Abstract
Yersinia enterocolitica target effector Yop proteins into the cytosol of eukaryotic cells by a mechanism requiring the type III machinery. LcrG and LcrV have been suggested to fulfill essential functions during the type III targeting of effector Yops. It is reported here that knockout mutations of lcrG caused mutant yersiniae to prematurely secrete Yops into the extracellular medium without abolishing the type III targeting mechanism (Los phenotype [loss of type III targeting specificity]). Knockout mutations in lcrV reduced type III targeting of mutant yersiniae but did not promote secretion into the extracellular medium (Not [no type III targeting]). However, knockout mutations in both genes caused ΔlcrGV yersiniae to display a Los phenotype similar to that of strains carrying knockout mutations in lcrG alone. LcrG binding to LcrV resulted in the formation of soluble LcrGV complexes in the bacterial cytoplasm. Membrane-associated, bacterial-surface-displayed or -secreted LcrG could not be detected. Most of LcrV was located in the bacterial cytoplasm; however, small amounts were secreted into the extracellular medium. These data support a model whereby LcrG may act as a negative regulator of type III targeting in the bacterial cytoplasm, an activity that is modulated by LcrG binding to LcrV. No support could be gathered for the hypothesis whereby LcrG and LcrV may act as a bacterial surface receptor for host cells, allowing effector Yop translocation across the eukaryotic plasma membrane.
Pathogenic yersiniae, e.g., Yersinia pestis, Y. pseudotuberculosis, and Y. enterocolitica, attach to mammalian cells and inject cytotoxic proteins across the plasma membrane into the eukaryotic cytosol (51). During infection, this mechanism allows yersiniae to escape phagocytic killing and establish residence within the lymphoid tissues of an infected host (42). Yersinia type III secretion machines catalyze the transport of cytotoxic proteins across the bacterial double-membrane envelope (36). Once yersiniae enter their host and are exposed to a 37°C environment, type III machinery components are expressed and assembled in the bacterial envelope (19, 22, 23, 62). Secretion can be induced by the removal of calcium ions from the media (11), causing Y. enterocolitica to massively export 14 different proteins (YopBDEHMNOPQRT, LcrV, YscM1, and YscM2) into the extracellular medium (36) (Y. pestis and Y. pseudotuberculosis express only YscM1 [LcrQ] but not YscM2 [49].) During host infection, the high concentration of calcium (1.8 mM) within extracellular fluids prevents the induction of type III secretion (11, 33). Bacterial attachment to host cells provides an inducing signal for type III machines (45), leading to the injection of YopEHMNOPT into the eukaryotic cytosol (type III targeting) (5, 20, 27, 37, 38, 43, 50). Some export substrates (YopBDR) are secreted into the extracellular medium (type III secretion) (30, 32), whereas another substrate, YopQ, is presumably associated with the bacterial envelope (25). Thus, in response to specific host signals, likely a receptor-ligand interaction between invading pathogens and immune cells, Yersinia modify their type III export pathways and direct bacterial virulence factors to several distinct destinations (32).
The genes specifying the type III machinery (ysc [Yop secretion]) and export substrates are located on a 70-kb virulence plasmid (11). Knockout mutations in any one of the 22 ysc genes (yscACDEFGIJKLNOPQRSTUVWXY) abrogate type III export under low-calcium conditions (1, 35), as well as type III targeting and secretion during infection (51). Knockout mutations in genes encoding effector Yops (yopEHMOPT) do not affect the type III pathway. In contrast, mutations that prevent the expression of YopD and LcrV abrogate type III targeting without affecting type III secretion (Not phenotype [no type III targeting]) (16, 32, 33, 44, 51). Knockout mutations in yopN, specifying another type III export substrate, cause mutant yersiniae to secrete most effector Yops into the extracellular medium (Los phenotype [loss of type III targeting specificity]) (30, 51). When incubated at 37°C in the presence of calcium, yopN mutants massively export type III substrates into the extracellular medium, thereby reducing the ability of mutant yersiniae to grow at elevated temperatures (temperature-sensitive or calcium-blind phenotype) (18, 65). Knockout mutations in several other regulatory genes, sycN, tyeA, and yscB, cause a similar phenotype (9, 14, 26, 28).
Previous work identified the lcrG gene as a member of the Yersinia low-calcium response pathway (4, 41, 48). Knockout mutations in lcrG cause mutant yersiniae to secrete Yops even in the presence of calcium (calcium-blind phenotype) (56). LcrG binds another Yersinia regulatory protein, LcrV, which is also encoded by the lcrGVHyopBD operon (4, 40). LcrG has been reported to be essential for the injection of effector Yops, since mutant Y. enterocolitica lacking a functional lcrG gene failed to inject YopE130-CyA as well as other reporter proteins into tissue culture cells (52). Fractionation experiments of bacterial cultures suggested that LcrG is located intracellularly and is at least partly associated with the membrane envelope (39, 40). In addition, Y. pestis has been reported to secrete small amounts of LcrG in the extracellular medium, and some LcrG is thought to be associated with the surface of Y. enterocolitica (7, 56). LcrV is secreted by the Yersinia type III pathway (33, 39, 40). Yersiniae carrying knockout mutations in lcrV did not display defects in calcium regulation since Yop secretion occurred only in the absence and not in the presence of calcium (33, 44, 47, 52, 55). Following induction of the type III pathway by temperature shift and calcium chelation, lcrV mutants express significantly less Yop proteins than wild-type yersiniae (33, 55). Overexpression of LcrV in wild-type Yersinia strains caused a calcium-blind phenotype similar to that observed for lcrG mutant strains (33, 39).
Immunofluorescence microscopy experiments suggested that LcrV may aggregate on the surface of yersiniae that have been induced for type III secretion (16, 44). It is not yet clear whether these aggregates represent the ordered assembly of a filamentous structure composed of LcrV or whether the immunofluorescent signal is caused by LcrV molecules that are secreted by the type III pathway. Antiserum or monoclonal antibody raised against purified LcrV has been reported to disrupt the type III targeting of effector Yops by Y. pseudotuberculosis into eukaryotic cells (44). However, the addition of anti-LcrV during tissue culture infection with Y. pestis showed no inhibition of type III targeting (16). Using an osmotic disruption of eukaryotic cells and cell fractionation, Straley and coworkers suggested that some LcrV molecules are injected into the cytosol of HeLa cells via a pathway that does not require type III genes (16, 17). The location of LcrV was also investigated by fractionating infected HeLa tissue cultures with the digitonin technique (see below) (33). Some LcrV was found to be secreted into the extracellular medium, but LcrV could not be detected in the cytosol of HeLa cells.
Several models for the function of LcrG and LcrV during infection have been proposed. Straley and coworkers advanced a model whereby LcrG may regulate the activity of the type III machinery by interacting directly with the membrane-embedded secretion apparatus (39). Further, the regulatory function of LcrG could be controlled by its binding to LcrV (titration hypothesis) (39). Wolf-Watz and coworkers proposed that secreted LcrV protein is involved in the assembly or function of an “injectisome” complex that allows translocation of effector Yops beyond the plasma membrane of eukaryotic cells (44). Cornelis and coworkers observed binding of LcrG to proteoglycans on the surface of tissue culture cells (7). Treatment with protease, heparinase, or xyloside, an inhibitor of glycosaminoglycan decorations of eukaryotic surface proteins, reduced LcrG binding to tissue culture cells (7). Further, binding of LcrG, as well as Yersinia injection of effector Yops into tissue culture, was competitively inhibited by the addition of heparin, dextran sulfate, and chondroitin sulfate B (7). Cornelis and colleagues proposed that LcrG functions as a bacterial receptor for eukaryotic cells (6, 7).
