Abstract
Introduction
Actinic keratosis (AK) is the most common precancerous skin condition caused by long-term UV exposure. Given the high recurrence rate of 15%–53%, identifying safe and effective treatment options is warranted. AVX001, a cytosolic phospholipase A2α (cPLA2α) enzyme inhibitor, is a novel anti-inflammatory drug for field-directed, self-administered, topical therapy of AK.
Methods and analysis
This study is a single-centre, randomised, vehicle-controlled, double-blind, parallel-group hybrid clinical trial in adults with multiple AK lesions Olsen grade 1 or 2. The hybrid design combines decentralised participant tasks and assessments with conventional in-clinic visits. Recruitment using targeted advertising on social media and eligibility prescreening are conducted via the Studies&Me online recruitment platform. Participants (n=60) are randomly assigned to 1 of 3 treatment arms: AVX001 gel 1%, AVX001 gel 3% or vehicle gel. The trial consists of a 4-week treatment period with daily field-directed topical application of the gel and an 8-week follow-up period. Participants attend in-clinic visits at baseline, week 4 and week 12. The remote participant trial tasks include questionnaires and upload of smartphone-obtained photos of the treated skin area using a study-specific web-based app. Both remote and in-clinic assessments of safety and efficacy will be performed. The primary objective is to evaluate the local tolerability of daily application of AVX001 gel (1% or 3%) compared with vehicle gel. Secondary objectives include safety, efficacy, dose–response efficacy relationship, treatment satisfaction and cosmetic outcome. Exploratory objectives include evaluations of tolerability and efficacy assessed by dermatologists using smartphone photos uploaded by participants, comparisons of in-clinic and remote assessments and assessment of AK-related skin changes by non-invasive optical imaging.
Ethics and dissemination
Approved by the Ethics Committee of the Capital Region of Denmark (H-21018064) and the Danish Medicines Agency (2021032485). Results will be submitted for publication in peer-reviewed scientific journals.
Trial registration numbers
2021-000934-32; NCT05164393.
Keywords: dermatology, adult dermatology, clinical trials, dermatological tumours, protocols & guidelines, adverse events
Strengths and limitations of this study.
This study employs for the first time a hybrid trial design in participants with actinic keratoses, using a combination of decentralised trial activities, remote assessments and conventional in-clinic visits.
This study uses online recruitment strategies for the first time in this patient group to reach a wider, more diverse and digitally literate population and reduce recruitment time.
The use of a web-based study app that participants access using their own smartphone enables participants to continuously register any side effects and adverse events throughout the trial.
Participation requires a certain level of digital literacy; given the demographic of patients affected by actinic keratosis, some participants might find it challenging to perform study tasks using their smartphone which can introduce selection bias.
Remote assessments of actinic keratosis clearance and local skin reactions using smartphone photographs have not yet been validated, which could result in discrepant endpoints.
Introduction
Actinic keratosis (AK) is a common skin condition most commonly caused by long-term sun exposure. Inflammation, genetic damage, oxidative stress, immunosuppression and abnormal cell proliferation caused by excessive skin exposure to UV radiation are among the main mechanisms involved in AK formation.1 2 AK lesions are presented as dry, rough and scaly patches that may itch or become irritated.3 4 The condition is more prevalent in fair-skinned populations, with around 40% prevalence reported in Australia4 and the Netherlands.5 AK can undergo malignant transformation into squamous cell carcinoma, an invasive type of skin cancer. Despite a low annual transformation rate of up to 0.53%,6 7 the high prevalence and the difficulty of predicting the progression of individual AK lesions necessitates the development of novel, field-directed topical treatments.
Although solitary lesions can occur, AK tends to appear in clusters on sun-exposed areas. In addition to their high recurrence rate of 15%–53%,6 subclinical lesions may exist in the surrounding sun-exposed area (field cancerisation) and can develop into primary tumours. While cryotherapy is the most commonly used in-office procedure for management of AK,1 2 field-directed treatments can address not just the clinically apparent lesions but also subclinical dysplasia.8 AVX001 is an anti-inflammatory compound that attenuates inflammation and abnormal keratinocyte hyperproliferation by inhibiting cytosolic phospholipase A2α (cPLA2α).9 10 The first-in-man dose escalation trial showed that 4-week topical treatment with AVX001 was well tolerated up to 5% concentration.11 AVX001 has been developed as a gel formulation and is intended for field-directed topical application.
