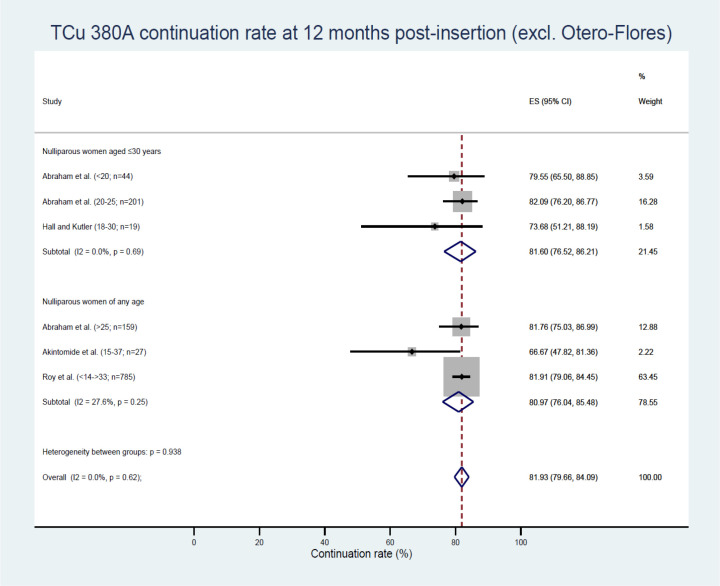
Three studies—Abraham et al19, Hall and Kutler34 and Otero-Flores et al44—reported on IUDs in women aged ≤30 involving the Copper T380A IUD (TCu 380A or Cu T380A).19 34 44 The TCu 380A data obtained from Otero-Flores et al44 was an outlier, with 30.7% reported as the continuation rate at 12 months.44 This was much lower than for the other two studies with a pooled estimate of 81.60% (95% CI 76.52% to 86.21%)19 34 (figure 2). When the Otero-Flores et al data were included in this TCu 380A meta-analysis, nulliparous women ≤30 years of age at 12 months had a continuation rate of 66.98% (95% CI 32.09% to 93.90%) (figure 3).
Figure 2.
TCu 380A continuation rates (excluding Otero-Flores). ES, effect size.
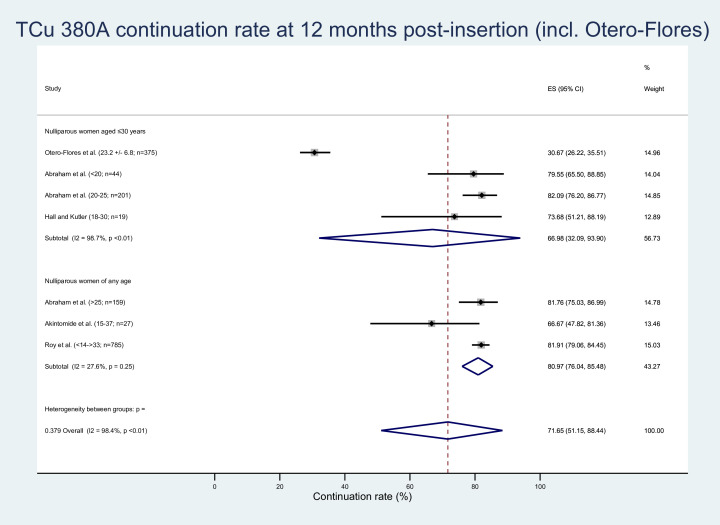
Figure 3.
TCu 380A continuation rates (including Otero-Flores). ES, effect size.
Continuation was also higher with age at 12 and 24 months when nulliparous TCu 380A IUD users aged <20 and 20–25 were compared (table 3).19
Studies of IUD types currently available in the UK involving nulliparous women of all ages
Five studies reporting data pertaining to seven population subgroups were amenable to meta-analysis examining the proportion of women continuing to use the TCu 380A IUD at 12 months post insertion.19 30 34 44 45 The pooled estimated continuation rate of the Copper T380A IUD type in nulliparous women of all ages from four studies was 81.93% (95% CI 79.66% to 84.09%).19 30 34 45 Additionally, statistical heterogeneity was found to be low/absent but was not statistically significant (tau2=0.0, I2=0.0%, p=0.62). Sensitivity analysis confirmed that the overall ES was largely robust to the exclusion of individual studies (−1.01% to +0.21% change in ES; see online supplemental material 4).
