Abstract
Introduction
As its indications expand, reverse total shoulder arthroplasty (rTSA) utilization continues to increase. Though relatively uncommon, instability following rTSA can be associated with significant morbidity and need for subsequent revision and treatment. This case control study aims to characterize factors leading to instability after rTSA, especially in those with no previous shoulder surgery.
Methods
194 rTSAs performed within the study period with appropriate operative indications and follow-up were included. Risk factors used in analysis included age, gender, BMI, ASA class, Charlson comorbidity index (CCI), glenosphere, tray, and liner size. Data was analyzed using a hierarchical binary logistical regression to create a predictive model for instability.
Results
Seven patients sustained a post-operative dislocation. Mean time to dislocation was 60.4 weeks. Five required open reduction with placement of either a larger humeral tray or polyethylene spacer. One required open reduction with osteophyte removal, and one was converted to a resection arthroplasty. Dislocators were more likely to have a larger BMI (p = 0.002), higher ASA classification (p = 0.09), and larger liner size (p = 0.01).
Conclusion
This study demonstrates a large series of patients successfully treated with rTSA. Dislocations were an uncommon complication, but were clearly associated with higher patient BMI, ASA classification, and increased liner size.
Keywords: Arthroplasty, Replacement, Shoulder; Shoulder; Shoulder prosthesis; Operative instability; Risk factors; Comorbidity; Dislocation; Retrospective studies; Exactech equinoxe
1. Introduction
In the United States, the utilization of reverse total shoulder arthroplasty (rTSA) has increased significantly since its inception.1,2 Although initially utilized for patients with rotator cuff arthropathy (RCA), indications have expanded to include patients with rheumatoid arthritis, proximal humerus fractures, chronic rotator cuff tear, osteoarthritis with severe glenoid wear, and revision of unsatisfactory or failed anatomic total shoulder arthroplasties (TSA).1,3, 4, 5 As rTSA use expands, a thorough understanding of its potential complications is important. Complications are uncommon, but include infection, mechanical failure, neurovascular injury, scapular notching, scapular stress fractures, and instability.6 Post-operative instability in the glenohumeral joint leads to dislocation and need for subsequent closed reduction, open reduction, or surgical revision. Although post-operative instability comprises approximately half of all complications in some studies, its causes and management remain poorly understood.1,4,5
A number of factors have been previously described as contributing to rTSA instability including soft tissue tensioning, mechanical factors, impingement secondary to body habitus, prosthetic malalignment, and soft tissue interposition.7 To remove intraoperative variability that cannot be accounted for in previous studies, the aim of this study is to examine a large database of rTSAs performed at a single institution by a single surgeon with a single implant system to characterize significant risk factors for post-operative instability. We investigated patient-based risk factors including age, gender, BMI, ASA classification, and Charlson comorbidity index (CCI); as well as implant-based risk factors including glenosphere, humeral tray, and liner size.
2. Methods
Institutional Review Board approval was obtained to retrospectively review the surgical database for rTSA procedures between 2009 and 2018 at a single academic institution to look for instances of dislocation. Consent and permission to enroll in the clinical database were obtained from all patients. All patients over the age of 18 years who had a rTSA procedure during the study time frame were included, totaling 358 individual cases. Persons with a history of previous ipsilateral shoulder surgery, prior ipsilateral proximal humerus fracture, acute proximal humerus fracture at time of operation, fracture malunion, or completed with other concomitant procedures such as extensive bone grafting of the glenoid were excluded from the study. With these criteria applied, 194 participants were enrolled in the study and included in risk factor analysis.
Indications for rTSA surgical intervention included but were not limited to osteoarthritis, rotator cuff arthropathy, and avascular necrosis. All procedures were performed by a single attending surgeon through a deltopectoral approach without subscapularis tendon repair. The same reverse shoulder arthroplasty system was used in all cases [Exactech Equinoxe® (Gainesville, FL)]. A pre-operative interscalene block was performed for all cases by the anesthesia team. No muscle paralysis was employed during the procedure to allow for a more natural soft tissue tension. Patients were discharged home in a sling and were then seen in clinic for post-operative follow-up at one week, two weeks, four weeks, three months, six months, one year, and then yearly visits thereafter. Radiographs were obtained post-operatively and at yearly follow-up. Patients were encouraged to remain in the sling until the four-week mark, at which point they could begin to wean themselves out as tolerated. Passive and gentle active-assist range of motion with internal rotation to the abdomen and external rotation to 30° was started within the first two weeks with no internal rotation behind the back or shoulder extension. Patients began formal physical therapy with deltoid isometric strengthening at the four-week mark. At three months, full range of motion was encouraged with increased deltoid and rotator cuff strengthening exercises progressing to endurance. Aggressive scapular stabilization and resumption of throwing and racquet sports was expected at the six-month mark with full return to all activity by eight months.