We wish to understand which Yersinia genes are needed for type III secretion and type III targeting (Yop translocation and Yop injection are commonly used terms for the same reaction). To analyze the function of LcrG and LcrV on type III targeting of effector Yops, we have generated lcrG, lcrV, and lcrGV mutant strains. As previously reported (55), lcrG and lcrGV mutant yersiniae displayed a calcium-blind phenotype for Yop secretion during growth in artificial medium, whereas the lcrV strain showed calcium regulation of Yop secretion. In contrast to previous reports (53), fractionation of infected HeLa tissue cultures with the digitonin technique suggests that lcrG mutants display a Los phenotype and are capable of injecting effector Yops into HeLa cells. Tissue culture infections with the lcrV mutant result in a Not phenotype (33). The lcrGV mutant displayed a Los phenotype similar to the lcrG mutant strain, suggesting that the role of lcrG in type III targeting is epistatic over that of lcrV. Biochemical studies suggest that LcrG is required for the type III secretion of LcrV. LcrG, as well as LcrG-LcrV complexes, was observed to be soluble in the bacterial cytoplasm. Membrane-associated LcrG-LcrV, bacterial-surface-exposed LcrG, or secreted LcrG could not be detected. These results support the previously proposed model that LcrG and LcrV function as regulators of the type III targeting pathway in the bacterial cytoplasm (39). The repression of type III targeting that is mediated by LcrG appears to be regulated by LcrG binding to LcrV. The Not phenotype of lcrV yersiniae may be caused by LcrG-mediated repression of the type III pathway rather than by a defect in targeting, i.e., the transport of effector Yops across the eukaryotic plasma membrane.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Y. enterocolitica strains W22703 (wild type) (12), CT1 (ΔlcrV) (33), VTL1 (ΔyopN) (30), and KUM1 (ΔyscV) (8) have been described elsewhere. Y. enterocolitica strains MC2 (ΔlcrG), NIA6 (ΔyscD), and CT130 (ΔyscN) were constructed by allelic exchange using the suicide plasmid pLC28 (8). The lcrG mutation is comprised of a stop codon, an EcoRI site, and a single nucleotide frameshift (TGAATTCA) inserted following codon 9 of lcrG. ΔlcrG was constructed from two PCR products amplified with the primers LcrG-Sac (5′-AAGAGCTCAGAAGTACAAAGAATCGTTCC-3′) and LcrG-EcoRI-1 (5′-AAGAATTCACTACATATTCATCAAAATGGGAAGAT-3′), as well as LcrG-EcoRI-2 (5′-AAGAATTCACAAAACGCTTAAACAGGCAG-3′) and LcrG-PstI (5′-AACTGCAGTATCGAGACTATTTTTTTTTC-3′). The PCR products were cut with SacI-EcoRI or EcoRI-PstI, fused at the EcoRI site, and cloned between the SacI-PstI sites of pLC28. The yscD mutation is comprised of a stop codon, a BamHI site, and a single nucleotide frameshift inserted following codon 9 of yscD. ΔyscD was constructed from two PCR products amplified with the primers Ysc07-Xba (5′-AATCTAGATGACTACCTAATAGCAAGAGT-3′) and Ysc09-Bam1 (5′-AAGGATCCTCACCAACTCACAATACGC-3′), as well as Ysc08-Xho (5′-AACTCGAGGTGTCATCGAGGTTTACCTC-3′) and Ysc10-Bam2 (5′-AAGGTACCTCTGTCGTTTTTATCAAGGGA-3′). The PCR products were cut with XbaI-BamHI or BamHI-XhoI, fused at the BamHI site, and cloned between the XbaI-XhoI sites of pLC28. The yscN mutant allele was designed to replace codons 51 to 390 of yscN with a unique BglII site (AGA TCT). ΔyscN was constructed from two PCR products with the primers YscN-delta-XbaI (5′-AATCTAGACACTCCAGATCGCATTAATCC-3′) and YscN-delta-BglII (5′-AAAGATCTTAAGTAACATAACTCACCGATGC-3′), as well as BglII-YscN-delta (5′-AAAGATCTCAAATCGGGGAGTACCAGAA-3′), and YscN-SalI (5′-AAGTCGACATAATACTCTCTACGCGGTC-3′). The PCR products were cut with XbaI-BglII or BglII-SalI, fused at the BglII site, and cloned between the XbaI-SalI sites of pLC28. The ΔlcrGV mutation was designed to replace codons 10 to 96 of lcrG and codons 1 to 141 of lcrV with a unique EcoRI site (GAATTC). ΔlcrGV was constructed from two PCR products amplified with the primers LcrG-SacI (5′-AAGAGCTCAGAAGTACAAAGAATCGTTCC-3′) and LcrG-EcoRI-1 (5′-AAGAATTCACTACATATTCATCAAAATGGGAAGAT-3′), as well as LcrV-EcoRI (5′-AAGAATTCAATGAATCATCATGGTGATGAA-3′) and LcrV-PstI (5′-AACTGCAGTGAGTGTCTGTCGTCTCTTG-3′). The PCR products were cut with SacI-EcoRI or EcoRI-PstI, fused at the EcoRI site, and cloned between the SacI-PstI sites of pLC28.
Allelic exchange following mating between Escherichia coli S17-1(pLC28) (15), and Y. enterocolitica W22703 has been previously described (8). The lcrG open reading frame was PCR amplified with two primers carrying abutted NdeI and BamHI restriction sites: LcrG-Nde (5′-AACATATGAAATCTTCCCATTTTGATGA-3′) and LcrG-Bam (5′-AAGGATCCTTAAATAATTTGCCCTCGCATCA-3′). The PCR product was digested with NdeI/BamHI and cloned between the NdeI and BamHI sites of the low-copy-number plasmid pDA255 (3) to generate pCT60. pVL48 was generated by inserting a PCR fragment, amplified with the primers LcrG-Kpn (5′-AAGGTACGAAATCTTCCCATTTTGATGA-3′) and LcrG-Bam between the KpnI and BamHI sites of pDA255 (3). Expression of lcrG and gst-lcrG is under the control of the tac promoter. The lacIq allele is also cloned on the low-copy-number vector, and Y. enterocolitica transformants were induced to express lcrG and gst-lcrG by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Other plasmids used in this study are listed in Table 1.
TABLE 1.
Plasmids used in this work
| Plasmid | Encoded protein (promoter)a | Vector | Antibiotic resistanceb | Source or reference |
|---|---|---|---|---|
| pKD2 | LcrV-DHFRHis6 (T7)* | pET9a | Km | This work |
| pKD3 | LcrV1–200-DHFRHis6 (T7)* | pET9a | Km | This work |
| pKD4 | LcrV1–100-DHFRHis6 (T7)* | pET9a | Km | This work |
| pKD5 | LcrV1–50-DHFRHis6 (T7)* | pET9a | Km | This work |
| pKD6 | LcrV1–15-DHFRHis6 (T7)* | pET9a | Km | This work |
| pKD7 | LcrVΔ2–14-DHFRHis6 (T7)* | pET9a | Km | This work |
| pKD8 | LcrV-NPT (lcrG)* | pHSG576 | Cm | This work |
| pKD14 | LcrV-GST (sycE)* | pHSG576 | Cm | This work |
| pKD15 | FLAG-LcrV (tac)* | pHSG576 | Cm | This work |
| pKD16 | GST-LcrV201–326 (tac)* | pHSG576 | Cm | This work |
| pKD17 | GST-LcrV201–274 (tac)* | pHSG576 | Cm | This work |
| pKD18 | GST-LcrV275–326 (tac)* | pHSG576 | Cm | This work |
| pKD19 | GST-LcrV101–326 (tac)* | pHSG576 | Cm | This work |
| pKD20 | GST-LcrV101–274 (tac)* | pHSG576 | Cm | This work |
| pKD21 | GST-LcrV151–274 (tac)* | pHSG576 | Cm | This work |
| pKD22 | GST-LcrV51–326 (tac)* | pHSG576 | Cm | This work |
| pKD23 | GST-LcrV51–274 (tac)* | pHSG576 | Cm | This work |
| pKD24 | GST-LcrV151–326 (tac)* | pHSG576 | Cm | This work |
| pCT60 | LcrG (tac) | pHSG576 | Cm | This work |
| pVL48 | GST-LcrG (tac) | pHSG576 | Cm | This work |
| pGEX-2TK | GST (tac) | pHSG576 | Cm | 29 |
| pVL47 | GST-LcrV (tac) | pHSG576 | Cm | 33 |
| pVL69 | LcrV-Flag (tac) | pHSG576 | Cm | This work |
| pLC15 | YopQ-DHFRHis6 (T7) | pET9a | Km | 8 |
| pHSG576 | Low-copy cloning vector | Cm | 59 | |
| pET9a | T7 polymerase expression | Km | 58 |
For entries indicated with an asterisk, the primer sequences can be obtained from the authors upon request.
Km, resistance to 50 μg of kanamycin per ml; Cm, resistance to 20 μg of chloramphenicol per ml.
Secretion and targeting assays.
Measurements for the secretion of Yop proteins during bacterial growth in tryptic soy broth (TSB) supplemented with either 5 mM calcium or 5 mM EGTA have been previously described (8). Targeting of Yop proteins into the cytosol of HeLa cells was determined by fractionating tissue cultures by the digitonin technique (30). Briefly, overnight cultures of yersiniae were diluted 1:20 into 30 ml of fresh Luria broth and grown for 2 h at 26°C with shaking. Bacteria were sedimented at 8,000 × g for 10 min and suspended in phosphate-buffered saline (PBS). HeLa cells were grown to 80% confluence in 75-cm2 tissue culture flasks with Dulbecco modified Eagle medium (DMEM) and 10% fetal bovine serum. Prior to infection, cells were washed twice with PBS, covered with 10 ml of DMEM, and warmed to 37°C for 30 min. Aliquots of HeLa cells were counted, and each flask was infected with yersiniae at a multiplicity of infection of 10 and incubated for 3 h at 37°C with 5% CO2. Culture media were removed and centrifuged at 32,000 × g for 15 min to separate soluble proteins from nonadherent bacteria in the sediment. HeLa cells, as well as adherent bacteria, were scraped off the flasks into 10 ml of digitonin in PBS and placed on a rotary shaker for 20 min. Samples were centrifuged at 32,500 × g for 15 min. A 7-ml aliquot was withdrawn and precipitated with methanol-chloroform, while the remaining supernatant was discarded. The sediment was suspended in 10 ml of PBS, and a 7-ml aliquot was precipitated with methanol-chloroform. Protein precipitates were solubilized in sample buffer, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and analyzed by immunoblotting with specific antiserum. Immunoreactive species were quantified as chemiluminescent signals on X-ray film by using laser densitometry scanning.