The estimated TCu 380A continuation rate in nulliparous women of all ages remained good at 71.65% (95% CI 51.15% to 88.44%; tau2=0.299, I2=98.4%, p=<0.01) when the Otero-Flores et al data were included44 (figure 3). An LFK index value of 6.77 identified major Doi plot asymmetry consistent with publication bias (see online supplemental material 8).
bmjopen-2021-060606supp008.pdf (162.7KB, pdf)
Individual studies showed the TCu 380A had higher discontinuation related to bleeding/pain and expulsion34 44 46 when compared with IUDs of smaller size or those with flexible arms30 44 (table 3).
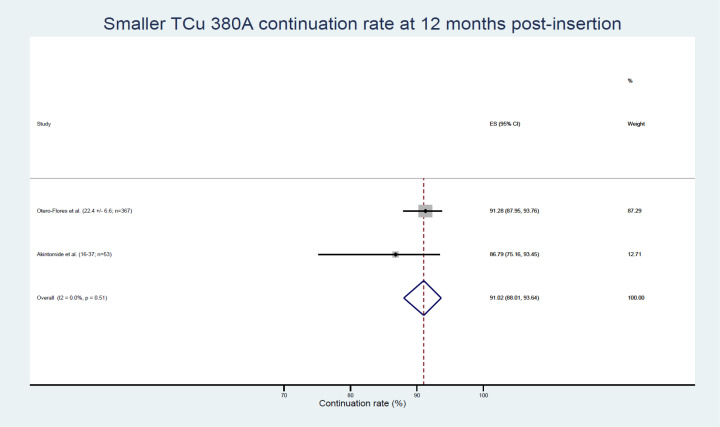
The highest continuation rates at 12 months were reported with smaller sized IUDs—the Copper 380A Nul (TCu 380A Nul: 91.3%), Multiload Copper 375 sl (ML Cu 375 sl: 89%) and Mini TT380 slimline (86.8%) (table 3). These data were obtained from only two studies whose participants were aged 15–37.30 44 Meta-analysis of continuation rate data on the TCu 380A Nul/Mini TT380 slimline IUD type gave a weighted average of 91.02% (95% CI 88.01% to 93.64%) (figure 4). These smaller IUDs were also associated with the lowest rates of removals for bleeding/pain (3.80%–6.68%) and expulsion (1.87%–3.77%) reported in nulliparous women at 12 months (table 3).
Figure 4.
Smaller TCu 380A continuation rates. ES, effect size.
Studies of IUD types comparable to those in the UK involving nulliparous women of all ages
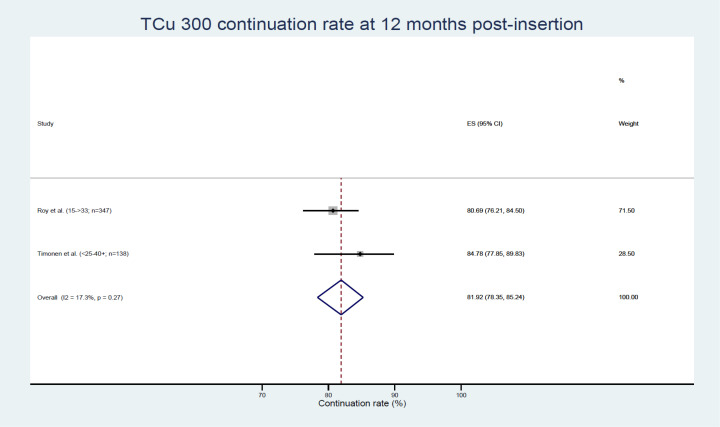
Two studies reporting data pertaining to two population subgroups were amenable to meta-analysis examining the proportion of women continuing to use the Copper T300 IUD (TCu 300) at 12 months post insertion, with an overall ES of 81.92% (95% CI 78.35% to 85.24%, see figure 5).45 47
Figure 5.
TCu 300 continuation rates. ES, effect size.
