Abstract
Osteonecrosis associated with the use of glucocorticoids is a severe, potentially debilitating complication. In broader terms, it commonly involves the femoral head with secondary hip osteoarthritis. Osteonecrosis can also be caused by trauma and other non-traumatic factors besides steroid treatment. Nonetheless, glucocorticoid use is frequently observed in clinical settings in which this represents a common therapeutic option, including general practice, rheumatology and clinical immunology, among others. The pathogenesis involves genetic components, vascular impairment, adipocyte hypertrophy, and increased intraosseous pressure, ultimately leading to marrow and bone ischemia and necrosis and the process rapidly becomes irreversible. Osteonecrosis manifests with pain and impaired motility while the diagnosis is usually made with magnetic resonance imaging allowing early detection and potentially (dependent on the patient's needs for steroids and stage) timely management with conservative options, followed by joint replacement at late stages. In this review we discuss the pathogenesis, risk factors, diagnosis, staging, and management of this complication associated with glucocorticoid treatment.
Keywords: Safety, Adverse event, Chronic inflammation, Aseptic osteonecrosis, Prosthesis
1. Introduction
Almost immediately after the introduction of cortisone for the treatment of rheumatoid arthritis in 1950s, the medical community became aware of associated potential complications involving the bone [1] beyond reduced bone density leading to osteoporosis and fractures. Indeed, collapse of the femoral and humeral heads was described following both systemic and intra-articular steroid administration [[2], [3], [4], [5]]. Since these lesions were not induced by trauma nor supported by infections it was coined ‘aseptic osteonecrosis’. A direct hormonal effect on osteoblasts was hypothesized as the causative mechanism [6]. With the growing use of systemic glucocorticoids, increased awareness of complications, and improvement of imaging techniques, steroid-induced osteonecrosis was further described in case series and has been later referred to as ‘avascular’ or ‘ischemic necrosis’ [[7], [8], [9], [10], [11]]. The word osteonecrosis derives from an inability to supply nutrients to crucial areas of bones, resulting in bone death, commonly involving the femoral head with secondary hip osteoarthritis. Osteonecrosis can be caused by both trauma, the most common cause accounting for more than 20% of cases [12], and non-traumatic factors. The latter particularly includes excessive and/or chronic alcohol intake, but is also associated with tobacco use, sickle cell disease, congenital or acquired coagulopathies, genetic factors, hematological diseases, radiation, and Caisson disease. However, among non-traumatic factors, the most common cause of osteonecrosis is glucocorticoid use [13,14], with a prevalence that varies from 3 to 38% [15] accounting for 5–25% of patients with atraumatic osteonecrosis [16]. Further, among patients with a history of high-dose corticosteroid treatment, 5% developed osteonecrosis [16]. It is well known that corticosteroids induce osteoporosis by inhibiting osteoblastogenesis and promoting osteoblast and osteocyte apoptosis, while they enhance osteoclast differentiation, maturation, and lifespan [17]. The risk of vertebral fractures related to osteoporosis in patients under oral glucocorticoids tripled, whereas the risk of hip fracture doubled. This also depends on the duration and dosage of steroids, reaching an incidence rate of 16 at ≥5 mg per day and an average fracture incidence rate of 13.4 at cumulative doses ≥5.4 g [17], While the proportion of patients with osteoporosis who develop osteonecrosis is unclear, it is acknowledged that osteoporosis may be associated to osteonecrosis [18]. Further, some osteoporosis drugs, bisphosphonates and denosumab, have been associated with an increased risk of osteonecrosis of the jaw, with an incidence of 0.01% [19].
Among patients with no history of trauma nor steroid use, around 30% of cases of osteonecrosis were alcohol-associated [20]. The prevalence was 10% in sickle cell disease (with a cumulative incidence of 22%) [21], around 20% after radiation therapy, reduced until 3% after optimization of radiation techniques [22], and 17–27% were idiopathic [20]. Thrombophilia is another risk factor: Factor V Leiden mutations are present in around 10% of patients with osteonecrosis, 3–4% have a mutation of the prothrombin gene, anti-thrombin III deficiency is present in around 10%, while protein C and S deficiency are detected in 2-13% of cases [23].
2. Pathogenesis
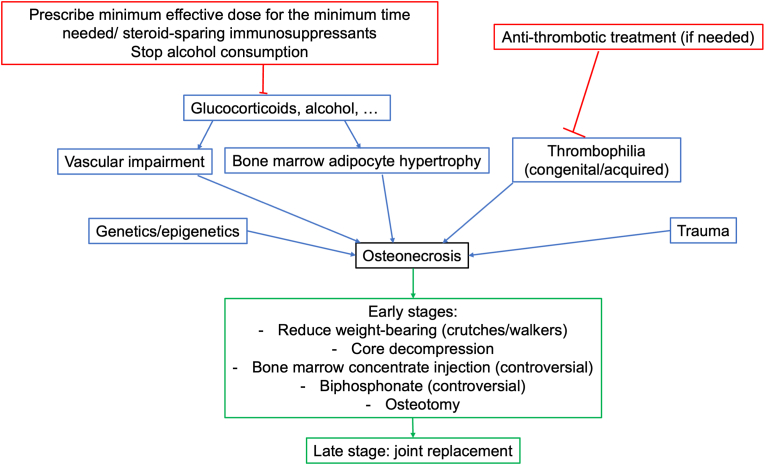
The pathogenesis of steroid-induced osteonecrosis, as well as non-traumatic osteonecrosis by other causes, remains to be elucidated at the molecular level but the ultimate mechanism is compromised blood flow with failure to deliver nutrients to the affected bone, favored by numerous preexisting factors [13], including individual susceptibility and vascular damage, mechanical stress, increased intraosseous pressure, adipocyte dysfunction, and defects in apoptosis and coagulation [14]. A representation of the main risk factors, measures to be taken for prevention, and proposed treatments in early and late stages are illustrated in Fig. 1.