Cell fractionations.
Overnight cultures of Y. enterocolitica W22703 were diluted 1:50 into 800 ml of fresh TSB media and grown for 2 h at 26°C and then for 3 h at 37°C in the presence or absence of calcium. Cells were harvested at 6,000 × g for 15 min and suspended in 10 ml of HEPES buffer (20 mM HEPES, 100 mM potassium acetate, 2 mM magnesium acetate, 1 mM dithiothreitol [DTT]; pH 7.5). Bacteria were broken in a French pressure cell at 14,000 lb/in2, and intact cells were removed by centrifugation at 6,000 × g for 10 min. A 3-ml aliquot of crude bacterial extract was removed with the supernatant and subjected to ultracentrifugation at 100,000 × g for 30 min. The supernatant (S) was removed, and the membrane pellet was suspended in 3 ml of HEPES buffer (P). At each fractionation step, aliquots were withdrawn and mixed with an equal volume of sample buffer. Samples were separated on by SDS–15% PAGE and analyzed by immunoblotting.
Protease protection.
Overnight cultures of Y. enterocolitica W22703 were diluted 1:50 into fresh TSB media and grown for 2 h at 26°C and for 3 h at 37°C in the presence of 5 mM calcium or 5 mM EGTA. Four 1-ml aliquots of cultures were incubated with or without 20 μg proteinase K per ml, 1% SDS, or 1 mM phenylmethylsulfonyl fluoride (PMSF) and incubated at 37°C for 30 min. Proteolysis was quenched by the addition of 1 mM PMSF to all reactions. Proteinase K and SDS were added so that each sample contained the same reagents. Proteins were precipitated with chloroform-methanol, dried, solubilized in 100 μl of equal parts buffer B (6 M urea, 0.1 M NaH2PO4, 0.01 M Tris-HCl; pH 8.0)/sample buffer, and analyzed by immunoblotting.
Purification of glutathione S-transferase (GST)–LcrG.
Overnight cultures of Y. enterocolitica MC2(pVL48) were diluted 1:50 into fresh TSB media supplemented with 20 μg of chloramphenicol per ml. Bacteria were grown and induced by incubation for 2 h at 26°C and for 3 h at 37°C. Cells from 500 ml of culture were harvested by centrifugation at 6,000 × g for 15 min. The cell pellet was suspended in 10 ml of F buffer (50 mM Tris-HCl, 20% sucrose, 1 mM DTT; pH 7.0), and bacteria were broken by a single passage through a French pressure cell at 14,000 lb/in2. Unbroken cells and debris were removed by centrifugation at 32,500 × g for 15 min. The supernatant was subjected to affinity chromatography on glutathione-Sepharose preequilibrated with F buffer. The column was washed with 30 volumes of wash buffer (50 mM Tris-HCl, 150 mM NaCl; pH 7.5), and proteins were eluted with 4 ml of wash buffer containing 10 mM glutathione. Eluted proteins were mixed with an equal volume of sample buffer containing 3 M urea and analyzed by immunoblotting. To determine the enrichment of proteins during the purification procedure, equal volumes of load and eluate fractions were subjected to immunoblotting and chemiluminescent signals quantified using an AlphaScanner instrument.
Protein-binding assays.
Full-length and truncated sequences of LcrV were fused to either the C terminus of GST or the N terminus of His6-tagged dihydrofolate reductase (DHFRHis6). LcrV sequences for GST fusions were PCR amplified with appended 5′-KpnI and 3′-BamHI sites. The resulting fragments were subcloned into pCR2.1 (Invitrogen) and digested with KpnI and BamHI. Fragments were inserted into a previously described derivative of pHSG575 (59). For DHFRHis6 proteins, LcrV sequences were PCR amplified with appended 5′-NdeI and 3′-KpnI sites. Resulting fragments were inserted into a previously described plasmid (8). To create an insert for the first 15 codons, appropriate primers were annealed and directly inserted into the expression vector containing DHFRHis6. Fusion proteins were expressed and affinity purified from the E. coli cytoplasm. Fusion proteins were separated by SDS–15% PAGE, in duplicate. One SDS-PAGE gel was Coomassie brilliant blue stained. The second SDS-PAGE gel was electroblotted onto polyvinylidene difluoride (PVDF) membrane and incubated with purified 32P-labeled GST-LcrG. The filter membranes were then analyzed on a PhosphorImager instrument for radioactive signals, which are reported as arbitrary units per picomoles. Glutathione-Sepharose beads containing immobilized GST-LcrG were used for 32P-labeling with 3′-5′-cyclic AMP (cAMP)-dependent heart muscle protein kinase (Sigma). GST-LcrG (2.5 to 5 μg immobilized on 50 μl of 50% slurry of glutathione-Sepharose) in 100 μl of 20 mM Tris-HCl–100 mM NaCl–12 mM MgCl2–1 mM DTT–0.1 mM cAMP was incubated with 100 to 500 μCi of [γ-32P] ATP (800 μCi mmol−1) and 100 U of kinase (100 U kinase = 1.4 μg of kinase in 20 mM Tris-HCl–100 mM NaCl–12 mM MgCl2–1 mM DTT–20% [vol/vol] glycerol). After optimal labeling, typically for 1 h at 37°C, the beads were washed three times with 1 ml of 20 mM Tris-HCl–100 mM NaCl–20% glycerol and 32P-labeled GST-LcrG was eluted with 50 μl of 20 mM Tris-HCl–100 mM NaCl–20% glycerol–10 mM glutathione. Aliquots were analyzed by scintillation counting and SDS-PAGE–autoradiography to determine the specific activity.
RESULTS
lcrG mutants are calcium blind and fail to secrete LcrV.
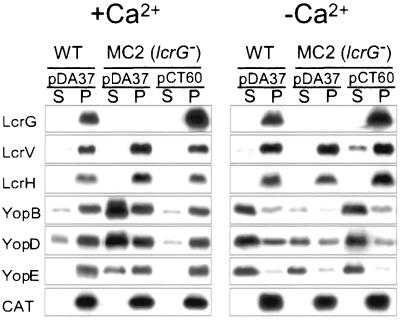
The lcrG mutant strain Y. enterocolitica MC2 was generated by inserting a stop codon and frameshift mutation after codon 9 of the Y. enterocolitica W22703 (wild-type) lcrG gene. Wild-type and lcrG mutant yersiniae were grown in TSB at 37°C in the presence of 5 mM calcium (Fig. 1, +Ca2+) or 5 mM EGTA (−Ca2+). Cultures were centrifuged, and the extracellular medium was separated from the bacterial pellet. Proteins in both fractions were precipitated with trichloroacetic acid (TCA), separated by SDS-PAGE, and analyzed by immunoblotting (Fig. 1). When induced by the chelation of calcium, the type III machines of wild-type yersiniae secreted YopBDE into the extracellular medium. In the presence of calcium, yersiniae secreted only small amounts of YopBDE via the type III pathway (11% YopB, 16% YopD, and 3% YopE). In contrast, the lcrG mutant strain MC2 exported 73% YopB, 58% YopD, and 53% YopE (percentage of the total amount of Yop protein in the supernatant and sediment) in the presence of calcium. This result corroborates previous observations that lcrG mutations abolish the calcium regulation of the Yersinia type III pathway (calcium-blind phenotype) (56). Wild-type yersiniae secreted 8% of LcrV in the presence and 23% of LcrV in the absence of calcium. lcrG mutant yersiniae failed to secrete LcrV in either the presence or the absence of calcium. Following centrifugation of Yersinia cultures at 25,000 × g, LcrG sedimented with the bacteria into the pellet fraction, suggesting that LcrG is not secreted by the type III pathway. lcrGVH and yopBD form a polycistronic operon that is expressed at a low level when calcium is added to the culture medium (4) (Fig. 1). The lcrG mutant strain MC2 expressed the lcrGVHyopBD operon at a higher level than for the wild-type yersiniae. Chloramphenicol acetyltransferase (CAT) served as a control for proper fractionation of a cytoplasmic protein. Transformation of Y. enterocolitica MC2 with pCT60, encoding wild-type lcrG, reduced the calcium-blind phenotype, as well as defects in LcrV secretion and lcrGVH and yopBD regulation.
