Abstract
Introduction
Several biomaterials can be used in ear surgery to pack the middle ear or support the graft. The absorbable gelatin sponge is the most widely used, but it may produce fibrosis and impair ventilation of the middle ear.
Objective
This experimental study aimed to investigate the inflammatory effects of the sugarcane biopolymer sponge (BP) in the rat middle ear compared with absorbable gelatin sponge (AGS).
Materials and methods
Prospective experimental study design. Thirty adult female Wistar rats were allocated to receive the BP sponge into the right ear and AGS into the left ear. Animals were randomly killed at 4 and 12 weeks post-procedure. Qualitative histological assessments were performed to evaluate the inflammatory reaction in the tympanic bullae.
Results
The BP sponge caused inflammation more intense and persistent than AGS. The BP was not absorbed during the experiment. Fibrosis was observed only in the ears with AGS. There were thickening of the mucosa and neoangiogenesis in the group of AGS.
Conclusion
Despite inflammation, the BP sponge produced less fibrosis and neoangiogenesis compared to AGS. The sponge BP appeared to be a non-absorbable biomaterial in the middle ear.
Keywords: Biocompatible materials, Otologic surgical procedures, Gelatine sponge, absorbable, Biopolymers
Resumo
Introdução
Existem diversos biomateriais que podem ser utilizados na cirurgia otológica para preencher a cavidade da orelha média ou dar suporte a enxertos. A esponja de gelatina absorvível é a mais utilizada, mas pode provocar fibrose e prejudicar a ventilação da orelha média.
Objetivo
Investigar os efeitos da reação inflamatória provocada pela esponja do biopolímero da cana-de-açúcar (BP) comparada a esponja de gelatina absorvível (EGA) na mucosa da orelha média de ratos.
Materiais e métodos
Estudo experimental prospectivo. A esponja do BP foi implantada na orelha direita e a EGA na orelha esquerda de 30 ratos Wistar fêmeas. Os animais foram sacrificados com 4 e 12 semanas após o procedimento. Avaliação histológica qualitativa foi realizada para verificar a reação inflamatória na bula timpânica.
Resultados
A esponja do BP provocou exsudato inflamatório mais intenso e persistente que a EGA. O BP não foi absorvido durante o tempo de observação. Traves de fibrose foram observadas apenas nos ouvidos com a EGA. Houve espessamento da mucosa e neoangiogênese no grupo da EGA.
Conclusão
Apesar da reação inflamatória, a esponja do BP provocou menos fibrose e neoangiogênese quando comparada a EGA. A esponja do BP comportou-se como um biomaterial não-absorvível na orelha média.
Palavras-chave: Materiais biocompatíveis, Prática diária do otorrinolaringologista, Esponja de gelatina absorvível, Biopolímeros
Introduction
Tympanoplasty and tympanomastoidectomy are common otologic surgical procedures in ENT practices. In most of the techniques used, besides the graft material for tympanic membrane (TM) perforation closure, it is necessary to use a supporting or packing material in the cavity of the middle ear. The support material must be safe for the patient, biocompatible and should not cause any mucosal reaction, which could compromise middle ear ventilation.1 Ideally it should be conformable to the tympanic cavity and maintain the graft stability long enough for healing.1 After healing, the material may or may not be absorbed by the body.
Several types of support materials may be used in tympanoplasty and tympanomastoidectomy procedures. There are nonabsorbable materials (such as silicone) that require revision surgery to be removed. Absorbable materials can be manufactured from hyaluronic acid, synthetic materials, alternative materials (plants) or gelatine. Worldwide, the material most commonly used to provide stability to the tympanic graft is a hemostatic absorbable gelatine sponge, derived from pig dermis. However, studies in the medical literature challenge their use in otology. Significant submucosal fibrosis of the middle ear of rats was found in contact areas with this hemostatic sponge.2 One study compared three substances: Nasopore® (polyurethane membrane), Sepragel® (hyaluronic acid polymer) and Gelfoam® (hemostatic absorbable sponge) that were inoculated into the tympanic bullae of rats. After 3 days, an increased inflammatory reaction was noted in the Gelfoam® group, compared to the other groups; and after 20 days there was a greater degree of subepithelial thickening and fibrosis in this group.3
The middle ear mucosa is very reactive. Tonnaer et al. inoculated various substances (bacteria, hemocyanin, charcoal or saline) in the tympanic bulla of rats by a transtympanic route.4 The authors concluded that they could provoke an acute otitis media with any of the tested substances (except saline) by contact of the substance with the middle ear mucosa.4 The difficulty in finding a biocompatible material that would cause minimal damage to mucosa has stimulated the search for new biomaterials.
The sugarcane biopolymer is a macromolecule produced by the bacterium Zoogloea sp. when this organism is grown in a culture medium rich in molasses from sugarcane.5 It has been shown to be biocompatible in membranous form in several studies conducted in different sites.6 Silva et al.7 conducted an experimental study using the biopolymer as a tympanic graft fixed on the external surface of TM perforation, and noted the closure of the tympanic perforation in most cases. Mayer et al.8 studied the inflammatory reaction of the sugarcane biopolymer membrane in the middle ear of rats. These authors noted the presence of exudate and mucosal thickening that regressed over time.
