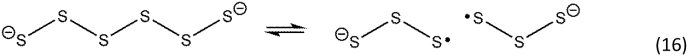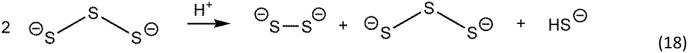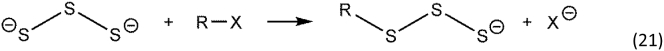Abstract
It has become apparent that hydrogen sulfide (H2S), hydropersulfides (RSSH) and other polysulfide species are all intimately linked biochemically. Indeed, at least some of the biological activity attributed to hydrogen sulfide (H2S) may actually be due to its conversion to RSSH and derived polysulfur species (and vice-versa). The unique chemistry associated with the hydropersulfide functional group (-SSH) predicts that it possesses possible protective properties that can help a cell contend with oxidative and/or electrophilic stress. However, since RSSH and polysulfides possess chemical properties akin to disulfides (RSSR), they can also be sources of oxidative/electrophilic stress/signaling as well. Herein are discussed the unique chemistry, possible biochemistry and the physiological implications of RSSH (and polysulfides), especially as it pertains to their putative cellular protection properties against a variety of stresses and/or as possible stressors/signaling agents themselves.
Keywords: Hydrogen sulfide, Hydropersulfides, Polysulfides, S-nitrosothiol
Abbreviations: CARS, cysteinyl-tRNA synthetase; RSSH, alkyl hydropersulfide; RSSR, dialkyl disulfide; RSH, alkyl thiol; GPx, glutathione peroxidase; RSS·, perthiyl radical; RS·, thiyl radical; PSSH, sulfurated thiol protein or protein hydropersulfide; Sec, selenocysteine; GSH, glutathione; GST, glutathione transferase; Grx, glutaredoxin; RSOH, sulfenic acid; RS-NO, S-nitrosothiol; RSS-NO, S-nitrosopersulfide; GS-NO, S-nitrosoglutathione; NAC, N-acetylcysteine
1. Introduction
Interest in nitric oxide (NO) as a small-molecule bioregulator in the early 1990's led to the eventual interest in other endogenously produced di- or tri-atomic species as possible physiological signaling agents [for a review, see [1]]. Thus, carbon monoxide (CO) and hydrogen sulfide (H2S) have also been determined to possess important physiological signaling properties and along with dioxygen (O2) can be grouped together as a class of signaling species sometimes referred to as “gasotransmitters” (an unfortunate, misleading and inaccurate term since all of these species are solutes and not gasses when acting physiologically). Importantly, all of these small-molecule species appear to have an integrated signaling relationship in that they can control the actions of each other due to a commonality in targets and/or binding sites and, for some, the fact that they (themselves or via derived species) can react with one another [for example [2]]. Among these species, H2S is unique in that it has dissociable protons (and therefore can easily exist in an ionic state) and is inherently nucleophilic (i.e., will react with, for example, electrophilic sulfur species). The reaction of H2S with other oxidized sulfur species (e.g., disulfides, RSSR) allows for the eventual generation of a plethora of sulfur exchange products. The simplest of these products is an alkyl hydropersulfide (RSSH) (Reaction 1). It should be mentioned that Reaction 1 as written is uphill thermodynamically [3] and thus the equilibrium, in a purely chemical system, lies predominantly to the left.
| (1) |
Further reactions associated with RSSH species are possible (if not likely), generating other higher order polysulfur species [e.g. [4]]. Importantly, the biosynthesis of H2S via some routes can involve RSSH intermediacy (i.e., H2S is generated via the reverse of Reaction 1) [e.g. [5,6]]. However, in spite of the existence of RSSH species (i.e., cysteine hydropersulfide, Cys-SSH) in some H2S generating pathways, the possible biological utility beyond serving as a possible precursor for H2S generation has been relatively ignored until recently. It is now proposed that RSSH species possess unique and important chemical properties that predict its possible use as a protective agent against oxidative and/or electrophilic stress (among other possible roles) (vide infra). Therefore, in order to provide a chemical basis for predicting the possible biological functions/utility of RSSH species, the chemical properties of the –SSH functional group will be discussed first.
1.1. General RSSH chemistry and comparison with thiols and disulfides
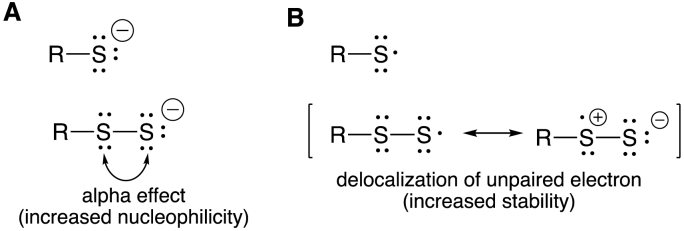
The physiological utility of RSSH will be wholly dependent on the chemistry associated with the –SSH functional group and its distinction from other sulfur (or chalcogen) species/redox states. Since RSSH are ultimately derived from the corresponding alkyl thiols (RSH) in biological systems, it may be useful to first examine the biochemical utility of thiols and derived species and discuss the difference an additional sulfur atom can make (i.e., RSSH versus RSH, note: herein, RSH and RSSH will be used to denote both the protonated RSH/RSSH species as well as the corresponding conjugate bases, RS−/RSS− unless otherwise noted). In general, RSH can act as a nucleophile (e.g., as in cysteine proteases), a reductant (e.g., as in glutathione peroxidase (GPx)), and a metal ligand (e.g., as in Zn-finger proteins) [7]. All of these properties are due to the chemistry associated with the reactivity of the lone-pair electrons on the sulfur atom. The –SSH functional group also possesses lone pairs of electrons which can have analogous chemical/biochemical properties to those found with the –SH functional group. That is, RSSH species are known to be nucleophiles [e.g. [3,8,9]], reductants [e.g. [[10], [11], [12]]] and metal ligands [e.g. [13,14]]. Generally speaking, however, the nucleophilic and reducing power of RSSH exceed that of RSH. Due to an alpha effect (the effect associated with lone pair electrons on adjacent atoms), RSSH species possess significantly greater nucleophilicity compared to RSH [9]. Moreover, RSSH is a superior one-electron reductant compared to RSH since the one-electron oxidized RSSH species, the perthiyl radical (RSS·), is stabilized by delocalization of the unpaired electron (an effect not possible with the thiyl (RS·) species) [10,11,15]. The effects responsible for the increased reactivity associated with RSSH versus RSH are depicted schematically in Fig. 1.
Fig. 1.
A) Alpha effect associated with increased nucleophilicity of RSSH/RSS− over RSH/RS−. B) Delocalization of unpaired electron of RSS· responsible for increased stability over RS·.
Thus, it can be surmised that the –SSH functional group is hyperactive compared to –SH with regards to the above-mentioned reactivity (i.e., nucleophilicity, reducing ability and metal ligation – at least for some metals). Indeed, the –SSH group can be compared to the selenol functional group (-SeH, which denotes both the protonated and unprotonated, -Se-, species) in that -SeH has superior nucleophilicity and reducing abilities compared to the corresponding –SH containing species. In this regard, Cys-SSH and/or associated sulfurated thiol proteins (PSSH) can be considered to be ‘fleeting’ selenocysteine (Sec) and/or selenoprotein analogs [16] (note: RSSH/PSSH can be considered fleeting due to the existence of the reverse of Reaction 1). Moreover, the inherent reactivity of the –SSH functional group as a nucleophile and reductant has led to favorable comparisons to numerous biological protective/antioxidant systems such as ascorbate, α-tocopherol, glutathione (GSH), glutathione transferase (GST), glutaredoxin (Grx) and glutathione peroxidase (GPx) [17].