FIG. 1.
lcrG mutant yersiniae secrete Yops in the presence of calcium. Y. enterocolitica W22703 (wild type) and the isogenic lcrG mutant, strain MC2, were transformed with pDA37 (vector control) or pCT60 (wild-type lcrG) and grown in the presence of 5 mM calcium or 5 mM EGTA for 2 h at 37°C. Cultures were centrifuged, and the supernatant (S) was separated from the cell pellet (P). Proteins in each sample were precipitated with TCA, solubilized in sample buffer, and analyzed by SDS-PAGE, followed by immunoblotting with antisera raised against LcrG, LcrV, LcrH, YopB, YopD, YopE, and CAT. Y. enterocolitica W22703 secretes more YopBDE in the absence than in the presence of calcium. In contrast, the lcrG mutant strain displays a calcium-blind phenotype and secretes YopBDE in the presence or absence of calcium. Further, the lcrG mutant failed to secrete LcrV. Transformation of lcrG mutant cells with pCT60 restored the wild-type phenotype.
Upon fractionation of Yersinia cultures, Skrzypek et al. as well as Nilles et al., found that Y. pestis secretes significant amounts of LcrG into the extracellular medium (40, 55, 56). Skrzypek et al. reported that both in the absence and in the presence of 2.5 mM Ca2+ lcrG mutant Y. pestis strains secrete LcrV into the extracellular medium (56). We do not know the reason for the discrepancy of these data with our results.
lcrG mutants display a Los phenotype during the infection of HeLa cells.
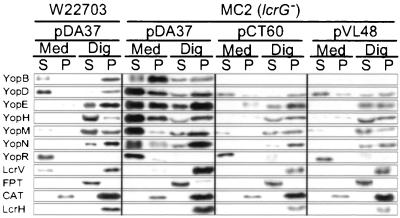
To examine the role of lcrG in type III targeting, HeLa cells were infected with wild-type Y. enterocolitica W22703 or the lcrG mutant strain MC2. The medium was decanted and centrifuged, separating nonadherent bacteria (P; pellet) from the extracellular medium (S; supernatant). HeLa cells with adherent yersiniae were extracted with digitonin, a detergent known to disrupt the cholesterol-containing plasma membrane of HeLa cells but not the bacterial envelope (30). Digitonin extracts were centrifuged to sediment bacteria, as well as HeLa cell debris (P), while the soluble contents of the eukaryotic cytosol remained in the supernatant (S). Proteins were precipitated with chloroform-methanol and analyzed by SDS-PAGE and immunoblotting (Fig. 2). As a control, digitonin extraction released farnesyl protein transferase (FPT) from the eukaryotic cytosol. LcrH and CAT, proteins that are located in the Yersinia cytoplasm (10), were not released and sedimented with the bacteria. Wild-type Yersinia targeted YopEHMN into the cytosol of HeLa cells and secreted YopBDR and small amounts of LcrV into the extracellular medium (Fig. 2) (30). The lcrG mutant appears to inject small amounts of YopBDEHMN into the cytosol of HeLa cells. Moreover, lcrG mutant yersiniae secreted large amounts of YopBDEHMNR into the extracellular medium, suggesting a Los phenotype (Fig. 2). The lcrG mutant strain overexpressed proteins associated with the type III pathway and failed to secrete LcrV into the extracellular medium. Transformation of strain MC2 with plasmids pCT60 or pVL48, encoding wild-type LcrG or GST-LcrG, respectively, restored the type III targeting specificity of effector Yops. The two- to threefold overexpression of LcrG or GST-LcrG from the IPTG-inducible tac promoter (see Fig. 1) reduced the expression of the Yersinia yop virulon and reduced the synthesis of YopB. The reduced amount of YopB did not interfere with the type III targeting of YopEHMN. This finding is consistent with the earlier report that YopB may be dispensable for the injection of effector Yops into eukaryotic cells (32). Our results differ from those of Sarker et al., who observed no type III targeting for lcrG mutant Y. enterocolitica using the Cya fusion approach (53). lcrG mutants generated 0.24 ± 0.21 nmol of cAMP per mg protein using YopE130-Cya as a reporter. yscN mutants (type III mutant control) generated 0.36 nmol of cAMP, while wild-type yersiniae generated 5.7 nmol of cAMP. We do not know the reason for the discrepancy of these data with our results.
FIG. 2.
lcrG mutant yersiniae display a Los phenotype and secrete effector Yops into the extracellular medium. HeLa cells were infected with Y. enterocolitica W22703 or MC2 (ΔlcrG) harboring plasmids pDA37 (vector control), pCT60 (lcrG), or pVL48 (gst-lcrG). After incubation for 3 h at 37°C, the tissue culture medium (Med) was decanted and centrifuged to separate secreted proteins from those present within nonadherent bacteria. HeLa cells, as well as adherent yersiniae, were extracted with digitonin (Dig), a detergent that solubilizes the eukaryotic plasma membrane but not the bacterial envelope. Extracts were centrifuged to separate proteins solubilized from the HeLa cytoplasm from those that sediment with the bacteria. Proteins were precipitated with chloroform-methanol and analyzed by immunoblotting. Y. enterocolitica MC2 displayed a loss of targeting specificity (Los) and secreted large amounts of YopB, YopD, YopE, YopH, YopM, and YopN into the culture medium. The Los phenotype was complemented by transforming MC2 cells with either pCT60 or pVL48. As a control for proper fractionation, FPT is located in the cytosol of HeLa cells and is solubilized by digitonin extraction. LcrH and CAT reside in the bacterial cytoplasm and are not solubilized by digitonin extraction.
lcrGV mutants display a Los phenotype during the infection of HeLa cells.
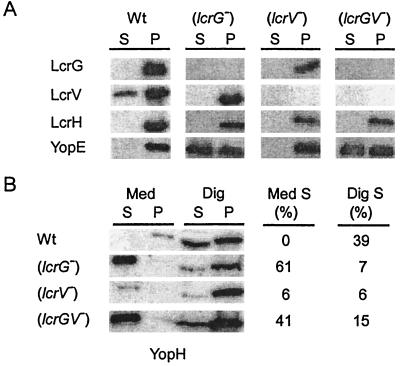
The lcrGV mutant strain KLD1 was generated by replacing lcrG codons 10 to 96 and lcrV codons 1 to 141 with an EcoRI site. The double-mutant strain was grown in TSB at 37°C in the presence of 5 mM calcium, and type III secretion was analyzed by immunoblotting (Fig. 3A). As expected, Y. enterocolitica KLD1 (ΔlcrGV) did not express LcrG and LcrV, while strains MC2 (ΔlcrG) and CT1 (ΔlcrV) displayed the expected defects in expression of LcrG and LcrV, respectively. Further, the lcrGV mutant strain secreted YopE (and several other Yops) in the presence of calcium, a result consistent with the previously reported calcium-blind phenotype of lcrGV double-mutant strains (55). Transformation of KLD1 with plasmids expressing either LcrG or LcrV caused the mutant yersiniae to display the same phenotype as lcrV or lcrG single-mutant strains, respectively (data not shown). During the infection of HeLa cells, the lcrV mutant strain CT1 expressed fewer effector Yops than wild-type or lcrG mutant yersiniae (33). Strain CT1 injected reduced amounts of YopH into the eukaryotic cytosol and secreted only small amounts of YopH into the extracellular medium (33) (Fig. 3B). Infection of HeLa cells with the lcrGV mutant strain KLD1 revealed an increase in YopH expression, an increase in YopH secretion into the extracellular medium, and an increase in YopH injection into the cytosol of eukaryotic cells. Together, these data suggest that the lcrGV mutant strain displays a Los phenotype. Further, LcrV may not be required for the injection of effector Yops into HeLa cells, and the Not phenotype of lcrV mutant strains can be explained as the LcrG-mediated repression of the yop virulon.
FIG. 3.
lcrGV mutant yersiniae display a calcium-blind and Los phenotype. (A) Y. enterocolitica W22703 (Wt) and the isogenic mutant strains MC2 (lcrG), CT1 (lcrV), and KLD1 (lcrGV) were grown in the presence of calcium for 2 h at 37°C. Cultures were centrifuged, and the supernatant (S) was separated from the cell pellet (P). Protein in each sample was precipitated with TCA, solubilized in sample buffer, and analyzed by SDS-PAGE, followed by immunoblotting with antisera raised against LcrG, LcrV, LcrH, and YopE. The percentage of secreted YopE is indicated. (B) HeLa cells were infected with Y. enterocolitica strains MC2 (lcrG), CT1 (lcrV), and KLD1 (lcrGV) and incubated for 3 h at 37°C. The tissue culture medium (Med) was decanted and centrifuged to separate secreted proteins from those present within nonadherent bacteria. HeLa cells, as well as adherent yersiniae, were extracted with digitonin (Dig), a detergent that solubilizes the eukaryotic plasma membrane but not the bacterial envelope. Extracts were centrifuged to separate proteins solubilized from the HeLa cytoplasm from those that sediment with the bacteria. Proteins were precipitated with chloroform-methanol and analyzed by immunoblotting with antiserum raised against purified YopH. The percentage of YopH secreted into the extracellular medium (Med S) or targeted into the cytosol of HeLa cells (Dig S) is indicated.
