Summary
Background
Adipose tissue is a source of multiple factors that modulate systemic insulin sensitivity and cardiovascular risk. Taurine is obtained from the diet but it is less known that it is endogenously synthesized by cysteine dioxygenase type 1 (CDO1). CDO1 exerts a role in adipose tissue from rodent models, but the potential translational value in humans is not available in the literature.
Methods
CDO1 gene expression was analysed in visceral and subcutaneous adipose tissue samples in association with metabolic traits in participants with different degrees of obesity in four independent cohorts. CDO1 was also evaluated in isolated human adipocytes in vitro. Mechanistically, CDO1gene knockdown (KD) of human preadipocytes and adipose-derived mesenchymal stem cells (ASC52telo) (using lentiviral particles) was also evaluated. Mitochondrial respiratory function of adipocytes was evaluated using Seahorse.
Findings
Both visceral (VAT) and subcutaneous adipose tissue (SAT) CDO1 mRNA was associated with gene expression markers of adipose tissue function in the four cohorts. Higher CDO1 expression was linked to decreased fasting triglycerides and blood HbA1c even after adjusting by age, BMI and sex. In addition, CDO1 mRNA positively correlated with the expression of genes involved in adipogenesis and negatively with different inflammatory markers. Both VAT and SAT CDO1 mRNA was mainly expressed in adipocytes and significantly increased during adipocyte differentiation, but attenuated under inflammatory conditions. Mechanistically, CDO1 gene KD reduced taurine biosynthesis, evidencing lower CDO1 activity. In both human preadipocytes and ASC52telo cells, CDO1 gene KD resulted in decreased gene expression markers of adipogenesis (ADIPOQ, FABP4, FASN, SLC2A4, CEBPA) and increased inflammatory genes (TNF and IL6) during adipocyte differentiation. Of note, CDO1 gene KD led to decreased mitochondrial respiratory function in parallel to decreased expression of mitochondrial function-, but not biogenesis-related genes.
Interpretation
Current findings show the relevance of CDO1 in adipose tissue physiology, suggesting its contribution to an improved systemic metabolic profile.
Funding
This work was partially supported by research grants PI16/01173, PI19/01712, PI20/01090 and PI21/01361 from the Instituto de Salud Carlos III from Spain, Fondo Europeo de Desarrollo Regional (FEDER) funds, and VII Spanish Diabetes Association grants to Basic Diabetes Research Projects led by young researchers.
Keywords: Adipogenesis, Adipose tissue, Taurine, Obesity, Inflammation
Research in context.
Evidence before this study
Taurine exerts beneficial effects on systemic metabolism, improving obesity-associated metabolic disturbances. In mice, taurine directly impacts on white adipose tissue, reducing adipocyte hypertrophy and inflammation and modulating adipogenesis. Of note, the key enzyme in taurine biosynthesis, cysteine dioxygenase type 1 (CDO1), exerts an important role in adipocyte differentiation in mouse preadipocytes.
Added value of this study
In the present study, we reported that higher CDO1 expression in human adipose tissue was linked to decreased fasting triglycerides and blood HbA1c even after adjusting by age, BMI and sex. We also found that CDO1 mRNA positively correlated with the expression of genes involved in adipogenesis and negatively with different inflammatory markers. CDO1 gene KD in human preadipocytes and mesenchymal stem cells reduced taurine biosynthesis in parallel to decreased mitochondrial respiratory function and gene expression markers of adipogenesis, but increased inflammatory genes.
Implications of all the available evidence
Current findings show the relevance of CDO1 in human adipose tissue physiology, suggesting its contribution to an improved systemic metabolic profile.
Alt-text: Unlabelled box
Introduction
The global epidemic of obesity associated with metabolic disturbances and increased risk of type 2 diabetes and cardiovascular diseases has multiple dimensions.1 It is well known that subjects with obesity and similar BMI exhibit substantial heterogeneity in metabolic traits. The adipose tissue is a source of multiple factors that modulate systemic insulin sensitivity and cardiovascular risk. The search of novel factors is of utmost importance to try to modulate the long-term risk.
Taurine is a highly abundant free amino acid in mammals, reaching intracellular concentrations in the millimolar range, in contrast to other free amino acids that are found at micromolar range.2 Taurine is present in multiple food sources. It is less known that taurine is also endogenously produced from cysteine, participating in relevant processes for cellular homeostasis,3 and exerting beneficial effects on systemic metabolism. In animal models, taurine administration substantially decreases weight gain under a high-fat diet, increasing energy expenditure and improving obesity-associated metabolic disturbances.3, 4, 5, 6 Taurine directly impacts on white adipose tissue, enhancing thermogenic and oxidative pathways, reducing adipocyte hypertrophy and inflammation and modulating adipogenesis.3,6, 7, 8
Not only exogenous taurine seems important. The key enzyme in taurine biosynthesis, cysteine dioxygenase type 1 (CDO1), also exerts an important role in adipocyte differentiation, since CDO1 is highly expressed in fat depots4,9,10 and enhances the recruitment of PPARγ to the promoters of target genes.11
To the best of our knowledge CDO1 has not been yet studied in human adipose tissue. Here, we aimed to evaluate the possible link of CDO1 gene expression in human adipose tissue to different metabolic traits. We then explored CDO1 gene expression and activity (by evaluating taurine biosynthesis) in human adipocytes. Finally, we studied the possible mechanistic implications by downregulating CDO1 in human preadipocytes and ASC52telo cells.
Methods
Subjects’ recruitment for adipose tissue samples
Cohorts 1-3. Visceral (VAT) and subcutaneous (SAT) adipose tissue samples of a group of 299 (cohort 1), 150 (cohort 2) and 65 (cohort 3) participants with normal body weight and different degrees of obesity (with body mass index (BMI) within 20 and 68 kg/m2) were analysed. All these subjects were recruited at the Endocrinology Service of the Hospital of Girona “Dr Josep Trueta”. Based on previous studies,12,13 these cohorts could be considered to be representative of the general population. All subjects were of Caucasian origin and reported that their body weight had been stable for at least three months before the study. Subjects were studied in the post-absorptive state. BMI was calculated as weight (in kg) divided by height (in m) squared.
Cohort 4. Consecutively, 30 morbidly obese (BMI>35 kg/m2) subjects were recruited at the Endocrinology Service of the Hospital Dr Josep Trueta. This cohort is exclusively representative of the subjects with severe obesity. All subjects were of Caucasian origin and reported a body weight stable for at least three months before the study. Subjects were studied in the post-absorptive state.
The following exclusion criteria were considered in all cohorts (Cohorts 1-4): i) no systemic disease other than obesity; ii) free of any infections in the previous month before the study; iii) no liver diseases (specifically tumoral disease and HCV infection) and thyroid dysfunction, which were specifically excluded by biochemical work-up.
Adipose tissue handling
Adipose tissue samples were obtained from SAT and VAT depots during elective surgical procedures (cholecystectomy, surgery of abdominal hernia and gastric by-pass surgery). Both SAT and VAT samples were collected from the abdomen, following standard procedures. Samples of adipose tissue were immediately transported to the laboratory (5-10 min). The handling of tissue was carried out under strictly aseptic conditions. Adipose tissue samples were washed in PBS, cut off with forceps and scalpel into small pieces (100 mg), and immediately flash-frozen in liquid nitrogen before stored at −80°C. The isolation of adipocyte and stromal vascular fraction cells (SVF) was performed from 12 SAT and 14 VAT non-frozen adipose tissue samples. These samples were washed three to four times with phosphate-buffered saline (PBS) and suspended in an equal volume of PBS supplemented with 1% penicillin-streptomycin and 0.1% collagenase type I prewarmed to 37°C. The tissue was placed in a shaking water bath at 37°C with continuous agitation for 60 minutes and centrifuged for 5 minutes at 300 to 500g at room temperature. The supernatant, containing mature adipocytes, was recollected. The pellet was identified as the SVF. Isolated mature adipocytes and SVF stored at −80°C for gene expression analysis.
Analytical methods
Serum glucose concentrations were measured in duplicate by the glucose oxidase method using a Beckman glucose analyser II (Beckman Instruments, Brea, California). Intra- and inter-assay coefficients of variation were less than 4%. Roche Hitachi Cobas c711 instrument (Roche, Barcelona, Spain) was used to do HDL cholesterol and total serum triglycerides determinations. HDL cholesterol was quantified by a homogeneous enzymatic colorimetric assay through the cholesterol esterase/cholesterol oxidase/ peroxidase reaction (Cobas HDLC3). Serum fasting triglycerides were measured by an enzymatic, colorimetric method with glycerol phosphate oxidase and peroxidase (Cobas TRIGL). C-reactive protein (ultrasensitive assay; 110 Beckman, Fullerton, CA, United States) was determined by a routine laboratory test.
