Abstract
Objective
The peRson-cEntred Support Programme EndoCrine Therapy intervention is a complex intervention encompassing a person-centred support programme for patients with breast cancer being treated with endocrine therapy (ET). The aim of this study was to explore the feasibility of the trial design and patient acceptability of the intervention and outcome measures and to provide data to estimate the parameters required to design the final intervention.
Design
A controlled before-and-after design following the Consolidated Standards of Reporting Trials 2010 statement for feasibility trials.
Setting
A surgical outpatient clinic in Sweden.
Participants
Forty-one patients (aged 47–85) with breast cancer who were treated with ET.
Interventions
Eligible patients were assigned to the control group or intervention group, which included individual education material, an individualised learning plan and a personalised reminder letter using a person-centred approach. The intervention could be delivered as a telephone or digital follow-up during a 12-week follow-up.
Outcome measures
The aims were to determine the recruitment rate, assess the rate of retention, explore whether the intervention was delivered according to the protocol, assess the preferred form of educational support, rate of education sessions, length per education session and length between each education session, determine the distribution of education materials and assess completion rates of patient-reported instruments, including the General Self-efficacy Scale, the Quality of Care from the Patient’s Perspective Questionnaire and the Memorial Symptom Assessment Scale.
Results
Eighty-six per cent of the patients in the intervention group completed the intervention and questionnaires 3 months after their inclusion. The call attendance was 90%. During the intervention, the contact nurse complied with the intervention protocol. For self-efficacy, symptoms and quality of care, there were no differences in effect size between the control and intervention groups.
Conclusions
This intervention seems to be feasible and acceptable among patients.
Keywords: Breast tumours, Quality in health care, QUALITATIVE RESEARCH
Strengths and limitations of this study.
This is the first study to investigate the feasibility of a person-centred support model for patients with breast cancer treated with endocrine therapy.
This study uses the Consolidated Standards of Reporting Trials 2010 statement for feasibility trials.
This study reports the recruitment rate, assesses the rate of retention, explores whether the intervention was delivered according to the protocol, assesses the preferred form of educational support, rate of education sessions, length per education session and length between each education session, determines the distribution of education materials and assesses completion rates of patient-reported instruments.
Due to the COVID-19 pandemic, face-to-face sessions were restricted.
Background
For women diagnosed with hormone receptor-positive breast cancer, endocrine therapy (ET), that is, the use of tamoxifen or aromatase inhibitors, is recommended for at least 5 years to reduce recurrence and rates of mortality.1 A previous study reported that up to 91% of patients experience side effects from ET,2 such as sleeping difficulties, hot flashes3 4 and musculoskeletal symptoms.5 Difficulties in managing these side effects have been reported to be obstacles to staying in treatment.6 Other challenges that have been identified include older age,7 medicine costs or a general dislike of taking a regular medicine.8 As ET is a long-lasting treatment, women may request support in managing challenges.9 To manage challenges with ET, a partnership with healthcare professionals could be appropriate, as a previous study10 identified that women with ET want to be able to manage their treatment but need guidance to do so.
Regarding the management of ET-related symptoms, previous studies have investigated the effect of symptom management interventions for patients prescribed ET.11–13 A study identified management needs for ET symptoms, emotional needs and needs for information acquisition and found that patients’ relationship with healthcare providers was important.12 A combination of information with cognitive–behavioural therapy to manage the side effects of tamoxifen showed successful results for the development of management skills among patients who were unable to stay in treatment.11 Furthermore, training intervention with a physiotherapist or personal trainer followed by adapted training at home could be effective. However, a problem with this intervention was programme adherence, as patients reported difficulty meeting the training goal in frequency and intensity due to other demands in life14 Additionally, training has not been found to have an effect on musculoskeletal symptoms in patients treated with Aromatase inhibitors (AIs).15 Managing a disease and its additional challenges requires self-care knowledge and skills gained from a partnership with healthcare professionals.16 Self-care involves the ability to both care for oneself and to achieve, promote and maintain optimal health.17 A common feature of self-care and person-centred care (PCC) is the ability to view humans as the agent and the subject of action.18 19 To include aspects of treatment important to the individual patient, such as different side effects, healthcare structures, fear of side effects and lack of management skills and for support, a person-centred support programme was developed.10 As self-care requires knowledge and skills,16 PCC could be appropriate for use in a support programme. Self-care requisites are described as all elements that individuals need at all stages in life to care for themselves, that is, air, food and water; self-care requisites also depend on how individuals react to illness.16 PCC can be a preferable way of identifying those requisites, as they can be identified in the narratives and used in the patient–healthcare provider partnership.19 Patients are often motivated to engage in self-care, as they have personal interest in acquiring requisite knowledge and skills for performing self-care operations to reach their intended health goals.16 It has been shown that when self-care capabilities increase,20 self-efficacy and adherence to ET also increase.21 22 Self-efficacy constitutes the self-image of the person and affects how people experience and behave in specific situations.23 Previous studies using PCC have improved patients’ self-efficacy.24–26
It is important for patients to not only identify accurate information but also assess and integrate the information to gain increased knowledge, self-efficacy and self-care skills.10 Moreover, in addition to the emotional needs identified by Kim et al,12 it is important to assess the amount of needed information and to explore patients’ understanding of diagnosis and treatment.27 For written health education materials to be effective, the patient must be able to apply the new information to her own life. This can be achieved by providing understandable examples and presenting the information, so the patient sees its relevance to her situation,28 as the ultimate reason for educating patients is to improve health.29
In Sweden, all patients are allocated a contact nurse when diagnosed with breast cancer. The contact nurse functions as the main point of contact during the patient’s cancer treatments to reduce fragmented care and to strengthen patient involvement in care.30 It has been suggested that contact nurses have a positive impact on care. Contact nurses aim to improve communication between patients and their healthcare professionals, as well as improve the care process.31 However, it has been reported that other factors seem to decrease contact nurses’ ability to provide the care they are meant to. Named reasons are challenges regarding the lack of information to patients and lack of supportive care resources. Although the patients had a contact nurse, the patients reported how they lacked the possibility to influence decisions about their care.32
A previous study developed a person-centred support programme in collaboration among patients, healthcare professionals, researchers and managers with ET experience10 that needed to be tested in a feasibility study using the Template for Intervention Description and Replication (TIDieR) checklist33 and the Consolidated Standards of Reporting Trials (CONSORT) 2010 statement.34 Previous studies have used feasibility studies prior to conducting a study in a larger setting.9 35 The intervention was developed to encourage patients to be more actively involved in their care and well-being as partners with their contact nurse.10 It has been stated that an intervention could be considered complex due to the behaviours required by those delivering the intervention,36 that is, a contact nurse. The complexity is caused by the context in which the intervention is to be implemented rather than the number of parts of the intervention.37 It has been reported that complex interventions require engagements with the care context stakeholders, that is, patients and contact nurses, to be able to identify if the intervention could be acceptable, operable, cost-effective, possible to scale up and transferable across contexts. The development phases were identified, including developing or using an existing complex intervention, feasibility, evaluation and implementation.38
Aim
In this feasibility trial, the aim was to explore the feasibility of the study design and the patient acceptability of the peRson-cEntred Support Programme EndoCrine Therapy intervention and outcome measures and to provide data to estimate the parameters required to design the final intervention.
