ABSTRACT
Background
Anemia and suboptimal gestational weight gain (GWG) are associated with adverse maternal and birth outcomes. Limited research indicates that balanced energy-protein (BEP) supplements reduce the incidence of inadequate GWG.
Objectives
We assessed the efficacy of a micronutrient-fortified BEP supplement on the secondary outcomes of anemia, GWG, GWG rate, and GWG in relation to the Institute of Medicine (IOM)’s recommendations, as compared with an iron–folic acid (IFA) tablet.
Methods
We conducted a randomized controlled trial in Burkina Faso, among pregnant women (15–40 y old) enrolled at <21 weeks of gestation. Women received either BEP and IFA (intervention) or IFA (control). Hemoglobin (g/dL) concentrations were measured at baseline and the third antenatal care visit (ANC), whereas maternal weight was measured at baseline and all subsequent ∼7-weekly ANCs. GWG (kg) was calculated as a woman's last weight measurement (at ∼36 weeks of gestation) minus weight at enrollment, whereas GWG rate (kg/wk) was GWG divided by the time between the first and last weight measurements. GWG adequacy (%) was computed as GWG divided by the IOM's recommendation. Binary outcomes included severely inadequate, inadequate, and excessive GWG. Statistical analyses followed the intention-to-treat principle. Linear regression and probability models were fitted for the continuous and binary outcomes, respectively, adjusting for baseline measurements.
Results
Women in the BEP group tended to have higher, but nonsignificantly different, GWG (0.28 kg; 95% CI: −0.05, 0.58 kg; P = 0.099). Furthermore, there were no significant differences in prenatal anemia prevalence, GWG rate, GWG adequacy, or incidence of inadequate or excessive GWG. Findings were robust to model adjustments and complete case and per protocol analyses.
Conclusions
This trial does not provide evidence that fortified BEP supplementation reduces maternal anemia or increases GWG, as compared with IFA. In conjunction with the small, but positive, effects of maternal BEP supplementation on birth outcomes, our findings warrant the investigation of additional biochemical and postnatal outcomes.
This trial was registered at clinicaltrials.gov as NCT03533712.
Keywords: anemia, balanced energy-protein, Burkina Faso, gestational weight gain, iron–folic acid, multiple micronutrients, randomized controlled trial
Introduction
Maternal gestational weight gain (GWG) is a cumulative measure reflecting the altering physiology of the mother (fat and fat-free mass deposition, as well as breast tissue, blood volume, and extracellular fluid expansion), the gravid uterus, the placental weight, and the developing fetus (fat and fat-free mass, as well as the amniotic fluid accretion) (1). Suboptimal GWG, a modifiable factor by both preconception and antenatal care (2, 3), has been related to adverse maternal and birth outcomes. Low GWG is associated with an increased prevalence of low birth weight and small-for-gestational age (SGA) at birth, and greater GWG is associated with a higher prevalence of large-for-gestational age and macrosomia (4–6). Furthermore, both lower and higher GWG have been associated with an increased risk of preterm birth (4). In addition, women who gain excessive weight during pregnancy may experience various adverse maternal outcomes, including the progression of gestational diabetes, complications during labor, increased prevalence of cesarean delivery, and subsequent maternal postpartum weight retention, obesity, and cardiovascular disorders (7, 8). However, high-quality interventional or epidemiologic GWG data remain scarce for low- and middle-income countries (LMICs) (9).
In 2009, the Institute of Medicine (IOM), now called the National Academy of Medicine, re-examined their GWG recommendations, stratified by prepregnancy maternal BMI (in kg/m2) (1). At present, no universally accepted GWG references exist (10); therefore, the IOM's recommendations, which are based entirely on studies conducted in high-income countries, are widely used in LMICs. Two recent studies using data from Demographic and Health Surveys estimated that the mean total GWG in sub-Saharan Africa was 6.5 kg (95% CI: 6.0, 7.0 kg) (11) or 6.6 kg (95% CI: 3.4, 9.9 kg) (12), which is about half the IOM's minimum recommendations of 11.5 kg for normal-weight and 12.5 kg for underweight women. Attaining optimal GWG may not only improve immediate newborn outcomes, but may also confer many potential long-term benefits. Prior evidence has shown that optimal GWG is associated with decreased risks of infant mortality, childhood overweight, as well as adulthood obesity (13, 14).
To date, 2 systematic reviews have assessed the effectiveness of prenatal nutritional supplements on GWG. A 2018 Cochrane review indicated no difference in GWG, when small quantity lipid-based nutrient supplements (SQ-LNSs) were compared against iron–folic acid (IFA) or multiple micronutrient (MMN) supplementation; however, IFA was more effective than SQ-LNSs in terms of reducing maternal anemia (15). Similarly, a 2015 Cochrane review of the limited number of (fortified) balanced energy-protein (BEP) supplementation trials reported a null effect on GWG, but highlighted the very-low-quality evidence (16). Two other systematic reviews have suggested that prenatal anemia is associated with an increased risk of adverse pregnancy outcomes, such as preterm birth and low birth weight (17, 18). To our knowledge, no study has assessed the effect of MMN-fortified BEP supplements on maternal anemia, because most prior BEP trials aimed to cover energy and macronutrient requirements only (19).
Using data from the MIcronutriments pour la SAnté de la Mère et de l'Enfant (MISAME)-III randomized controlled trial (RCT) among pregnant women in rural Burkina Faso (20), we assessed the efficacy of prenatal fortified BEP supplementation, as compared with IFA tablets (i.e., the standard of care), on maternal anemia, absolute GWG (both prespecified secondary study outcomes), GWG rate, and adequacy of GWG based on the IOM's recommendations (1). The results from this analysis will contribute to understanding the previously reported modest effects of fortified BEP supplements on birth outcomes observed in the MISAME-III trial (e.g., 50.1 g increase in birth weight; 95% CI: 8.11, 92.0 g) (21), and inform on effective prenatal nutritional interventions to achieve optimal GWG in LMICs.
Methods
Our research was reported using the CONSORT 2010 checklist (22).
Study setting
The prenatal phase of the MISAME-III study (NCT03533712) was conducted between the first enrollment on 30 October, 2019 and the last delivery on 7 August, 2021 in the catchment areas of 6 rural health centers of the health district of Houndé, Tuy Province, in the Hauts-Bassins region of Burkina Faso. In the preceding MISAME-I (23) and MISAME-II (24) RCTs, 48.4% and 45.5% of pregnant women were anemic [hemoglobin (Hb) < 11 g/dL] at baseline, respectively. Malaria transmission is perennial, with seasonal variations. The usual diet during pregnancy is nondiverse (25), predominantly maize-based with a complement of leafy vegetables (26), and consequently dietary micronutrient intakes are inadequate to cover the Estimated Average Requirements (EARs) (27). Moreover, among a subsample of MISAME-III women, the mean daily energy intake of the base diet (i.e., excluding supplements) was estimated to be ∼1940 kcal in both trial arms at the end of the preharvest season (27).
Study design, participants, and enrollment procedures
The MISAME-III protocol was published previously (20). In brief, the study was a community-based, nonblinded, individually randomized 2 × 2 factorial RCT, with directly observed daily supplement intake.
Women aged between 15 and 40 y and living in the study villages were identified through a census in the study area (n = 10,165). A network of 142 locally trained community support staff visited all eligible women at their homes every 5 wk to identify pregnancy early, by screening for self-reported amenorrhea. Women suspected to be pregnant were referred to the health center for a urine pregnancy test. Once gestation was preliminarily confirmed, the MISAME-III study purpose and procedures were explained in the local language: Bwamu, Mooré, or Dioula. Before randomization, we excluded women who intended to leave the study area during their pregnancy, planned to deliver outside the study area, or mothers who had a peanut allergy.
