Summary
Background
Dementia after the age of 80 years (late-life) is increasingly common due to vascular and non-vascular risk factors. Identifying individuals at higher risk of late-life dementia remains a global priority.
Methods
In prospective study of 958 ambulant community-dwelling older women (≥70 years), lateral spine images (LSI) captured in 1998 (baseline) from a bone density machine were used to assess abdominal aortic calcification (AAC). AAC was classified into established categories (low, moderate and extensive). Cardiovascular risk factors and apolipoprotein E (APOE) genotyping were evaluated. Incident 14.5-year late-life dementia was identified from linked hospital and mortality records.
Findings
At baseline women were 75.0 ± 2.6 years, 44.7% had low AAC, 36.4% had moderate AAC and 18.9% had extensive AAC. Over 14.5- years, 150 (15.7%) women had a late-life dementia hospitalisation (n = 132) and/or death (n = 58). Compared to those with low AAC, women with moderate and extensive AAC were more likely to suffer late-life dementia hospitalisations (9.3%, 15.5%, 18.3%, respectively) and deaths (2.8%, 8.3%, 9.4%, respectively). After adjustment for cardiovascular risk factors and APOE, women with moderate and extensive AAC had twice the relative hazards of late-life dementia (moderate, aHR 2.03 95%CI 1.38–2.97; extensive, aHR 2.10 95%CI 1.33–3.32), compared to women with low AAC.
Interpretation
In community-dwelling older women, those with more advanced AAC had higher risk of late-life dementia, independent of cardiovascular risk factors and APOE genotype. Given the widespread use of bone density testing, simultaneously capturing AAC information may be a novel, non-invasive, scalable approach to identify older women at risk of late-life dementia.
Funding
Kidney Health Australia, Healthway Health Promotion Foundation of Western Australia, Sir Charles Gairdner Hospital Research Advisory Committee Grant, National Health and Medical Research Council of Australia.
Keywords: Aging, Imaging, Vascular disease, Dementia, Epidemiology
Abbreviations: AAC, abdominal aortic calcification; AAC24, abdominal aortic calcification 24 scale scores; AUC, area under the curve; AD, Alzheimer's disease; ASVD, atherosclerotic vascular disease; CAC, coronary artery calcification; CVD, cardiovascular disease; DXA, dual-energy X-ray absorptiometry; IDI, integrated discrimination improvement; LSI, lateral spine imaging; NRI, net reclassification improvement; ROC, receiver operator characteristics; APOE, apolipoprotein E; FRS, Framingham General Cardiovascular Risk Scores
Research in context.
Evidence before this study
Despite strong links between vascular disease and dementia risk there is a paucity of data on whether vascular calcification, and in particular extra-coronary vascular calcifications may be a marker of later life dementia risk. The abdominal aorta is one of the first sites where vascular calcification is seen and abdominal aortic calcification (AAC) is common in older men and women. To date, two studies in middle-aged and older men and women have found the presence and extent of AAC is associated with poorer cognitive function. To our knowledge no study has investigated the association between AAC and dementia risk.
Added value of this study
Abdominal aortic calcification assessed on images from bone density machines identifies older women at a higher risk of late-life dementia, independent of other cardiovascular risk factors and APOE genotype. This study suggests that capturing these lateral spine images at the time of bone density testing may be an easy and cost-effective way to identify women at higher risk of developing late-life dementia.
Implications of all the available evidence
Vascular brain aging is increasingly being recognised as a major driver of dementias, particularly late-life dementia. Screening for a stable marker of advanced vascular disease, abdominal aortic calcification at the time of bone density testing may be a promising approach to identify older women at higher risk of cognitive decline and developing late-life dementia. Early identification may provide a window of opportunity to enable dietary, lifestyle and cardiovascular risk management modifications to prevent disease progression.
Alt-text: Unlabelled box
Introduction
Dementia, in particular Alzheimer's disease (AD), is recognised as having an extended asymptomatic prodromal period of up to 20 years, characterised by gradual accumulation of neuropathological disease processes.1 This transitional phase provides a target for the implementation of interventions before the onset of clinically significant impairment which typically signals irreversible neurodegeneration. However, identifying people at high risk of developing dementia during this preclinical phase remains challenging and is a global priority. It is increasingly recognised that a substantial overlap between cardiovascular disease and both vascular dementia and AD exists with many shared modifiable risk factors such as hypertension, obesity, smoking, and hypercholesterolaemia.2 Putative mechanisms underlying the association between vascular factors and neurocognitive health include: small vessel disease (e.g. microinfarcts and axonal pathology),3 regional hypo-perfusion,4 atherosclerosis5 and arteriosclerosis.6 Noteworthy, abdominal aortic calcification (AAC) is a stable marker of generalised advanced atherosclerosis,7 with associated blood vessel stiffening resulting in higher pressures being transmitted into small vessels in other organs such as the brain leading to end-organ damage.8
Bone density testing with dual-energy X-ray absorptiometry machines (DXA) are widely used in osteoporosis screening. These DXA machines can also easily and rapidly capture lateral thoracolumbar spine images at a fraction of the cost and radiation exposure of standard thoracolumbar radiographs to detect vertebral fractures. These lateral spine images (LSI) can also be used to identify and semi-quantify AAC using the 24-point Kaupilla scale (AAC24).9 The presence and extent of AAC is associated with coronary artery calcifications10 and carotid atherosclerosis.11 Furthermore, the presence and extent of AAC is associated with substantially higher cardiovascular risk and poorer long-term prognosis in older men and women.12
We therefore hypothesised that AAC assessed on images captured using widely available bone density machines would be a stable marker of accumulated vascular damage and as such associated with higher risk of late-life dementia, independent of established CVD risk factors and apolipoprotein E (APOE) genotype.
Materials and methods
Study population
Ambulant community-dwelling older Australian women (≥70 years) were recruited to a five-year prospective, randomized, controlled trial of oral calcium supplements to prevent osteoporotic fractures commencing in 1998.13 Women had an expected survival beyond five-years, were ambulant, and not receiving any medication (including hormone replacement therapy) known to affect bone metabolism. Baseline disease burden and medications were comparable between study participants and the general population of similar age, albeit a higher socio-economic status.13 Over five-years, participants received 1.2 g of elemental calcium (calcium carbonate) daily or a matching placebo. Upon completion, women were subsequently enrolled in a further 10-years of observational follow-up; The Perth Longitudinal Study of Aging Women (PLSAW) registered retrospectively in the Australian New Zealand Clinical Trials Registry (ACTRN12615000750583).
Lateral spine images were captured during bone density testing (DXA) performed at baseline visits (1998/1999) in 1053 of the 1460 women. Cardiovascular risk factors and APOE genotypes14 were determined in 973 of these women. After excluding dementia events (n = 15) occurring before 80 years, 958 women were included (Supplementary Figure 1). Baseline characteristics for the 477 women not included in this study due to missing data are presented in Supplementary Table 1. As expected, women who were excluded from this study (due to withdrawing/not attending the year 1 clinic visit for AAC and/or missing covariates) were slightly older, had a higher CVD risk score with a higher incidence of dementia-related hospitalisations. Written informed consent was obtained from all participants, including follow-up from electronic health records. The Human Ethics Committee of the University of Western Australia approved the study protocol and consent form (05/06/004/H50). The Human Research Ethics Committee of the Western Australian Department of Health approved the data linkage study (#2009/24). The manuscript complies with the STROBE reporting guidelines for observational studies.