Methods
Study design
This was a feasibility trial using a controlled before-and-after design39 to investigate the feasibility of the intervention, a person-centred support programme aimed at empowering patients prescribed ET to manage ET-related symptoms and problems. Allocation was based on inclusion time and not on patient preferences.
Patient and public involvement
Patients and healthcare professionals were involved in the design and development of the person-centred support programme.10 However, there was no patient involvement in the evaluation of the person-centred support programme presented in this study.
Participants
Between September 2020 and June 2021, 66 potential female patients from 1 outpatient clinic at 1 university hospital in Sweden were identified as eligible for inclusion when starting ET. Patients in the control group were included from September 2020 to December 2021, while patients in the intervention group were included from December 2021 to March 2021. The inclusion criteria were women>18 years of age who had been diagnosed with breast cancer and treated with ET after surgery. Patients receiving adjuvant chemotherapy were excluded as the study aimed to investigate an intervention targeting patients treated with ET. All patients were contacted by a contact nurse and were invited by telephone to participate in the study approximately 3 weeks after their surgery when prescribed ET (table 1). In the online supplemental files 1 and 2, the CONSORT flow diagrams for the usual care (UC) group and person-centred support programme group are available.
Table 1.
Demographic and clinical characteristics of the participants in the control group (n=20) and intervention group (n=21) in the peRson-cEntred Support Programme EndoCrine Therapy project
| Demographic characteristics | Control group, n=20 | Intervention group, n=21 |
| Median age, years (range) | 65 (50–85) | 66 (47–79) |
| Civil status, n (%) | ||
| Married/cohabiting | 12 (63%) | 16 (76%) |
| Single | 8 (37%) | 5 (23%) |
| Ancestral homeland, n (%) | ||
| Sweden | 16 (80%) | 18 (86%) |
| Scandinavian countries | 1 (5%) | 1 (5%) |
| Europe | 1 (5%) | 2 (10%) |
| Outside Europe | 1 (5%) | 0 (0%) |
| Education, n (%) | ||
| University | 9 (45%) | 10 (48%) |
| High school | 8 (40%) | 8 (38%) |
| Elementary school | 3 (15%) | 3 (14%) |
| Radiation therapy, n (%) | 16 (80%) | 21 (100%) |
| Tumour size, median mm (range) | 14 (4–45) | 12 (1–19) |
| Breast surgery | ||
| Mastectomy | 4 (20%) | 2 (10%) |
| Partial mastectomy | 15 (75%) | 19 (90%) |
| Axillary lymph node dissection | 1 (0.5%) | 0 (0%) |
| Tamoxifen, n (%) | 9 (45%) | 9 (43%) |
| Aromatase inhibitor, n (%) | 11 (55%) | 12 (57) |
bmjopen-2022-060946supp001.pdf (252.3KB, pdf)
All patients were given verbal and written information about the study, and after agreeing to have an informed consent form sent to them by mail, they all provided written informed consent. If the patient agreed to participate, she sent the informed consent form back using a prepaid envelope.
Control group
UC involves patients being allocated a contact nurse (an experienced undergraduate nurse or postgraduate nurse in surgical care), as the Swedish Patient Act40 gives patients a statutory right to permanent contact with a healthcare professional. Internationally, the role is called clinical nurse specialist41 and is identified as a valuable resource in cancer care.42
Patients can contact the contact nurse all weekdays by telephone or by using a nationwide digital tool, 1177.se.43 All patients receive written information as a brochure or a digital ‘My care and rehabilitation plan’ when diagnosed with breast cancer. Support in UC aims to give patients information about their state of health, available methods for examinations, care and treatments, as well as information about at which time point she can expect to receive care and permanent contact with the healthcare provider. The contact nurse writes down the information that is available before surgery, such as tumour characteristics and surgery preparations. The patient can also write down questions to bring to upcoming appointments. UC is based on patients’ initiative to make contact.
Intervention group
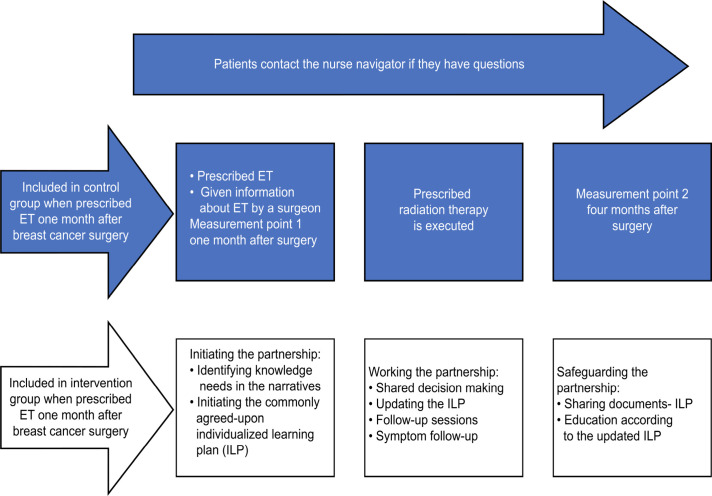
The intervention was provided in a surgical outpatient clinic in western Sweden from December 2020 to June 2021. The goal of the intervention was to empower patients prescribed ET to manage ET-related symptoms and problems. In addition to UC, a 12-week intervention was offered to the participants in the intervention group as described in a previous study.10 Figure 1 shows the care and measurement chain for the control and intervention groups.
Figure 1.
The care and measurement chain for the control and intervention groups. Both groups received the content in the blue area (usual care). ET, endocrine therapy.