It needs to be stressed that RSSH is oxidized with respect to RSH (i.e., the formal oxidation state of the sulfur of RSH is 2- (i.e., RS2−H) while the formal oxidation state of the internal sulfur of RSSH is 1- (i.e., RS1−SH)). Indeed, one mechanism of RSSH generation in a biological system requires an oxidized thiol species, RSSR, to react with H2S (Reaction 1). Other oxidized thiols such as sulfenic acids (RSOH) are also capable of reacting with H2S to generate RSSH [3] (Reaction 2).
| RSOH + H2S → RSSH + H2O | (2) |
Alternatively, inorganic oxidized sulfur species (oxidized H2S or oxoacids of sulfur) can be formed which react with RSH to give RSSH species [18]. Regardless, formation of RSSH from RSH and H2S requires an oxidation at some point in the process. Considering the requirement for an oxidation step somewhere in RSSH generation from RSH, it would be expected that RSSH formation may be particularly prevalent under biological oxidative stress conditions (this is discussed in further detail below).
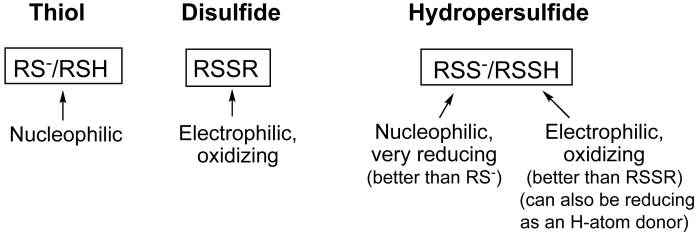
The reducing and nucleophilic properties of RSSH are predominantly due to the anionic RSS− species (although RSSH is also reported to be a good reductant via H-atom donation [11,19,20]). The pKa values of RSSH are reported to be 2–4 units lower than the corresponding RSH [e.g. [3,19]]. Therefore, at physiological pH the anionic RSS− species should be the predominate equilibrium species for most RSSHs. However, there is no doubt that potentially consequential levels of the protonated RSSH can also be present since it is in a dynamic equilibrium with the anionic RSS− species. The chemistry of the protonated RSSH can be distinct from RSS− since disulfides are known to be electrophilic (consider the predominant biochemistry associated with RSSR, for example). In fact, it would be expected that RSSH can be a superior electrophile compared to RSSR since nucleophilic attack at the internal sulfur of RSSH (the bolded sulfur atom) may be predicted to be more favorable compared to nucleophilic attack at either of the sulfur atoms of RSSR due to the fact that HS− is typically a weaker base than RS− (e.g., the pKa of H2S is 7, while the pKa of a representative thiol on cysteine (Cys-SH) is 8.3) and therefore is a superior leaving group for this reaction. Also, attack at either sulfur atom of RSSH would be sterically less hindered compared to nucleophilic attack on RSSR sulfur atoms due to the small size of a proton versus an alkyl, R, group. Thus, by all accounts the protonated RSSH species should be an excellent electrophile, likely superior to RSSR in this regard. Fig. 2 depicts the dichotomous chemical behavior associated with RSSH and the comparison with RSH and RSSR. The biological implications of all the aspects of RSSH chemistry will be discussed later.
Fig. 2.
Chemical reactivities of thiols (RSH), disulfides (RSSR) and hydropersulfides (RSSH).
It is also worth noting that polysulfur species such as RSnR (n > 2) have been reported to react with H2O under mildly basic conditions to give the corresponding polysulfide and sulfenic acid (Reaction 3) [e.g. [21]].
| (3) |
Interestingly, RSSR disulfides can also react with H2O to give RSH and sulfenic acid (RSOH) (Reaction 3 with n = 2), but this only occurs appreciably under highly basic conditions and is not likely to be biologically important at physiological pH due to the lack of nucleophilic hydroxide (HO−) (although enzyme-mediated hydrolysis is possible) [22]. Due to the known electrophilicity of RSOH (e.g., RSOH reacts readily with nucleophilic sulfur species [23]) (Reaction 4), this chemistry can contribute to the complexity of possible sulfur exchange products generated in biological systems.
| RSnOH + R’Sx’H → RSnSx’R’ + H2O | (4) |
1.2. Reaction of RSSH with S-Nitrosothiols: Transnitrosation
Transnitrosation describes the transfer of a nitrosonium group (NO+) from one nucleophile to another. This reaction occurs via the attack of a nucleophile at the electrophilic nitrogen of X–NO followed by displacement/loss of the X group. In the context of this review, the transnitrosation reaction of interest involves the transfer of NO+ from one sulfur to another. The transnitrosation reaction between RSH and an S-nitrosothiol (RS-NO) occurs readily and is reversible (Reaction 5) [e.g. [24]].
| (5) |
As a more potent nucleophile, RSSH species should also readily participate in transnitrosation reactions with RS-NO to give an S-nitrosopersulfide (RSS-NO) (Reaction 6).
| (6) |
Although the transnitrosation reaction between RSSH and RS-NO is also presumably reversible (Reaction 6 shown as being reversible), there is another competing reaction pathway available to RSS-NO that is not readily available to RS-NO. Due to the stability of the two radical products, homolysis of the S–N bond of RSS-NO readily occurs, giving RSS· and NO (Reaction 7).
| (7) |
As mentioned above, the stability of RSS· can be explained by the delocalization of the unpaired electron which lowers the energy of this radical intermediate. An analogous homolytic reaction cannot occur as readily for RS-NO due to the higher energy associated with RS· species (the rationale for the relative stability of RSS· versus RS· is schematically depicted in Fig. 1). Thus, combining Reactions 6 and 7 gives Reaction 8, which indicates that RSSH can degrade R’S–NO, generating R’SH and releasing NO.
| RSSH + R’S–NO → RSS· + NO + R’SH | (8) |
Consistent with the existence of Reaction 8, several reports indicate that the reaction of RSSH with, for example, the S-nitrosothiol of glutathione (GS-NO) results in the generation of NO as well as the formation of GSH and the corresponding tetrasulfide RSSSSR, which would have resulted from the simple dimerization of RSS· (Reaction 9) [11,25].
| 2 RSS· → RSSSSR | (9) |
The physiological implications of Reaction 8 are significant and will be discussed further below.
1.3. Biological utility/effects of RSSH as a reductant/nucleophile
As mentioned above, RSSH is oxidized with respect to RSH and should be more prevalent under cellular oxidizing conditions (i.e., oxidative stress). The generation of RSSH species under cellular oxidative stress conditions may have evolved since this would mean that a strong reductant/nucleophile can be readily generated under potentially deleterious oxidizing conditions. The fact that an oxidized species is a stronger reductant/nucleophile than the molecule from which it was derived is typically unexpected (although not unheard of, consider for example oxidation of ammonia (NH3) to hydrazine (NH2NH2) or hydroxylamine (NH2OH)) and it may make physiological sense that this phenomenon is exploited to generate a strong reductant/nucleophile, RSSH, from RSH under electrophilic/oxidative stress conditions as a protective mechanism [e.g., [7]]. However, as alluded to above, RSSH is also an oxidized species and therefore has the potential to itself promote an oxidative stress (vide infra).