Fig. 1.
Schematic representation of the main risk factors for osteonecrosis (blue boxes), measures to be taken for prevention (red boxes), and proposed treatments in early and late stages (green boxes).
2.1. Genetics and epigenetics
A genetic component has been recognized and may explain why only some patients taking systemic glucocorticoids develop the disease. Polymorphisms in genes involved in corticosteroid metabolism can influence the risk for steroid-induced osteonecrosis, as in the case of the ATP-binding cassette subfamily B member 1 (ABCB1), a gene encoding for a transport protein which plays a vital role in absorption and distribution of a broad range of drugs. Single nucleotide polymorphisms (SNP) of this gene modify drug distribution and pharmacokinetics and may affect individual sensitivity and side effects for systemic glucocorticoids. A significant association was observed between ABCB1 3435 T and 2677 T/A polymorphisms and steroid-induced osteonecrosis. In fact, the occurrence of the disease was significantly lower in patients with these polymorphisms, possibly for their influence on glucocorticoid-induced osteocyte apoptosis [24].
Other SNPs with an impact on steroid-induced osteonecrosis have been demonstrated for cytochrome P450 and associated with lower risk [25], whereas different IL1B polymorphisms influence the risk for steroid-induced osteonecrosis [26]. Metalloproteinase 2, 8, and 10 polymorphisms contribute to susceptibility [27,28], as RETN (coding for resistin, an adipokine involved in metabolic disorders) and ApoA 5 and APOE (involved in lipid metabolism) SNP [[29], [30], [31]]. In contrast, metalloproteinase 9 SNPs appears to reduce the risk [32], similar to the gene for the tissue inhibitor of metalloprotease-4 [33], while the plasminogen activator inhibitor-1 (PAI-1) SNP has been demonstrated to be associated with an increased risk of steroid-induced osteonecrosis [34]. Interestingly, the long-chain RNA p53-induced transcript (LncRNA LINC-PINT) has been reported to impact on the risk. When it is overexpressed, cell proliferation is inhibited, and apoptosis is increased. LINC-PINT also regulates transforming growth factor beta (TGF-β) and Wnt/β-catenin pathways. SNP of this gene are related to several diseases, as cancer and increased susceptibility to steroid-induced osteonecrosis [35] (Table 1).
Table 1.
Genes in which polymorphisms influence steroid-induced osteonecrosis risk.
| Gene | Risk | Ref. |
|---|---|---|
| ATP-binding cassette subfamily B member 1 (ABCB1) | Reduced | [24] |
| Cytochrome P450 | Reduced | [25] |
| IL1B | Increased or reduced | [26] |
| Metalloproteinase 2, 8, and 10 | Increased | [27,28] |
| Metalloproteinase 9 | Reduced | [32] |
| RETN | Increased | [29] |
| APOA5 | Increased | [30] |
| APOE | Increased | [31] |
| TIMP4 | Reduced | [33] |
| PAI-1 | Increased | [34] |
| LncRNA LINC-PINT | Increased | [35] |
| VEGF | Increased | [13] |
| NO | Increased | [13] |
Epigenetic mechanisms have also been observed to impact the risk associated with systemic glucocorticoids. Significant methylation level differences between patients and controls were found in ABCB1 gene, with patients having higher methylation levels [25]. Micro RNAs (MIR) were also found to be implicated in risk of diseases, and the transcripts of MIR17HG and MIR155HG gene variants, which regulate bone metabolism among other functions, are associated with steroid-induced osteonecrosis [36].
2.2. Vascular impairment
Steroid-induced osteonecrosis, in particular when affecting the femoral head, is often considered a compartment syndrome with several factors increasing intra-osseous pressure. As the vessels vascularizing the epiphysis are housed in the closed chamber of the femoral head, filled with marrow cells, an increase of pressure in this area leads to vascular compression, ischemia, and ultimately marrow and bone necrosis [13]. Further, glucocorticoids can damage endothelial cells. In fact, they alter endothelial function with increased peripheral resistance and systemic hypertension [14]. They also contribute to susceptibility to thrombi formation by decreasing tissue plasminogen activator activity and increasing PAI-1 activity. With the release of vasoactive mediators as endothelin-1, noradrenalin and bradykinin, glucocorticoids modulate vascular responsiveness and induce vasoconstriction [37].
2.3. Fat cells and intraosseous pressure
Systemic glucocorticoids promote adipogenesis with the differentiation of pre-adipocytes and mesenchymal stem cells to mature adipocytes [13]. In fact, through the corticosteroid receptor, glucocorticoids activate several pathways, including the Wnt, TGFβ/bone morphogenetic protein (BMP) superfamily and Notch signaling pathways, targeting transcription factors as peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT-enhancer-binding proteins (C/EBPs) for adipogenesis, and promoting post-transcriptional modifications, which induce the dysfunction of bone marrow mesenchymal stem cells [38]. Further, systemic glucocorticoids contribute to adipocyte hypertrophy [39] and to systemic hyperlipidemia, which contributes to formation of deposits in the vascular sinusoids, therefore reducing blood flow [14]. The bone marrow is therefore composed largely by adipocytes at the expense of hematopoietic cells and the increase in volume leads to increased intraosseous pressure, which compresses venous sinusoids, induces intravascular coagulation impairing blood flow and causing ischemia [13].