One form of the sugarcane biopolymer is a non-porous laminar sheet that was experimentally evaluated to replace or fix in place the graft in TM perforation repair procedures. Another form is a sugarcane biopolymer sponge that is porous and dense. In contact with water, this biomaterial expands only slightly and becomes crumbly over time. This type of material could be used as support for the graft or even as packing material for the tympanic cavity and ear canal. The present study aims to evaluate the inflammatory reaction of the sponge form of the sugarcane biopolymer when it contacts the middle ear mucosa, and to compare it with that of absorbable gelatine sponge, marketed as Gelfoam®. The early inflammatory response will be analyzed through the characterization of the exudate and submucosal edema. The delayed inflammatory response will also be assessed by evaluating for neoangiogenesis and fibrosis, as well as any chronic exudate.
Materials and methods
In the present study, 30 healthy Wistar female albino rats (Rattus norvegicus albinus), weighing 211-290 g (mean 247.25 g), about four months old, were used. We excluded from the study any rat with an abnormality of the middle ear or the tympanic membrane (perforation or extensive myringosclerosis).
The selected animals were operated and followed-up at the Center of Experimental Surgery. The animals were kept in a vivarium with constantly controlled temperature (22 ± 2 °C) in collective cages with sawdust in its floor with five animals before the experiment, and in individual cages after the experiment. The animals were under illumination for 12 hours/day. The rats were fed ad libitum with industrialized ration (Labina®).
Study model
This is a prospective, controlled, analytical, experimental study.
Procedures
For the procedure, the rats underwent general anesthesia, using ketamine chlorydrate (5 mg/100 g body weight) IM, xylazine chlorydrate (2 mg/100 g body weight) IM, and atropine (0.16 ml/100 g body weight) SC. Antibiotic prophylaxis was administered with cephalothin (1.3 mg/100 g body weight) IM.
Arbitrarily, all right ears were allocated to the sugarcane sponge biopolymer (BP) group, and all left ears of the same rats formed the absorbable gelatine sponge, Gelfoam® (GF) group.
All animals underwent otomicroscopy with a surgical microscope (MC-M31 column microscope, DF Vasconcelos®). After otomicroscopy with magnification ×10, the included rats underwent ear surgery.
Ear surgery
The surgical procedure was performed on a surgical table with complete asepsis and antiseptic precautions. After anesthesia and shaving the retroauricular and cervical regions, a ventrolateral 2 cm incision was performed with a #15 scalpel blade 0.5 cm from the external ear orifice (Fig. 1). Then, the muscle plane was retracted to avoid unnecessary trauma, thus allowing the visualization of the tendon of insertion of the digastric muscle at the base of the skull. In this position, the posterior portion of the tympanic bulla is deeply located (Fig. 2). After the retroauricular exposure, the tympanic bulla was perforated gently with the tip of a needle 40 × 1.20 mm. Then, with the alligator forceps, the corresponding material was introduced. In the same rat, the biopolymer was placed on the right side, and Gelfoam® on the left side. The size of the biomaterials was standardized (5 mm long × 1 mm thick, Fig. 3). After the introduction of the biomaterials, the muscle layer was repositioned, and the skin was sutured with 4-0 nylon. All surgery procedures were performed by the same investigator to avoid variations in technique. After 15 days, the rat skin naturally expels the suture, obviating the need to remove it.
Fig. 1.
Wistar rat in lateral decubitus. Surgical incision in the retroauricular region of the left ear. A, anterior; P, posterior.
Fig. 2.
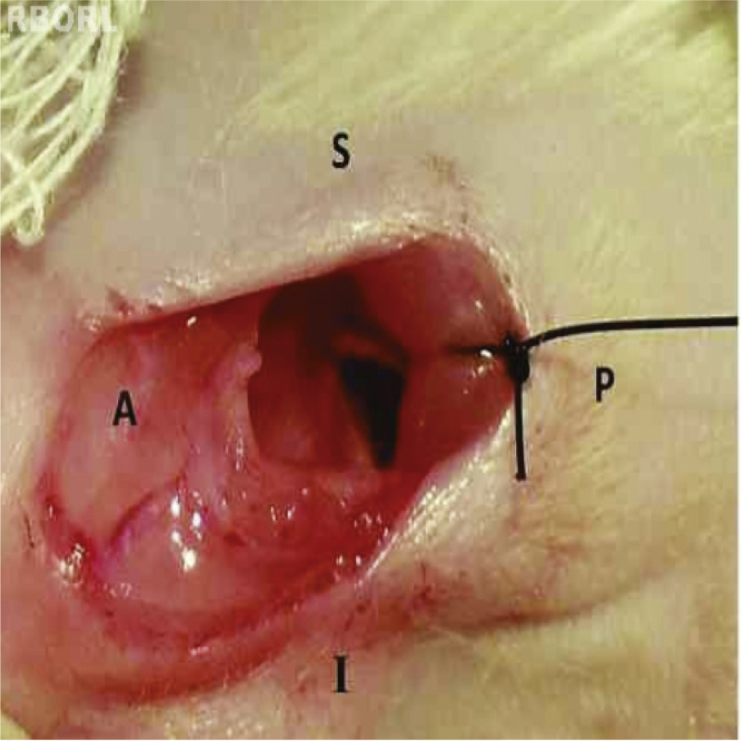
Exposure of the location of the tympanic bulla, deep to the cervical muscles on the left side of the rat. A, anterior; P, posterior; S, superior; I, inferior.
