Abstract
The fight against the COVID-19 epidemic significantly raises the global demand for personal protective equipment, especially disposable face masks (DFMs). The discarded DFMs may become a potential source of microplastics (MPs), which has attracted much attention. In this work, we identified the detailed source of MPs released from DFMs with laser direct infrared spectroscopy. Polypropylene (PP) and polyurethane (PU) accounted for 24.5% and 57.1% of released MPs, respectively. The melt-blown fabric was a dominant MPs source, however, previous studies underestimated the contribution of mask rope. The captured polyethylene terephthalate (PET), polyamide (PA), polyethylene (PE), and polystyrene (PS) in airborne only shared 18.4% of released MPs. To deepen the understanding of MPs release from medical mask into the aquatic environment, we investigated the effects of environmental factors on MPs release. Based on regression analysis, the effects of temperature, incubation time, and wearing time significantly affect the release of MPs. Besides, acidity, alkalinity, sodium chloride, and humic acid also contributed to the MPs release through corroding, swelling, or repulsion of fibers. Based on the exposure of medical mask to simulated environments, the number of released MPs followed the order: seawater > simulated gut-fluid > freshwater > pure water. Considering the risk of MPs released from DFMs to the environment, we innovatively established a novel flotation removal system combined with cocoamidopropyl betaine, achieving 86% removal efficiency of MPs in water. This work shed the light on the MPs release from DFMs and proposed a removal strategy for the control of MPs pollution.
Keywords: Microplastics, Release behavior, Disposable face masks, COVID-19 epidemic, Flotation removal, Personal protective equipment
Graphical abstract
1. Introduction
Low production costs and versatile applications were the main reasons for the extensive use of plastics. Global plastic production reached 367 million tons in 2020, and it would continue to maintain at a high level (Huang et al., 2022). However, the mismanagement of plastic wastes leads to a globally pervasive issue. Evidence showed that only 9% of waste plastic was recycled, while the majority was landfilled (Neo et al., 2022). Meanwhile, during the COVID-19 epidemic, the global demand for personal protective equipment persistently increased, arising potential environmental issues (Zhao et al., 2022). Most countries have encouraged people to wear disposable face masks (DFMs) as an effective strategy for preventing virus transmission. It was estimated that China manufactured 450 million DFMs per day in 2020 (Tabatabaei et al., 2021), and the world consumed 129 billion DFMs per day during the COVID-19 epidemic (Li et al., 2022). DFMs effectively prevent the spread of COVID-19, however, due to the lack of guidance, discarded DFMs by the public might result in severe plastic pollution (Kutralam-Muniasamy et al., 2022). Every year, roughly 1.59 billion masks penetrate the marine ecosystem, according to a survey by OceansAsia (Mghili et al., 2022). These plastic wastes might become persistent pollutants in soil and aquatic environment, threatening the ecosystem and public health.
Under the effects of aging, mechanical abrasion, and microbial colonization, considerable secondary microplastics (MPs) derived from waste plastics appeared in the natural environment. Organisms may eat MPs with a diameter of less than 5 mm mistakenly, which was proved by the evidence of the existence of MPs in the digestive tract of mussels and seabirds (Alimba and Faggio, 2019). With the accumulation of MPs in the food chain, polyethylene terephthalate (PET), polyethylene (PE), and polystyrene (PS) MPs were found in human blood (Leslie et al., 2022). Meanwhile, the positive correlation between MPs and inflammatory bowel disease further revealed the threats of MPs to human health (Yan et al., 2022b). MPs have high surface areas and abundant functional groups, inducing the adsorption of hazardous heavy metals, organic pollutants, and pathogens, and the toxicity can be aggravated (Shen et al., 2021b; Wang et al., 2021a). Moreover, ubiquitous MPs can obstruct the photosynthesis of algae and seriously interfere with the carbon fixation capacity of the ocean (Shen et al., 2020). However, present efforts on MPs removal are still faced with challenges, and source control of MPs might be a feasible alternative (Zhang et al., 2021a). The rise of medical wastes created during the COVID-19 outbreak has outstripped current disposal capacity, and the mismanaged DFMs have become a potential source of MPs (Mghili et al., 2022).
Medical DFMs consist of layers of polypropylene (PP) non-woven fabric, melt-blown fabric, and hot-air cotton. Chen et al. revealed that most MPs were released from the melt-blown fabric, and the average amount of released MPs reached 1246 particles/piece (Chen et al., 2021b). The release process of MPs from discarded DFMs in water was studied, and the release kinetics of MPs was well described by the Elovich equation (Liang et al., 2022). The ingestion of PP MPs from DFMs might lead to a significant decline in the fecundity of aquatic organisms (Sun et al., 2021). However, a few researches focused on the mask rope made with polyurethane (PU) and polyamide (PA), and it can be deduced that the mask rope could be a source of PU and PA MPs (Chen et al., 2021a). Wang et al. claimed that the effect of UV weathering could break the fiber structures and contribute to the release of MPs (Wang et al., 2021b). The complicated environmental factors in the aquatic environment might drive the release of MPs from the face mask (Gao et al., 2021b). Investigations on the effect environmental factors on MPs release are of significance for revealing the ecological risks of the discarded face masks. In addition, almost all studies called for the ensuring of environmental management of disposable mask, while the treatment strategy for the MPs contamination from masks was rarely provided. An efficient, green, and cost-saving treatment was required. Froth flotation is mainly used for beneficiation and has great potential in the removal of MPs (Zhang et al., 2021b).