After written informed consent was obtained, women (pregnancy not yet confirmed by an ultrasound) were randomly assigned to receive either a daily fortified BEP supplement and IFA tablet (intervention group) or a daily IFA tablet alone (control group) during pregnancy. The stratified randomization scheme per health center was generated by an external research analyst before the start of the study with Stata version 15.1 (StataCorp), in permuted blocks of 8 (4 control, 4 intervention). The allocation was coded with the letter A for the prenatal control arm and the letter B for the prenatal intervention arm. Randomization codes were concealed in sequentially numbered sealed opaque envelopes by project employees, who were not in direct contact with enrolled women. The project midwives, who enrolled participants, assigned women to a trial arm by drawing a sealed envelope containing the A/B letter code. MISAME-III enrollment ran from 30 October, 2019 to 12 December, 2020. Within 14 d of enrollment, a woman's pregnancy was definitively confirmed by an ultrasound. Gestational age (GA) was estimated by measuring crown–rump length (7–13 weeks of gestation) or by calculating the mean of 3–4 measurements: biparietal diameter, head circumference, abdominal circumference, and femur length (12–26 weeks of gestation) (23). Postrandomization, we excluded nonpregnant women, mothers with a GA ≥ 21 completed weeks, and multifetal pregnancies (i.e., not meeting the a priori–defined study inclusion criteria) (28).
Trained village-based project workers visited 10–25 pregnant women per day to ensure the directly observed intake of BEP supplements and IFA tablets. When women had a short and scheduled absence from home, BEP supplements and IFA tablets were given to the mother in advance (thus, counted as nonobserved intakes for the respective days). The home visitors also encouraged pregnant women to attend ≥4 scheduled ANC visits every ∼7 wk. All serious adverse events (e.g., miscarriage and stillbirth) were recorded on a case-by-case basis, and verbal autopsies were conducted by the MISAME-III physician for maternal or infant deaths that occurred outside a health center.
Because the fortified BEP supplement and IFA tablet were identifiable, it was not possible to blind study mothers or community support staff. However, the RCT's physician and midwives responsible for measuring the prenatal secondary maternal outcomes might be deemed partially blinded (although access was permitted to the allocation code in the enrollment file). Researchers who managed, cleaned, and analyzed MISAME-III data were not blinded.
Study supplements
In 2016, the Bill & Melinda Gates Foundation convened an expert group to recommend the optimal nutritional composition of the BEP supplement (29). In a formative study, the most preferred and suitable MMN-fortified BEP supplement was selected for administration in the MISAME-III efficacy trial (30, 31). The BEP supplement is an LNS in the form of an energy-dense peanut paste fortified with MMNs. The BEP is made by Nutriset and is ready-to-consume, does not require a cold chain, and has a long shelf life. On average, the 72-g fortified BEP provided 393 kcal and consisted of 36% lipids, 20% protein, and 32% carbohydrates. Furthermore, the MMN content alone covered at least the IOM's daily EARs of micronutrients for pregnant women, except for calcium, phosphorus, and magnesium (32). Supplemental Table 1 provides the complete nutritional composition of the MMN-fortified BEP.
Women in the intervention group daily received a fortified BEP supplement and an IFA tablet {65 mg Fe (form: FeH2O5S) and 400 μg folic acid [form: C19H19N7O6; Tolerable Upper Intake Level from fortified food or supplements, not including folate from food: 1000 μg/d (33)]}, whereas women in the control group daily received an IFA tablet only (Sidhaant Life Sciences), in accordance with Burkina Faso's national health protocol (i.e., standard of care). Following Burkinabe guidelines, all enrolled women received malaria prophylaxis (3 oral doses of sulfadoxine-pyrimethamine) at the relevant ANC visits.
Data collection and measures
At enrollment (i.e., first ANC visit), we measured maternal height, weight, midupper arm circumference (MUAC) in duplicate, and Hb concentration. Maternal weight and MUAC were measured again, in duplicate, at each subsequent ANC visit. Hb concentration was assessed again between 19 and 34 weeks of gestation (i.e., third ANC visit). Furthermore, a comprehensive socioeconomic and demographic questionnaire was administered at baseline (20).
Maternal height was measured to the nearest 1 cm using a ShorrBoard® Infant/Child/Adult (Weigh and Measure) and weight to the nearest 100 g with a Seca 876 scale (Seca); and the accuracy of the scales was verified weekly. Maternal MUAC was measured to the nearest 1 mm using a Seca 212 measuring tape (Seca). Pregnant women's Hb concentration was measured by spectrophotometry with a HemoCue® Hb 201+ (HemoCue); and a weekly calibration check was made with the use of a HemoCue Control Cuvette. The study's physician performed transabdominal ultrasound fetal biometry within 2 wk of enrollment. Pregnancy was confirmed and GA was estimated using a portable diagnostic imaging and full-color, flow-mapping SonoSite M-Turbo (Fujifilm SonoSite Inc.). Concurrently, maternal subscapular and tricipital skinfold measurements were taken in triplicate using a Harpenden caliper.
MISAME-III data were collected using SurveySolutions version 21.5 (The World Bank) on tablets by the project physician and midwives; these data were transferred to a central Ghent University server weekly. Questionnaire assignments were sent once a week to the field team including preloaded data collected at a previous ANC visit to lower the amount of incorrect data. Furthermore, we programmed generic validation codes to avoid the entry of implausible values and to improve the quality of data collection in the field. In addition, biweekly data quality checks were conducted and missing or inconsistent data were sent back to the field for revision. The quality of ultrasound images and estimation of GA were checked for >10% of the examinations regularly by an external gynecologist, using a quality checklist and scoring sheet. The trained project workers collected daily data on fortified BEP and IFA compliance in both prenatal study arms via smartphone-assisted personal interviewing programmed in CSPro version 7.3.1 (U.S. Census Bureau, ICF, and Serpro) . Six supervisors performed monthly lot quality assurance sampling schemes of each home visitor's work on a random day (34).
All data collection forms are available on the study's website: https://misame3.ugent.be/resources.
Ethics
The study protocol was approved by the Ethics Committee of Ghent University Hospital in Belgium (B670201734334) and the ethics committee of Centre Muraz in Burkina Faso (N°2018–22/MS/SG/CM/CEI). An independent Data and Safety Monitoring Board (DSMB), comprising an endocrinologist, 2 pediatricians, a gynecologist, and an ethicist of both Belgian and Burkinabe nationalities, was established before the start of the efficacy trial. The DSMB conducted remote safety reviews for adverse and serious events at 9 and 20 mo after the start of enrollment.
Statistical analysis
Analyses were recorded in the MISAME-III statistical analysis plan that was validated on 24 October, 2019 and published online on 3 November, 2020 on the study's website: https://www.misame3.ugent.be/resource-files/MISAME-III_SAP_v1_102019.pdf. For consistency and comparability of study findings, the present analyses followed the analytical procedures used to assess the efficacy of the prenatal fortified BEP intervention on birth outcomes (21).
The MISAME-III efficacy trial specified 1 primary prenatal outcome: SGA [<10th percentile of the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) newborn size standards (35)]. Secondary maternal outcomes of the prenatal BEP intervention included anemia (Hb < 11 g/dL) at the third ANC visit and GWG (kg) between the first and last ANC visits (or before delivery). Furthermore, we estimated the GWG rate, defined as the absolute GWG divided by the time interval between the first and last maternal weight measurements, and the expected weight gain for each woman at the time of their last observed weight measurement using the following IOM 2009 formula (1):
 |
(1) |
The expected total weight gain during the first trimester was assumed to be 2 kg for underweight (BMI < 18.5) and normal-weight women (BMI = 18.5–24.9), 1 kg for overweight women (BMI = 25–29.9), and 0.5 kg for obese women (BMI ≥ 30); and the recommended rates of GWG for the second and third trimesters were 0.51, 0.42, 0.28, and 0.22 kg/wk for underweight, normal-weight, overweight, and obese women, respectively (1). Finally, the percentage adequacy of GWG was calculated by dividing the actual GWG by the expected GWG at the last observed weight measurement, multiplied by 100. This is a continuous measure that has been used in previous GWG studies in Africa (36, 37). Following Liu et al. (37), we further categorized the percentage adequacy of GWG into binary outcome measures. Inadequate GWG was defined as a percentage adequacy of GWG < 90%, severely inadequate GWG as a percentage adequacy of GWG < 70%, and excessive GWG as a percentage adequacy of GWG ≥ 125%. The cutoffs 90% and 125% correspond to the lower and upper limits of the recommended total weight gain during pregnancy by the IOM's guideline. The recommended range is 12.5–18 kg for women who are underweight (BMI < 18.5), 11.5–16 kg for normal weight (BMI = 18.5–24.9), 7–11.5 kg for overweight (BMI = 25–29.9), and 5–9 kg for obese (BMI ≥ 30) (1).