Patients were separated into two cohorts for statistical analysis – those with post-operative dislocation events and those without. Several variables were evaluated as possible independent risk factors for dislocation including age, gender, BMI, ASA classification, CCI, glenosphere size, liner size, and tray size. Demographic data and patient-based risk factors were entered into a database and tracked throughout the study (Table 1). To protect against a Type I error, prior to conducting logistic regression, Chi square analyses were performed to assess the relationship between potential predictor variables and dislocations. Chi square analysis and binary logistic regression were then used for quantitative statistical analysis to create a predictive model for dislocation events following rTSA. Variables with a probability value of 0.10 or less were included in the regression model. The predictive power of the final logistic regression model was assessed with a Hosmer-Lemeshow test and assigned a Nagelkerke R2 value. The Nagelkerke R2 is a coefficient between zero and one that provides an estimate of the predictive value of the model. Coefficients closer to one indicate a stronger association.8
Table 1.
Patient demographics and classifications.a.
| Patient Variables | All rTSAs |
rTSA Dislocation |
|
|---|---|---|---|
| (N=194) | Yes (n=7) | No (n=187) | |
| Male, n (%) | 118 (61%) | 4 (57%) | 114 (61%) |
| Female, n (%) | 76 (39%) | 3 (43%) | 73 (39%) |
| Mean age, y (range) | 68.0 (39.0–93.0) | 67.5 (56.3–83.1) | 68.0 (39.0–93.0) |
| Mean BMI (range) | 31.1 (16.9–83.3) | 34.3 (23.1–39.7) | 30.9 (16.9–83.3) |
| CCI (range) | 3.5 (0–8) | 3.41, 2, 3, 4, 5, 6, 7, 8 | 3.5 (0–8) |
| ASA Classification (range) | 2.71, 2, 3, 4 | 3.13,4 | 2.71, 2, 3, 4 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI; body mass index; CCI, Charlson Comorbidity Index; rTSA, reverse total shoulder arthroplasty.
3. Results
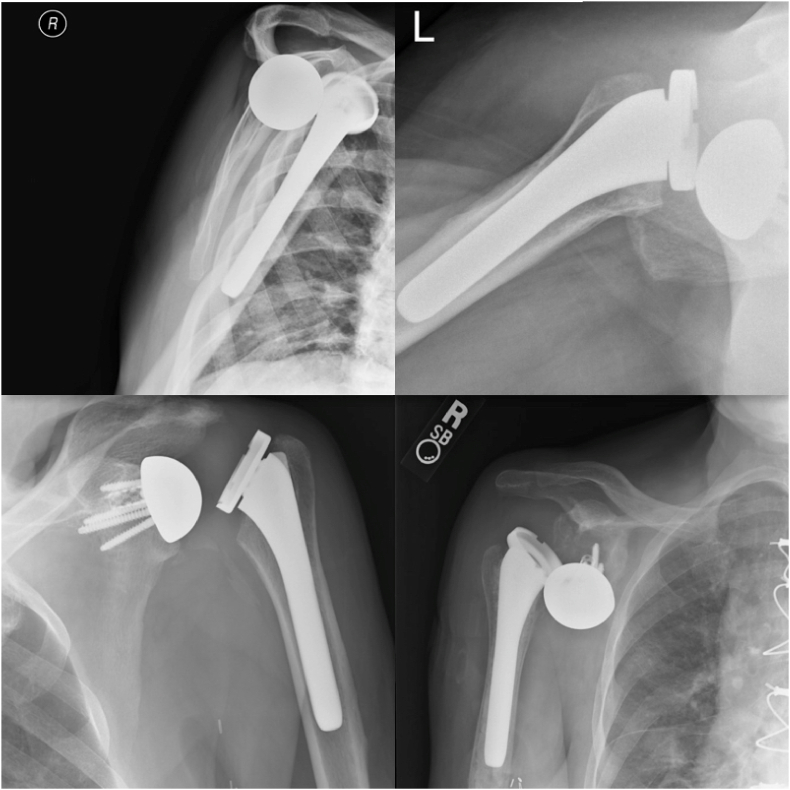
Of the 194 patients, seven (3.6%) experienced dislocations following rTSA (Table 2). Dislocations were defined with either radiographic evidence or clinical instability and required either a closed reduction, open reduction, or revision surgery (Fig. 1). This cohort included four males (57%) and three females (43%), with a mean age of 67.5 ± 8.9 years (range, 56.3–83.1 years). Four of the seven dislocators had a BMI score between 38 and 40 while three had a BMI of less than 35. The mean BMI score was 34.3 ± 6.6 kg/m2 (range, 23.1–39.7 kg/m2).