Decentralised clinical trials are a relatively new phenomenon that leverages new digital technologies and has the potential to transform clinical trial conduct. With the growing popularity of smartphones and digital literacy in all age groups, the inclusion of decentralised elements in the trial design will facilitate a more dynamic and even real-time data acquisition. The decentralised approach can provide several advantages for both study staff and participants, including remote monitoring of participant engagement and adherence, immediate and complete reporting of side effects and time-saving and cost-effective remote assessments.12 13 Compared with conventional clinical trials, decentralised trials can be conducted with fewer study sites involved, thereby allowing a better oversight by the principal investigator. Online recruitment strategies and remote prescreening procedures can accelerate recruitment and attract a more diverse population.14 The hybrid trial design is particularly suited for dermatological early-phase trials; previous studies on atopic dermatitis, where in-clinic efficacy and safety assessments were supplemented by blinded remote evaluations, demonstrated high agreement between in-clinic and photograph-based assessments.13 15 16
The aim of this phase I/IIa trial is to evaluate the tolerability, safety and efficacy of daily field-directed topical applications of AVX001 gel in two different concentrations (1% or 3%) in comparison to vehicle control, in participants with multiple AK lesions. The trial will be conducted in a hybrid setting, combining visits to the study clinic with remote trial-related tasks and assessments enabled by the use of a study-specific web-based application (hereafter referred to as the Study App), which the participants can access using their own smartphone.
Methods
Trial design
The study is a single-centre, randomised, vehicle-controlled, double-blind, parallel-group hybrid clinical trial. The hybrid trial design combines decentralised procedures with in-clinic visits. The decentralised element consists of trial-related tasks (questionnaires and photo upload) conducted by participants using a Study App, online recruitment strategies and remote, image-based dermatological assessments. The in-clinic visits will take place at the Department of Dermatology, Copenhagen University Hospital, Bispebjerg, Denmark.
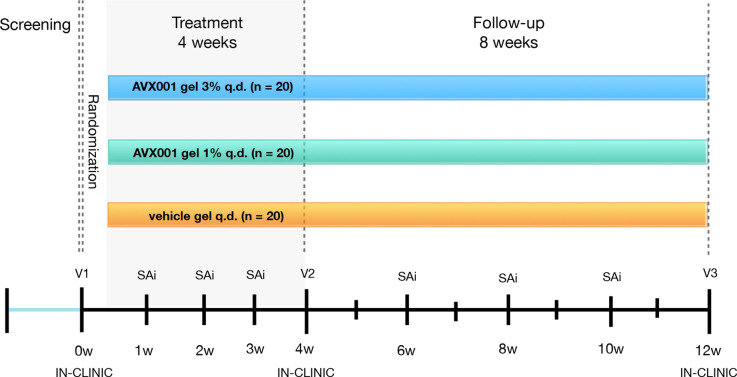
An overview of the trial design is shown in figure 1. Participants will be randomly assigned to one of three treatment arms; AVX001 gel 1%, AVX001 gel 3% or vehicle gel. The total trial duration for each participant will be around 13 weeks, which includes a prescreening period, a 4-week treatment period, and an 8-week follow-up period. Participants will attend an in-clinic visit at baseline, end of treatment (EOT), and end of study (EOS). In between the visits to the clinic, participants will undertake scheduled Study App interactions on a weekly to biweekly basis.
Figure 1.
Overview of trial design. q.d., one time per day; SAi, remote study APP interactions; V, visit; w, week.
Eligibility criteria
Men and women aged 18 years or above who have a clinical AK diagnosis confirmed by the principal investigator are eligible to participate if they are able to comply with the inclusion/exclusion criteria. Key inclusion and exclusion criteria are shown in table 1. A comprehensive list of the inclusion/exclusion criteria may be found in the supplemental material.
Table 1.