Only singleton pregnancies were included in the analysis. All analyses were conducted by the intention-to-treat (ITT) principle to reduce potential bias arising from missing data. Therefore, before analyses, we performed multiple imputation of missing maternal Hb concentration (g/dL), maternal weight before delivery (kg), and gestational duration (wk) under the “missing at random” assumption. Fifty imputations of missing continuous outcome data for cases lost to follow-up were run to estimate the regression coefficients, based on the following predictors at baseline: maternal height (cm), maternal weight (kg), MUAC (mm), Hb (g/dL), and age (y) at inclusion; GA at baseline (wk); primiparity; and month of inclusion. Anemia and GWG adequacy variables were calculated from the imputed continuous data.
Descriptive data are presented as percentages or means ± SDs. Unadjusted and adjusted group differences were estimated by fitting linear regression models for the continuous outcomes, to estimate the mean group difference, and using linear probability models with robust variance estimators for the binary outcomes, to estimate risk differences in percentage points (pp). All models were adjusted for the baseline value of the outcome of interest [i.e., either Hb or maternal weight at study enrollment, thus an ANCOVA (38, 39)] and contained health center and randomization block as fixed effects to account for clustering by the study design. Adjusted models in addition contained potential baseline prognostic factors of maternal outcomes, including maternal height (cm), MUAC (mm), and age (y) at inclusion; GA at baseline (wk); and primiparity. We did not adjust for any other sociodemographic variables, owing to balanced baseline characteristics across the prenatal study groups (i.e., < |2.5| pp difference).
To assess the robustness of the primary findings, we conducted the following sensitivity analyses: 1) complete case analysis (i.e., excluding women who were lost to follow-up for birth outcomes); and 2) per protocol analysis restricting the intervention sample to women with fortified BEP compliance of ≥75%. The strict compliance rate was calculated by dividing the total number of BEP supplements effectively taken under direct observation of a trained home visitor by the theoretical maximum number of prenatal BEP supplements allowed (i.e., the number of days between study inclusion and delivery). Moreover, to assess the potential underestimation of absolute GWG, we replicated our complete case analysis among mothers who had a baseline weight measurement taken at <14 weeks of gestation (i.e., first trimester). In addition, for complete cases, we used the INTERGROWTH-21st GWG standards to derive GWG z scores (40). Because the currently available GWG equation for recommended weight gain is for normal-weight women with a GA between 14 and 40 wk, we restricted this outcome to normal-weight women with a GA of ≤40 completed weeks at their last ANC visit.
Statistical significance was set at P < 0.05 for all 2-sided tests. All analyses were conducted with Stata version 16.1 (StataCorp).
Results
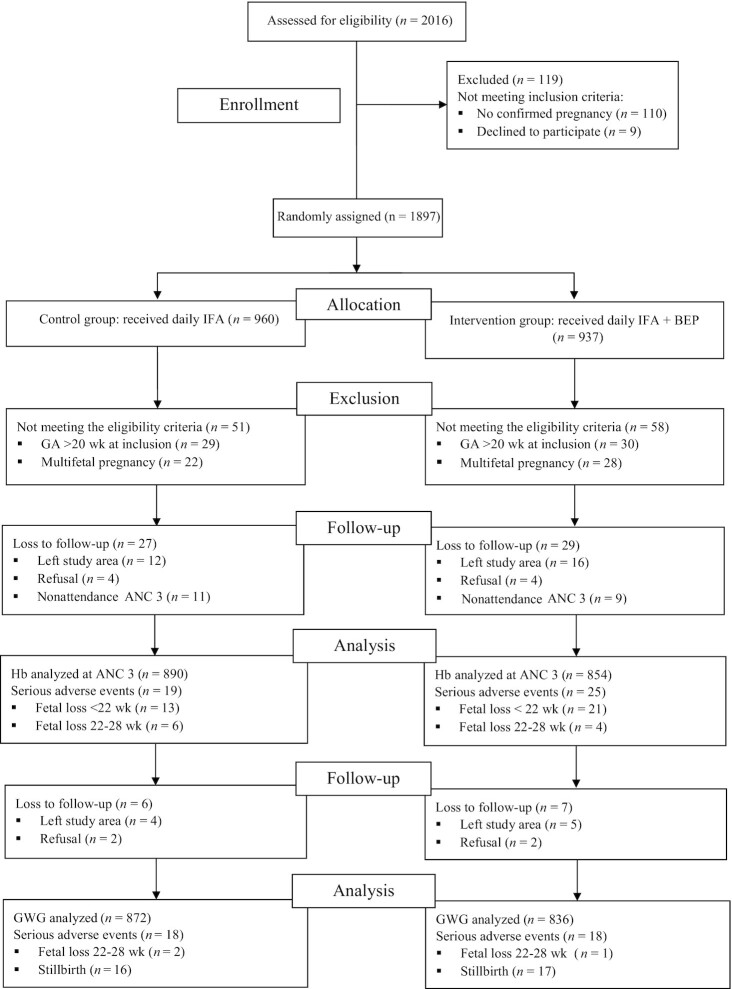
Between October 2019 and December 2020, 2016 women were assessed for eligibility, of whom 1897 were randomly assigned (960 control, 937 intervention) and 119 excluded for not meeting the trial's inclusion criteria. Subsequent ultrasounds, to confirm urine pregnancy test results and to estimate GA, led to postrandomization exclusion of a further 59 women who were at ≥21 weeks of gestation at inclusion and 50 women with a multifetal pregnancy (Figure 1). Table 1 presents the baseline characteristics of mothers included in the ITT analyses of GWG (909 control, 879 intervention). The prenatal trial arms were well balanced regarding household, maternal, and pregnancy characteristics (i.e., < |2.5| pp differences across groups). At baseline, 7.1% of mothers were underweight, 10.8% were overweight, 1.7% were obese, 37.7% were anemic, and the mean ± SD GA was 11.5 ± 4.06 wk. Of the 1788 women with sociodemographic data, 27 control (2.97%) and 29 intervention arm (3.30%) mothers either were lost to follow-up or failed to attend their third ANC visit, whereas in total 22 control (2.42%) and 27 intervention arm mothers (3.07%) were lost to follow-up before delivery (there were 1659 complete cases) (Figure 1).
FIGURE 1.
MISAME-III (Micronutriments pour la Santé de la Mère et de l'Enfant study 3) trial flowchart. ANC, antenatal care; BEP, balanced energy-protein; GWG, gestational weight gain; Hb, hemoglobin; IFA, iron–folic acid.
TABLE 1.
