Summary
Background
Acute rheumatic fever (ARF) and rheumatic heart disease (RHD) remain an inequitable cause of avoidable suffering and early death in many countries, including among Indigenous Māori and Pacific populations in New Zealand. There is a lack of robust evidence on interventions to prevent ARF. This study aimed to identify modifiable risk factors, with the goal of producing evidence to support policies and programs to decrease rates of ARF.
Methods
A case-control study was undertaken in New Zealand using hospitalised, first episode ARF cases meeting a standard case-definition. Population controls (ratio of 3:1) were matched by age, ethnicity, socioeconomic deprivation, location, sex, and recruitment month. A comprehensive, pre-tested questionnaire was administered face-to-face by trained interviewers.
Findings
The study included 124 cases and 372 controls. Multivariable analysis identified strong associations between ARF and household crowding (OR 3·88; 95%CI 1·68-8·98) and barriers to accessing primary health care (OR 2·07; 95% CI 1·08-4·00), as well as a high intake of sugar-sweetened beverages (OR 2·00; 1·13-3·54). There was a marked five-fold higher ARF risk for those with a family history of ARF (OR 4·97; 95% CI 2·53-9·77). ARF risk was elevated following self-reported skin infection (aOR 2·53; 1·44-4·42) and sore throat (aOR 2·33; 1·49-3·62).
Interpretation
These globally relevant findings direct attention to the critical importance of household crowding and access to primary health care as strong modifiable causal factors in the development of ARF. They also support a greater focus on the role of managing skin infections in ARF prevention.
Funding
This research was funded by the Health Research Council of New Zealand (HRC) Rheumatic Fever Research Partnership (supported by the New Zealand Ministry of Health, Te Puni Kōkiri, Cure Kids, Heart Foundation, and HRC) award number 13/959.
Keywords: Acute rheumatic fever, Risk factors, Case-control study, New Zealand, Group A streptococcal infections, Social determinants, Housing, Crowding, Primary healthcare, Māori, Pacific peoples
Research in context.
Evidence before this study
We conducted a structured review of the published literature focusing on risk factors for ARF, RHD, and GAS infections (with full results reported in the published study protocol). It was restricted to studies that used robust epidemiological designs (cohort, case-control, cross-sectional, controlled trials). This scope included studies referenced in Medline and Embase plus those found by a manual search of references identified in these studies. We also drew on one recent published systematic review of social determinants for ARF, RHD and GAS infection. This search found an almost complete lack of high-quality studies with none covering a full range of host and environmental risk factors and no intervention studies. The modifiable risk factors consistently identified were exposure to poverty and household crowding. The literature identified GAS pharyngitis as a known trigger for ARF. There was one recent record linkage study supporting a role for GAS skin infections. There was also some evidence for susceptibility having a familial and genetic component.
Added value of this study
Our study has identified important modifiable risk factors for ARF, notably household crowding and barriers to accessing primary health care. It used tight matching to control for the effects of established sociodemographic risk factors and family history, allowing identification of the contribution of specific modifiable environmental exposures and health service factors to the risk of disease. Findings also demonstrated that a preceding skin infection was associated with an increased risk of ARF.
Implications of all the available evidence
Findings from this study show the importance of minimizing household crowding as an intervention to reduce the incidence of ARF in the many countries where this disease remains an endemic public health problem. Results also support the importance of access to primary health care and strengthen the evidence for effective treatment of skin infections to reduce ARF. Findings also show that there are likely to be benefits in targeting prevention interventions to populations with the highest rates of ARF/RHD and potentially incorporating family history of disease in this process. The identified association between ARF and the number of sugar-sweetened drinks consumed each day requires further investigation.
Alt-text: Unlabelled box
Introduction
Acute rheumatic fever (ARF) is an immune-mediated disease that occurs as a delayed sequelae to group A streptococcus (GAS) infection.1,2 ARF and its complication rheumatic heart disease (RHD) have significant effects on health, often resulting in chronic illness and premature death.2 ARF and RHD cause a large worldwide burden of morbidity and mortality with an estimated 34 million people living with RHD in 2015.3
In most high-income countries, improvements in living conditions, and the introduction of antimicrobial drugs to treat GAS pharyngitis, are thought to have led to ARF virtually disappearing.4 However, ARF remains an important disease in New Zealand, where there are particularly high rates among indigenous Māori and Pacific populations. While New Zealand Europeans rarely develop ARF, Māori children in the peak age group, 5-14 years, have a rate of 36 cases per 100,000 while Pacific children have a rate of 80 cases per 100,000.5 These rates are similar to those observed in the first half of the 20th century in New Zealand among the non-indigenous population and currently in low- and middle-income countries.3,4
A range of environmental, host, and microbial risk factors interact to influence ARF risk and outcomes.6,7 However, knowledge gaps remain regarding the risk factors for ARF, limiting the ability to develop and implement effective interventions.8 An etiological link has been proposed between ARF development and poverty, with associated risk factors including poor housing conditions (e.g. cold, damp, mould),9,10 overcrowding,9 and bed sharing.11 However, these links remain uncertain due to limited evidence.
GAS pharyngitis is a known trigger for ARF, and GAS skin infections are also proposed to cause ARF either directly or in combination with GAS pharyngitis.12 GAS pharyngitis is highly infectious and close proximity to others is a risk factor for GAS transmission.13 A lack of washing facilities and resources may also contribute to a greater GAS transmission risk.14
Effective treatment of GAS infections could interrupt the development of ARF. Accordingly, access to suitable primary care services to diagnose and treat GAS throat and skin infections should be a protective factor.15 Several aspects of nutrition may contribute to ARF risk, including overall nutritional status and intake of micronutrients.16 Associations between dental caries and ARF were shown as early as the 1930s.17 While familial linkages for ARF are known,18 inherited genetic risk variants are poorly understood.19, 20, 21 For many of these potential risk factors confounding is possible through their strong association with poverty.