LcrG binds LcrV in the cytoplasm of yersiniae.
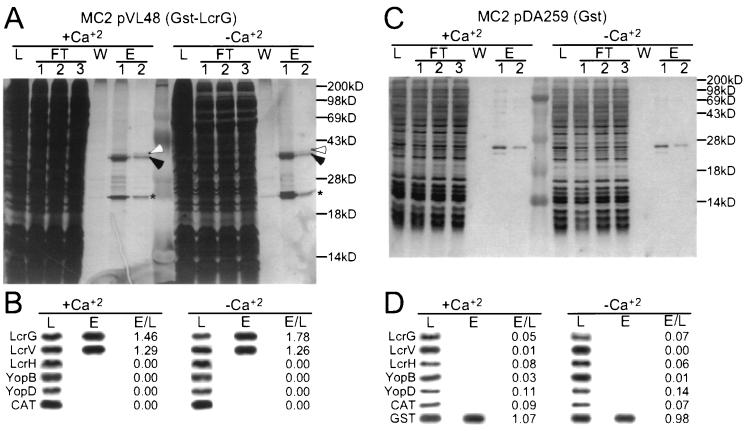
Plasmid pVL48 encodes a fusion between GST and LcrG. Expression of GST-LcrG is controlled by the IPTG-inducible tac promoter. Transformation of pVL48 into Y. enterocolitica MC2 and induction with IPTG reversed the calcium-blind phenotype, as well as defects in LcrV secretion and lcrGVH and yopBD repression (Fig. 1). This result suggests that GST-LcrG may function similar to wild-type LcrG in complementing the defects of the lcrG mutant strain. Y. enterocolitica MC2(pVL48) was grown in the presence or absence of calcium. The bacteria were harvested by centrifugation and disrupted in a French pressure cell. Unbroken cells and insoluble material were removed by centrifugation, and the supernatant was subjected to affinity chromatography on glutathione-Sepharose. Collected fractions were analyzed on silver-stained SDS-PAGE gels (Fig. 4A). To analyze the collected fractions further, samples were subjected to immunoblotting (Fig. 4B). Purification and enrichment of Yersinia proteins was monitored by measuring immunoreactive signals in the eluate (E) and dividing them by those present in crude extracts (L; lysate). An E/L quotient 1.46 was observed for GST-LcrG, indicating enrichment of this polypeptide during affinity chromatography (E/L = 1.78 when cells were grown in the absence of calcium). The E/L quotient of 1.29 (1.26) revealed that LcrV had also been enriched and copurified with GST-LcrG. In contrast, YopB and YopD, proteins that have been proposed to interact with LcrV (54), failed to bind GST-LcrG or -LcrV (E/L = 0). CAT did not copurify with GST-LcrG/LcrV. As a control for the specific binding of LcrV to GST-LcrG, Yersinia extracts were subjected to affinity chromatography on glutathione-Sepharose containing bound GST. LcrG, LcrV, LcrH, YopB, and YopD did not bind to GST, suggesting that the observed coelution of GST-LcrG and LcrV is caused by the direct interaction between LcrV and LcrG.
FIG. 4.
GST-LcrG binds to LcrV. (A) Y. enterocolitica MC2 (lcrG)/pVL48 (gst-lcrG) was grown at 37°C and induced for type III secretion by the chelation of calcium ions. Expression of GST-LcrG was induced by the addition of 1 mM 1PTG to the culture medium. Cells (1012 CFU) were harvested by centrifugation and lysed in a French pressure cell, insoluble material was removed by centrifugation at 100,000 × g, and the supernatant (L, load) was subjected to affinity chromatography on glutathione-Sepharose. Flowthrough (FT), wash (W), and eluate (E) fractions of 1 ml were collected and analyzed by silver-stained SDS-PAGE. Arrowheads indicate the positions of GST-LcrG (Black) and LcrV (white). The asterisk indicates the migration of an unknown Yersinia protein that binds to glutathione-Sepharose. (B) The load (L) and eluate (E; fraction 1) of the affinity chromatography analyses were analyzed by immunoblotting with antisera (LcrG, LcrV, LcrH, YopB, YopD, and CAT) and quantified. Results are reported as the ratio of signal intensity observed for the eluate fraction divided by that of the load fraction (E/L). The migration of molecular mass markers (M) is indicated in kilodaltons. The positions of GST-LcrG (filled arrowhead) and LcrV (open arrowhead) are indicated. As a control for the specific binding of LcrV to GST-LcrG, Y. enterocolitica expressing GST alone was subjected to affinity chromatography and analyzed by silver-stained SDS-PAGE (D) and immunoblotting (C).
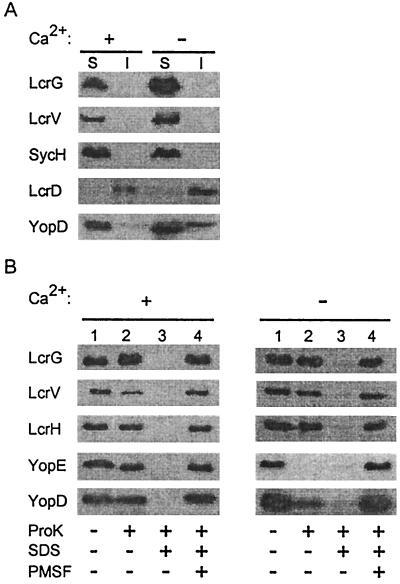
To examine the subcellular location of LcrG, wild-type yersiniae were grown in the presence of 5 mM calcium or 5 mM EGTA, harvested by centrifugation, and lysed in a French pressure cell. Unbroken cells were removed by slow-speed centrifugation, and bacterial extracts were analyzed by ultracentrifugation, separating soluble cytoplasmic proteins from the insoluble membrane envelope in the sediment. Proteins in both fractions were precipitated with TCA and analyzed by immunoblotting (Fig. 5A). Cytoplasmic SycH chaperone remained in the supernatant (64), whereas almost all of the inner membrane protein LcrD (YscV) (46) sedimented into the pellet. Both LcrG and LcrV remained in the supernatant, suggesting that these polypeptides do not associate with the membrane envelope of yersiniae. In contrast, 34% of the YopD sedimented into the pellet fraction during the centrifugation of extracts obtained from low-calcium-induced yersiniae but not during the centrifugation of extracts from uninduced bacteria.
FIG. 5.
LcrG is located intracellularly. (A) Cell fractionation of yersiniae. Y. enterocolitica W22703 (wild type) was grown in the presence of 5 mM calcium (+Ca2+) or 5 mM EGTA (−Ca2+) for 3 h at 37°C. Bacteria were lysed in a French pressure cell. Unbroken cells were removed by low-speed centrifugation, and crude extracts were subjected to ultracentrifugation at 100,000 × g. The supernatant (S), containing soluble cytoplasmic contents, was separated from the insoluble (membrane) sediment (I). Samples were analyzed by SDS-PAGE and immunoblotting with antibodies raised against LcrG, LcrV, LcrH, YopE, and YopD. (B) Protease protection assay. Y. enterocolitica W22703 was grown in the presence or absence of calcium. Four 6-ml culture aliquots (108 CFU/ml) were incubated at 37°C for 30 min with or without 20 μg of proteinase K per ml, 1% SDS, or 1 mM PMSF as indicated. Samples were precipitated with chloroform-methanol, dried, solubilized in sample buffer, and analyzed by SDS-PAGE and immunoblotting.
We sought to determine whether LcrG and LcrV are accessible to protease digestion on the surface of yersiniae. Y. enterocolitica W22703 was grown in the presence or absence of calcium and incubated with 20 μg of proteinase K/ml of culture. The addition of proteinase K to the extracellular medium of yersiniae grown in the presence of calcium digested some LcrV and small amounts of YopD, but proteinase K did not digest YopE, LcrG, and LcrH (Fig. 5B). The addition of proteinase K to the extracellular medium of yersiniae grown in the absence of calcium digested all or most of the YopE and YopD, as well as some of the LcrV. In contrast, LcrG and LcrH were not digested by extracellular proteinase K. The addition of SDS dissolved the membrane envelope of yersiniae, allowing access of protease and digestion of all proteins examined. Protease digestion was quenched when proteinase K was added together with PMSF, a known serine protease inhibitor. These results suggest that LcrG and LcrH are located intracellularly, protected from extracellular protease by the membrane envelope of yersiniae, whereas secreted LcrV, YopD, and YopE molecules are accessible to extracellular protease. As reported in Fig. 1, some LcrV is secreted and protease accessible when the yersiniae are grown in the presence of calcium.
LcrG and the type III machinery are required for the secretion of LcrV.