Baseline cardiovascular risk factor assessment
All women provided their previous medical history whilst current medications were verified by their primary health care provider, where possible. The International Classification of Primary Care–Plus (ICPC-Plus) method15 was adopted, enabling aggregation of different terms for similar pathologic entities as defined by the ICD-10 coding system. Information was used to determine prevalent diabetes (T89001-90009) and atherosclerotic vascular disease (ASVD). Prevalent ASVD included coronary heart disease (ICD-9-CM codes 410-414); heart failure (ICD-9-CM code 428); cerebrovascular disease excluding haemorrhage (ICD-9-CM codes 433-438); and peripheral arterial disease (ICD-9-CM codes 440-444).16 Cardiovascular medications included antihypertensives, statins and low dose aspirin. Former/current smoker was defined as smoking >1 cigarette/d for >3 months at any time during the participants' life. Weight was assessed using digital scales with participants. Height was assessed using a stadiometer and the body mass index (BMI) was calculated in kg/m2. Blood pressure was measured (average of three measurements) on the right arm with a mercury column manometer with participants seated upright and rested for five minutes. Mean arterial pressure was calculated using the following equation= [(2 x diastolic blood pressure) + systolic blood pressure]/3. Renal function measurements were available in 867/958 (90.5%) of the older adult women with estimated glomerular filtration (eGFR) rate calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation using serum creatinine.16 Genetyping for APOE was performed by Polymerase Chain Reaction (PCR) amplification17 with oligonucleotide primers.18 Alcohol intake was obtained from a questionnaire (none, <10 standard drinks p/w or ≥10 standard drinks p/w). The 10-year estimated Framingham General Cardiovascular Risk Scores (FRS) was calculated using BMI and included age, sex, previous diabetes, smoking status and systolic blood pressure.19
Abdominal aortic calcification 24 scores (AAC24)
Lateral spine images were collected in a convenience sample subgroup of the original cohort in 1998 (baseline), then the majority women had LSI (82%) captured in 1999 (Year 1). All AAC scores from 0 to 24 were derived from digitally enhanced DXA-derived lateral single-energy images of the thoraco-lumbar spine (Hologic 4500A, Marlborough, MA, USA). A single experienced investigator (JTS) read all images using an established technique 20 blinded to outcomes. For studies of DXA-derived images, the reported intra-rater reliability for AAC-24 readings have been very high (intraclass correlation coefficient: 0.91 to 0.95).21 AAC24 scoring system records AAC relative to each vertebral height (L1–L4); 0 (no calcification), 1 (≤1/3 of the aortic wall), 2 (>1/3 to ≤2/3 of the aortic wall) or 3 (>2/3 of the aortic wall) for both the anterior and posterior aortic walls, giving a maximum possible score of up to 24. More than 99.5% of the images were of sufficient quality to assess AAC. Extent of AAC24 was categorized using published groupings of AAC (from DXA) testing the association with asymptomatic and clinical cardiovascular disease outcomes7,22,23; low (AAC24 score 0 or 1), moderate (AAC24 score 2–5) and extensive (AAC24 score ≥6).
Late-life dementia outcomes
Dementia outcomes over 14.5- years were tracked through the Western Australian Data Linkage System (Department of Health Western Australia) and retrieved from the Western Australia Hospital Morbidity Data Collection (HMDC). Records were obtained for each individual from 1998 until 2013. Codes were identified using the International Classification of Diseases, Injuries and Causes of Death Clinical Modification (ICD-9-CM) or the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification (ICD-10-AM).24,25 The primary outcome of the study was late-life (after the age of 80 years) dementia events (hospitalisation and/or death) as a previous study found including death data improved identification of those with dementia.26 Dementia codes included; Alzheimer's disease (ICD-9-CM 331.0, ICD-10-AM F00, G30), vascular dementias (ICD-9-CM 290.4, ICD-10-AM F01) and unspecified dementias (F03)27; also considered individually as secondary outcomes. Events were defined using the principal or additional discharge diagnosis codes from hospital morbidity data collection. To increase identification of dementia cases we used the linked coded multiple causes of death data (dementia codes as above) or Parts 1 and 2 of the death certificate where coded cause of death data was not yet available.28 Studies have used this methodology to investigate the aetiology and consequences of dementia.28
Statistical analysis
The association between AAC categories with dementia hospitalisation and/or death over 14.5- years was assessed in unadjusted and multivariable-adjusted Cox regression analyses. For the primary analyses, we treated non-dementia deaths as censored. This approach means that the HRs can be interpreted as the risk of dementia for any time during follow-up assuming that a woman stays alive for that long. Given the advanced age of these women we also performed competing-risks analyses based on Fine and Gray's proportional subhazards model29 to account for the competing risk of non-dementia mortality. Proportional hazards assumptions were tested via Schoenfeld residuals. P-values of >0.05 were recorded for all analysis indicating proportional hazards assumptions were not violated. The multivariable adjusted model included treatment code (calcium or placebo), the FRS, cardiovascular medications (statins and low dose aspirin), prevalent ASVD, APOE genotypes (ℇ2/3, ℇ2/4, ℇ3/3, ℇ3/4, ℇ4/4) and alcohol intake (none, <10 standard drinks p/w or ≥10 standard drinks p/w). Kaplan–Meier survival curves were analysed to identify group differences in AAC categories with respect to time to late-life dementia hospitalisation and/or death. The prognostic utility of the extent of AAC over APOE genotype and CVD estimated risk and medications was assessed using standard tests to measure discrimination such as receiver operator characteristics (ROC), net risk reclassification improvement (NRI) and integrated discrimination improvement (IDI). P-values <0.05 in two-tailed testing were considered statistically significant for primary outcomes. All analyses were undertaken using IBM SPSS Statistics Version 25 (2012, Armonk, NY:IBM Corp) and STATA Version 13 (StataCorp LP, College Station, TX).
Additional analysis
We undertook analysis replacing estimated CVD risk with the individual variables used to derive the estimated Framingham risk score (age, BMI, previous diabetes, smoking history and systolic blood pressure) to the multivariable-adjusted model. We also included eGFR as a covariate to the multivariable-adjusted model due to its potential link to vascular calcification and/or dementia. We examined the relationship between AAC and any dementia event where we excluded women with incident haemorrhagic or ischemic strokes (I60–I69) prior to dementia events; given such events are also associated with greater risk of dementia. As carriage of the APOE ℇ4 allele is known to be associated with increased risk of dementia,30 the relationship between AAC and dementia events in individuals with or without the presence of the APOE ℇ4 allele was investigated. Additionally, we dichotomised AAC and APOE genotypes into four groups (low AAC and no APOE ℇ4 allele; low AAC and an APOE ℇ4 allele; moderate to extensive AAC and no APOE ℇ4 allele; and moderate to extensive AAC and an APOE ℇ4 allele) and examined its relationship with late-life dementia events in multivariable-adjusted Cox models.