Two recent structured reviews found few rigorous epidemiological studies of potentially modifiable risk factors for ARF in the published literature.6,7 These reports include few high quality case-control studies internationally and none have been conducted in New Zealand. Accordingly, the aim of this study was to identify modifiable risk factors for ARF, with the goal of producing robust evidence to support policies and programs to decrease rates of this disease.
Methods
Study design
The acute rheumatic fever risk factors study (RF RISK) was a population-based matched case-control study (using a dynamic population and concurrent sampling)22 that was carried out in 11 District Health Board (DHB) regions within New Zealand between 2013 and 2016. The population of New Zealand in 2013 was 4·50 million with Māori 16·4% of the total, Pacific Peoples 8·0%, and New Zealand Europeans and other ethnic groups 75·6%. Full details of the study design and methods have been published.6
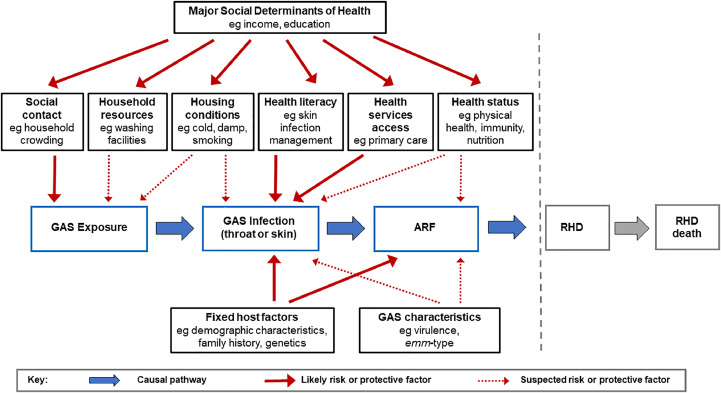
The choice of risk and protective factors to investigate was based on an extensive review of the literature.6 We also developed a hypothesised causal pathway for how these factors operate to increase or decrease the risk of ARF and used this to identify and group exposures for inclusion in the data collection and analysis (Figure 1). We categorised these factors as: environmental exposures (including social contacts, housing conditions, and resources); health services access (including use, barriers, and health literacy); health status (including physical and oral health, and nutrition); social determinants (including deprivation level of household and main caregiver); predisposing factors (including family history and demographic characteristics). Potential initiating infections were included as mediating factors on the causal pathway to ARF.
Figure 1.
Causal pathway from GAS exposure to ARF and RHD showing major hypothesised groups of risk and protective factors (updated from protocol paper6).
Study cases
Incident cases met the standard New Zealand case-definition for ARF (definite or probable, excluding chorea or indolent carditis only).23 They were all recruited following hospitalisation and within four weeks of admission for a first episode of ARF, aged less than 20 years, and residing in the North Island of New Zealand, where more than 95% of national cases occurred.5 A case-review panel of clinicians determined eligibility of 138 potential cases by reviewing clinical case records. Following review 124 cases classified as definite or probable ARF were included.
Study controls
Population controls were identified from the New Zealand Health Survey (NZHS) cohort who had consented to further follow-up. The NZHS is a rolling population-based survey with 14,000 participants annually.6 Controls (372, ratio of 3:1 case) were recruited and individually matched to each case by age (within two years), ethnicity (prioritised),6 socioeconomic deprivation (NZDep13) quintile,6 and region (DHB). Prioritised ethnicity grouping allocates individuals to a single ethnic group based on a prioritised order of Māori, Pacific Peoples, Asian, and European/Other.6 The NZDep13 score is an area-based measure of socioeconomic deprivation derived from national census data.6 Where the available pool of controls allowed, they were also matched on sex and this was achieved in most (77.2%) instances. Matching effectively occurred by time-period, as controls were selected and interviews conducted within one-to-four weeks of the case interview, to control for possible seasonal effects. Controls that had ever had ARF/RHD were excluded.
Data collection
Following recruitment, cases and controls (or their parent/caregiver if <16 years) were interviewed face-to-face using a pre-tested questionnaire that focused on exposure status mainly during the four weeks prior to illness or interview (Supplement 1). The study questionnaire drew on existing questionnaires where appropriate to maximise comparability.6 Additional data were obtained from linked records (Table 2).6
Table 2.
Univariable association between individual risk factors and ARF, adjusted for sociodemographic matching variables.