Baseline characteristics of study participants, by MISAME-III trial arm1
| Characteristics | IFA (n = 909) | IFA + BEP (n = 879) |
|---|---|---|
| Health center catchment area | ||
| Boni | 22.0 | 21.8 |
| Dohoun | 10.5 | 11.0 |
| Dougoumato II | 18.9 | 17.5 |
| Karaba | 10.2 | 10.7 |
| Kari | 18.4 | 18.7 |
| Koumbia | 20.0 | 20.3 |
| Household | ||
| Asset index (range: 0–10 points) | 4.51 ± 1.74 | 4.67 ± 1.75 |
| Household food insecurity2 | 53.9 | 55.5 |
| Improved primary water source3 | 62.2 | 62.7 |
| Improved sanitation facility4 | 59.3 | 60.6 |
| Household size, n | 6.19 ± 4.45 | 6.20 ± 4.21 |
| Polygamous households | 31.8 | 32.7 |
| Head of household | ||
| Age, y | 33.4 ± 9.16 | 33.8 ± 9.33 |
| Male | 99.7 | 99.8 |
| Completed primary education | 59.8 | 59.2 |
| Maternal | ||
| Age, y | 25.1 ± 6.20 | 25.0 ± 6.18 |
| Ethnic group | ||
| Bwaba | 57.3 | 57.6 |
| Mossi | 35.3 | 34.5 |
| Other | 7.37 | 7.96 |
| Religion | ||
| Muslim | 42.1 | 42.3 |
| Animist | 23.4 | 22.8 |
| Protestant | 16.2 | 18.4 |
| Catholic | 14.4 | 13.1 |
| No religion, no animist | 3.85 | 3.19 |
| Completed primary education | 42.4 | 41.4 |
| Weight, kg | 57.9 ± 8.65 | 58.4 ± 8.69 |
| Height, cm | 162 ± 5.915 | 163 ± 6.05 |
| BMI, kg/m2 | 22.0 ± 2.87 | 22.0 ± 2.87 |
| <18.5 | 7.05 | 7.17 |
| 25–29.9 | 10.3 | 11.3 |
| ≥30 | 1.54 | 1.82 |
| Midupper arm circumference, mm | 262 ± 26.8 | 262 ± 26.4 |
| Subscapular skinfold, mm | 11.9 ± 5.47 | 12.1 ± 5.58 |
| Tricipital skinfold, mm | 11.8 ± 4.76 | 12.0 ± 4.86 |
| Hb, g/dL | 11.4 ± 1.47 | 11.3 ± 1.52 |
| Anemia (Hb < 11 g/dL) | 36.7 | 38.7 |
| Severe anemia (Hb < 7 g/dL) | 0.22 | 0.23 |
| Gestational age, wk | 11.4 ± 4.08 | 11.5 ± 4.04 |
| Trimester of gestation | ||
| First | 63.1 | 62.0 |
| Second | 36.9 | 38.0 |
| Parity | ||
| 0 | 21.8 | 23.1 |
| 1–2 | 35.9 | 33.4 |
| ≥3 | 42.4 | 43.5 |
Values are percentages or means ± SDs. BEP, balanced energy-protein; Hb, hemoglobin; IFA, iron–folic acid; MISAME-III, Micronutriments pour la Santé de la Mère et de l'Enfant study 3.
Assessed using Food and Nutrition Technical Assistance Project (FANTA)/USAID's Household Food Insecurity Access Scale (72).
Protected well, borehole, pipe, or bottled water were considered improved water sources.
Flush toilet connected to local sewage or septic tank, or pit latrine with slab and/or ventilation were considered as improved sanitation facilities.
The height of 1 woman with a physical disability could not be measured.
The third ANC visit was completed at (mean ± SD) 26.6 ± 3.43 and 26.4 ± 3.36 weeks of gestation for women in the intervention and control groups, respectively. Our unadjusted ITT analyses (3.21% of observations were imputed) of a combined daily BEP supplement and IFA tablet indicated a nonsignificant difference in maternal Hb concentration (0.02 g/dL; 95% CI: −0.09, 0.14 g/dL; P = 0.701), anemia (1.01 pp; 95% CI: −3.60, 5.60 pp; P = 0.665), or severe anemia prevalence (0.12 pp; 95% CI: −0.11, 0.35 pp; P = 0.319) at ANC 3, as compared with an IFA tablet alone (Table 2). These findings were confirmed (P > 0.05) by adjusting the regression models for prognostic factors of maternal anemia at enrollment (Table 2), by complete case analyses (Supplemental Table 2), and by per protocol analyses (Supplemental Table 3).
TABLE 2.
Efficacy of prenatal fortified BEP supplementation on maternal outcomes1
| Women's characteristics | IFA (n = 890) | IFA + BEP (n = 854) | Unadjusted ∆ (95% CI) | P | Adjusted ∆ (95% CI) | P |
|---|---|---|---|---|---|---|
| Hb at ANC 3, g/dL | 10.9 ± 1.28 | 11.0 ± 1.24 | 0.02 (−0.09, 0.14)2 | 0.701 | 0.03 (−0.08, 0.14)2 | 0.604 |
| Anemia (Hb < 11 g/dL) at ANC 3 | 47.8 | 49.4 | 1.01 (−3.60, 5.60)2 | 0.665 | 0.67 (−3.89, 5.23)2 | 0.774 |
| Severe anemia (Hb < 7 g/dL) at ANC 3 | 0 | 0.12 | 0.12 (−0.11, 0.35)2 | 0.319 | 0.11 (−0.11, 0.33)2 | 0.321 |
| GWG,3 kg | 6.00 ± 3.52 | 6.27 ± 3.52 | 0.28 (−0.05, 0.60)2 | 0.099 | 0.27 (−0.05, 0.58)2 | 0.095 |
| GWG rate,3 kg/wk | 0.261 ± 0.155 | 0.274 ± 0.189 | 0.013 (−0.004, 0.030)2 | 0.141 | 0.013 (−0.004, 0.029)2 | 0.128 |
| GWG adequacy,3,4 % | 57.0 | 60.0 | 2.88 (−0.84, 6.60)5 | 0.129 | 3.09 (−0.60, 6.78)5 | 0.101 |
| Inadequate GWG (<90%)3,4 | 86.2 | 83.4 | −2.81 (−6.20, 0.57)5 | 0.103 | −2.97 (−6.34, 0.40)5 | 0.084 |
| Severely inadequate GWG (<70%)3,4 | 68.3 | 64.7 | −3.43 (−7.87, 1.01)5 | 0.130 | −3.72 (−8.12, 0.68)5 | 0.097 |
| Excessive GWG (≥125%)3,4 | 3.05 | 4.23 | 1.15 (−0.63, 2.93)5 | 0.205 | 1.19 (−0.57, 2.95)5 | 0.184 |
Values are percentages or means ± SDs unless otherwise indicated. ANC, antenatal care; BEP, balanced energy-protein; GWG, gestational weight gain; Hb, hemoglobin; IFA, iron–folic acid; MUAC, midupper arm circumference.
Unadjusted and adjusted group differences were estimated by fitting linear regression models for the continuous outcomes, to estimate the mean group difference, and using linear probability models with robust variance estimation for the binary outcomes, to estimate risk differences in percentage points. All models were adjusted for the baseline outcome [i.e., Hb (g/dL) or weight (kg)] and contained health center and randomization block as fixed effects to account for clustering by the study design. Adjusted models in addition contained a set of a priori–determined known prognostic factors of outcomes including maternal age, primiparity, gestational age, height, and MUAC at study enrollment.
n = 872 for IFA group GWG measures; n = 836 for IFA + BEP group GWG measures.
Expected GWG gain during the first trimester was assumed to be 2 kg for underweight [BMI (in kg/m2) <18.5] and normal-weight women (BMI = 18.5–24.9), 1 kg for overweight women (BMI = 25–29.9), and 0.5 kg for obese women (BMI ≥ 30); and the recommended rates of GWG for the second and third trimesters were 0.51, 0.42, 0.28, and 0.22 kg/wk for underweight, normal-weight, overweight, and obese women, respectively. GWG adequacy percentage was calculated by dividing the actual GWG by the expected GWG at the last observed weight measurement, then multiplying by 100.
Unadjusted and adjusted group differences were estimated by fitting linear regression models for continuous GWG adequacy, to estimate the mean group difference, and using linear probability models with robust variance estimation for the binary GWG adequacy outcomes, to estimate risk differences in percentage points. All models contained health center and randomization block as fixed effects to account for clustering by the study design. Adjusted models in addition contained a set of a priori–determined known prognostic factors of outcomes including maternal age, primiparity, and MUAC at study enrollment.