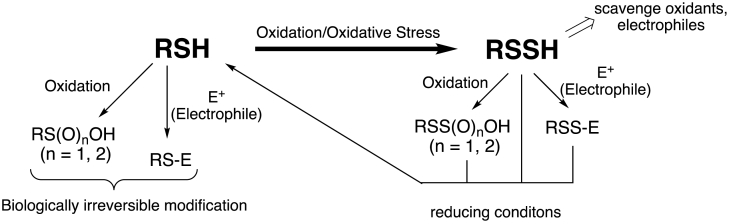
RSSH can protect a cell from oxidative/electrophilic stress by several possible mechanisms. As a good reductant and nucleophile, RSSH can scavenge potentially deleterious oxidants and electrophiles. That is, RSSH can quench oxidizing radical chain reactions, thus limiting cellular damage, akin to the effects of other antioxidants such as ascorbate or α-tocopherol. RSSH can also react with and intercept electrophilic species akin to the actions of GSH and other GSH-requiring processes (e.g., Grx, GPx or GST) [17]. Alternatively, the –SSH functional group contained in proteins (PSSH) is proposed to protect the cysteine thiol from irreversible oxidative or electrophilic damage [e.g. [26]]. This aspect of RSSH-mediated protection occurs since PSSH contains a reactive and yet sacrificial sulfur atom that can be modified (i.e., oxidative modification) and then later reduced off to generate the corresponding protein thiol (PSH). This idea is not necessarily new as another oxidized thiol species has also been touted as being protected as well; glutathionlylation of thiol proteins (PSSG formation) has been proposed to protect protein cysteines from irreversible oxidative damage. However, the proposed mechanism of protein thiol protection via PSSH is distinct from the protection afforded PSSG. Glutathionlylated proteins are proposed to be resistant to oxidative modification due to their lesser general reactivity with oxidants/electrophiles compared to the corresponding PSH and, after the oxidative stress is resolved, can be readily reduced back to the original PSH species [27]. PSSH species, on the other hand, are susceptible to oxidation and/or electrophilic modification but the oxidized/modified sulfur atom can be readily removed reductively to regenerate PSH when the stress has resolved. Fig. 3 outlines the above-mentioned mechanisms of protection by RSSH (or PSSH) species.
Fig. 3.
Possible mechanisms of RSH protection via conversion to RSSH. (Note: RSSH can be PSSH in these mechanisms).
To be sure, there are numerous reports indicating that RSSH/PSSH formation can be protective in biological systems. For example, Paul and Snyder [87] proposed that PSSH formation may protect against over-oxidation and permanent damage (although no mechanism for this proposal was forwarded). Krishnan and coworkers [81] also reported that sulfuration of the protein phosphatase PTP1B could offer protection from irreversible oxidation. Greiner and coworkers [79] subsequently showed that sulfane sulfur addition to the active site cysteine of the protein phosphatase PTEN results in protection from subsequent irreversible H2O2-mediated modification. Ida et al., [86] found that increased levels of persulfides in cells protect from H2O2 toxicity and further investigation of RSSH-mediated protection revealed that increased intracellular RSSH levels in cells also protect from electrophile-mediated toxicity in both eukaryotes [4] and prokaryotes [85]. Ezerina and coworkers [80] also provide strong evidence that the ‘antioxidant’ and protective properties of N-acetylcysteine (NAC) may actually due to formation of the hydropersulfides, an effect that has been further expounded [28]. Polysulfides (including RSSH species) are reported to be potent anti-inflammatory agents capable of desensitizing macrophages to TLR signaling [29]. Furthermore, sulfuration of glycogen synthase kinase 3β inhibits Tau hyperphosphorylation and thus is neuroprotective against Alzheimer's disease [30]. The antioxidant properties of RSSH species have also been shown to protect against oxidative stress-mediated toxicity in myoblasts [31] and against myocardial ischemia-reperfusion injury [32]. Finally, an RSSH donor compound was found to possess potent anti-inflammatory and antioxidant properties in ATDC5 cells [33].
1.4. Oxidative properties of RSSH/polysulfides
Clearly, the chemical properties of RSSH predicts that it has the potential to provide the cellular protection discussed above. However, it needs to be stressed that although RSSH is a good reductant/nucleophile (mostly as the anionic RSS− species), it is also a good oxidant/electrophile when protonated, likely better than RSSR (vide supra). Thus, the protonated RSSH could be capable of eliciting an oxidative stress via conversion of PSH to PSSH or PSSR via Reactions 10 or 11.
| (10) |
| (11) |
Assuming the modified thiol is crucial to the activity of PSH, the PSSH and/or PSSR species are likely to be inactive (there are only a few instances where PSSH maintains activity [e.g. [34]]), thus this modification can be deleterious to fundamental cellular biochemistry and therefore can represent a toxicity. Interestingly, it was found that numerous cell types treated with cysteine trisulfide (Cys-SSS-Cys, also known as thiocystine) results in an increase in intracellular levels of RSSH species (e.g., GSSH and Cys-SSH) [35]. The increase in RSSH (in this case, Cys-SSH) via treatment with Cys-SSS-Cys was presumably due to Reaction 12 occurring intracellularly.
| (12) |
Importantly, the increase in overall intracellular RSSH also resulted in the cellular export of Cys-SSH into the cell culture media [35], consistent with the idea that RSSH species can be potentially deleterious. That is, since Cys-SSH is an oxidized sulfur species, it is hypothesized that export of Cys-SSH protects cells from an oxidative stress when levels get too high, analogous to the idea that GSSG export helps regulate thiol redox homeostasis [e.g. [36]]. Alternatively, it is proposed that exported Cys-SSH can also serve as an extracellular protectant that can scavenge otherwise toxic oxidants/electrophiles prior to interacting with cells. Finally, it can be speculated that in the vascular system extracellular and circulating Cys-SSH can serve to release NO from circulating S-nitroso albumin (via Reaction 8), a species reported to be a source of labile NO that elicits venodilation [37].
One interesting aspect of RSSH export is that Cys-SSH is exported in spite of the fact that GSSH is in much higher intracellular concentration (as much as 3–4 times higher). Thus, it is curious that a relatively minor component of the overall RSSH load in these treated cells was exported, while a major RSSH species, GSSH, remains intracellular. If the –SSH functional group were deleterious, it may be expected that the majority RSSH species, in this case GSSH, would also be, if not preferentially, exported. The reason for this is not currently known but it is speculated that GSSH can be sequestered intracellularly (akin to, for example, GSSG [82]), not allowing it to elicit an oxidative stress. Clearly there can be other possible explanations and this is likely to be a topic of a significant research focus in the future.
Although Cys-SSS-Cys is considered to be a donor of RSSH via Reaction 12, it needs to be stressed that Cys-SSS-Cys, along with RSSH species are oxidized with respect to RSH/PSH and therefore can potentially elicit an oxidative stress. An example of RSSH/polysulfide-mediated oxidative/electrophilic stress was reported by Switzer and coworkers [84]. They found that cells treated with the Cys-SSH-donor and polysulfur species Cys-SSS-Cys results in cellular toxicity in human cells by eliciting an endoplasmic reticulum stress (ER-stress). Interestingly, however, pre-reacting Cys-SSS-Cys with H2S, which will generate Cys-SSH via Reaction 13 [4], prior to the treatment of cells did not induce an ER-stress and actually results in protection against electrophilic stress. Thus, while Cys-SSH can be protective, the precursor polysulfur species Cys-SSS-Cys can cause an oxidative stress.
| (13) |
Although the electrophilic/oxidative properties associated with RSSH or polysulfides can be deleterious to cells, these species can also activate protective cellular machinery. The transcription factor Nrf2, when activated, elicits cellular protection against oxidative/electrophilic stress via the induced expression of protective genes as well as suppressing the induction of pro-inflammatory cytokines [38,39]. The regulation of Nrf2 involves Keap1, which serves as an E3 ubiquitin ligase substrate recognition subunit that targets Nrf2. Under normal conditions the Keap1-Cul3 E3 ligase complex ubiquitinates Nrf2 leading to rapid proteosomal degradation. However, under oxidative/electrophilic stress Keap1 thiols are oxidatively/electrophilically modified leading to a loss of ubiquitination activity and consequent Nrf2 activation. Not surprisingly, as an electrophile and oxidizing agent, RSSH is capable of activating Nrf2, leading to cellular protection from electrophiles [40].