| Exposure (in 4 weeks prior to interview and based on questionnaire data unless otherwise stated) | Cases (n = 124) |
Controls (n = 372) |
aOR (95% CI)⁎ | ||||
|---|---|---|---|---|---|---|---|
| n | %⁎⁎ | n | %⁎⁎ | ||||
| Potential initiating infections, self-reported | |||||||
| Throat infection | Yes | 60 | 48·4 | 106 | 28·5 | 2·33 (1·49-3·62) | |
| No | 59 | 47·6 | 263 | 70·7 | |||
| Skin infection | Yes | 27 | 21·8 | 37 | 9.9 | 2·53 (1·44-4·42) | |
| No | 96 | 77·4 | 335 | 90·1 | |||
| Throat and skin infection | Yes | 13 | 10·5 | 9 | 2·4 | 15·32 (3·37-69·60) | |
| No throat or skin infections | 46 | 37.1 | 237 | 63.7 | |||
| Scabies | Yes | 7 | 5·6 | 5 | 1·3 | 5·90 (1·74-20·04) | |
| No | 117 | 94·4 | 364 | 97·8 | |||
| Environmental exposures, including social contacts and housing | |||||||
| Housing tenure | Rented | 97 | 78·2 | 234 | 62·9 | 3·48 (1·81-6·70) | |
| Owned by occupant | 17 | 13·7 | 104 | 28·0 | |||
| Exposure to damp and mould in the home | 1-3 indicators | 75 | 60·5 | 141 | 37·9 | 3.03 (1.88-4.92) | |
| None | 49 | 39·5 | 230 | 61·8 | |||
| Exposure to cold in the home | 1-4 indicators | 92 | 74·2 | 231 | 62·1 | 1·99 (1·22-3·33) | |
| None | 32 | 25·8 | 139 | 37·4 | |||
| Structural household crowding (American Crowding Index) | Crowded | 31 | 25·0 | 25 | 6·7 | 6·04(3·03-12·04) | |
| Uncrowded | 93 | 75·0 | 342 | 91·9 | |||
| Functional household crowding (Sharing a sleeping room to stay warm) | Yes | 34 | 27·4 | 46 | 12·4 | 3·26 (1·78-5·97) | |
| No | 90 | 72·6 | 321 | 86·3 | |||
| Number of people usually sharing child's bed | ≥ 1 person | 65 | 52·4 | 143 | 38·4 | 2.28 (1.44-3.60) | |
| No one | 59 | 47·6 | 229 | 61·6 | |||
| Limited hot water for bath or shower | 1-2 indicators | 28 | 22·6 | 41 | 11·0 | 2.45 (1.29-4.74) | |
| None | 74 | 59·7 | 291 | 78·2 | |||
| Smoker living in house | Yes | 74 | 59·7 | 181 | 48·7 | 1·78 (1·12-2·81) | |
| No | 50 | 40·3 | 191 | 51·3 | |||
| Number of social gatherings outside of home | 4-9 activities | 54 | 43.5 | 212 | 57.0 | 0.59 (0.30-0.89) | |
| 0-3 activities | 70 | 56.5 | 160 | 43.0 | |||
| Health services access, use, and health literacy | |||||||
| Barriers to accessing primary health care in last 12 months (composite of 5 questions) | 1-5 barriers | 63 | 50·8 | 117 | 31·5 | 2·12 (1·36-3·32) | |
| None | 61 | 49·2 | 255 | 68·5 | |||
| Child attended a school with a sore throat management programme (linked school and health records) | Yes | 44 | 35·5 | 108 | 29·0 | 1.94 (1.06-3.55) | |
| No | 67 | 54·0 | 232 | 62·4 | |||
| Seen dental care worker when required in last 12 months | Yes | 14 | 11·3 | 34 | 9·1 | 1·18 (0·59-2·33) | |
| No | 109 | 87.9 | 337 | 90·6 | |||
| ARF health literacy (composite of 3 questions) | 2-3 indicators | 18 | 14·5 | 83 | 22·3 | 0·52 (0·28-0·95) | |
| 0-1 indicators | 106 | 85·5 | 287 | 77·2 | |||
| Health status, oral health, and nutrition | |||||||
| General health prior to illness/interview (self-rated or parent-rated on 5-point scale) | Poor/fair | 9 | 7·3 | 7 | 1.9 | 4.06 (1.36-12.10) | |
| Good/very good/excellent | 115 | 92·7 | 365 | 98·1 | |||
| Tonsils or adenoids removed at any time | Yes | 3 | 2·4 | 26 | 7.0 | 0·31 (0·09-1·08) | |
| No | 120 | 96·8 | 344 | 92·5 | |||
| Average fruit servings eaten per day | <1 servings | 33 | 26.6 | 71 | 19.1 | 1·40 (0·84-2·32) | |
| 1-4+ servings | 91 | 73.4 | 301 | 80.9 | |||
| Average vegetable servings eaten per day | <1 servings | 41 | 33·1 | 85 | 22·8 | 1·66(1·02-2·69) | |
| 1-4+ servings | 83 | 66.9 | 287 | 77·2 | |||
| Number of sugar-sweetened beverages (can or large glass) per day | 1-9 beverages | 71 | 57.3 | 136 | 36.6 | 2·34 (1·50-3·66) | |
| None | 53 | 42·7 | 230 | 61·8 | |||
| Poor oral health based on decayed, missing, filled permanent and deciduous teeth (linked dental records) | 1-10+ | 82 | 66·1 | 204 | 54·8 | 1.48 (0.93-2.38) | |
| No | 40 | 32·3 | 138 | 37·1 | |||
| Social determinants | |||||||
| NZiDep score of caregiver | ≥2 | 76 | 61·3 | 146 | 39·2 | 3·18 (1·92-5·26) | |
| <2 | 47 | 37·9 | 225 | 60·5 | |||
| Predisposing factors | |||||||
| Family history of ARF (blood relative diagnosed with ARF/RHD) | Yes | 56 | 45·2 | 73 | 19·6 | 3·73 (2·23-5·62) | |
| No | 64 | 51·6 | 292 | 78·5 | |||
| Number of grandparents with any Māori/Pacific ethnicity | 4 | 106 | 85.5 | 209 | 56·2 | 5.83 (3.15-10.81) | |
| 0-3 | 18 | 14·5 | 163 | 43·8 | |||
Adjusted odds ratio (aOR), adjusted by age, sex, ethnicity, deprivation (NZDep13), region (DHB); and 95% confidence interval (95%CI).
Missing values not show so percentages may add up to less than 100% .
Exposure measures
Composite measures that combined multiple questions were used for the following exposures: Exposure to damp and mould in the home; exposure to cold in the home; limited hot water for bathing/showering; number of social gatherings outside the home; barriers to accessing primary health care in the last 12 months; and ARF health literacy. A full list of questions included in each of the composite variables is available in Supplement 1.