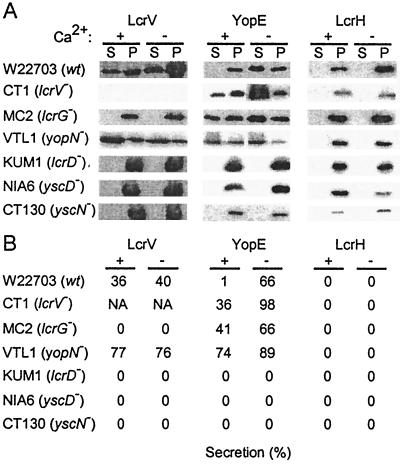
Previous work suggested that LcrV may be exported and injected into HeLa cells in a manner that does not depend the type III secretion machinery (16). Fractionation of Yersinia-infected HeLa cells revealed that small amounts of LcrV were secreted into the extracellular medium, while most immunoreactive species sedimented with the bacteria after digitonin extraction (33). To test whether LcrV secretion in the presence or absence of calcium required the type III pathway, we tested various mutant backgrounds. As expected, wild-type yersiniae secreted LcrV in the presence or absence of calcium, whereas YopE secretion occured only in the absence of calcium (Fig. 6). As a control, cytoplasmic LcrH was not secreted and sedimented with yersiniae into the pellet fraction. The yopN mutant strain VTL1 was defective in calcium regulation and secreted large amounts of YopE even in the presence of calcium. Secretion of YopE and LcrV was abolished in Yersinia mutants that failed to express the type III machinery components LcrD, YscD, and YscN. These results suggest that the secretion of LcrV requires LcrG, as well as expression of type III machinery components.
FIG. 6.
LcrV is secreted via the type III pathway. (A) Y. enterocolitica wild-type strain W22703 (wt) and isogenic mutant strains CT1 (lcrV), MC2 (lcrG), VTL1 (yopN), KUM1 (yscV), NIA6 (yscD), and CT130 (yscN) were grown in presence of 5 mM calcium (+Ca2+) or 5 mM EGTA (−Ca2+) at 37°C. Cultures were centrifuged, and the supernatant (S) was separated from the cell pellet (P). Protein in each sample was precipitated with TCA, solubilized in sample buffer, and analyzed by immunoblotting with antisera raised against LcrV, YopE, and LcrH. (B) Immunoblots were quantified, and the amount of secreted polypeptide is recorded as a percentage.
LcrG binds to the C-terminal region of LcrV.
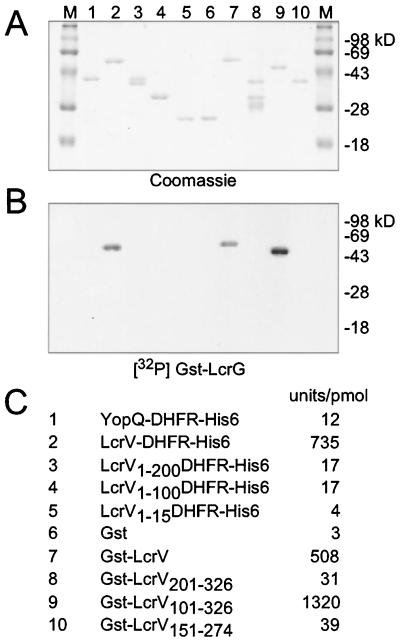
To identify the LcrV binding site of LcrG, GST and DHFRHis6 fusions to LcrV were expressed in E. coli and purified. After separation by SDS-PAGE and electrotransfer to a PVDF membrane, LcrV fusions were incubated with 32P-labeled GST-LcrG, and binding was quantified by use of a PhosphorImager (Fig. 7). Fusion of full-length LcrV (amino acids 1 to 326) to the N terminus of DHFR or the C terminus of GST did not interfere with the binding to GST-LcrG, suggesting that a free N or C terminus of LcrV is not required for binding. Truncating the C terminus of LcrV generated the hybrids LcrV1–200-, LcrV1–100-, LcrV1–50-, and LcrV1–15-DHFRHis6, all of which failed to bind GST-LcrG. In contrast, truncation at the N terminus of LcrV, deleting residues 1 to 100 (GST-LcrV101–326), did not interfere with the binding of GST-LcrG. Additional truncations at the N and C termini of GST-LcrV101–326 abolished binding. Thus, LcrV residues 100 to 326 are necessary and sufficient for the binding of LcrV to GST-LcrG.
FIG. 7.
Binding of Gst-LcrG to LcrV sequences. LcrV gene sequences were fused to the 3′-GST gene sequences or to the 5′ end of a gene encoding DHFRHis6. Recombinant proteins were purified, separated by SDS-PAGE, and electroblotted onto PVDF membrane in duplicate. One membrane was stained with Coomassie brilliant blue (A), whereas the other was incubated with 32P-labeled GST-LcrG. Binding was quantified on a PhosphorImager instrument (B) and is reported in arbitrary units per picomoles of LcrV fusion protein (C). The migration of molecular mass markers (M) is indicated in kilodaltons.
LcrV secretion signals.
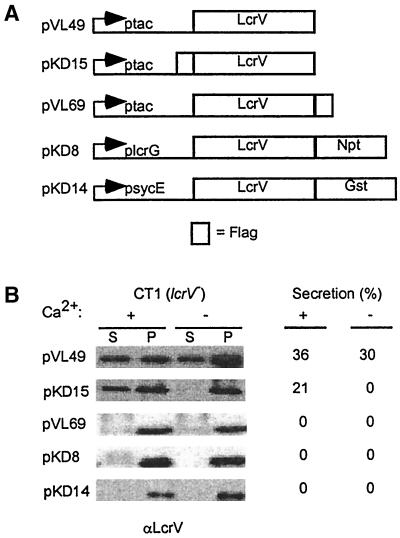
Fusion of LcrV to the N terminus of neomycin phosphotransferase (NPT) prevented type III secretion of the hybrid proteins (Fig. 8). Similar results were observed when LcrV was fused to the N terminus of GST. Appending amino acids (FLAG sequence) to the C terminus of LcrV also abrogated type III secretion of the epitope tagged protein. In contrast, when the FLAG sequence was appended to the N terminus of LcrV, 21% of the epitope-tagged protein was secreted in the presence of calcium, whereas no secretion occured in the absence of calcium. All truncations of the N- or C-terminal sequences abrogated LcrV secretion via the low-calcium-induced type III pathway (data not shown).
FIG. 8.
Fusion of reporter sequences to LcrV. (A) The coding sequences of the NPT gene (npt), GST (gst), or the FLAG epitope tag (empty box) were fused to coding sequences for either the N or the C terminus of LcrV. Recombinant genes were cloned on a low-copy-number plasmid and expressed from the IPTG-inducible promoter in Y. enterocolitica CT1 (lcrV). (B) Yersinia strains were grown in presence of 5 mM calcium (+Ca2+) or 5 mM EGTA (−Ca2+) for 3 h at 37°C. Cultures were centrifuged, and the supernatant (S) was separated from the cell pellet (P). Protein in each sample was precipitated with TCA, solubilized in sample buffer, and analyzed by SDS-PAGE and immunoblotting with antiserum raised against LcrV.
DISCUSSION
Straley and colleagues characterized LcrG as a regulator of the type III pathway, controlling the synthesis of Yops when yersiniae are grown in the presence of calcium (56). The same group also showed that the regulatory activity of LcrG is controlled via the binding to LcrV (39, 40). Since the amount of LcrV in the bacterial cytoplasm is dependent on the expression of the lcrGVHyopBD operon, as well as on the type III export of LcrV, LcrG may act as a sensor for the assembly and function of the type III pathway. The data reported here corroborate this model. LcrV secretion appears to require the presence of LcrG. Although it is tempting to speculate that LcrG binding to the C-terminal end of LcrV may allow initiation of the polypeptide into the type III pathway, none of our data provide definitive proof for this notion. Once LcrV is exported by the type III pathway, the liberated LcrG may bind to other LcrV molecules or, if intracellular LcrV concentrations drop below a certain threshold, unbound LcrG may somehow control the expression of yop genes. In this model LcrG and LcrV appear to function as a regulatory switch for the expression of Yops prior to the attachment of yersiniae to host cells and the formation of a type III injection device.
There are several unusual features in the recognition of LcrV secretion substrate. (i) LcrV secretion via the type III pathway is not tightly regulated by calcium as is observed for YopE. Consistent with this notion is the observation that yersiniae secrete LcrV into the extracellular medium during tissue culture infections, i.e., when the concentration of extracellular calcium is 1.8 mM (33). (ii) Unlike typical secretion chaperones (SycE and SycH) (64), LcrG binds to the C-terminal domain of the secretion substrate (53; also, our results). (iii) LcrV does not harbor the secretion signals that have been identified in all other Yops examined thus far (2, 3, 8, 34, 54, 57). (iv) In contrast to other Yops, LcrV signal recognition does not tolerate C-terminal fusions (16, 44; also, our results); however, some insertions at the N-terminal end are permitted.