1.5. RSSH and regulation of RS-NOs
RS-NO formation on PSH (making PS-NO) can alter the activity, function and properties of the protein and therefore is implicated in the etiology of numerous diseases and proposed to be an important part of NO signaling [e.g. [[41], [42], [43], [44]]]. Thus, the synthesis, degradation and fate of RS-NO moieties are important research topics. Since RS-NOs are oxidized with respect to RSH and, like RSSH formation, can also be considered an aspect of cellular oxidative stress (although they are somewhat distinct and often referred to as a ‘nitrosative’ stress when at excessive levels) [e.g. [45]]. Therefore, the reaction of RSSH with RS-NO can have important (patho)physiological implications. As discussed above, RSSH can participate in transnitrosation reactions leading to the degradation of RS-NO with consequent release of NO (i.e., Reaction 8). An important aspect of this mechanism of RS-NO degradation is the release of NO since it has been proposed that RS-NO species can be storage forms or reservoirs of NO that can be released/utilized at opportune times [e.g. [[46], [47], [48], [49]]]. However, in many cases a reasonable physiological mechanism for NO release from RS-NO is not established. Although there are numerous pathways for the biochemical degradation of RS-NOs that do not generate NO [e.g. [[50], [51], [52], [53]]] there are only a few currently proposed mechanisms that are capable of releasing NO as part of the degradation process. NO release from RS-NO can occur photochemically [54,55] or via a metal-mediated process (i.e., via a copper-mediated process) [55]. However, these chemical mechanisms of NO generation from RS-NO degradation are not likely to be physiologically relevant [e.g. [56]]. Thus, the prevalence of RSSH, especially under oxidative stress conditions, provides at least one possible physiologically relevant pathway for NO release from RS-NO species. Indeed, since RS-NO species are thought to be factors in some diseases, this process has been proposed to be an important aspect of the presumed protective actions of RSSH [56]. Importantly, further experimental examination of the RSSH/RS-NO reaction has shown that NO release is the predominant reaction pathway (as opposed to a possible competing HNO-forming pathway that has been shown to occur in the analogous RSH/RS-NO reaction (Reaction 14) [57]) and can lead to the relaxation of vascular tissue, thus providing a biological context for this process [25].
| RS(S)H + R’S–NO → RS(S)SR’ + HNO | (14) |
Thus, the proposal that RSSH may control RS-NO levels has, at the very least, reasonable chemical rationale/precedence and importantly may provide some credence to proposals that RS-NO can be storage forms for NO. Regardless, RSSH-mediated degradation of RS-NO species may be an important aspect of RS-NO regulation, especially since both species, RSSH and RS-NO, should be more prevalent under cellular stress conditions.
1.6. The fate of RSS·
When RSSH acts as a one-electron reductant capable of reacting with and quenching one-electron oxidants (such as those in damaging biological radical chain reactions) (Reaction 15), the immediate sulfur-containing product will be RSS·.
| RSS−/RSSH + X· → RSS· + X−/X–H (X· = one-electron oxidant) | (15) |
Also, in the case of RSSH-mediated degradation of RS-NO and the release of NO, RSS· is produced (Reaction 8). Therefore, it is worth addressing at this point the possible biochemical fate/reactivity of RSS·. It has already been discussed how RSS· is chemically more stable than the corresponding RS· species (justifying the increased reducing power of RSSH over RSH, Fig. 1). The lack of reactivity of RSS· (at least with respect to it acting as a potent oxidant) can be seen from the ability of RSSH to act as an antioxidant where RSSH can quench oxidation chemistry [19,20]. The fact that RSS· does not carry on oxidation chemistry (i.e., act as an intermediate in radical chain processes) is a testament to its lack of oxidative reactivity. Furthermore, as a paramagnetic species with an unpaired electron, RSS· does not appear to have a great propensity to react with O2 (another paramagnetic species). However, it should be noted that Everett and coworkers [60] have reported that radiolytic formation of RSS· in a solution containing O2 can result in low to moderate yields of sulfate (SO42−). Clearly RSS· has no affinity for paramagnetic NO since NO is readily released from RSS-NO due to the very low bond dissociation energy of approximately 3–4 kcal/mol [11,25]. Under purely chemical conditions (i.e., in a simplified system devoid of possible reactive species that can be present in a biological milieu), RSS· will simply dimerize to RSSSSR (Reaction 9). However, since Reaction 9 is second order in RSS·, the likelihood of this reaction occurring in biological systems may be small since concentrations of RSS· are likely to be low and other reaction pathways for RSS· may exist. The ultimate fate of RSS· becomes an important question since it may have physiological consequences. Although there currently is no established answer for this question, possible fates for RSS· can be gleaned from the chemical properties of RSS·. A calculated reduction potential for the RSS·/RSS− couple is reported to be 0.68 V (pH 7) [58]. For comparison, the reduction potentials for the ascorbyl radical anion (Asc·-)/ascorbate (Asc−) and the tocopheroxyl radical (Toc-O·),H+/tocopherol (Toc-OH) couples are 0.282 and 0.500 V respectively (both pH 7) [59]. These values predict that either ascorbate (vitamin C) or α-tocopherol (vitamin E) are both thermodynamically capable of reducing RSS· to RSS−. Indeed, RSS· generated via pulse irradiation of RSSSR has been reported to react with Asc− with a fairly high rate constant of 4.1 x 106 M−1s−1 [60]. There may also be biological reductases capable of reducing RSS· to RSS−, akin to what is known for Asc·- reduction to Asc− [e.g. [61]]. Thus, like ascorbate and tocopherols, RSS· may be recycled back to RSSH rather than simply dimerizing to RSSSSR. Regardless, further work will need to be done to address this possibility.
One possible biological fate of RS· is reaction with, for example, R’SH to generate a radical anion (R’SSR·-), which is a potent reductant capable of reducing O2 to superoxide (O2−) [62]. Thus, it is conceivable that RSS· may also react with H2S/HS− or RSH/RS− in an analogous fashion. That is, RSS· may react with R’SH to give the corresponding trisulfide radical anion (RSSSR’·-). To be sure RSSSR·- species have been speculated to be in equilibrium with RS− and RSS· (or RS· and RSS−), lending credence to the idea that this reaction can occur [60]. The chemical properties of RSSSR·- are currently not established, thus the physiological implications of this possible reaction remain to be determined.
1.7. Inorganic polysulfides
In addition to RSSH and longer polysulfide species (R(S)nSH), inorganic polysulfide analogs are also formed with the general formula of H2Sn (n = 2–9). While they share some properties of RSSH, inorganic polysulfides have additional chemical features that may have biochemical or biological importance. Similar to RSSH, H2Sx are stronger acids compared to their monothiol analogue, H2S, and the anionic species predominate at physiological pH [63]. Additionally, inorganic polysulfides retain the radical stabilization effects observed in RSSH (Fig. 1). This is reflected in the high one-electron reduction potential of S32− and S42−, indicating that polysulfides are excellent reducing agents that can initiate or quench radical chain reactions [64]. The stability of polysulfide radicals is further demonstrated by the one-electron reduction of the heme group in indoleamine 2,3-dioxygenase by polysulfides, which results in enzymatic activation and cellular oxidation [65]. Therefore, the one-electron reducing ability of polysulfides can promote either cellular antioxidant or pro-oxidant effects. Similar to hydropersulfides and alkyl polysulfides, inorganic polysulfides spontaneously homolyze to form radical anions, highlighting the stability of these radical species (e.g., Reaction 16) [63].
While alkyl hydropersulfide radicals appear to be fairly non-reactive with O2, inorganic polysulfides slowly autoxidize to form thiosulfate and the spontaneous formation of radical polysulfide species may be involved in this process [66].
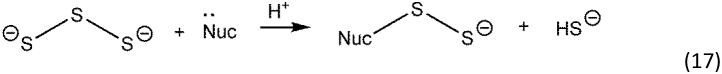
As well as forming radical species, inorganic polysulfides react as electrophiles in the presence of other nucleophiles as in Reaction 17.