Structural household crowding was measured using the American Crowding Index. This index states that crowding occurs if there is more than one person per room, severe crowding occurs if there are more than 1.5 persons per room (excluding bathrooms, balconies, porches, foyers, hallways and half-rooms). Functional crowding was defined as the case or control sharing a sleeping room just to stay warm.6
Statistical analysis
Statistical analysis was conducted using SAS 9.4 (SAS Institute, Cary, NC). Cochran-Mantel-Haenszel tests were used to test for associations between exposure and case status for all sociodemographic variables except mean age, for which a Student's T-test was used. Conditional logistic regression was used to investigate the independent association between each risk factor of interest (Table 2) and ARF development. Adjusted Odds Ratios (aOR) for occurrence of ARF were estimated in models including all the matching variables of age (years), ethnicity (prioritised, Māori/Pacific/Other), socioeconomic deprivation (NZDep13 levels 1-10), region (11 DHBs that were participating), and sex (male/female).
The multivariable model was adjusted for all the risk factor variables included in the full model, the matching variables (age, sex, ethnicity, deprivation (NZDep13), and region), and an additional deprivation measure (the caregiver's individual deprivation score, NZiDep).6 These risk factor variables were identified a priori in the study protocol6 and refined by stepwise regression, with collinear or non-significant variables removed. Note that in some cases non-significance was caused by very closely related exposure variables being included in the model (e.g. damp and mould); in this case the model was run including each variable separately and the exposure variable with the strongest association was chosen. Exposure variables included in the model were: Housing tenure (rented versus owner-occupied); exposure to damp and mould in the home (damp/mould versus none); exposure to cold in the home (cold versus not cold); limited hot water for bath or shower (yes/no); living with a current smoker (yes/no); structural household crowding (crowded versus uncrowded); functional household crowding (yes/no); number of people usually sharing the bed (shared versus unshared); and social gatherings outside the home (4-9 activities versus 0-3). Also included in the model were barriers to accessing primary health care (barriers versus no barriers); the number of sugar-sweetened beverages consumed per day (1-9 versus none); a family history of ARF/RHD (any blood relative); and number of grandparents with any Māori or Pacific ethnicity (4 versus 0-3). Recent sore throat and skin infection were excluded from the model as they were considered mediating events on the causal pathway to ARF. In the multivariable model we imputed missing data using mean imputation, i.e. missing observations for a certain variable were replaced with the mean of the non-missing observations for that variable obtained for other cases or controls (see Supplement Table 2 for frequency of missing values).
Ethical approval
The New Zealand Health and Disability Ethics Committee (HDEC) approved this study (reference number 14/NTA/53). Informed consent to participate was required (from the parent or legal guardian in the case of children aged less than 16 years). The ethics, study design, and operation were also reviewed by a Māori Steering Group and a Pacific Steering Group (see acknowledgements).
Role of the funding source
The sponsor of the study was not involved in the study design, data collection, data analysis, data interpretation, or writing of the report. The authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Sociodemographic characteristics
There were 496 participants (124 cases and 372 controls) (Table 1). Males were a higher proportion of cases (65·3% of cases) than controls (53·8%). Almost all cases were either Māori (41·9%) or Pacific (57·3%).
Table 1.
Sociodemographic characteristics of ARF cases and healthy controls.
| Variable | Cases |
Controls |
|||
|---|---|---|---|---|---|
| n = 124 | % | n = 372 | % | P* | |
| Age (years) | |||||
| <5 | 1 | 0·8 | 7 | 1·9 | |
| 5-9 | 36 | 31·5 | 135 | 36·3 | |
| 10-12 | 49 | 33·9 | 123 | 33·1 | |
| 13-15 | 31 | 27·4 | 86 | 23·1 | |
| 16-19 | 7 | 6·5 | 21 | 5·7 | |
| (mean, SD) | 11·0 | (95% CI: 10·5-11·5), SD 2·0 | 10·5 | (95% CI: 10·2-10·9), SD 3·2 | 0·162 |
| Sex | |||||
| Male | 81 | 65·3 | 200 | 53·8 | |
| Female | 43 | 34·7 | 172 | 46·2 | 0·025 |
| Ethnicity (prioritised6) | |||||
| Māori | 52 | 41·9 | 164 | 44·1 | |
| Pacific | 71 | 57·3 | 204 | 54·8 | |
| NZ European | 1 | 0·8 | 3 | 0·8 | |
| Other | 0 | 0·0 | 1 | 0·3 | 0·912 |
| Socioeconomic deprivation (NZDep136) | |||||
| 1-2 (least deprived) | 2 | 1·6 | 5 | 1·3 | |
| 3-4 | 7 | 5·7 | 14 | 3·8 | |
| 5-6 | 12 | 9·7 | 29 | 7·8 | |
| 7-8 | 20 | 16·1 | 79 | 21·2 | |
| 9-10 (most deprived) | 83 | 66·9 | 245 | 65·9 | 0·654 |
| Region (District Health Board) | |||||
| Auckland | 21 | 16·9 | 62 | 16·7 | |
| Bay of Plenty | 7 | 5·7 | 22 | 5·9 | |
| Capital and Coast | 2 | 1·6 | 9 | 2·4 | |
| Counties-Manukau | 52 | 41·9 | 155 | 41·7 | |
| Hawke's Bay | 3 | 2·4 | 9 | 2·4 | |
| Hutt Valley | 3 | 2·4 | 6 | 1·6 | |
| Lakes | 6 | 4·8 | 15 | 4·0 | |
| Northland | 5 | 4·0 | 15 | 4·0 | |
| Tairawhiti | 3 | 2·4 | 10 | 2·7 | |
| Waikato | 10 | 8·1 | 31 | 8·3 | |
| Waitemata | 12 | 9·7 | 38 | 10·2 | 0·999 |
Determined using Cochran-Mantel-Haenszel test for all variables except mean age, which was determined using a Student's t-test.