Table 2.
Summary of patient dislocators.a.
| Patient | Age (y) | Sex | Diagnosis | BMI (kg/m2) | Time from rTSA to dislocation (wks) | ASA Class | CCI | Original Implant Size (Glenosphere, Humeral Tray, Liner) (mm) | Revision Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 56.3 | M | CTA | 23.1 | 25.0 | 3 | 1 | 38, 0, 2.5 | 48 mm glenosphere and 2.5 mm liner |
| 2 | 67.3 | M | RCD | 39.7 | 32.4 | 3 | 2 | 38, 0, 2.5 | 2.5 mm liner |
| 3 | 58.5 | F | GHOA | 38.1 | 27.0 | 3 | 2 | 38, 0, 0 | 5 mm tray |
| 4 | 71.8 | F | CTA | 39.2 | 36.4 | 3 | 8 | 38, 0, 2.5 | 10 mm tray |
| 5 | 66.0 | F | AVN | 27.1 | 74.1 | 4 | 5 | 38, 0, 0 | Resection arthroplasty |
| 6 | 69.2 | M | RCD | 38.4 | 102.9 | 3 | 2 | 42, 5, 2.5 | 10 mm tray |
| 7 | 83.1 | M | CTA | 34.8 | 125.1 | 3 | 4 | 42, 5, 2.5 | Osteophyte debridement |
Abbreviations: ASA, American Society of Anesthesiologists; AVN, avascular necrosis; BMI, body mass index; CCI, Charlson Comorbidity Index; CTA, rotator cuff tear arthropathy; F, female; GHOA, glenohumeral osteoarthritis; M, male; RCD, rotator cuff deficiency; rTSA, reverse total shoulder arthroplasty.
Fig. 1.
Radiographic evidence of patient shoulder dislocation.1.
All seven of the rTSA instability events dislocated anteriorly and were taken back into the operating room for a revision surgery following unsuccessful attempts at closed reduction or continued episodes of dislocation after initial reduction. Five required open reduction with placement of either a larger humeral tray3 or a larger polyethylene spacer.2 One required open reduction with removal of a newly formed osteophyte from the greater tubercle that was impinging upon range of motion. One rTSA was found to have a completely dissociated glenosphere with insufficient glenoid bone stock for repair upon revision and the surgeon elected to remove all hardware, converting the rTSA to a resection arthroplasty. Of the revisions, six remained stable and only one went on to further dislocate, at which point the patient elected to live with the dislocating shoulder instead of further surgical intervention. The mean time from rTSA to initial dislocation event in the seven patients was 60.4 ± 40.6 weeks.
Numerous variables were analyzed for possible inclusion in logistic regression to evaluate risk of dislocation. Patient variables analyzed included BMI, CCI, ASA, sex, and age at surgery, while surgical variables included glenosphere size, tray size, and liner size. Variables with a Chi square probability value of 0.10 or less were included in the regression model. Three of these variables met the cut-off value. BMI was significantly related to dislocation (X25 = 7.20, p = 0.002). Four of the seven dislocators had a BMI score between 38 and 40 while three had a BMI of less than 35. Liner size was also significant (X21 = 6.63, fisher's exact test p = 0.010). There were five dislocations with liner size 2.5 and two for liner size 0.0. ASA classification approached significance (X23 = 5.95, fisher's exact test p = 0.090). Six dislocators were ASA class three and one was ASA class four. Table 3 presents the Pearson Chi Square values, degrees of freedom, and p-values for each of the variables entered into the logistic regression analysis.
