Abstract
Introduction
Long pulse width stimulation (LPWS; 120–150 ms) has the potential to stimulate denervated muscles and to restore muscle size in denervated people with spinal cord injury (SCI). We will determine if testosterone treatment (TT)+LPWS would increase skeletal muscle size, leg lean mass and improve overall metabolic health in persons with SCI with denervation. We hypothesise that the 1-year TT+LPWS will upregulate protein synthesis pathways, downregulate protein degradation pathways and increase overall mitochondrial health.
Methods and analysis
Twenty-four male participants (aged 18–70 years with chronic SCI) with denervation of both knee extensor muscles and tolerance to the LPWS paradigm will be randomised into either TT+neuromuscular electrical stimulation via telehealth or TT+LPWS. The training sessions will be twice weekly for 1 year. Measurements will be conducted 1 week prior training (baseline; week 0), 6 months following training (postintervention 1) and 1 week after the end of 1 year of training (postintervention 2). Measurements will include body composition assessment using anthropometry, dual X-ray absorptiometry and MRI to measure size of different muscle groups. Metabolic profile will include measuring of basal metabolic rate, followed by blood drawn to measure fasting biomarkers similar to hemoglobin A1c, lipid panels, C reactive protein, interleukin-6 and free fatty acids and then intravenous glucose tolerance test to test for insulin sensitivity and glucose effectiveness. Finally, muscle biopsy will be captured to measure protein expression and intracellular signalling; and mitochondrial electron transport chain function. The participants will fill out 3 days dietary record to monitor their energy intake on a weekly basis.
Ethics and dissemination
The study was approved by Institutional Review Board of the McGuire Research Institute (ID # 02189). Dissemination plans will include the Veteran Health Administration and its practitioners, the national SCI/D services office, the general healthcare community and the veteran population, as well as the entire SCI community via submitting quarterly letters or peer-review articles.
Trial registration number
Keywords: neuromuscular disease, neurobiology, neurophysiology
Strengths and limitations of this study.
Long pulse width stimulation (LPWS) is safe to be administered in persons with spinal cord injury (SCI) with lower motor neuron injury.
Training twice weekly may encourage long-term compliance and adherence.
Testosterone treatment may serve as an alternative therapy for denervated muscles.
Recruitment and retaining for a duration of 12 months is rather challenging in this subpopulation with SCI.
Currently, LPWS stimulators are not clinically or commercially available in North America.
Background
Spinal cord injury (SCI) is a devastating medical condition that increases one’s risk for type II diabetes mellitus, dyslipidaemia and cardiovascular disease.1–5 Prevalence of individuals with SCI has been estimated to be 250 000–400 000 with a 14% growth since 1988.6 7 The Department of Veterans Affairs cares for >46 000 veterans with SCI-related disability. Due to advances in healthcare, individuals with SCI are now expected to have similar lifespans to able-bodied controls. The national aggregate direct costs of SCI in the USA have increased with a concomitant decline in mortality over the first year after SCI.8 Medical care costs per case may exceed US$1 million for acute stabilisation and rehabilitation, with annual charges thereafter ranging from US$41 000 to US$182 000.6–8 The estimated lifetime costs can exceed US$12 million.8 Secondary health-related consequences challenge the productivity, quality of life and well-being of those with SCI, but may be reversible with appropriate exercise or pharmaceutical interventions that minimise SCI-related secondary disorders and favourably influence healthcare costs after SCI.
Previous work demonstrated that surface neuromuscular electrical stimulation (NMES, ie, stimulation of intact axonal branches) evokes whole thigh and knee extensors muscle hypertrophy following exercise-induced resistance training (RT) with ankle weights in individuals with chronic SCI.9–12 Evoking skeletal muscle hypertrophy is vital for several activities of cellular and whole body metabolism.13–15 Skeletal muscles serve as a large paracrine gland that controls the interplay between the musculoskeletal system and other physiological systems.13–15 Studies documented that skeletal muscle releases important myokines that may regulate atrophic pathways, bone and endocrine glands.14 15 Twelve weeks of training increased skeletal muscle hypertrophy by >40% and improved glucose tolerance many years after injury.10 Ryan et al noted an improvement in mitochondrial capacity by 25% following 16 weeks twice-weekly NMES-RT.11 NMES-RT may increase mitochondrial capacity to use fat as a source of energy during exercise and further improve insulin sensitivity.12 Twelve weeks of twice-weekly NMES-RT can elicit ~35% increase in skeletal muscle size, decreased intramuscular fat (IMF) and visceral adipose tissue, increased insulin sensitivity and increased insulin growth factors-1 (IGF-1) by 25%.12 However, despite the benefits of NMES applications, approximately 25% of the SCI population cannot benefit from the standard NMES (pulse duration <1000 µs or 1 ms) because of lower motor neurons (LMN) denervation.16 17
Today, there are no stimulation protocols that may train the muscle following LMN denervation, because of increasing the depth of penetration following deleterious atrophy, subcutaneous fat thickness and excessive infiltration of IMF that may diminish the spread of the current density to activate the target muscles.16 Standard surface NMES (pulse duration of 150–1000 µs which directly stimulates axonal branches or peripheral nerves) fails to activate the denervated muscles because of an increase in the minimum time required, chronaxie, as demonstrated on the strength-duration curve.16 This requires higher current charges for the direct depolarisation of the muscle fibres. The limited pulse duration is inversely associated with an increase in the current amplitude required to activate the muscle. Most commercially available stimulators have amplitudes that do not exceed 200 mA because of an increased risk of skin irritation and burns, especially in individuals with SCI. This low amplitude of the current is unlikely to cause muscle activation following denervation in persons with SCI. This, subsequently, results in failure maintaining skeletal muscle vitality following LMN denervation as well as maintaining the integrity of other physiological systems.
Long pulse width stimulation (LPWS) has the capacity to penetrate deeply and activate muscle fibres directly without reliance on stimulating the denervated peripheral nerves.18–20 The European project, Research and Innovation Staff Exchange, has introduced long pulse width (LPW) NMES to restore muscle size following denervation in people with SCI.21–24 The effects of home-based functional electrical stimulation, introducing an LPW (120–150 ms) at an amplitude of 250 mA for 5 days per week has been studied for 2 years in 25 persons with SCI with complete LMN denervation.21–24 The trial showed an increase (24%) in knee extensor cross-sectional area following the first year and an additional 7% in the second year, respectively, with no changes in the hamstring muscles.21–24 However, the safety and the feasibility of application of LPWS has not been determined within the US population with SCI or generally in North America.25 Successful completion of this trial will provide a safe and feasible rehabilitation approach that likely enhances muscle hypertrophy in persons with SCI with LMN denervation.