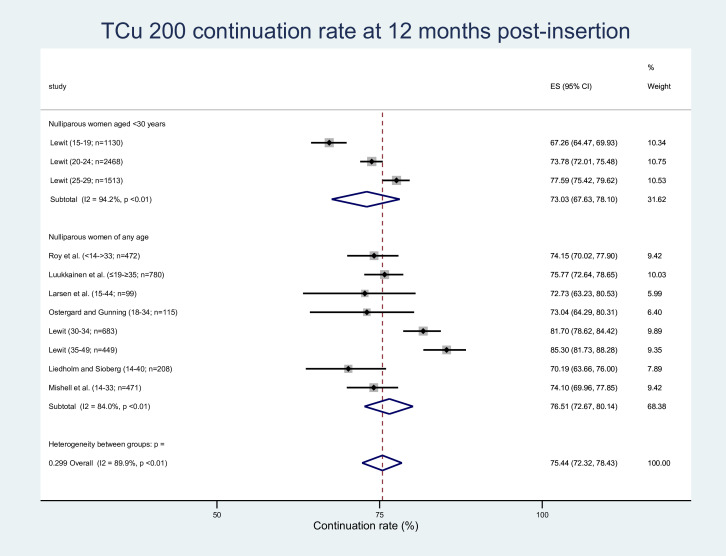
Seven studies reporting data pertaining to 11 population subgroups were amenable to meta-analysis examining the proportion of women continuing to use the Copper T200 IUD (TCu 200 or Cu T200) at 12 months post insertion, with a weighted average of 75.44% (95% CI 72.32% to 78.43%, see figure 6).36–38 40 41 43 45 These studies were also amenable to meta-analysis examining the proportion of women discontinuing the TCu 200 at 12 months post insertion due to bleeding and/or pain, expulsion and pregnancy (see online supplemental material 9). For these meta-analyses, nulliparous women aged <30 years compared with nulliparous women of any age were less likely to continue to use the TCu 200 at 12 months (73.03% (95% CI 67.63% to 78.10%) vs 76.51% (95% CI 72.67% to 80.14%)), and less likely to discontinue the TCu 200 due to bleeding and/or pain (7.05% (95% CI 5.59% to 8.65%) vs 12.77% (95% CI 8.48 to 17.78%)). Nulliparous women aged <30 years compared with nulliparous women of any age were however more likely to discontinue the TCu 200 due to expulsion (10.52% (95% CI 7.17% to 14.41%) vs 4.93% (95% CI 2.93% to 7.39%)) and pregnancy (2.19% (95% CI 1.47% to 3.05%) vs 1.15% (95% CI 0.54% to 1.95%)). The overlapping confidence intervals for these two ESs suggest the difference in effect is not statistically significant, and therefore may or may not be clinically significant. Statistical heterogeneity values for overall TCu 200 continuation rates as well as discontinuation rates for bleeding/pain and expulsion were tau2=0.012, I2=89.9%, p=<0.01; tau2=0.025 I2=93.2%, p=<0.01; and tau2=0.018, I2=96.3%, p=<0.01 respectively (see figure 6 and online supplemental material 9). Sensitivity analyses confirmed that the overall ESs were largely robust due to the exclusion of individual studies (see online supplemental material 4). In all cases, their LFK index values identified major Doi plot asymmetry consistent with publication bias (see online supplemental material 8).
Figure 6.
TCu 200 continuation rates. ES, effect size.
bmjopen-2021-060606supp009.pdf (290.1KB, pdf)
Continuation rates were seen to progressively improve with age where Lewit37 reported rates in nulliparous TCu 200 users by age groups 15–19, 20–24, 25–29, 30–34 and 35–4937 (table 3).
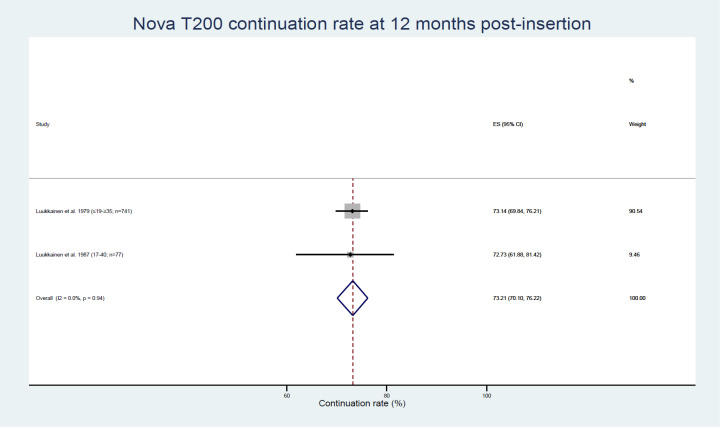
Two studies reporting data pertaining to two population subgroups were amenable to meta-analysis examining the proportion of women continuing to use the Nova T200 at 12 months post insertion, with a weighted average of 73.21% (95% CI 70.10% to 76.22%, see figure 7).39 40
Figure 7.
Nova T200 continuation rates. ES, effect size.
Studies also showed that IUDs with flexible arms (Nova T, Multiload) were associated with higher continuation and lower removal rates for bleeding/pain, expulsion and pregnancy when compared with IUDs with rigid arms (Cu T or TCu)31 39 44 (table 3).