Table 3.
Pearson chi square and P values for variables entered into logistic regression.a.
| Variable | X2 Value | df | Asymptotic Significance (2-sided) |
|---|---|---|---|
| BMI | 7.20 | 5 | 0.002* |
| Liner Size | 6.63 | 1 | 0.010* |
| ASA Classification | 5.95 | 3 | 0.090 |
*p < 0.01.
Prior to conducting the logistic regression, an analysis was performed suggesting the assumption of multicollinearity was met (tolerance = 0.97). With this assumption met, BMI, liner size, and ASA classifications were entered into the logistic regression equation. Prediction of dislocation was significantly better than the null model (omnibus test of model: X25 = 13.23, p = 0.022, Hosmer & Lemeshow p = 0.824, nonsignificant, indicating a good fit of the data to the model). Nagelkerke R2 for the model was 0.25, indicating moderate predictive power. Table 4 presents the classification statistics for the model. The results presented show that the model correctly predicted 57.1% of dislocators and 90.4% of non-dislocators. Of the three independent variables in the final model, only liner size reached significance at the p < 0.05 level. With BMI and ASA held constant, the odds of dislocation occurring increased by 14% with each unit of increase in liner size (95% CI 0.25–0.80). Table 5 shows the odds ratios and p-values for each variable and for the final model with all three variables included.
Table 4.
Observed and predicted frequencies for dislocation by logistic regression.a.
| Observed | Predicted by Logistic Regression |
Percentage Correct | ||
|---|---|---|---|---|
| No dislocation | Dislocation | |||
| No dislocation | 187 | 169 | 18 | 90.4 |
| Dislocation | 7 | 3 | 4 | 57.1 |
| Overall % correct | 89.2 | |||
Data predicted by Logistic Regression analysis. Logistic Regression predicted 169 “no dislocations” and 18 “dislocations” in the observed group that did not dislocate for an accuracy of 90.4% correctly predicted to not dislocate. Likewise, Logistic Regression predicted three “no dislocations” and four “dislocations” in the observed group that dislocated post-operatively, accurately predicting 57.1% of all dislocations. This Logistic Regression analysis was overall 89.2% accurate in correctly predicting either “no dislocation” or “dislocation” as compared to the observed results.
Table 5.
Results for logistic regression.a.
| Odds Ratio (Exp B) |
95% C.I. for Exp B |
||||
|---|---|---|---|---|---|
| Independent Variables | Model # 1 | Model #2 | Model #3 | Lower | Upper |
| Liner Size | 0.15* (p = 0.02) | 0.13*(p = 0.02) | 0.14* (p = 0.02) | 0.25 | 0.80 |
| BMI | 1.05 (p = 0.20) | 1.05 (p = 0.27) | 0.96 | 1.15 | |
| ASA Classification | 0.29 (p = 0.32) | 0.03 | 3.39 | ||
| Constant | 0.10 | 0.24 | 0.10 | ||
| Nagelkerke (pseudo R2) | 0.11 | 0.13 | 0.25 | ||
| N | 194 | 194 | 194 | ||
*p < 0.05.
4. Discussion
Although rTSA instability continues to be a common topic of research, no clear consensus of contributing factors exists. Factors previously described as contributing to rTSA instability include surgical elements like soft tissue tensioning, mechanical failure, and impingement.7,9 Additional studies hypothesize that patient-specific factors such as age, BMI, gender, and previous surgical history also influence post-operative instability by affecting soft tissues.1,10 Our study sought to add to the current literature regarding potential risk factors for instability after rTSA. A summary of studies reporting risk factors for instability following rTSA can be found in Table 6. Where previous studies11,12 included patients with history of prior shoulder surgery and proximal humerus fractures, we excluded those high risk candidates to look at what might contribute to instability in a more low risk patient without previous complications. We found that larger BMI, higher ASA classification, and increased liner size lead to higher rates of rTSA implant dislocation (p < 0.05). Interestingly, our study found that average time to dislocation following rTSA was greatly increased compared to others that included high risk patients in their studies.1,10, 11, 12 We attribute this finding to the patients in our study having fewer previous comorbidities and a generally more anatomically and structurally intact shoulder at the time of operation. Whether or not a history of previous shoulder surgery is directly responsible for earlier dislocation times following rTSA is an area of research worth continuing.