2.4. Bone marrow ischemia
As microvascular coagulation occurs at the early phases of osteonecrosis, factors modulating coagulation cascade and fibrinolysis can act as protective or risk factors. Congenital or acquired coagulopathies can promote osteonecrosis [40] and anti-coagulants have been proposed for the prevention of steroid-induced osteonecrosis [41]. Intravascular coagulation induces failure of the vascular supply, ischemia, hypoxia, marrow necrosis and promotes angiogenesis by release of vascular endothelial growth factor (VEGF) and nitric oxide (NO), two genes in which polymorphisms have been reported to increase the risk of osteonecrosis [13]. Also, exosomes derived from hypoxia-preconditioned bone marrow mesenchymal stem cells, but not from cells under normal oxygen pressure, exert therapeutic effects on steroid-induced osteonecrosis by promoting cell proliferation, migration, VEGF expression, and angiogenesis [42].
Hematopoietic cells are more sensitive to ischemia and die within 12 h, whilst adipocytes remain alive for 48 h and osteocytes for 24–72 h of complete ischemia. However, not all ischemic lesions lead to osteonecrosis and if perfusion is restored and dead bone is substituted by new tissue, depending on genetic predisposition and coagulative state, tissue can be repaired [43].
If the process progresses, there is the formation of a focal sequestrum surrounded by a fibrous membrane, which makes the course of the disease irreversible [13] and this histological finding allows the definite diagnosis of osteonecrosis [43].
2.5. Reparative attempts and fracture
The necrotic bone can be recognized as a foreign body and thus induce an immune reaction, depending on the amount of presented antigens, mainly glycoproteins and byproducts from ischemic bone marrow. In glucocorticoid-induced osteonecrosis, osteocyte apoptosis is prevalent, particularly in the subchondral area and cell swelling and inflammation usually do not occur [1]. A fibrovascular tissue encapsulating dead bone develops, but this reparative tissue cannot enter the dead marrow and no real repair occurs. The capsule progressively ossifies, and dead adipocytes release fatty acids which saponify with extracellular calcium, leading to formation of insoluble soaps. The resulting tissue does not have the strength and cannot bear weight as healthy bone. Fractures can occur at the subchondral portion or at the necrotic margin, leading to femoral head collapse and ultimately to osteoarthritis of the joint [13].
3. Risk factors
Osteonecrosis is commonly associated with higher cumulative doses and longer treatment courses with systemic glucocorticoids, but it has also been described after intra-articular injections [44], topical administration [45,46] or low-dose, short-term oral steroids [47]. No major gender differences have been identified [48]. Detailed discussion of dose and age have been discussed elsewhere [14,49].
Patients with systemic lupus erythematosus (SLE), solid organ transplants, hematological diseases, multiple sclerosis, and recent neurosurgery have higher prevalence of steroid induced osteonecrosis [49]. Severe acute respiratory syndrome and SARS-CoV-2 infections (i.e., COVID-19) also puts patients at risk for developing osteonecrosis [[49], [50], [51], [52]]. In one study in SLE, a prednisone-equivalent dosage greater than 30–40 mg/daily or a cumulative dose ≥12 g/year conferred a significant risk of osteonecrosis while in patients undergoing kidney transplantation, the dose of systemic glucocorticoids in the first weeks was more important than the total cumulative dose. The risk is higher if the steroid dose is greater than 5000 mg during the first 3 months [49]. Further, the number of osteonecrosis sites was directly related to the dosage of systemic glucocorticoids. A peak dose of more than 200 mg, or a cumulative methylprednisolone-equivalent dose of more than 4000 mg seems to be a significant risk factor for multifocal osteonecrosis with both epiphyseal and diaphyseal lesions [49]. Steroids with a longer half-life pose a greater risk and it should be emphasized that much lower doses of steroids have also been associated with osteonecrosis [14,49]. Markers for bone resorption and formation do not appear to predict the development of osteonecrosis [53].
4. Clinical manifestations and diagnosis
Once clinical features appear, osteonecrosis lesions are generally long established. The most common symptoms at presentation include pain, inability to bear weight, and limitation in range of motion of the joint. If the femoral head is involved, pain is located at the hip but can radiate to the groin, thigh, or knee [14]. The new onset of a persistent joint pain exacerbated by movements with reduced range of motion, in patients under glucocorticoids should raise the suspicion of osteonecrosis [1].
The diagnosis of osteonecrosis is performed by combining clinical evaluation and adequate imaging. In 2017, the Association Research Circulation Osseous (ARCO) Committee developed classification criteria for clinical trial standardization on steroid-associated osteonecrosis of the femoral head through Delphi surveys. These were to be applied to patients clinically diagnosed with osteonecrosis by symptoms, signs, imaging, and/or histological examination and included: 1) a history of systemic glucocorticoid use >2 g of prednisolone equivalent within a 3-month period; 2) diagnosis within 2 years after systemic glucocorticoid usage; 3) no other risk factor(s) besides systemic glucocorticoid, including a history of trauma, alcohol abuse, hereditary coagulopathies, Caisson disease, radiation therapy involving the femoral head, non-glucocorticoid chemotherapeutics for cancer, sickle cell disease, or Gaucher's disease [54]. But here again the individual history of a patient's steroid use must be considered as part of differential diagnosis.
5. Imaging and staging
The first imaging test to be performed is usually an X-ray but at early stages signs are frequently missed and radiographs appear normal. When the femoral head collapses due to subchondral fracture, crescent sign can be appreciated and is pathognomonic for the disease [55]. The first classification and staging system for hip osteonecrosis was developed by Ficat and Arlet in 1960 and it is based on X-rays. It is still widely used by orthopedic surgeons and in most studies the four-stage version is used, after several modifications. Stage I is characterized by normal plain radiographs, stage II shows diffuse sclerosis and cysts, representing attempts of bone repair, stage III presents the crescentic sign, subchondral fractures that appear as radiolucent crescent parallel to bone cortex, while stage IV appears when the femoral head collapses with flattening, destruction, and hip osteoarthritis [56]. This staging system has been questioned since it is based on radiographs only with poor inter-observer reliability and intra-observer reproducibility, with low kappa statistic values [57,58].