We previously attempted to combine NMES-RT with testosterone treatment (TT) to attenuate cardiometabolic risk factors after SCI.26 27 TT is an Food and Drug Administration-approved therapy used to treat hypogonadism and often results in significant improvement of muscle strength and fat-free mass (FFM) in hypogonadal men.28 29 In rats with complete SCI, TT has been shown to attenuate muscle atrophy and the decline in oxidative and glycolytic enzymatic activities.30 Additionally, 60% of men with SCI have low T levels in the first 6 months after SCI.29 Administering TT may attenuate the effects of denervation on the paralysed muscle. However, applications of TT in chronic models of denervation have not been established in clinical population with SCI. The role of TT on muscle size independent of the changes in the peripheral nervous system has not been investigated. It is possible to assume that may result from upregulation of the androgen receptors that may lead to proliferation of satellite cells.31
We hypothesise that 1 year of TT+LPWS will result in a significant knee extensor muscle hypertrophy of 25% or more.25 This will be associated with a significant increase in leg lean mass (>10%) and concomitant improvement in overall cardiometabolic profile by 20%–30%. Previous studies suggest that LPWS may require 5 days per week up 2 years to restore muscle size in persons with SCI with LMN denervation.20–24 Clinically, this is not feasible considering the barriers related to dressing, bowel and bladder movements, transportation and loading and unloading of wheelchairs. We are hypothesising that the addition of TT to LPWS may facilitate the increase in leg lean mass and allow optimisation of the stimulation protocol in just 1 year. Therefore, this approach of combining both physical and pharmacological interventions may allow twice-weekly LPWS to be highly effective in restoring muscle size.
The goal of this randomised prospective controlled study is to investigate the effects of 1 year of TT+LPWS versus TT+standard NMES on muscle size (primary outcome variable), leg lean mass and percentage IMF, metabolic profile (basal metabolic rate (BMR), carbohydrate and lipid profiles) and protein synthesis and degradation pathways as well as mitochondrial health. Carbohydrate profile will include resting plasma glucose and insulin, glucose effectiveness (Sg) and insulin sensitivity (Si), whereas the lipid profile will include the entire analysis of the lipid panel. We hypothesise that the 1-year TT+LPWS protocol will upregulate protein synthesis pathways, downregulate protein degradation pathways and increase overall mitochondrial health. Three specific aims will address these hypotheses. Aim 1 will assess the effects of TT+LPWS compared with TT+standard NMES as a control group) on the size of thigh skeletal muscle, IMF and leg lean mass. Aim 2 will determine the association between the changes in skeletal muscle size, leg lean mass and the metabolic profile as determined by measuring BMR, serum lipids and carbohydrate profile. Aim 3 will investigate the cellular mechanisms (protein and messenger RNA (mRNA) expressions) responsible for evoking skeletal muscle hypertrophy following TT+LPWS. This study is novel because it provides a feasible rehabilitation intervention by combining two approaches, which are likely to improve the quality of life in persons with SCI with LMN denervation. If proven successful, the intervention will be translated into clinical practice for persons with SCI. The long-term goal is to develop a rehabilitation strategy to mitigate the deleterious changes in muscle size and lower leg lean mass in persons with denervation following SCI.
Methods
Study design
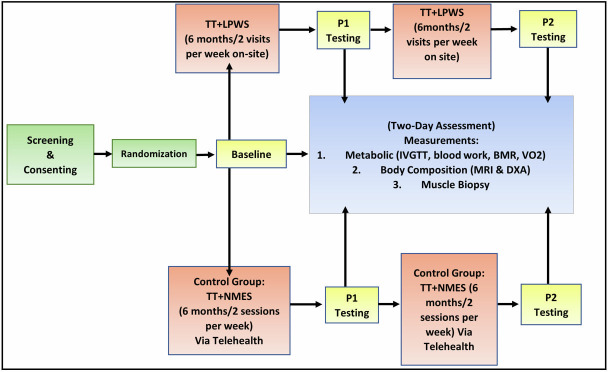
The entire study design and timeline is presented in figure 1. Twenty-four participants will be randomly assigned to either 1 year of TT+LPWS group (n=12) or a TT+standard NMES control group (n=12). Testosterone transdermal patches (Tp; 4–8 mg/day) will be replaced daily on alternating skin sites at bedtime for 1 year. Participants will be block randomised based on the degree of denervation (lower or higher than 50% of compound muscle action potential (CMAP) of the standard normal femoral nerve values). Both groups (TT+LPWS and TT+standard NMES) will undergo 1 year of supervised bilateral (by alternating one leg at a time) progressive RT, twice weekly, using ankle weights. For the TT+standard NMES group, supervised training at home will be performed using our established telehealth programme. This programme has proven to be successful in administering electrically evoked RT in persons with motor complete SCI.
Figure 1.
The study timeline (table 4) and procedure are highlighted. After screening and consent, participants will be randomised into one of two testing groups. Each participant will undergo baseline testing before beginning TT+LPWS or control TT+NMES. Each group will be tested for metabolic, body and muscle composition (P1) after a 6-month period. Each group will then complete another 6 months of electrical stimulation exercise training followed by another testing (P2). BMR, basal metabolic rate; DXA, dual-energy X-ray absorptiometry; IVGTT, intravenous glucose tolerance test; LPWS, long pulse width stimulation; NMES, neuromuscular electrical stimulation; TT, testosterone treatment.
A Stimulette Den 2x stimulator (Schuhfried, Vienna, Austria and approved only for research use in our site) is used to perform the exercise training session.25 A detailed information about the stimulator can be found in the following link (https://www.anatomicalconcepts.com/stimulette-den2x/). The progression of the stimulation parameters will be set as shown in tables 1 and 2. Each session consists of 4 sets of 10 repetitions and will last for 40–50 min. Both legs will be alternatively trained starting with the right leg and then followed with the left leg. This approach was adopted to avoid possible muscle fatigue (see table 2) that may result from electrically stimulating the denervated knee extensor muscle groups. Once the participant completes 40 repetitions of knee extension, ankle weights will be gradually increased by 2 lbs (0.907 kg) (see table 2 for details). All training procedures will be conducted with the participants sitting in their wheelchairs with enough space to clear their foot off the ground.9–12 26 27 32 For the control group (TT+standard NMES), over a 1-year period, participants will have the option to perform a home-based training using our established videoconference telehealth system to monitor their training using VA video connect similar to previous work.33 The parameters using NMES will be as follows: the direct current at 450 µs will be turned up to 200 mA for 10 times/set, which is unlikely to cause either twitches or tetanic contraction of the stimulated muscle in persons with SCI with American Spinal Injury Impairment Scale (AIS) classification A or B. However, we cannot rule the possibility of inducing muscle contraction in persons with partial denervation similar to persons with SCI with an AIS classification C. Study visits will be limited to once a month throughout the 1-year period to refill their 30 days stock of TT patches. For TT+LPWS group, participants will be trained under supervision in order to perform their LPWS training sessions.
Table 1.
Example of progression of the stimulation parameters over 1-year period for TT+LPWS group
| Months of training | Pulse width (ms) | Interpulse interval (ms) | Frequency (Hz) | Amplitude of current (mA) | Weight (lbs) |
| 1–3 | 120–150 | 400 | 2 | Up to 200 | 0 |
| 4–6 | 90–120 | 400 | 15–25 | Up to 200 | 0 |
| 6–9 | 60–90 | 100–400 | 25–30 | Up to 200 | 2 lbs/40 reps/session |
| 9–12 | 30–60 | 10–12 | 25–30 | Up to 200 | 2 lbs/40 reps/session |
LPWS, long pulse width stimulation; reps, repetitions; TT, testosterone treatment.
Table 2.