Table 6.
Summary of Previously Reported Risk Factors for Instability Following rTSA.
| Author | Year | Study Type | Sample Size | Dislocations | Identified Risk Factors |
|---|---|---|---|---|---|
| Abdelfattah et al.7 | 2018 | Case Series | 1426 | 43 | Deltoid dysfunction, soft tissue impingement |
| Chalmers et al.1 | 2014 | Case Series | 385 | 11 | BMI>30, male gender, subscapularis insufficiency, prior surgery |
| Cheung et al.12 | 2018 | Retrospective Cohort | 119 | 11 | Male gender, previous surgery, preoperative diagnoses of fracture sequelae |
| Guarrella et al.21 | 2021 | Retrospective Cohort | 1035 | 31 | Younger age, decreased bony lateralization |
| Gutierrez et al.9 | 2008 | Biomechanical Analysis | N/A | N/A | Lower implant compressive force, smaller glenohumeral socket depth |
| Kohan et al.10 | 2017 | Case Series | 1055 | 29 | Inadequate soft tissue tensioning, impingement or liner failure |
| Padegimas et al.11 | 2016 | Case Control | 510 | 15 | Male gender, increased BMI, preoperative diagnoses of fracture sequelae, prior surgery |
Another important topic of debate regarding rTSA instability is soft tissue tensioning, specifically the subscapularis tendon. In an often cited study, Edwards et al.4 showed that patients undergoing a rTSA with irreparable subscapularis tendons demonstrated higher rates of postoperative instability. However, as the authors acknowledge, patients that sustained postoperative dislocations all had complex primary diagnoses including proximal humeral nonunion, failed prior arthroplasty, or previous fixed dislocation. This contributed to the exclusion criteria in our patient population so that we could analyze instability outcomes in a more controlled environment since the surgical technique and implant hardware did not require subscapularis repair. Furthermore, additional studies have shown equivalent outcomes between patients who undergo rTSA with and without subscapularis tendon repair.3,13,14 This study adds to existing data in which a large group of patients undergoing rTSA with no subscapularis tendon repair report a dislocation rate well within the accepted limits of 0–8%.1
One other biomechanical attribute worth discussing is the implant hardware and its characteristics. The acceptably low dislocation rate in this study could also have been influenced by implant characteristics. As Friedman et al. explains, implants with a medialized glenoid component and a lateralized humeral component are likely to possess increased stability in the setting of an unrepaired subscapularis tendon.13 The Equinoxe® [Exactech (Gainesville, FL)] implant system used in this study includes a medialized glenoid and lateralized humeral component, eliminating the need to repair the subscapularis tendon. The relatively lateral position of the humeral prosthesis leads to increased deltoid wrapping and more anatomic rotator cuff tensioning, even with an absent subscapularis, which both contribute to stability.13,15,16 In addition, Friedman et al. points out that with a lateralized humeral stem implant, subscapularis tendon repair may actually be biomechanically unfavorable.13 After rTSA, the subscapularis muscle loses its normal function as a relative abductor of the shoulder and becomes an adductor since the moment arm of a repaired subscapularis is inferior to the relatively preserved center of rotation in the glenohumeral joint. Thus, an intact post-operative subscapularis opposes the deltoid in abduction and the posterior cuff musculature in external rotation.13 This leads to an increase in work required by the deltoid and posterior cuff, as well as increased joint reaction force at the glenohumeral interface. Looking at the other half of a rTSA implant system, larger glenosphere size has been shown to increase abduction range of motion, but limited evidence exists demonstrating its effect on stability.17 Our study did not find any correlation between glenosphere or humeral tray size and an increased rate of instability. This study gives further evidence that acceptably low dislocation rates are achievable without subscapularis repair when an implant with a lateralized humeral component is used.