Furthermore, the last maternal weight measurement was taken at (mean ± SD) 35.5 ± 4.35 weeks of gestation in the combined BEP and IFA arm and at 35.6 ± 4.51 weeks of gestation for the IFA-only arm. Our unadjusted ITT analyses (2.87% of observations were imputed) did not show a significant difference across study arms for total GWG (0.28 kg; 95% CI: −0.05, 0.60 kg; P = 0.099), GWG rate (13 g/wk; 95% CI: −4, 30 g/wk; P = 0.141), GWG adequacy (2.88 pp; 95% CI: −0.84, 6.60 pp; P = 0.129), and inadequate GWG (−2.81 pp; 95% CI: −6.20, 0.57 pp; P = 0.103), severely inadequate GWG (−3.43 pp; 95% CI: −7.87, 1.01 pp; P = 0.130), or excessive GWG prevalence (1.15 pp; 95% CI: −0.63, 2.93 pp; P = 0.205) (Table 2). Our main findings were confirmed (P > 0.05) by adjusting the regression model for prognostic factors of maternal GWG at baseline (Table 2) and per protocol analysis (Supplemental Table 3). Nevertheless, complete case analyses indicated small, but significant, differences in absolute GWG and GWG adequacy (Supplemental Table 2). Restricting our analysis to only women with a baseline weight measurement in the first trimester of gestation did not change our results. To summarize, mean ± SD absolute GWG was 6.25 ± 3.62 kg in the control arm (n = 600) and 6.56 ± 3.64 kg (n = 583) in the intervention arm; and the unadjusted mean difference was 0.30 kg (95% CI: −0.09, 0.71 kg; P = 0.143).
Lastly, among women with a normal BMI at baseline and a GA ≤ 280 d at their last weight measurement, the mean ± SD of GWG z scores were −1.52 ± 1.26 SD in the IFA arm (n = 584) and −1.48 ± 1.27 SD in the fortified BEP and IFA arm (n = 524); and the unadjusted mean difference was 0.05 SD (95% CI: −0.09, 0.21 SD; P = 0.480).
Discussion
In the MISAME-III trial, we found that pregnant women who received a daily fortified BEP supplement and IFA tablet did not have different (midline) Hb concentrations, total GWG, GWG rates, or prevalence of (severely) inadequate GWG and excessive GWG, as compared with those women who received an IFA tablet only.
The absence of an increase in prenatal Hb and GWG in the BEP group was unexpected (41), given that the mean energy and iron contents of the fortified BEP supplement were 393 kcal/d and 22 mg/d (and mean BEP and IFA compliance were both >80%), respectively (21). The lack of efficacy on Hb concentration might be explained by the BEP's zinc (15 mg/d) (42) and calcium (500 mg/d) competing with or blocking the mucosal uptake of iron in the gut (43), respectively. Another clarification might be that the maximum Hb response was already achieved by the elemental iron (∼22.4 mg/d) intake from the IFA tablets over follow-up (44). Moreover, despite all women receiving daily IFA tablets, anemia prevalence in fact increased on average by ∼10 pp during pregnancy, which is probably due to physiologic hemodilution in the second trimester (45).
A cross-sectional substudy in MISAME-III indicated that BEP supplementation increased energy and macro- and micronutrient intakes and filled nutrient gaps without displacing food intakes among pregnant women (27). Correspondingly, a longitudinal follow-up study in MISAME-III reported that usual prenatal dietary diversity did not differ across trial arms (25). Hence, it is unlikely that the observed null effects on Hb and GWG are due to BEP supplements displacing nutrient or food group intakes. The effect of macronutrient supplementation is nevertheless dependent on prior maternal energy deficits, whereas >90% of mothers in MISAME-III were normal weight or overweight (including obese) at enrollment and thus potentially not (sufficiently) vulnerable to macronutrient deficiencies [i.e., WHO antenatal care guidelines suggest use of BEP where the population-level prevalence of low BMI (<18.5) is >20%] (2).
Limited research has examined the effectiveness or efficacy of prenatal MMN or (fortified) BEP supplementation on GWG, and the results are heterogeneous (16). Our findings were similar to maternal supplementation interventions administering either SQ-LNSs in Bangladesh [mean GWG rate: 0.29 kg/wk (control) compared with 0.30 kg/wk] (46), Corn Soya Blend in Cambodia (mean GWG: 8.1 kg compared with 8.5 kg) (47), or an LNS and fortified tea in The Gambia (mid- and late-pregnancy GWG differences: both P > 0.05) (48). Similarly, an RCT in Ghana reported that there were no differences in GWG measures between pregnant women who received either SQ-LNSs or MMN supplements and those who received IFA supplements (e.g., mean GWG: 7.2 kg or 7.7 kg compared with 7.3 kg) (36). Moreover, an RCT conducted in Mexico reported that MMN supplements did not increase weight gain during pregnancy when compared with iron supplements alone (mean GWG: 7.6 kg compared with 7.3 kg) (49). In contrast, 2 large RCTs conducted in Tanzania among HIV-negative women (50) and HIV-infected women (51) demonstrated that prenatal MMN supplements were significantly associated with a 253-g (95% CI: 177, 388 g) and 304-g (95% CI: 17, 590 g) greater total (and third-trimester) GWG than placebo, respectively. Moreover, using the IOM's recommendations, the GWG adequacy difference between the MMN and placebo arms was 2.3 pp (95% CI: 0.3, 4.2 pp) (37). In MISAME-III, we enrolled a substantially smaller sample of pregnant women than did Liu et al. (37) (n = 7573); however, our BEP intervention also indicated mean increases of similar magnitudes for absolute GWG and GWG adequacy, i.e., 276 g (95% CI: −5.4, 597 g) and 2.9 pp (−0.84, 6.6 pp), respectively. Furthermore, when a prenatal MMN-fortified milk-based product was compared against a nonfortified powdered milk in Chile, an increase in maternal weight gain was also observed (mean GWG: 12.3 kg compared with 11.3 kg) (52). Correspondingly, a BEP intervention in Thailand concluded that providing 13 g protein and 350 kcal during the third trimester significantly improved maternal weight gain (mean GWG rate: 0.45 kg/wk compared with 0.28 kg/wk) (53). In the multicountry Women First trial, preconception SQ-LNS supplementation increased maternal weight gain, as compared with the same supplement commenced late in the first trimester of pregnancy or not at all (mean GWG: 6.9 kg compared with 6.4 kg compared with 6.2 kg) (54). The discrepancies between trial findings might be due to the differences in study population and intervention doses and timing (16). To illustrate, MMN trials in Nepal and Bangladesh have brought up the concern that single RDA regimens may be suboptimal in settings of widespread undernutrition and have not resulted in micronutrient deficiency corrections (42, 55).
Our current result that fortified BEP supplementation was not significantly associated with improvements in measures of GWG (i.e., potential mediating factor) is consistent with the previously reported MISAME-III finding of modest effects on anthropometric birth outcomes (21). Overall, an estimated 50% of absolute GWG is ascribed to the feto-placental unit; 25% to blood volume expansion, extravascular fluid, and breast tissue; and the remaining 25% to maternal fat stores (1). Early pregnancy weight gain is slow and primarily due to maternal fat deposition, total body water accretion, and placental and other tissue development, whereas later pregnancy weight gain is thought to be more related to fetal growth (1). Previous studies have suggested that GWG during the first and second trimesters has a stronger effect on birth weight than GWG that occurs in the third trimester (56). Nevertheless, GWG in late pregnancy was associated with higher placental and birth weights in rural Bangladesh (57).
In addition, we report that GWG was ∼6 kg in both intervention and control arms, ∼85% of the study participants experienced inadequate GWG, and ∼65% experienced severely inadequate GWG during pregnancy. This finding is consistent with a recent meta-analysis of studies from sub-Saharan Africa, in which the authors reported that the percentage of inadequate GWG, as defined according to the IOM's recommendation, was >60% in 8 of the 16 studies (6). Furthermore, observational studies in rural Malawi (58), Niger (59), Bangladesh (60, 61), and peri-urban India (62) reported that 72%, 63%, 74% and 54%, and 40% of pregnant women had inadequate GWG, respectively, whereas RCTs conducted in Ghana (36), Vietnam (63), and Tanzania (37) reported that 63%, 62%, and ∼50% of mothers experienced inadequate GWG, respectively. Nonetheless, in MISAME-III only ∼7% and ∼12% of women were underweight and overweight (including obese) at baseline, respectively, which is substantially lower than most GWG studies conducted in sub-Saharan Africa (6). We therefore hypothesize that in LMICs, inadequate GWG might be more prevalent among normal-weight women, because the range of the IOM's recommendation for these mothers is slightly narrower than for underweight women, and the minimum acceptable GWG is greater than for overweight and obese women (1).