Progression of the current amplitude (mA) of the stimulation parameters, and ankle weights of the right leg from week 1 to week 45 following LPWS protocol in the trial
| Repetitions | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Week 1 | ||||||||||||
| Pulse width: 200 ms | Set 1 | Amp (mA)-set 1 | 160 | 160 | 160 | 160 | 160 | 165 | 165 | 165 | 165 | 165 |
| Pulse interval: 400 ms | Set 2 | Amp (mA)-set 2 | 170 | 170 | 170 | 170 | 170 | 175 | 175 | 175 | 175 | 175 |
| Frequency: 1.66 Hz | Set 3 | Amp (mA)-set 3 | 180 | 180 | 180 | 180 | 180 | 185 | 185 | 185 | 185 | 185 |
| Ankle weights: 0 lbs | Set 4 | Amp (mA)-set 4 | 190 | 190 | 190 | 190 | 190 | 195 | 195 | 195 | 195 | 195 |
| Week 45 | ||||||||||||
| Pulse width: 35 ms | Set 1 | Amp (mA)-set 1 | 200 | 172 | 148 | 163 | 165 | 166 | 169 | 182 | 200 | 173 |
| Pulse interval: 400 ms | Set 2 | Amp (mA)-set 2 | 191 | 183 | 173 | 180 | 179 | 200 | 170 | 185 | 187 | 187 |
| Frequency: 25 Hz | Set 3 | Amp (mA)-set 3 | 200 | 177 | 163 | 194 | 200 | 199 | 200 | 200 | 170 | 191 |
| Ankle weights: 2 lbs | Set 4 | Amp (mA)-set 4 | 200 | 173 | 177 | 173 | 196 | 200 | 195 | 178 | 200 | 200 |
The highlighted region represents the amplitude in week 45.
Note the amplitude of the current increased approximately by 19% in week 1 from set 1 to set 4, this reflects the increase in muscle fatigue of the denervated knee extensor muscle group during one training session. This fatigue pattern decreased to only 9% in week 45 (ie, last training bout) following 12 months of training in one of the participants.
LPWS, long pulse width stimulation.
Planned outcomes and specific aims
Muscle size as measured by MRI
The two-day period is measured at the baseline prior to training and repeated at 6 months (post-intervention (1) and after 1 year (post-intervention (2)). The 2-day assessment includes an estimation of body composition, anthropometry and dual X-ray absorptiometry (DXA), MRI scans will be obtained for thigh skeletal muscles to determine muscle and IMF CSAs.33–42 The aim of these measurements is to compare the effects of TT+LPWS with TT+standard NMES (control group) on the size of thigh skeletal muscle, IMF and leg lean mass (specific aim 1).
Basal metabolic rate and metabolic profile
After obtaining the previous measurements, participants will be escorted to a nearby hotel for dinner, and overnight stay. After an overnight fast, the subject will be gently awakened at 06:00 hours to measure BMR.26 35 At 06.30 hours, an intravenous line will be placed and blood will be drawn for serum total T and IGF-1 concentrations at 06.30, 07.00 and 07.30 hours.26 Resting blood pressure and fasting metabolic markers will be obtained including hemoglobin A1c, as well as lipid panels, C reactive protein (CRP), interleukin (IL)-6, tumor necrosis factor (TNF)-α and free fatty acids (FFA). This will be followed by a 3-hour intravenous glucose tolerance test (IVGTT), which will begin at 08:00 hours and terminate at 11:00 hours. The aim of these measurements is to determine the association between the changes in skeletal muscle size, IMF, leg lean mass and the metabolic profile as determined by measuring BMR, lipid panel and carbohydrate profile.26 27 32 We hypothesise that the increase in lean mass following TT+LPWS will increase BMR and improve both carbohydrate and lipid profiles as well as associated with decrease in FFA and inflammatory biomarkers (specific aim 2).
During the IVGTT, a dietitian will meet with each participant individually to instruct on how to follow a standard diet pattern during the 1-year intervention (45% carbohydrate, 35% fat and 25% protein) to avoid any confounding effects on our measurements.12 26 27 32 All participants will be asked to maintain a 3-day food record monitoring their energy intake during the course of the study. The diaries will be evaluated weekly by the dietitian to provide monthly feedback. All participants will meet with the dietitian 3 times during the study to determine their adherence to the diet pattern throughout the study.
Mitochondrial electron transport chain and signalling pathways
The third aim is to investigate the cellular mechanisms responsible for evoking skeletal muscle hypertrophy following TT+LPWS compared with TT+standard NMES.27 Participants will undergo three muscle biopsies of the right vastus lateralis muscle at baseline, 6 and 12 months postintervention to measure gene and protein expression and perform mitochondrial enzymatic assays.43 44 Considering the limited muscle tissue in persons with denervation atrophy, four biopsy samples of the vastus lateralis muscle (total: 25–50 mg wet wt) will be obtained by a 14-gauge Tru-Cut needle using a sterile technique and local anaesthesia (2% lidocaine). The biopsy samples will be frozen in liquid nitrogen and stored at −70°C until further analysis. We hypothesise that the TT+LPWS will upregulate protein synthesis and downregulate protein degradation pathways, gene expression and mitochondrial enzymatic electron transport chain (ETC) activities compared with the TT+standard NMES group.
Screening and consenting
Prior to the 2-day assessment and training, we will perform multiple neurophysiological tests to confirm LMN denervation of the knee extensor muscle group. The first technique is to place the surface NMES electrodes on knee extensor muscle group and increase the current (30 Hz, 450 µs) gradually up to 200 mA. If the knee extensors show visible elicited contraction, then the participant will be disqualified from the study. If there is no response, participant will then be escorted to the electromyography (EMG) laboratory and further testing for evidence documenting muscle denervation will be performed by a trained electromyographer. Briefly, participants will undergo a femoral nerve motor conduction study with recording of the CMAP from the vastus medialis (VM) with supramaximal femoral nerve stimulation just below the inguinal ligament. The participant will undergo needle EMG of the VM to determine the presence and intensity of spontaneous muscle fibre fibrillation potentials to quantify the level of denervation. A monopolar EMG needle electrode will be inserted into the VM muscle to record spontaneous intramuscular activity in the resting muscle.
Each participant will provide a written informed consent that was approved by the local institutional review board (IRB) (see online supplemental file 1). Determination of a subject’s capacity to consent will be made by the investigator. Input may be solicited from an SCI primary provider who knows the potential subjects. Potential subjects will have the full study verbally explained to them. If they voice an interest in the study, they will have the written consent form provided. Potential subjects will be allowed sufficient time and opportunity to read the consent alone and have all questions and concerns answered. Emphasis will be placed on explaining all study procedures. Family members will be included in the discussion as desired by the subjects. They will be told that participation is voluntary and that they can withdraw at any time with no impact on the care they receive from the VA. They will be allowed to take consent home for additional time prior to making decisions. They can also discuss the study with their primary provider. All signed consents will be scanned into the VA computerised patient record system and attached to a note indicating enrolment in study. In addition to the entered consent form, progress notes of their active participation will also be entered on a weekly basis.
bmjopen-2022-064748supp001.pdf (406.6KB, pdf)
After informed consent, each subject will undergo a complete physical examination by a physiatrist board certified in SCI medicine, including neurological assessment and AIS examination. Participants will be evaluated every 3 months after treatment initiation and then annually to assess any adverse effects and to check compliance. Testosterone measurements will be acquired every 4 weeks during the intervention to determine the serum level and the dose will be adjusted to allow ~30% increase from baseline. A prostatic abnormality on digital rectal examination will also lead to exclusion. An increase in serum or plasma prostate-specific antigen (PSA) of 1.4 ng/mL above baseline will result in immediate cessation of TT.