In addition to soft tissue tensioning, several studies have focused on patient-specific factors, associating male gender and BMI over 30 with an increased likelihood of instability.1,10 In a multicenter study of 510 rTSAs performed by multiple surgeons utilizing different implant systems, Padegimas et al. associated male gender, history of open shoulder surgery, and fracture sequelae with post-operative instability.11 Cheung et al. utilized a similar design of a single institution study with rTSAs performed by two surgeons. The study found that male gender, prior open reductions, proximal humerus or tuberosity nonunion, and absence of subscapularis repairs were associated with instability.12
The risk factors identified that lead to rTSA instability share a similar trend with overall reverse shoulder replacement arthroplasty morbidity. Patients with higher BMI and therefore higher risk of medical comorbidity have been associated with increased instability events.1,10 It is possible that having higher BMI and ASA classification predisposes the patient to higher stresses during recovery due to excessive soft tissue impingement around the shoulder joint that ultimately leads to instability and dislocation, as seen in other major joint arthroplasties.18,19
With the elimination of multiple surgeons and implant variability, our results indicate that a larger liner size in the implant system increases the probability of dislocation. Though an adequate liner size is necessary for proper implant fit and function, perhaps the larger liner size in a patient with no prior shoulder surgery predisposes the patient to instability by placing an increased amount of post-operative tensioning and stress on the shoulder's adductors that ultimately progresses to an anterior dislocation.20 It would be useful to compare a population of patients with no prior shoulder surgery against those with a history of shoulder surgery to determine whether liner size has a correlation to dislocation between the two.
Though rTSA technique is becoming more popular among shoulder specialists, studies analyzing post-operative complications and treatments are hindered by a generally small sample size. We used a cohort of procedures from a wide study period and proliferative surgeon. This provided a larger sample size, allowing the variables of interest in our analysis a higher statistical power. Additionally, this study eliminated confounding variables that are prevalent in other studies reviewing similar rTSA instability events. The use of a single surgeon, institute, and implant system gave us the unique benefit of eliminating different surgical preferences, techniques, and instrumental variations seen in other studies.
The limitations of this study are like any retrospective study. Data quality depends on the accuracy and availability of the medical record. Our results may not be reflective of a more junior surgeon or one who does not exclusively perform shoulder surgery as the results presented are reflective of a shoulder specialist with considerable personal experience performing complex shoulder surgeries. It should also be noted that the results of this study may only apply to the Equinoxe® or biomechanically similar implant systems.
5. Conclusion
Post-operative instability is an uncommon but challenging complication of reverse total shoulder arthroplasty. Patients with increased BMI, higher ASA classification, and the use of larger liners lead to an increased probability of instability events.
Author contributions
Margaret A. Sinkler: Conceptualization, Writing – Original draft preparation. Joshua D. Dolan: Conceptualization, Data curation, Formal analysis, Writing – Review & Editing. Drew Henderson: Conceptualization, Writing – Original draft preparation. Michael J. Steflik: Resources, Data curation, Project administration. Frank D. Lewis: Methodology, Software, Formal Analysis. Stephen A. Parada: Supervision, Project administration. Lynn A. Crosby: Investigation.
Funding
This research received no specific grant from any funding agency in the public, commercial, or non-for-profit sectors.
Informed consent
Written informed consent was obtained from all subjects before the study.
Ethical approval
Ethical approval for this study was obtained from Augusta University (AU) Committee A IRB (ID#: Pro00001781).
Additional disclosures
Dr. Stephen Parada is a research consultant for Exactech, Inc.
Level III Prognostic study
Case-Control Study.
Acknowledgements
None.
Footnotes
Some patients were able to reduce their dislocations at home and therefore did not have radiographic evidence of dislocation in clinic. Starting top left and moving in a clockwise fashion is Patient 7, Patient 2, Patient 5, and Patient 1.
No additional sources of funding for research and/or publication.