Fig. 3.

Materials studied. GF, Gelfoam (absorbable gelatine sponge); BP, sponge of sugarcane biopolymer.
The operated rats were subdivided in two subgroups by random allotment: T1 time–rats sacrificed at 4 weeks, and T2 time–rats sacrificed 12 weeks after surgery. At these time intervals the animals were euthanized painlessly by intraperitoneal administration of sodium thiopental followed by a lethal dose of barbiturate by intracardiac injection. After euthanasia, their tympanic bullae were removed for study.
Preparation and histological analysis
The tympanic bullae were isolated and prepared for histological study. The fixation was performed in 10% buffered formalin and decalcification in 5% nitric acid for 24 hours. In order to improve fixation, a 2-mm hole was made in the posterior part of the tympanic bulla for introduction of the buffered formalin. The material was dehydrated in an increasing ethanol series of 70%, 80%, 90% and 100%, one hour each, and then subjected to a diaphanization process in xylene, and finally embedded in paraffin. With the specimens already embedded in blocks of paraffin, 5-μm slices were obtained in a microtome (Spencer AO) with a 50-micron interval. The slices were made in a transverse plane to the TM.
Then the material was stained with haematoxylin-eosin (HE). After the processing of all slides, we selected those whose section was situated at the level of the end of the malleus handle, corresponding to mesotympanum, and whose section was situated at the level of the long process of the incus, corresponding to epitympanum. The selected sections were subjected to qualitative analysis by an experienced pathologist. In the analysis, the morphology of the mucosa and TM of the bullae, type and intensity of the inflammatory reaction, presence of fibrosis, and behavior of biopolymer, compared to Gelfoam, were observed.
The description of histological findings was subdivided according to the following parameters: TM or mucosal thickness, cell type in the inflammatory infiltrate, and submucosal neovascularization and fibrosis.
The TM thickness was rated as absent, mild, moderate or severe. The intensity of inflammation was described based on the observation of exudate cellularity and extent of the process through the tympanic bulla cavity. The intensity of the inflammatory activity was rated as follows: null–absence of inflammatory signals; mild–exudate with little cell infiltration, with a reaction involving up to 1/3 of the tympanic bulla lumen (epitympanum or mesotympanum); moderate–exudate with moderate cell infiltration, with a reaction involving between 1/3 and 2/3 of the bulla lumen (epitympanum and mesotympanum); intense–exudate with extensive cell infiltration, with a reaction involving more than 2/3 of the bulla accompanied by signs of necrosis.
The inflammatory infiltrate was also analyzed for cellularity. If there were more lymphocytes and plasma cells, it was rated as chronic and lymphomononuclear (LMN). If there was greater number of neutrophils, the infiltrate was rated as acute and polymorphonuclear (PMN). If there was as much LMN as PLM, it was considered as a subacute exudate.
Neoangiogenesis occurs from pre-existing vessels. In a late phase of inflammation, there is vasodilatation and increased vascular permeability and degradation of the basement membrane with endothelial cell migration toward the angiogenic stimulus. The presence or absence of this behaviour of mucosal inflammation was evaluated.
Presence of fibrosis is also part of the late phase of inflammation, and was characterized by presence of fibroblasts and collagen deposition in the extracellular matrix. The subepithelial fibrosis in the bulla was rated as mild, moderate or severe, depending on the extension of the process into the tympanic bulla.
In assessing the degree of absorption of the material into the tympanic bulla, we considered whether there was any sign of persistence of the material in the slides investigated.
Statistical analysis
The data obtained were categorized and presented descriptively and analytically. All data were grouped into tables. In the comparative statistical analysis, we checked whether there were differences between biomaterials. We also compared data between T1 and T2 times for each group separately. The Fisher's exact test was used due to the small number of observations. Results whose descriptive levels (p values) were <0.05 were considered statistically significant. The statistical calculations were performed using SPSS software for Windows version 18.0–Statistical Package for the Social Sciences.
Ethical considerations
This study followed the principles governing the Code of Ethics and the experimental animal protection laws, according to the abiding principles in Brazil, especially Law No. 9,605–art. 32, and Decree No. 3,179 - art. 17, dated September 21, 1999, which deal with the issue of using animals for scientific purposes. Furthermore, the study had full approval from the Ethics Committee for Animal Use, and was registered under protocol No. 23076.020776/2010-48, in agreement with the fact that the death of the animals used in this study is justified, because there are no alternative resources for the realization of our scientific procedure. The use of general anesthesia before any procedure aimed to avoid pain and reduce animal stress. During the experiment, the experimental animals were followed-up by veterinary officers of the Centre for Experimental Surgery.