If the incoming nucleophile is another polysulfide, a disproportionation reaction occurs, and polymer chains of different length are generated concomitantly with sulfide. Thus, polysulfides in solution will form linear polysulfide chains of varying length, as in Reaction 18.
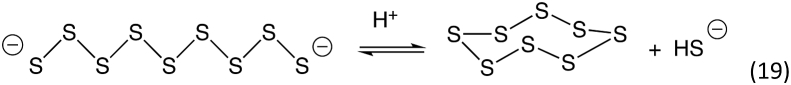
Analogous to the disproportionation reaction, longer chain polysulfides can undergo an internal cyclization reaction to form elemental sulfur species (which is often S8 but can exist as other homocyclic structures as well, e.g., S6 and greater [67]) and sulfide (Reaction 19).
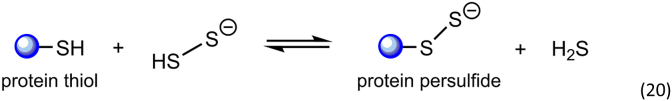
If the incoming nucleophile is a thiol, polysulfides can potentially generate protein and other small molecule hydropersulfides and polysulfides. For example, protein thiols can react with hydrogen persulfide to generate a protein persulfide and sulfide (Reaction 20):
Similar to hydropersulfide nucleophilicity being greater than that of thiols, inorganic polysulfides are more nucleophilic than sulfide [68]. As such, polysulfides are predicted to function as nucleophiles, as in Reaction 21 [ [69]].
Therefore, inorganic polysulfides are highly reactive cellular species that can form radicals, react as either nucleophiles or electrophiles, are capable of cyclizing and can serve as a source of H2S. This chemical diversity indicates that multiple biochemical and cellular effects can be elicited in response to polysulfide generation.
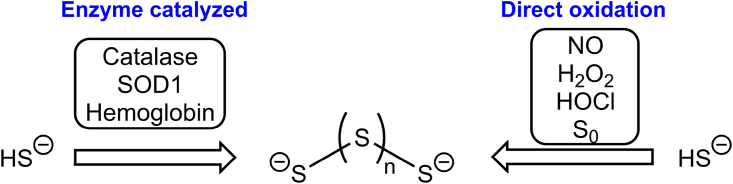
While the exact cellular sources have not yet been identified, a number of biochemical reactions have been described that produce inorganic polysulfides. There are two general pathways to generate polysulfides: 1) the oxidation of sulfide (Fig. 4) and 2) the decomposition of alkyl polysulfides.
Fig. 4.
Pathways of inorganic polysulfide generation from sulfide oxidation.
Enzyme catalyzed sulfide oxidation to persulfide has been demonstrated for hemoglobin, catalase and SOD1 [[70], [71], [72]]. In addition to enzyme-catalyzed sulfide oxidation, 3-mercaptopyruvate sulfurtransferase catalyzes the formation of polysulfides from 3-mercaptopyruvate [73]. Similarly, cysteinyl-tRNA synthetase (CARS) form polysulfides from multiple cycles of cysteine oxidation [74]. The generation or degradation of polysulfides by metalloproteins is an active area of investigation and additional enzymes involved in polysulfide metabolism are likely to be discovered. Finally, Olson and coworkers [83] have shown that ubiquinone (CoQ) and related quinones are capable of oxidizing H2S to polysulfides and thiosulfate in O2-dependent processes. The ubiquitous nature of CoQ in biological membranes indicates that the generation of polysulfides via H2S oxidation can occur in numerous locations and can be a common event.
In addition to enzyme-catalyzed sulfide oxidation, sulfide is directly oxidized to polysulfides by NO [75,76], hydrogen peroxide [77], hypochlorous acid [78]] and elemental sulfur [63]. Interestingly, the direct oxidation of sulfide by H2O2 is a relatively slow process (k = 0.73 M−1s−1, pH 7.4, 37 °C [77]). The reaction of sulfide with elemental sulfur is the reverse of Reaction 19 and is a traditional synthetic route for inorganic polysulfides [63]. Therefore, H2S may be a critical source of polysulfides [79] and implies that deficient sulfide signaling may limit polysulfide formation and reactivity.
Another potential route to inorganic polysulfides is the decomposition of alkyl polysulfides by cellular thiols. For example, dialkyl polysulfides (RSnSR; n > 1) can react with glutathione to form hydrogen persulfide and mixed glutathione disulfide (Reaction 22).
Therefore, inorganic polysulfides are interchangeable, highly reactive biomolecules, capable of inducing or limiting cellular oxidation events.
2. Summary
The chemical properties of RSSH predict that is can act as a reductant/nucleophile or an oxidant/electrophile depending on its protonation state (Fig. 2) and it is likely that both properties can be observed in biological systems. To date, numerous reports indicate that RSSH can be protective, possibly due to their reducing and nucleophilic properties which can either scavenge deleterious oxidants/electrophiles. Alternatively, PSSH can protect from irreversible protein damage since electrophilic or oxidative modification of the sacrificial sulfur atom of PSSH still allow reduction back to active PSH. More recently, it is proposed that RSSH can degrade RS-NO species with subsequent liberation of NO. This phenomenon may have multiple purposes. For example, RSSH-mediated degradation of RS-NO may be important in the regulation of RS-NO levels and thus important to their (patho)physiology. Moreover, RSSH-mediated degradation of RS-NO liberates NO, allowing for the possibility that RS-NO species can be storage forms or reservoirs for NO. Although the chemistry of these interactions is clear, the relevance of this chemistry to biology still requires experimental verification and this will be the challenge for researchers in the future as this field progresses.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
C.H.S. is supported by British Heart Foundation project grant PG/19/33/34385.
Data availability
No data was used for the research described in the article.