Differentiation of human pre-adipocytes
Isolated human subcutaneous preadipocytes (Zen-Bio Inc., Research Triangle Park, NC) were plated on T-75 cell culture flasks and cultured at 37˚C and 5% CO2 in DMEM/nutrient mix F-12 medium (1:1, vol/vol) supplemented with 10 U/ml penicillin/streptomycin, 10% foetal bovine serum (FBS), 1% HEPES, and 1% glutamine (all from GIBCO, Invitrogen S.A, Barcelona, Spain). One week later, the isolated and expanded human sc preadipocytes were cultured (∼40,000 cells/cm2) in 12-well plates with preadipocytes medium (Zen-Bio) composed of DMEM/nutrient mix F-12 medium (1:1, vol/vol), HEPES, FBS, penicillin, and streptomycin in a humidified 37 C incubator with 5% CO2. Twenty-four hours after plating, cells were checked for complete confluence (d 0), and differentiation was induced using differentiation medium (Zen-Bio) composed of preadipocytes medium, human insulin, dexamethasone, isobutylmethylxanthine, and PPARγ agonists (rosiglitazone). After 7 day (d7), differentiation medium was replaced with fresh adipocyte medium (Zen-Bio) composed of DMEM/nutrient mix F-12 medium (1:1, vol/vol), HEPES, FBS, biotin, pantothenate, human insulin, dexamethasone, penicillin, streptomycin, and amphotericin. Fourteen days after the initiation of differentiation, cells appeared rounded with large lipid droplets apparent in the cytoplasm. Cells were then considered mature adipocytes, harvested, and stored at −80°C for RNA purification. For time course experiment, cells were harvested and stored at −80°C for RNA purification at day 0, 2, 5, 7, 9, 12 and 14.
Treatments with Lipopolysaccharide (LPS)
Primary human subcutaneous preadipocytes (Zen-Bio Inc., Research Triangle Park, NC) were treated with Lipopolysaccharides from Escherichia coli O26:B6 (#L8274, Sigma-Aldrich). Treatments were performed during the differentiation process (at day 0 and day 7) or at the end of differentiation for 96h, at dose 1 µg/ml. PBS was used as control/vehicle condition.
Lipid droplet quantification
Lipid droplet (LDs) area was quantified with Fiji software.14 The area occupied by lipid droplets was selected and quantified using threshold plugin, after sharpening pixel intensity of lipid droplets. Values obtained were expressed as the lipid droplet percentage area with respect to total image.
Oil red O staining
Intracellular lipid accumulation was measured by oil red O staining. For oil red O staining, cells were washed twice with PBS, fixed in 4% formaldehyde for 1 h, and stained for 30 min with 0.2% oil red O solution in 60% isopropanol. Cells were then washed several times with water, and excess water was evaporated by placing the stained cultures at ∼32°C. To determine the extent of adipose conversion, 0.2 mL of isopropanol was added to the stained culture dish. The extracted dye was immediately removed by gentle pipetting, and its optical density was monitored spectrophotometrically at 500 nm by using a multiwell plate reader.
Differentiation of human immortalized adipose-derived mesenchymal stem cells
Human telomerase reverse transcriptase immortalized adipose‐derived MSC (ASC52telo, SCRC‐4000, ATCC, LGC Standards SLU, Barcelona, Spain) cells were cultured in Mesenchymal Stem Cell Basal Medium (ATCC PCS-500-030) plus FBS (2%), rhFGF basic (5 ng/ml), rhFGF acidic (5 ng/ml), rhEGF (5 ng/ml), L-Alanyl-l-Glutamine (2.4 mM) and G418 (0.2 mg/ml) at 37°C in a 5% CO2 in air atmosphere. Adipocyte differentiation was performed as previously reported.15 Briefly, ASC52telo cells were cultured in three repetitive cycles of 72 h in adipogenic differentiation medium composed of DMEM/Nutrient Mix F‐12 medium, FBS (10%), penicillin, streptomycin, human insulin (10 μg/mL), dexamethasone (1 μmol/L), isobutylmethylxanthine (0.5 mmol/L), and PPARγ agonists (rosiglitazone, 1 μmol/L), followed by 72 h in adipogenic maintenance medium composed of DMEM/Nutrient Mix F‐12 medium, FBS (10%), penicillin, streptomycin, and human insulin (10 μg/mL). For time course experiment, cells were harvested and stored at -80°C for RNA purification at day 0, 2, 5, 7, 9, 12 and 15.
Short hairpin (sh) RNA-mediated knockdown of CDO1 gene
Gene knockdown (KD) was performed using CDO1-targeted and control (scrambled, SRC) shRNA lentiviral particles (sc-91642-V and sc-108080, Santa Cruz Biotechnology, CA, USA) following the manufacturer instructions in human subcutaneous preadipocytes and ASC52telo cells at 80% of cell confluence. In human preadipocytes, adipocyte differentiation started 24 h after lentiviral transfection and no antibiotic selection was performed. In ASC52telo cells, positive cells harboring shRNA cassette for CDO1 were selected by puromycin dihydrochloride (3 μg/mL) selection 60 h after infection.
RNA-seq
RNA purification was performed using RNeasy-Tissue Mini-Kit (QIAgen). Total RNA was quantified by Qubit® RNA BR Assay kit (Thermo Fisher Scientific) and the integrity was checked by using the RNA Kit (15NT) on 5300 Fragment Analyzer System (Agilent).
The RNASeq libraries were prepared with Illumina® TruSeq® Stranded Total RNA Sample Preparation kit following the manufacturer´s recommendations with some modifications. Briefly, in function of availability 100-500ng of total RNA was rRNA depleted using the RiboZero Magnetic Gold Kit and fragmented by divalent cations. The strand specificity was achieved during the second strand synthesis performed in the presence of dUTP. The cDNA was adenylated and ligated to Illumina platform compatible IDT adaptors with unique dual indexes with unique molecular identifiers (Integrated DNA Technologies), for paired end sequencing. The ligation products were enriched with 15 PCR cycles and the final library was validated on an Agilent 2100 Bioanalyzer with the DNA 7500 assay (Agilent). The libraries were sequenced on NovaSeq 6000 (Illumina) in a fraction of sequencing flow cell with a read length of 2×101bp following the manufacturer's protocol for dual indexing. Image analysis, base calling and quality scoring of the run were processed using the manufacturer's software Real Time Analysis (RTA v3.4.4) and followed by generation of FASTQ sequence files.
RNA-seq reads were mapped against human reference genome (GRCh38) using STAR software version 2.5.3a16 with ENCODE parameters. Genes were quantified using RSEM version 1.3.017 with default parameters and using the annotation file from GENCODE version 29. Only protein-coding genes that were expressed >1 cpm in at least 10 samples were considered for the association of gene expression with clinical variables. We used the ‘limma-voom’ R package18 with a model adjusting by BMI, sex and age and correcting by the ‘sgof’19 multiple testing method, as detailed in statistical analysis section. RNA-seq data were deposited in GEO with the accession number GSE213058.
RNA expression
RNA purification was performed using RNeasy Lipid Tissue Mini Kit (QIAgen, Izasa SA, Barcelona, Spain) and the integrity was checked by Agilent Bioanalyzer (Agilent Technologies, Palo Alto, CA). Gene expression was assessed by real time PCR using a LightCycler® 480 Real-Time PCR System (Roche Diagnostics SL, Barcelona, Spain), using TaqMan® technology suitable for relative genetic expression quantification. The RT-PCR reaction was performed in a final volume of 12 μl. The cycle program consisted of an initial denaturing of 10 min at 95°C then 40 cycles of 15 s denaturizing phase at 95°C and 1 min annealing and extension phase at 60°C. A threshold cycle (Ct value) was obtained for each amplification curve and then a ΔCt was first calculated by subtracting the Ct value for human cyclophilin A (PPIA) RNA from the Ct value for each sample. Fold changes compared with the endogenous control were then determined by calculating 2−ΔCt, so that gene expression results are expressed as expression ratio relative to PPIA gene expression according to the manufacturer's guidelines. TaqMan® primer/probe sets (Thermo Fisher Scientific, Waltham, MA, USA) used were as follows: Peptidylprolyl isomerase A (cyclophilin A) (4333763, PPIA as endogenous control), cysteine dioxygenase type 1 (CDO1, Hs02797011_m1), adiponectin (ADIPOQ, Hs00605917_m1), peroxisome proliferator-activated receptor gamma (PPARG, Hs00234592_m1), fatty acid synthase (FASN, Hs00188012_m1), CCAAT/enhancer binding protein alpha (CEBPA, Hs00269972_s1), solute carrier family 2 member 4 (SLC2A4, Hs00168966_m1), fatty acid binding protein 4, adipocyte (FABP4, Hs01086177_m1), leptin (LEP, Hs00174877_m1), PPARG coactivator 1 alpha (PPARGC1A, Hs00173304_m1), cytochrome c oxidase subunit 5B (COX5B, Hs00426950_g1), succinate dehydrogenase complex flavoprotein subunit A (SDHA, Hs07291714_mH), ubiquinol-cytochrome c reductase core protein 1 (UQCRC1, Hs00163415_m1), voltage dependent anion channel 1 (VDAC1, Hs04978484_m1), uncoupling protein 1 (UCP1, Hs01084772_m1), cell death-inducing DFFA-like effector a (CIDEA, Hs00154455_m1), interleukin 6 (interferon, beta 2) (IL6, Hs00985639_m1), tumor necrosis factor (TNF, Hs00174128_m1) and C-X-C motif chemokine ligand 8 (CXCL8, Hs00174103_m1).