Results
Of the total of 30 rats in the study, 15 rats were randomly selected for T1, and 15 for T2 group. In the T1 group, two animals died due to anesthetic complications during surgery. Of the 13 operated rats, 6 were lostdue to problems such as bilateral secondary infection or failure of the histotechnical processing. Of the 7 remaining rats, two had unilateral otitis (left side) and remained in the study. Thus, at the end of the first month of observation of the experiment, we obtained 7 right ears (BP group) and 5 left ears (GF group).
Among the 15 rats sacrificed at T2, two left ears were excluded due to secondary infection and two others due to the low quality of the histotechnical processing. Thus, in the GF group, 11 ears remained. The 36 ears studied were distributed as follows: T1–7 in BP group and 5 in GF group; T2–15 in BP group and 11 in GF group.
The description of the histological findings was subdivided according to the following parameters: TM and mucosal thickness, cell type in the inflammatory infiltrate, neovascularization and severity of the submucosal fibrosis. To characterize the sample studied, we present in Table 1, Table 2 the absolute (N) and relative (percentage) frequencies of classes in each qualitative variable.
Table 1.
Histological findings in the tympanic bulla of rats after one month of the experiment (T1).
| Histological findings | Muc. Th. | TM. Th. | LMN | PMN | Neoangio | Fibrosis |
|---|---|---|---|---|---|---|
| BP (n = 7) | ||||||
| Absent | 1 (14.28%) | 3 (42.86%) | – | – | 1 (14.28%) | – |
| Light | 3 (42.86%) | 4 (57.14%) | 1 (14.28%) | 1 (14.28%) | 6 (85.71%) | 5 (71.43%) |
| Moderate | – | – | 4 (57.14%) | 3 (42.86%) | – | 2 (28.57%) |
| Intense | 3 (42.86%) | – | 2 (28.57%) | 3 (42.86%) | – | – |
| GF (n = 5) | ||||||
| Absent | 2 (40.0%) | 3 (60.0%) | – | 5 (100%) | – | – |
| Light | 1 (20.0%) | – | 4 (80.0%) | – | 4 (80.0%) | 1 (20.0%) |
| Moderate | 1 (20.0%) | 1 (20.0%) | 1 (20.0%) | – | 1 (20.0%) | 3 (60.0%) |
| Intense | 1 (20.0%) | 1 (20.0%) | – | – | – | 1 (20.0%) |
BP, biopolymer; GF, Gelfoam®; Muc.Th., mucosal thickening; TM.Th., tympanic membrane thickening; LMN, lymphmononuclear infiltrate; PMN, polymorphonuclear infiltrate; Neoangio, neoangiogenesis.
Table 2.
Histological findings in the tympanic bulla of rats after 3 months of the experiment (T2).
| Histological findings | Muc. Th. | TM. Th. | LMN | PMN | Neoangio | Fibrosis |
|---|---|---|---|---|---|---|
| BP (n = 15) | ||||||
| Absent | 1 (6.66%) | 8 (53.33%) | – | – | 5 (33.33%) | 1 (6.66%) |
| Light | 5 (33.33%) | 5 (33.33%) | – | 1 (6.66%) | 8 (53.33%) | 8 (53.33%) |
| Moderate | 4 (26.66%) | 1 (6.66%) | 12 (80.0%) | 6 (40.0%) | 2 (13.33%) | 5 (33.33%) |
| Intense | 5 (33.33%) | 1 (6.66%) | 3 (20.0%) | 8 (53.33%) | – | 1 (6.66%) |
| GF (n = 11) | ||||||
| Absent | 1 (9.09%) | 5 (45.45%) | – | 9 (81.81%) | – | – |
| Light | 1 (9.09%) | 3 (27.27%) | 7 (63.63%) | 1 (9.09%) | 3 (27.27%) | 4 (36.36%) |
| Moderate | 3 (27.27%) | 3 (27.27%) | 4 (36.36%) | – | 7 (63.63%) | 3 (27.27%) |
| Intense | 6 (54.54%) | – | – | 1 (9.09%) | 1 (9.09%) | 4 (36.36%) |
BP, biopolymer; GF, Gelfoam®; Muc.Th., mucosal thickening; TM.Th., tympanic membrane thickening; LMN, lymphmononuclear infiltrate; PMN, polymorphonuclear infiltrate; Neoangio, neoangiogenesis.
To compare the groups with respect to the histological findings, we applied the Fisher's exact test, due to the small number of observations. For the statistical analysis, the histological results rated as absent or mild were pooled and compared to the pooled outcome of the data rated as moderate or severe.
Table 3, Table 4 list the statistical analysis of the data. These tables show a comparison between the biomaterials.
Table 3.