References
- 1.Fukuto J.M., Carrington S.J., Tantillo D.J., Harrison J.G., Ignarro L.J., Freeman B.A., Chen A., Wink D.A. Small molecule signaling agents: the integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide and their derived species. Chem. Res. Toxicol. 2012;25:769–793. doi: 10.1021/tx2005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukuto J.M., Collins M.D. Interactive endogenous small molecule (gaseous) signaling: implications for teratogenesis. Curr. Pharmaceut. Des. 2007;13(29):2952–2978. doi: 10.2174/138161207782110525. [DOI] [PubMed] [Google Scholar]
- 3.Cuevasanta E., Lange M., Bonanata J., Coitino E.L., Ferrer-Sueta G., Filipovic M.R., Alvarez B. Reaction of hydrogen sulfide with disulfide and sulfenic acid to form the strongly nucleophilic persulfide. J. Biol. Chem. 2015;290:26866–26880. doi: 10.1074/jbc.M115.672816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianco C.L., Akaike T., Ida T., Nagy P., Bogdandi V., Toscano J.P., Kumagai Y., Henderson C.F., Goddu R.N., Lin J., Fukuto J.M. The reaction of hydrogen sulfide with disulfides: formation of a stable trisulfide and implications for biological systems. Br. J. Pharmacol. 2019;176:671–683. doi: 10.1111/bph.14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stipanuk M.H., Beck P.W. Characterization of the enzymatic capacity for cysteine desulphhydration in liver and kidney in the rat. Biochem. J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stipanuk M.H. Metabolism of sulfur-containing amino acids. Annu. Rev. Nutr. 1986;6:179–209. doi: 10.1146/annurev.nu.06.070186.001143. [DOI] [PubMed] [Google Scholar]
- 7.Fukuto J.M., Ignarro L.J., Nagy P., Wink D.A., Kevil C.G., Feelisch M., Cortese-Krott M.M., Bianco C.L., Kumagai Y., Hobbs A.J., Lin J., Ida T., Akaike T. Biological hydropersulfides and related polysulfides – a new concept and perspective in redox biology. FEBS Lett. 2018;592:2140–2152. doi: 10.1002/1873-3468.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saund S.S., Sosa V., Henriquez S., Nguyen Q.N.N., Bianco C.L., Soeda S., Millikin R., White C., Le H., Ono K., Tantillo D.J., Kumagai Y., Akaike T., Lin J., Fukuto J.M. The chemical biology of hydropersulfides (RSSH): chemical stability, reactivity and redox roles. Arch. Biochem. Biophys. 2015;588:15–24. doi: 10.1016/j.abb.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benchoam D., Semelak J.A., Cuevasanta E., Mastrogiovanni M., Grassano J.S., Ferrer-Sueta G., Zeida A., Trujillo M., Moller M.N., Estrin D.A., Alvarez B. Acidity and nucleophilic reactivity of glutathione persulfide. J. Biol. Chem. 2020;295:15466–15481. doi: 10.1074/jbc.RA120.014728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francoleon N.E., Carrington S.J., Fukuto J.M. The reaction of H2S with oxidized thiols: generation of persulfides and implications to H2S biology. Arch. Biochem. Biophys. 2011;516:146–154. doi: 10.1016/j.abb.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Bianco C.L., Chavez T.A., Sosa V., Saund S.A., Nguyen Q.N.N., Tantillo D.J., Ichimura A.S., Toscano J.P., Fukuto J.M. The chemical biology of the persulfide (RSSH)/perthiyl (RSS·) redox couple and possible role in biological redox signaling. Free Radic. Biol. Med. 2016;101:20–31. doi: 10.1016/j.freeradbiomed.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galardon E., Huguet F., Herrero C., Ricoux R., Artaud I., Padovani D. Reactions of persulfides with heme cofactor of oxidized myoglobin and microperoxidase 11: reduction and coordination. Dalton Trans. 2017;46:7939–7946. doi: 10.1039/c7dt01638g. [DOI] [PubMed] [Google Scholar]
- 13.Bamford V.A., Bruno S., Rasmussen T., Appia-Ayme C., Chessman M.R., Berks B.C., Hemmings A.M. Structural basis for the oxidation of thiosulfate by a sulfur cycle enzyme. EMBO J. 2002;21:5599–5610. doi: 10.1093/emboj/cdf566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dambe T., Quentmeier A., Rother D., Friedrich C., Scheidig A.J. Structure of the cytochrome complex SoxXA of Paracoccus pantotrophus, a heme enzyme initiating chemotrophic sulfur oxidation. J. Struct. Biol. 2005;152:229–234. doi: 10.1016/j.jsb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez L., Suarez-Vega V., McGinity C., Khodada V.S., Toscano J.P., Nagy P., Lin J., Works C., Fukuto J.M. The reactions of hydropersulfides (RSSH) with myoglobin. Arch. Biochem. Biophys. 2020;687 doi: 10.1016/j.abb.2020.108391. [DOI] [PubMed] [Google Scholar]
- 16.Fukuto J.M., Lin J., Khodade V.S., Toscano J.P. Predicting the possible physiological/biological utility of the hydropersulfide functional group based on its chemistry: similarities between hydropersulfides and selenols. Antioxidants Redox Signal. 2020;33:1295–1307. doi: 10.1089/ars.2020.8079. [DOI] [PubMed] [Google Scholar]
- 17.Fukuto J.M., Hobbs A.J. A comparison of the chemical biology of hydropersulfides (RSSH) with other protective biological antioxidants and nucleophiles. Nitric Oxide. 2021;107:46–57. doi: 10.1016/j.niox.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Scrivner O., Kumar M.R., Sorokolet K., Wong A., Kebaara B., Farmer P.J. Characterization of endogenous and extruded and small oxoacids of sulfur (SOS) in cell culture. ACS Chem. Biol. 2021;16:1413–1424. doi: 10.1021/acschembio.1c00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everett S.A., Wardman P. Perthiols as antioxidants: radical-scavenging and prooxidative mechanisms. Methods Enzymol. 1995;251:55–69. doi: 10.1016/0076-6879(95)51110-5. [DOI] [PubMed] [Google Scholar]
- 20.Chauvin J.-P.R., Griesser M., Pratt D.A. Hydropersulfides: H-atom transfer agents par excellence. J. Am. Chem. Soc. 2017;139:6484–6493. doi: 10.1021/jacs.7b02571. [DOI] [PubMed] [Google Scholar]
- 21.Sawa T., Takata T., Matsunaga T., Ihara H., Motohashi H., Akaike T. Chemical biology of reactive sulfur species: hydrolysis-driven equilibrium of polysulfides as a determinant of physiological functions. Antioxidants Redox Signal. 2022;36:327–336. doi: 10.1089/ars.2021.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kettenhofen N.J., Wood M.J. Formation, reactivity, and detection of protein sulfenic acids. Chem. Res. Toxicol. 2010;23:1633–1646. doi: 10.1021/tx100237w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta V., Carroll K.S. Sulfenic acid chemistry, detectionand cellular lifetime. Biochim. Biophys. Acta. 2014;1840:847–875. doi: 10.1016/j.bbagen.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogg N. The kinetics of S-transnitrosation – a reversible second order reaction. Anal. Biochem. 1999;272:257–262. doi: 10.1006/abio.1999.4199. [DOI] [PubMed] [Google Scholar]
- 25.Zarenkiewicz J., Perez-Ternero C., Kojasoy V., McGinity C., Khodade V.S., Lin J., Tantillo D.J., Toscano J.P., Hobbs A.J., Fukuto J.M. The reactionof hydropersulfides (RSSH) with S-nitrosothiols and the biological/physiological implications. Free Radic. Biol. Med. 2022;188:459–467. doi: 10.1016/j.freeradbiomed.2022.06.245. [DOI] [PubMed] [Google Scholar]
- 26.Millikin R., Bianco C.L., White C., Saund S.S., Henriquez S., Sosa V., Akaike T., Kumagai Y., Soeda S., Toscano J.P., Lin J., Fukuto J.M. The chemical biology of protein hydropersulfides: studies of a possible protective function of biological hydropersulfide generation. Free Radic. Biol. Med. 2016;97:136–147. doi: 10.1016/j.freeradbiomed.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalle-Donne I., Milzani A., Gagliano N., Colombo R., Giustarini D., Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxidants Redox Signal. 2008;10:445–473. doi: 10.1089/ars.2007.1716. [DOI] [PubMed] [Google Scholar]