Ethical standards
All participants provided written informed consent. Ethics approval was granted by the Human Ethics Committee of the University of Western Australia. CAIFOS and PLSAW studies were retrospectively registered on the Australian New Zealand Clinical Trials Registry (CAIFOS trial registration number #ACTRN12615000750583 and PLSAW trial registration number #ACTRN12617000640303) and complied with the Declaration of Helsinki. Human ethics approval for the use of linked data was provided by the Human Research Ethics Committee of the Western Australian Department of Health (project number #2009/24).
Role of the funding source
None of the funding agencies had any role in the conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Results
Study recruitment overview is provided in Supplementary Figure 1. There were 15 women presenting with dementia hospitalisation or death before aged 80 years and were excluded. 150 women had a late-life dementia hospitalisation or death (132 women with late-life dementia hospitalisation and 58 with late-life dementia death). Of these, 72 women had an Alzheimer's disease event, 109 had an unspecified dementia event and 7 had a vascular dementia event. At baseline, women who later sustained a late-life dementia event were more likely to be older, have a lower BMI, were less likely to have been randomised to calcium supplementation, have higher estimated 10-year CVD risk, and were more likely to have an APOE ℇ4 allele (Table 1). 44.7% of women had low AAC, 36.4% had moderate AAC and 18.9% had extensive AAC. Baseline characteristics presented by AAC extent can be found in Supplementary Table 2.
Table 1.
Baseline characteristics of the study population stratified by development of late-life dementia.
| Whole cohort | No late-life dementia | Late-life dementia | P-value | |
|---|---|---|---|---|
| Number | 958 | 808 | 150 | |
| Age, years | 75.0 ± 2.6 | 74.8 ± 2.5 | 76.0 ± 2.8 | <0.001 |
| Body mass index, kg/m2 | 27.1 ± 4.4 | 27.3 ± 4.5 | 26.4 ± 4.0 | 0.021 |
| Ever smoked, yes (%) | 340 (35.5) | 271 (33.5) | 69 (46.0) | 0.003 |
| Systolic blood pressure, mmHg | 137.4 ± 18.0 | 137.2 ± 17.2 | 138.4 ± 22.1 | 0.463 |
| Antihypertensive medication, yes (%) | 403 (42.1) | 339 (42.0) | 64 (42.7) | 0.871 |
| Diabetes, yes (%) | 57 (5.9) | 41 (5.1) | 16 (10.7) | 0.008 |
| Estimated CVD risk (Framingham), % | 21.8 ± 10.7 | 21.4 ± 10.1 | 24.0 ± 13.5 | 0.006 |
| Statins medication, yes (%) | 182 (19.1) | 154 (19.1) | 28 (18.7) | 0.910 |
| Low dose aspirin, yes (%) | 189 (19.7) | 152 (18.8) | 37 (24.7) | 0.098 |
| Previous ASVD, yes (%) | 105 (11.0) | 88 (10.9) | 17 (11.3) | 0.873 |
| Alcohol | 0.754 | |||
| None (%) | 171 (17.8) | 141 (17.5) | 30 (20.0) | |
| <10 standard drinks p/w (%) | 631 (65.9) | 535 (66.2) | 96 (64.0) | |
| ≥10 standard drinks p/w (%) | 156 (16.3) | 132 (16.3) | 24 (16.0) | |
| Randomisation, calcium (%) | 465 (48.5) | 404 (50.0) | 61 (40.7) | 0.036 |
| APOE genotypes | <0.001 | |||
| APOEℇ2/3, yes (%) | 160 (16.7) | 145 (17.9) | 15 (10.0) | |
| APOEℇ2/4, yes (%) | 18 (1.9) | 16 (2.0) | 2 (1.3) | |
| APOEℇ3/3, yes (%) | 581 (60.6) | 496 (61.4) | 85 (56.7) | |
| APOEℇ3/4, yes (%) | 186 (19.4) | 146 (18.1) | 40 (26.7) | |
| APOEℇ4/4, yes (%) | 13 (1.4) | 5 (0.6) | 8 (5.3) | |
| Abdominal aortic calcification | <0.001 | |||
| Low (AAC24 0 or 1) | 428 (44.7) | 384 (47.5) | 44 (29.3) | |
| Moderate (AAC24 2–5) | 349 (36.4) | 277 (34.3) | 72 (48.0) | |
| Extensive (AAC24 ≥6) | 181 (18.9) | 147 (18.2) | 34 (22.7) |
Data expressed as mean ± SD or number and (%). Abbreviations: mmHg, millimetres mercury; ASVD, atherosclerotic vascular disease; APOE, apolipoprotein E. P-values obtained using ANOVA or Chi Square test where appropriate.
AAC categories and late-life dementia
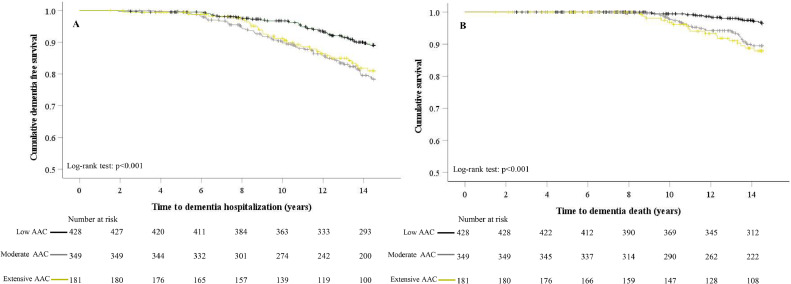
Mean ± SD follow-up time for a late-life dementia hospitalisation (total person years 12,089) and/or death (total person years 12,381) was 12.6 ± 3.0 and 12.9 ± 2.9 y, respectively. Women in moderate to extensive AAC groups had greater cumulative event rates in Kaplan–Meier curves for hospitalisations at around 6-7 years, and deaths at around 9-10 years (Figure 1). After multivariable-adjustment, women with moderate AAC were at a 2- and 3-fold increased relative hazard of late-life dementia hospitalisations or deaths compared to those with low AAC (Table 2). Women with extensive AAC had a 2- and 4-fold increase in the adjusted relative hazard of late-life dementia hospitalisation or death (Table 2). When investigating the types of dementia, women with moderate to extensive AAC were at a 2-fold increased relative hazard of late-life AD and unspecified dementia hospitalisations or deaths compared to those with low AAC, in the unadjusted and multivariable-adjusted model (Supplementary Table 3). Due to only 7 women having vascular dementia (1 with low AAC, 5 with moderate AAC and 1 with extensive AAC) no further analyses of this outcome was undertaken.
Figure 1.
Kaplan Meier Survival curves for late-life dementia (A) hospitalisations and (B) deaths by severity of abdominal aortic calcification (AAC) categories. Black line- low AAC, grey line- moderate AAC and mustard line- extensive AAC.
Table 2.