Randomisation and allocation
Randomisation will be done at the end of the 2-day assessment period using a random number generator computer program (baseline). Participants will be block randomised based on the degree of denervation (lower or higher than 50% of CMAP of the standard normal femoral nerve values. Standard NMES (30 Hz, 450 µs and amplitude of current (mA) as tolerated) was added for the control group (TT+NMES) with the attempt to blind our participants to study design. Also, it is unclear whether standard NMES as such short pulse duration would have any effects on the denervated muscles (table 3).
Table 3.
The use of random number generators to plan randomisation of 24 participants with SCI with LMN injury into either TT+LPWS (n=12) or TT+NMES (n=12; control group) for the entire study
| Subject ID | Randomisation | Assignment | Order in the group |
| 1 | 1 | TT+LPWS | 1 |
| 2 | 1 | TT+LPWS | 2 |
| 3 | 0 | TT+NMES | 1C |
| 4 | 0 | TT+LPWS | 2C |
| 5 | 0 | TT+NMES | 3C |
| 6 | 0 | TT+NMES | 4C |
| 7 | 1 | TT+LPWS | 3 |
| 8 | 1 | TT+NMES | 4 |
| 9 | 1 | TT+LPWS | 5 |
| 10 | 0 | TT+NMES | 5C |
| 11 | 0 | TT+NMES | 6C |
| 12 | 1 | TT+LPWS | 6 |
| 13 | 1 | TT+LPWS | 7 |
| 14 | 0 | TT+NMES | 7C |
| 15 | 0 | TT+NMES | 8C |
| 16 | 1 | TT+LPWS | 8 |
| 17 | 0 | TT+NMES | 9C |
| 18 | 1 | TT+LPWS | 9 |
| 19 | 0 | TT+NMES | 10C |
| 20 | 1 | TT+LPWS | 10 |
| 21 | 1 | TT+LPWS | 11 |
| 22 | 0 | TT+NMES | 11C |
| 23 | 0 | TT+NMES | 12C |
| 24 | 1 | TT+LPWS | 12 |
In the randomisation column, 1 refers to TT+LPWS and 0 refers to TT+NMES. C refers to control group.
LMN, lower motor neurons; LPWS, long pulse width stimulation; NMES, neuromuscular electrical stimulation; SCI, spinal cord injury; TT, testosterone treatment.
Measurements
Body composition assessment
Body mass index
Each participant will be asked to void his bladder and then will propel onto a wheelchair weighing scale. After weighing the participant and his wheelchair (1), he will be helped to transfer to an adjustable mat and his/her wheelchair will be weighted empty (2). The weight of each participant will be determined by subtracting (2) from (1) (kg). The height will be determined in the supine position. Two smooth wooden boards will be placed at the participant’s head and heels and the distance between them will be taken as the height to the nearest cm. Every effort will be taken to maintain the knees in an extended position. Body mass index (kg/m2) will be calculated as weight (kg) divided by height2 (m2).26 27
Dual energy X-ray absorptiometry
iDXA will be used to measure body composition including regional and total fat mass (FM), FFM and bone mineral density (BMD). Total body and regional scans (arms, trunk and legs) will be performed using an iDXA scanner (Lunar, Madison, Wisconsin, USA) bone densitometer to determine regional BMD and T-scores for hips and knees.36 41 42 We will perform testing after lower extremity elevation for at least 20 min to minimise fluid shift. All scans will be performed and analysed by a trained, certified DXA operator. The subject will be assisted to lie on a padded table and both legs will be strapped proximal to the knees and the ankles. The arms and legs will be positioned to ensure proper alignment and the ability to lie still for 10 min during the scan. Total and regional (%FM and FFM) will be determined using total and regional DXA software. The coefficient of variability of two repeated scans is <3%.42
Magnetic resonance imaging
MRI will be performed using a 1.5 T magnet (GE).34–41 The skeletal muscle CSAs will be determined at baseline, 6 months (midintervention) and 1 year after starting the intervention (postintervention). Both lower limbs will be strapped together using a soft Thera-band to avoid any movement inside the magnet. Participants will be instructed to lie still inside the magnet and they will be provided with earplugs to protect their ears against the magnet noise. The duration of the whole scan including the preparation time should not exceed 10 min. Images of both thighs will be collected using the following scanning parameters (repetition time, 500; echo time, 14; field of view, 20 cm; matrix, 256×256). Transaxial images, 8 mm thick and 4 mm apart, will be taken from the hip joint to the knee joint using a localised coil. Images will be downloaded and analysed using X-vessel software.12 26 27 32 34
Testosterone and PSA concentration
The serum testosterone concentration and PSA levels will be measured at the beginning, every 4 weeks and 1 year after intervention. Intravenous line will be placed after overnight fast, serum total T and PSA concentrations will be measured in duplicates.41 45 46 Samples will be sent for analysis using a standard procedure assays.
Metabolic studies
BMR and respiratory exchange ratio
After an overnight fast for 10–12 hours, participants will be kept in a dark room for 20–30 min to attain a resting state during which BMR will be measured by using a canopy. The gasses (VCO2 and VO2) collected will be used to determine the respiratory exchange ratio. This will help to determine the changes in the percentage of substrate utilisation (% fat vs % carbohydrate) after the interventions.12 26
Serum total, free testosterone and IGF-1
The plasma T and IGF-1 will be measured in the morning (2 mL/sample).26 38 41 The analysis of total T will be performed by radioimmunoassay (RIA) after sample extraction and column chromatography. The interassay coefficient of variation (CV) is 12.5% or less for all quality control samples analysed. Plasma IGF-I and insulin growth factor binding protein-3 (IGFBP-3), concentrations will be measured by immunoluminometric assay (Quest Diagnostics, Madison, New Jersey, USA) and RIA (Diagnostics Systems Laboratories, Webster, Texas, USA), respectively. Intra-assay precision of IGF-1 is 4.6% at 50 ng/mL and 3.6% at 168 ng/mL.
Blood lipids
Each subject will have fasting lipid profiles (high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, total cholesterol and triglyceride) assessed, with total cholesterol:HDL-C ratios used as the criterion variable. Concurrent with the IVGTT and following a 12-hour fast, 10 mL of blood will be collected from the indwelling venous catheter and lipids determined by standard analyses procedures.26 35 41
Inflammatory biomarkers
Before starting the IVGTT and following a 12-hour fast, 10 mL of blood will be collected from the indwelling venous catheter and CRP, IL-6, TNF-α and FFA will be determined by standard procedures using commercially available assay kits.26 47
Intravenous glucose tolerance test
An IVGTT will be used to determine insulin sensitivity and glucose effectiveness.26 48 Each subject will undergo an IVGTT test 3 times. After a 10-hour to 12-hour fast, an indwelling catheter with an intravenous saline drip (0.9% NaCl) will be placed in an antecubital vein, and another intravenous line will be placed in a contralateral hand vein to facilitate infusion of glucose and blood sampling during the IVGTT. Glucose samples will be taken at –6, –4, −2, 0, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 35, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160 and 180 min after the rapid glucose injection (0.3 g/kg intravenously over 30 s at time zero). Twenty minutes after the glucose injection, a bolus of insulin (0.02 U/kg) will be injected to determine insulin sensitivity. Plasma glucose will be measured by the Autoanalyzer glucose oxidase method and plasma insulin concentrations will be determined by commercial RIA using single-antibody kits. The SI (glucose disposal rate per unit of secreted insulin per unit time) and SG (glucose-mediated glucose disposal rate) will be calculated from a least-squares fitting of the temporal pattern of glucose and insulin using the MINMOD program.34 The CV is approximately 15%. KG, a measure of glucose tolerance, is calculated as the least square slope of the natural log of absolute glucose concentration between 5 and 20 min after the glucose bolus.34 The homeostatic model of assessment of insulin resistance will be calculated and insulin sensitivity will be determined using Matsuda and DeFronzo formula.49 50
Muscle biopsies
Protein content
Muscle biopsy samples will be homogenised on ice using the appropriate buffers. Equal amounts of protein will be resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis after which proteins will be electrophoretically transferred to a polyvinylidene fluoride membrane. Western blot analysis will then be performed to determine the protein concentrations as described in preliminary data and previously by our lab.27 43 After blocking, membranes will be probed with primary antibodies for activated pathways including Adenosine monophosphate kinase (AMPK), p-AMPK PGC-1α (phosphorylated-AMPK peroxisome-proliferator-activated receptor-γ coactivator-1 alpha), IGF-1, Akt, p-Akt, mammalian target of rapamycin (mTOR) and protein degradation pathways (Forkhead box O (FOXO)1/3, atrogin-1, Muscle RING-finger protein-1 (MURF)), followed by incubation with the appropriate secondary antibody. Western blot analysis will be quantified by scanning with a GS-800 densitometer. Optical densities of the western blot analysis will be measured using image-analysis software (Molecular Analyst; Bio-Rad).