Comparison between groups of biomaterials in relation to histological findings in the tympanic bulla of rats after 1 month of experiment (T1).
| Histological findings | Group–T1 |
P value | |
|---|---|---|---|
| BP (n = 7) | GF (n = 5) | ||
| Muc. Th. | |||
| Absent/Light | 4 (57.1%) | 3 (60.0%) | > 0.999. |
| Moderate/Severe | 3 (42.9%) | 2 (40.0%) | |
| TM. Th. | |||
| Absent/Light | 7(100%) | 3 (60.0%) | 0.152. |
| Moderate/Severe | 0(0%) | 2 (40.0%) | |
| LMN | |||
| Absent/Light | 1(14.3%) | 4 (80.0%) | 0.062. |
| Moderate/Severe | 6 (85.7%) | 1 (20.0%) | |
| PMN | |||
| Absent/Light | 1(14.3%) | 5 (100%) | 0.015a |
| Moderate/Severe | 6 (85.7%) | 0 (0%) | |
| Neoangio | |||
| Absent/Light | 7(100%) | 4 (80.0%) | 0.417. |
| Moderate/Severe | 0 (0%) | 1 (20.0%) | |
| Fibrosis | |||
| Absent/Light | 5 (71.4%) | 1 (20.0%) | 0.234. |
| Moderate/Severe | 2(28.6%) | 4 (80.0%) | |
Statistically significant (p < 0.05).
Table 4.
Comparison between groups of biomaterials in relation to histological findings in the tympanic bulla of rats after 3 months of experiment (T2).
| Histological findings | Group–T2 |
P value | |
|---|---|---|---|
| BP (n = 15) | GF (n = 11) | ||
| Muc. Th. | |||
| Absent/Light | 6 (40.0%) | 2 (18.2%) | 0.234. |
| Moderate/Severe | 9 (60.0%) | 9 (81.8%) | |
| TM. Th. | |||
| Absent/Light | 13 (86.7%) | 8 (72.7%) | 0.620. |
| Moderate/Severe | 2 (13.3%) | 3 (27.3%) | |
| LMN | |||
| Absent/Light | 0 (0%) | 7 (63.6%) | 0.001a |
| Moderate/Severe | 15 (100%) | 4 (36.4%) | |
| PMN | |||
| Absent/Light | 1 (6.7%) | 10 (90.9%) | 0.001a |
| Moderate/Severe | 14 (93.3%) | 1 (9.1%) | |
| Neoangio | |||
| Absent/Light | 13 (86.7%) | 3 (27.3%) | 0.004a |
| Moderate/Severe | 2 (13.3%) | 8 (72.7%) | |
| Fibrosis | |||
| Absent/Light | 9 (60.0%) | 4 (36.4%) | 0.234. |
| Moderate/Severe | 6 (40.0%) | 7 (63.6%) | |
Statistically significant (p < 0.05).
There was no statistical difference between the analyzed parameters when comparing the results in T1 and T2 only for BP group, as well as when this comparison were performed in T1 and T2 for GF group.
Regarding the presence of the implant after 3 months of the experiment, BP was seen in all the tympanic bullae. GF was seen in only one tympanic bulla. Bone neoformation was observed in two bullae with GF and in three bullae with BP.
The fibrogenic response pattern was different between the two groups. In BP implants, fibrosis, when observed, was mild in most cases. On the other hand, some cases in the GF implants developed, fibrous adhesions, with the air spaces fully occupied. These adhesions were observed in this group both at one and three months after the surgery.
Discussion
The use of biomaterials in otologic surgery is a common practice in otorhinolaryngology. After the introduction of packing materials into the tympanic cavity in the 50s by Zollner and Wullstein, the use of biomaterials became popular.9, 10 Depending on the indication and surgical technique proposed to the patient, materials were developed to be used as implants in the replacement of the ossicles of the middle ear, oval window sealants, hemostasis materials, ventilation tubes, support materials for graft and packing material for tympanic cavity or external ear canal.1 The support and packing materials are indicated in most tympanoplasty and mastoidectomy procedures.
Currently, Gelfoam® is the absorbable biomaterial most used in otologic surgery. Initially this sponge was used as a hemostatic agent in neurosurgery. The expandability of the material in contact with fluid and its absorption by the body in the medium term resulted in Gelfoam being used to occupy space in various surgical areas. In 1951, Blaine11 described its use not only as a hemostatic agent, but also as a replacement of destroyed tissues. Since the 50s, Gelfoam® has been used in various otologic operations, but, since the 1960s, some studies noted deleterious effects on the middle ear. Schuknecht12, 13 in two reports, documented the occurrence of hearing loss after stapedectomy surgery and concluded that this failure was secondary to the use of Gelfoam® in the oval window.
The middle ear mucosa and TM, when in contact with some harmful material, suffer subepithelial oedema and hyperplasia, evidenced by histological thickening. In the present study, we observed, both in T1 and T2 of our experiment, that none of the biomaterials tested caused a significant TM thickening. Laurent et al.14 also observed no changes in TM in experimental surgeries using GF.