The current study has several strengths. First, MISAME-III used an individually randomized design with an IFA control group (i.e., the standard of care). Second, we examined the effect of BEP supplements on absolute GWG, GWG rate, and GWG in relation to the IOM's recommendations. In contrast, previous epidemiologic studies of GWG have often used either total GWG in kilograms or mean GWG rate only, which may be biased owing to their correlation with gestation duration (64). By including the IOM's adequacy ratios and INTERGROWTH-21st GWG z scores (by definition independent of pregnancy length), among normal-weight mothers the observed (nonsignificant) small increase in total GWG in the fortified BEP arm could be explained independently from the supplement's known efficacy on gestational duration (i.e., on average 1.4 d, translating to an ∼84-g increase among normal-weight women) (21). Third, in our RCT, GA was determined using ultrasound, the gold-standard method, rather than the error-prone last menstrual period (e.g., recall bias, irregular menses) (62).
However, our study has some limitations that warrant caution. First, the efficacy of prenatal BEP supplementation on third-trimester anemia prevalence (i.e., when RBC volume is more proportional to the hydremia of pregnancy) could not be assessed, because maternal Hb concentrations were not measured before delivery. Second, the IOM's recommendations might still lead to misclassification if women gain well above or below the assumed first-trimester weight gain (65). Third, because prepregnancy BMI is one of the main determinants of GWG, findings on total weight gain or GWG rate from different studies might not be comparable if there were large differences in prepregnancy BMI across studies (4, 66). Fourth, because this RCT enrolled participants after their pregnancies were confirmed, prepregnancy BMI was not available in this study. Therefore, because prepregnancy BMI is required to calculate the recommended weight gain based on the IOM and INTERGROWTH-21st recommendations, we used BMI measured in the late first or early second trimester (the latter excluded in the sensitivity analyses) as a substitute for prepregnancy BMI given that weight gain during the first trimester is minimal (67). Fifth, the last maternal weight measurement was taken at ∼36 weeks of gestation, rather than before delivery (at ∼40 weeks of gestation); hence total GWG, and subsequently GWG adequacy, were likely underestimated. We believe that the potential measurement error for GWG is not differential across study arms, resulting in unbiased results for efficacy. Sixth, our use of total pregnancy weight gain measures to summarize GWG is not sensitive to a mother's weight gain pattern/timing (68) or more granular changes in maternal body composition (e.g., fat-free mass) (57). Lastly, the hypotheses that increased GWG might reflect lower amounts of physical work during pregnancy in LMICs (69) or lower (subclinical) inflammation and greater placental angiogenesis as a consequence of BEP (70, 71), and thus better fetal nourishment, could not be tested because neither maternal physical activity, nor biological markers, were measured at baseline in the MISAME-III trial.
In conclusion, our results indicate that the provision of daily fortified BEP supplements to pregnant women did not improve absolute GWG, GWG rate, GWG in relation to the IOM's recommendations, or GWG z scores. Future randomized interventions might assess whether (preconception) environments conducive to adequate GWG allow the mother to be more nutritionally replete, permitting any additional nutrients from supplementation to support fetal growth and development.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all the women from Boni, Dohoun, Karaba, Dougoumato II, Koumbia, and Kari who participated in the study; the data collection team; and Henri Somé from AFRICSanté. We thank Nutriset (France) for donating the BEP supplements. The authors’ responsibilities were as follows—PK, CL, LH, BdK, GH-C, and LCT: designed the study; BdK, LCT, GH-C, AC, MO, and LH: conducted the research; GH-C: analyzed the data and performed the statistical analysis; GH-C and KT: developed the first draft and revised the manuscript; LCT, BdK, AA, TD-C, PK, CL, and LH: critically reviewed the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by Bill & Melinda Gates Foundation grant OPP1175213 (to PK). The funder had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ANC, antenatal care; BEP, balanced energy-protein; DSMB, Data and Safety Monitoring Board; EAR, Estimated Average Requirement; GA, gestational age; GWG, gestational weight gain; Hb, hemoglobin; IFA, iron–folic acid; INTERGROWTH-21st, International Fetal and Newborn Growth Consortium for the 21st Century; IOM, Institute of Medicine; ITT, intention-to-treat; LMIC, low- and middle-income country; LNS, lipid-based nutrient supplement; MISAME, MIcronutriments pour la SAnté de la Mère et de l'Enfant; MMN, multiple micronutrient; MUAC, midupper arm circumference; pp, percentage point; RCT, randomized controlled trial; SGA, small-for-gestational age; SQ-LNS, small-quantity lipid-based nutrient supplement.
Contributor Information
Giles Hanley-Cook, Department of Food Technology, Safety and Health, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium.
Laeticia C Toe, Department of Food Technology, Safety and Health, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium; Nutrition and Metabolic Diseases Unit, Health Sciences Research Institute (IRSS), Bobo-Dioulasso, Burkina Faso.
Kokeb Tesfamariam, Department of Food Technology, Safety and Health, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium.
Brenda de Kok, Department of Food Technology, Safety and Health, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium.
Alemayehu Argaw, Department of Food Technology, Safety and Health, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium.
Anderson Compaoré, AFRICSanté (Health Research and Expertise Training Agency for Africa), Bobo-Dioulasso, Burkina Faso.
Moctar Ouédraogo, AFRICSanté (Health Research and Expertise Training Agency for Africa), Bobo-Dioulasso, Burkina Faso.
Trenton Dailey-Chwalibóg, Department of Food Technology, Safety and Health, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium.
Patrick Kolsteren, Department of Food Technology, Safety and Health, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium.
Carl Lachat, Department of Food Technology, Safety and Health, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium.
Lieven Huybregts, Department of Food Technology, Safety and Health, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium; Poverty, Health, and Nutrition Division, International Food Policy Research Institute (IFPRI), Washington, DC, USA.
Data Availability
The informed consent form does not allow sharing of personal data outside the research team. Requests to access data need to be directed to the ethics committee of Ghent University Hospital through ethisch.comite@uzgent.be. Supporting study documents, including the study protocol and questionnaires, are publicly available on the study's website: https://misame3.ugent.be.