Key inclusion and exclusion criteria
| Key inclusion criteria | Key exclusion criteria |
| Can present a skin area located in face, neck or chest of ≥25 cm2 with 4–8 actinic keratosis lesions | Actinic keratosis lesions classified as Olsen grade 3 in target area |
| Actinic keratosis lesions in target area of severity grade 1 or 2 as defined by the Olsen clinical criteria for actinic keratosis | Atypical actinic keratosis lesions in the target area, including suspected squamous cell carcinoma and basal cell carcinoma |
| Have a suitable smartphone to complete the trial tasks (Android operating system: Android 8.1 or higher; iPhone with iOS 12.4 or higher), and able and willing to follow the trial procedures by using the Study App | Any dermatological condition in the target area that can be exacerbated by treatment or affect assessments |
| Women must either be of non-childbearing potential or must be using a highly effective method of contraception for the duration of the study | Received lesion-directed or field-directed therapy within 2 cm of the target skin area within 1 month prior to baseline visit, including topical drugs, destructive therapies or field ablation treatments |
Interventions
The trial products will be administered as a gel formulation for topical application. Participants will receive either a gel containing 1% AVX001, 3% AVX001 or the vehicle gel without the active ingredient. The trial products will be provided in tubes containing 5 g of gel (manufactured by Bioglan AB, Sweden). The gel should be applied topically in doses of 0.5 g per day (one fingertip unit) on the target area. During the baseline visit, participants will be instructed on how to apply the gel, and the first application will be supervised by site staff. Hereafter, participants will be instructed to apply the gel in the evening before bedtime for the following 4 weeks. The investigator will use a plastic sheet overlay to map the treatment area. The plastic sheet will be provided to the participant to help them apply the treatment in the correct area.
To improve treatment adherence, participants will be asked via a treatment questionnaire if they have applied the treatment as prescribed. Trial support staff will be notified of participants who report having missed treatment applications, and will reach out to provide support.
Participants will also receive weekly notifications to open a new tube with trial product and a reminder to return them at the EOT visit. Adherence will be assessed by weighing the tubes before and after, hereby measuring the total amount of trial product used by each participant.
Discontinuation of the trial product is required in the event of pregnancy or if an adverse event (AE) contraindicates further application.
Objectives and endpoints
The primary objective is to evaluate the local tolerability of daily application of AVX001 gel (1% or 3%) compared with application of the vehicle gel during 4 weeks of field-directed treatment in participants with AK.
The secondary objectives are to evaluate the safety, efficacy, dose–response efficacy relationship, treatment satisfaction and cosmetic outcome of daily application of AVX001 gel (1% or 3%) compared with vehicle gel.
The exploratory objectives are to evaluate tolerability and efficacy as assessed remotely using smartphone photos uploaded by the participants, to compare remote and in-clinic assessments of tolerability and efficacy and to evaluate AK-related skin changes by non-invasive optical imaging. Primary, secondary, and exploratory endpoints are listed in table 2, along with information on whether the assessments will be performed remotely or in-clinic.
Table 2.