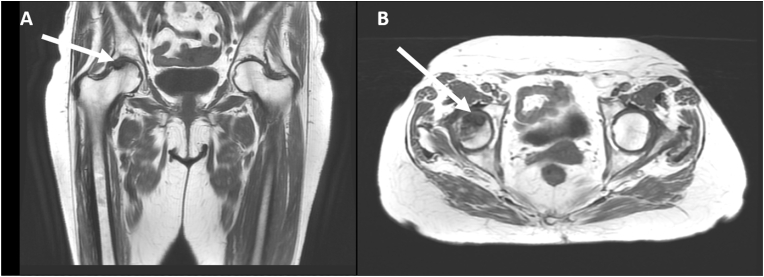
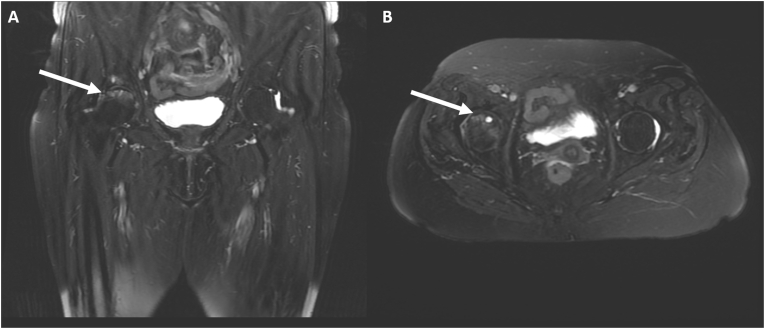
With the introduction of magnetic resonance imaging (MRI), osteonecrosis has become identifiable at early stages and MRI is now considered the most sensitive and specific diagnostic technique. Viable zones have low signal intensity on T1-weighted images and higher signal intensity on T2-weighted images, whilst necrotic areas are hypointense in both T1-and T2-weighted sequences. The so-called double line sign is a diagnostic alteration on T2-weighted images visible at the interface between viable and necrotic tissue. It is formed by an outer low signal rim corresponding to sclerotic bone and by an inner hyperintense rim, corresponding to fibrovascular tissue (Fig. 2 and Fig. 3). Contrast with gadolinium may be of further help in early stages for differentiating hypervascularized viable tissue from hypovascularized necrotic tissue. Advanced osteonecrosis shows low intensity or more tissue features within the same area. Fractures typically appear as hypointense rim on T1-weighted images, with surrounding edema on T2-weighted images [59]. In contrast, the classification proposed by Steinberg in 1995 implemented plain radiograph imaging with bone scans and MRI [60] (Table 2). This classification also was criticized for the scarce intra- and inter-agreement on all stages [61].
Fig. 2.
T1-weighted coronal (A) and axial (B) images of a woman with steroid-induced osteonecrosis showing an area of low signal intensity in the anterior-superior right femoral head.
Fig. 3.
T2-weighted fat suppressed coronal (A) and axial (B) image of a woman with steroid-induced osteonecrosis showing a crescentic area of subchondral edema of the anterior part of the right femoral head, associated with a low signal intensity peripheral rim. A subchondral cyst may also be seen.
Table 2.
Steinberg staging system for hip osteonecrosis [60].
| Stage | Imaging |
|---|---|
| 0 | Normal imaging |
| I | Normal radiographs. Abnormal bone scan and/or MRI |
| A- Mild (<15% femoral head involvement) | |
| B- Moderate (15–30% femoral head involvement) | |
| C- Severe (>30% femoral head involvement) | |
| II | Cystic and sclerotic changes |
| A- Mild (<15% femoral head involvement) | |
| B- Moderate (15–30% femoral head involvement) | |
| C- Severe (>30% femoral head involvement) | |
| III | Subchondral collapse without femoral head flattening |
| A- Mild (<15% femoral head involvement) | |
| B- Moderate (15–30% femoral head involvement) | |
| C- Severe (>30% femoral head involvement) | |
| IV | Femoral head flattening/collapse |
| A- Mild (<15% femoral head involvement) | |
| B- Moderate (15–30% femoral head involvement) | |
| C- Severe (>30% femoral head involvement) | |
| V | Joint space narrowing and/or acetabular changes |
| A- Mild | |
| B- Moderate | |
| C- Severe | |
| VI | Advanced disease |
The most reliable classification is considered the Japanese Investigation Committee, which is based on the location of the necrotic lesion. In fact, this is an important factor that affects prognosis, independently of the lesion size. The three stages of this classification include: (A) lesions occupying the medial one-third or less of the weight-bearing portion, (B) lesions occupying the medial two-thirds or less of the weight-bearing portion, (C) lesions occupying more than the medial two-thirds of the weight-bearing portion, with stage C1 for lesions not extending laterally and stage C2 for lesions extending laterally to the acetabular edge [56,62]. The underlying concept is that the location of necrosis in relation to the acetabular weight-bearing region is a factor impacting prognosis. This is relevant in the early stages of disease, when lesions may be small and underestimated. On one hand, if lesions involve a significant area of the weight-bearing portion of the bone, these can rapidly progress and lead to bone collapse while if the weight-bearing portion is not involved or is minimally affected, lesions can stabilize without collapsing. The cumulative survival rate, with collapse as the endpoint, is better represented by this classification compared to others, with stage A having 100% survival at 10 years, stage B 94%, stage C1 32%, and stage C2 18%. The survival rates with hip arthroplasty as endpoint are 100% for stage A and B, 60% for stage C1, and 42% for stage C2 [56,[62], [63], [64]].