- 28.Pedre B., Barayeu U., Ezerina D., Dick T. The mechanism of action of N-acetylcysteine (NAC): the emerging tole of H2S and sulfane sulfur species. Pharmacol. Therapeut. 2021;228 doi: 10.1016/j.pharmthera.2021.107916. [DOI] [PubMed] [Google Scholar]
- 29.Zhang T., Ono K., Tsutsuki H., Ihara H., Islam W., Akaike T., Sawa T. Enhanced cellular polysulfides negatively regulate TLR4 signaling and mitigate lethal endotoxin shock. Cell Chem. Biol. 2019;26:1–13. doi: 10.1016/j.chembiol.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Giovinazzo D., Bursac B., Sbodio J.I., Nalluru S., Vignane T., Snowman A.M., Albacarys L.M., Sedlak T.W., Torregrossa R., Whiteman M., Filipovic M.R., Snyder S.H., Paul B.D. Proc. Natl. Acad. Sci. U.S.A. 2021;118 doi: 10.1073/pnas.2017225118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khodade V.S., Aggarwal S.C., Pharoah B.M., Paolocci N., Toscano J.P. Alkylsulfenyl thiocarbonates: precursors to hydropersulfides potently attenuate oxidative stress. Chem. Sci. 2021;12:8252–8259. doi: 10.1039/d1sc01550h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khodade V.S., Pharoah B.M., Paolocci N., Toscano J.P. Alkylamine-substituted perthiocarbamates: dual precusros to hydropersulfide and carbonyl sulfide with cardioprotective actions. J. Am. Chem. Soc. 2020;142:4309–4316. doi: 10.1021/jacs.9b12180. [DOI] [PubMed] [Google Scholar]
- 33.Trummer M., Galardon E., Fischer A., Toegel S., Mayer B., Steiner G., Kloesch B. Characterization of the inducible and slow -releasing hydrogen sulfide and persulfide donor P*: insights into hydrogen sulfide signaling. Antioxidants. 2021;10:1049. doi: 10.3390/antiox10071049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandiver M.S., Paul B.D., Xu R., Karuppagounder S., Rao F., Snowman A.M., Ko H.S., Lee Y.I., Dawson V.L., Dawson T.M., Sen N., Snyder S.H. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 2013;4:1626. doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin J., Akiyama M., Bica F., Long F.T., Henderson C.F., oddu R.N., Suarez V., Baker B., Ida T., Shinkai Y., Nagy P., Akaike T., Fukuto J.M., Kumagai Y. The uptake and release of polysulfur cysteine species by cells: physiological and toxicological implications. Chem. Res. Toxicol. 2019;32:447–455. doi: 10.1021/acs.chemrestox.8b00340. [DOI] [PubMed] [Google Scholar]
- 36.Ballatori N., Krance S.M., Marchan R., Hammond C.L. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol. Aspect. Med. 2009;30:13–28. doi: 10.1016/j.mam.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orie N.N., Vallance P., Jones D.P., Moore K.P. S-Nitroso-albumin carries a thiol labile pool of nitric oxide, which causes venodilation in the rat. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H916–H923. doi: 10.1152/ajpheart.01014.2004. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki T., Yamamoto M. Stress-sensing mechanism and physiological roles of the Keap1-Nrf2 system during cellular stress. J. Biol. Chem. 2017;292:16817–16824. doi: 10.1074/jbc.R117.800169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018;98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koike S., Nishimoto S., Ogasawara Y. Cysteine persulfides and polysulfides produced by exchange reactions with H2S protect SH-SY5Y cells from methylglyoxal-induced toxicity through Nrf2 activation. Redox Biol. 2017;12:530–539. doi: 10.1016/j.redox.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernando V., Zheng X., Walia Y., Sharma V., Letson J., Furuta S. S-Nitrosylation: an emerging paradigm of redox signaling. Antioxidants. 2019;8:404. doi: 10.3390/antiox8090404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marozkina N.V., Gaston G. S-Nitrosylation signaling regulates cellular protein interactions. Biochim. Biophys. Acta. 2012;1820:722–729. doi: 10.1016/j.bbagen.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hess Dt, Matsumoto A., Kim S.-O., Marshall H.E., Stamler J.S. Protein S-nitrosylation: purview and parameters. Nature Rev. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y., Deng Y., Yang X., Xue H., Lang Y. The relationship between protein S-nitrosylation and human disease: a review. Neurochem. Res. 2020;45:2815–2827. doi: 10.1007/s11064-020-03136-6. [DOI] [PubMed] [Google Scholar]
- 45.Thomas D.D., Ridnour L.A., Isenberg J.S., Flores-Santana W., Switzer C.H., Donzellie S., Hussain P., Vecoli C., Paolocci N., Ambs S., Colton C., Harris C., Roberts D.D., Wink D.A. The chemical biology of nitric oxide. Implications in cellular signaling. Free Radic. Biol. Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez J., Maloney R.E., Rassaf T., Bryan N.A., Feelisch M. Chemical nature of nitric oxide storage forms in rat vascular tissue. Proc. Natl. Acad. Sci. U.S.A. 2003;100:336–341. doi: 10.1073/pnas.0234600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng E.S.M., Kubes P. The physiology of S-nitrosothiols: carrier molecules for nitric oxide. Can. J. Physiol. Pharmacol. 2003;81:759–764. doi: 10.1139/y03-078. [DOI] [PubMed] [Google Scholar]
- 48.Rayner B.S., Wu B.-J., Raftery M., Stocker R., Wittig P.K. Human S-nitroso Oxymyoglobin is a store of vasoactive nitric oxide. J. Biol. Chem. 2005;280:9985–9993. doi: 10.1074/jbc.M410564200. [DOI] [PubMed] [Google Scholar]
- 49.Singel D.J., Stamler J.S. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu. Rev. Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 50.Jensen D.E., Belka G.K., DuBois G.C. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isozyme. Biochem. J. 1998;331:659–668. doi: 10.1042/bj3310659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu L., Hausladen A., Zeng M., Que L., Heitman J., Stamler J.S. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:491–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 52.Sengupta R., Holmgren A. Thioredoxin and thioredoxin reductase in relation to reversible S-nitrosylation. Antioxidants Redox Signal. 2013;18:259–269. doi: 10.1089/ars.2012.4716. [DOI] [PubMed] [Google Scholar]
- 53.Ren X., Sengupta R., Lu J., Lundberg J.O., Holmgren A. Characterization of mammalian glutaredoxin isoforms as de-nitrosylases. FEBS Lett. 2019;593:1799–1806. doi: 10.1002/1873-3468.13454. [DOI] [PubMed] [Google Scholar]
- 54.Sexton D.J., Muruganandam A., McKenney D.J., Mutus B. Visible light photochemical release of nitric oxide from S-nitrosoglutathione: potential photochemotherapeutic applications. Photochem. Photobiol. 1994;59:463–467. doi: 10.1111/j.1751-1097.1994.tb05065.x. [DOI] [PubMed] [Google Scholar]
- 55.Singh R.J., Hogg N., Joseph J., Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols. J. Biol. Chem. 1996;271:18596–18603. doi: 10.1074/jbc.271.31.18596. [DOI] [PubMed] [Google Scholar]
- 56.Fukuto J.M., Perez-Ternero C., Zarenkiewicz J., Lin J., Hobbs A.J., Toscano J.P. Hydropersulfides (RSSH) and nitric oxide (NO) signaling: possible effects on S-nitrosothiols (RS-NO) Antioxidants. 2022;11:169. doi: 10.3390/antiox11010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong P.S.-Y., Hyun J., Fukuto J.M., Shiroda F.N., DeMaster E.G., Nagasawa H.T. The reaction between nitrosothiols and thiols: generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry. 1998;37(16):5362–5371. doi: 10.1021/bi973153g. [DOI] [PubMed] [Google Scholar]