Cox proportional hazards regression for late-life dementia events by extent of abdominal aortic calcification.
| Number (%) | Unadjusted HR (95% CI) | Multivariable-adjusted HR (95% CI)# | |
|---|---|---|---|
| Any late-life dementia event | |||
| Low AAC (AAC24 score 0 or 1) | 44 (10.3) | 1 (reference) | 1 (reference) |
| Moderate AAC (AAC24 score 2–5) | 72 (22.6) | 2.19 (1.51–3.19)* | 2.03 (1.38–2.97)* |
| Extensive AAC (AAC24 score ≥6) | 34 (18.8) | 2.06 (1.32–3.22)* | 2.10 (1.33–3.32)* |
| P for trend | 0.001 | 0.001 | |
| Any late-life dementia hospitalisation | |||
| Low AAC (AAC24 score 0 or 1) | 40 (9.3) | 1 (reference) | 1 (reference) |
| Moderate AAC (AAC24 score 2–5) | 64 (18.3) | 2.14 (1.44–3.17)* | 1.95 (1.30–2.91)* |
| Extensive AAC (AAC24 score ≥6) | 28 (15.5) | 1.86 (1.15–3.01)* | 1.92 (1.17–3.15)* |
| P for trend | 0.008 | 0.006 | |
| Any late-life dementia death | |||
| Low AAC (AAC24 score 0 or 1) | 12 (2.8) | 1 (reference) | 1 (reference) |
| Moderate AAC (AAC24 score 2–5) | 29 (8.3) | 3.18 (1.62–6.24)* | 3.09 (1.56–6.10)* |
| Extensive AAC (AAC24 score ≥6) | 17 (9.4) | 3.78 (1.80–7.91)* | 3.88 (1.82–8.28)* |
| P for trend | <0.001 | <0.001 | |
Abbreviations: AAC; abdominal aortic calcification, ASVD; atherosclerotic vascular disease, HR; hazard ratio.
Cox proportional hazards regression analyses were adjusted for General Framingham Risk Score plus treatment code (calcium or placebo), alcohol intake, prevalent ASVD, prescription of statin medications, use of low dose aspirin and Apolipoprotein E genotype. Trend test performed using the median values for each AAC severity category.
indicates p-values<0.05 compared to women with no-low abdominal aortic calcification.
The addition of the AAC categories to models accounting for estimated cardiovascular risk, cardiovascular medications, alcohol intake and APOE genotype led to improvements in the area under the curve for late-life dementia deaths and events (Table 3). Category-free net reclassification improvements indicated the addition of AAC led to 33–49% being correctly reclassified, predominantly women who suffered a dementia hospitalisation or death being correctly moved up in risk. The Hosmer-Lemeshow test (p = 0.842) also indicated that our models analyzing the relationship between AAC and any dementia-related event was well calibrated (Supplementary Figure 2).
Table 3.
Analyses of the area under the curve, net reclassification improvement and integrated discrimination indices.
| AUC | p- value# | Category-free NRI | Net events correctly reclassified higher | Net non-events correctly reclassified lower | IDI, p-value | |
|---|---|---|---|---|---|---|
| Late-life dementia, (n = 150) | ||||||
| Full model | 0.635 | |||||
| Full model + AAC severity | 0.678* | 0.018 | 0.383 | 30.7% | 7.7% | 0.011, p = 0.006 |
| Late-life dementia hospitalisations, (n = 132) | ||||||
| Full model | 0.667 | |||||
| Full model + AAC severity | 0.697 | 0.056 | 0.366 | 30.3% | 6.3% | 0.007, p = 0.029 |
| Dementia deaths, (n = 58) | ||||||
| Full model | 0.631 | |||||
| Full model + AAC severity | 0.711* | 0.014 | 0.492 | 41.4% | 7.8% | 0.013, p = 0.007 |
Abbreviations: AAC; abdominal aortic calcification, ASVD; atherosclerotic vascular disease, AUC; area under the curve, IDI; integrated discrimination indices, NRI; net reclassification improvement. #Compared to full model were including General Framingham Risk Score plus treatment code (calcium or placebo), alcohol intake, prevalent ASVD, prescription of statin medications, use of low dose aspirin and APOE genotype.
significantly different from full model.
Additional analyses
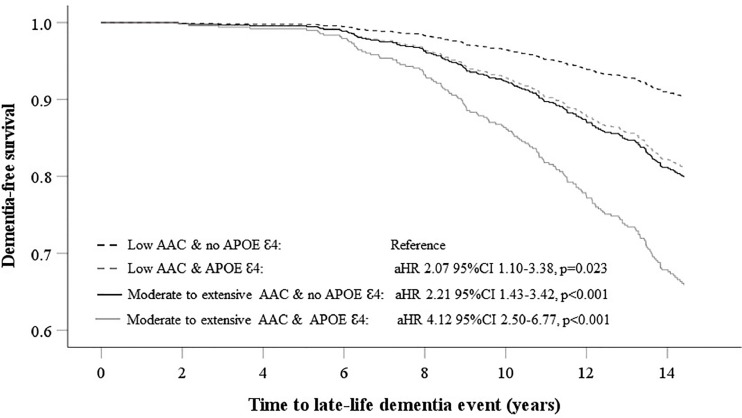
Including individual variables used to compute FRS to the multivariable-adjusted model did not alter the relationship between AAC categories, dementia-related events and deaths (Supplementary Table 4). For dementia-related hospitalisations, this relationship was slightly attenuated for women with extensive AAC (p = 0.056). Competing risks analyses for non-dementia deaths did not substantially affect the point estimates for the AAC groups (Supplementary Table 5). Including eGFR to the multivariable-adjusted Cox model also did not result in substantive changes to the observed associations between AAC and late-life dementia events (Supplementary Table 6). Point estimates for the relationship between AAC categories (moderate AAC HR 2.06 95%CI 1.35–3.15, p < 0.001; extensive AAC HR 2.31 95%CI 1.39–3.83, p < 0.001) and all dementia events remained similar when we excluded women with incident haemorrhagic or ischemic CVAs (n = 125). In women who did not carry an APOE ℇ4 allele (n = 741), those with moderate AAC (n = 265, HR 2.36 95%CI 1.48–3.75, p < 0.001) and extensive AAC (n = 142, HR 2.07 95%CI 1.17–3.64, p = 0.012) had greater risk for any dementia-related event, compared to women with low AAC (n = 334). However, in women carrying an APOE ℇ4 allele (n = 217), AAC status was not associated with significantly increased dementia-related events despite similar aHRs. Here, compared to women with low AAC (n = 94), those with moderate AAC (n = 84) and extensive AAC (n = 39) had HR's for dementia-related events of 1.81 95%CI 0.93–3.53, p = 0.081 and 1.89 95%CI 0.85–4.17, p = 0.115 respectively. Finally, in a multivariable-adjusted analysis, compared to women with low AAC and no APOE ℇ4 allele, those with low AAC and an APOE ℇ4 allele (n = 94), moderate to extensive AAC and no APOE ℇ4 allele (n = 407), and moderate to extensive AAC and a APOE ℇ4 allele had 207%, 221% and 412% greater relative hazards for any late-life dementia event (Figure 2).
Figure 2.
Multivariable-adjusted Cox regression for late-life dementia events (hospitalisation and/or death) dichotomised by severity of abdominal aortic calcification (AAC) and the presence of the APOEℇ4 gene.