Discussion
Findings and interpretation
Evidence on IUDs currently used in nulliparous women aged under 30 is limited. These findings estimate the continuation rate for the recommended TCu 380A IUD11 to be 81% at 12 months post insertion based on four studies involving young nulliparous women.19 30 34 45 This was the same estimate for the TCu 300 based on two studies.45 47 Smaller sized and flexible IUDs had higher continuation rates of 86%–91% in this group of women, based on two studies, as well as fewer removals for bleeding/pain and expulsion compared with the TCu 380A or IUDs of the same rigid design or size.30 44 Lower continuation rates of 75% and 73% were obtained for the Cu T200 and Nova T200 based on eight studies.36–41 43 45
The study by Otero-Flores et al was the only reported RCT solely involving IUDs currently used in the UK with nulliparous women aged ≤30.44 Over a thousand nulliparous women aged 15–30 were randomised to receive three different IUDs: TCu 380A (width 32 mm), TCu 380A Nul (width 23 mm) and ML Cu 375 sl (width≤20 mm), the latter two being primarily designed for nulliparous women. The TCu 380A overall rate of discontinuation (69.3%) and bleeding/pain as a reason for discontinuation (61.6%) were significantly higher than for TCu 380A Nul (8.7% and 3.81%) and ML Cu 375 sl (11.0% and 6.68%), as well as significantly different from rates reported by other included studies involving the TCu 380A. This could be because the TCu 380A considerably differs in size from the TCu 380A Nul and ML Cu 375 sl IUDs, and Otero-Flores et al also exclusively involved nulligravid participants (as opposed to nulliparous).
Sivin and Stern46 was the only other RCT involving a TCu 380A that reported separately on nulliparous users.46 However, their TCu 380A discontinuation and bleeding/pain rates, 44.3% and 21.9%, respectively, were obtained at 2 years and their participants were aged <20–35+ years.
The disparity in discontinuation rates reported by Otero-Flores et al44 and Sivin and Stern46 suggests that the findings by Otero-Flores et al may be unreliable. But it may in fact be inappropriate to directly compare other studies’ TCu 380A data, including that of Sivin and Stern, to Otero-Flores et al’s data. Their studies’ designs as well as participants’ ages, gravidity/parity, environments and reported durations of use were not the same. Otero-Flores et al’s participants were younger (≤30 years), exclusively nulligravid, ‘highly educated’ and based in a Mexico city with free access to healthcare in the millenial era, with the study being single-(patient) blinded. This contrasts with most studies involving the TCu 380A or similar IUDs where participants were more likely to be aged 30 years or older and parous with unspecified educational attainment. The Sivin and Stern study population were living and accessing healthcare (which was not stated to have been free) across the USA, in the late 1970s (over two decades earlier than the Otero-Flores et al’s study, and not long after the Dalkon Shield era), with the study being double-blinded. Other explanations for the disparity could be that the modern younger nulligravid cohort may be less tolerant of unwanted IUD effects, and that some contraceptive research may be less likely to acknowledge participants’ reasons and wishes for early IUD discontinuation.49
The TCu 200 IUD was ≥33 mm in width and/or height so perhaps larger than a standard-sized TCu 380A.50 IUD size may contribute to pain, which may explain TCu 200’s lower continuation rates compared with the TCu 380A. However the TCu 300, of the same design and size as the TCu 200,47 unexpectedly had a higher continuation rate than the TCu 200. This is because higher copper content has been associated with more bleeding which contributes to early discontinuation.51 The TCu 300 data were limited to two studies that both had total MMAT scores of 7,45 47 whereas the TCu 200 data had been obtained from seven studies with MMAT scores of 7,37 38 41 45 639 and 5,43 respectively.
Strengths and limitations
This is the first systematic review to explore IUD types in younger aged nulliparous women. It has included all observational studies that provided information on IUD continuation or reasons for discontinuation in this user group. Non-restriction to RCTs may be considered a limitation, but a realist approach of expanding the inclusion criteria where RCT evidence is lacking could be commendable and more representative of routine practice. Using the MMAT, the quality of reviewed and included studies in this systematic review was good overall.