6. Management
Steroid-induced osteonecrosis often affects young individuals and efforts must be made for a conservative treatment of the joint, as replacements usually have a limited duration. The first approach is reducing weight bearing and crutches and walkers are recommended [1]. When the osteonecrotic area is small, treatment can be avoided as usually lesions do not increase in size, regardless of stage progression. At early stages, when osteonecrosis may be reversible and the weight-bearing portion is not involved, core decompression may be performed. Injection of bone marrow aspirate concentrate combined with core decompression may be beneficial but remains controversial and very much dependent on stage and on need for continued or future steroid usage. Further, osteotomies to move the necrotic area from a weight-bearing to a non-weight-bearing region may preserve osteonecrotic joints in the selected candidates [43]. Another approach involves bisphosphonates, which proved beneficial in some studies in delaying femoral head collapse and in postponing surgery [1], but their use remains controversial. At later stages, when the joint is irreversibly damaged leading to development of osteoarthritis, total joint replacement will be required [1].
7. Conclusions
Steroid-induced osteonecrosis is a severe, debilitating disease occurring in patients treated with glucocorticoids. Diagnosis based on an MRI, which allows early detection and management. Treatment is conservative (if possible), but in more advanced stages total joint replacement is required. Our understanding of the mechanisms of damage in patients taking systemic glucocorticoids remains elusive and a more focused research effort may allow us to identify cases with higher risk of osteonecrosis. Most importantly, steroids should be used only when indicated and for the lowest possible dose and duration.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Credit author statement
Francesca Motta: investigation, methodology, reviewing and editing; Suraj Timilsina: conceptualization, investigation, editing and reviewing; M. Eric Gershwin: conceptualization, visualization, editing, reviewing and supervision; Carlo Selmi: visualization, editing, reviewing and supervision.
IL: interleukin; RETN: gene coding for resistin; APOA5: gene coding for Apolipoprotein A5; APOE: gene coding for Apolipoprotein E; TIMP4: gene coding for tissue inhibitors of metalloprotease-4; PAI-1: gene coding for plasminogen activator inhibitor-1. LncRNA LINC-PINT: long-chain RNA p53-induced transcript: VEGF: gene coding for vascular endothelial growth factor; NO: gene coding for nitric oxide.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: MEG is the Advisory Editor of Journal of Translational Autoimmunity. CS is in the Editorial Board.
Contributor Information
M. Eric Gershwin, Email: megershwin@ucdavis.edu.
Carlo Selmi, Email: carlo.selmi@hunimed.eu.
Data availability
No data was used for the research described in the article.
References
- 1.Weinstein R.S. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41:183–190. doi: 10.1007/S12020-011-9580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloch-Michel H., Benoist M., Peyron J. [Aseptic osteonecrosis in the course of corticotherapy of pemphigus] Rev Rhum Mal Osteoartic. 1959;26:648–659. https://pubmed-ncbi-nlm-nih-gov.humanitas.idm.oclc.org/13801518/ (accessed June 21, 2022) [PubMed] [Google Scholar]
- 3.Brahic J., Delmont J., Chamlian A., Brusquet Y. [A case of aseptic osteonecrosis of both femur heads during supplementary cortisone-like treatment for Sheehan's disease] Marseille Med. 1962;99:730–732. https://pubmed-ncbi-nlm-nih-gov.humanitas.idm.oclc.org/14014881/ (accessed June 21, 2022) [PubMed] [Google Scholar]
- 4.Ruhlin C.W. Aseptic necrosis of femoral heads associated with steroid therapy. J. Maine Med. Assoc. 1962;53:280–281. https://pubmed-ncbi-nlm-nih-gov.humanitas.idm.oclc.org/13975471/ (accessed June 21, 2022) [PubMed] [Google Scholar]
- 5.Schwob R.A., Duparc J., Lambert P., Bernheim R. [Late acropathia ulcero-mutilans, aseptic osteonecrosis of the femur head, osteolytic coxitis following intra-articular corticotherapy] Rev Rhum Mal Osteoartic. 1962;29:569–575. https://pubmed-ncbi-nlm-nih-gov.humanitas.idm.oclc.org/13992450/ (accessed June 21, 2022) [PubMed] [Google Scholar]
- 6.Boksenbaum M., Mendelson C.G. Aseptic necrosis of the femoral head associated with steroid therapy. JAMA. 1963;184:262–265. doi: 10.1001/JAMA.1963.03700170054007. [DOI] [PubMed] [Google Scholar]
- 7.Dyreborg E., Pilgaard S. Osteonecrosis in three young men previously treated with steroid for aplastic anaemia. Acta Orthop. Scand. 1974;45:199–205. doi: 10.3109/17453677408989140. [DOI] [PubMed] [Google Scholar]
- 8.Svahn T., Elmstedt E., Lewander R. Avascular necrosis of the bone in 99 renal allograft recipients. Scand. J. Urol. Nephrol. 1975;9:135–137. https://pubmed-ncbi-nlm-nih-gov.humanitas.idm.oclc.org/781808/ (accessed June 21, 2022) [PubMed] [Google Scholar]
- 9.Taylor L.J. Multifocal avascular necrosis after short-term high-dose steroid therapy. A report of three cases. J Bone Joint Surg Br. 1984;66:431–433. doi: 10.1302/0301-620X.66B3.6725356. [DOI] [PubMed] [Google Scholar]