We assumed that some environmental factors might accelerate the release of MPs from face masks. In this work, the effects of incubation time, temperature, pH, mask type, wearing time, sodium chloride concentration, and humic acid concentration on MPs release were systematically discussed. Regression analysis was performed to analyze the relationship between these factors and MPs release. The size, shape, and type of MPs was recorded to identify the detailed source of MPs from DFMs. We further simulated the MPs release in freshwater, seawater, and digestive tract of aquatic organism, revealing the risk of MPs release from DFMs. The type of MPs was accurately identified and characterized by laser direct infrared spectroscopy (LDIR) (Yan et al., 2022a). Most papers mentioned the MPs release from DFMs but few works provided the solution for the MPs pollution. After revealing the risk of MPs release, we sought to mitigate MPs contamination by DFMs in water. In order to treat wastewater contaminated with DFMs, we proposed an innovative process of froth flotation combined with green surfactants for the removal of MPs, which can be applied to the remediation of localized MPs contamination. This work provided a novel insight into the MPs release from DFMs and shed the light on remediating MPs pollution from DFMs.
2. Experimental
2.1. Materials and chemicals
Three types of DFMs, including medical mask, N95 mask, and activated carbon mask, were purchased from Baiyi Weishi Co., Ltd, China. The medical face mask contained two non-woven fabric layers and one melt-blown fabric layer. In contrast, N95 masks have one more layer of non-woven fabrics, and the activated carbon masks have an active charcoal cover over the outer non-woven fabrics. Analytically pure sodium hydroxide (NaOH), hydrochloric acid (HCl), sodium chloride (NaCl), humic acid (HA), calcium chloride (CaCl2), and sodium taurocholate (STC) were purchased from Macklin Co., Ltd, China. Ultrapure water was provided by China Resources C'estbon Beverage (China) Co. Ltd. Cocoamidopropyl betaine (CAPB) of 85% purity was purchased from Jinya Technology Co., Ltd.
2.2. MPs release experiment
A new mask was immersed in 200 mL ultrapure water in a conical flask. The conical flask was placed in a shaking incubator (ZQPL-200) for 24 h, and the speed was controlled at 220 r/min. 400 mL ultrapure water was used to rinse the mask and the flask. Subsequently, the MPs in the water were filtered with a 0.22 μm membrane. The membrane was dried at room temperature and prepared for counting MPs. The quantities, size, shape, and color of MPs on the membrane were recorded using an optical microscope. Meanwhile, we compared the release of MPs from the normal medical mask, the N95 mask, and the activated carbon mask, and the results of released MPs were similar. In the subsequent experiment, the effects of pH (3–11), temperature (15–40 °C), HA concentration (0–100 mg/L), NaCl concentration (0–500 mg/L), incubation duration (1–7 days), and wearing time (0–8 h), on the MPs release from the normal medical masks were investigated (Jiang et al., 2022b). Salinity and organic matter might affect the surface properties of MPs (Wang et al., 2021a). According to the recommendation of the US Centers for Disease Control and Prevention that DFMs should be worn within 8 h, the DFMs used in this study for detection of MPs release were worn no more than 8 h. After washing the face and nose with alcohol, a lab staff was assigned to wear a new mask while sitting in the same position in a constant-temperature laboratory. In the mask-wearing experiment, the lab staff was not allowed to walk around and maintained a steady and normal breathing rate to rule out other causes. To control the test error, each experiment was repeated three times. Nitrile gloves and laboratory coats were worn to reduce errors. Glass containers were immersed with the ultrapure water before and after the experiments, and aluminum foil was used to cover the top of the glass container. Regarding the blank group, we only put 200 mL of ultrapure water in the conical flask and repeated the release experiments of MPs without face mask. The average number of MPs in the blank group was 4 (Fig. S1), which did not significantly affect the MPs release tests.
2.3. Mask exposure in environments
We studied the migration of MPs from DFMs in freshwater, seawater, and a simulated digestive tract of aquatic organisms. The freshwater was collected in Xiangjiang River, China, and the natural seawater was collected in Weihai, China. We also considered that the mask might be ingested by aquatic organisms, and the simulated digestive tract of aquatic organisms was prepared by 0.1 M CaCl2, 15.5 mM sodium taurocholate (STC), and pH = 7 (Wang et al., 2021a). The freshwater and seawater were filtered to remove suspended solids before the exposure experiment. The release experiments were controlled at 18 °C. Other incubation steps and MPs collection processes were conducted according to the steps in the Section 2.2. To control the test error, each experiment was repeated three times.
2.4. Characterizations
An optical microscope was used to observe the MPs that were filtered from the leachate of mask, and the images were recorded by a digital camera module in microscope. The magnification of this microscope was one hundred times (100×). The smallest microplastic size that can be identified was 10 μm. A counting method was applied to determine the quantity of MPs on the membrane (diameter 5 cm) (De Falco et al., 2018). 21 images were taken along two orthogonal diameters of each filter membrane. Every micrograph represented a rectangular area on the filter membrane, equal to 3 mm2. The number of MPs was calculated according to Eq. (1).
| (1) |
where N was the number of MPs; A i represented the image area (3 mm2); C a was the average amount of MPs in the images; A T was the total area of the filter membrane (1384.7 mm2).
The detailed type, shape, and size of MPs (20–500 μm) were characterized by the laser direct infrared imaging (LDIR) analyses using an 8700 imaging system (Agilent Technologies, California, USA). Before the analysis of LDIR, the membrane sample should be rinsed by ethanol and treated by ultrasound. The ethanol solution is concentrated and added dropwise onto a high-reflection glass. To further identify the MPs sources, we obtained images of the layers of non-woven fabric, melt-blown fabric, and mask strap using a scanning electron microscope (SEM; MIRA3 TESCAN, Czech Republic). A zeta potential analyzer (ZetaPALS, Bruker, USA) was used to detect the electronegativity change on MPs surface. Fourier transform infrared spectroscopy (FT-IR, Nicolet 6700, ThermoFisher Scientific, USA) were employed to analyze the composition of mask materials.