Discussion
To our knowledge this is the first study to report an association between AAC and the risk of future late-life dementia. In older women at high risk of late-life dementia, widely available bone density machines may be an novel inexpensive, safe, and non-invasive way to identify individuals with accumulated vascular damage at substantively higher risk of dementia hospitalisations and deaths. Specifically, we demonstrated that over half of the older women with AAC were at 2 to 4 times the risk of late-life dementia hospitalisation and deaths over the next 15- years. Importantly, both extent of AAC and APOE ℇ4 genotype provided complimentary prognostic information in older women suggesting this may be novel way to identify high risk older women. Given recent advances in the automation of AAC this offers a cheap, rapid and safe way to screen for late-life dementia risk. Noteworthy, the addition of AAC to estimated CVD risk, medication and APOE ℇ4 genotype demonstrated improved discrimination for predicting these late-life dementia events in addition to these established risk factors. These findings suggest that a combination of genetics and vascular imaging can identify a high-risk population where implementation of lifestyle31 and/or pharmaceutical32 disease modifying interventions has the potential to prevent late-life dementia.
While findings that cardiovascular risk measures are associated with dementia risk are not new, the gradients of risk observed in this study across the AAC categories warrant particular attention with over 55% of the women being at 2–4 times the risk of late-life dementia. When compared to coronary artery calcification these gradients of risk are substantially higher. For example, Akira and colleagues found that in the 6293 participants of the Multi-Ethnic Study of Atherosclerosis (free of cardiovascular disease and noticeable cognitive deficit) that the adjusted HR were 1.23 for people with CAC scores of 1–400, 1.35 for those with CAC scores 400–1000 and 1.71 for those with CAC scores >1000. Results were attenuated for all groups except CAC >1000 when interim strokes were excluded.33 Other also report that coronary artery calcification was not associated with dementia risk. However, in minimally adjusted models, aortic arch calcification, intracranial carotid calcification and extracranial carotid calcification were associated and only extracranial carotid calcification remained significant after adjusting for APOE ℇ4 genotype and cardiovascular risk factors.34 A recent systematic review and meta-analysis also found that pooled HR of clinical coronary artery disease with incident mild cognitive impairment or dementia in six studies (n = 70,060 individuals) was 1.51.35 Similar, modest increases in dementia risk are seen with carotid ultrasound measures of atherosclerosis.36,37 Collectively, these findings suggests that AAC may be a better marker of future dementia risk than measures of coronary or carotid atherosclerosis.
Presumably this may be due to AAC being more strongly related to increasing aortic stiffness8,38 and raised central blood pressure.39 Alternative explanations include that AAC is thought to precede CAC40 and has been shown to be an independent predictor of CAC incidence and long-term progression.41; Interestingly the Multiethnic Study of Atherosclerosis found AAC and CAC predicted hard coronary heart disease and hard CVD independent of risk factors and one another, whilst only AAC was independently associated with CVD mortality and was more strongly associated with all-cause mortality.42 Whilst traditional cardiovascular risk factors such as lipids and blood pressure are related to the development and progression of both CAC and AAC,41 the progression of AAC has also been shown to be associated with non-traditional CVD risk factors such as chronic kidney disease,43 rapid bone loss44 and low muscle mass and poor physical function.45 These conditions have all been shown to be associated with increased risk of cognitive deficits or decline.46, 47, 48 Taken together these studies suggest AAC, may be an early marker of systemic atherosclerotic vascular disease burden and capture non-traditional cardiovascular disease risk factors compared to CAC. Hence AAC is likely to add new information on the risk of late-life dementia compared to CAC alone. However, future studies directly comparing AAC and CAC with late-life dementia events are needed.
Early diagnosis and the implementation of intervention strategies to reduce risk factors are likely to reduce the burden of dementia.49 Previous research assessing both AAC and coronary artery calcification (CAC) have often relied on the use of CT scanning which limits the clinical utility of these measurements due to cost and patient radiation exposure.50,51 Our results demonstrate that extensive AAC, assessed on low radiation DXA-derived LSI are robustly associated with greater relative hazard of dementia hospitalisations and deaths. There is currently great interest in the automation of AAC assessment which would enhance its clinical utility.52 While this is the first study to investigate the relationship between AAC and late-life dementia risk over 14.5-years, previous studies have assessed the association between AAC with cognition and markers of brain health. Reis, Launer53 investigated the relationship between cognition and computed tomography (CT) generated AAC. An inverse relationship between AAC and measures of verbal learning and processing speed were recorded.53 Noteworthy, verbal memory (Rey Auditory Verbal Learning Test) is reported to be sensitive to the early markers of cognitive decline that characterise dementia and AD.54 Similarly, the Rotterdam study reported that mid-life (∼55 years of age) assessment of AAC was predictive of MRI determined white matter periventricular lesion burden detected 20- years subsequent to initial assessment, but not in those with MRI assessments only 5- years post initial AAC assessment.55 A cross sectional study of 1209 individuals (≥60 years) also reported that DXA assessed AAC was associated with poorer performance in global cognition, verbal memory, and language fluency.56 However, stratification by age (<75 years, ≥75 years) showed that AAC and cognition was only identified in individuals 60–75 years.56
These clinical findings from previous studies are in general supportive of the current findings. There appears to be a delay of about 5- years between the AAC measurement and the onset of clinical disease of a severity resulting in hospitalisation or death. Future work evaluating changes in neurophysiology during this time will hopefully contribute to interventions to prevent progression. In this regard, it should be noted that in this study participants with severe AAC at increased dementia risk were receiving similar exposure to cardiovascular disease medications as those who did not develop dementia. Perhaps, they would have benefited from more aggressive treatment based on their AAC score.
Mechanistically, in older individuals with dementia, cerebrovascular changes are a common neuropathologic feature. Some neuropathologic features of dementia include endothelial dysfunction, atherosclerosis, ischemia, haemorrhage and cerebral amyloid angiopathy.57 When considering cerebrovascular risk factors, there appears to be a relationship with neuropathologic correlates of dementia. For example, metabolic disease (hypertension, diabetes, hypercholesterolemia), smoking, and aging are all risk factors for vascular disease and the risk for AD-related dementias. Specifically, neuropathology may be impacted by hypoxia (caused by haemorrhage and ischemia), metabolic dysfunction (decreases glucose metabolism and mitochondrial function) and cerebrovascular haemodynamics (endothelial damage, higher blood pressure and tortuosity) (see57 for review). Noteworthy, AAC is associated with stiffening of blood vessels that can increase pressure transmitted into small vessels in other organs such as the brain that can lead to end-organ damage.8 As such, increased central blood pressure may increase dementia risk.58 Our results could also be due to the overlapping pathologies for which AAC is a marker of, and which are themselves risk factors for dementia and AD. It is particularly noteworthy, that CAC has also been linked smaller total brain volume59 and increased atrophy,60 increased white matter infarcts61 and lesions.50,59 Collectively, atherosclerotic calcification is likely to be a strong contributor towards the neuropathologic features of dementia and supports further investigation of subclinical measures of abdominal aortic calcification for disease risk stratification during aging. Regarding the presence of APOE ℇ4 allele, our findings were similar to previous epidemiological work30 identifying the important role for this genotype for increased risk of late life dementia. Further, it evidenced that that an increased AAC score is additional to genotype, being an equally important risk factor for late-life dementia.