Step 1: individual education material
Using a PCC approach,19 the contact nurse listened to patients’ narratives regarding their individual needs for knowledge and understanding, resources, goals and needs for support from the contact nurse. The timing of the supply of individual educational materials depended on the individual patient’s needs, resources and goals during the 12-week intervention. Mutual trust was demonstrated, and the relationship between the patient and her contact nurse was reinforced through the assessment of the commonly agreed on individualised learning plan (ILP).44
Step 2: an individualised learning plan (ILP)
An ILP was established depending on the individual patient’s needs for knowledge and understanding about ET and considering the patient’s resources, goals and needs for education material and support from the contact nurse. In combination with the individual educational materials (step 1), a follow-up plan was made using telephone and/or digital follow-up. Physical follow-ups were minimised as the COVID-19 pandemic was ongoing. The number of follow-up sessions and whether relatives were to be included during the 12-week intervention were agreed on between the patient and the contact nurse. Patients could also refuse all educational material and other materials and only use the contact nurse for support.
Step 3: a personalised reminder letter
The third part of the support programme was a personalised reminder letter after 3 months, including contact information and an invitation for patients to make contact if needed.
Education of the intervention nurse
The aim of the education was to increase the intervention nurse’s knowledge and understanding of ET, its problems and symptom management using PCC. Microteaching45 46 sessions and seminars were used; the microteaching sessions were adapted to the specific needs of knowledge about ET, side effects,10 pedagogy47 and PCC,19 48 and the chosen approach was intended to help the contact nurse take responsibility for her own learning, that is, student-centred learning.49 Additionally, practical exercises were used, as the contact nurse was able to practice her knowledge and understanding in a care setting and reflect on it, and the intervention nurse’s curiosity was used as a motivator to gain knowledge.47 A full description of the education of the intervention nurse is reported in the online supplemental file 3.
Data collection
Data were collected from September 2020 to June 2021. Feasibility outcomes were collected during the whole study period by the intervention nurse and were documented directly after every session in a trial log to secure the data collection.50 The trial log contained a summary of the results of the feasibility criteria using Excel (Microsoft Excel, V.16.50).
The three questionnaires were distributed by mail to patients in the control group (between September 2020 and December 2020) and the intervention group (December 2020 and March 2021). These three questionnaires were distributed at baseline, that is, at the start of the intervention and ET and 3 months after the start of the intervention.
The first questionnaire was the General Self-Efficacy (GSE), a 10-item (short form) psychometric scale that assesses optimistic self-beliefs to cope with a variety of demands in life. The GSE is a validated instrument that has been translated into Swedish51 and has previously been used with patients with breast cancer.52 The total score is the mean value of respondents’ answers to all items. High scores imply higher self-efficacy.
The second questionnaire was the Quality of Patient Perspective (QPP), a 45-item instrument that measures satisfaction in four dimensions: medical-technical competence, physical–technical conditions, identity-oriented approach and sociocultural atmosphere.53 54 Moreover, to identify patients’ views of whether the healthcare was adapted to their needs rather than healthcare routines, three items (“I was given the possibility to tell the medical staff how I experienced my situation; I was given the opportunity to participate in the planning of my care/treatment; I received the information I needed to be able to participate in decisions about my own care and treatment”) that were previously used by the Swedish SOM Institute were added.55 To calculate the execution index, each question is scored in terms of actual experience and subjective importance, each on a 4-point Likert scale. The execution index score ranges from 1 to 7, where 1 is inadequate quality of care from the patient perspective and 7 is good quality of care.56
The last questionnaire was the Memorial Symptom Assessment Scale (MSAS), a 32-question instrument for patients to rate their symptoms on a 5-point Likert scale.57 58 The instrument has been validated in Swedish patients with breast cancer58 and has previously been used with patients with breast cancer.52 The total MSAS Score is the average of the symptom scores for all 32 symptoms. Each symptom score is an average of the dimensions and includes the number of symptoms, how often patients experienced them, the severity of the symptoms and the cause of distress.
Feasibility outcomes
In this study, feasibility outcomes were defined as the primary outcome. Craig et al36 described several challenging variables that can affect an intervention’s results and conclusions. The feasibility classification (process, resources, scientific) and feasibility criteria reported by Thabane and Lancaster59 and Lancaster et al50 were used to collect feasibility data. Based on the recommendations for feasibility studies and an expected attrition rate of 20%, the sample size was set to 20 participants in each group.60 To determine whether the chosen feasibility criteria were successful,59 criteria for success were stated according to the CONSORT 2010 statement.34
The intervention process was assessed with the feasibility criteria as follows:
Recruitment was studied to determine whether the patients were willing to participate in this study. It has been suggested that the loss of participants should be less than 15%.61 The criterion was determined to be successful if the percentage rates of recruitment were >70%.
Retention was studied to determine whether the patients were willing to remain for the entire study period, that is, 12 weeks. The criterion was determined to be successful if the percentage rates of retention were >70%.
Compliance with the intervention protocol was studied to determine if the patients were offered the three parts of the planned intervention, that is, education materials, learning plan and personalised letter. The criterion was determined to be successful if all three parts of the intervention were offered.
The resources used in the intervention were assessed with the feasibility criteria as follows:
The form of educational support was studied to determine the preferred form of educational support during the intervention period, that is, 12 weeks. The criterion was determined to be successful if one of the three forms of educational support (face to face, telephone and digital) were requested by the patients.
The number of educational sessions was studied to determine how many educational opportunities the patient used during the intervention period, that is, 12 weeks. The criterion was determined to be successful if no more than four education sessions were used by each patient.
The length per education session was studied to determine how much time the patient spent in each education session. The criterion was determined to be successful if <45 min was used per education session. The time was clocked by the intervention nurse.
The length between each education session was studied to determine how often women wanted to have education opportunities. The criterion was determined to be successful if there were no more than 4 weeks between each education session.
The distribution of education materials was studied to determine how much intervention materials the patients received during the study. The criterion was determined to be successful if the distribution of education materials was >70%.
The scientific challenges of the intervention were assessed with the following feasibility criteria:
The completion rate of questionnaires was studied to determine if the patient was willing to answer the questionnaires, that is, at baseline and 12 weeks. The criterion was determined to be successful if the percentage rates of patient completion of questionnaires were >70% at baseline and 12 weeks.