Measurement of taurine levels
Taurine levels in cell culture were measured using Taurine Assay Kit (colorimetric) (K988-100, BioVision, Inc., CA, USA), strictly following the manufacturer's instructions.
Mitochondrial respiration
Mitochondrial respiratory function was assessed in controla and CDO1 gene KD ASC52telo cells by means of a Seahorse XFp Extracellular Flux Analyzer (Seahorse Bioscience, Agilent Technologies) using Seahorse XFp Cell Mito Stress Test Kit according to manufacturer's instructions. Cells were cultured for 48 h, followed by 60 min of culture with XF base medium supplemented with 1 mM pyruvate, 2 mM glutamine, and 10 mM glucose in a CO2 free incubator. Oxygen consumption rate (OCR) was normalized to the total cell number.
Statistical analyses
Statistical analyses were performed using SPSS 12.0 software. Unless otherwise stated, descriptive results of continuous variables are expressed as mean and SD for Gaussian variables or median and interquartile range for non-Gaussian variables. Gaussianity was evaluated using the Kolmogorov-Smirnov test. Since most of gene expression parameters, (including CDO1 gene expression) were non-Gaussian variables, the relation between variables was analysed by simple correlation using the nonparametric spearman correlation coefficient. One factor ANOVA was used to compare Gaussian variables according to obesity in human cohorts, and nonparametric test (Mann Whitney test) to compare non-Gaussian variables. Unpaired t-test and nonparametric test (Mann Whitney test) were used to analyse in vitro experiments. Levels of statistical significance were set at P<0.05.
Differential expression gene analyses were performed on gene counts using the “limma” R package.18 First, low expressed genes were filtered, so that only genes with more than 10 reads in at least 2 samples were selected. RNA-seq data were then normalized for RNA composition using the trimmed mean of M-value (TMM) as implemented in edgeR package.20 Normalized counts were then converted to log2 count per million (logCPM) with associated precision weights to account for variations in precision between different observations using the “voom” function with donor's age, BMI, sex, and education years as covariates. A robust linear regression model adjusted for the previous covariates was then fitted to the data using the “lmFit” function with the option method=“robust”, to limit the influence of outlying samples. Finally, an empirical Bayes method was applied to borrow information between genes with the “eBayes” function. P-values were adjusted for multiple comparisons using a Sequential Goodness of Fit21 as implemented in the “SGoF” R package. Unlike FDR methods, which decrease their statistical power as the number of test increases, SGoF methods increase their power with increasing number of tests. SGoF has proven to behave particularly better than FDR methods with high number of tests and low sample size, which is the case of omics large datasets. The functional roles of differentially expressed genes were characterized using over-representation analyses based on the Reactome and KEGG databases using ConsensusPathDB.22 Pathway significance was assessed using a hypergeometric test and a Storey procedure (qvalue) was applied for multiple testing correction. Statistical significance was set at qvalue<0.1.
Ethics
Samples and data from patients included in cohort 1 and cohort 2 were provided by the FATBANK platform promoted by the CIBEROBN and coordinated by the IDIBGI Biobank (Biobanc IDIBGI, B.0000872), integrated in the Spanish National Biobanks Network and they were processed following standard operating procedures with the appropriate approval of the Ethics, External Scientific and FATBANK Internal Scientific Committees (approval numbers: 009/19 for cohort 1 and 43/09 for cohort 2). The protocols of cohort 3 and cohort 4 were revised, validated and approved by the Ethics committee of the Hospital Dr Josep Trueta (approval numbers: 2009 046 for cohort 3 and 143_19 for cohort 4). In all cohorts, the purpose of the study was explained to participants and they signed written informed consent before being enrolled in the study.
Role of funders
The funders did not have any role in study design, data collection, data analyses, interpretation, or writing of report.
Results
CDO1 gene expression is associated with markers of adipose tissue function
Both visceral (VAT) and subcutaneous adipose tissue (SAT) CDO1 mRNA was associated with gene expression markers of adipose tissue function in the four cohorts.
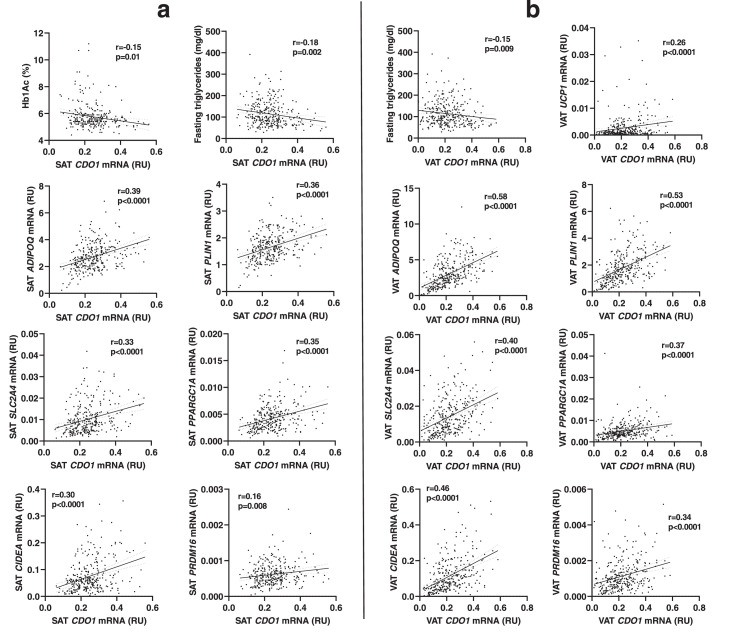
In cohort 1, in which samples from subjects with different degree of obesity were evaluated, no significant differences of VAT and SAT CDO1 gene expression according to obesity were found (Table 1). SAT CDO1 gene expression was negatively correlated with age, HbA1c, fasting triglycerides, and positively with HDL-cholesterol, and gene expression markers of adipogenesis (ADIPOQ, FASN, PLIN1, SLC2A4) and thermogenesis and catabolism-related processes (PRDM16, PPARGC1A, CIDEA) (Table 2 and Figure 1a). Multiple linear regression analysis indicated that these associations were maintained after adjusting for age, gender and BMI (Table 3). Specifically, SAT CDO1 gene expression was an important factor contributing to decreased HbA1c (P=0.04) and fasting triglycerides (P=0.03), but increased HDL-cholesterol (P=0.02), and expression of relevant genes for adipose tissue physiology [CIDEA (P<0.0001), PPARGC1A (<0.0001), PRDM16 (P=0.003), SLC2A4 (<0.0001), ADIPOQ (<0.0001), FASN (<0.0001) and PLIN1 (<0.0001)] after adjusting for sex, age, and BMI (Table 3).
Table 1.
Anthropometric, clinical parameters and VAT and SAT CDO1 mRNA levels according to obesity in cohort 1.
| Non-obese | Obese | P-value | |
|---|---|---|---|
| N | 112 | 187 | |
| Sex (men/women) | 15/97 | 39/148 | |
| Age (years) | 47.08 ± 11.4 | 47.8 ± 10.6 | 0.5 |
| BMI (kg/m2) | 24.6 ± 3.1 | 42.5 ± 7.2 | <0.0001 |
| Fasting glucose (mg/dl)a | 90 (85-97.7) | 96 (88-113) | 0.03 |
| HbA1c (%) | 5.5 ± 0.68 | 5.9 ± 1.02 | <0.0001 |
| HDL-cholesterol (mg/dl) | 61.57 ± 16.1 | 50.1 ± 13.9 | <0.0001 |
| Fasting triglycerides (mg/dl)a | 86.5 (58.5-114.2) | 117 (82.2-158.7) | <0.0001 |
| hsCRP (mg/dl)a | 0.35 (0.11-0.68) | 0.73 (0.34-1.45) | <0.0001 |
| VAT CDO1 (RU)a | 0.20 (0.14-0.31) | 0.21 (0.13-0.29) | 0.7 |
| SAT CDO1 (RU)a | 0.25 (0.19-0.31) | 0.23 (0.17-0.28) | 0.07 |
Median and interquartile range; R.U., relative gene expression units to PPIA (endogenous control) gene expression.
Table 2.