Strengths of this study include the use of DXA-derived LSI for AAC measurement assessed by a highly experienced investigator (JTS) blinded to clinical data. This work considered a wealth of comorbidity and covariate information for inclusion in each analysis, including CVD risk factors, medications, and APOE genotyping. We have also demonstrated that the degree of AAC was a strong contributor in addition to other cardiovascular and dementia associated covariates in our multivariable-adjusted model. The use of high-quality objective administrative data from two sources with an 18-year lookback period and 15- years of follow up, reduce the risk of selection and misclassification bias. Adopting ICD coding, based on clinical diagnosis, for the categorisation of dementia outcomes also reduces potential bias in dementia diagnosis. Nevertheless, this study has limitations. The major limitation is the reliance on linked Hospital discharge administrative data which has been shown to have high accuracy (96.7%) but low sensitivity (21.2%) compared to chart review; although adding this data increases dementia ascertainment compared to death certificates alone.28 In other Australian administrative data, the ICD-10 dementia diagnoses from hospital records has been shown substantial agreement (k = 0.71) with a sensitivity of 67% and a positive predictive value of 76%.62 In the current study the 15-year incidence in these older women was 15.7% which is similar to the incidence reported in the Australian Longitudinal Study on Women's Health. This study followed women aged 70 years or older in 1996 and investigated multiple administrative data sources to identify dementia cases including aged care assessments data, hospital admissions data for three states (Queensland, New South Wales, and South Australia), pharmaceutical data, death records and self-reported survey data (six surveys which occurred at 3-year intervals). Over the 16- years of follow-up, 20.4% of women were identified as having dementia in at least one of the data sources with aged care data identifying the most cases (79.3%), followed by hospital admission data (55.8%), pharmaceutical records (34.6%), death records (31.0%) and self-reported survey data with 18.5%.63 Importantly, if dementia diagnosis was missed, this would have biased the results towards the null. Additionally, due to the observational nature of our study, causality cannot be established. Another potential limitation is that the AAC observed may be due to non-atherosclerotic processes i.e. medial calcifications or a combination atherosclerotic and medial calcification as the localisation and underlying pathophysiology of the calcifications of the abdominal aorta is poorly understood. This is due to limited histological studies and current conventional imaging methodologies not being able to localise calcification within the different layers of the arterial wall.64 Despite considering a range of cardiovascular and dementia-related risk factors, there is the potential for residual confounding. DXA generated images for the quantification of AAC were only captured in 70% of the original cohort, potentially biasing results. As AAC was assessed in women ≥70 year who were healthy ambulant community-dwelling older women not on bone active medications, we cannot exclude the risk of selection bias. Of note, the prevalence of women with the APOE ℇ4 genotype in our study was 23%, which is similar to the 22–28% of individuals presenting with at least one APOE ℇ4 allele in other cohorts from Australia.65,66 Furthermore, as AAC was assessed in women ≥70 years, this could be on the fringe as an ideal pre-clinical dementia screening tool for early intervention.1 We had limited power to examine the associations of AAC for specific types of dementia. Finally, findings may not be generalised to younger women or men. Consequently, it will be important to replicate these findings in other cohorts.
In conclusion, this study identified an association between AAC and the subsequent development of late-life dementia. Significant increases in risk for hospitalisation and death from dementia were observed with increasing severity of AAC, in addition to shorter times to each of these events. Several proposed mechanisms and pathways which are impacted by an individual's cardiovascular risk profile likely contribute to the relationship between AAC and dementia. Therefore, understanding the impact of shared risk factors could allow for targeted disease modifying strategies. To this end, lateral spine imaging to detect AAC as well as spine fractures during bone density scanning, has potential for use as an early screening and assessment tool to identify women at higher risk of dementia, allowing for the implementation of early lifestyle intervention strategies in at risk populations. Such studies should be a high priority for long-term randomised controlled trials.
Contributors
MS, TP, SML, JMH, JRL conceived and designed the study; RLP, WHL, DPK, SML, JTS, KZ, JMH, JRL acquired funding; RLP, KZ, WHL, JRL, JTS collected the data; MS, TP, JTS, SML, JRL analyzed the data; MS, TP SML, JRL prepared the manuscript with input from all authors; MS, TP, JRL had the primary responsibility for the final content. RLP, JTS, CPB, WHL, KZ, DPK and JMH provided intellectual input and edited the paper. All authors read and approved the final manuscript.
Data sharing statement
The data that support the findings of this study are available from the corresponding author upon reasonable request in-line with governing ethical considerations.
Declaration of interests
DPK has received a grant from Solarea Bio, Amgen and royalties from Wolters Kluwer. DPK sits on the Scientific Advisory Boards of Solarea Bio, Pfizer and Reneo and has participated on the Data Safety Monitoring Board for the AgNovos Healthcare osteoporosis treatment trial. All other authors declare no conflicts of interest.
Acknowledgements
The authors wish to thank the staff at the Western Australia Data Linkage Branch, Hospital Morbidity Data Collection and Registry of Births, Deaths and Marriages for their work on providing the data for this study.
Funding
The study was supported by Kidney Health Australia grant S07 10, Healthway Health Promotion Foundation of Western Australia, Sir Charles Gairdner Hospital Research Advisory Committee Grant and by project grants 254627, 303169 and 572604 from the National Health and Medical Research Council of Australia. The salary of MS is supported by a Royal Perth Hospital Research Foundation Fellowship (RPHRF CAF 00/21) and an Emerging Leader Fellowship from the Western Australian Future Health Research and Innovation Fund. The salary of CPB is supported by a Royal Perth Hospital Research Foundation ‘Lawrie Beilin’ Career Advancement Fellowship (ID: CAF 127/2020). The salary of JMH is supported by a NHMRC of Australia Senior Research Fellowship (ID: 1116973). The salary of JRL is supported by a National Heart Foundation Future Leader Fellowship (ID: 102817). DPK is supported by a grant from the National Institute of Arthritis, Musculoskeletal and Skin Diseases (R01 AR 41398). None of the funding agencies had any role in the conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100502.