The estimated treatment effect was studied to determine if the selected instruments were appropriate to measure patients’ self-efficacy, quality of care and symptoms. The criterion was determined to be successful if there were any changes in the self-report measures between the first and second points of measurement.
Patients included in the study responded to the questionnaires and returned the questionnaires in a prepaid envelope.
Analysis
To analyse demographic variables, we used descriptive statistics (numbers, percentages, means, ranges). We calculated the percentage rates of recruitment, retention and completion of questionnaires. We calculated the number, median and range of educational sessions, distribution of education materials, length per education session and length between each education session. As the study was a feasibility test, no hypothesis testing was applied,62 but p values were calculated and presented to evaluate their relevance in a forthcoming Randomized Controlled Trial (RCT). Descriptive statistical analyses and the Mann-Whitney U test were performed to identify the experience of symptoms, satisfaction with care and perceived self-efficacy. P values below 0.05 were considered statistically significant, and all analyses were performed with IBM SPSS Statistics V.27 (IBM SPSS Statistics for Macintosh, V.27.0).
Results
Participant demographics
In the control group, the median age was 65 years, 80% of the participants were born in Sweden, 63% were cohabiting and 45% were prescribed tamoxifen. In the intervention group, the median age was 66 years, 86% were born in Sweden, 76% were cohabiting and 43% were prescribed tamoxifen (table 1).
Feasibility classification and criteria
Feasibility outcomes are presented in line with the CONSORT 2010 statement as follows.
Recruitment
In the control group, 22 eligible patients were screened, and 20 were approached at the clinic, of whom 20 consented to participate (100%). In the intervention group, 44 patients were screened, of whom 24 were approached and 21 consented to participate (88%) (table 1), and patients were enrolled from December 2020 to April 2021. Of the three patients who did not consent to participate in the intervention group, two indicated the number of questions in the questionnaires to be a reason for not participating. One patient gave no reason for not participating.
Retention
In the intervention group, 20 patients completed the questionnaire (95%). One patient dropped out from the intervention because the study reminded her about breast cancer surgery, which she was trying to forget.
Compliance with the intervention protocol
In the first session, the patients’ needs for knowledge and understanding, resources, goals and support from the contact nurse were identified in their narratives. Education material was offered accordingly using a written agreement between the patient and contact nurse and documented in the ILP. Patients decided with the contact nurse whether they needed knowledge. If they needed knowledge, they stated when they wanted the education materials and which parts. Their need for knowledge ranged between having everything sent after the first session and having some of the education material sent at the end of the intervention. Patients could state that they did not want any education material at the start of the intervention but would reevaluate their needs during the 12 weeks of the intervention. However, since the ILP was sent home with the patients, any changes in the plan had to be documented by the patient herself. Two patients received the education materials sent to them but did not want to read it, just to have it if they wanted to read it later. Seven patients did not want the intervention for the full 12-week period (33%) but stated that they would make contact if they needed further information during the intervention. One patient wanted her partner to be included. Two patients in the intervention group did not answer the telephone at the scheduled session, making the call attendance 90%. One patient rescheduled a session for personal reasons. In total, 33% of the patients did not want follow-up sessions during the full 12-week intervention. As 90% of the patients wanted all educational materials, 10% of the patients used only the contact nurse for support, and 100% of the patients received a personalised reminder letter (table 2).
Table 2.
Resource needs for the intervention
| The peRson-cEntred Support Programme EndoCrine Therapy | |
| Distributed educational material | |
| Individual educational material, part 1, n | 21 |
| Individual educational material, part 2, n | 20 |
| Individual educational material, part 3, n | 19 |
| Individual educational material, part 4, n | 19 |
| Individual educational material | |
| Information about tamoxifen or aromatase inhibitors, n | 20 |
| Additional educational material from patient needs: | |
| Complementary medicine, n | 1 |
| Sleep advice, n | 1 |
| Recommendations about internet sites: | |
| Sleep advice, n | 2 |
| Form of education and educational sessions per patient | |
| Face to face (n=0), median (range) | 0 (–) |
| Telephone (n=20), median (range) | 3 (2–4) |
| Digital (n=1), median (range) | 1 (1) |
| Length (min) per session | |
| Telephone (n=20), median (range) | 20 (5–60) |
| Digital (n=1), median (range) | 30 (30–45) |
| Length of time (weeks) between each session | |
| Telephone follow-up education sessions, weeks, median (range) | 4 (1–6) |
| Digital meeting follow-up sessions, weeks, median (range) | 4 (–) |
| Follow-up educational session | |
| Time from 1st session to 2nd session, weeks, median (range) | 2 (1–8) |
| Time from 2nd session to 3rd session, weeks, median (range) | 4 (2–8) |
| Time from 3rd session to 4th session, weeks, median (range) | 4 (2–5) |
Contact information and an invitation for patients (100%) to make contact if needed were sent after 12 weeks in the personalised letter. None of the patients made contact after the 12-week intervention, as shown in table 2.
Resources
None of the patients wanted to have face-to-face sessions as educational support. In fact, several of the patients stated that it was important to not have to come for appointments at the hospital. Reasons for not wanting to come to the hospital were related to the COVID-19 pandemic as well as to perceptions of appointments at the hospital being time-consuming. All patients but one preferred telephone sessions. If a patient had asked for a face-to-face follow-up session, this would have been managed accordingly, with arrangements made to ensure safety in the context of the COVID-19 pandemic. Face-to-face meetings at the hospital with patients were not prohibited but restricted. However, no patient–contact nurse pairs participated in a face-to-face session; had they done so, both the patient and the contact nurse would have had to wear face masks, and the contact nurse would have also had to wear a plastic face shield to prevent transmission of the COVID-19 virus.
Number of educational sessions
The number of educational sessions ranged between two and four sessions (table 2).
Length per education session
Telephone support sessions ranged between 5 and 60 min, and digital support sessions ranged between 30 and 45 min (table 2) and were clocked by the intervention nurse.
Length between education sessions
The length between follow-up sessions ranged between 1 and 6 weeks (table 2).
Distribution of education materials
All patients (100%) wanted part 1 of the individual education material. A further description of the distribution is shown in table 2.