Straley and coworkers proposed that LcrG may physically block the type III pathway (16). In this scenario, one could expect LcrG to somehow be associated with the type III machinery or perhaps to sediment with membranes. A membrane association could not be observed in the fractionation experiments reported here (Fig. 5A). We do not know the mechanism of LcrG-mediated regulation. Since LcrG appears to be a soluble protein, it is conceivable that LcrG may regulate gene expression directly, for example, at the level of transcriptional or posttranscriptional control. We believe that LcrG binding to surface proteoglycans of eukaryotic cells (7) may not play a role during infection, since LcrG does not appear to be positioned on the surface of Y. enterocolitica W22703. LcrG binding to heparin can also be thought of as an affinity for negative charge, a phenomenon that is often observed for nucleotide-binding proteins.
It is reported here that lcrG mutant Yersinia are capable of injecting effector Yops into HeLa cells but are defective for the specificity of the type III targeting reaction (Los phenotype). In contrast to the Not phenotype of lcrV mutants (16, 33, 44), lcrGV double mutants display a Los phenotype, suggesting that LcrV may not be essential for the type III targeting mechanism. Earlier work reported that yopD mutants display a Not phenotype. However, this defect of yopD mutants can be complemented by gst-yopD fusions, encoding a hybrid protein that cannot be secreted by the type III pathway (32). The same study suggested also that yopB may not be essential for the type III targeting mechanism. Taken together with the results presented here, these observations lead us to assume that the protein products encoded by the lcrGVHyopBD operon are not directly involved in effector Yop translocation (type III targeting) (30a, 32, 33; D. M. Anderson, K. Ramamurthi, C. Tam, and O. Schneewind, unpublished results). This view is currently under debate. Some of the above mentioned results are controversial as other groups have observed yopB to be essential for effector Yop translocation (5, 21). Further, YopB, YopD, and presumably LcrV form a complex that inserts into lipid bilayers, generating a voltage-conducting pore (21, 60). Cornelis and Wolf-Watz advanced a model whereby YopB, YopD, and LcrV represent a translocation pore through which effector Yops must be transported into the cytosol of eukaryotic cells (13). If so, what is the mechanism that couples type III secretion and effector Yop translocation? Earlier work from the same group suggested that effector Yops may first be secreted by the type III machinery to subsequently engage the translocator complex in a second membrane transport step (54, 57). We have thought of the two-step model as an unlikely scenario given that extracellular transport intermediates (effector Yops) have not yet been detected (30, 51). As an alternative model, Lee et al. proposed that the type III secretion machinery may form a continuous protein conduit through which effector Yops travel (30). Hoiczyk and Blobel advanced a similar model as YscF-containing needle complexes of Y. enterocolitica appear responsible for the formation of a type III targeting conduit between bacteria and eukarytotic cells (24). yopD knockout mutations did not interfere with the formation of the YscF polymer or with the penetration of these needle structures into eukaryotic cells (24).
What might be the role of the lcrGVHyopBD-encoded protein products on type III targeting? Earlier work and this study advance a model whereby intrabacterial LcrG, LcrV, LcrH, and YopD exert a regulatory function for the type III targeting mechanism (4, 33, 39, 40, 55, 56, 63). Data presented here and elsewhere suggest that LcrG may act as a repressor of type III targeting and that this activity can be regulated by LcrG binding to LcrV (16, 33, 39, 40, 53). Further, LcrG is required for LcrV secretion via the type III pathway. YopB and YopD follow the same pathway, and LcrH (SycD) appears to function as a secretion chaperone for these polypeptides (61). The secretion of YopB, YopD, and LcrV may serve a regulatory function, priming Yersinia for the type III targeting reaction (35). Furthermore, secreted YopB-YopD-LcrV complexes could exert a pathogenic role, damaging the plasma membranes of host cells. It has been reported that yersiniae respond to three host signals with type III targeting: glutamate, serum proteins, and the intracellular calcium concentration of eukaryotic cells (Lee et al., unpublished). Knockout mutations in yopD, lcrH, and yscM1 and -2 abrogate the Yersinia requirement for the glutamate signal (Lee et al., unpublished). Knockout mutations in lcrG, but not in lcrV, promote effector Yop secretion in the presence but not in the absence of glutamate and serum protein signals (55; Lee et al., unpublished). These studies, together with the data reported here, lead us to assume that the lcrGVHyopBD operon may function as a regulator of the type III pathway. In the absence of specific host signals yopD and lcrH may control the synthesis of secretion substrates, presumably by binding to yop mRNA (Anderson et al., unpublished), while lcrG and lcrV control the activity of the type III machinery by a hitherto-unknown mechanism. We are well aware of the speculative nature of this proposal.
ACKNOWLEDGMENTS
We thank Mailin Chu for strain MC1, Christina Tam for pCT60 and CT1, and Nigha Truong for NIA6. We thank members of our laboratory for critical reading of the manuscript.
K.L.D. was supported by Microbial Pathogenesis Training Grant AI07323 from the Public Health Service to the Department of Microbiology and Immunology at UCLA School of Medicine. V.T.L. acknowledges fellowship support from the National Science Foundation and the Warsaw Family Foundation. This work was supported by U.S. Public Health Service grant AI42797 from the NIH-NIAID Infectious Diseases Branch to O.S.
REFERENCES
- 1.Allaoui A, Schulte R, Cornelis G R. Mutational analysis of the Yersinia enterocolitica virC operon: characterization of yscE, F, G, I, J, K required for Yop secretion and yscH encoding YopR. Mol Microbiol. 1995;18:343–355. doi: 10.1111/j.1365-2958.1995.mmi_18020343.x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D M, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- 3.Anderson D M, Schneewind O. Yersinia enterocolitica type III secretion: an mRNA signal that couples translation and secretion of YopQ. Mol Microbiol. 1999;31:1139–1148. doi: 10.1046/j.1365-2958.1999.01254.x. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann T, Hakansson S, Forsberg A, Norlander L, Macellaro A, Backman A, Bolin I, Wolf-Watz H. Analysis of V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of lcrH and lcrV. J Bacteriol. 1991;173:1607–1616. doi: 10.1128/jb.173.5.1607-1616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boland A, Sory M-P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5–1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd A P, Grosdent N, Totemeyer S, Geuijen C, Bleves S, Iriarte M, Lambermont I, Octave J-N, Cornelis G R. Yersinia enterocolitica can deliver Yop proteins into a wide range of cell types: development of a delivery system for heterologous proteins. Eur J Cell Biol. 2000;79:659–671. doi: 10.1078/0171-9335-00098. [DOI] [PubMed] [Google Scholar]
- 7.Boyd A P, Sory M-P, Iriarte M I, Cornelis G R. Heparin interferes with translocation of Yop proteins into HeLa cells and binds to LcrG, a regulatory component of the Yersinia Yop apparatus. Mol Microbiol. 1998;27:425–436. doi: 10.1046/j.1365-2958.1998.00691.x. [DOI] [PubMed] [Google Scholar]
- 8.Cheng L W, Anderson D M, Schneewind O. Two independent type III secretion mechanisms for YopE in Yersinia enterolitica. Mol Microbiol. 1997;24:757–765. doi: 10.1046/j.1365-2958.1997.3831750.x. [DOI] [PubMed] [Google Scholar]
- 9.Cheng L W, Schneewind O. Yersinia enterocolitica TyeA, an intracellular regulator of the type III machinery, is required for the specific targeting of YopT, YopH, YopM, and YopN into the cytosol of eukaryotic cells. J Bacteriol. 2000;182:3183–3190. doi: 10.1128/jb.182.11.3183-3190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng L W, Schneewind O. Yersinia enterocolitica type III secretion: on the role of SycE in targeting YopE into HeLa cells. J Biol Chem. 1999;274:22102–22108. doi: 10.1074/jbc.274.31.22102. [DOI] [PubMed] [Google Scholar]
- 11.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M-P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelis G R, Colson C. Restriction of DNA in Yersinia enterocolitica detected by the recipient ability for a derepressed R factor from Escherichia coli. J Gen Microbiol. 1975;87:285–291. doi: 10.1099/00221287-87-2-285. [DOI] [PubMed] [Google Scholar]
- 13.Cornelis G R, Wolf-Watz H. The Yersinia yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 14.Day J B, Plano G V. A complex composed of SycN and YscB functions as a specific chaperone for YopN in Yersinia pestis. Mol Microbiol. 1998;30:777–789. doi: 10.1046/j.1365-2958.1998.01110.x. [DOI] [PubMed] [Google Scholar]
- 15.de Lorenzo V, Eltis E, Kessler B, Timmis K N. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123:17–24. doi: 10.1016/0378-1119(93)90533-9. [DOI] [PubMed] [Google Scholar]