Bivariate correlations between VAT and SAT CDO1 mRNA and clinical and anthropometric parameters, and adipose tissue gene expression in cohort 1. These correlations were analysed using the nonparametric Spearman correlation coefficient.
| VAT CDO1 (RU) |
SAT CDO1 (RU) |
|||
|---|---|---|---|---|
| r | P | r | P | |
| Age (years) | −0.03 | 0.6 | −0.15 | 0.01 |
| BMI (kg/m2) | −0.05 | 0.4 | −0.07 | 0.2 |
| Fasting glucose (mg/dl) | −0.03 | 0.6 | −0.06 | 0.3 |
| HbA1c (%) | 0.01 | 0.8 | −0.15 | 0.01 |
| HDL-cholesterol (mg/dl) | 0.11 | 0.06 | 0.14 | 0.02 |
| Fasting triglycerides (mg/dl) | −0.15 | 0.009 | −0.18 | 0.002 |
| hsCRP (mg/dl) | −0.05 | 0.4 | −0.02 | 0.7 |
| CIDEA (RU) | 0.46 | <0.0001 | 0.30 | <0.0001 |
| PPARGC1A (RU) | 0.37 | <0.0001 | 0.35 | <0.0001 |
| UCP1 (RU) | 0.26 | <0.0001 | −0.04 | 0.5 |
| PRDM16 (RU) | 0.34 | <0.0001 | 0.16 | 0.008 |
| SLC2A4 (RU) | 0.40 | <0.0001 | 0.33 | <0.0001 |
| ADIPOQ (RU) | 0.58 | <0.0001 | 0.39 | <0.0001 |
| FASN (RU) | 0.43 | <0.0001 | 0.36 | <0.0001 |
| PLIN1 (RU) | 0.53 | <0.0001 | 0.36 | <0.0001 |
R.U., relative gene expression units to PPIA (endogenous control) gene expression.
Figure 1.
(a) Bivariate correlations (Spearman correlation coefficient) between SAT CDO1 mRNA and HbA1c, fasting triglycerides, and expression of ADIPOQ, PLIN1, SLC2A4, PPARGC1A, CIDEA and PRDM16 genes in SAT (cohort 1). (b) Bivariate correlations between VAT CDO1 mRNA and fasting triglycerides, and expression of UCP1, ADIPOQ, PLIN1, SLC2A4, PPARGC1A, CIDEA and PRDM16 genes in VAT (cohort 1).
Table 3.
Multiple linear regression analysis to predict the impact of SAT CDO1 mRNA on metabolic parameters and on SAT gene expressions in cohort 1.
| HbA1c (%) |
HDL-cholesterol (mg/dl) |
Fasting triglycerides (mg/dl) |
||||
|---|---|---|---|---|---|---|
| t | P | t | P | t | P | |
| Age (years) | 4.09 | <0.0001 | 1.77 | 0.07 | 2.25 | 0.02 |
| Gender | 0.26 | 0.78 | 4.87 | <0.0001 | −2.94 | 0.003 |
| BMI (kg/m2) | 3.85 | <0.0001 | −6.57 | <0.0001 | 2.93 | 0.004 |
| SAT CDO1 (RU) | −2.05 | 0.04 | 2.29 | 0.02 | −2.09 | 0.03 |
| Adjusted R2 | 0.116 | 0.218 | 0.091 | |||
| ANOVA P | <0.0001 | <0.0001 | <0.0001 | |||
| SAT CIDEA (RU) |
SAT PPARGC1A (RU) |
SAT PRDM16 (RU) |
||||
|---|---|---|---|---|---|---|
| t | P | t | P | t | P | |
| Age (years) | −1.37 | 0.17 | 0.33 | 0.7 | 1.74 | 0.08 |
| Gender | −1.41 | 0.16 | −0.46 | 0.6 | −0.42 | 0.67 |
| BMI (kg/m2) | −8.96 | <0.0001 | −2.44 | 0.01 | −1.76 | 0.08 |
| SAT CDO1 (RU) | 5.54 | <0.0001 | 5.92 | <0.0001 | 2.95 | 0.003 |
| Adjusted R2 | 0.296 | 0.122 | 0.034 | |||
| ANOVA P | <0.0001 | <0.0001 | 0.007 | |||
| SAT SLC2A4 (RU) |
SAT ADIPOQ (RU) |
SAT FASN (RU) |
||||
|---|---|---|---|---|---|---|
| t | P | t | P | t | P | |
| Age (years) | −1.64 | 0.10 | 0.18 | 0.86 | −1.77 | 0.07 |
| Gender | 1.63 | 0.11 | 2.41 | 0.02 | −0.63 | 0.53 |
| BMI (kg/m2) | −7.82 | <0.0001 | −5.14 | <0.0001 | −7.71 | <0.0001 |
| SAT CDO1 (RU) | 4.51 | <0.0001 | 6.48 | <0.0001 | 4.54 | <0.0001 |
| Adjusted R2 | 0.251 | 0.220 | 0.237 | |||
| ANOVA P | <0.0001 | <0.0001 | <0.0001 | |||
| SAT PLIN1 (RU) |
||||||
|---|---|---|---|---|---|---|
| t | P | |||||
| Age (years) | −0.59 | 0.55 | ||||
| Gender | 1.88 | 0.06 | ||||
| BMI (kg/m2) | −2.12 | 0.03 | ||||
| SAT CDO1 (RU) | 6.31 | <0.0001 | ||||
| Adjusted R2 | 0.151 | |||||
| ANOVA P | <0.0001 | |||||
R.U., relative gene expression units to PPIA (endogenous control) gene expression.
VAT CDO1 gene expression also was negatively correlated with fasting triglycerides, and positively with ADIPOQ, FASN, PLIN1, SLC2A4, UCP1, PRDM16, PPARGC1A, CIDEA (Table 2 and Figure 1b). Again, these associations were maintained after adjusting for age, gender and BMI (Table 4). Multiple linear regression analysis indicated that VAT CDO1 gene expression was an important factor contributing to decreased fasting triglycerides (P=0.03), and increased gene expression of CIDEA (P<0.0001), PPARGC1A (<0.0001), UCP1 (P=0.005), PRDM16 (P<0.0001), SLC2A4 (<0.0001), ADIPOQ (<0.0001), FASN (<0.0001) and PLIN1 (<0.0001)] after adjusting for sex, age, and BMI (Table 4).
Table 4.
Multiple linear regression analysis to predict the impact of VAT CDO1 mRNA on metabolic parameters and on VAT gene expressions in cohort 1.
| Fasting triglycerides (mg/dl) |
VAT CIDEA (RU) |
VAT PPARGC1A (RU) |
||||
|---|---|---|---|---|---|---|
| t | P | t | P | t | P | |
| Age (years) | 2.83 | 0.005 | −0.86 | 0.39 | 0.53 | 0.59 |
| Gender | −3.53 | <0.0001 | 1.61 | 0.11 | −0.63 | 0.52 |
| BMI (kg/m2) | 2.82 | 0.005 | −8.11 | <0.0001 | 0.61 | 0.54 |
| VAT CDO1 (RU) | −2.12 | 0.03 | 8.61 | <0.0001 | 4.91 | <0.0001 |
| Adjusted R2 | 0.099 | 0.334 | 0.064 | |||
| ANOVA P | <0.0001 | <0.0001 | <0.0001 | |||
| VAT UCP1 (RU) |
VAT PRDM16 (RU) |
VAT SLC2A4 (RU) |
||||
|---|---|---|---|---|---|---|
| t | P | t | P | t | P | |
| Age (years) | 1.56 | 0.11 | 1.33 | 0.18 | −1.29 | 0.19 |
| Gender | 1.15 | 0.25 | −1.35 | 0.17 | 3.98 | <0.0001 |
| BMI (kg/m2) | 0.03 | 0.97 | 1.91 | 0.06 | −8.26 | <0.0001 |
| VAT CDO1 (RU) | 2.84 | 0.005 | 6.25 | <0.0001 | 6.94 | <0.0001 |
| Adjusted R2 | 0.027 | 0.118 | 0.323 | |||
| ANOVA P | 0.017 | <0.0001 | <0.0001 | |||
| VAT ADIPOQ (RU) |
VAT FASN (RU) |
VAT PLIN1 (RU) |
||||
|---|---|---|---|---|---|---|
| t | P | t | P | t | P | |
| Age (years) | −0.51 | 0.61 | −0.61 | 0.54 | 1.17 | 0.24 |
| Gender | 3.91 | <0.0001 | −0.03 | 0.97 | 2.36 | 0.02 |
| BMI (kg/m2) | −6.21 | <0.0001 | −7.29 | <0.0001 | −2.24 | 0.02 |
| VAT CDO1 (RU) | 9.87 | <0.0001 | 7.11 | <0.0001 | 8.79 | <0.0001 |
| Adjusted R2 | 0.349 | 0.256 | 0.234 | |||
| ANOVA P | <0.0001 | <0.0001 | <0.0001 | |||
R.U., relative gene expression units to PPIA (endogenous control) gene expression.