References
- 1. National Academy of Medicine, National Research Council . Weight gain during pregnancy: reexamining the guidelines. Rasmussen KM, Yaktine AL, editors. Vol. 12. Washington (DC): The National Academies Press; 2009. [PubMed] [Google Scholar]
- 2. World Health Organization . WHO recommendations on antenatal care for a positive pregnancy experience [Internet]. Geneva, Switzerland: WHO; 2016. Available from: https://www.who.int/publications/i/item/9789241549912 (Accessed 2022 Jan 14). [PubMed] [Google Scholar]
- 3. Stephenson J, Heslehurst N, Hall J, Schoenaker D, Hutchinson J, Cade JEet al. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet. 2018;391(10132):1830–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Voerman E, Santos S, Inskip H, Amiano P, Barros H, Charles MAet al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA. 2019;321(17):1702–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MHet al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317(21):2207–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asefa F, Cummins A, Dessie Y, Hayen A, Foureur M. Gestational weight gain and its effect on birth outcomes in sub-Saharan Africa: systematic review and meta-analysis. PLoS One. 2020;15(4):e0231889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nehring I, Schmoll S, Beyerlein A, Hauner H, von Kries R. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr. 2011;94(5):1225–31. [DOI] [PubMed] [Google Scholar]
- 8. Goldstein RF, Abell SK, Ranasinha S, Misso ML, Boyle JA, Harrison CLet al. Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018;16(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Victora CG, Christian P, Vidaletti LP, Gatica-Domínguez G, Menon P, Black RE. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet. 2021;397(10282):1388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hermanussen M. Pregnant women need local references for gestational weight gain—an editorial. Eur J Clin Nutr. 2022;76(6):781–2. [DOI] [PubMed] [Google Scholar]
- 11. Coffey D. Prepregnancy body mass and weight gain during pregnancy in India and sub-Saharan Africa. Proc Natl Acad Sci U S A. 2015;112(11):3302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang D, Wang M, Darling AM, Perumal N, Liu E, Danaei Get al. Gestational weight gain in low-income and middle-income countries: a modelling analysis using nationally representative data. BMJ Glob Health. 2020;5(11):e003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li N, Liu E, Guo J, Pan L, Li B, Wang Pet al. Maternal prepregnancy body mass index and gestational weight gain on offspring overweight in early infancy. PLoS One. 2013;8(10):e77809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Champion ML, Harper LM. Gestational weight gain: update on outcomes and interventions. Curr Diab Rep. 2020;20(3):11. [DOI] [PubMed] [Google Scholar]
- 15. Das JK, Hoodbhoy Z, Salam RA, Bhutta AZ, Valenzuela-Rubio NG, Weise Prinzo Zet al. Lipid-based nutrient supplements for maternal, birth, and infant developmental outcomes. Cochrane Database Syst Rev. 2018;8(8):CD012610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ota E, Hori H, Mori R, Tobe-Gai R, Farrar D. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database Syst Rev. 2015;(6):CD000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2015;(7):CD004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WWet al. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2013;346:f3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lassi ZS, Padhani ZA, Rabbani A, Rind F, Salam RA, Das JKet al. Impact of dietary interventions during pregnancy on maternal, neonatal, and child outcomes in low- and middle-income countries. Nutrients. 2020;12(2):531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vanslambrouck K, de Kok B, Toe LC, De Cock N, Ouedraogo M, Dailey-Chwalibóg Tet al. Effect of balanced energy-protein supplementation during pregnancy and lactation on birth outcomes and infant growth in rural Burkina Faso: study protocol for a randomised controlled trial. BMJ Open. 2021;11(3):e038393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Kok B, Toe LC, Hanley-Cook G, Argaw A, Ouédraogo M, Compaoré Aet al. Prenatal fortified balanced energy-protein supplementation and birth outcomes in rural Burkina Faso: a randomised controlled efficacy trial. PLoS Med. 2022;19(5):e1004002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roberfroid D, Huybregts L, Lanou H, Henry MC, Meda N, Menten Jet al. Effects of maternal multiple micronutrient supplementation on fetal growth: a double-blind randomized controlled trial in rural Burkina Faso. Am J Clin Nutr. 2008;88(5):1330–40. [DOI] [PubMed] [Google Scholar]
- 24. Huybregts L, Roberfroid D, Lanou H, Menten J, Meda N, Van Camp Jet al. Prenatal food supplementation fortified with multiple micronutrients increases birth length: a randomized controlled trial in rural Burkina Faso. Am J Clin Nutr. 2009;90(6):1593–600. [DOI] [PubMed] [Google Scholar]
- 25. Hanley-Cook GT, Argaw A, de Kok B, Toe LC, Dailey-Chwalibóg T, Ouédraogo Met al. Seasonality and day-to-day variability of dietary diversity: longitudinal study of pregnant women enrolled in a randomized controlled efficacy trial in rural Burkina Faso. J Nutr. 2022;152(9):2145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huybregts L, Roberfroid D, Kolsteren P, Van Camp J. Dietary behaviour, food and nutrient intake of pregnant women in a rural community in Burkina Faso. Matern Child Nutr. 2009;5(3):211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Kok B, Argaw A, Hanley-Cook G, Toe LC, Ouédraogo M, Dailey-Chwalibóg Tet al. Fortified balanced energy-protein supplements increase nutrient adequacy without displacing food intake in pregnant women in rural Burkina Faso. J Nutr. 2021;151(12):3831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fergusson D, Aaron SD, Guyatt G, Hébert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325(7365):652–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bill & Melinda Gates Foundation . Framework and specifications for the nutritional composition of a food supplement for pregnant and lactating women (PLW) in undernourished and low-income settings. [Internet]. Seattle, WA: Gates Open Research; 2017. Available from: https://gatesopenresearch.org/documents/3-1498 (Accessed 2022 Feb 2). [Google Scholar]
- 30. Jones L, de Kok B, Moore K, de Pee S, Bedford J, Vanslambrouck Ket al. Acceptability of 12 fortified balanced energy protein supplements - insights from Burkina Faso. Matern Child Nutr. 2021;17(1):e13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Kok B, Moore K, Jones L, Vanslambrouck K, Toe LC, Ouédraogo Met al. Home consumption of two fortified balanced energy protein supplements by pregnant women in Burkina Faso. Matern Child Nutr. 2021;17(3):e13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Food and Nutrition Board, Institute of Medicine, National Academies . Dietary Reference Intakes (DRIs): Estimated Average Requirements[Internet]. 2011. Available from: https://www.nal.usda.gov/sites/default/files/fnic_uploads/recommended_intakes_individuals.pdf. [Google Scholar]
- 33. Allen LH, Carriquiry AL, Murphy SP. Perspective: proposed harmonized nutrient reference values for populations. Adv Nutr. 2020;11(3):469–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Valadez JJ, Brown LD, Vargas WV, Morley D. Using lot quality assurance sampling to assess measurements for growth monitoring in a developing country's primary health care system. Int J Epidemiol. 1996;25(2):381–7. [DOI] [PubMed] [Google Scholar]
- 35. Villar J, Ismail LC, Victora CG, Ohuma EO, Bertino E, Altman DGet al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–68. [DOI] [PubMed] [Google Scholar]
- 36. Adu-Afarwuah S, Lartey A, Okronipa H, Ashorn P, Ashorn U, Zeilani Met al. Maternal supplementation with small-quantity lipid-based nutrient supplements compared with multiple micronutrients, but not with iron and folic acid, reduces the prevalence of low gestational weight gain in semi-urban Ghana: a randomized controlled trial. J Nutr. 2017;147(4):697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu E, Wang D, Darling AM, Perumal N, Wang M, Urassa Wet al. Multivitamin supplementation is associated with greater adequacy of gestational weight gain among pregnant women in Tanzania. J Nutr. 2022;152(4):1091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tennant PWG, Arnold KF, Ellison GTH, Gilthorpe MS. Analyses of ‘change scores’ do not estimate causal effects in observational data. Int J Epidemiol. 2021;Jun 7; (Epub ahead of print; doi: 10.1093/ije/dyab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Senn S. Change from baseline and analysis of covariance revisited. Stat Med. 2006;25(24):4334–44. [DOI] [PubMed] [Google Scholar]