Completion rate of questionnaires
In the control group, 95% completed the first questionnaires at baseline, as 1 patient questionnaire was lost in the mail. At 3 months, 95% of patients responded to their surveys. In the intervention group, 100% responded to the first questionnaires and 86% responded to the follow-up questionnaires after 3 months. At the first measurement point, two reminder messages were sent to three patients in the intervention group before one patient was recorded as a dropout. At the second measurement point, one reminder message was sent to six patients 3 weeks after the questionnaires were sent. A second reminder message was sent approximately 2 weeks later to five patients, and as two patients did not return their questionnaires, they were recorded as dropouts. Two patients in the intervention group did not answer the telephone at the scheduled session, making the call attendance 90%. One patient rescheduled a session for personal reasons.
Estimated treatment effect
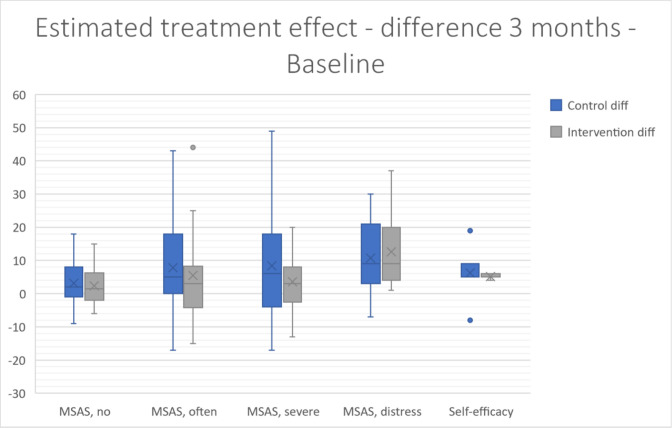
Differences between the control and intervention groups in perceived self-efficacy (0.5 and 0, p=0.731) and reported number of symptoms according to the MSAS (2 and 1, p=0.724) after 3 months were observed (figure 2). Median differences at baseline and 3 months in the control group and intervention group are also reported in the online supplemental file 4.
Figure 2.
Baseline and 3-month difference measures in the control and intervention groups for self-efficacy and reported symptoms. MSAS, Memorial Symptom Assessment Scale; no, number of symptoms; often, how often the patient had a symptom; severe, how severe the symptom usually experienced by the patient was; distress, how much the experienced symptom distressed or bothered the patient.
Quality of care was measured using the QPP, and these results are shown in the online supplemental file 5. Overall, the patients in the control group had higher quality of care index scores than patients in the intervention group.
Discussion
The results show that the intervention was feasible regarding the classification process and resources. However, it was less feasible regarding scientific challenges. The recruitment methods used seem to be accurate and feasible for an RCT with the aim of empowering patients prescribed ET to manage ET-related symptoms and problems. As a before-and-after design was used, the risk of contamination between groups was minor, as the intervention nurse had minor clinical contact with the control group.
The most common problems reported by trial investigators were a lack of adherence to the trial protocol, difficulties with recruitment, data collection and the intervention itself63; however, during the intervention, the contact nurse succeeded in adhering to the trial protocol. Moreover, a weakness in interventions has been reported to be a lack of a theoretical approach64; to address this challenge, the present study was founded on a theoretical model.10 Modelling was used to identify pitfalls and barriers.65 The contact nurse in the intervention pretested the intervention protocol in clinical meetings with patients before launching the support programme. It is crucial for healthcare professionals to have deep insight into the theory and selected methods to increase patients’ required knowledge about ET since no one will adhere to a protocol if the protocol is of no significance.47 Moreover, a healthcare context is referred to as a complex adaptive system, as it contains a collection of individuals with the freedom to act in a way that is not always predictable and changes depending on the context. Moreover, a complex adaptive system has fuzzy boundaries, and healthcare professionals’ priorities depend on, for example, their private lives and things they would not sacrifice to follow a study protocol.66 These challenges need to be addressed, as the intervention is to be applied in a care setting.
Furthermore, as the follow-up is flexible and the patients decided, in a partnership with the contact nurse, how many educational sessions were needed, the patient’s individuality and resources were a focus, and no patient needed to acquire education than necessary. This needed flexibility is another component making the intervention a complex intervention. Moreover, the COVID-19 pandemic has increased the use of digital follow-up, which seems to be well liked among patients.67–69 Furthermore, face-to-face sessions were not prohibited during the intervention but were restricted due to the COVID-19 pandemic. If a patient would have asked for a face-to-face session, this would have been managed to not put the patient, fellow patients, or the contact nurse or other healthcare professionals in danger. However, we cannot specifically state that patients would prefer telephone sessions under other circumstances, but telephone follow-up seems to be suitable, as patients indicated physical appointments to be time-consuming. A previous study also used telephone follow-up to increase confidence in controlling illness in patients with chronic obstructive pulmonary disease with positive results in controlling symptoms (p=0.028),70 and telephone follow-ups were found to be well liked among registered nurses.71 A previous study using PCC also allowed patients to decide the number of follow-up sessions.72 Thus, this approach could be a preferable way to administer the intervention and could also be more cost-effective, as patients do not need to attend more sessions than needed; however, it needs to be evaluated further. Furthermore, not all healthcare professionals have a PCC approach, which might affect the responses to the questionnaires and the interpretation of the results. To manage this, the whole care chain needs to structure its work according to PCC, as in a previous study.24
Moreover, the intervention protocol ranged over 12 weeks, as this period has been identified as the most troublesome for patients with ET,73 and a previous study identified that the start of the ET period could be preferable for an intervention.35 As 67% of the patients wanted education during the full 12-week intervention, 12 weeks is indicated to be a suitable length for a support programme in a future RCT. However, an optional follow-up session after 6 months, when the patients have more experience with ET, could be appropriate, but measures would need to be taken to help patients stay focused on ET when responding to the questionnaires. A later session could also be preferable for patients who do not want to be educated during the first months undergoing ET.35
To address scientific challenges, two measurement points were used, baseline and 3 months after being prescribed ET. In an RCT, additional measurement points could be added at 6 and 12 months. However, there were no differences in self-efficacy between the control and intervention groups; rather, both the control group and the intervention had high self-efficacy scores at baseline, indicating that the ceiling level was reached. Higher education implies higher self-efficacy74 (p=0.017).75 In the present study, 45% of the patients in the control group and 48% of the patients in the intervention group had university education, indicating that the GES may not be suitable as an instrument. General self-efficacy has been increased using PCC in a previous study in patients with acute coronary syndrome,72 indicating that patients with breast cancer could also benefit from PCC. This is of importance, as low self-efficacy has been identified as a predictor of terminating ET prematurely76 due to beliefs about its low influence on health or low satisfaction with involvement in healthcare.76
The study had some limitations. As the COVID-19 pandemic was ongoing, there were restrictions on the patients’ ability to have face-to-face sessions with the trial leader. However, several patients stated that they would participate only if there were no mandatory sessions at the hospital. The patients also had the possibility of having their sessions using a digital conference system. As the intervention contact nurse and the participants almost never met in person, their relationships could have been affected. However, a partnership was established between the patient and the trial leader using a PCC protocol. This might have decreased the effect of not meeting in person. In a future RCT, it will be crucial for patients to have face-to-face relationships with the intervention contact nurse with whom they will build partnerships. This study did not identify when the intervention should stop, as it was decided before the intervention that it should last for 12 weeks. It might have been important for the patients in the intervention to have given this important information. However, seven of the 21 patients did not use the full 12-week intervention, which implies that a 12-week support programme is suitable. No patient actively asked for longer follow-up. All patients were allocated a contact nurse whom they could contact after the intervention if further questions were answered.