2.5. Statistical analysis
Besides, we conducted regression analysis to analyze the relationship of MPs abundance with pH, temperature, HA concentration, NaCl concentration, incubation duration, and wearing time. Tests were considered significant at p ≤ 0.05.
2.6. MPs removal strategy
The leachate of normal medical mask was collected for the subsequent removal. A self-designed flotation system was consisted of a glass column, a spherical air stone, and an air pump (5 W). A 1 L beaker was placed on the bottom of the flotation column to collect the overflowing MPs. Before flotation, we mixed CAPB with the leachate to improve the flotation efficiency. The conditioning duration was controlled at 5 min to ensure the full contact of MPs and surfactant (Gao et al., 2021a). Then the mixed solution and 800 mL of rinsing water were transferred to the flotation column. Air bubbles can carry the MPs to the foam layer, which can be easily scraped away. The remaining MPs and the removed MPs were collected and counted by the microscope to evaluate the removal efficiency. Furthermore, the effect of CAPB concentration (0–4 mg/L) on removal efficiency was studied. We employed flotation kinetics to describe the flotation process of with CAPB conditioning and without CAPB conditioning. The removed MPs in different time intervals were collected, and the flotation kinetics were fitted by a first-order model with a rectangular distribution of floatability according to the Eq. (2) (Ni et al., 2018). The parameters of the models were calculated using Origin (Version 2021) based on the nonlinear least-square optimization method. or k would increase if the MPs hydrophobicity was enhanced, or the flotation conditions improved. To prevent the error, each test was repeated for three times.
| (2) |
where was removal ratio at time t, %. was ultimate removal ratio, %. k was flotation rate constant, s−1.
3. Results and discussion
3.1. Identification of MPs
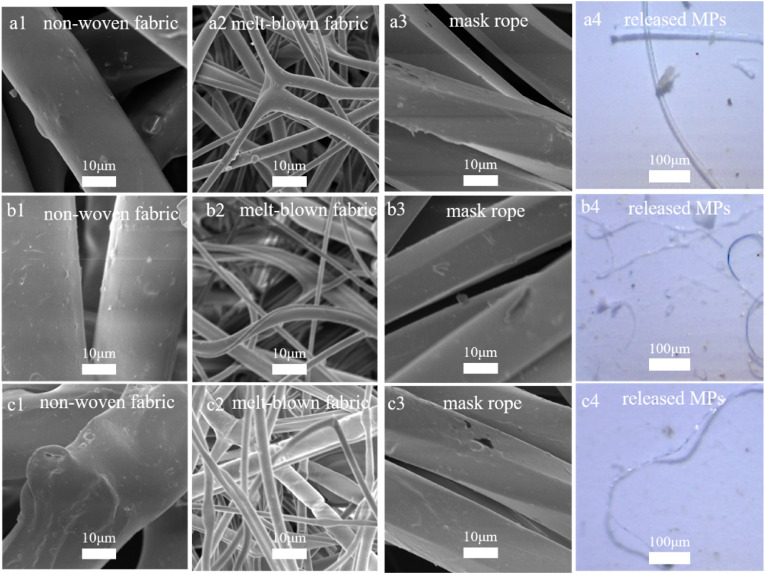
We first characterized the structure of DFMs. According to the SEM images of DFMs, the diameter of fibers in non-woven fabric and mask rope were closed to 20 μm, while the diameter of finer fiber in melt-blown fabric ranged from 1 to 2 μm (Fig. 1 ). Medical face masks, N95 masks, and activated carbon masks followed the above size distribution of fibers. The entanglement of fibers was exhibited in each layer of masks, and the fibers of mask rope were bundled. Fibers experienced scratching and breaking on their surfaces, even in new masks, which might promote the release of MPs (O'Brien et al., 2020). The fibers in the microscope images were transparent or blue, and the diameter of these fibers was closed to that in the melt-blown fabric, consistent with the previous report (Shen et al., 2021a). Similar release results were obtained when treating the mask rope and non-woven fabric solely (Fig. S2), despite their coarser fiber structure according to the SEM images. Therefore, the breaking and peeling of MPs from mask rope and the non-woven fabric were possible, however, the previous work also regarded the melt-blown fabric as the main source of MPs. In addition to fibers, MPs may migrate out of masks as finer particles (Fig. 1). However, after 24 h, the number of MPs released from the medical face mask, the N95 mask, and the activated carbon mask were 1447 ± 218, 1339 ± 166, and 1600 ± 237 items/(piece·d), respectively. The type of DFMs might not significantly affect the release of MPs, and the previous work also confirmed this conclusion (Chen et al., 2021b).
Fig. 1.
SEM images of medical face masks (a1–a3), N95 masks (b1–b3), and activated carbon masks (c1–c3). Microscope images of MPs released from medical face masks (a4), N95 masks (b4), and activated carbon masks (c4).