- 58.Koppenol W.H., Bounds P.L. Signaling by sulfur-containing molecules. Quantitative aspects. Arch. Biochem. Biophys. 2017;617:3–8. doi: 10.1016/j.abb.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Buettner G.R. The pecking order of free radicals and antioxidants: lipid peroxidation, α-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 60.Everett S.A., Schoneich C., Stewart J.H., Asmus K.-D. Perthiyl radicals, trisulfide radical ions, and sulfate formation. A combined photolysis and radiolysis study on redox processes with organic di- and trisulfides. J. Phys. Chem. A. 1992;96:306–314. [Google Scholar]
- 61.Linster C.L., Van Schaftingen E. Vitamin C, biosynthesis, recycling and degradation in mammals. FEBS J. 2007;274:1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 62.Winterbourn C.C. Are free rdicals involved in thiol-based signaling. Free Radic. Biol. Med. 2015;80:164–170. doi: 10.1016/j.freeradbiomed.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 63.Steudel R. In: Elemental Sulfur and Sulfur-Rich Compounds II. Stuedel R., editor. Vol. 231. Springer; Berlin, Heidelberg: 2003. Inorganic polysulfides Sn2- and radical anions Sn·- (Topics in Current Chemistry). [DOI] [Google Scholar]
- 64.Li H., Tang X., Pang J.H., Wu X., Yeow E.K.L., Wu J., Chiba S. Polysulfide anions as visible light photoredox catalysts for aryl cross-couplings. J. Am. Chem. Soc. 2021;143:481–487. doi: 10.1021/jacs.0c11968. [DOI] [PubMed] [Google Scholar]
- 65.Nelp M.T., Zheng V., Davis K.M., Stiedel K.J.E., Groves J.T. Potent activation of indolamine 2,3-dioxygenase by polysulfides. J. Am. Chem. Soc. 2019;141:15288–15300. doi: 10.1021/jacs.9b07338. [DOI] [PubMed] [Google Scholar]
- 66.Kleinjan W.E., de Keizer A., Janssen A.J.H. Kinetics of the chemical oxidation of polysulfide anions in aqueous solution. Water Res. 2005;39:4093–4100. doi: 10.1016/j.watres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Steudel R. Homocyclic sulfur molecules. Top. Curr. Chem. 1982;102:149–176. doi: 10.1007/3-540-11345-2_11. [DOI] [PubMed] [Google Scholar]
- 68.Ling M., Zhng L., Zheng T., feng J., Guo J., Mai L., Liu G. Nucleophilic substitution between polysulfides and binders unexpectedly stabilizing lithium sulfur battery. Nano Energy. 2017;38:82–90. [Google Scholar]
- 69.Steudel R., Kustos M. In: King R.B., editor. Vol. 7. Wiley; Chichester: 1994. p. 4009. (Encyclopedia of Inorganic Chemistry). [Google Scholar]
- 70.Vivitsky V., Yadav P.K., An S., Seravalli J., Cho U.-S., Banerjee R. Structural and mechanistic insight into hemoglobin-catalyzed hydreogen sulfide oxidation and the fate of polysulfide products. J. Biol. Chem. 2017;292:5584–5592. doi: 10.1074/jbc.M117.774943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olson K.R., Gao Y., DeLeon E.R., Arif M., Arif F., Arora N., Straub K.D. Catalase as a sulfide-sulfur oxido-reductase: an ancient (and modern?) regulator of reactive sulfur species (RSS) Redox Biol. 2017;12:325–339. doi: 10.1016/j.redox.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olson K.R., Gao Y., Arif F., Arora K., Patel S., DeLeon E.R., Sutton T.R., Feelisch M., Cortese-Krott M.M., Straub K.D. Metabolism of hydrogen sulfide (H2S) and production of reactive sulfur species (RSS) by superoxide dismutase. Redox Biol. 2018;15:74–85. doi: 10.1016/j.redox.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kimura Y., Toyofuku Y., Koike S., Shibuya N., Nagahara N., Lefer D., Ogasawara Y., Kimura H. Identification of H2S3 and H2S produced by 3-mercaptopyruvate sulfurase in the brain. Sci. Rep. 2015;5 doi: 10.1038/srep14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akaike T., Ida T., Wei F.-W., Nishida M., Kumagai Y., Alam M., Ihara H., Sawa T., Matsunaga T., Kasamatsu S., Nishimura A., Morita M., Tomizawa K., Nishimura A., Watanabe S., Inaba K., Shima H., Tanuma N., Jung M., Fujii S., Watanabe Y., Ohmuraya M., Nagy P., Feelisch M., Fukuto J.M., Motohashi H. Cysteinyl-tRNA synthase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017;8 1177:1–15. doi: 10.1038/s41467-017-01311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eberhardt M., Dux M., Namer B., Miljkovic J., Cordasic N., Will C., Kichko T.I., de la Roches J., Fischer M., Suarez S.A., Bikiel D., Dorsch K., Leffler A., Babes A., Lampert A., Lennerz J.K., Jacobi J., Marti M.A., Doctorovich F., Hogestatt E.D., Zygmunt P.M., Ivanocic-Burmazovic I., Messlinger K., Reeh P., Filipovic M.R. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signaling pathway. Nat. Commun. 2014;5:4381. doi: 10.1038/ncomms5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cortese-Krott M.M., Kuhnle G.G.C., Dyson A., Fernandez B.O., Grman M., DuMond J.F., Barrow M.P., McLeod G., Nakagawa H., Ondrias K., Nagy P., King S.B., Saavedra J.E., Keefer L.K., Singer M., Keim M., Butler A.R., Feelisch M. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc. Natl. Acad. Sci. U.S.A. 2015;112:E4651–E4660. doi: 10.1073/pnas.1509277112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carballal S., Trujillo M., Cuevasanta E., Bartesaghi S., Moller M.N., Foljes L.K., Garcia-Bereguiain M.A., Gutierrez-Merino C., Warman P., Denicola A., Radi R., Alvarez B. Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radic. Biol. Med. 2011;50:196–205. doi: 10.1016/j.freeradbiomed.2010.10.705. [DOI] [PubMed] [Google Scholar]
- 78.Nagy P., Winterbourne C.C. Rapid reaction of hydrogen sulfide with the neutrophil oxidant hypochlorous acid to generate polysulfides. Chem. Res. Toxicol. 2010;23:1541–1543. doi: 10.1021/tx100266a. [DOI] [PubMed] [Google Scholar]
- 79.Greiner R., Palinkas Z., Basell K., Becher D., Antelmann H., Nagy P., Dick T.P. Polysulfides link H2S to protein thiol oxidation. Antioxidants Redox Signal. 2013;19:1749–1765. doi: 10.1089/ars.2012.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ezerina D., Takano Y., Hanaoka K., Urano Y., Dick T.P. N-acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular H2S and sulfane sulfur production. Cell Chem. Biol. 2018;25:447–459. doi: 10.1016/j.chembiol.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krishnan N., Fu C., Pappin D., Tonks N.K. H2S-induced sulfhydration of PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 2012;4:ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morgan B., Ezerina D., Amoako T.N.E., Riemer J., Seedorf M., Dick T.P. Multiple glutathione disulfide removal pathways mediate cytosolic redox homeostasis. Nat. Chem. Biol. 2013;9:119–125. doi: 10.1038/nchembio.1142. [DOI] [PubMed] [Google Scholar]
- 83.Olson K.R., Clear K.J., Derry P.J., Gao Y., Ma Z., Wu G., Kent T.A., Straub K.D. Coenzyme Q10 and related quinones oxidize H2S to polysulfides and thiosulfate. Free Radic. Biol. Med. 2022;182:119–131. doi: 10.1016/j.freeradbiomed.2022.02.018. [DOI] [PubMed] [Google Scholar]
- 84.Switzer C.H., Guttzeit S., Eykyn T.R., Eaton P. Cysteine trisulfide oxidizes protein thiols and induces electrophilic stress in human cells. Redox Biol. 2021;47 doi: 10.1016/j.redox.2021.102155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Henderson, CF, Bica, I, Long, FT, Irwin, DD, Stull, CH, Baker, BW, Suarez-Vega, V, Taugher, ZM, Fletes, ED, Bartleson, JM, Humphrey, ML, Alvarez, L, Akiyama, M, Kumagai, T, Fukuto JM and Lin, J, Cysteine trisulfide protects E. coli from electrophile-induced death through the generation of cysteine hydropersulfide, Chem. Res. Toxicol., 33, 678-686. [DOI] [PubMed]
- 86.Ida T., Sawa T., Tsuchiya Y., Watanabe Y., Kumagai Y., Suematsu M., Motohashi H., Matsunaga T., Yamamoto M., Ono K., Devarie-Baez N.O., Xian M., Fukuto J.M., Akaike T. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. U.S.A. 2014;111:7606–7611. doi: 10.1073/pnas.1321232111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paul B.D., Snyder S.H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.