Secondary and exploratory endpoints
| Outcome measure | Type of assessment | Time period |
| Primary endpoint | ||
| Proportion of participants with LSR>2 | In-clinic | Baseline to EOS |
| Secondary endpoints | ||
| Assessment of safety based on frequency of SAEs | In-clinic and remote | Baseline to EOS |
| Assessment of safety based on frequency of AEs | In-clinic and remote | Baseline to EOS |
| Assessment of safety based on skin examinations | In-clinic | Baseline to EOS |
| Assessment of safety based on blood pressure (vital sign) | In-clinic | Baseline to EOS |
| Assessment of safety based on pulse (vital sign) | In-clinic | Baseline to EOS |
| Assessment of safety based on temperature (vital sign) | In-clinic | Baseline to EOS |
| Proportion of participants who experience LSR grades 1, 2, 3 and 4 | In-clinic | Baseline to EOT, Baseline to EOS |
| Proportion of participants experiencing a clinically visible clearance of the target area of >50% | In-clinic | Baseline to EOT/early termination, Baseline to EOS |
| Recurrence rate of AKs after treatment clearance | In-clinic | Between EOT and EOS |
| Appearance of new lesions in the target area | In-clinic | Baseline to EOS |
| Participant satisfaction with the AVX001 gel, assessed by the TSQM | Remote | Week 2 and EOT |
| Proportion of participants with a cosmetic outcome grade <2, as assessed using the Cosmetic Scoring Tool | In-clinic and remote | Baseline to EOS |
| Cosmetic outcome of target area as evaluated by participants by comparing the status at EOS | Remote | Baseline and EOS |
| Exploratory endpoints | ||
| Changes in AK-FAS as evaluated by the central assessors (remote dermatologists) on the smartphone photos taken by the participants | Remote | Baseline to EOT, Baseline to EOS |
| Level of agreement between AK-FAS in-clinic and AK-FAS performed remotely by central assessors (dermatologists) using smartphone photos taken by the participants | In-clinic and remote | Baseline to EOT, Baseline to EOS |
| Proportion of participants presenting with an LSR>2, as evaluated by the central assessors (remote dermatologists) on the smartphone photos taken by the participants | Remote | Baseline to EOT, Baseline to EOS |
| Proportion of participants who experience LSR grades 1, 2, 3 and 4, as evaluated by the central assessors (remote dermatologists) on the smartphone photos taken by the participants | Remote | Baseline to EOS |
| Time to reach a clinically visible clearance of target area of >50% for all enrolled participants performed from remote by central Assessors (remote dermatologists) using smartphone photos taken by the subjects | Remote | Baseline to EOS |
| Presence of AK-related skin changes evaluated by non-invasive optical imaging | In-clinic | Baseline and EOS |
AE, adverse event; AK-FAS, Actinic Keratosis Field Assessment Scale; EOS, end of study; EOT, end of treatment; LSR, local skin reaction; SAE, serious adverse event; TSQM, Treatment Satisfaction Questionnaire for Medication.
Sample size
The sample size calculation is based on the primary endpoint defined as the proportion of participants that have experienced local skin reaction (LSR)>2 at any time between baseline and EOS. Based on the assumption that 10% of participants experience LSR>2 during the trial, including 18 completing participants in each arm results in a 95% CI of 1.0% to 33.3% computed with the exact (Clopper-Pearson) CI formula. Assuming a dropout rate of 10%, the sample size required is 20 in each of the three treatment arms.
Recruitment
Participant recruitment will mainly be done via the Studies&Me recruitment platform. The online platform uses social media and online tools for recruitment. Advertisement will be done through social media. Potential candidates who are interested in participating are directed to a landing page where they can sign up by answering a generic questionnaire. On the landing page, candidates are informed about the trial at a high level, and will be asked to consent to terms, conditions and data usages in prequalification. Interested candidates are directed to a prequalifying questionnaire, and will be asked to upload two photos of their lesions. Central assessors (board-certified remote dermatologists) will pre-evaluate potential candidates remotely based on the uploaded photos and prequalifying questionnaire. Qualified candidates will receive an invitation to join the trial via email.
Informed consent
Once the qualified candidate has signed up for the trial, they will be asked to review the informed consent form and additional educational material, including a video explaining the purpose of the trial and the implications of participating. If the candidate wishes to participate, they will be asked to book their first visit with the investigator. At the first visit, the informed consent conversation will take place.
Screening
Once informed consent has been obtained, the participant will be screened for eligibility. Screening includes a baseline questionnaire, a skin type questionnaire,17 and questionnaires regarding concomitant medications and concurrent procedures. The investigator will also administer a pregnancy test for female participants of childbearing potential.
Randomisation, treatment allocation and blinding
At the baseline visit, the investigator will initiate randomisation via the decentralised clinical trial (DCT) platform. The Interactive Response Technology system will be used to control randomisation and assign the required kit numbers for the participants. Participants will be randomised into one of the three trial arms (allocation ratio 1:1:1), defining their treatment allocation. No stratification is performed. Both participants, site staff, central assessors and Studies&Me personnel will be blinded to the assigned treatment for the duration of the study. The randomisation code list connecting the randomisation numbers with the different treatment arms will be generated by the provider of the DCT platform and sent directly to the third party pharmacy in charge of handling the trial products.