Conclusion
This intervention seems to be feasible regarding its process and resources and acceptable among patients, as 95% completed the 12-week support programme and 86% responded to the 3-month questionnaire. A telephone follow-up intervention seems to be the preferable way to administer the intervention. However, for self-efficacy and symptoms, there were no differences in effect size between the control and intervention groups, indicating that the intervention was less feasible regarding scientific challenges.
Supplementary Material
Acknowledgments
We thank all patients for sharing their personal experiences of life with endocrine therapy. We would also like to thank the staff of the surgical outpatient care unit for contributing to finding eligible patients to include in this study.
Footnotes
Contributors: SAK contributed to conceptualisation, formal analysis, funding acquisition, methodology, visualisation, writing—original draft. IH contributed to supervision, conceptualisation, methodology, funding acquisition, writing—review & editing. ROB contributed to supervision, conceptualisation, methodology, writing—review & editing. CW contributed to supervision, conceptualisation, methodology, writing—review & editing. SAK is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: SAK has received honoraria from the R&D Council in Gothenburg and Bohuslän (VGFOUGSB-932984). IH is currently receiving a grant from the Bröstcancerforbundet, grant number N/A. The remaining authors have no funding sources to declare.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Swedish Ethical Review Authority (approval no 2020-03239). Participants gave informed consent to participate in the study before taking part.
References
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771–84. 10.1016/S0140-6736(11)60993-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkowitz MJ, Thompson CK, Zibecchi LT, et al. How patients experience endocrine therapy for breast cancer: an online survey of side effects, adherence, and medical team support. J Cancer Surviv 2021;15:29–39. 10.1007/s11764-020-00908-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahlstedt Karlsson S, Wallengren C, Olofsson Bagge R, et al. Women's coping strategies during the first three months of adjuvant endocrine therapy for breast cancer. Nurs Open 2020;7:605–12. 10.1002/nop2.430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moon Z, Hunter MS, Moss-Morris R, et al. Factors related to the experience of menopausal symptoms in women prescribed tamoxifen. J Psychosom Obstet Gynaecol 2017;38:226–35. 10.1080/0167482X.2016.1216963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nabieva N, Fehm T, Häberle L, et al. Influence of side-effects on early therapy persistence with letrozole in post-menopausal patients with early breast cancer: results of the prospective EvAluate-TM study. Eur J Cancer 2018;96:82–90. 10.1016/j.ejca.2018.03.020 [DOI] [PubMed] [Google Scholar]
- 6.Bluethmann SM, Murphy CC, Tiro JA, et al. Deconstructing decisions to initiate, maintain, or discontinue adjuvant endocrine therapy in breast cancer survivors: a mixed-methods study. Oncol Nurs Forum 2017;44:E101–10. 10.1188/17.ONF.E101-E110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavazza M, Banks H, Ercolanoni M, et al. Factors influencing adherence to adjuvant endocrine therapy in breast cancer-treated women: using real-world data to inform a switch from acute to chronic disease management. Breast Cancer Res Treat 2020;183:189–99. 10.1007/s10549-020-05748-6 [DOI] [PubMed] [Google Scholar]
- 8.Shinn EH, Broderick G, Fellman B, et al. Simulating time-dependent patterns of nonadherence by patients with breast cancer to adjuvant oral endocrine therapy. JCO Clin Cancer Inform 2019;3:1–9. 10.1200/CCI.18.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Meglio A, Soldato D, Presti D, et al. Lifestyle and quality of life in patients with early-stage breast cancer receiving adjuvant endocrine therapy. Curr Opin Oncol 2021;33:553-573. 10.1097/CCO.0000000000000781 [DOI] [PubMed] [Google Scholar]
- 10.Ahlstedt Karlsson S, Henoch I, Olofsson Bagge R, et al. An intervention mapping-based support program that empowers patients with endocrine therapy management. Eval Program Plann 2022;92:102071. 10.1016/j.evalprogplan.2022.102071 [DOI] [PubMed] [Google Scholar]
- 11.Moon Z, Moss-Morris R, Hunter MS, et al. Development of a self-management intervention to improve tamoxifen adherence in breast cancer survivors using an intervention mapping framework. Support Care Cancer 2021;29:3329–38. 10.1007/s00520-020-05850-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SH, Park S, Kim SJ, et al. Self-management needs of breast cancer survivors after treatment: results from a focus group interview. Cancer Nurs 2020;43:78–85. 10.1097/NCC.0000000000000641 [DOI] [PubMed] [Google Scholar]
- 13.Shelby RA, Dorfman CS, Bosworth HB, et al. Testing a behavioral intervention to improve adherence to adjuvant endocrine therapy (aet). Contemp Clin Trials 2019;76:120–31. 10.1016/j.cct.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duijts SFA, van Beurden M, Oldenburg HSA, et al. Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: results of a randomized, controlled, multicenter trial. J Clin Oncol 2012;30:4124–33. 10.1200/JCO.2012.41.8525 [DOI] [PubMed] [Google Scholar]
- 15.Roberts KE, Rickett K, Feng S, et al. Exercise therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer. Cochrane Database Syst Rev 2020;1:CD012988. 10.1002/14651858.CD012988.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLauglin Renpenning KT S. G. self-care theory in nursing. In: Selected papers of Dorothea Orem. New York: Springer Publishing Company, 2003. [Google Scholar]