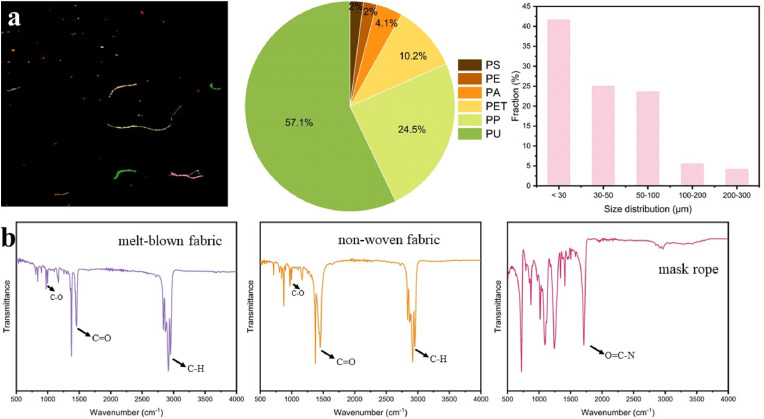
To obtain more detailed information about MPs type, we employed LDIR analysis for the leachate of a used medical face mask (Fig. 2 a). MPs existed mainly as fibers and particles. PU MPs accounted for 57.1% of particle samples, and PP MPs (24.5%) were another dominant MPs type. These two main MPs were speculated to come from non-woven/melt-blown fabrics. After 8 h of wearing, a small amount of polyethylene (PE), PA, polystyrene (PS), and polyethylene glycol terephthalate (PET) in airborne contributed to 18.3% of MPs in the leachate of a used face mask. LDIR only analyzed the MPs of 20–500 μm, and the most common sizes of MPs are 20–100 μm. In the FT-IR spectra, the peaks at 2850–2950 cm−1, 1650–1850 cm−1, and 1000–1200 cm−1 corresponded to C–H stretching of PP, C O bonds and C–O stretching of additives (Fig. 2b) (Yao et al., 2022). Therefore, the non-woven/melt-blown fabrics were made of PP. Evidence showed the structure of PU in mask rope, supported by the amide groups (O C–N) at 1650 cm−1 (Wang et al., 2020). The dominant view held that the PP melt-blown fabrics were the main MPs source (Chen et al., 2021b). However, the PU MPs release from mask rope was more significant. In addition, there was a risk of release of PS, PA, PE, and PET in the mask to the environment.
Fig. 2.
LDIR results of MPs released from medical face masks (a) and FT-IR spectrum of medical face mask (b).
3.2. The effects of temperature, incubation time, and wearing time
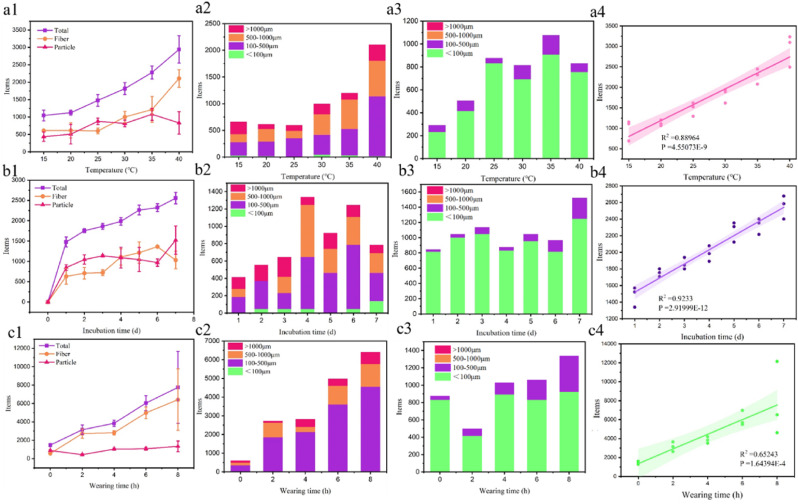
Different factors, such as the wearing time of DFMs, incubation temperature and time of the discarded DFMs, may cause distinct release levels of MPs. Firstly, we recorded the number of released MPs and the size distribution under different temperatures, as shown in Fig. 3 a1 to a3. Temperature could be regarded as a crucial driving factor for the release of MPs. The total amount of released MPs increased from 1043 ± 155 to 2940 ± 392 items/(piece·d) as the temperature increased from 15 °C to 40 °C. The number of released MPs maintained stably at the temperature of 15 °C–20 °C, and the release of MPs was significantly increased when the temperature further increased. An increase of temperature from 15 °C to 45 °C resulted in a 3.5-fold increase in fibers and a 1.9-fold increase in particles. According to the size distribution, fibers of 100–500 μm accounted for the largest proportion of the released MPs, followed by fibers of 500–1000 μm and larger than 1000 μm. However, finer MPs (less than 100 μm) were predominant in particles, and particles larger than 500 μm were rarely observed. Besides, a significant correlation was observed between temperature and MPs number (R2 = 0.889, p = 4.55E-9). High temperature (70–100 °C) might cause chain scission, surface cracking, and fragmentation of plastics (Liu et al., 2022). The temperature of 40 °C could only strengthen the surface fluidity and swelling of plastic fiber (De Falco et al., 2018). Natural environments with high temperature might aggravate the risk of MPs release from masks.
Fig. 3.
The effect of temperature (a1–a4), incubation time (b1–b4), and wearing time (c1–c4) on the number of total released MPs, size distribution of fiber, size distribution of particle, and related regression analysis.
In terms of incubation time, the number of released MPs reached 1477 ± 122 items/piece on the first day, and the release rate of MPs became slower in the remaining 6 days. The release of MPs still did not obtain an equilibrium in 7 days. The ratio of particles to fibers remained close with increased incubation time. The MPs of 100–500 μm and 500–1000 μm dominated in fibers. Interestingly, the finer plastic fiber (≤100 μm) gradually appeared and was more numerous than large fiber (≥1000 μm) after 7 days. Fiber required time to break into a smaller one (Julienne et al., 2019). The fraction of medium particle (100–500 μm) slightly increased during the incubation time. Long incubation time indicated a strong mechanical abrasion because the mask was in the shaker during the incubation. The kinetics of MPs release could be well described by the Elovich equation model due to high R2 value (Table S1), which was supported by the finding of Liang et al. (2022). The detailed calculation was described in the Supplementary Materials. Besides, we identified strong correlation (R2 = 0.923, p value = 2.920E-12) between incubation time and the release of MPs.