Unblinding before the end of the trial is restricted to emergency situations and should only be used under circumstances where emergency treatment requires knowledge of the trial product received, or where obtaining this information is required for regulatory or pharmacovigilance reasons. Emergency unblinding can be performed by the investigator via the DCT platform. In the event of emergency unblinding, this will be documented in the DCT platform along with an explanatory description for unblinding. Any participant who has had their treatment unblinded, prior to end of trial, will be withdrawn from the trial.
Patient and public involvement
Patients were involved in the conception phase of the study to better understand pain points in the disease-specific patient journey, in order to design an overall better participant experience. Learnings from patient interviews provided insights into how patients perceive and speak about the disease (terminology). This allowed us to tailor and target our online recruitment strategies to make the recruitment process more efficient. We furthermore plan to involve patient focus groups in the planning and development of the lay summary of the results that will be shared with the public and the participants.
Data collection
DCT platform
The data collected in this trial will be captured in a DCT platform, which is customised to the study to facilitate data collection. It is used to capture and store all data from the investigator and central assessors, and is integrated with the Study App, hereby replacing the conventional case report form.
Study App interactions
During the course of the trial, remote activities will be performed via the Study App, which the participants can access through their private smartphones. The Study App is customised to suit the needs of the present trial. In between the in-clinic visits, participants will use the Study App to upload photos of treated skin area for remote assessment by the central assessors. The first photo of the treatment area will be taken by the investigator using the participant’s smartphone during the baseline visit. The investigator will create an outline of the treatment area to support the remote assessment. At the baseline visit, the investigator will instruct the participants on how to complete the tasks in the Study App, and provide guidance on how to take the photos of the treated skin area. Instructions for taking the photos will furthermore be provided in the Study App and in a Q&A handout. If the central assessors evaluate that the photo quality is unsatisfactory, they will report it through the DCT platform, which will trigger a notification for the trial support staff at Studies&Me. Trial support will then reach out to the participant and request a new photo upload.
Participants will also be prompted to complete questionnaires related to LSRs and other signs and symptoms of AEs, and report treatment adherence using the Study App. At weeks 2 and 4, they will be asked to use the Study App to fill out the Treatment Satisfaction Questionnaire for Medication (TSQM V.1.4). The TSQM is a generic, psychometrically valid instrument suited for the assessment of patient-reported effectiveness, side effects, convenience and satisfaction where disease-specific patient-reported outcome questionnaires are lacking.18 Before the EOS visit, they will be asked to evaluate the cosmetic outcome of the target area with the current status in weeks 11 and 12.
The participants may report signs and symptoms in the Study App at any time during the treatment period. This will trigger a notification for the investigator. In between visits, participants will have the possibility to contact the site staff to address signs and symptoms. This participant-centred approach will allow AE reporting in real-time, improving the ongoing collection of safety data during the trial.
In-clinic visits
The participants will have three in-clinic visits during the trial. At the baseline visit, the investigator will create a map of the lesions in the target area using a transparent plastic template. At the subsequent visits, any progress will be marked with a different colour.
At each in-clinic visit, the investigator will clinically assess LSRs, cosmetic outcome, AK lesions, AK field damage and evaluate subclinical changes using non-invasive imaging.
Skin reactions in the target area will be scored using the LSR scale developed for AK.19 The scale is used to measure multiple components of possible skin responses to the trial product. It measures presence and severity (0–4) of six clinical characteristics that are commonly observed LSRs in relation to topical therapies (erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation and erosion/ulceration).
The cosmetic outcome will be evaluated using a cosmetic scoring tool designed for comparison before and after treatment in mild-to-moderate AK.20 The tool rates the cosmetic appearance of the skin surface, pigmentation, degree of scarring and atrophy.