- 10.Massardo L., Jacobelli S., Leissner M., González M., Villarroel L., Rivero S. High-dose intravenous methylprednisolone therapy associated with osteonecrosis in patients with systemic lupus erythematosus. Lupus. 1992;1:401–405. doi: 10.1177/096120339200100610. [DOI] [PubMed] [Google Scholar]
- 11.Zizic T.M., Marcoux C., Hungerford D.S., Dansereau J.v., Stevens M.B. Corticosteroid therapy associated with ischemic necrosis of bone in systemic lupus erythematosus. Am. J. Med. 1985;79:596–604. doi: 10.1016/0002-9343(85)90057-9. [DOI] [PubMed] [Google Scholar]
- 12.Bergman J., Nordström A., Nordström P. Epidemiology of osteonecrosis among older adults in Sweden. Osteoporos. Int. 2019;30:965–973. doi: 10.1007/S00198-018-04826-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui Q., Jo W.L., Koo K.H., Cheng E.Y., Drescher W., Goodman S.B., Ha Y.C., Hernigou P., Jones L.C., Kim S.Y., Lee K.S., Lee M.S., Lee Y.J., Mont M.A., Sugano N., Taliaferro J., Yamamoto T., Zhao D. ARCO consensus on the pathogenesis of non-traumatic osteonecrosis of the femoral head. J. Kor. Med. Sci. 2021;36:1–15. doi: 10.3346/JKMS.2021.36.E65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang C., Greenspan A., Gershwin M.E. The pathogenesis, diagnosis and clinical manifestations of steroid-induced osteonecrosis. J. Autoimmun. 2020;110 doi: 10.1016/J.JAUT.2020.102460. [DOI] [PubMed] [Google Scholar]
- 15.Assouline-Dayan Y., Chang C., Greenspan A., Shoenfeld Y., Gershwin M.E. Pathogenesis and natural history of osteonecrosis. Semin. Arthritis Rheum. 2002;32:94–124. doi: 10.1053/SARH.2002.33724B. [DOI] [PubMed] [Google Scholar]
- 16.Xie X.H., Wang X.L., Yang H.L., Zhao D.W., Qin L. Steroid-associated osteonecrosis: epidemiology, pathophysiology, animal model, prevention, and potential treatments (an overview) J Orthop Translat. 2015;3:58–70. doi: 10.1016/J.JOT.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chotiyarnwong P., McCloskey E.v. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat. Rev. Endocrinol. 2020;16:437–447. doi: 10.1038/S41574-020-0341-0. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi A., Umehara J., Kamimura M., Aizawa T., Itoi E. Obesity is a risk factor for osteoarthritis progression and spontaneous osteoporosis is a risk for the development of spontaneous osteonecrosis in patients with medial meniscus posterior root tear. J. Orthop. Sci. 2021;26:844–849. doi: 10.1016/J.JOS.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Mücke T., Krestan C.R., Mitchell D.A., Kirschke J.S., Wutzl A. Bisphosphonate and medication-related osteonecrosis of the jaw: a review. Semin. Muscoskel. Radiol. 2016;20:305–314. doi: 10.1055/S-0036-1592367. [DOI] [PubMed] [Google Scholar]
- 20.Sato R., Ando W., Fukushima W., Sakai T., Hamada H., Takao M., Ito K., Sugano N. Epidemiological study of osteonecrosis of the femoral head using the national registry of designated intractable diseases in Japan. Mod. Rheumatol. 2022;32:808–814. doi: 10.1093/MR/ROAB047. [DOI] [PubMed] [Google Scholar]
- 21.Adesina O.O., Neumayr L.D. Osteonecrosis in sickle cell disease: an update on risk factors, diagnosis, and management. Hematol Am Soc Hematol Educ Prog. 2019;2019:351–358. doi: 10.1182/HEMATOLOGY.2019000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu S.H., Tang J.S., Shen X.Y., Niu Z.X., Xiao J.L. Osteoradionecrosis of the hip, a troublesome complication of radiation therapy: case series and systematic review. Front. Med. 2022;9 doi: 10.3389/FMED.2022.858929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezus E., Tamba B.I., Badescu M.C., Popescu D., Bratoiu I., Rezus C. Osteonecrosis of the femoral head in patients with hypercoagulability-from pathophysiology to therapeutic implications. Int. J. Mol. Sci. 2021;22 doi: 10.3390/IJMS22136801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Z., Hua Y., Liu J., Zuo D., Wang H., Chen Q., Zheng L., Cai Z. Association of ABCB1/MDR1 polymorphisms in patients with glucocorticoid-induced osteonecrosis of the femoral head: evidence for a meta-analysis. Gene. 2015;569:34–40. doi: 10.1016/J.GENE.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Huang R., Zhan Q., Hu W., Yang R., Cheng N., Han Y., Yue X. Association of ABCB1 and CYP450 gene polymorphisms and their DNA methylation status with steroid-induced osteonecrosis of the femoral head in the Chinese population. Genet. Test. Mol. Biomarkers. 2020;24:789–797. doi: 10.1089/GTMB.2020.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Y., Zhang Y., Wu J., Sun Y., Xiong Z., Niu F., Lei L., Du S., Chen P., Yang Z. Genetic polymorphisms in IL1B predict susceptibility to steroid-induced osteonecrosis of the femoral head in Chinese Han population. Osteoporos. Int. 2019;30 doi: 10.1007/S00198-019-04835-9. [DOI] [PubMed] [Google Scholar]