According to the results of LDIR, the MPs in airborne were easily captured by breathing and accumulated on mask. However, the number of captured PET, PE, PS and PA MPs was limited. The effect of wearing time accelerated the release of MPs, and the number of released MPs increased to 7755 ± 122 items/(piece·d) after wearing for 8 h. The contribution of the fiber MPs was significant for the increasing number of MPs, but the released particle MPs only fluctuated with wearing time. The fibers of 100–500 μm accounted for 62.9%–75.4% of released fiber MPs and increased by an incredible 1041.3% after wearing for 8 h. The ten-fold increase of medium particle (100–500 μm) confirmed the debonding of medium particle during the wearing time, while finer particle almost remained unchanged. The effects of body temperature (37 °C) and mechanical abrasion of breathing on MPs release were significant. The positive correlation between wearing time and MPs release suggested that, in aquatic environment, there would be more MPs released from the discarded DFMs.
3.3. The effects of aqueous environment
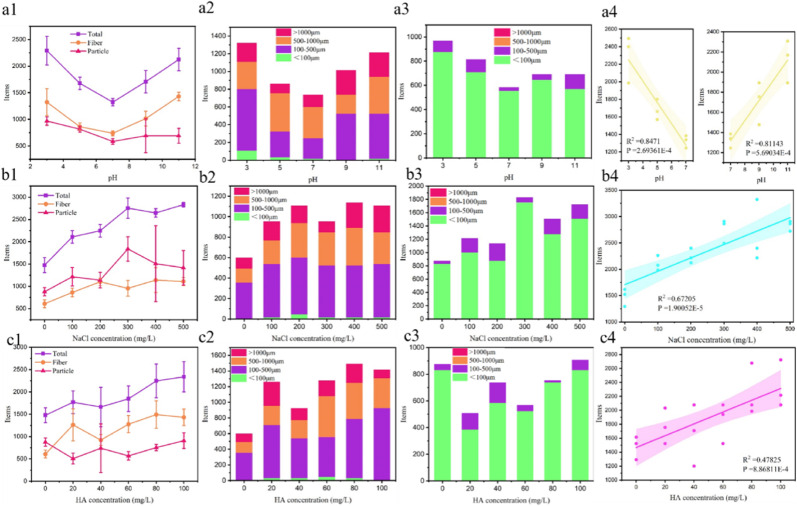
In addition to the above factors, the exposure of DFMs to environments also involved the interaction with aqueous species, such as H+, OH−, NaCl, and HA. The number of released MPs dropped from 2293 ± 271 to 1324 ± 71 items/(piece·d) when pH increased from 3 to 7 (Fig. 4 a1). As the environment became alkaline (pH = 7–11), more MPs were released. It seemed that acid and alkaline environments both induced the MPs release because the backbone chain of polymer could be attacked by OH− and H3O+ species in the alkaline and acid environments (Lim and Thian, 2022). The hydrolysis of O C–N groups in mask rope made by PU was possible. Besides, both finer fibers and particles (≤100 μm) were also more abundant in a more acid environment. Alkalinity induced DFMs to release more medium fibers and particles (100–500 μm). The surface potentials of the released MPs were listed in Fig. S3. The more negative potential on MPs surface caused by alkalinity leads to a greater electrostatic repulsion between MPs. The regression analysis manifested the relationship between MPs release and acidity/alkalinity, the risk of MPs release might be intensified in acid or alkaline industrial wastewater.
Fig. 4.
The effect of pH (a1–a4), NaCl concentration (b1–b4), and HA concentration (c1–c4) on the number of total released MPs, size distribution of fiber, size distribution of particle, and related regression analysis.
In theory, the presence of NaCl could not corrode plastic. However, we accidently discovered that the higher NaCl concentration significantly correlated with the release of MPs (Fig. 4 b1). The regression results also confirmed the statistical significance. Besides, the releases of particle MPs and fiber MPs were both promoted. The face mask could release approximately two times of MPs in the solution of 500 mg/L NaCl than that in pure water. The medium fibers (100–500 μm) and finer particles (≤100 μm) were the largest proportion of released MPs. The melt-blown fibers bonded together insecurely through the melt blowing process (Wu et al., 2022). However, the exposure to the NaCl solution might result in the debonding between fibers, and polymer composites also suffered from the aging effect of salt water on mechanical properties (Shreepannaga et al., 2022). It could be speculated that the incubation in salt water might decrease the stability of the fiber network in DFMs and therefore, release more MPs.
In the natural environment, HA accounted for nearly 50% of dissolved organic matter as a complex structured polyelectrolyte molecule (Shabbir et al., 2022). The HA concentration was significantly correlated with the release of MPs (Fig. 4 c4). 1431 ± 185 items/(piece·d) of fiber MPs and 909 ± 175 items/(piece·d) of particle MPs were released from a mask in the solution of 100 mg/L HA. Likewise, HA promoted the migration of MPs from DFMs. Fiber MPs mainly existed as the size of 100–500 μm and 500–1000 μm, while the finer fibers (≤100 μm) were rarely discovered. The dosage of HA resulted in a weak acid environment. Meanwhile, HA could enhance the transport of MPs by increasing electrostatic repulsion and steric repulsion (Rong et al., 2022). MPs were negatively charged in the natural environment, and the surface potential of released MPs became more negative with the increasing HA concentration (Fig. S4).