The tympanic membrane is composed of three layers, and its innermost layer is contiguous with the tympanic bulla mucosa, being subject to the same injuries and with a similar inflammatory response. Mayer et al.8 observed a significant TM thickening after inoculation of material through a perforated eardrum. The authors noted that this finding resulted not only from the presence of the material, but also from the handling of TM itself, and concluded that the methodology for application of the material in the experimental surgery of the tympanic bulla may influence the results.8 Therefore, our study used the retrobullar route, already described by other authors, which causes no damage to TM.
In our study, although not statistically different, the GF group in T2 had the highest percentage of severe mucosal thickening in the tympanic bulla (81.85%). This trend for a more intense mucosal thickening secondary to GF use was expected, since other experimental studies have reported it previously. Krupala et al.15 found a thickening with mucosal inflammation in 9 of 10 animals in which GF was used as support material. Jang et al.16 found significant mucosal thickening, when compared GF versus Interceed®. In their study, the authors performed myringotomy procedures with scarification of the mucosa before the procedure; and after 3 weeks, the histological findings revealed adhesions and mucosal thickening in 7 of 10 ears with the use of GF.16 After eight weeks of observation, Bahadir et al.17 found moderate mucosal thickening in rats treated with inoculation of Gelfoam per transtympanic route. When a scarification of the mucosa in contact with the GF was performed, the authors found a much more exuberant mucosal thickening; 6 rats exhibited a moderate, and 4 a severe, grade of thickening in a total of 10 animals.16
An inflammatory infiltrate was present in all cases, but with a difference regarding to the cellularity of the process. In ears packed with BP, a greater inflammatory reaction was noted, with the presence of both polymorphonuclear (PMN) and lymphocytic (LMN) infiltration. The PMN infiltrate was present around the BP sponge, and the LMN infiltrate in the underlying mucosa. On the other hand, in the ears packed with GF, the LMN infiltrate represented most cases and there was no PNM exudate, with a statistically significant difference between biomaterials.
The amount of exudate was also significantly different between groups, since 85.7% of the BP exudate were considered of moderate to severe at the beginning of the experiment, unlike GF, which caused mild or absent inflammatory reaction in most cases. The inflammatory reaction caused by BP sponge remained different from GF throughout the experiment. In T2, Table 4 shows that in most cases BP maintained a moderate to intense inflammatory response, both in PMN as in LMN exudate, while the group of ears treated with PG showed mild or no reaction, with statistically significant difference. There was no change in inflammatory intensity over time; BP group remained with the most intense reaction and GF group with the lightest reaction.
In other studies, BP also induced an important inflammatory response. In the form of the membrane used in the tympanic bulla of rats, BP led to the formation of a subacute exudate, rated as moderate to severe in 33.3% of cases.8 As vascular replacement, BP induced inflammatory reaction with the presence of neutrophils, lymphocytes, and fibrosis.18 In subaponeurotic tissue, BP was tested as a pubovaginal sling.19 Severe inflammatory reaction with the presence of PMN and giant cells around the biomaterial was reported.19 In the present study, the inflammatory infiltrate proved important, especially in terms of permeating the sponge; in the bulla region, contralateral to BP, there was no exudate. Finally, it remains unknown to what extent this persistent inflammatory response may impair the ventilation and the scarring process of the tympanic bulla.
The degradation of BP was slower than GF's: this also contributed to the maintenance of the immune stimulus. The chemical composition of BP was defined as a cellulose polysaccharide composed of different monomeric bases, namely: glucose 87.57%, xylose 8.58%, ribose 1.68%, glucuronic acid 0.83%, mannose 0.82%, arabinose 0.37%, galactose 0.13%, fucose 0.01%, and rhamnose 0.01%.5 Although, in theoretical terms, all these elements are easily degraded in the body, the persistence of an intense inflammatory reaction and the continued presence of BP sponge 3 months after surgery in in vivo experiments demonstrate a a level of inflamatory response not observed in the sponge derived from pig dermis. One possibility could be that BP is of plant origin, in contrast to the animal origin of GF, or perhaps there is some immunological difficulty of the rodent species. The persistence of BP in the body may have applicability in other situations; this product may become a non-absorbable support material. Future biomolecular studies could be performed to better assess this property.
Despite the presence of a persistent inflammatory exudate, the BP sponge does not seem to induce neovascularization and fibrosis in the same proportion as the GF sponge. Table 4 shows that neovascularization was absent or mild in 86.7% in the tympanic bullae with BP, but was moderate to severe in 72.7% in the GF group, a statistically significant difference. The neovascularization occurs as an endothelial proliferation in the presence of an inflammatory stimulus. The immune reaction caused by GF allowed the vessels to dilate with the formation of new vascular meshes, which probably point to an organization of the inflammatory process. This intense neovascularization in ears treated with GF may be related to a higher rate of fibrosis at a later time. After all, in our result and in the literature, there is a greater tendency to fibrosis in the middle ear exposed to GF.