The investigator will grade the individual AK lesions in the target area using the Olsen clinical classification scheme21 and the target area with the Actinic Keratosis Field Assessment Scale (AK-FAS) at each visit. The Olsen scale is the most commonly used classification system for clinical evaluation of AK, grading lesions from I to III based on lesion thickness and degree of hyperkeratosis. The AK-FAS is a validated scale for grading disease severity of the entire field affected by AK, including an estimate of the actinically damaged area, the presence of hyperkeratosis, and signs of sun damage.22 At EOT and EOS visits, the investigator will assess whether a visible clearance of >50% of target area is reached.
Subclinical changes in each AK lesion will be monitored using a non-invasive laser-based imaging modality, optical coherence tomography (OCT). A commercially available OCT system (VivoSight Dx; Michelson Diagnostics, Kent, UK) with an optical resolution of <7.5 µm laterally and <5 µm axially will be used. At the EOS visit, the investigator will assess the recurrence rate and appearance of new AK lesions in the area.
Furthermore, the investigator will perform safety assessments including vital signs, skin examinations and evaluations of AEs and local symptoms at each visit.
Remote assessments
The photos taken by the participants will be reviewed remotely by the central assessors, which consist of four board-certified dermatologists. To reduce inter-rater variability, the same dermatologist will assess the same participant’s photos throughout the trial. The central assessors have received both general and study-specific training in AK rating using images. They will be supporting the assessment of efficacy and safety endpoints by performing remote, image-based dermatological evaluations of the target lesions throughout the trial. The central assessors will score the appearance of the photographic target lesions using the AK-FAS, LSR scale, as well as evaluate the cosmetic outcome and time to reach a visible clearance of target area of >50%. If the central assessors identify LSR>2 or any signs of AEs in the photos, the investigator will be notified in order to evaluate and, if necessary, follow-up with the participant.
In between the visits, the investigator will monitor AEs using the self-reported information provided by the participants in the Study App.
Data management
To ensure a clean and consistent database for statistical analysis, the DCT platform is configured to contain date ranges and validation checks to maintain ongoing quality assurance of entered data. Ongoing and prior to database lock, a database review will be performed, and any discrepancies or missing data will be queried and resolved.
Statistical analysis
For analysis purposes, the trial population will be divided into (i) a Full Analysis Set including all randomised participants; (ii) a Safety Set including all randomised participants who have had at least one application with trial product and (iii) a Per Protocol Set including all randomised participants who fulfil the protocol in terms of eligibility, treatment adherence and outcome assessments. All the analyses of endpoints specified in the protocol will be performed according to a statistical analysis plan, which will be finalised before breaking the randomisation code.
The primary endpoint will be calculated by counting the number of participants with LSR>2 at any time between baseline and EOS divided by the number of participants included in the Full Analysis Set for each treatment arm. For efficacy analysis, proportion of subjects with treatment success (clinically visible clearance of target areas of >50%) are compared between treatment and vehicle, based on Fisher’s exact test. The treatment satisfaction and subject-reported cosmetic outcome will be compared between treatment arms with the non-parametric Mann-Whitney-Wilcoxon (Wilcoxon rank sum) test. Changes in cosmetic outcome between groups will be analysed with Fisher’s exact test. AEs and other safety parameters will be summarised and listed.
Monitoring
The trial will be monitored on an ongoing basis to verify that (i) the rights and well-being of the participants are protected; (ii) the reported trial data are accurate, complete and if applicable verifiable from source documents and (iii) the conduct of the trial is in compliance with the currently approved protocol/amendment(s), the International Council for Harmonisation Good Clinical Practice guideline (ICH GCP), and all applicable regulatory requirements. Monitoring will be performed using a systematic, risk-based approach. Both centralised and onsite monitoring will be used.
Ethics and dissemination
The sponsor of this trial is Coegin Pharma AB (Lund, Sweden). The trial is conducted by Studies&Me according to the current version of the ICH GCP guideline.23 It has been approved by the Danish Medicines Agency (2021032485) and the Ethics Committee of the Capital Region of Denmark (H-21018064).
Data protection
The Recruitment Platform used for prequalifying complies with the General Data Protection Regulation, and any candidate who has signed up can at any time contact Studies&Me to have their data deleted. All data, including personal information about participants, will be collected in the DCT platform, which will be secured with role-based data access restrictions and an audit trail. At the end of the trial, pseudonymised data will be transferred to the sponsor.