Real-time quantitative PCR
Quantification of mRNA levels by RT quantitative PCR (real-time qPCR) in muscle biopsy samples will follow well-established procedures in our lab. Briefly, frozen muscle biopsy samples will be added to Trizol reagent and immediately homogenised then centrifuged to separate the chloroform and aqueous phases. mRNA will be extracted using commercially available kits. Residual genomic DNA will be removed by the column DNAse I digestion. complementary DNA libraries will be synthesised by reverse transcription of total RNA using commercially available kits. Expression of individual target genes (IGF-1, PGC-1α, AMPK, Akt, mTOR) will be evaluated by qPCR using 18S RNA or housekeeping genes as loading controls. The effects of interventions on mRNA expression levels will be expressed as fold-change, where fold-change is calculated using the 2−∆∆Ct method.30 43
Mitochondrial ETC activities
The assays will be performed using fresh cholate-treated skeletal muscle homogenates. ETC complex activities will be measured spectrophotometrically as specific donor-acceptor oxidoreductase activities in 0.1 M phosphate buffer (HP 8453 and Lambda 35 UV/VIS). Rotenone-sensitive NADH cytochrome c reductase will measure complexes I and III. NADH coenzyme Q reductase will be measured as the rotenone-sensitive oxidation of NADH with decylubiquinone as acceptor, and assesses complex I. NADH ferricyanide reductase measures NADH dehydrogenase in complex I. Cytochrome c oxidase will be measured as the oxidation of reduced cytochrome c and expressed as the first-order rate constant.50 51 Flurometric measurements will be conducted to measure the oxidative and glycolytic enzymes’ activities including citrate synthase and succinate dehydrogenase.27 32 44 50–52
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Statistical analyses
Means and SD or frequencies and percentages will be reported for all data. Similar summaries will be provided for all outcome and biomarker data separately at baseline, 6 and 12 months. A repeated-measures analysis of variance will be used to analyse the primary study outcome, skeletal muscle size, with treatment, time and the interaction of these variables included in the model. A first-order autoregressive correlation structure will be used to capture the dependency within subjects’ outcomes. A specific contrast will be used to determine if the change from baseline for the treatment group is different than that of the control group. A model selection procedure will be performed due to the relatively large number of predictor variables compared with the number of subjects for specific aims 2 and 3. A penalised random effects model will be used to suggest the important predictors for each outcome separately. These include each specific predictor mentioned in the specific aims as well as injury characteristics.53 A parsimonious model will be chosen by the Bayesian information criterion. Since the aforementioned model is well-suited for model selection, but results in biased parameter estimates, an unpenalised random effects model will be fitted based on the parsimonious model and used for inference. Study period will be included in all analyses, and a time by group interaction will be included for the analyses associated with specific aim 3.
All statistics will be performed with R (V.3.3.0) with the ‘glmmLasso’ package. Prior to any statistical analysis, any of the biomarkers may be transformed to ensure all statistical assumptions are met. A simulation study was used to determine the power achieved from the proposed study if 5% of subjects were assumed to drop out at the 12-month observation point. One thousand datasets were created with Cohen’s d varying between 0.5 and 2.0, with the anticipated 24 subjects being equally allocated to the treatment and control groups and the subjects’ outcomes having a correlation of 0.25. A linear mixed-effects model was used to analyse the simulated data using a one-sided test at α=0.05. Results of the study show that this design will have the power to detect differences of d=1.28 with 80% power and d=1.48 with 90% power. An intent-to-treat approach will be used to deal with missing data. If the missing data are due to drop out, the statistical methods are valid as long as the data mechanism is considered missing at random or missing completely at random.
Recruitment strategy
The following timeline and research activities (table 4) will be implemented to meet our recruitment goals. Our goal is to recruit, train and test eight participants per year. We plan to have four participants recruited quarterly and we will recruit an additional four participants every 6 months using the following timeline to meet our sample size. Approximately two to three subjects will be screened every other month. They will then be randomised to either group. The goal is to recruit eight participants per year (four participants every 6 months) to finish data collection in 4 years. Table 5 highlights the number of participants currently enrolled, completed or withdrew from the current trial.
Table 4.
Timeline of research activities across the 4-year study duration
| Research activities | Year 1 | Year 2 | Year 3 | Year 4 | ||||
| Months | 1–6 | 6–12 | 1–6 | 6–12 | 1–6 | 6–12 | 1–6 | 6–12 |
| Equipment and supply purchase |
|
|||||||
| Recruitment (n=4 per quarter) |
|
|
|
|
|
|
||
| Screen and enrol subjects |
|
|
|
|
|
|
||
| Data collection |
|
|
|
|
|
|
||
| Progress report |
|
|
|
|
||||
| Data entry/coding/cleaning |
|
|
|
|
|
|
||
| Data analysis and interpretation |
|
|
|
|
|
|
|
|
| Presentation/Publications |
|
|
|
|||||
Table 5.
The number of persons who were recruited, completed or withdrew from the study
| Subject ID | TT+LPWS/NMES | Baseline 1 | Postintervention | Postintervention 2 | Sex | LOI | TSI (years) | AIS | Classification |
| 001 | TT+LPWS | C | C | C | M | T9 | 2 | A | Paraplegia |
| 002 | TT+LPWS | C | C | X | M | T7 | 5 | C | Paraplegia |
| 003 | TT+NMES | C | C | C | M | T11 | 2 | A | Paraplegia |
| 004 | TT+LPWS | C | C | X | M | T11 | 19 | A | Paraplegia |
| 005 | Withdrew | X | X | X | M | T11 | 12 | A | Paraplegia |
| 006 | TT+NMES | C | C | C | M | T11 | 20 | A | Paraplegia |
| 007 | Withdrew | X | X | X | M | T10 | 2.5 | B | Paraplegia |
| 008 | TT+NMES | C | C | C | M | T10 | 12 | A | Paraplegia |
| 009 | TT+LPWS | C | C | C | M | T12 | 14 | A | Paraplegia |
| 010 | TT+LPWS | C | C | O | M | T12 | 6 | A | Paraplegia |
| 011 | TT+NMES | C | C | O | M | T6 | 8 | A | Paraplegia |
| 012 | TT+NMES | C | O | O | M | T11 | 28 | A | Paraplegia |
AIS, American Spinal Injury Impairment Scale; C, completed the study; LOI, level of injury; LPWS, long pulse width stimulation; M, male; NMES, neuromuscular electrical stimulation; O, still ongoing; TSI, Time since injury; TT, testosterone treatment; X, withdrew from the study.