These associations led us to explore the correlation between markers of the adipogenesis and thermogenesis in SAT and VAT. Of note, we found that most of these correlations were positive, except the correlations between expression of UCP1 and some adipogenic (ADIPOQ, PLIN1 and FASN) genes in SAT (Supplemental Table 1).
To strengthen these findings, VAT and SAT CDO1 gene expression was analysed in two additional independent cohorts (Supplemental Table 2). Similar to in cohort 1, SAT CDO1 mRNA was negatively correlated with HbA1c in cohort 3 (Supplemental Table 3), and both VAT and SAT CDO1 gene expression were associated with gene expression markers of adipogenesis (ADIPOQ, SLC2A4, CIDEA, and FASN only in VAT), but also with thermogenic-related gene expression (PPARGC1A and UCP1) in cohort 3 and cohort 4 (Supplemental Table 3). In VAT, CDO1 was negatively correlated to TNF gene expression (Supplemental Table 3).
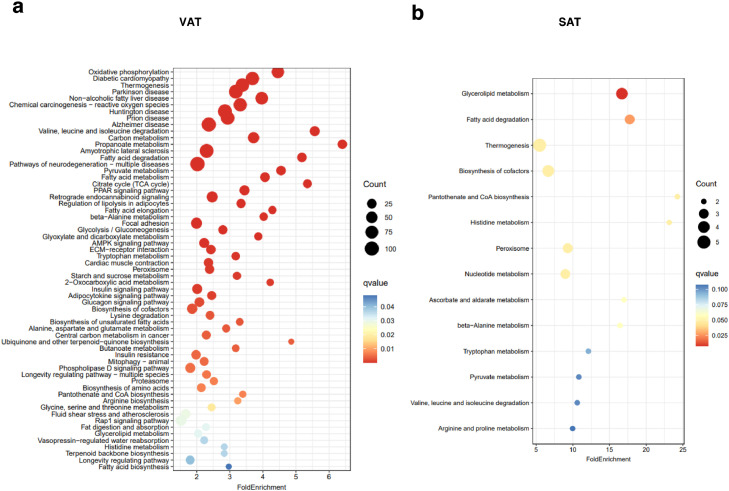
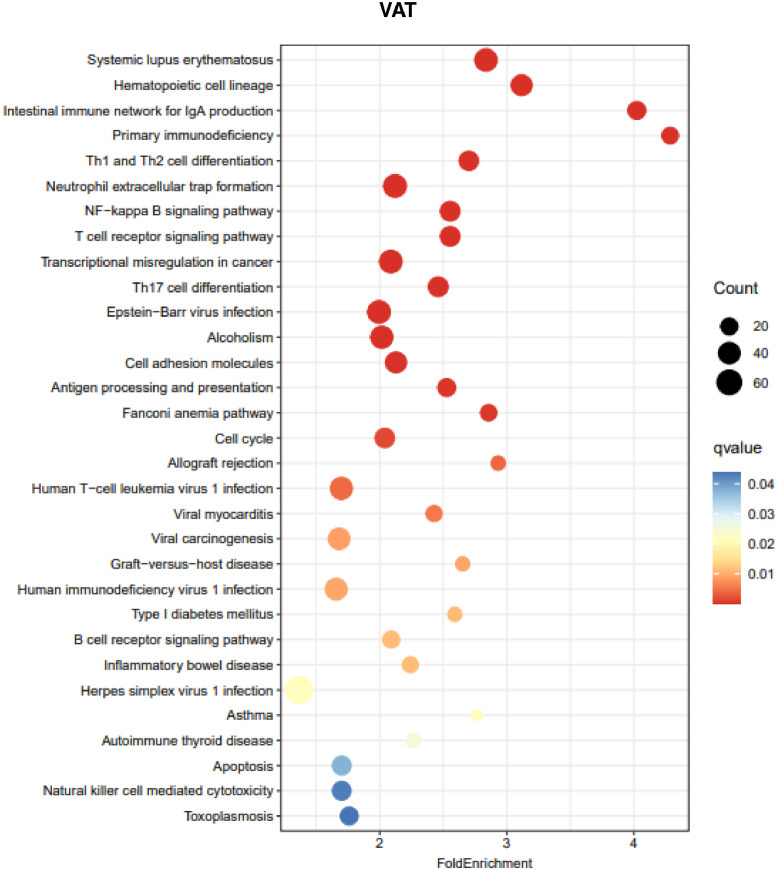
Next, to study the relevance of CDO1 in human adipose tissue, and taking advantage from unpublished data from a recent RNAseq analysis performed in human subcutaneous and visceral adipose tissue (Cohort 4, N=30, Supplemental Table 4), bivariate correlations between CDO1 mRNA and all transcriptome adjusting by age, sex and BMI, and KEGG- and Reactome-pathways enrichment analysis were performed as detailed in methods. Of note, VAT CDO1 mRNA was positively associated with important pathways for adipose tissue physiology, including fatty acid metabolism (biosynthesis and degradation), PPAR signaling, thermogenesis, AMPK signaling, adipocytokine and insulin signaling, insulin action, oxidative phosphorylation, glycerolipid metabolism and mitochondrial activity and function (q value<0.04, Figure 2a, Suppl. Figure 1), and negatively with pathways related to inflammation, immune response and cellular senescence (q value<0.01, Figure 3, Suppl. Figure 2). Compared to VAT, fewer associations were found in SAT (Figure 2b). However, SAT CDO1 mRNA was also positively associated with important pathways for adipose tissue physiology, including glycerolipid metabolism, fatty acid degradation and thermogenesis (q value<0.04, Figure 2b).
Figure 2.
(a-b)CDO1 mRNA levels was positively correlated with transcripts from adipose tissue function-related KEGG pathways after adjusting by age, BMI and gender in VAT (a) and SAT (b) (cohort 4).
Figure 3.
CDO1 mRNA levels was negatively correlated with transcripts from adipose tissue inflammation and senescence-related KEGG pathways after adjusting by age, BMI and gender in VAT (cohort 4).
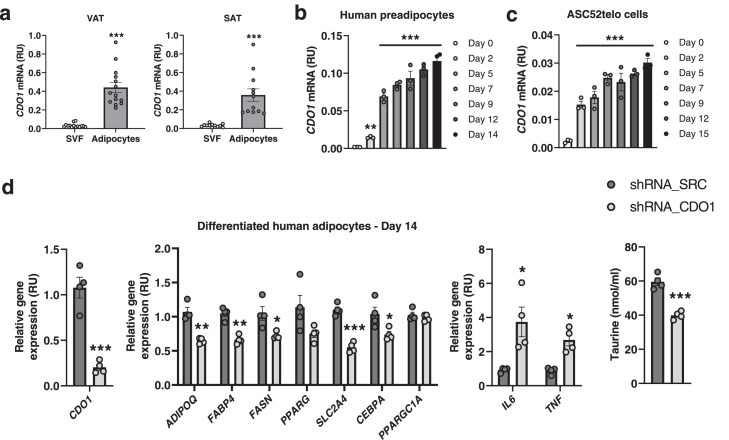
CDO1 gene is highly expressed in human adipocytes, but inhibited under inflammatory conditions
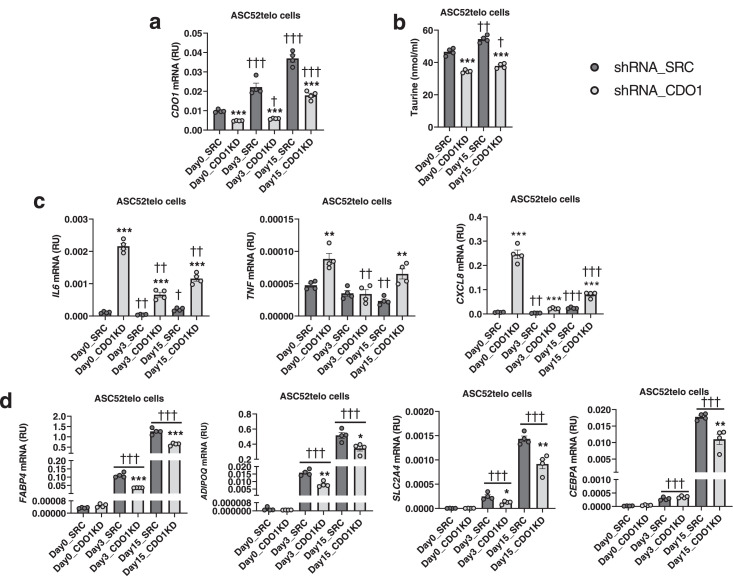
Evaluating adipose tissue cells, we found that CDO1 gene was mainly expressed in adipocytes compared to stromal vascular cell fraction in both visceral (VAT) and subcutaneous (SAT) adipose tissue (Figure 4a). During adipocyte differentiation of human preadipocytes (Figure 4b) or adipose-derived immortalized mesenchymal stem cells (ACS52telo, Figure 4c), CDO1 mRNA levels were significantly increased, achieving the highest amount in the fully differentiated adipocytes (at day14/15, Figures 4b-c). In addition, similar to adipogenic genes, CDO1 gene expression was significantly attenuated under inflammatory conditions, achieved after lipopolysaccharide (LPS, 1µg/ml) administration, during human adipocyte differentiation (Suppl. Figure 3a) and in fully differentiated human adipocytes during 96h (Suppl. Figure 3b). These results indicate that CDO1 gene expression in adipocytes and adipose tissue might be regulated through a similar mechanism to that of adipogenic genes, and suggest a possible role of CDO1 in human adipogenesis. To functionally investigate the association between CDO1 and adipogenesis, CDO1 gene knockdown (KD) experiments using lentiviral particles were performed.