Preceding infection analysis
The first section of Table 2 shows the association of ARF with potential initiating infections in the four weeks prior to the interview. Nearly half (48·4%) of cases but only 28·5% of controls reported a sore throat (age, sex, ethnicity, deprivation, region-adjusted OR (aOR) with 95% confidence interval; 2·33; 1·49-3·62). Cases were significantly more likely than controls to report a definite or probable skin infection (aOR 2·53; 1·44-4·42). The highest risk was seen when the child reported both a skin infection and a sore throat (aOR 15·32; 3·37-69·60). There was also an elevated risk of ARF associated with having scabies, although this was based on small numbers (n = 7 cases, n = 5 controls) of participants (aOR 5·90; 1·74-20·04).
Univariable risk factor analysis
Table 2 shows the results of the adjusted univariable analysis for the association of ARF with a range of potential risk factors, mostly during the four weeks prior to the interview.
ARF risk was associated with housing tenure, conditions, and exposures. There was a strong association with structural household crowding (aOR 6·04; 3·03-12·04) and functional crowding (aOR 3·26; 1·78-5·97). ARF risk was also associated with rental housing (aOR 3·48; 1·81-6·70), exposure to cold (aOR 1·99; 1·22-3·33), and exposure to damp and mould in the home (aOR 3.03; 1.88-4.92). Risk was also increased for children having a smoker living in the same house (aOR 1·78; 1·12-2·81).
There was little difference between cases and controls in terms of the frequency with which they usually had a bath or shower (79·0% of cases and 76·3% of controls reported at least one bath or shower each day). However, ARF was associated with a lack of hot water for having a bath or shower (aOR 2.45; 1.29-4.74).
ARF risk was associated with reported barriers to primary health care access within the last 12 months (aOR 2·12; 1·36-3·32) but not with reported access barriers to dental care. Knowledge of ARF appeared protective (aOR 0.52; 0.28-0.95), but attending a school with a sore throat management programme (a major ARF intervention in New Zealand24) was not (aOR 1.94; 1.06-3.55).
The risk of ARF was associated with reported poor general health prior to the onset of ARF symptoms or interview (OR: 4·06 (1·36-12·10)), although an overwhelming majority of subjects reported their prior health as good to excellent. There was an elevated risk of ARF associated with the number of sugar-sweetened beverages (including fruit juice) consumed, with 57·3% of cases but 36.6% of controls consuming one or more drinks per day (aOR 2·34; 1·50-3·66).
Almost half of all cases (45·2%) had a blood relative who had ever been diagnosed with ARF or RHD, while it was 19·6% among controls (aOR 3·73; 2·23-5·62). Most cases (85·5%) reported that all their grandparents were of New Zealand Māori or Pacific ethnicity, while that proportion was lower (56·2%) among controls (aOR 5.83 ;(3.15-10.81).
Multivariable risk factor analysis
Table 3 reports results of the multivariable analysis. ARF remained strongly associated with household crowding (aOR 3·88; 1·68-8·98) and barriers to accessing primary health care (aOR 2·07; 1·08-4·00). Barriers were: being unable to book an appointment within 24 hours; the cost of the appointment and prescription; a lack of transport to attend; and a lack of childcare for other children preventing attendance.
Table 3.
Multivariable association between risk factors and ARF, adjusted for sociodemographic matching variables and all other risk factors included in model (n = 496).
| Risk factor | Units | aOR | 95% CI* |
|---|---|---|---|
| Grandparents with any Māori or Pacific ethnicity | 4 versus 0-3 | 5·79 | (2·60-12·88) |
| Family history of ARF/RHD (blood relative) | Yes versus No | 4·97 | (2·53-9·77) |
| Structural household crowding (American Crowding Index) | Crowded versus uncrowded |
3·88 | (1·68-8·98) |
| Barriers to accessing primary health care | Barriers versus no barriers | 2·07 | (1·08-4·00) |
| Number of sugar-sweetened beverages per day | 1-9 versus none | 2·00 | (1·13-3·54) |
| Functional household crowding (Sharing sleeping room to stay warm) | Yes versus No | 1·86 | (0·78-4·41) |
| Limited hot water for bath or shower | Yes versus No | 1·77 | (0·78-4·02) |
| Number of people usually sharing child's bed | Shared versus not shared |
1·73 | (0·94-3·17) |
| Smoker living in house | Yes versus No | 1·28 | (0·67-2·45) |
| Exposure to damp and mould in the home | Damp and mould versus none | 1·27 | (0·64-2·52) |
| Housing tenure | Rented versus owned | 1·22 | (0·56-2·70) |
| Poor oral health (based on dental records) | Decayed, missing, filled teeth versus none |
1·14 | (0·58-2·24) |
| Exposure to cold in the home | Cold versus not cold | 1·11 | (0·51-2·41) |
| Social gatherings outside the home | 4-9 social activities versus 0-3 | 0·81 | (0·47-1·41) |
Adjusted odds ratio (aOR), with other matching variables included in model: age, sex, ethnicity, deprivation (NZDep13), region (DHB), plus NZiDep (composite of 8 questions), plus other risk factors listed in this table; and 95% confidence interval (95% CI).
In addition, ARF was strongly associated with having a family history of ARF and RHD (aOR 4·97; 2·53-9·77) as was having four grandparents of Māori or Pacific ethnicity (aOR 5·79; 2·60-12·88). Finally, the risk of ARF was associated with a high intake of sugar-sweetened beverages (aOR 2·00; 1·13-3·54). Several other important potential modifiable risk factors were included in the model but were not associated with the risk of ARF.