- 17.Richard AA, Shea K. Delineation of self-care and associated concepts. J Nurs Scholarsh 2011;43:255–64. 10.1111/j.1547-5069.2011.01404.x [DOI] [PubMed] [Google Scholar]
- 18.Denyes MJ, Orem DE, Bekel G, et al. Self-care: a foundational science. Nurs Sci Q 2001;14:48–54. 10.1177/089431840101400113 [DOI] [PubMed] [Google Scholar]
- 19.Ekman I, Swedberg K, Taft C, et al. Person-centered care--ready for prime time. Eur J Cardiovasc Nurs 2011;10:248–51. 10.1016/j.ejcnurse.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 20.Bağ E, Mollaoğlu M. The evaluation of self-care and self-efficacy in patients undergoing hemodialysis. J Eval Clin Pract 2010;16:605–10. 10.1111/j.1365-2753.2009.01214.x [DOI] [PubMed] [Google Scholar]
- 21.Toivonen KI, Williamson TM, Carlson LE, et al. Potentially modifiable factors associated with adherence to adjuvant endocrine therapy among breast cancer survivors: a systematic review. Cancers 2020;13. doi: 10.3390/cancers13010107. [Epub ahead of print: 31 12 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon Z, Moss-Morris R, Hunter MS, et al. Barriers and facilitators of adjuvant hormone therapy adherence and persistence in women with breast cancer: a systematic review. Patient Prefer Adherence 2017;11:305–22. 10.2147/PPA.S126651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Self-efficay BA. The exercise of control. New York: WH Freeman, 1997. [Google Scholar]
- 24.Fors A, Taft C, Ulin K, et al. Person-centred care improves self-efficacy to control symptoms after acute coronary syndrome: a randomized controlled trial. Eur J Cardiovasc Nurs 2016;15:186–94. 10.1177/1474515115623437 [DOI] [PubMed] [Google Scholar]
- 25.Ali L, Wallström S, Ekman I, et al. Effects of person-centred care via telephone on self-efficacy in patients with chronic obstructive pulmonary disease: subgroup analysis of a randomized controlled trial. Nurs Open 2021;8:927-935. 10.1002/nop2.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf A, Fors A, Ulin K, et al. An eHealth diary and symptom-tracking tool combined with person-centered care for improving self-efficacy after a diagnosis of acute coronary syndrome: a substudy of a randomized controlled trial. J Med Internet Res 2016;18:e40. 10.2196/jmir.4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wittenberg E, Goldsmith J, Parnell TA. Development of a communication and health literacy curriculum: optimizing the informal cancer caregiver role. Psychooncology 2020;29:766–74. 10.1002/pon.5341 [DOI] [PubMed] [Google Scholar]
- 28.Bernier MJ. Developing and evaluating printed education materials: a prescriptive model for quality. Orthop Nurs 1993;12:39–46. 10.1097/00006416-199311000-00008 [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann T, Worrall L. Designing effective written health education materials: considerations for health professionals. Disabil Rehabil 2004;26:1166–73. 10.1080/09638280410001724816 [DOI] [PubMed] [Google Scholar]
- 30.Swedish Government Official Reports SOU, (in Swedish) . En nationell cancerstrategi för framtiden; 2009: 11.
- 31.Pautasso FF, Zelmanowicz AdeM, Flores CD, et al. Role of the nurse navigator: integrative review. Rev Gaucha Enferm 2018;39:e20170102. 10.1590/1983-1447.2018.2017-0102 [DOI] [PubMed] [Google Scholar]
- 32.Westman B, Ullgren H, Olofsson A, et al. Patient-reported perceptions of care after the introduction of a new advanced cancer nursing role in Sweden. Eur J Oncol Nurs 2019;41:41–8. 10.1016/j.ejon.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 34.Eldridge SM, Chan CL, Campbell MJ, et al. Consort 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud 2016;2:64. 10.1186/s40814-016-0105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon Z, Moss-Morris R, Hunter MS, et al. Acceptability and feasibility of a self-management intervention for women prescribed tamoxifen. Health Educ J 2019;78:901–15. 10.1177/0017896919853856 [DOI] [Google Scholar]
- 36.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new medical research council guidance. Int J Nurs Stud 2013;50:587–92. 10.1016/j.ijnurstu.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 37.Moore GF, Evans RE, Hawkins J, et al. From complex social interventions to interventions in complex social systems: future directions and unresolved questions for intervention development and evaluation. Evaluation 2019;25:23–45. 10.1177/1356389018803219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of medical Research Council guidance. BMJ 2021;374:n2061. 10.1136/bmj.n2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sedgwick P. Before and after study designs. BMJ 2014;349:g5074. 10.1136/bmj.g5074 [DOI] [PubMed] [Google Scholar]
- 40.SFS . Patient act; 2014. [Accessed 18 May 2021].
- 41.Schober M, Lehwaldt D, Rogers M. Guidelines on advanced practice nursing; 2020.
- 42.Kerr H, Donovan M, McSorley O. Evaluation of the role of the clinical nurse specialist in cancer care: an integrative literature review. Eur J Cancer Care 2021;30:e13415. 10.1111/ecc.13415 [DOI] [PubMed] [Google Scholar]
- 43.Sjukdomar & besvär/Cancer/ Råd och stöd vid cancerKontaktsjuksköterskan – ditt stöd i vården vid cancer (21-07-21). Available: http://www.1177.se
- 44.Granger BB, Britten N, Swedberg K, et al. Dumping adherence: a person-centred response for primary care. Fam Pract 2020;37:862–4. 10.1093/fampra/cmaa060 [DOI] [PubMed] [Google Scholar]
- 45.Higgins A, Nicholl H. The experiences of lecturers and students in the use of microteaching as a teaching strategy. Nurse Educ Pract 2003;3:220–7. 10.1016/S1471-5953(02)00106-3 [DOI] [PubMed] [Google Scholar]
- 46.Remesh A, Microteaching RA. Microteaching, an efficient technique for learning effective teaching. J Res Med Sci 2013;18:158–63. [PMC free article] [PubMed] [Google Scholar]
- 47.Dewey J. Democray and education; 2016.