- 40. Ismail LC, Bishop DC, Pang R, Ohuma EO, Kac G, Abrams Bet al. Gestational weight gain standards based on women enrolled in the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project: a prospective longitudinal cohort study. BMJ. 2016;352:i555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tielemans MJ, Garcia AH, Santos AP, Bramer WM, Luksa N, Luvizotto MJet al. Macronutrient composition and gestational weight gain: a systematic review. Am J Clin Nutr. 2016;103(1):83–99. [DOI] [PubMed] [Google Scholar]
- 42. Christian P, Shrestha J, LeClerq SC, Khatry SK, Jiang T, Wagner Tet al. Supplementation with micronutrients in addition to iron and folic acid does not further improve the hematologic status of pregnant women in rural Nepal. J Nutr. 2003;133(11):3492–8. [DOI] [PubMed] [Google Scholar]
- 43. Cook JD, Dassenko SA, Whittaker P. Calcium supplementation: effect on iron absorption. Am J Clin Nutr. 1991;53(1):106–11. [DOI] [PubMed] [Google Scholar]
- 44. Persson LÅ, Arifeen S, Ekström EC, Rasmussen KM, Frongillo EA, Yunus M. Effects of prenatal micronutrient and early food supplementation on maternal hemoglobin, birth weight, and infant mortality among children in Bangladesh: the MINIMat randomized trial. JAMA. 2012;307(19):2050–9. [DOI] [PubMed] [Google Scholar]
- 45. Roberfroid D, Huybregts L, Habicht J-P, Lanou H, Henry M-C, Meda Net al. Randomized controlled trial of 2 prenatal iron supplements: is there a dose-response relation with maternal hemoglobin?. Am J Clin Nutr. 2011;93(5):1012–18. [DOI] [PubMed] [Google Scholar]
- 46. Matias SL, Mridha MK, Paul RR, Hussain S, Vosti SA, Arnold CDet al. Prenatal lipid-based nutrient supplements affect maternal anthropometric indicators only in certain subgroups of rural Bangladeshi women. J Nutr. 2016;146(9):1775–82. [DOI] [PubMed] [Google Scholar]
- 47. Janmohamed A, Karakochuk CD, Boungnasiri S, Chapman GE, Janssen PA, Brant Ret al. Prenatal supplementation with Corn Soya Blend Plus reduces the risk of maternal anemia in late gestation and lowers the rate of preterm birth but does not significantly improve maternal weight gain and birth anthropometric measurements in rural Cambodian women: a randomized trial. Am J Clin Nutr. 2016;103(2):559–66. [DOI] [PubMed] [Google Scholar]
- 48. Prentice AM, Cole TJ, Foord FA, Lamb WH, Whitehead RG. Increased birthweight after prenatal dietary supplementation of rural African women. Am J Clin Nutr. 1987;46(6):912–25. [DOI] [PubMed] [Google Scholar]
- 49. Ramakrishnan U, González-Cossío T, Neufeld LM, Rivera J, Martorell R. Effect of prenatal multiple micronutrient supplements on maternal weight and skinfold changes: a randomized double-blind clinical trial in Mexico. Food Nutr Bull. 2005;26(3):273–80. [DOI] [PubMed] [Google Scholar]
- 50. Changamire FT, Mwiru RS, Peterson KE, Msamanga GI, Spiegelman D, Petraro Pet al. Effect of multivitamin supplements on weight gain during pregnancy among HIV-negative women in Tanzania. Matern Child Nutr. 2015;11(3):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Villamor E, Msamanga G, Spiegelman D, Antelman G, Peterson KE, Hunter DJet al. Effect of multivitamin and vitamin A supplements on weight gain during pregnancy among HIV-1-infected women. Am J Clin Nutr. 2002;76(5):1082–90. [DOI] [PubMed] [Google Scholar]
- 52. Mardones-Santander F, Rosso P, Stekel A, Ahumada E, Llaguno S, Pizarro Fet al. Effect of a milk-based food supplement on maternal nutritional status and fetal growth in underweight Chilean women. Am J Clin Nutr. 1988;47(3):413–19. [DOI] [PubMed] [Google Scholar]
- 53. Tontisirin K, Booranasubkajorn U, Hongsumarn A, Thewtong D. Formulation and evaluation of supplementary foods for Thai pregnant women. Am J Clin Nutr. 1986;43(6):931–9. [DOI] [PubMed] [Google Scholar]
- 54. Hambidge KM, Westcott JE, Garcés A, Figueroa L, Goudar SS, Dhaded SMet al. A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: the Women First trial. Am J Clin Nutr. 2019;109(2):457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schulze KJ, Mehra S, Shaikh S, Ali H, Shamim AA, Wu LSFet al. Antenatal multiple micronutrient supplementation compared to iron–folic acid affects micronutrient status but does not eliminate deficiencies in a randomized controlled trial among pregnant women of rural Bangladesh. J Nutr. 2019;149(7):1260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Darling AM, Werler MM, Cantonwine DE, Fawzi WW, McElrath TF. Timing and amount of gestational weight gain in association with adverse birth outcomes. Epidemiology. 2019;30(5):695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gernand AD, Christian P, Paul RR, Shaikh S, Labrique AB, Schulze KJet al. Maternal weight and body composition during pregnancy are associated with placental and birth weight in rural Bangladesh. J Nutr. 2012;142(11):2010–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gondwe A, Ashorn P, Ashorn U, Dewey KG, Maleta K, Nkhoma Met al. Pre-pregnancy body mass index (BMI) and maternal gestational weight gain are positively associated with birth outcomes in rural Malawi. PLoS One. 2018;13(10):e0206035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ouédraogo CT, Wessells KR, Young RR, Faye MT, Hess SY. Prevalence and determinants of gestational weight gain among pregnant women in Niger. Mater Child Nutr. 2020;16(1):e12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kac G, Arnold CD, Matias SL, Mridha MK, Dewey KG. Gestational weight gain and newborn anthropometric outcomes in rural Bangladesh. Matern Child Nutr. 2019;15(4):e12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hasan SMT, Rahman S, Locks LM, Rahman M, Hore SK, Saqeeb KNet al. Magnitude and determinants of inadequate third-trimester weight gain in rural Bangladesh. PLoS One. 2018;13(4):e0196190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ranadip Chowdhury N, Shankar Choudhary T, Dhabhai N, Mittal P, Dewan R, Kaur Jet al. Gestational weight gain and pregnancy outcomes: findings from North Indian pregnancy cohort. Matern Child Nutr. 2022;18(1):e13238. [Google Scholar]
- 63. Tran NT, Nguyen LT, Berde Y, Low YL, Tey SL, Huynh DTT. Maternal nutritional adequacy and gestational weight gain and their associations with birth outcomes among Vietnamese women. BMC Pregnancy Childbirth. 2019;19:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hutcheon JA, Bodnar LM, Joseph KS, Abrams B, Simhan HN, Platt RW. The bias in current measures of gestational weight gain. Paediatr Perinat Epidemiol. 2012;26(2):109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bodnar LM, Hutcheon JA, Parisi SM, Pugh SJ, Abrams B. Comparison of gestational weight gain z-scores and traditional weight gain measures in relation to perinatal outcomes. Paediatr Perinat Epidemiol. 2015;29(1):11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cantor AG, Jungbauer RM, McDonagh M, Blazina I, Marshall NE, Weeks Cet al. Counseling and behavioral interventions for healthy weight and weight gain in pregnancy: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(20):2094–109. [DOI] [PubMed] [Google Scholar]
- 67. Fattah C, Farah N, Barry SC, O'Connor N, Stuart B, Turner MJ. Maternal weight and body composition in the first trimester of pregnancy. Acta Obstet Gynecol Scand. 2010;89(7):952–5. [DOI] [PubMed] [Google Scholar]
- 68. Hutcheon JA, Bodnar LM. Good practices for observational studies of maternal weight and weight gain in pregnancy. Paediatr Perinat Epidemiol. 2018;32(2):152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Quyen PN, Nga HT, Chaffee B, Ngu T, King JC. Effect of maternal prenatal food supplementation, gestational weight gain, and breast-feeding on infant growth during the first 24 months of life in rural Vietnam. PLoS One. 2020;15(6):e0233671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang D, Darling AM, McDonald CR, Perumal N, Liu E, Wang Met al. Plasma concentrations of leptin at mid-pregnancy are associated with gestational weight gain among pregnant women in Tanzania: a prospective cohort study. BMC Pregnancy Childbirth. 2021;21(1):675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Darling AM, McDonald CR, Conroy AL, Hayford KT, Liles WC, Wang Met al. Angiogenic and inflammatory biomarkers in midpregnancy and small-for-gestational-age outcomes in Tanzania. Am J Obstet Gynecol. 2014;211(5):509.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for measurement of food access: indicator guide (v.3). Washington (DC): Food and Nutrition Technical Assistance Project and FHI 360; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The informed consent form does not allow sharing of personal data outside the research team. Requests to access data need to be directed to the ethics committee of Ghent University Hospital through ethisch.comite@uzgent.be. Supporting study documents, including the study protocol and questionnaires, are publicly available on the study's website: https://misame3.ugent.be.