Discussion
The current trial will provide a novel rehabilitation strategy, combining TT+LPWS to determine the effects of mitigating muscle loss following LMN denervation. Previously, our group was successful in attempting to combine electrically evoked RT with TT to attenuate cardiometabolic risk factors in persons with SCI.26 Establishing the LPWS rehabilitation protocol is a rigorous process and may require several attempts to refine and establish the best stimulation protocol necessary for stimulation of LMN denervation.21 25 The addition of TT may provide additional benefits of increasing lean mass and accelerate the actions of LPWS. Previous research attempts indicate that TT may increase lean mass and BMR in persons with SCI.28 This is the first clinical attempt to determine the role of TT on muscle size and lean mass in persons with LMN denervation independent of neural structures.
This is a promising combination of physical and pharmacological therapies that are likely to improve body composition and other cellular functions which may improve other cardiometabolic health biomarkers. Several of the mechanisms involved in increasing muscle size, including proteins and gene expression will be studied,27 30 31 43 which will allow us to design specific interventions that target specific abnormalities at the cellular level which have the potential to impact body composition and metabolic adaptations in future studies.
The consequences of LMN denervation on skin, muscle mass and bone mass have been previously highlighted.17–20 25 Applications of LPWS required the use of two large carbon electrodes soaked with gel and placed inside wet conductive spongy pads and strapped to participant’s thigh with Velcro straps. The large carbon electrodes ensure adequate dissipation of the energy density to the target muscles.25 Following 3 years, we did not report a single incidence following applications of LPWS. We have administered LPWS to persons with T7 SCI with LMN that occurred as result of vascular infraction. LPWS (120–150 ms) has the potential to safely stimulate denervated muscles and to restore muscle size in people with SCI. The previous paradigm has focused on daily activation (5 days per week) of the denervated muscles without applying progressive loading of applying ankle weights. Daily training is not a clinically feasible approach in persons with SCI. However, the downside of conducting LPWS twice weekly is that the current dose may not be sufficient to induce strong potent stimuli to upregulate protein synthesis and evoke muscle hypertrophy similar to what reported in the innervated muscles.27 Future studies that incorporate telehealth supervision may facilitate increasing the frequency of training to 4× per week for home-based training.33 Moreover, previous trials did not focus on enhancing the neuromuscular homeostasis by promoting the increase in lean mass independent of LMN denervation. Administering TT may likely increase lean mass and BMR28 and reduce the catabolic pathways that hinder anabolic profile and muscle hypertrophy in persons with SCI.26 30
Considering the small size of this SCI subpopulation and limited access to them, we sought to collect three levels of measurements that address body composition, metabolic profile and cellular adaptations in response to exercise and TT. Historically, changes in body composition are associated with changes in metabolic profile.34 35 For example, loss in lean mass has been associated with decreases in BMR.34 Waist circumference, a proxy-index of visceral adiposity, has been linked to altered cardiometabolic profile.54 Electrically evoked RT with TT resulted in robust muscle hypertrophy and 14% increases in BMR.26 The use of TT in the current study may result in changes in whole, regional body composition as well as non-stimulated muscles and lead to increase in whole-body lean mass, which subsequently resulted in increasing BMR. Therefore, using DXA to measure body composition assessment is rather an important measurement that may help controlling for whole and regional changes as well as explaining the findings of the current study.41 42 The cellular mechanisms responsible for these adaptations are not well-studied.27 31 32 44 Therefore, the cellular mechanisms may provide additional insights on whether denervated muscles responds differently from the paralysed innervated muscles as far as signalling pathways and mitochondrial activities.
Recruitment is by far the largest challenge to complete the current trial. Another concern is the ability to recruit the suggested sample size and to retain participants to finish the 1-year study; transportation is the greatest impediment to subject compliance for this population. We have been attempting to provide transportation and allow for subjects’ personalised schedules. Providing financial reimbursement, social interaction during training sessions and access to the data at the conclusion of the study are additional methods to be used to reduce subject attrition.
COVID-19 is currently another unavoidable challenge55 56; it negatively impacts the study’s designated time frame for recruitment process. COVID-19 amplified the limitations in terms of finding, recruiting, working with and transporting participants. Most of them are concerned about the 1 year commitment and of coming to the centre on weekly basis, since it increases the risk of being exposed to the virus. However, for safety, the COVID-19 guidelines and precautions are followed like washing hands frequently, wearing a face mask and a face shield, maintaining physical distance and limiting contact with people who may be infected. These are effective ways in preventing the spread of COVID-19, which are adhered and followed at the Hunter Holmes McGuire VA Medical Center.
Few participants might not be qualified for either TT or LPWS protocol. Those with hyperphysiological testosterone level >800 ng/dL are excluded from the study. For example, we had a non-randomised participant who was considered as a screen failure due to his testosterone level of 1724.6 ng/dL. The participant relied on commercially available non-prescribed testosterone boosters to maintain his muscle mass. Another factor is the extensive skin irritation, rashes or itching as result of TT patches applications. Therefore, participants may not be complying with patches applications on a regular basis due to skin discomfort and irritation. However, patch compliance is monitored by counting the number of returned patches per month throughout the course of the trial. Neuropathic pain is one of the factors that kept participants from being compliant with the study protocol of applying LPWS.57 Participant noted difficulty in tolerating amplitude of the current >100 mA. Despite the neuropathic pain, the LPWS did not aggravate it and participants were able to finish the 12 months of the study. The initial inclusion criteria primarily targeted those with LMN below the level of T10 AIS classification A or B; however, during the course of the trial we have screened several participants either with T6 level of injury (as result of vascular infarction) or with AIS classification C. These participants should be considered based on the limited size of the population with LMN denervation compared with the entire SCI population. However, it may be unlikely that participants with AIS classification C may not tolerate the intensity of the LPWS. Women will not be included in the current study because administering TT is neither appropriate nor safe. They are also at risk of virilizing actions of testosterone; therefore, we were planning to limit this trial only to men with SCI. No vulnerable populations will be included and persons under 18 years will be excluded.
Dissemination and implementation plan
The target audiences for dissemination of the results from this study include the Veteran Health Adminstration and its practitioners, the national SCI/D services office, the general healthcare community and the veteran population. We will share our findings with the SCI community. Our SCI/D services and Paralyzed Veteran Affairs (PVA) publish a quarterly newsletter that is sent to SCI practitioners across the VA system. We will inform the VA community of the impact of these findings. We will report our findings to the scientific community via the American Congress of Rehab Medicine, American College of Sports Medicine and American Spinal Cord Injury Association. We will target peer-review journals to publish our reports.
Data Safety and Monitoring Board
The Data Safety Monitoring Board (DSMB) will meet annually to review the protocol prior to data collection to evaluate subjects’ safety, data quality and study progress and execution. The DSMB will review protocol for any major concern prior to implementation. They will also evaluate safety, study conduct and scientific validity and integrity of the trial. They will also assess the performance of overall study operations and any other relevant issues, as necessary. The report will include the following:
Evidence of efficacy according to pre-established statistical guidelines.