In the descriptive results, we noted not only the submucosal fibrosis of the GF group, but also trabeculae of fibrosis crossing the cavity of the tympanic bulla. In the 1980s, otologists in Sweden found adhesions with trabeculae of connective tissue in the middle ear in reoperations of tympanoplasty and in programmed revision mastoidecomy surgeries.20 There was a suspicion that the hemostatic sponge used as support could be the cause of these changes. In experimental surgery with rats, Swedish researchers found tympanic membrane retraction with synechiae and fibrosis in the middle ear in the presence of Gelfoam after 2 months.20 These authors also observed new bone formation and submucosal thickening in both the promontory and the hypotympanum after 3 months of experiment.20 Hellstrom et al. concluded that Gelfoam® could be involved in the failure of some otologic surgeries.20
The presence of moderate or severe fibrosis in 80% and 63.6% in T1 and T2, respectively, is consistent with studies in theliterature implanted GF.Rates a high as 90% of cases with moderate to severe fibrosis have been reported.3, 16, 20, 21, 22 In other studies, 60% of the cases exhibited fibrosis, but there are also papers that noted this change in only 20% of cases with GF.2, 15, 17
Due to its ability to cause endothelial fibrosis, GF is widely used as microparticle for embolization in hemodynamic procedures.23 However, the occurrence of fibrosis is unwanted in the middle ear surgery. To function properly, the tympanic cavity should be well aerated and with the ossicles free to vibrate. The occurrence of adhesions, mucosal fibrosis or the presence of chronic exudate affect the sound transmission and can cause secondary infection or neotympanum retraction. The ideal biomaterial should not cause inflammatory reaction which would result in an increase of connective tissue.
The BP sponge caused no significant fibrosis, but there was an inflammatory exudate which could compromise the healing process in otologic surgery. So far, an ideal biomaterial for use in contact with the mucosa of the middle ear has not been identified, since this region is hyperresponsive to any stimulus.1, 4 However, some authors question how to use the support or packing material. The amount of material used, the preservation of the mucosa and the use of steroid drops during surgery seem to favour a better outcome.17, 24, 25
BP is a polymer derived from sugarcane, a low cost and abundant biomass in Brazil, capable of changing its natural state to gel, membrane or sponge simply through physical phenomena, without need of adding chemically active products. BP is biocompatible in vitro; but more studies are needed to ensure a satisfactory end result in ear surgery.
Conclusion
The sugarcane biopolymer sponge caused a more intense inflammatory reaction with exudate, compared to absorbable gelatine sponge. There was little neovascularization and mild fibrosis in the sugarcane biopolymer sponge group, compared to absorbable gelatine sponge group. Further studies may elucidate whether this behaviour of the sugarcane biopolymer may be useful in otologic operations.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
To Camila Sartechi, for his help in the statistical analysis of this study. To Adriana Amorim, Med. Vet., by her care for the experimental animals.
Footnotes
Please cite this article as: Bunzen DL, Lins N, Leal MC, Lira MMM, Caldas Neto SS. Middle ear packing materials: comparison between absorbable hemostatic gelatine sponge and sugarcane biopolymer sponge in rats. Braz J Otorhinolaryngol. 2014;80:237-44.
References
- 1.Shen Y., Teh B.M., Friedland P.L., Eikelboom R.H., Atlas M.D. To pack or not to pack?. A contemporary review of middle ear packing agents. Laryngoscope. 2011;121:1040–1048. doi: 10.1002/lary.21470. [DOI] [PubMed] [Google Scholar]
- 2.Liening D.A., Lundy L., Silberberg B., Finstuen K. A comparison of the biocompatibility of three absorbable hemostatic agents in the rat middle ear. Otolaryngol Head Neck Surg. 1997;116:454–457. doi: 10.1016/S0194-59989770294-8. [DOI] [PubMed] [Google Scholar]
- 3.Dogru S., Haholu A., Gungor A., Kucukodaci Z., Cincik H., Ozdemir T., et al. Histologic analysis of the effects of three different support materials within rat middle ear. Otolaryngol Head Neck Surg. 2009;140:177–182. doi: 10.1016/j.otohns.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Tonnaer E.L.G.M., Ingels K.J.A.O., Rijkers G.T., Curfs J.H.A.J. Antigenic as well as nonantigenic stimuli induce similar middle ear responses in the rat. Laryngoscope. 2003;113:322–327. doi: 10.1097/00005537-200302000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Paterson-Beedle M., Kennedy J.F., Melo F.A.D., Lloyd L.L.M.V. A cellulosic exopolysaccharide produced from sugarcane molasses by a Zoogloea sp. Carbohydrate Polymers. 2000;42:375–383. [Google Scholar]