Figure 4.
(a) CDO1 mRNA levels in VAT and SAT cell fractions. ***P<0.001 compared to SVF (Unpaired t-test). (b-c)CDO1 mRNA during adipocyte differentiation in a time course experiment in human preadipocytes (b) and in ACS52telo cells (c). **P<0.01 and ***P<0.005 compared to day 0 (Mann Whitney test). (d) Effects of CDO1 gene KD on CDO1, adipogenic (ADIPOQ, FABP4, FASN, PPARG, SLC2A4, CEBPA)-related, mitochondrial (PPARGC1A)-related and inflammatory (IL6, TNF)-related gene expression, and on taurine levels in culture medium in human preadipocytes at day 14 of adipocyte differentiation. *P<0.05, **P<0.01 and ***P<0.005 compared to shRNA_SRC (Mann Whitney test).
CDO1 gene KD impairs human adipogenesis and reduces adipocyte taurine biosynthesis
During differentiation of human preadipocytes, CDO1 gene KD resulted in decreased expression of adipogenesis (ADIPOQ, FABP4, FASN, SLC2A4, CEBPA)-related genes, but increased inflammatory (TNF and IL6) gene expression (Figure 4d). As expected, CDO1 gene KD also reduced taurine biosynthesis, evidencing lower CDO1 activity (Figure 4d). Interestingly, CDO1 gene KD also attenuated taurine biosynthesis in non-differentiated (Day 0) and in fully differentiated adipocyte (Day 15) ASC52telo cells (Figures 5a-b), in parallel to an important induction of inflammatory genes, especially at day 0 (Figures 5c), and decreased expression of adipogenic genes at day 3 and 15 of adipocyte differentiation process (Figures 5d) and reduced lipid droplet area percentage (Suppl. Figure 4a) and intracellular lipid accumulation measured by Oil Red O staining (Suppl. Figure 4b).
Figure 5.
(a-d) Effects of CDO1 gene KD on CDO1 mRNA levels (a), taurine levels in culture medium (b), and inflammatory (IL6, TNF, CXCL8) (c) and adipogenic (FABP4, ADIPOQ, SLC2A4, CEBPA) (d) mRNA levels in ACS52telo cells at day 0, 3 and 15 of adipocyte differentiation. *P<0.05, **P<0.01 and ***P<0.005 compared to shRNA_SRC. †P<0.05, ††P<0.01 and †††P<0.005 compared to day 0 (Mann Whitney test).
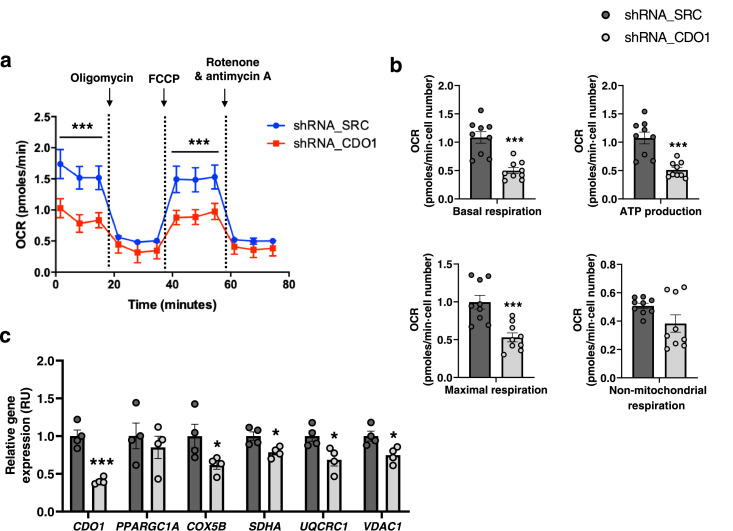
CDO1 gene KD reduces mitochondrial respiration in adipocyte precursor cells
CDO1 gene KD in ASC52telo cells resulted in a significant decrease in mitochondrial respiration, including basal and maximal respiration and oxygen consumption for ATP production, without significant effects on non-mitochondrial respiration (Figure 6a-b). Next, to examine if this reduction in mitochondrial respiration is caused by decreased mitochondrial biogenesis or function, expression of peroxisome proliferator-activated receptor-γ coactivator 1-alpha (PPARGC1A), a key regulator of mitochondrial biogenesis,23,24 and expression of mitochondrial respiration- and function-related genes, such as cytochrome c oxidase subunit 5B (COX5B), succinate dehydrogenase complex flavoprotein subunit A (SDHA) and ubiquinol-cytochrome c reductase core protein 1 (UQCRC1) (three relevant enzymes of mitochondrial respiratory chain), and voltage dependent anion channel 1 (VDAC1), the major component of the outer mitochondrial membrane that regulates mitochondrial functions, were analysed. Interestingly, while CDO1 gene knockdown (KD) in ACS52telo cells did not impact on expression of PPARGC1A gene, it led to decreased expression of relevant genes for mitochondrial respiration and function (COX5B, SDHA, UQCRC1, VDAC1) (Figure 6c), indicating that CDO1 KD affected mitochondrial function, but not biogenesis.
Figure 6.
(a-c) The impact of CDO1 gene KD on mitochondrial respiratory function in ACS52telo cells, including basal respiration, ATP production, maximal respiration and non-mitochondrial respiration (a-b) and expression of CDO1, mitochondrial biogenesis (PPARGC1A)- and respiratory function (COX5B, SDHA, UQCRC1, VDAC1)-related genes (c). *P<0.05 and ***P<0.005 compared to shRNA_SRC (Mann Whitney test).
Discussion
Recent studies that have evaluated the extreme phenotypes/mRNA in plasma or in adipose tissue of subjects with obesity, did not capture CDO1 as a differentiated expressed gene.25,26 Our target approach in four different cohorts and in vitro functional analyses has uncovered the contribution of CDO1 to systemic metabolic traits.