Insurance and liability
Participants will be covered by the public patient compensation scheme in Denmark. The sponsor has clinical trial liability insurance.
Dissemination
Results of the trial will be submitted for publication in an appropriate, peer-reviewed scientific journal. A plain-language summary of the results will be made available to the public on the Studies&Me website (www.studiesandme.com), and will also be provided to the participants. The trial results are to be uploaded to EudraCT within 1 year from the end of trial, and will be reported in www.clinicaltrialsregister.eu, www.clinicaltrials.gov and national data registries in accordance with applicable law and regulations after clinical trial completion or premature termination.
Timeline
First Participant First Visit took place in November 2021, and Last Participant Last Visit is expected in March 2022.
bmjopen-2022-061012supp001.pdf (47.9KB, pdf)
Supplementary Material
Footnotes
Contributors: The authors MH, TD, JK, BJ, ADA, KK, ACM and JRZ have substantially contributed to the conception and design of the work. The following authors have contributed to the design of: acquisition (VKO, MH, IM and CPL); analysis (API) or interpretation of data for the work (ER, MLME, MH, VKO, TD, JK, BJ, FJA, AJF, ADA, KK, ACM and JRZ). All authors have been drafting the work or revising it critically for important intellectual content; finally approved the version to be published; agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This work is funded by Coegin Pharma AB.
Competing interests: ADA, KK, ACM, CPL, JRZ, API, MLME and ER were employed at Studies&Me for the present work, a clinical research organisation that has been contracted by Coegin Pharma to conduct the present study; TD is the CEO of the sponsor company Coegin Pharma; TD, BJ, AJF and FJA are shareholders and stock option holders of Coegin Pharma; BJ, AJF and FJA have received consulting fees from Coegin Pharma with patents planned; JK is a board member at Coegin Pharma and has received consulting fees from Coegin Pharma; IM has received consulting fees from Studies&Me; VKO has been employed at LEO Pharma AB and has received research grants from Innovation Fund Denmark; MH has received research grants from Studies&Me, LEO Pharma, Lutronic, Mirai Medical and Venus Concept and equipment from Cherry Imaging, Cynosure, Lutronic, Venus Concept, Perfaction Technologies, MiraDry Sientra and Mirai Medical; MH has delivered lectures/teaching for Galderma Nordic.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Costa C, Scalvenzi M, Ayala F, et al. How to treat actinic keratosis? An update. J Dermatol Case Rep 2015;9:29–35. 10.3315/jdcr.2015.1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashim PW, Chen T, Rigel D, et al. Actinic keratosis: current therapies and insights into new treatments. J Drugs Dermatol 2019;18:s161–6. [PubMed] [Google Scholar]
- 3.Gupta AK, Paquet M, Villanueva E, et al. Interventions for actinic keratoses. Cochrane Database Syst Rev 2012;12:CD004415. 10.1002/14651858.CD004415.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soyer HP, Prow TW, Jemec GBE. Actinic Keratosis. In: Karger AG, ed. Current problems in dermatology. 46, 2014. https://www.karger.com/Book/Home/262049 [Google Scholar]
- 5.Flohil SC, van der Leest RJT, Dowlatshahi EA, et al. Prevalence of actinic keratosis and its risk factors in the general population: the Rotterdam study. J Invest Dermatol 2013;133:1971–8. 10.1038/jid.2013.134 [DOI] [PubMed] [Google Scholar]
- 6.Werner RN, Sammain A, Erdmann R, et al. The natural history of actinic keratosis: a systematic review. Br J Dermatol 2013;169:502–18. 10.1111/bjd.12420 [DOI] [PubMed] [Google Scholar]
- 7.Dréno B, Amici JM, Basset‐Seguin N, et al. Management of actinic keratosis: a practical report and treatment algorithm from AKT eam TM expert clinicians. J Eur Acad Dermatol Venereol 2014;28:1141–9. 10.1111/jdv.12434 [DOI] [PubMed] [Google Scholar]