3.4. MPs release in simulated environments
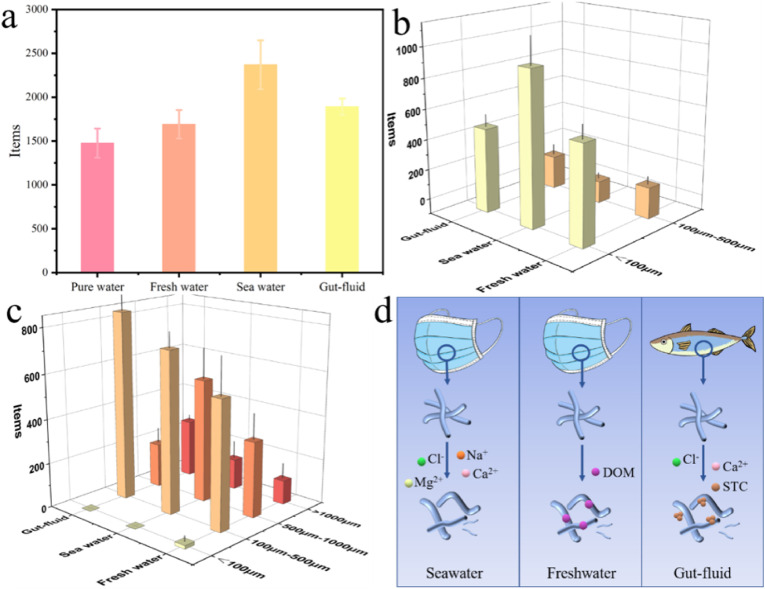
Affected by many environmental factors, face masks could release more MPs to the aquatic environment. We simulated the MPs release in three environments, and the release duration was controlled within 24 h. The number of total released MPs followed the order: sea water > simulated gut-fluid > fresh water > pure water (Fig. 5 a). According to the size distribution of particle MPs, particle MPs of 100–500 μm were released equally in three environments, but particle MPs less than 100 μm tended to be released more in seawater, followed by fresh water and simulated gut-fluid (Fig. 5b). A face mask in sea water released 1415 ± 289 items/(piece·d) of fiber, which reached nearly 235% of the MPs release in pure water (Fig. 5c). The medium fibers (100–500 μm) and lager fibers (>1000 μm) mostly released in the digestive tract of aquatic organisms. The release of finer fiber (<100 μm) was only observed in the freshwater.
Fig. 5.
The total number (a), particle distribution (b), fiber distribution (c), and schematic diagram (d) of released MPs from medical face masks in freshwater, seawater, and simulated gut-fluid.
Previous researches have studied the release of MPs from face masks in pure water, but the risk of MPs release in natural environments might be higher. The high concentration of ions in seawater might weaken the stability of fiber network in mask. Besides, we have discovered that some dissolved organic matters in the freshwater might lead to the swelling of fibers and the enhanced migration of MPs from the masks (Hahladakis et al., 2018). When the mask fragments are accidently eaten by aquatic organisms, such as fish, the MPs release could be prompted by surfactants and salt in the digestive tract. The presence of bile-salt colloids, represented by STC (Voparil and Mayer, 2004), might result in the swelling and debonding of fibers. Numerous MPs from DFMs were released into aquatic environments with the discharge of hazardous additives and pathogen in DFMs.
3.5. MPs removal
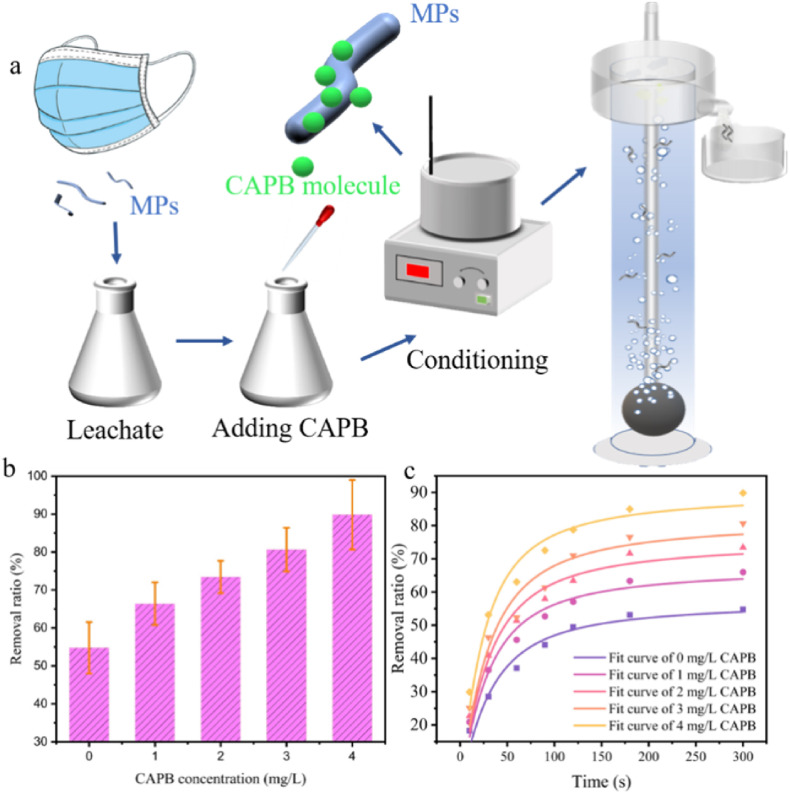
Above studies manifest conspicuous MPs release from DFMs in aqueous environment. Meanwhile, with the spread of the COVID-19 epidemic and mismanagement of medical waste, more DFMs were discarded in the environment. Due to significant threats, it is imperative to remove the released MPs from aqueous environment. Our previous work proves that froth flotation is an effective method for MPs removal in aqueous environment (Zhang et al., 2021b; Jiang et al., 2022b). A tentative process for removing the released MPs from DFMs by froth flotation was proposed. Froth flotation was conducted combined with CAPB conditioning to treat the wastewater polluted by DFMs. Due to its good foaming ability and environmental friendliness, CAPB is mainly used as a foaming agent and antistatic agent. CAPB can lower the surface tension and significantly enhance the abundance of air bubbles in the flotation process (Tackie-Otoo and Ayoub Mohammed, 2020). On the other hand, the long chain structure of CAPB might adhere on MPs surface through hydrophobic attraction or electrostatic interaction. As shown in Fig. 6 a, MPs were fully mixed with CAPB solution, and the fiber floated with air bubbles. Without CAPB, only 32% of MPs in the leachate could be removed, indicating the low efficiency of air flotation (Fig. 6b). However, when the CAPB concentration increased to 4 mg/L, the removal ratio of MPs reached 86.6%. The flotation process was well fitted by the first-order model based on the qualified R2 (0.93–0.96). The results of froth flotation suggested the values of and k rose with the increasing CAPB concentration (Jiang et al., 2022b). Under the effect of CAPB (4.00 mg/L), the removal ratio reached 80.3% in the initial 2 min, and it became constant when the duration was further extended.