The present study reveals CDO1 as a new key player in human adipogenesis and in adipocyte taurine biosynthesis. The downregulation of CDO1 in human preadipocytes or adipose-mesenchymal stem cells resulted in a significant reduction in gene expression markers of adipogenesis in parallel to increased proinflammatory cytokines and attenuated taurine levels in the culture medium. Translating these findings into a more physiological context, we found that adipose tissue CDO1 mRNA was positively correlated to adipogenesis-related gene expression, but negatively to inflammation-related gene expression (only in VAT). Current findings in humans were in agreement with previous studies in mice, showing the association between CDO1 and adipogenesis in 3T3-L1 mouse cell line and in mouse bone marrow-derived mesenchymal stem cells,11 and the contribution of CDO1 to adipose taurine biosynthesis.4,10 In human adipose tissue, we also found that CDO1 was positively correlated with thermogenic- and catabolic-related gene expression (UCP1, PRDM16, CIDEA and PPARGC1A), suggesting a possible induction of adipose tissue-disseminated beige/brite adipocytes27 associated to CDO1 mRNA levels and taurine biosynthesis. In fact, recent studies demonstrated that taurine has direct effects inducing adipocyte thermogenesis and promoting browning of white adipose tissue, which results in a significant reduction of body weight and fat mass.3,28,29 In line with this, reduced adipose tissue CDO1 mRNA was observed in genetic- and diet-induced obesity mice models4 and in human visceral adipose tissue from morbidly obese subjects (current study). Considering that expression of adipogenic genes in adipose tissue is also decreased in morbid obesity, as consequence of obesity-associated adipose tissue dysfunction,12,30, 31, 32, 33 the positive correlation between mRNA expression of CDO1 and adipogenic genes would be in consonance with the anti-obesity effects of CDO1 activity. In addition, CDO1 gene knockdown in human adipocyte precursor cells impairs mitochondrial respiration, a relevant process in adipogenesis and adipocyte function.34, 35, 36 Of note, gene expression analysis indicated that CDO1 KD affected mitochondrial function, but not biogenesis. In agreement with this finding, the relevance of taurine on mitochondrial function and biology has been previously substantiated.37
Interestingly, most of adipogenic gene expression were positively correlated with expression of thermogenic- and catabolic-related genes. In line with these findings, previous studies reported a positive correlation between adipogenic and thermogenic related-genes in human adipose tissue,38, 39, 40 suggesting a coexistence of white and brite/brown adipocytes, as recently demonstrated in a single-cell transcriptome study of mesenchymal progenitor cells from human adipose tissue.41
Another interesting finding of the current study is the observed proinflammatory effects of CDO1 gene knockdown in human adipocytes and in ASC52 telo cells, and the negative association between systemic (hsCRP) or tissue inflammation (TNF) and CDO1 gene expression in human visceral adipose tissue. These observations might be explained by the previously reported anti-inflammatory activity of taurine in adipose tissue7,42 or attenuating systemic inflammation.43
Adipose tissue CDO1 gene expression also was negatively associated to fasting triglycerides and HbA1c levels, but positively to HDL-cholesterol, suggesting that adipose CDO1 activity might exert a positive role in the prevention of obesity-associated metabolic disturbances, as consequence of the CDO1-linked improvement of adipose tissue adipogenesis. Healthy expansion of adipose tissue, characterized by increased adipogenesis, enhanced capacity to store and catabolize lipid excess and attenuated inflammation, is required to maintain good metabolic health in obesity.44, 45, 46 Otherwise, since in situations of adipose tissue dysfunction (increased adipose tissue inflammation and decreased adipogenesis) CDO1 mRNA levels were downregulated, the negative association between CDO1 mRNA and HbA1c or fasting triglycerides could be explained by the negative impact of adipose tissue dysfunction on obesity-associated metabolic disturbances.44, 45, 46 These findings suggest that CDO1 gene expression in adipose tissue might be regulated through a similar mechanism to that of adipogenic genes. Supporting this idea and in line with previous studies in mouse 3T3-L1 cells,10,11 current observations show how CDO1 gene expression was significantly increased in response to adipogenic stimuli in human cells, but decreased in response to anti-adipogenic factors, such as inflammatory conditions. At the cellular level, current data also indicated that CDO1 gene expression is associated with adipocyte function. While adequate adipocyte function is inversely associated with inflammation, excess of fat accumulation in adipocytes led to a phenotype of adipocyte hypertrophy, characterized by increased inflammation and reduced adipogenic markers.44, 45, 46
From a translational perspective and compared to exogenous taurine administration, the induction of CDO1 expression or activity to enhance endogenous taurine biosynthesis in adipose tissue could constitute a more precise and specific therapeutic approach to improve adipose tissue dysfunction and obesity-associated metabolic disturbances.
The lack of diversity in the cohorts (we only included subjects of Caucasian origin) should be considered as an important limitation to generalise the study findings. Another limitation of current study was the absence of in vivo experiments, for instance to further investigate the impact of specific adipose tissue CDO1 induction on obesity-associated adipose tissue dysfunction.
To sum up, altogether these data indicate that CDO1 might be a new important player in human adipose tissue, which could act at three different levels. Directly, CDO1 improves adipogenesis in white adipocytes by enhancing PPARγ transactivation,11 and indirectly, CDO1-produced taurine in adipocytes might has autocrine and paracrine effects on adipose tissue, enhancing thermogenic pathway in beige/brite adipocytes and attenuating visceral adipose tissue inflammation.
Contributors
JL, JM-P, NO-C, FO and FC participated in this study acquiring and analysing data; JMFR participated in this study analysing data and contributed to the discussion and reviewed the manuscript; JMMN contributed to research study design, conducting experiments, acquiring and analysing data, and writing the manuscript. JL, JM-P, NO-C, JMFR and JMMN have directly accessed and verified the underlying data reported in the manuscript. All authors have read and approved the final version of the manuscript.
Data sharing statement
All data generated or analysed during this study are included in this published article (and its supplementary information files). RNA-seq data collected for the study are available on GEO [GEO Accession viewer (nih.gov)] with the accession number GSE213058.
Declaration of interests
The authors declared no conflict of interest.
Acknowledgements
We acknowledge the technical assistance of Marc Dabad (CNAG-CRG), Beatriz Martín-Mur (CNAG-CRG) and Dr Anna Esteve-Codina (CNAG-CRG) in RNAseq data processing and uploading to GEO. We want to particularly acknowledge the patients, the FATBANK platform promoted by the CIBEROBN and the IDIBGI Biobank (Biobanc IDIBGI, B.0000872), integrated in the Spanish National Biobanks Network, for their collaboration and coordination. This work was partially supported by research grants PI16/01173, PI19/01712, PI20/01090 and PI21/01361 from the Instituto de Salud Carlos III from Spain, co-financed with Fondo Europeo de Desarrollo Regional (FEDER) funds, and VII Spanish Diabetes Association grants to Basic Diabetes Research Projects led by young researchers. CIBEROBN Fisiopatología de la Obesidad y Nutrición is an initiative from the Instituto de Salud Carlos III from Spain.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104302.
Contributor Information
José Manuel Fernández-Real, Email: jmfreal@idibgi.org.
José María Moreno-Navarrete, Email: jmoreno@idibgi.org.
Appendix. Supplementary materials
Supplementary Figure 1.CDO1 mRNA levels were positively correlated with transcripts from adipose tissue function-related Reactome pathways after adjusting by age, BMI and gender in VAT (cohort 4). BMI, body mass index.
Supplementary Figure 2.CDO1 mRNA levels were negatively correlated with transcripts from adipose tissue inflammation and senescence-related Reactome pathways after adjusting by age, BMI and gender in VAT (cohort 4). BMI, body mass index.
Supplementary Figure 3. (a-b) Effects of LPS (1µg/ml) administration on expression of CDO1, inflammatory (IL6, TNF)- and adipogenic (ADIPOQ, FABP4, SLC2A4) genes in human preadipocytes during adipocyte differentiation at day 14 (a) and in human differentiated adipocytes after 96 h (b). *P<0.05, ⁎⁎P<0.01 and ⁎⁎⁎P<0.005 compared to vehicle (Mann Whitney test).
Supplementary Figure 4. (a-b) Effects of CDO1 gene KD on intracellular lipid accumulation, visually assessed at 10x magnifications and quantified with Fiji software at day 14 of ASC52telo cell adipocyte differentiation as detailed in Methods (a) and measured by Oil Red O staining at day 9 of ASC52telo cell adipocyte differentiation (b). *P<0.05 and ⁎⁎⁎P<0.005 compared to shRNA_SRC (Mann Whitney test).