Articles for inclusion were unfortunately limited to publications in the English language. There was an absence of studies on IUDs currently available in the UK and solely involving women aged under 30. This warranted including all ages if women under 30 years were involved, and up to (≤) 30 years for the TCu 380A data and meta-analysis because of the ages of the Hall and Kutler study participants (18–30 years). Many studies did not report all the required information, hence some included studies had missing information (table 3). Most studies did not differentiate between nulligravid and nulliparous participants, many age ranges were not specific (eg, ≤19–≥35), while some reports, for example, Sivin and Stern,46 were a combination of individual studies. Similarly, it appeared common for older studies to only state numbers (rather than rates or percentages), or only graphically depict data on continuation rates or unwanted effects. It is also not unusual for a systematic review to include such studies, for example, Hubacher7, and to calculate or measure rates accordingly, as has been done in this review. These are potential limitations which are not considered to impact the validity of the review. All mitigating actions that were taken have also been appropriately stated.
Relevance of findings
IUD use in young nulliparous women has been established to be safe, effective and acceptable.52–54 It is recommended that women are provided with the most appropriate IUD types for their uterine cavity size. Uterine cavity width (measurable using a cavimeter or ultrasonography, not routinely practised) in addition to uterine length (routinely measured using a hysterome) should be recognised as influencing IUD type choice.29 55–57 This systematic review suggests which IUD types may be more suitable for younger aged nulliparous women and emphasises the need for further research.
Recommendations
Strengthening the evidence for contraceptive choice and continuation is needed to improve sexual health in younger aged women. Prospective observational studies that include various IUD designs and types, and detailed reporting of users’ experiences could facilitate a better understanding of early IUD discontinuation and reasons for discontinuation based on IUD types. Studies designed to overcome the challenges of recruiting large numbers from varied demographic backgrounds, significant loss to follow-up, and time or funding constraints are also likely to yield data widely applicable to IUC provision in and outside the UK.
Conclusion
Research is lacking on outcomes with the IUD types currently in use by young nulliparous women in the UK. Available evidence estimates a continuation rate of 81% at 12 months for the recommended standard-sized TCu 380A IUD in these women. More studies are needed to better estimate continuation rates for smaller sized and flexible IUDs in this user group.
Supplementary Material
Acknowledgments
The authors are immensely grateful to the following for their expertise and support that greatly assisted this research: Diana Mansour, Consultant Community Gynaecologist, Newcastle upon Tyne Hospitals NHS Foundation Trust; Jill Shawe, Professor of Women’s Health, University of Plymouth; Judith Stephenson, Margaret Pyke Professor of Sexual & Reproductive Health, University College London; Mark Chambers, Electronic Services Librarian, Newcastle upon Tyne Hospitals NHS Foundation Trust; and Nataliya Brima, PhD Fellow, Kings College London.
Footnotes
Twitter: @midwifeAliJ
Contributors: HA: Research idea, study design, protocol, searches, first reviewer, data summary, writing—original draft, review and editing, funding application for open access publishing, project administration and guarantor. AJ: Second reviewer, supervision, writing—review and editing, project administration. PB: Searches, writing—review and editing. MM: Meta-analysis, writing—original draft, review and editing. JR: Contributed to research idea, study design, protocol, funding applications, and project administration, as well as supervision and writing—review and editing. All authors approved the final version.
Funding: This work was supported by the British Medical Association’s Foundation for Medical Research in the form of a Lift into Research 2019 grant to HA. JR is part-funded by the National Insitute of Health Research (NIHR) Applied Research Collaboration North East and North Cumbria, funded by the National Institute for Health Research (NIHR) Applied Research Collaboration (ARC) North East and North Cumbria (NIHR200173). The views expressed are those of the author(s) and not necessarily those of the British Medical Association's Foundation for Medical Research nor NIHR ARC.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Department of Health & Social Care: National Statistics . Abortion statistics, England and Wales: 2020, 2021. Available: https://www.gov.uk/government/statistics/abortion-statistics-for-england-and-wales-2020 [Accessed 20 Dec 2021].
- 2.NHS Digital . Statistics on sexual and reproductive health services (contraception): data (tables 6 and 7), 2021. Available: https://digital.nhs.uk/data-and-information/publications/statistical/sexual-and-reproductive-health-services/2020-21/data-tables [Accessed 23 Dec 2021].
- 3.BMJ Group and the Royal Pharmaceutical Society of Great Britain . British National formulary, 2021. Available: https://bnf.nice.org.uk/medicinal-forms/intra-uterine-contraceptive-devices-copper.html [Accessed 20 Dec 2021].