Appendix. Supplementary materials
References
- 1.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 2.Qiu C. Preventing Alzheimer's disease by targeting vascular risk factors: hope and gap. J Alzheimers Dis. 2012;32(3):721–731. doi: 10.3233/JAD-2012-120922. [DOI] [PubMed] [Google Scholar]
- 3.Wang R, Laveskog A, Laukka EJ, et al. MRI load of cerebral microvascular lesions and neurodegeneration, cognitive decline, and dementia. Neurology. 2018;91(16):e1487–e1e97. doi: 10.1212/WNL.0000000000006355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18(7):419–434. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore longitudinal study of aging cohort. Ann Neurol. 2010;68(2):231–240. doi: 10.1002/ana.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Oijen M, de Jong FJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Atherosclerosis and risk for dementia. Ann Neurol. 2007;61(5):403–410. doi: 10.1002/ana.21073. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JR, Schousboe JT, Lim WH, et al. Abdominal aortic calcification identified on lateral spine images from bone densitometers are a marker of generalized atherosclerosis in elderly women. Arterioscler Thromb Vasc Biol. 2016;36(1):166–173. doi: 10.1161/ATVBAHA.115.306383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsao CW, Himali JJ, Beiser AS, et al. Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology. 2016;86(7):619–626. doi: 10.1212/WNL.0000000000002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PWF. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132(2):245–250. doi: 10.1016/s0021-9150(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 10.Schousboe JT, Claflin D, Barrett-Connor E. Association of coronary aortic calcium with abdominal aortic calcium detected on lateral dual energy x-ray absorptiometry spine images. Am J Cardiol. 2009;104(3):299–304. doi: 10.1016/j.amjcard.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis JR, Schousboe JT, Lim WH, et al. Abdominal aortic calcification identified on lateral spine images from bone densitometers are a marker of generalized atherosclerosis in elderly women. Arterioscler Thromb Vasc Biol. 2016;36(1):166–173. doi: 10.1161/ATVBAHA.115.306383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leow K, Szulc P, Schousboe JT, et al. Prognostic value of abdominal aortic calcification: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. 2021;10(2) doi: 10.1161/JAHA.120.017205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med. 2006;166(8):869–875. doi: 10.1001/archinte.166.8.869. [DOI] [PubMed] [Google Scholar]
- 14.Dick I, Devine A, Marangou A, et al. Apolipoprotein E4 is associated with reduced calcaneal quantitative ultrasound measurements and bone mineral density in elderly women. Bone. 2002;31(4):497–502. doi: 10.1016/s8756-3282(02)00851-7. [DOI] [PubMed] [Google Scholar]
- 15.Britt H, Scahill S, Miller G. ICPC PLUS for community health? A feasibility study. Health Inf Manage. 1997;27(4):171–175. doi: 10.1177/183335839802700406. [DOI] [PubMed] [Google Scholar]
- 16.Lewis JR, Lim W, Dhaliwal SS, et al. Estimated glomerular filtration rate as an independent predictor of atherosclerotic vascular disease in older women. BMC Nephrol. 2012;13(1):58. doi: 10.1186/1471-2369-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545–548. [PubMed] [Google Scholar]
- 18.Wenham P, Price W, Blundell G. Apolipoprotein E genotyping by one-stage PCR. Lancet North Am Ed. 1991;337(8750):1158–1159. doi: 10.1016/0140-6736(91)92823-k. [DOI] [PubMed] [Google Scholar]
- 19.D'Agostino RB, Sr., Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 20.Schousboe JT, Wilson KE, Kiel DP. Detection of abdominal aortic calcification with lateral spine imaging using DXA. J Clin Densitom. 2006;9(3):302–308. doi: 10.1016/j.jocd.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Schousboe JT, Lewis JR, Kiel DP. Abdominal aortic calcification on dual-energy X-ray absorptiometry: methods of assessment and clinical significance. Bone. 2017;104:91–100. doi: 10.1016/j.bone.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Schousboe JT, Taylor BC, Kiel DP, Ensrud KE, Wilson KE, McCloskey EV. Abdominal aortic calcification detected on lateral spine images from a bone densitometer predicts incident myocardial infarction or stroke in older women. J Bone Miner Res. 2008;23(3):409–416. doi: 10.1359/jbmr.071024. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JR, Schousboe JT, Lim WH, et al. Long-term atherosclerotic vascular disease risk and prognosis in elderly women with abdominal aortic calcification on lateral spine images captured during bone density testing: a prospective study. J Bone Miner Res. 2018;33(6):1001–1010. doi: 10.1002/jbmr.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization . World Health Organization; Geneva: 1977. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death: Based on the Recommendations of the Ninth Revision Conference, 1975, and Adopted by the Twenty-Ninth World Health Assembly. 1975 Revision. ed. [Google Scholar]
- 25.World Health Organization . 2nd ed. World Health Organization; Geneva: 2004. ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision. [Google Scholar]
- 26.Zilkens RR, Spilsbury K, Bruce DG, Semmens JB. Clinical epidemiology and in-patient hospital use in the last year of life (1990–2005) of 29,884 Western Australians with dementia. J Alzheimers Dis. 2009;17(2):399–407. doi: 10.3233/JAD-2009-1057. [DOI] [PubMed] [Google Scholar]
- 27.Zilkens RR, Bruce DG, Duke J, Spilsbury K, Semmens JB. Severe psychiatric disorders in mid-life and risk of dementia in late- life (age 65-84 years): a population based case-control study. Curr Alzheimer Res. 2014;11(7):681–693. doi: 10.2174/1567205011666140812115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zilkens RR, Spilsbury K, Bruce DG, Semmens JB. Linkage of hospital and death records increased identification of dementia cases and death rate estimates. Neuroepidemiology. 2009;32(1):61–69. doi: 10.1159/000170908. [DOI] [PubMed] [Google Scholar]
- 29.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc. 1999;94:496–509. [Google Scholar]
- 30.Pankratz N, Byder L, Halter C, et al. Presence of an APOE4 allele results in significantly earlier onset of Parkinson's disease and a higher risk with dementia. Movement Disord. 2006;21(1):45–49. doi: 10.1002/mds.20663. [DOI] [PubMed] [Google Scholar]
- 31.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet North Am Ed. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 32.Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer's disease drug development pipeline: 2019. Alzheimer's Dement. 2019;5:272–293. doi: 10.1016/j.trci.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujiyoshi A, Jacobs DR, Jr, Fitzpatrick AL, et al. Coronary artery calcium and risk of dementia in MESA (Multi-Ethnic Study of Atherosclerosis) Circulation. 2017;10(5) doi: 10.1161/CIRCIMAGING.116.005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bos D, Vernooij MW, de Bruijn RF, et al. Atherosclerotic calcification is related to a higher risk of dementia and cognitive decline. Alzheimer's Dement. 2015;11(6) doi: 10.1016/j.jalz.2014.05.1758. 639-47. e1. [DOI] [PubMed] [Google Scholar]