References
- 1.Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 2.Learn DB, Fried VA, Thomas EL. Taurine and hypotaurine content of human leukocytes. J Leukoc Biol. 1990;48:174–182. [PubMed] [Google Scholar]
- 3.Guo YY, Li BY, Peng WQ, Guo L, Tang QQ. Taurine-mediated browning of white adipose tissue is involved in its anti-obesity effect in mice. J Biol Chem. 2019;294:15014–15024. doi: 10.1074/jbc.RA119.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuboyama-Kasaoka N, Shozawa C, Sano K, et al. Taurine (2-Aminoethanesulfonic Acid) deficiency creates a vicious circle promoting obesity. Endocrinology. 2006;147:3276–3284. doi: 10.1210/en.2005-1007. [DOI] [PubMed] [Google Scholar]
- 5.Zhao D, Lv Q, Yang J, et al. Taurine improves lipid metabolism and skeletal muscle sensitivity to insulin in rats fed with high sugar and high fat diet. Adv. Exp. Med. Biol. 2019;1155:133–146. doi: 10.1007/978-981-13-8023-5_12. [DOI] [PubMed] [Google Scholar]
- 6.Murakami S. The physiological and pathophysiological roles of taurine in adipose tissue in relation to obesity. Life Sci. 2017;186:80–86. doi: 10.1016/j.lfs.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 7.De Carvalho FG, Brandao CFC, Muñoz VR, et al. Taurine supplementation in conjunction with exercise modulated cytokines and improved subcutaneous white adipose tissue plasticity in obese women. Amino Acids. 2021;53:1391–1403. doi: 10.1007/s00726-021-03041-4. [DOI] [PubMed] [Google Scholar]
- 8.De Carvalho FG, Brandao CFC, Batitucci G, et al. Taurine supplementation associated with exercise increases mitochondrial activity and fatty acid oxidation gene expression in the subcutaneous white adipose tissue of obese women. Clin Nutr. 2021;40:2180–2187. doi: 10.1016/j.clnu.2020.09.044. [DOI] [PubMed] [Google Scholar]
- 9.Ide T, Kushiro M, Takahashi Y, Shinohara K, Cha S. mRNA expression of enzymes involved in taurine biosynthesis in rat adipose tissues. Metabolism. 2002;51:1191–1197. doi: 10.1053/meta.2002.34036. [DOI] [PubMed] [Google Scholar]
- 10.Ueki I, Stipanuk MH. 3T3-L1 adipocytes and rat adipose tissue have a high capacity for taurine synthesis by the cysteine dioxygenase/cysteinesulfinate decarboxylase and cysteamine dioxygenase pathways. J Nutr. 2009;139:207–214. doi: 10.3945/jn.108.099085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng P, Chen Y, Ji N, et al. Cysteine dioxygenase type 1 promotes adipogenesis via interaction with peroxisome proliferator-activated receptor gamma. Biochem Biophys Res Commun. 2015;458:123–127. doi: 10.1016/j.bbrc.2015.01.080. [DOI] [PubMed] [Google Scholar]
- 12.Ortega FJ, Mayas D, Moreno-Navarrete JM, et al. The gene expression of the main lipogenic enzymes is downregulated in visceral adipose tissue of obese subjects. Obesity (Silver Spring) 2010;18:13–20. doi: 10.1038/oby.2009.202. [DOI] [PubMed] [Google Scholar]
- 13.Moreno-Navarrete JM, Jove M, Ortega F, et al. Metabolomics uncovers the role of adipose tissue PDXK in adipogenesis and systemic insulin sensitivity. Diabetologia. 2016;59:822–832. doi: 10.1007/s00125-016-3863-1. [DOI] [PubMed] [Google Scholar]
- 14.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comas F, Latorre J, Ortega F, et al. Permanent cystathionine-β-Synthase gene knockdown promotes inflammation and oxidative stress in immortalized human adipose-derived mesenchymal stem cells, enhancing their adipogenic capacity. Redox Biol. 2021;42:101668. doi: 10.1016/j.redox.2020.101668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro-Conde I, de Uña-Alvarez J. sgof: An R Package for Multiple Testing Problems. R J. 2014;6:96–113. doi: 10.32614/RJ-2014-027. [DOI] [Google Scholar]
- 20.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carvajal-Rodríguez A, de Uña-Alvarez J, Rolán-Alvarez E. A new multitest correction (SGoF) that increases its statistical power when increasing the number of tests. BMC Bioinformatics. 2009;10:209. doi: 10.1186/1471-2105-10-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamburov A, Stelzl U, Lehrach H, Herwig R. The ConsensusPathDB interaction database: 2013 update. Nucleic Acids Res. 2013;41:D793–D800. doi: 10.1093/nar/gks1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 24.Millay DP, Olson EN. Making muscle or mitochondria by selective splicing of PGC-1α. Cell Metab. 2013;17:3–4. doi: 10.1016/j.cmet.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seyres D, Cabassi A, Lambourne JJ, et al. Transcriptional, epigenetic and metabolic signatures in cardiometabolic syndrome defined by extreme phenotypes. Clin Epigenetics. 2022;14:39. doi: 10.1186/s13148-022-01257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das SK, Ma L, Sharma NK. Adipose tissue gene expression and metabolic health of obese adults. Int J Obes (Lond) 2015;39:869–873. doi: 10.1038/ijo.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barreau C, Labit E, Guissard C, et al. Regionalization of browning revealed by whole subcutaneous adipose tissue imaging. Obesity. 2016;24:1081–1089. doi: 10.1002/oby.21455. [DOI] [PubMed] [Google Scholar]
- 28.Wen C, Li F, Zhang L, et al. Taurine is involved in energy metabolism in muscles, adipose tissue, and the liver. Mol Nutr Food Res. 2019;63:1800536. doi: 10.1002/mnfr.201800536. [DOI] [PubMed] [Google Scholar]
- 29.Kim KS, Jang MJ, Fang S, et al. Anti-obesity effect of taurine through inhibition of adipogenesis in white fat tissue but not in brown fat tissue in a high-fat diet-induced obese mouse model. Amino Acids. 2019;51:245–254. doi: 10.1007/s00726-018-2659-7. [DOI] [PubMed] [Google Scholar]
- 30.Ortega FJ, Mercader JM, Moreno-Navarrete JM, et al. Surgery-induced weight loss is associated with the downregulation of genes targeted by MicroRNAs in adipose tissue. J Clin Endocrinol Metab. 2015;100:E1467–E1476. doi: 10.1210/jc.2015-2357. [DOI] [PubMed] [Google Scholar]
- 31.Honecker J, Ruschke S, Seeliger C, et al. Transcriptome and fatty-acid signatures of adipocyte hypertrophy and its non-invasive MR-based characterization in human adipose tissue. EBioMedicine. 2022;79 doi: 10.1016/j.ebiom.2022.104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carruthers NJ, Strieder-Barboza C, Caruso JA, et al. The human type 2 diabetes-specific visceral adipose tissue proteome and transcriptome in obesity. Sci Rep. 2021;11:17394. doi: 10.1038/s41598-021-96995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Q, Fu Z, Gong Y, et al. Metabolic health status contributes to transcriptome alternation in human visceral adipose tissue during obesity. Obesity (Silver Spring) 2020;28:2153–2162. doi: 10.1002/oby.22950. [DOI] [PubMed] [Google Scholar]
- 34.Yin X, Lanza IR, Swain JM, Sarr MG, Nair KS, Jensen MD. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J Clin Endocrinol Metab. 2014;99:E209–E216. doi: 10.1210/jc.2013-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Pauw A, Tejerina S, Raes M, Keijer J, Arnould T. Mitochondrial (dys)function in adipocyte (de)differentiation and systemic metabolic alterations. Am J Pathol. 2009;175:927–939. doi: 10.2353/ajpath.2009.081155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Marsboom G, Toth PT, Rehman J. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS One. 2013;8:e77077. doi: 10.1371/journal.pone.0077077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jong CJ, Sandal P, Schaffer SW. The role of taurine in mitochondria health: more than just an antioxidant. Molecules. 2021;26:4913. doi: 10.3390/molecules26164913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreno-Navarrete JM, Moreno M, Ortega F, et al. CISD1 in association with obesity-associated dysfunctional adipogenesis in human visceral adipose tissue. Obesity. 2016;24:139–147. doi: 10.1002/oby.21334. [DOI] [PubMed] [Google Scholar]
- 39.Moreno-Navarrete JM, Ortega F, Moreno M, Xifra G, Ricart W, Fernández-Real JM. PRDM16 sustains white fat gene expression profile in human adipocytes in direct relation with insulin action. Mol Cell Endocrinol. 2015;405:84–93. doi: 10.1016/j.mce.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 40.Lim J, Park HS, Kim J, et al. Depot-specific UCP1 expression in human white adipose tissue and its association with obesity-related markers. Int J Obes (Lond) 2020;44:697–706. doi: 10.1038/s41366-020-0528-4. [DOI] [PubMed] [Google Scholar]
- 41.Min SY, Desai A, Yang Z, et al. Diverse repertoire of human adipocyte subtypes develops from transcriptionally distinct mesenchymal progenitor cells. Proc Natl Acad Sci U S A. 2019;116:17970–17979. doi: 10.1073/pnas.1906512116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin S, Hirai S, Yamaguchi Y, et al. Taurine improves obesity-induced inflammatory responses and modulates the unbalanced phenotype of adipose tissue macrophages. Mol Nutr Food Res. 2013;57:2155–2165. doi: 10.1002/mnfr.201300150. [DOI] [PubMed] [Google Scholar]
- 43.Rosa FT, Freitas EC, Deminice R, Jordão AA, Marchini JS. Oxidative stress and inflammation in obesity after taurine supplementation: a double-blind, placebo-controlled study. Eur J Nutr. 2014;53:823–830. doi: 10.1007/s00394-013-0586-7. [DOI] [PubMed] [Google Scholar]
- 44.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome–an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Kim JY, Van De Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vishvanath L, Gupta RK. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Invest. 2019;129:4022–4031. doi: 10.1172/JCI129191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1.CDO1 mRNA levels were positively correlated with transcripts from adipose tissue function-related Reactome pathways after adjusting by age, BMI and gender in VAT (cohort 4). BMI, body mass index.
Supplementary Figure 2.CDO1 mRNA levels were negatively correlated with transcripts from adipose tissue inflammation and senescence-related Reactome pathways after adjusting by age, BMI and gender in VAT (cohort 4). BMI, body mass index.
Supplementary Figure 3. (a-b) Effects of LPS (1µg/ml) administration on expression of CDO1, inflammatory (IL6, TNF)- and adipogenic (ADIPOQ, FABP4, SLC2A4) genes in human preadipocytes during adipocyte differentiation at day 14 (a) and in human differentiated adipocytes after 96 h (b). *P<0.05, ⁎⁎P<0.01 and ⁎⁎⁎P<0.005 compared to vehicle (Mann Whitney test).
Supplementary Figure 4. (a-b) Effects of CDO1 gene KD on intracellular lipid accumulation, visually assessed at 10x magnifications and quantified with Fiji software at day 14 of ASC52telo cell adipocyte differentiation as detailed in Methods (a) and measured by Oil Red O staining at day 9 of ASC52telo cell adipocyte differentiation (b). *P<0.05 and ⁎⁎⁎P<0.005 compared to shRNA_SRC (Mann Whitney test).