- 4.Teal SB, Sheeder J. IUD use in adolescent mothers: retention, failure and reasons for discontinuation. Contraception 2012;85:270–4. 10.1016/j.contraception.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 5.Akintomide H, Brima N, Mansour DJ, et al. Copper IUD continuation, unwanted effects and cost consequences at 1 year in users aged under 30 - a secondary analysis of the EURAS-IUD study. Eur J Contracept Reprod Health Care 2021;26:175–83. 10.1080/13625187.2021.1879783 [DOI] [PubMed] [Google Scholar]
- 6.Bateson D, Harvey C, Trinh L, et al. User characteristics, experiences and continuation rates of copper intrauterine device use in a cohort of Australian women. Aust N Z J Obstet Gynaecol 2016;56:655–61. 10.1111/ajo.12534 [DOI] [PubMed] [Google Scholar]
- 7.Hubacher D. Copper intrauterine device use by nulliparous women: review of side effects. Contraception 2007;75:S8–11. 10.1016/j.contraception.2006.12.005 [DOI] [PubMed] [Google Scholar]
- 8.Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol 2014;123:585–92. 10.1097/AOG.0000000000000144 [DOI] [PubMed] [Google Scholar]
- 9.O'Brien PA, Kulier R, Helmerhorst FM, et al. Copper-containing, framed intrauterine devices for contraception: a systematic review of randomized controlled trials. Contraception 2008;77:318–27. 10.1016/j.contraception.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence . Long-acting reversible contraception: clinical guideline, 2015. Available: https://www.nice.org.uk/guidance/cg30 [Accessed 20 Dec 2021]. [PubMed]
- 11.Clinical Effectiveness Unit . FSRH clinical guideline: intrauterine contraception, 2015 (Amended September 2019). Available: https://www.fsrh.org/standards-and-guidance/documents/ceuguidanceintrauterinecontraception/ [Accessed 20 Dec 2021].
- 12.Akintomide H, Barnes P, Brima N, et al. Copper intrauterine contraception discontinuation in nulliparous and young women (CRD42019120969), 2019. PROSPERO - International prospective register of systematic reviews. Available: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019120969 [Accessed 25 Aug 2022].
- 13.Hong QN, Pluye P, Fabregues S, et al. Mixed methods appraisal tool (MMAT), version 2018, 2018. Available: http://mixedmethodsappraisaltoolpublic.pbworks.com/w/file/fetch/127916259/MMAT_2018_criteria-manual_2018-08-01_ENG.pdf [Accessed 20 Dec 2021].
- 14.Borges Migliavaca C, Stein C, Colpani V, et al. How are systematic reviews of prevalence conducted? A methodological study. BMC Med Res Methodol 2020;20:96. 10.1186/s12874-020-00975-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions. version 6.3, 2022. www.training.cochrane.org/handbook [Google Scholar]
- 16.Rücker G, Schwarzer G, Carpenter JR, et al. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol 2008;8:79. 10.1186/1471-2288-8-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc 2018;16:195–203. 10.1097/XEB.0000000000000141 [DOI] [PubMed] [Google Scholar]
- 18.The Contraception Priority Setting Partnership . The ‘Top 10’ unanswered research priorities for contraceptive care, in The Contraception Priority Setting Partnership Report; 2018. https://www.fsrh.org/documents/fsrh-contraception-psp-report-2018-jla/ [Accessed 25 Aug 2022].
- 19.Abraham M, Zhao Q, Peipert JF. Young age, Nulliparity, and continuation of long-acting reversible contraceptive methods. Obstet Gynecol 2015;126:823–9. 10.1097/AOG.0000000000001036 [DOI] [PubMed] [Google Scholar]
- 20.Garbers S, Haines-Stephan J, Lipton Y, et al. Continuation of copper-containing intrauterine devices at 6 months. Contraception 2013;87:101–6. 10.1016/j.contraception.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 21.Goldstuck ND. Clinical evaluation of the combined multiload copper 250-mini IUD in selected nulliparous women. Contracept Deliv Syst 1980;1:379–87. [PubMed] [Google Scholar]
- 22.Lete I, Morales P, de Pablo JL. Use of intrauterine contraceptive devices in nulliparous women: personal experience over a 12-year period. Eur J Contracept Reprod Health Care 1998;3:190–3. 10.3109/13625189809167252 [DOI] [PubMed] [Google Scholar]