- 48.Ekman I. Personcentrering inom hälso- och sjukvård. Från filosofi till praktik. In: Liber AB, 2014. [Google Scholar]
- 49.Neill GM, McMahon T. Student–centered learning: what does it mean for students and lecturers? In: Emerging issues in the practice of university learning and teaching, 2005. [Google Scholar]
- 50.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307–12 10.1111/j.2002.384.doc.x [DOI] [PubMed] [Google Scholar]
- 51.Löve J, Moore CD, Hensing G. Validation of the Swedish translation of the general self-efficacy scale. Qual Life Res 2012;21:1249–53. 10.1007/s11136-011-0030-5 [DOI] [PubMed] [Google Scholar]
- 52.Thieme M, Einenkel J, Zenger M, et al. Optimism, pessimism and self-efficacy in female cancer patients. Jpn J Clin Oncol 2017;47:849–55. 10.1093/jjco/hyx079 [DOI] [PubMed] [Google Scholar]
- 53.Wilde B, Starrin B, Larsson G, et al. Quality of care from a patient perspective--a grounded theory study. Scand J Caring Sci 1993;7:113–20. 10.1111/j.1471-6712.1993.tb00180.x [DOI] [PubMed] [Google Scholar]
- 54.Wilde-Larsson B, Larsson G. Patients' views on quality of care and attitudes towards re-visiting providers. Int J Health Care Qual Assur 2009;22:600–11. 10.1108/09526860910986867 [DOI] [PubMed] [Google Scholar]
- 55.Wallström SE, I; Taft C. Svenskarnas syn på personcentrering i vården. SOM-institutets sjuttonde forskarantologi (red Andersson, Ohlsson, Oscarsson och Oskarsson); 2017.
- 56.Wilde-Larsson B, Larsson G. Reviderat åtgärdsindex. PM 2016:1. Halmstad, Sweden: Improveit Digital Solutions AB; 2016. [Google Scholar]
- 57.Portenoy RK, Thaler HT, Kornblith AB, et al. The memorial symptom assessment scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer 1994;30A:1326–36. 10.1016/0959-8049(94)90182-1 [DOI] [PubMed] [Google Scholar]
- 58.Browall M, Kenne Sarenmalm E, Nasic S, et al. Validity and reliability of the Swedish version of the memorial symptom assessment scale (MSAS): an instrument for the evaluation of symptom prevalence, characteristics, and distress. J Pain Symptom Manage 2013;46:131–41. 10.1016/j.jpainsymman.2012.07.023 [DOI] [PubMed] [Google Scholar]
- 59.Thabane L, Lancaster G. A guide to the reporting of protocols of pilot and feasibility trials. Pilot Feasibility Stud 2019;5:37. 10.1186/s40814-019-0423-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 2005;4:287–91. 10.1002/pst.185 [DOI] [Google Scholar]
- 61.Zelle DM, Kok T, Dontje ML, et al. The role of diet and physical activity in post-transplant weight gain after renal transplantation. Clin Transplant 2013;27:E484–90. 10.1111/ctr.12149 [DOI] [PubMed] [Google Scholar]
- 62.Eldridge SM, Lancaster GA, Campbell MJ, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS One 2016;11:e0150205. 10.1371/journal.pone.0150205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eldridge SM, Ashby D, Feder GS, et al. Lessons for cluster randomized trials in the twenty-first century: a systematic review of trials in primary care. Clin Trials 2004;1:80–90. 10.1191/1740774504cn006rr [DOI] [PubMed] [Google Scholar]
- 64.Heiney SP, Parker PD, Felder TM, et al. A systematic review of interventions to improve adherence to endocrine therapy. Breast Cancer Res Treat 2019;173:499–510. 10.1007/s10549-018-5012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rowlands G, Sims J, Kerry S. A lesson learnt: the importance of modelling in randomized controlled trials for complex interventions in primary care. Fam Pract 2005;22:132–9. 10.1093/fampra/cmh704 [DOI] [PubMed] [Google Scholar]
- 66.Plsek PE, Greenhalgh T. Complexity science: the challenge of complexity in health care. BMJ 2001;323:625–8. 10.1136/bmj.323.7313.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gunasekeran DV, Tham Y-C, Ting DSW, et al. Digital health during COVID-19: lessons from operationalising new models of care in ophthalmology. Lancet Digit Health 2021;3:e124–34. 10.1016/S2589-7500(20)30287-9 [DOI] [PubMed] [Google Scholar]
- 68.Budd J, Miller BS, Manning EM, et al. Digital technologies in the public-health response to COVID-19. Nat Med 2020;26:1183–92. 10.1038/s41591-020-1011-4 [DOI] [PubMed] [Google Scholar]
- 69.Vargo D, Zhu L, Benwell B, et al. Digital technology use during COVID ‐19 pandemic: A rapid review. Hum Behav Emerg Technol 2021;3:13–24. 10.1002/hbe2.242 [DOI] [Google Scholar]
- 70.Ali L, Wallström S, Ekman I, et al. Effects of person-centred care via telephone on self-efficacy in patients with chronic obstructive pulmonary disease: subgroup analysis of a randomized controlled trial. Nurs Open 2021;8:927–35. 10.1002/nop2.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boström E, Ali L, Fors A, et al. Registered nurses' experiences of communication with patients when practising person-centred care over the phone: a qualitative interview study. BMC Nurs 2020;19:54. 10.1186/s12912-020-00448-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fors A, Ekman I, Taft C, et al. Person-centred care after acute coronary syndrome, from hospital to primary care - A randomised controlled trial. Int J Cardiol 2015;187:693–9. 10.1016/j.ijcard.2015.03.336 [DOI] [PubMed] [Google Scholar]
- 73.Fontein DBY, Nortier JWR, Liefers GJ, et al. High non-compliance in the use of letrozole after 2.5 years of extended adjuvant endocrine therapy. results from the ideal randomized trial. Eur J Surg Oncol 2012;38:110–7. 10.1016/j.ejso.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 74.Foster C, Breckons M, Cotterell P, et al. Cancer survivors' self-efficacy to self-manage in the year following primary treatment. J Cancer Surviv 2015;9:11–19. 10.1007/s11764-014-0384-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sherer M, Maddux JE, Mercandante B, et al. The self-efficacy scale: construction and validation. Psychol Rep 1982;51:663–71. 10.2466/pr0.1982.51.2.663 [DOI] [Google Scholar]
- 76.Lambert LK, Balneaves LG, Howard AF, et al. Understanding adjuvant endocrine therapy persistence in breast cancer survivors. BMC Cancer 2018;18:732. 10.1186/s12885-018-4644-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-060946supp001.pdf (252.3KB, pdf)
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.