- 27.Du J., Jin T., Cao Y., Chen J., Guo Y., Sun M., Li J., Zhang X., Wang G., Wang J. Association between genetic polymorphisms of MMP8 and the risk of steroid-induced osteonecrosis of the femoral head in the population of northern China. Medicine. 2016;95 doi: 10.1097/MD.0000000000004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J., Tian Y., An F., Wang J., Liu C., Wu H., Cao Y., Wang G. MMP2 and MMP10 polymorphisms are related to steroid-induced osteonecrosis of the femoral head among Chinese han population. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/8298193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.An F., Zhang L., Gao H., Wang J., Liu C., Tian Y., Ma C., Zhao J., Wang K., Wang J. Variants in RETN gene are associated with steroid-induced osteonecrosis of the femoral head risk among Han Chinese people. J. Orthop. Surg. Res. 2020;15 doi: 10.1186/S13018-020-1557-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui Y., Kaisaierjiang A., Cao P., Wu Z.Y., Lv Q. Association of apolipoprotein A5 genetic polymorphisms with steroid-induced osteonecrosis of femoral head in a Chinese Han population. Diagn. Pathol. 2014;9 doi: 10.1186/S13000-014-0229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan L., Li W., Wang X., Yang G., Yu H., Sun S. The relationship between genetic polymorphisms in apolipoprotein E (ApoE) gene and osteonecrosis of the femoral head induced by steroid in Chinese Han population. Genes Genomi. 2018;40:225–231. doi: 10.1007/S13258-017-0625-5. [DOI] [PubMed] [Google Scholar]
- 32.Du J., Liu W., Jin T., Zhao Z., Bai R., Xue H., Chen J., Sun M., Zhang X., Wang G., Wang J. A single-nucleotide polymorphism in MMP9 is associated with decreased risk of steroid-induced osteonecrosis of the femoral head. Oncotarget. 2016;7:68434–68441. doi: 10.18632/ONCOTARGET.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J., An F., Cao Y., Gao H., Sun M., Ma C., Wu H., Zhang B., Liu W., Wang J. Association of TIMP4 gene variants with steroid-induced osteonecrosis of the femoral head in the population of northern China. PeerJ. 2019;7 doi: 10.7717/PEERJ.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y., Wang R., Li S., Kong X., Wang Z., Chen W., Lin N. Genetic polymorphisms in plasminogen activator inhibitor-1 predict susceptibility to steroid-induced osteonecrosis of the femoral head in Chinese population. Diagn. Pathol. 2013;8 doi: 10.1186/1746-1596-8-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun M., Cao Y., Wang T., Liu T., An F., Wu H., Wang J. Association between LINC-PINT and LINC00599 gene polymorphism and the risk of steroid-induced osteonecrosis of the femoral head in the population of northern China. Steroids. 2021;173 doi: 10.1016/J.STEROIDS.2021.108886. [DOI] [PubMed] [Google Scholar]
- 36.Liu T., Cao Y., Han C., An F., Wang T., Sun M., Ma C., Dong Q., Wang J. Association of MIR17HG and MIR155HG gene variants with steroid-induced osteonecrosis of the femoral head in the population of northern China. J. Orthop. Surg. Res. 2021;16 doi: 10.1186/S13018-021-02669-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerachian M.A., Séguin C., Harvey E.J. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J. Steroid Biochem. Mol. Biol. 2009;114:121–128. doi: 10.1016/J.JSBMB.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han L., Wang B., Wang R., Gong S., Chen G., Xu W. The shift in the balance between osteoblastogenesis and adipogenesis of mesenchymal stem cells mediated by glucocorticoid receptor. Stem Cell Res. Ther. 2019;10 doi: 10.1186/S13287-019-1498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peckett A.J., Wright D.C., Riddell M.C. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism. 2011;60:1500–1510. doi: 10.1016/J.METABOL.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Cohen-Rosenblum A., Cui Q. Osteonecrosis of the femoral head. Orthop. Clin. N. Am. 2019;50:139–149. doi: 10.1016/J.OCL.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Nagasawa K., Tada Y., Koarada S., Tsukamoto H., Horiuchi T., Yoshizawa S., Murai K., Ueda A., Haruta Y., Ohta A. Prevention of steroid-induced osteonecrosis of femoral head in systemic lupus erythematosus by anti-coagulant. Lupus. 2006;15:354–357. doi: 10.1191/0961203306LU2311OA. [DOI] [PubMed] [Google Scholar]
- 42.Yuan N., Ge Z., Ji W., Li J. Exosomes secreted from hypoxia-preconditioned mesenchymal stem cells prevent steroid-induced osteonecrosis of the femoral head by promoting angiogenesis in rats. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/6655225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hines J.T., Jo W.L., Cui Q., Mont M.A., Koo K.H., Cheng E.Y., Goodman S.B., Ha Y.C., Hernigou P., Jones L.C., Kim S.Y., Sakai T., Sugano N., Yamamoto T., Lee M.S., Zhao D., Drescher W., Kim T.Y., Lee Y.K., Yoon B.H., Baek S.H., Ando W., Kim H.S., Park J.W. Osteonecrosis of the femoral head: an updated review of ARCO on pathogenesis, staging and treatment. J. Kor. Med. Sci. 2021;36:1–15. doi: 10.3346/JKMS.2021.36.E177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Omari A.A., Aleshawi A.J., Marei O.A., Younes H.M.B., Alawneh K.Z., Alquran E., Mohaidat Z.M. Avascular necrosis of the femoral head after single steroid intra-articular injection. Eur. J. Orthop. Surg. Traumatol. 2020;30:193–197. doi: 10.1007/S00590-019-02555-8. [DOI] [PubMed] [Google Scholar]
- 45.el Maghraoui A., Tabache F., Bezza A., Ghafir D., Ohayon V., Archane M.I. Femoral head osteonecrosis after topical corticosteroid therapy. Clin. Exp. Rheumatol. 2001;19:233. https://pubmed-ncbi-nlm-nih-gov.humanitas.idm.oclc.org/11326500/ (accessed June 23, 2022) [PubMed] [Google Scholar]