Discussion
This study has identified important modifiable risk factors for ARF, notably household crowding and barriers to accessing primary health care. A major advance is its use of tight matching to control for the effects of established sociodemographic risk factors and adjusting for family history, allowing identification of the contribution of specific environmental exposures and health service factors to the risk of disease. These results also add to our understanding of the pathogenesis of ARF by showing a link to preceding GAS skin infection. Findings are also consistent with a parallel study in New Zealand looking at risk factors for GAS throat and skin infection that also found an association with household crowding and barriers to accessing primary health care.25
Results from this study show the importance of a supply of suitable, well-maintained, housing as a key strategy to minimise household crowding and reduce the incidence of ARF in the many countries where this disease remains an endemic public health problem. Findings suggest that it may also be important to focus on improving housing conditions more generally, by reducing exposure to damp, mould, and cold, ensuring enough hot water for washing, and reducing bed sharing, and functional crowding (sharing a sleeping room to stay warm), although these factors require more investigation.
The importance of adequate access to primary health care, which provides an opportunity to effectively treat GAS pharyngitis and skin infections to reduce ARF, is strengthened by findings from this study. The widespread availability of affordable comprehensive care clinics in the United States (Baltimore), Cuba, and Costa Rica coincided with significant reductions on ARF rates in those countries.26, 27, 28 ARF remains relatively common in populations where access to health care is a known public health problem. Findings from this study add to other research evidence from New Zealand that effective treatment of skin infection in children may provide an important intervention for preventing ARF.12,25,29 By contrast, the lack of a protective effect for children attending a school with a sore throat management programme adds to other evidence questioning the effectiveness of this intervention (based on short-courses of oral antibiotics), which has not so far resulted in consistent declines in ARF.12,24
These results have reinforced the contribution of genetic and intergenerational factors to the risk of ARF. Familial ARF has been reported.9,18 In New Zealand, Māori and Pacific children have 12 and 24 times the risk of developing ARF compared with New Zealand European children, even after controlling for socioeconomic deprivation.5,30 This study has found a markedly high risk amongst cases where all grandparents had Māori or Pacific ethnicity. Furthermore, cases with a self-reported family history had a five-fold increase in risk even after accounting for modifiable environmental risk factors. Similar associations were found between these familial factors and the risk of GAS skin infection.25 However, it is important to note that ARF has historically been observed in all ethnic groups living in disadvantaged circumstances, internationally and in New Zealand, highlighting the importance of environmental risk factors.4,31
These findings suggest there are benefits in targeting prevention interventions to populations with the highest rates of ARF/RHD (e.g. Māori and Pacific Peoples in New Zealand) and potentially incorporating family history of disease in this selection process. Such interventions include access to improved housing and to prompt accessible treatment for sore throats and skin infections with support to ensure adherence. There are also opportunities to improve prevention of RHD by better detection of preceding ARF, which is often not diagnosed.32 Research from Uganda33 and New Zealand34 has also shown a familial risk of RHD, supporting screening for undetected RHD in family members of ARF patients. Regular penicillin treatment can prevent progression of latent RHD detected by such screening.35
This study has also identified an association between ARF and the number of sugar-sweetened drinks consumed each day. People in New Zealand have high daily intakes of total sugars,36 and sugar-sweetened beverages are readily available and affordable. This association requires further investigation to assess whether reducing the intake of sugar-sweetened drinks could lower ARF/RHD risk in children.
This study also produced a number of negative results that are helpful in suggesting that some hypothesised risk factors and associated interventions are unlikely to contribute to reducing ARF. Notable among these findings were the lack of ARF association with social gatherings outside the house and markers of poor oral health (such as accumulated dental caries experience in the deciduous and permanent teeth).
This study has several important strengths and limitations. Firstly, the research design had a strong theoretical framework to investigate risk factors based on a structured literature review and hypothesised causal pathway.6 There was rigorous case review by a panel of experienced clinicians using well-established ARF diagnostic criteria to ensure that the included cases had a high probability of being true ARF cases. Close matching and measurement and adjustment for multiple potential confounding factors through multivariable analysis should have mitigated the effects of confounding bias. However, it is possible that there may have been some residual confounding due to unmeasured or inaccurately measured exposures. In addition, some recall bias is possible, particularly because cases were interviewed shortly (a median of 21 days) after the onset of illness and controls did not have a similar stimulus to recall. There is also likely to be some social desirability bias with questions on risks for children (e.g. smoking indoors), which could result in differential reporting between cases and controls.
Although case recruitment for this study occurred at hospitals, it can be regarded as population-based because the standard of care in New Zealand is that all diagnosed cases of ARF are hospitalised.23 However, we know that a large proportion of ARF cases are not diagnosed, because many of those presenting with RHD have no prior history of ARF.32 It is possible that these unrecognised cases have different risk factors to those who are diagnosed.
Not all findings of this study will be generalisable to other countries. New Zealand is a high-income country, though many children and families live in relative poverty, so some findings may not be applicable to low- and middle-income countries where most ARF cases occur. Similarly, some housing factors, such as cold, damp and mould, may be more relevant in temperate countries like New Zealand than tropical regions where ARF is concentrated (though even in New Zealand ARF has only a modest degree of seasonality).6
Māori and Pacific Peoples continue to suffer high rates of ARF in New Zealand.5 Our study findings direct attention to the critical importance of household crowding, access to primary health care, and family history as likely causal factors in the development of ARF, which therefore should be the target of interventions to reduce highly inequitable ARF rates. These major findings should be generalizable to other countries and further demonstrate the urgent need to address poor housing conditions as a key measure to improve child health and reduce unacceptable health disparities.