Evidence of study-related adverse events.
Data quality, completeness and timeliness.
Performance of the study.
Adequacy of compliance with goals for recruitment and retention, including those related to the participation of women.
Adherence to the protocol.
Factors that might affect the study outcome or compromise the confidentiality of the trial data (such as protocol violations).
Factors external to the study such as scientific or therapeutic developments that may impact participant safety or the ethics of the study.
Trial status
It is currently an active trial that has been open for enrolment since 1 July 2018 with anticipated completion date of 30 August 2023. The study was approved by McGuire VA IRB and R&D since 8 May 2018. Recruitment started on 1 July 2018 and will end by 30 June 2023.
Access to data
Data will be available on email communication with the principal investigator following completion of the study and receiving prior approval for data sharing from the appropriate regulatory bodies.
Supplementary Material
Acknowledgments
We would like to thank all the participants with spinal cord injury who contributed their time and effort to complete the current work.
Footnotes
Contributors: All authors contributed to the current work and they were involved in several aspects of the protocol. ASG: design of the study, secured funding, IRB approval, data management; RK: IRB coordination, recruitment, scheduling, training participants, data management; MA: training participants and data management; RG: administering IVGTT and responsible for all analysis of the blood work; JR: responsible for conducting muscle biopsy; LG, TC, TLL: responsible for medically screening patients and determine their eligibility to participate in all aspects of the study; DCi: design of the study and oversee protocol completion; DCa: administering electrodiagnostic testing to confirm denervation; EL: design of the study and responsible for all mitochondria work; CC: design of the study and responsible for all intracellular signalling and mRNA studies; RA: design of the study, prescribe testosterone treatment and monitor testosterone dose throughout the trial.
Funding: The work was supported by the Department of Veterans Affairs-VA Merit Programme (application # 1 I01 RX002649-01A; protocol ID: B2649-R) to ASG.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data will be available on email communication with the principal investigator following completion of the study and receiving prior approval for data sharing from the appropriate regulatory.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
Not applicable.
References
- 1.Gater DR. Obesity after spinal cord injury. Phys Med Rehabil Clin N Am 2007;18:333–51. 10.1016/j.pmr.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 2.Gater D. Pathophysiology of obesity after spinal cord injury. Top Spinal Cord Inj Rehabil 2007;12:20–34. 10.1310/sci1204-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver FM, Collins EG, Kurichi J, et al. Prevalence of obesity and high blood pressure in veterans with spinal cord injuries and disorders: a retrospective review. Am J Phys Med Rehabil 2007;86:22–9. 10.1097/phm.0b013e31802b8937 [DOI] [PubMed] [Google Scholar]
- 4.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism 1994;43:749–56. 10.1016/0026-0495(94)90126-0 [DOI] [PubMed] [Google Scholar]
- 5.Lavela SL, Weaver FM, Goldstein B, et al. Diabetes mellitus in individuals with spinal cord injury or disorder. J Spinal Cord Med 2006;29:387–95. 10.1080/10790268.2006.11753887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeVivo MJ, Go BK, Jackson AB. Overview of the national spinal cord injury statistical center database. J Spinal Cord Med 2002;25:335–8. 10.1080/10790268.2002.11753637 [DOI] [PubMed] [Google Scholar]
- 7.Strauss DJ, Devivo MJ, Paculdo DR, et al. Trends in life expectancy after spinal cord injury. Arch Phys Med Rehabil 2006;87:1079–85. 10.1016/j.apmr.2006.04.022 [DOI] [PubMed] [Google Scholar]
- 8.DeVivo MJ. Causes and costs of spinal cord injury in the United States. Spinal Cord 1997;35:809–13. 10.1038/sj.sc.3100501 [DOI] [PubMed] [Google Scholar]
- 9.Dudley GA, Castro MJ, Rogers S, et al. A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol 1999;80:394–6. 10.1007/s004210050609 [DOI] [PubMed] [Google Scholar]
- 10.Mahoney ET, Bickel CS, Elder C, et al. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch Phys Med Rehabil 2005;86:1502–4. 10.1016/j.apmr.2004.12.021 [DOI] [PubMed] [Google Scholar]
- 11.Ryan TE, Brizendine JT, Backus D, et al. Electrically induced resistance training in individuals with motor complete spinal cord injury. Arch Phys Med Rehabil 2013;94:2166–73. 10.1016/j.apmr.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 12.Gorgey AS, Mather KJ, Cupp HR, et al. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc 2012;44:165–74. 10.1249/MSS.0b013e31822672aa [DOI] [PubMed] [Google Scholar]
- 13.Kanzleiter T, Rath M, Görgens SW, et al. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem Biophys Res Commun 2014;450:1089–94. 10.1016/j.bbrc.2014.06.123 [DOI] [PubMed] [Google Scholar]
- 14.Pedersen BK, Febbraio MA, Muscles FMA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012;8:457–65. 10.1038/nrendo.2012.49 [DOI] [PubMed] [Google Scholar]
- 15.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 2008;88:1379–406. 10.1152/physrev.90100.2007 [DOI] [PubMed] [Google Scholar]
- 16.Boncompagni S. Severe muscle atrophy due to spinal cord injury can be reversed in complete absence of peripheral nerves. Eur J Transl Myol 2012;22:161–200. 10.4081/bam.2012.4.161 [DOI] [Google Scholar]
- 17.Carraro U, Boncompagni S, Gobbo V, et al. Persistent muscle fiber regeneration in long term denervation. past, present, future. Eur J Transl Myol 2015;25:77. 10.4081/bam.2015.2.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurz A, Volk GF, Arnold D, et al. Selective electrical surface stimulation to support functional recovery in the early phase after unilateral acute facial nerve or vocal fold paralysis. Front Neurol 2022;13:869900. 10.3389/fneur.2022.869900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold D, Thielker J, Klingner CM, et al. Selective surface electrostimulation of the denervated zygomaticus muscle. Diagnostics 2021;11:188. 10.3390/diagnostics11020188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kern H, Carraro U. Home-based functional electrical stimulation of human permanent denervated muscles: a narrative review on diagnostics, managements, results and Byproducts revisited 2020. Diagnostics 2020;10:529. 10.3390/diagnostics10080529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kern H, Hofer C, Strohhofer M, et al. Standing up with denervated muscles in humans using functional electrical stimulation. Artif Organs 1999;23:447–52. 10.1046/j.1525-1594.1999.06376.x [DOI] [PubMed] [Google Scholar]
- 22.Carraro U, Rossini K, Zanin ME. Induced myogenesis in long-term permanent denervation: perspective role in functional electrical stimulation of denervated legs in humans. BAM-PADOVA 2002;12:53–64. [Google Scholar]
- 23.Hofer C, Mayr W, Stöhr H, et al. A stimulator for functional activation of denervated muscles. Artif Organs 2002;26:276–9. 10.1046/j.1525-1594.2002.06951.x [DOI] [PubMed] [Google Scholar]
- 24.Kern H, Hofer C, Mödlin M, et al. Denervated muscles in humans: limitations and problems of currently used functional electrical stimulation training protocols. Artif Organs 2002;26:216–8. 10.1046/j.1525-1594.2002.06933.x [DOI] [PubMed] [Google Scholar]