- 46.Egger A.C., Ballock R.T. Osteonecrosis of the femoral head in an adolescent on long-term inhalational corticosteroids. Case Rep Pediatr. 2017:1–4. doi: 10.1155/2017/6969787. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dilisio M.F. Osteonecrosis following short-term, low-dose oral corticosteroids: a population-based study of 24 million patients. Orthopedics. 2014;37 doi: 10.3928/01477447-20140626-54. [DOI] [PubMed] [Google Scholar]
- 48.Zhao R., Wang H., Wang X., Feng F. Steroid therapy and the risk of osteonecrosis in SARS patients: a dose-response meta-analysis. Osteoporos. Int. 2017;28:1027–1034. doi: 10.1007/S00198-016-3824-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powell C., Chang C., Naguwa S.M., Cheema G., Gershwin M.E. Steroid induced osteonecrosis: an analysis of steroid dosing risk. Autoimmun. Rev. 2010;9:721–743. doi: 10.1016/J.AUTREV.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwala S.R., Vijayvargiya M., Sawant T. Secondary osteonecrosis of the knee as a part of long COVID-19 syndrome: a case series. BMJ Case Rep. 2022;15 doi: 10.1136/BCR-2021-248583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sulewski A., Sieroń D., Szyluk K., Lukoszek D., Christe A., Dabrowski M., Kubaszewski Ł. Avascular necrosis bone complication after active COVID-19 infection: preliminary results. Medicina. 2021;57 doi: 10.3390/MEDICINA57121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agarwala S.R., Vijayvargiya M., Pandey P. Avascular necrosis as a part of “long COVID-19. BMJ Case Rep. 2021;14 doi: 10.1136/BCR-2021-242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan M.H.M., Chan P.K.S., Griffith J.F., Chan I.H.S., Lit L.C.W., Wong C.K., Antonio G.E., Liu E.Y.M., Hui D.S.C., Suen M.W.M., Ahuja A.T., Sung J.J.Y., Lam C.W.K. Steroid-induced osteonecrosis in severe acute respiratory syndrome: a retrospective analysis of biochemical markers of bone metabolism and corticosteroid therapy. Pathology. 2006;38:229–235. doi: 10.1080/00313020600696231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoon B.H., Jones L.C., Chen C.H., Cheng E.Y., Cui Q., Drescher W., Fukushima W., Gangji V., Goodman S.B., Ha Y.C., Hernigou P., Hungerford M., Iorio R., Jo W.L., Khanduja V., Kim H., Kim S.Y., Kim T.Y., Lee H.Y., Lee M.S., Lee Y.K., Lee Y.J., Mont M.A., Sakai T., Sugano N., Takao M., Yamamoto T., Koo K.H. Etiologic classification criteria of ARCO on femoral head osteonecrosis Part 1: glucocorticoid-associated osteonecrosis. J. Arthroplasty. 2019;34:163–168. doi: 10.1016/J.ARTH.2018.09.005. e1. [DOI] [PubMed] [Google Scholar]
- 55.Fu W., Liu B., Wang B., Zhao D. Early diagnosis and treatment of steroid-induced osteonecrosis of the femoral head. Int. Orthop. 2019;43:1083–1087. doi: 10.1007/S00264-018-4011-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sultan A.A., Mohamed N., Samuel L.T., Chughtai M., Sodhi N., Krebs V.E., Stearns K.L., Molloy R.M., Mont M.A. Classification systems of hip osteonecrosis: an updated review. Int. Orthop. 2019;43:1089–1095. doi: 10.1007/S00264-018-4018-4. [DOI] [PubMed] [Google Scholar]
- 57.Schmitt-Sody M., Kirchhoff C., Mayer W., Goebel M., Jansson V. Avascular necrosis of the femoral head: inter- and intraobserver variations of Ficat and ARCO classifications. Int. Orthop. 2008;32:283–287. doi: 10.1007/S00264-007-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith S.W., Meyer R.A., Connor P.M., Smith S.E., Hanley E.N. Interobserver reliability and intraobserver reproducibility of the modified Ficat classification system of osteonecrosis of the femoral head. J Bone Joint Surg Am. 1996;78:1702–1706. doi: 10.2106/00004623-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 59.Saini A., Saifuddin A. MRI of osteonecrosis. Clin. Radiol. 2004;59:1079–1093. doi: 10.1016/J.CRAD.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 60.Steinberg M., Hayken G., Steinberg D. A quantitative system for staging avascular necrosis. Bone Joint Surg Br. 1995;77:34–41. [PubMed] [Google Scholar]
- 61.Plakseychuk A.Y., Shah M., Varitimidis S.E., Rubash H.E., Sotereanos D. Classification of osteonecrosis of the femoral head. Reliability, reproducibility, and prognostic value. Clin. Orthop. Relat. Res. 2001;386:34–41. doi: 10.1097/00003086-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Takashima K., Sakai T., Hamada H., Takao M., Sugano N. Which classification system is most useful for classifying osteonecrosis of the femoral head? Clin. Orthop. Relat. Res. 2018;476:1240–1249. doi: 10.1007/S11999.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishii T., Sugano N., Ohzono K., Sakai T., Sato Y., Yoshikawa H. Significance of lesion size and location in the prediction of collapse of osteonecrosis of the femoral head: a new three-dimensional quantification using magnetic resonance imaging. J. Orthop. Res. 2002;20:130–136. doi: 10.1016/S0736-0266(01)00063-8. [DOI] [PubMed] [Google Scholar]
- 64.Hernigou P., Poignard A., Nogier A., Manicom O. Fate of very small asymptomatic stage-I osteonecrotic lesions of the hip. J Bone Joint Surg Am. 2004;86:2589–2593. doi: 10.2106/00004623-200412000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.