References
- 1.Chalmers P.N., Rahman Z., Romeo A.A., Nicholson G.P. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737–744. doi: 10.1016/j.jse.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Bengner U., Johnell O., Redlund-Johnell I. Changes in the incidence of fracture of the upper end of the humerus during a 30-year period. A study of 2125 fractures. Clin Orthop Relat Res. 1988;(231):179–182. [PubMed] [Google Scholar]
- 3.Clark J.C., Ritchie J., Song F.S., et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36–41. doi: 10.1016/j.jse.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Edwards T.B., Williams M.D., Labriola J.E., Elkousy H.A., Gartsman G.M., O'Connor D.P. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):892–896. doi: 10.1016/j.jse.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Grammont P., Trouilloud P., Laffay J.P., Deries X. Etude et rÈalisation d'une nouvelle prothËse d'Èpaule. Rhumatologie. 1987;39:407–418. [Google Scholar]
- 6.Crosby L.A., Hamilton A., Twiss T. Scapula fractures after reverse total shoulder arthroplasty: classification and treatment. Clin Orthop Relat Res. 2011;469(9):2544–2549. doi: 10.1007/s11999-011-1881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdelfattah A., Otto R.J., Simon P., et al. Classification of instability after reverse shoulder arthroplasty guides surgical management and outcomes. J Shoulder Elbow Surg. 2018;27(4):e107–e118. doi: 10.1016/j.jse.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Nagelkerke N.J.D. A note on a general definition of the coefficient of determination. Biometrika. 1991;78(3):691–692. [Google Scholar]
- 9.Gutierrez S., Keller T.S., Levy J.C., Lee W.E., 3rd, Luo Z.P. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):670–676. doi: 10.1007/s11999-007-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohan E.M., Chalmers P.N., Salazar D., Keener J.D., Yamaguchi K., Chamberlain A.M. Dislocation following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(7):1238–1245. doi: 10.1016/j.jse.2016.12.073. [DOI] [PubMed] [Google Scholar]
- 11.Padegimas E.M., Zmistowski B.M., Restrepo C., et al. Instability after reverse total shoulder arthroplasty: which patients dislocate? Am J Orthoped. 2016;45(7):E444–E450. [PubMed] [Google Scholar]
- 12.Cheung E.V., Sarkissian E.J., Sox-Harris A., et al. Instability after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27(11):1946–1952. doi: 10.1016/j.jse.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Friedman R.J., Flurin P.H., Wright T.W., Zuckerman J.D., Roche C.P. Comparison of reverse total shoulder arthroplasty outcomes with and without subscapularis repair. J Shoulder Elbow Surg. 2017;26(4):662–668. doi: 10.1016/j.jse.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 14.Vourazeris J.D., Wright T.W., Struk A.M., King J.J., Farmer K.W. Primary reverse total shoulder arthroplasty outcomes in patients with subscapularis repair versus tenotomy. J Shoulder Elbow Surg. 2017;26(3):450–457. doi: 10.1016/j.jse.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Roche C.P., Diep P., Hamilton M., et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(4):284–293. 2013. [PubMed] [Google Scholar]
- 16.Roche C. American Academy of Orthopaedic Surgeons; Rosemont, IL: 2013. Kinematics and Biomechanics of Reverse Total Shoulder Arthroplasty. AAOS Orthopaedic Knowledge Update #4; pp. 45–54. [Google Scholar]
- 17.Langohr G.D., Giles J.W., Athwal G.S., Johnson J.A. The effect of glenosphere diameter in reverse shoulder arthroplasty on muscle force, joint load, and range of motion. J Shoulder Elbow Surg. 2015;24(6):972–979. doi: 10.1016/j.jse.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Sadr Azodi O., Adami J., Lindstrom D., Eriksson K.O., Wladis A., Bellocco R. High body mass index is associated with increased risk of implant dislocation following primary total hip replacement: 2,106 patients followed for up to 8 years. Acta Orthop. 2008;79(1):141–147. doi: 10.1080/17453670710014897. [DOI] [PubMed] [Google Scholar]
- 19.Elkins J.M., Daniel M., Pedersen D.R., et al. Morbid obesity may increase dislocation in total hip patients: a biomechanical analysis. Clin Orthop Relat Res. 2013;471(3):971–980. doi: 10.1007/s11999-012-2512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen M.L., Nayak A., Narayanan M.S., et al. Role of subscapularis repair on muscle force requirements with reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;73(Suppl 1):S21–S27. 2015. [PubMed] [Google Scholar]
- 21.Guarrella V., Chelli M., Domos P., Ascione F., Boileau P., Walch G. Risk factors for instability after reverse shoulder arthroplasty. Shoulder Elbow. 2021;13(1):51–57. doi: 10.1177/1758573219864266. [DOI] [PMC free article] [PubMed] [Google Scholar]