Contributors
M.G.B. took principal responsibility for initiating the study, preparing the funding application, study design, and manuscript submission and revision. J.G. took principal responsibility for study protocol development and study management. N.J.M. established procedures for collection of specimens in all study sites and advised on the immunological analyses. J. B. drafted the manuscript and advised on epidemiological and statistical analysis. J.O. managed specimen collection and extensively reviewed the literature for this paper. D.A.W. advised on the microbiological analyses. N.P., R.E., and J.Z. advised on and conducted the epidemiological and statistical analyses. T.R.M. advised on the genetic analyses. D.L., N.W., T.P., C.J., and F.C.M. contributed to case recruitment and review. W.M.T. advised on the dental component. All authors contributed to the design of the study and reviewed and approved this manuscript. The following authors have verified the data: J.B., J.Z., N.P., J.G.
Data sharing statement
The study protocol is provided in the protocol paper.6 Individual participant data will be made available when the remaining analyses have been completed, upon reasonable requests directed to the corresponding author.
Declaration of interests
We declare that we have no conflicts of interest.
Acknowledgements
This research was funded by the Health Research Council of New Zealand (HRC) Rheumatic Fever Research Partnership (supported by the New Zealand Ministry of Health, Te Puni Kōkiri, Cure Kids, Heart Foundation, and HRC) award number 13/959. We thank all the participants and their families for sharing their time and experiences to make this study possible. We also wish to acknowledge the Māori Steering Group (chaired by Jason Gurney) and the Pacific Steering Group (chaired by Teuila Percival) for helpful advice about the study design and operation; Clinicians, medical officers of health, nursing staff, support staff and other DHB staff who have assisted us with case recruitment; Laboratory staff in Labtests (Auckland), Southern Community Laboratories (Wellington), DHBs, and ESR who have assisted with specimen collection, processing and testing; Interviewing and support staff employed by CBG Health Research Limited; Homes.co.nz for provision of housing data.
In particular, we wish to acknowledge Professor Diana (Dinny) Lennon, the senior clinical author enabling this study, who died at the time of its completion. Early in her career as an infectious disease's paediatrician, she developed an understanding of epidemiology and the social determinants of child health. She was a passionate advocate for better outcomes, especially for Māori and Pacific children. We salute her contribution as New Zealand's leading rheumatic fever researcher for 30 years. At her funeral in 2018, Māori elders paid Dinny an everlasting tribute: “Kua hinga te totara i te wao nui a Tane – a great totara tree has fallen in the forest of Tane”.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100508.
Appendix. Supplementary materials
References
- 1.Parks T, Smeesters PR, Steer AC. Streptococcal skin infection and rheumatic heart disease. Curr Opin Infect Dis. 2012;25(2):145–153. doi: 10.1097/QCO.0b013e3283511d27. [DOI] [PubMed] [Google Scholar]
- 2.Carapetis JR, Beaton A, Cunningham MW, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers. 2016;2:15084. doi: 10.1038/nrdp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watkins DA, CO Johnson, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017;377(8):713–722. doi: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 4.Steer AC. Historical aspects of rheumatic fever. J Paediatr Child Health. 2015;51(1):21–27. doi: 10.1111/jpc.12808. [DOI] [PubMed] [Google Scholar]
- 5.Bennett J, Zhang J, Leung W, et al. Rising ethnic inequalities in acute rheumatic fever and rheumatic heart disease, New Zealand, 2000-2018. Emerg Infect Dis. 2021;27(1):36–46. doi: 10.3201/eid2701.191791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker MG, Gurney J, Oliver J, et al. Risk factors for acute rheumatic fever: literature review and protocol for a case-control study in New Zealand. Int J Environ Res Public Health. 2019;16(22):4515. doi: 10.3390/ijerph16224515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffey PM, Ralph AP, Krause VL. The role of social determinants of health in the risk and prevention of group A streptococcal infection, acute rheumatic fever and rheumatic heart disease: a systematic review. PLoS Negl Trop Dis. 2018;12(6):e0006577. doi: 10.1371/journal.pntd.0006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant PA, Robins-Browne R, Carapetis JR, Curtis N. Some of the people, some of the time: susceptibility to acute rheumatic fever. Circulation. 2009;119(5):742–753. doi: 10.1161/CIRCULATIONAHA.108.792135. [DOI] [PubMed] [Google Scholar]
- 9.Vlajinac H, Adanja B, Marinković J, Jarebinski M. Influence of socio-economic and other factors on rheumatic fever occurrence. Eur J Epidemiol. 1991;7(6):702–704. doi: 10.1007/BF00218687. [DOI] [PubMed] [Google Scholar]
- 10.Zaman MM, Yoshiike N, Chowdhury AH, et al. Socio-economic deprivation associated with acute rheumatic fever. A hospital-based case-control study in Bangladesh. Paediatr Perinat Epidemiol. 1997;11(3):322–332. doi: 10.1111/j.1365-3016.1997.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 11.Adanja B, Vlajinac H, Jarebinski M. Socioeconomic factors in the etiology of rheumatic fever. J Hyg Epidemiol Microbiol Immunol. 1988;32(3):329–335. [PubMed] [Google Scholar]