- 8.Lozzi F, Lanna C, Mazzeo M, et al. Investigational drugs currently in phase II clinical trials for actinic keratosis. Expert Opin Investig Drugs 2019;28:629–42. 10.1080/13543784.2019.1636030 [DOI] [PubMed] [Google Scholar]
- 9.Feuerherm AJ, Dennis EA, Johansen B. Cytosolic group IVA phospholipase A2 inhibitors, AVX001 and AVX002, ameliorate collagen-induced arthritis. Arthritis Res Ther 2019;21:29. 10.1186/s13075-018-1794-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashcroft FJ, Mahammad N, Midtun Flatekvål H, et al. cPLA2α Enzyme Inhibition Attenuates Inflammation and Keratinocyte Proliferation. Biomolecules 2020;10:E1402. 10.3390/biom10101402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omland SH, Habicht A, Damsbo P, et al. A randomized, double-blind, placebo-controlled, dose-escalation first-in-man study (phase 0) to assess the safety and efficacy of topical cytosolic phospholipase A2 inhibitor, AVX001, in patients with mild to moderate plaque psoriasis. J Eur Acad Dermatol Venereol 2017;31:1161–7. 10.1111/jdv.14128 [DOI] [PubMed] [Google Scholar]
- 12.Pfammatter AF, Mitsos A, Wang S, et al. Evaluating and improving recruitment and retention in an mHealth clinical trial: an example of iterating methods during a trial. Mhealth 2017;3:49. 10.21037/mhealth.2017.09.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali Z, Zibert JR, Thomsen SF. Virtual clinical trials: perspectives in dermatology. Dermatology 2020;236:375–82. 10.1159/000506418 [DOI] [PubMed] [Google Scholar]
- 14.Lane TS, Armin J, Gordon JS. Online recruitment methods for web-based and mobile health studies: a review of the literature. J Med Internet Res 2015;17:e183. 10.2196/jmir.4359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali Z, Anderson K, Chiriac A, et al. High adherence and low dropout rate in a virtual clinical study of atopic dermatitis through Weekly reward-based personalized genetic lifestyle reports. PLoS One 2020;15:e0235500. 10.1371/journal.pone.0235500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali Z, Chiriac A, Bjerre-Christensen T, et al. Mild to moderate atopic dermatitis severity can be reliably assessed using smartphone-photographs taken by the patient at home: a validation study. Skin Res Technol 2022;28:336–41. 10.1111/srt.13136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 1988;124:869–71. 10.1001/archderm.1988.01670060015008 [DOI] [PubMed] [Google Scholar]
- 18.Grada A, Feldman SR, Bragazzi NL, et al. Patient‐reported outcomes of topical therapies in actinic keratosis: a systematic review. Dermatol Ther 2021;34:e14833. 10.1111/dth.14833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen R, Marmur E, Anderson L, et al. A new, objective, quantitative scale for measuring local skin responses following topical actinic keratosis therapy with ingenol mebutate. Dermatol Ther 2014;4:207–19. 10.1007/s13555-014-0059-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinhold U, Dirschka T, Ostendorf R, et al. A randomized, double-blind, phase III, multicentre study to evaluate the safety and efficacy of BF-200 ALA (Ameluz(®)) vs. placebo in the field-directed treatment of mild-to-moderate actinic keratosis with photodynamic therapy (PDT) when using the BF-RhodoLED(®) lamp. Br J Dermatol 2016;175:696–705. 10.1111/bjd.14498 [DOI] [PubMed] [Google Scholar]
- 21.Olsen EA, Lisa Abernethy M, Kulp-Shorten C, et al. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J Am Acad Dermatol 1991;24:738–43. 10.1016/0190-9622(91)70113-G [DOI] [PubMed] [Google Scholar]
- 22.Dréno B, Cerio R, Dirschka T, et al. A novel actinic keratosis field assessment scale for grading actinic keratosis disease severity. Acta Derm Venereol 2017;97:1108–13. 10.2340/00015555-2710 [DOI] [PubMed] [Google Scholar]
- 23.EMA . ICH E6 (R2) Good clinical practice [Internet]. European Medicines Agency, 2018. Available: https://www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-061012supp001.pdf (47.9KB, pdf)