- 35.Xia C, Vonder M, Sidorenkov G, et al. The relationship of coronary artery calcium and clinical coronary artery disease with cognitive function: a systematic review and meta-analysis. J Atheroscler Thromb. 2020;27(9):934–958. doi: 10.5551/jat.52928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Norby FL, George KM, et al. Association of carotid intima-media thickness and other carotid ultrasound features with incident dementia in the ARIC-NCS. J Am Heart Assoc. 2021;10(9) doi: 10.1161/JAHA.120.020489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitagawa K, Miwa K, Yagita Y, Okazaki S, Sakaguchi M, Mochizuki H. Association between carotid stenosis or lacunar infarction and incident dementia in patients with vascular risk factors. Eur J Neurol. 2015;22(1):187–192. doi: 10.1111/ene.12553. [DOI] [PubMed] [Google Scholar]
- 38.Cui C, Sekikawa A, Kuller LH, et al. Aortic stiffness is associated with increased risk of incident dementia in older adults. J Alzheimers Dis. 2018;66(1):297–306. doi: 10.3233/JAD-180449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odink A, Mattace-Raso F, van der Lugt A, et al. The association of arterial stiffness and arterial calcification: the Rotterdam Study. J Hum Hypertens. 2008;22(3):205–207. doi: 10.1038/sj.jhh.1002315. [DOI] [PubMed] [Google Scholar]
- 40.Strong JP, Malcom GT, McMahan CA, et al. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA. 1999;281(8):727–735. doi: 10.1001/jama.281.8.727. [DOI] [PubMed] [Google Scholar]
- 41.Onuma OK, Pencina K, Qazi S, et al. Relation of risk factors and abdominal aortic calcium to progression of coronary artery calcium (from the Framingham Heart Study) Am J Cardiol. 2017;119(10):1584–1589. doi: 10.1016/j.amjcard.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Criqui MH, Denenberg JO, McClelland RL, et al. Abdominal aortic calcium, coronary artery calcium, and cardiovascular morbidity and mortality in the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34(7):1574–1579. doi: 10.1161/ATVBAHA.114.303268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanada S, Ando R, Naito S, et al. Assessment and significance of abdominal aortic calcification in chronic kidney disease. Nephrol Dialysis Transplant. 2010;25(6):1888–1895. doi: 10.1093/ndt/gfp728. [DOI] [PubMed] [Google Scholar]
- 44.Bagger Y, Tanko L, Alexandersen P, Qin G, Christiansen C, Group PERFS. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J Intern Med. 2006;259(6):598–605. doi: 10.1111/j.1365-2796.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 45.Szulc P, Chapurlat R. Rapid progression of aortic calcification in older men with low appendicular lean mass and poor physical function. J Nutr Health Aging. 2021;25(10):1217–1225. doi: 10.1007/s12603-021-1697-0. [DOI] [PubMed] [Google Scholar]
- 46.Lary CW, Rosen CJ, Kiel DP. Osteoporosis and dementia: establishing a link. J Bone Miner Res. 2021;36(11):2103. doi: 10.1002/jbmr.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beeri MS, Leugrans SE, Delbono O, Bennett DA, Buchman AS. Sarcopenia is associated with incident Alzheimer's dementia, mild cognitive impairment, and cognitive decline. J Am Geriatr Soc. 2021;69(7):1826–1835. doi: 10.1111/jgs.17206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jabbari B, Vaziri ND. The nature, consequences, and management of neurological disorders in chronic kidney disease. Hemodialysis Intl. 2018;22(2):150–160. doi: 10.1111/hdi.12587. [DOI] [PubMed] [Google Scholar]
- 49.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet North Am Ed. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bos D, Ikram MA, Elias-Smale SE, et al. Calcification in major vessel beds relates to vascular brain disease. Arterioscler Thromb Vasc Biol. 2011;31(10):2331–2337. doi: 10.1161/ATVBAHA.111.232728. [DOI] [PubMed] [Google Scholar]
- 51.Bos D, Vernooij MW, Elias-Smale SE, et al. Atherosclerotic calcification relates to cognitive function and to brain changes on magnetic resonance imaging. Alzheimers Dement. 2012;8(5 Suppl):S104–S111. doi: 10.1016/j.jalz.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Reid S, Schousboe JT, Kimelman D, Monchka BA, Jozani MJ, Leslie WD. Machine learning for automated abdominal aortic calcification scoring of DXA vertebral fracture assessment images: a pilot study. Bone. 2021;148 doi: 10.1016/j.bone.2021.115943. [DOI] [PubMed] [Google Scholar]
- 53.Reis JP, Launer LJ, Terry JG, et al. Subclinical atherosclerotic calcification and cognitive functioning in middle-aged adults: the CARDIA study. Atherosclerosis. 2013;231(1):72–77. doi: 10.1016/j.atherosclerosis.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allison SL, Jonaitis EM, Koscik RL, et al. Neurodegeneration, Alzheimer's disease biomarkers, and longitudinal verbal learning and memory performance in late middle age. Neurobiol Aging. 2021;102:151–160. doi: 10.1016/j.neurobiolaging.2021.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Leeuw FE, De Groot JC, Oudkerk M, et al. Aortic atherosclerosis at middle age predicts cerebral white matter lesions in the elderly. Stroke. 2000;31(2):425–429. doi: 10.1161/01.str.31.2.425. [DOI] [PubMed] [Google Scholar]
- 56.Wei J, Ali MK, Wang T, Xu H. Abdominal aortic calcification and cognitive function among older adults: cross-sectional analysis of National Health and Nutrition Examination Survey, 2013-2014. Int J Geriatr Psychiatry. 2021 doi: 10.1002/gps.5599. [DOI] [PubMed] [Google Scholar]
- 57.Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab. 2016;36(1):172–186. doi: 10.1038/jcbfm.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Odink AE, Mattace-Raso FU, van der Lugt A, et al. The association of arterial stiffness and arterial calcification: the Rotterdam study. J Hum Hypertens. 2008;22(3):205–207. doi: 10.1038/sj.jhh.1002315. [DOI] [PubMed] [Google Scholar]
- 59.Vidal JS, Sigurdsson S, Jonsdottir MK, et al. Coronary artery calcium, brain function and structure: the AGES-Reykjavik study. Stroke. 2010;41(5):891–897. doi: 10.1161/STROKEAHA.110.579581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kin T, Yamano S, Sakurai R, et al. Carotid atherosclerosis is associated with brain atrophy in Japanese elders. Gerontology. 2007;53(1):1–6. doi: 10.1159/000095385. [DOI] [PubMed] [Google Scholar]
- 61.Rosano C, Naydeck B, Kuller LH, Longstreth WT, Jr., Newman AB. Coronary artery calcium: associations with brain magnetic resonance imaging abnormalities and cognitive status. J Am Geriatr Soc. 2005;53(4):609–615. doi: 10.1111/j.1532-5415.2005.53208.x. [DOI] [PubMed] [Google Scholar]
- 62.Henderson T, Shepheard J, Sundararajan V. Quality of diagnosis and procedure coding in ICD-10 administrative data. Med Care. 2006:1011–1019. doi: 10.1097/01.mlr.0000228018.48783.34. [DOI] [PubMed] [Google Scholar]
- 63.Waller M, Mishra GD, Dobson AJ. Estimating the prevalence of dementia using multiple linked administrative health records and capture–recapture methodology. Emerg Themes Epidemiol. 2017;14(1):1–9. doi: 10.1186/s12982-017-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bartstra JW, Mali WPTM, Spiering W, de Jong PA. Abdominal aortic calcification: from ancient friend to modern foe. Eur J Prevent Cardiol. 2021;28(12):1386–1391. doi: 10.1177/2047487320919895. [DOI] [PubMed] [Google Scholar]
- 65.Martins RN, Clarnette R, Fisher C, et al. ApoE genotypes in Australia: roles in early and late onset Alzheimer's disease and Down's syndrome. Neuroreport. 1995;6(11):1513–1516. [PubMed] [Google Scholar]
- 66.Fowler C, Rainey-Smith SR, Bird S, et al. Fifteen years of the Australian Imaging, Biomarkers and Lifestyle (AIBL) study: progress and observations from 2,359 older adults spanning the spectrum from cognitive normality to Alzheimer’s disease. J Alzheimer’s Dis Rep. 2021;5(1):443–468. doi: 10.3233/ADR-210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.