- 23.Ogedengbe OK, Giwa-Osagie OF, Oye-Adeniran BA. A comparison of multiload with Copper-T IUDs in a family planning clinic in Lagos. Br J Fam Plann 1991;17:67–9. [Google Scholar]
- 24.Phillips SJ, Hofler LG, Modest AM, et al. Continuation of copper and levonorgestrel intrauterine devices: a retrospective cohort study. Am J Obstet Gynecol 2017;217:57.e1–57.e6. 10.1016/j.ajog.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sivin I, Tatum HJ. Four years of experience with the TCu 380A intrauterine contraceptive device. Fertil Steril 1981;36:159–63. [PubMed] [Google Scholar]
- 26.Teal SB, Romer SE, Goldthwaite LM, et al. Insertion characteristics of intrauterine devices in adolescents and young women: success, ancillary measures, and complications. Am J Obstet Gynecol 2015;213:515.e1–515.e5. 10.1016/j.ajog.2015.06.049 [DOI] [PubMed] [Google Scholar]
- 27.Hindle WH. Clinical evaluation and follow-up on 3,829 IUD procedures. Trans Pac Coast Obstet Gynecol Soc 1978;45:105–10. [PubMed] [Google Scholar]
- 28.Patnaik BP, Mishra KP. User satisfaction and retention of Cu-T (IUD) amongst rural women in Orissa. Health and Population: Perspectives and Issues 2003;26:52–8. [Google Scholar]
- 29.Petersen KR, Brooks L, Jacobsen N, et al. Clinical performance of intrauterine devices in nulligravidae: is the length of the endometrial cavity of significance? Acta Eur Fertil 1991;22:225–8. [PubMed] [Google Scholar]
- 30.Akintomide H, Barnes P, Brima N, et al. Higher discontinuation rate with a standard-sized compared to a small-sized ‘gold standard’ copper intrauterine device: a case-control review. BMJ Sex Reprod Health 2019;45:263–8. 10.1136/bmjsrh-2018-200296 [DOI] [PubMed] [Google Scholar]
- 31.Allonen H, Luukkainen T, Nielsen NC, et al. Two-year rates for nova T and copper T in a comparative study. Contraception 1980;21:321–34. 10.1016/S0010-7824(80)80011-4 [DOI] [PubMed] [Google Scholar]
- 32.Elkhateeb RR, Kishk E, Sanad A, et al. The acceptability of using IUDs among Egyptian nulliparous women: a cross-sectional study. BMC Womens Health 2020;20:1–6. 10.1186/s12905-020-00977-9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Fugere P. Five years experience of intrauterine contraception with the Nova-T. Contraception 1990;41:1–7. 10.1016/0010-7824(90)90121-B [DOI] [PubMed] [Google Scholar]
- 34.Hall AM, Kutler BA. Intrauterine contraception in nulliparous women: a prospective survey. J Fam Plann Reprod Health Care 2016;42:36–42. 10.1136/jfprhc-2014-101046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaislasuo J, Heikinheimo O, Lähteenmäki P, et al. Menstrual characteristics and ultrasonographic uterine cavity measurements predict bleeding and pain in nulligravid women using intrauterine contraception. Hum Reprod 2015;30:1580–8. 10.1093/humrep/dev102 [DOI] [PubMed] [Google Scholar]
- 36.Larsen S, Hansen MK, Jacobsen JC, et al. Comparison between two IUDs: Progestasert and cut 200. Contracept Deliv Syst 1981;2:281–6. [PubMed] [Google Scholar]
- 37.Lewit S. Two years of experience with the Copper-T: a research report. Stud Fam Plann 1973;4:171–2. 10.2307/1965330 [DOI] [PubMed] [Google Scholar]
- 38.Liedholm P, Sjöberg NO. Two years experience with copper-T 200 in a Swedish population-a comparison between nulliparous and parous women. Contraception 1974;10:55–61. 10.1016/0010-7824(74)90132-2 [DOI] [PubMed] [Google Scholar]
- 39.Luukkainen T, Nielsen N-C, Nygren K-G, et al. Randomized comparison of clinical performance of two copper-releasing IUDs, Nova-T and Copper-T-200, in Denmark, Finland and Sweden. Contraception 1979;19:1–9. 10.1016/S0010-7824(79)80003-7 [DOI] [PubMed] [Google Scholar]
- 40.Luukkainen T, Allonen H, Haukkamaa M, et al. Effective contraception with the levonorgestrel-releasing intrauterine device: 12-month report of a European multicenter study. Contraception 1987;36:169–79. 10.1016/0010-7824(87)90012-6 [DOI] [PubMed] [Google Scholar]