Fig. 6.
The process of froth flotation for the MPs removal in the mask leachate (a). The effect of CAPB concentration on removal efficiency (b). The flotation kinetics of MPs with CAPB conditioning and without conditioning (c).
To further clarify the potential effect of CAPB on water quality, we measured the total organic carbon (TOC) in the solution. If CAPB of 4.00 mg/L is used, the residual CAPB in the solution after flotation was 2.30 mg/L. The TOC in the effluent is 2.04 mg/L, as some of the CAPB and other organics were converted to foam and leave the flotation column. The green characteristic of biodegradable CAPB make the process more sustainable (Tackie-Otoo and Ayoub Mohammed, 2020). Next, we performed simple economic analysis to evaluate the feasibility of the proposed process. Considering the electricity and reagent consumption, the costs of the froth flotation was maintained within 0.01 USD/L. Compared with the other MPs removal strategy, such as density extraction (3.74 USD/L) and electrocoagulation (0.82 CNY/L) (Kedzierski et al., 2017; Xu et al., 2022), froth flotation seemed more cost-effective (Jiang et al., 2022a). Therefore, the proposed process can be a feasible strategy for MPs removal from the economic and environmental perspectives.
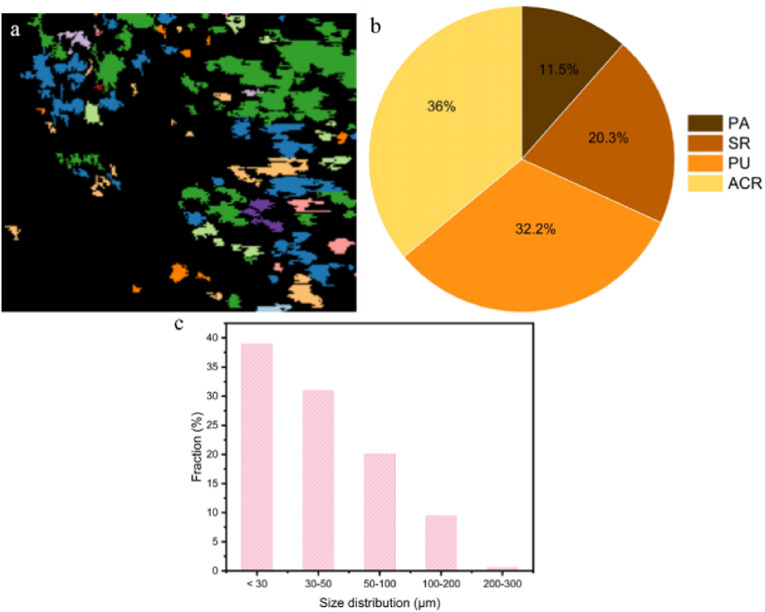
The residue MPs in the treated leachate were determined by LDIR, and the fraction of PU MPs significantly decreased (Fig. 7 ). PP MPs were completely removed. However, an increase of acrylate copolymer (ACR), and silicone resin (SR) might originate from the impurity of CAPB. Interestingly, the PA MPs from air were unaffected by the flotation removal and constitute 11.5% of particle, whereas other MPs from airborne, such as PET, PS, and PE, were completely removed by froth flotation. According to the size distribution, froth flotation could not efficiently remove the MPs less than 50 μm. More efforts are required to improve the removal efficiency of froth flotation.
Fig. 7.
The LDIR images (a), particle types (b), and size distribution (c) of the remaining MPs in the leachate after the flotation removal.
4. Conclusions
Melt-blown fabric, non-woven fabric, and mask rope were potential sources of MPs from DFMs. Similar MPs were released from different types of masks (medical masks, N95 masks, and activated carbon masks). A more accurate identification of MPs sources was proposed in this work. According to the LDIR results, PU and PP fibers account for 57.1% and 24.5% of the released fiber MPs, respectively. The released particle MPs were composed of PS, PA, PE, and PET. A medical mask released 1043 ± 155 items/(piece·d) of MPs in pure water. Exterior factors, including temperature, incubation time, and wearing time significantly promote the MPs release from DFMs. Besides, soluble species related to acidity, alkalinity, NaCl, and HA also contributed to the MPs release from masks. The mechanical abrasion, erosion, swelling, and debonding of fibers in masks aggravated MPs release. The medium fibers (100–500 μm) and finer particles (≤100 μm) were the dominant released MPs. We further simulate the MPs release from DFMs in freshwater, seawater, and gut-fluid. The exposure of DFMs in these three environments would lead to more released MPs. In terms of pollution control of the released MPs from DFMs, a froth flotation process combined with CAPB of 4 mg/L was innovatively proposed, and MPs of 86.6% were removed. The flotation kinetics confirmed that CAPB improved ultimate removal ratio and flotation rate. This work provided better understanding the real contribution of DFMs into the release of MPs to the aquatic environment.