- 25.Chandrasekaran S, Davis J, Bersch I, et al. Electrical stimulation and denervated muscles after spinal cord injury. Neural Regen Res 2020;15:1397–407. 10.4103/1673-5374.274326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorgey AS, Khalil RE, Gill R, et al. Low-dose testosterone and evoked resistance exercise after spinal cord injury on cardio-metabolic risk factors: an open-label randomized clinical trial. J Neurotrauma 2019;36:2631–45. 10.1089/neu.2018.6136 [DOI] [PubMed] [Google Scholar]
- 27.Gorgey AS, Graham ZA, Chen Q, et al. Sixteen weeks of testosterone with or without evoked resistance training on protein expression, fiber hypertrophy and mitochondrial health after spinal cord injury. J Appl Physiol 2020;128:1487–96. 10.1152/japplphysiol.00865.2019 [DOI] [PubMed] [Google Scholar]
- 28.Bauman WA, Cirnigliaro CM, La Fountaine MF, et al. A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm Metab Res 2011;43:574–9. 10.1055/s-0031-1280797 [DOI] [PubMed] [Google Scholar]
- 29.Kostovski E, Iversen PO, Birkeland K, et al. Decreased levels of testosterone and gonadotrophins in men with long-standing tetraplegia. Spinal Cord 2008;46:559–64. 10.1038/sc.2008.3 [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Zhao J, Zhao W, et al. Nandrolone normalizes determinants of muscle mass and fiber type after spinal cord injury. J Neurotrauma 2012;29:1663–75. 10.1089/neu.2011.2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, et al. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab 2004;89:5245–55. 10.1210/jc.2004-0084 [DOI] [PubMed] [Google Scholar]
- 32.Gorgey AS, Lai RE, Khalil RE, et al. Neuromuscular electrical stimulation resistance training enhances oxygen uptake and ventilatory efficiency independent of mitochondrial complexes after spinal cord injury: a randomized clinical trial. J Appl Physiol 2021;131:265–76. 10.1152/japplphysiol.01029.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorgey AS, Lester RM, Wade RC, et al. A feasibility pilot using telehealth videoconference monitoring of home-based NMES resistance training in persons with spinal cord injury. Spinal Cord Ser Cases 2017;3:17039. 10.1038/scsandc.2017.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorgey AS, Mather KJ, Poarch HJ, et al. Influence of motor complete spinal cord injury on visceral and subcutaneous adipose tissue measured by multi-axial magnetic resonance imaging. J Spinal Cord Med 2011;34:99–109. 10.1179/107902610X12911165975106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorgey AS, Gater DR. Regional and relative adiposity patterns in relation to carbohydrate and lipid metabolism in men with spinal cord injury. Appl Physiol Nutr Metab 2011;36:107–14. 10.1139/H10-091 [DOI] [PubMed] [Google Scholar]
- 36.Lester RM, Ghatas MP, Khan RM, et al. Prediction of thigh skeletal muscle mass using dual energy X-ray absorptiometry compared to magnetic resonance imaging after spinal cord injury. J Spinal Cord Med 2019;42:622–30. 10.1080/10790268.2019.1570438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogawa M, Lester R, Akima H, et al. Quantification of intermuscular and intramuscular adipose tissue using magnetic resonance imaging after neurodegenerative disorders. Neural Regen Res 2017;12:2100–5. 10.4103/1673-5374.221170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorgey A, Gater D. Insulin growth factor-1 may explain the variability in skeletal muscle size in spastic individuals with spinal cord injury. J Rehabil Res Dev 2012;49:373–80. 10.1682/jrrd.2011.04.0076 [DOI] [PubMed] [Google Scholar]
- 39.Edmunds KJ, Gíslason MK, Arnadottir ID, et al. Quantitative computed tomography and image analysis for advanced muscle assessment. Eur J Transl Myol 2016;26:6015. 10.4081/ejtm.2016.6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghatas MP, Lester RM, Khan MR, et al. Semi-automated segmentation of magnetic resonance images for thigh skeletal muscle and fat using threshold technique after spinal cord injury. Neural Regen Res 2018;13:1787–95. 10.4103/1673-5374.238623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abilmona SM, Sumrell RM, Gill RS, et al. Serum testosterone levels may influence body composition and cardiometabolic health in men with spinal cord injury. Spinal Cord 2019;57:229–39. 10.1038/s41393-018-0207-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorgey AS, Cirnigliaro CM, Bauman WA, et al. Estimates of the precision of regional and whole body composition by dual-energy X-ray absorptiometry in persons with chronic spinal cord injury. Spinal Cord 2018;56:987–95. 10.1038/s41393-018-0079-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graham ZA, Collier L, Peng Y, et al. A soluble activin receptor IIb fails to prevent muscle atrophy in a mouse model of spinal cord injury. J Neurotrauma 2016;33:1128–35. 10.1089/neu.2015.4058 [DOI] [PubMed] [Google Scholar]
- 44.O'Brien LC, Wade RC, Segal L, et al. Mitochondrial mass and activity as a function of body composition in individuals with spinal cord injury. Physiol Rep 2017;5:e13080. 10.14814/phy2.13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536–59. 10.1210/jc.2009-2354 [DOI] [PubMed] [Google Scholar]
- 46.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72. 10.1210/jcem.84.10.6079 [DOI] [PubMed] [Google Scholar]
- 47.Manns PJ, McCubbin JA, Williams DP. Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch Phys Med Rehabil 2005;86:1176–81. 10.1016/j.apmr.2004.11.020 [DOI] [PubMed] [Google Scholar]
- 48.Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes 1989;38:1512–27. 10.2337/diab.38.12.1512 [DOI] [PubMed] [Google Scholar]
- 49.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–70. 10.2337/diacare.22.9.1462 [DOI] [PubMed] [Google Scholar]
- 50.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 51.Brass EP, Hiatt WR, Gardner AW, et al. Decreased NADH dehydrogenase and ubiquinol-cytochrome c oxidoreductase in peripheral arterial disease. Am J Physiol Heart Circ Physiol 2001;280:H603–9. 10.1152/ajpheart.2001.280.2.H603 [DOI] [PubMed] [Google Scholar]
- 52.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 2011;1813:1269–78. 10.1016/j.bbamcr.2010.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groll A, Tutz G. Variable selection for generalized linear mixed models by L 1-penalized estimation. Stat Comput 2014;24:137–54. 10.1007/s11222-012-9359-z [DOI] [Google Scholar]
- 54.Gill S, Sumrell RM, Sima A, et al. Waist circumference cutoff identifying risks of obesity, metabolic syndrome, and cardiovascular disease in men with spinal cord injury. PLoS One 2020;15:e0236752. 10.1371/journal.pone.0236752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sessa C, Cortes J, Conte P, et al. The impact of COVID-19 on cancer care and oncology clinical research: an experts' perspective. ESMO Open 2022;7:100339. 10.1016/j.esmoop.2021.100339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elaraby A, Shahein M, Bekhet AH, et al. The COVID-19 pandemic impacts all domains of quality of life in Egyptians with spinal cord injury: a retrospective longitudinal study. Spinal Cord 2022;60:757–62. 10.1038/s41393-022-00775-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitsikostas D-D, Moka E, Orrillo E, et al. Neuropathic pain in neurologic disorders: a narrative review. Cureus 2022;14:e22419. 10.7759/cureus.22419 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-064748supp001.pdf (406.6KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data will be available on email communication with the principal investigator following completion of the study and receiving prior approval for data sharing from the appropriate regulatory.