- 6.Assis F.D., Melo D., Marques E. Citotoxicidade de biopolímero de cana-de-açúcar. An Fac Med Univ Fed Pernamb. 2004;49:73–77. [Google Scholar]
- 7.Silva D.B., Aguiar J.L.A., Marques A., Coelho A., Rolim Filho E.L. Miringoplastia com enxerto livre de membrana de biopolímero de cana-de-açúcar e fáscia autóloga em Chinchilla laniger. An Fac Med Univ Fed Pernamb. 2006;51:45–51. [Google Scholar]
- 8.Mayer D.L.B., Araújo J.G., de, Leal de C., Caldas Neto S., da S., Ataíde R.F., Mello R.J.V.de. Membrana do biopolímero da cana-de-açúcar: avaliação experimental na orelha média [Sugarcane biopolymer membrane: experimental evaluation in the middle ear] Braz J Otorhinolaryngol. 2011;77:44–50. doi: 10.1590/S1808-86942011000100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zollner F. The prognosis of the operative improvement of hearing in chronic middle ear infections. Ann Otol Rhinol Laryngol. 1957;66:907–917. doi: 10.1177/000348945706600401. [DOI] [PubMed] [Google Scholar]
- 10.Wullstein H. The restoration of the function of the middle ear, in chronic otitis media. Ann Otol Rhinol Laryngol. 1956;65:1021–1041. [PubMed] [Google Scholar]
- 11.Blaine G. Absorbable gelatin sponge in experimental surgery. Lancet. 1951;2:427–429. doi: 10.1016/s0140-6736(51)91693-5. [DOI] [PubMed] [Google Scholar]
- 12.Schuknecht H.F. Sensorineural hearing loss following stapedectomy. Acta Otolaryngol. 1962;54:336–340. doi: 10.3109/00016486209126953. [DOI] [PubMed] [Google Scholar]
- 13.Schuknecht H.F. Gelfoam as an implant in oval window following stapedectomy. Ann Otol Rhinol Laryngol. 1971;80:415–418. doi: 10.1177/000348947108000320. [DOI] [PubMed] [Google Scholar]
- 14.Laurent C., Hellström S., Stenfors L.E. Hyaluronic acid reduces connective tissue formation in middle ears filled with absorbable gelatin sponge: an experimental study. Am J Otolaryngol. 1986;7:181–186. doi: 10.1016/s0196-0709(86)80004-7. [DOI] [PubMed] [Google Scholar]
- 15.Krupala J.L., Gianoli G.J., Smith R.A. The efficacy of hyaluronic acid foam as a middle ear packing agent in experimental tympanoplasty. Am J Otol. 1998;19:546–550. [PubMed] [Google Scholar]
- 16.Jang C.H., Park H., Cho Y.B., Choi C.H. The effect of Interceed for reducing adhesion as a middle ear packing agent: an experimental study. Int J Pediatr Otorhinolaryngol. 2008;72:1517–1521. doi: 10.1016/j.ijporl.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Bahadir O., Aydin S., Caylan R. The effect on the middle-ear cavity of an absorbable gelatine sponge alone and with corticosteroids. Eur Arch Otorhinolaryngol. 2003;260:19–23. doi: 10.1007/s00405-002-0494-6. [DOI] [PubMed] [Google Scholar]
- 18.Aguiar J.L.A., Lins E.M., Marques S.R.B., Coelho A.R.B., Rossiter R.O., Melo R.J.V. Surgarcane biopolymer patch in femoral artery angioplasty on dogs. Acta Cir Bras. 2007;22:77–81. doi: 10.1590/s0102-86502007000700015. [DOI] [PubMed] [Google Scholar]
- 19.Lucena R. Utilização do biopolímero da cana-de-açúcar como novo material para sling pubovaginal: análise estereológica. Recife: Universidade Federal de Pernambuco. Tese. Recife: Universidade Federal de Pernambuco, Centro de Ciências da Saúde; 2007.
- 20.Hellström S., Salén B., Stenfors L.E. Absorbable gelatin sponge (Gelfoam) in otosurgery: one cause of undesirable postoperative results? Acta Otolaryngol. 1983;96:269–275. doi: 10.3109/00016488309132899. [DOI] [PubMed] [Google Scholar]
- 21.McGhee M.A., Dornhoffer J.L. The effect of gelfilm in the prevention of fibrosis in the middle ear of the animal model. Am J Otol. 1999;20:712–716. [PubMed] [Google Scholar]
- 22.Huang G., Chen X., Jiang H. Effects of NasoPore packing in the middle ear cavity of the guinea pig. Otolaryngol Head Neck Surg. 2011;145:131–136. doi: 10.1177/0194599811400834. [DOI] [PubMed] [Google Scholar]
- 23.Loffroy R., Guiu B., Cercueil J.-P., Krausé D. Endovascular therapeutic embolisation: an overview of occluding agents and their effects on embolised tissues. Curr Vasc Pharmacol. 2009;7:250–263. doi: 10.2174/157016109787455617. [DOI] [PubMed] [Google Scholar]
- 24.Park A.H., Jackson A., Hunter L., McGill L., Simonsen S.E., Alder S.C., et al. Cross-linked hydrogels for middle ear packing. Otol Neurotol. 2001;27:1170–1175. doi: 10.1097/01.mao.0000227893.50162.9e. [DOI] [PubMed] [Google Scholar]
- 25.Li G., Feghali J.G., Dinces E., McElveen J., Van de Water T.R. Evaluation of esterified hyaluronic acid as middle ear-packing material. Arch Otolaryngol Head Neck Surg. 2001;127:534–539. doi: 10.1001/archotol.127.5.534. [DOI] [PubMed] [Google Scholar]