- 12.Oliver J, Bennett J, Thomas S, et al. Preceding group A streptococcus skin and throat infections are individually associated with acute rheumatic fever: evidence from New Zealand. BMJ Global Health. 2021;6(12):e007038. doi: 10.1136/bmjgh-2021-007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danchin MH, Rogers S, Kelpie L, et al. Burden of acute sore throat and group A streptococcal pharyngitis in school-aged children and their families in Australia. Pediatrics. 2007;120(5):950–957. doi: 10.1542/peds.2006-3368. [DOI] [PubMed] [Google Scholar]
- 14.Luby SP, Agboatwalla M, Feikin DR, et al. Effect of handwashing on child health: a randomised controlled trial. Lancet. 2005;366(9481):225–233. doi: 10.1016/S0140-6736(05)66912-7. [DOI] [PubMed] [Google Scholar]
- 15.Chang C. Cutting edge issues in rheumatic fever. Clin Rev Allergy Immunol. 2012;42(2):213–237. doi: 10.1007/s12016-011-8271-1. [DOI] [PubMed] [Google Scholar]
- 16.Adanja BJ, Vlajinac HD, Marinkovic JP, Jarebinski MS. Rheumatic fever and diet. Isr J Med Sci. 1991;27(3):161–163. [PubMed] [Google Scholar]
- 17.Elzea CF. Nutrition and physical degeneration: a comparison of primitive and modern diets and their effects. Am J Public Health. 1939;29:1358–1359. [Google Scholar]
- 18.Kerdemelidis M, Lennon DR, Arroll B, Peat B, Jarman J. The primary prevention of rheumatic fever. J Paediatr Child Health. 2010;46(9):534–548. doi: 10.1111/j.1440-1754.2010.01854.x. [DOI] [PubMed] [Google Scholar]
- 19.Parks T, Mirabel MM, Kado J, et al. Association between a common immunoglobulin heavy chain allele and rheumatic heart disease risk in Oceania. Nat Commun. 2017;8:14946. doi: 10.1038/ncomms14946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray LA, D'Antoine HA, Tong SYC, et al. Genome-wide analysis of genetic risk factors for rheumatic heart disease in aboriginal australians provides support for pathogenic molecular mimicry. J Infect Dis. 2017;216(11):1460–1470. doi: 10.1093/infdis/jix497. [DOI] [PubMed] [Google Scholar]
- 21.Engel ME, Stander R, Vogel J, Adeyemo AA, Mayosi BM. Genetic susceptibility to acute rheumatic fever: a systematic review and meta-analysis of twin studies. PLoS One. 2011;6(9):e25326. doi: 10.1371/journal.pone.0025326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandenbroucke JP, Pearce N. Case-control studies: basic concepts. Int J Epidemiol. 2012;41(5):1480–1489. doi: 10.1093/ije/dys147. [DOI] [PubMed] [Google Scholar]
- 23.Heart Foundation of New Zealand . New Zealand Guidelines for Rheumatic Fever: Diagnosis, Management and Secondary Prevention of Acute Rheumatic Fever and Rheumatic Heart Disease: Heart Foundation of New Zealand. Heart Foundation of New Zealand; Auckland, New Zealand: 2014. [Google Scholar]
- 24.Jack SJ, Williamson DA, Galloway Y, et al. Primary prevention of rheumatic fever in the 21st century: evaluation of a national programme. Int J Epidemiol. 2018;47(5):1585–1593. doi: 10.1093/ije/dyy150. [DOI] [PubMed] [Google Scholar]
- 25.Bennett J, Moreland NJ, Oliver J, et al. Risk factors for group A streptococcal pharyngitis and skin infections: a case control study. Lancet Reg Health. 2022;22:In press. doi: 10.1016/j.lanwpc.2022.100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordet P, Lopez R, Dueñas A, Sarmiento L. Prevention and control of rheumatic fever and rheumatic heart disease: the Cuban experience (1986-1996-2002) Cardiovasc J Afr. 2008;19(3):135–140. [PMC free article] [PubMed] [Google Scholar]
- 27.Gordis L. Effectiveness of comprehensive-care programs in preventing rheumatic fever. N Engl J Med. 1973;289(7):331–335. doi: 10.1056/NEJM197308162890701. [DOI] [PubMed] [Google Scholar]
- 28.Arguedas A, Mohs E. Prevention of rheumatic fever in Costa Rica. J Pediatr. 1992;121(4):569–572. doi: 10.1016/s0022-3476(05)81146-1. [DOI] [PubMed] [Google Scholar]
- 29.Thomas S, Bennett J, Jack S, et al. Descriptive analysis of group A Streptococcus in skin swabs and acute rheumatic fever, Auckland, New Zealand, 2010–2016. Lancet Reg Health. 2021;8:100101. doi: 10.1016/j.lanwpc.2021.100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurney JK, Stanley J, Baker MG, Wilson NJ, Sarfati D. Estimating the risk of acute rheumatic fever in New Zealand by age, ethnicity and deprivation. Epidemiol Infect. 2016;144(14):3058–3067. doi: 10.1017/S0950268816001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanhope JM. New Zealand trends in rheumatic fever: 1885-1971. N Z Med J. 1975;82(551):297–299. [PubMed] [Google Scholar]
- 32.Oliver J, Robertson O, Zhang J, et al. Ethnically disparate disease progression and outcomes among acute rheumatic fever patients in New Zealand, 1989-2015. Emerg Infect Dis. 2021;27(7):1893–1902. doi: 10.3201/eid2707.203045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aliku T, Sable C, Scheel A, et al. Targeted echocardiographic screening for latent rheumatic heart disease in Northern Uganda: evaluating familial risk following identification of an index case. PLoS Negl Trop Dis. 2016;10(6):e0004727. doi: 10.1371/journal.pntd.0004727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Culliford-Semmens N, Tilton E, Wilson N, et al. Echocardiography for latent rheumatic heart disease in first degree relatives of children with acute rheumatic fever: implications for active case finding in family members. EClinicalMedicine. 2021 doi: 10.1016/j.eclinm.2021.100935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beaton A, Okello E, Rwebembera J, et al. Secondary antibiotic prophylaxis for latent rheumatic heart disease. N Engl J Med. 2021;386(3):230–240. doi: 10.1056/NEJMoa2102074. [DOI] [PubMed] [Google Scholar]
- 36.Cleghorn C, Blakely T, Mhurchu CN, Wilson N, Neal B, Eyles H. Estimating the health benefits and cost-savings of a cap on the size of single serve sugar-sweetened beverages. Prev Med. 2019;120:150–156. doi: 10.1016/j.ypmed.2019.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.