- 16.Fields K A, Nilles M L, Cowan C, Straley S C. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect Immun. 1999;67:5395–5408. doi: 10.1128/iai.67.10.5395-5408.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fields K A, Straley S C. LcrV of Yersinia pestis enters infected eukaryotic cells by a virulence plasmid-independent mechanism. Infect Immun. 1999;67:4801–4813. doi: 10.1128/iai.67.9.4801-4813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsberg A, Viitanen A-M, Skunik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 19.Goguen J D, Yother J, Straley S C. Genetic analysis of the low calcium response in Yersinia pestis Mud1(Ap lac) insertion mutants. J Bacteriol. 1984;160:842–848. doi: 10.1128/jb.160.3.842-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakansson S, Gaylov E, Rosqvist R, Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 21.Hakansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homble F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 22.Hoe N P, Goguen J D. Temperature sensing in Yersinia pestis: translation of the LcrF activator protein is thermally regulated. J Bacteriol. 1993;175:7901–7909. doi: 10.1128/jb.175.24.7901-7909.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoe N P, Minion F C, Goguen J D. Temperature sensing in Yersinia pestis: regulation of yopE transcription by lcrF. J Bacteriol. 1992;174:4275–4286. doi: 10.1128/jb.174.13.4275-4286.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoiczyk E, Blobel G. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc Natl Acad Sci USA. 2001;98:4669–4674. doi: 10.1073/pnas.071065798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmstrom A, Petterson J, Rosqvist R, Hakansson S, Tafazoli F, Fallman M, Magnusson K-E, Wolf-Watz H, Forsberg A. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 26.Iriarte M, Cornelis G R. Identification of SycN, YscX, and YscY, three new elements of the Yersinia yop virulon. J Bacteriol. 1999;181:675–680. doi: 10.1128/jb.181.2.675-680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iriarte M, Cornelis G R. YopT, a new Yersinia effector protein, affects the cytoskeleton of host cells. Mol Microbiol. 1998;29:915–929. doi: 10.1046/j.1365-2958.1998.00992.x. [DOI] [PubMed] [Google Scholar]
- 28.Iriarte M, Sory M-P, Boland A, Boyd A P, Mills S D, Lambermont I, Cornelis G R. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 1998;17:1907–1918. doi: 10.1093/emboj/17.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaelin W G, Krek W, Sellers W R, DeCaprio J A, Ajchenbaum F, Fuchs C S, Chittenden T, Li Y, Farnham P J, Blanar M A, Livingston D M, Flemington E K. Expression cloning of a cDNA encoding a retinoblastoma-binding protein with EF2-like properties. Cell. 1992;70:351–364. doi: 10.1016/0092-8674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- 30.Lee V T, Anderson D M, Schneewind O. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol Microbiol. 1998;28:593–601. doi: 10.1046/j.1365-2958.1998.00822.x. [DOI] [PubMed] [Google Scholar]
- 30a.Lee, V. T., S. K. Mazmanian, and O. Schneewind. A program of Yersinia enterocolitica type III secretion reactions is activated by specific signals. J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 31.Lee V T, Schneewind O. Type III machines and the pathogenesis of enteric infections caused by Yersinia and Salmonella spp. Immunol Rev. 1999;168:241–255. doi: 10.1111/j.1600-065x.1999.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee V T, Schneewind O. Type III machines of pathogenic yersiniae secrete virulence factors into the extracellular milieu. Mol Microbiol. 1999;31:1619–1629. doi: 10.1046/j.1365-2958.1999.01270.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee V T, Tam C, Schneewind O. Yersinia enterocolitica type III secretion. LcrV, a substrate for type III secretion, is required for toxin-targeting into the cytosol of HeLa cells. J Biol Chem. 2000;275:36869–36875. doi: 10.1074/jbc.M002467200. [DOI] [PubMed] [Google Scholar]
- 34.Michiels T, Cornelis G R. Secretion of hybrid proteins by the Yersinia Yop export system. J Bacteriol. 1991;173:1677–1685. doi: 10.1128/jb.173.5.1677-1685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michiels T, Vanooteghem J-C, Lambert de Rouvroit C, China B, Gustin A, Boudry P, Cornelis G R. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J Bacteriol. 1991;173:4994–5009. doi: 10.1128/jb.173.16.4994-5009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michiels T, Wattiau P, Brasseur R, Ruysschaert J-M, Cornelis G. Secretion of Yop proteins by yersiniae. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills S D, Boland A, Sory M-P, van der Smissen P, Kerbouch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nilles M L, Fields K A, Straley S C. The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG. J Bacteriol. 1998;180:3410–3420. doi: 10.1128/jb.180.13.3410-3420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nilles M L, Williams A W, Skrzypek E, Straley S C. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J Bacteriol. 1997;179:1307–1316. doi: 10.1128/jb.179.4.1307-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry R D, Harmon P A, Bowmer W S, Straley S C. A low-Ca2+ response operon encodes the V antigen of Yersinia pestis. Infect Immun. 1986;54:428–434. doi: 10.1128/iai.54.2.428-434.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Persson C, Carballeira N, Wolf-Watz H, Fallman M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesion. EMBO J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Persson C, Nordfelth R, Holmstrom A, Hakansson S, Rosqvist R, Wolf-Watz H. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol Microbiol. 1995;18:135–150. doi: 10.1111/j.1365-2958.1995.mmi_18010135.x. [DOI] [PubMed] [Google Scholar]
- 44.Petterson J, Holmstrom A, Hill J, Frithz-Lindsten E, von Euler-Matell A, Carlsson E, Titball R, Forsberg A, Wolf-Watz H. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol Microbiol. 1999;32:961–976. doi: 10.1046/j.1365-2958.1999.01408.x. [DOI] [PubMed] [Google Scholar]
- 45.Petterson J, Nordfelth R, Dubinina E, Bergman T, Gustafsson M, Magnusson K E, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 46.Plano G V, Barve S S, Straley S C. LcrD, a membrane-bound regulator of the Yersinia pestis low-calcium response. J Bacteriol. 1991;173:7293–7303. doi: 10.1128/jb.173.22.7293-7303.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price S B, Cowan C, Perry R D, Straley S C. The Yersinia pestis V antigen is a regulatory protein necessary for Ca2+-dependent growth and maximal expression of low-Ca2+ response virulence genes. J Bacteriol. 1991;173:2649–2657. doi: 10.1128/jb.173.8.2649-2657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price S B, Leung K Y, Barve S S, Straley S C. Molecular analysis of lcrGVH, the V antigen operon of Yersinia pestis. J Bacteriol. 1989;171:5646–5653. doi: 10.1128/jb.171.10.5646-5653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rimpilainen M, Forsberg A, Wolf-Watz H. A novel protein, LcrQ, involved in the low-calcium response of Yersinia pseudotuberculosis shows extensive homology to YopH. J Bacteriol. 1992;174:3355–3363. doi: 10.1128/jb.174.10.3355-3363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosqvist R, Forsberg A, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarker M R, Neyt C, Stainier I, Cornelis G R. The Yersinia yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J Bacteriol. 1998;180:1207–1214. doi: 10.1128/jb.180.5.1207-1214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarker M R, Sory M-P, Boyd A P, Iriarte M I, Cornelis G R. LcrG is required for efficient translocation of Yersinia Yop effector proteins into eukaryotic cells. Infect Immun. 1998;66:2976–2979. doi: 10.1128/iai.66.6.2976-2979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schesser K, Fritzh-Lindsten E, Wolf-Watz H. Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J Bacteriol. 1996;178:7227–7233. doi: 10.1128/jb.178.24.7227-7233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skrzypek E, Straley S C. Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J Bacteriol. 1995;177:2530–2542. doi: 10.1128/jb.177.9.2530-2542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skrzypek E, Straley S C. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J Bacteriol. 1993;175:3520–3528. doi: 10.1128/jb.175.11.3520-3528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sory M-P, Boland A, Lambermont I, Cornelis G R. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci USA. 1995;92:11998–12002. doi: 10.1073/pnas.92.26.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 59.Takeshita S, Sato M, Toba M, Masahashi W, Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for LacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61:63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- 60.Tardy F, Homble F, Neyt C, Wattiez R, Cornelis G R, Ruysschaert J-M, Cabiaux V. Yersinia enterocolitica type III secretion-translocation system: channel formation by secreted Yops. EMBO J. 1999;18:6793–6799. doi: 10.1093/emboj/18.23.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wattiau P, Bernier B, Deslee P, Michiels T, Cornelis G R. Individual chaperones required for Yop secretion by Yersinia. Proc Natl Acad Sci USA. 1994;91:10493–10497. doi: 10.1073/pnas.91.22.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wattiau P, Cornelis G R. Identification of DNA sequences recognized by VirF, the transcriptional activator of the Yersinia yop regulon. J Bacteriol. 1994;176:3878–3884. doi: 10.1128/jb.176.13.3878-3884.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams A W, Straley S C. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J Bacteriol. 1998;180:350–358. doi: 10.1128/jb.180.2.350-358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woestyn S, Sory M-P, Boland A, Lequenne O, Cornelis G R. The cytosolic SycE and SycH chaperones of Yersinia protect the region of YopE and YopH involved in translocation across eukaryotic cell membranes. Mol Microbiol. 1996;20:1261–1271. doi: 10.1111/j.1365-2958.1996.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 65.Yother J, Goguen J D. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J Bacteriol. 1985;164:704–711. doi: 10.1128/jb.164.2.704-711.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]