- 41.Mishell DR, Israel R, Freid N. A study of the copper T intrauterine contraceptive device (TCu 200) in nulliparous women. Am J Obstet Gynecol 1973;116:1092–6. 10.1016/0002-9378(73)90942-3 [DOI] [PubMed] [Google Scholar]
- 42.Nygren KG, Nielsen NC, Pyörälä T, et al. Intrauterine contraception with Nova-T and copper-T-200 during three years. Contraception 1981;24:529–42. 10.1016/0010-7824(81)90057-3 [DOI] [PubMed] [Google Scholar]
- 43.Ostergard DR, Gunning JE. Intrauterine contraception with the copper T-200 and the Dalkon shield in nulligravid women. J Reprod Med 1976;17:172–4. [PubMed] [Google Scholar]
- 44.Otero-Flores JB, Guerrero-Carreño FJ, Vázquez-Estrada LA. A comparative randomized study of three different IUDs in nulliparous Mexican women. Contraception 2003;67:273–6. 10.1016/S0010-7824(02)00519-X [DOI] [PubMed] [Google Scholar]
- 45.Roy S, Cooper D, Mishell DR. Experience with three different models of the copper T intrauterine contraceptive device in nulliparous women. Am J Obstet Gynecol 1974;119:414–7. 10.1016/0002-9378(74)90303-2 [DOI] [PubMed] [Google Scholar]
- 46.Sivin I, Stern J. Long-acting, more effective copper T IUDs: a summary of U.S. experience, 1970-75. Stud Fam Plann 1979;10:263–81. 10.2307/1965507 [DOI] [PubMed] [Google Scholar]
- 47.Timonen H, Toivonen J, Luukkainen T. Use-effectiveness of the copper-T300 during the first year. Am J Obstet Gynecol 1974;120:466–9. 10.1016/0002-9378(74)90622-X [DOI] [PubMed] [Google Scholar]
- 48.Page M, McKenzie J, Bossuyt P, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J 2021;372. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inoue K, Barratt A, Richters J. Does research into contraceptive method discontinuation address women's own reasons? A critical review. J Fam Plann Reprod Health Care 2015;41:292–9. 10.1136/jfprhc-2014-100976 [DOI] [PubMed] [Google Scholar]
- 50.Museum of New Zealand . IUD 'Copper-T200, Schering'. Object | Part of History Collection. Available: https://collections.tepapa.govt.nz/object/1340909 [Accessed 23 Dec 2021].
- 51.O'Brien P. The effects of increasing the copper load on IUD performance: a systematic review. Eur J Contracept Reprod Health Care 2004;9:93. [Google Scholar]
- 52.Jatlaoui TC, Riley HEM, Curtis KM. The safety of intrauterine devices among young women: a systematic review. Contraception 2017;95:17–39. 10.1016/j.contraception.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foran T, Butcher BE, Kovacs G, et al. Safety of insertion of the copper IUD and LNG-IUS in nulliparous women: a systematic review. Eur J Contracept Reprod Health Care 2018;23:379–86. 10.1080/13625187.2018.1526898 [DOI] [PubMed] [Google Scholar]
- 54.Bahamondes MV, Bahamondes L. Intrauterine device use is safe among nulligravidas and adolescent girls. Acta Obstet Gynecol Scand 2021;100:641–8. 10.1111/aogs.14097 [DOI] [PubMed] [Google Scholar]
- 55.Kurz KH, Tadesse E, Haspels AA. In vivo measurements of uterine cavities in 795 women of fertile age. Contraception 1984;29:495–510. 10.1016/S0010-7824(84)80011-6 [DOI] [PubMed] [Google Scholar]
- 56.Bahamondes MV, Monteiro I, Canteiro R, et al. Length of the endometrial cavity and intrauterine contraceptive device expulsion. Int J Gynaecol Obstet 2011;113:50–3. 10.1016/j.ijgo.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 57.Wildemeersch D, Hasskamp T, Nolte K, et al. A multicenter study assessing uterine cavity width in over 400 nulliparous women seeking IUD insertion using 2D and 3D sonography. Eur J Obstet Gynecol Reprod Biol 2016;206:232–8. 10.1016/j.ejogrb.2016.09.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-060606supp001.pdf (61.6KB, pdf)
bmjopen-2021-060606supp002.pdf (48.3KB, pdf)
bmjopen-2021-060606supp003.pdf (51.7KB, pdf)
bmjopen-2021-060606supp004.pdf (70.6KB, pdf)
bmjopen-2021-060606supp005.pdf (98.8KB, pdf)
bmjopen-2021-060606supp006.pdf (99.2KB, pdf)
bmjopen-2021-060606supp007.pdf (72KB, pdf)
bmjopen-2021-060606supp008.pdf (162.7KB, pdf)
bmjopen-2021-060606supp009.pdf (290.1KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.