Credit author statement
Hongru Jiang: Writing and Investigation. Jiming Su: Conceptualization and Methodology. Yingshuang Zhang: Editing and review. Kai Bian: Data processing. Zhiyi Wang: Editing and review. Hui Wang: Funding acquisition and supervision. Chongqing Wang: Editing and review.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (21878343).
Handling Editor: X. Cao
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chemosphere.2022.136748.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Alimba C.G., Faggio C. Microplastics in the marine environment: current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharmacol. 2019;68:61–74. doi: 10.1016/j.etap.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Chen R., Zhang D., Xu X., Yuan Y. Pyrolysis characteristics, kinetics, thermodynamics and volatile products of waste medical surgical mask rope by thermogravimetry and online thermogravimetry-Fourier transform infrared-mass spectrometry analysis. Fuel. 2021;295 doi: 10.1016/j.fuel.2021.120632. [DOI] [Google Scholar]
- Chen X., Chen X., Liu Q., Zhao Q., Xiong X., Wu C. Used disposable face masks are significant sources of microplastics to environment. Environ. Pollut. 2021;285 doi: 10.1016/j.envpol.2021.117485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Falco F., Gullo M.P., Gentile G., Di Pace E., Cocca M., Gelabert L., Brouta-Agnésa M., Rovira A., Escudero R., Villalba R., Mossotti R., Montarsolo A., Gavignano S., Tonin C., Avella M. Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ. Pollut. 2018;236:916–925. doi: 10.1016/j.envpol.2017.10.057. [DOI] [PubMed] [Google Scholar]
- Gao J., Sun W., Lyu F. Understanding the activation mechanism of Ca2+ ion in sodium oleate flotation of spodumene: a new perspective. Chem. Eng. Sci. 2021;244 doi: 10.1016/j.ces.2021.116742. [DOI] [Google Scholar]
- Gao L., Fu D., Zhao J., Wu W., Wang Z., Su Y., Peng L. Microplastics aged in various environmental media exhibited strong sorption to heavy metals in seawater. Mar. Pollut. Bull. 2021;169 doi: 10.1016/j.marpolbul.2021.112480. [DOI] [PubMed] [Google Scholar]
- Hahladakis J.N., Velis C.A., Weber R., Iacovidou E., Purnell P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018;344:179–199. doi: 10.1016/j.jhazmat.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Huang J., Veksha A., Chan W.P., Giannis A., Lisak G. Chemical recycling of plastic waste for sustainable material management: a prospective review on catalysts and processes. Renew. Sustain. Energy Rev. 2022;154 doi: 10.1016/j.rser.2021.111866. [DOI] [Google Scholar]
- Jiang H., Zhang Y., Bian K., Wang C., Xie X., Wang H., Zhao H. Is it possible to efficiently and sustainably remove microplastics from sediments using froth flotation? Chem. Eng. J. 2022;448 doi: 10.1016/j.cej.2022.137692. [DOI] [Google Scholar]
- Jiang H., Zhang Y., Bian K., Wang H., Wang C. Insight into the effect of aqueous species on microplastics removal by froth flotation: kinetics and mechanism. J. Environ. Chem. Eng. 2022;10 doi: 10.1016/j.jece.2022.107834. [DOI] [Google Scholar]
- Julienne F., Delorme N., Lagarde F. From macroplastics to microplastics: role of water in the fragmentation of polyethylene. Chemosphere. 2019;236 doi: 10.1016/j.chemosphere.2019.124409. [DOI] [PubMed] [Google Scholar]
- Kedzierski M., Le Tilly V., César G., Sire O., Bruzaud S. Efficient microplastics extraction from sand. A cost effective methodology based on sodium iodide recycling. Mar. Pollut. Bull. 2017;115:120–129. doi: 10.1016/j.marpolbul.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Kutralam-Muniasamy G., Pérez-Guevara F., Shruti V.C. A critical synthesis of current peer-reviewed literature on the environmental and human health impacts of COVID-19 PPE litter: new findings and next steps. J. Hazard. Mater. 2022;422 doi: 10.1016/j.jhazmat.2021.126945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie H.A., van Velzen M.J.M., Brandsma S.H., Vethaak A.D., Garcia-Vallejo J.J., Lamoree M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022;163 doi: 10.1016/j.envint.2022.107199. [DOI] [PubMed] [Google Scholar]
- Li B., Huang Y., Guo D., Liu Y., Liu Z., Han J.-C., Zhao J., Zhu X., Huang Y., Wang Z., Xing B. Environmental risks of disposable face masks during the pandemic of COVID-19: challenges and management. Sci. Total Environ. 2022;825 doi: 10.1016/j.scitotenv.2022.153880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Ji Y., Ge W., Wu J., Song N., Yin Z., Chai C. Release kinetics of microplastics from disposable face masks into the aqueous environment. Sci. Total Environ. 2022;816 doi: 10.1016/j.scitotenv.2021.151650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B.K.H., Thian E.S. Biodegradation of polymers in managing plastic waste — a review. Sci. Total Environ. 2022;813 doi: 10.1016/j.scitotenv.2021.151880. [DOI] [PubMed] [Google Scholar]
- Liu G., Wang J., Wang M., Ying R., Li X., Hu Z., Zhang Y. Disposable plastic materials release microplastics and harmful substances in hot water. Sci. Total Environ. 2022;818 doi: 10.1016/j.scitotenv.2021.151685. [DOI] [PubMed] [Google Scholar]
- Mghili B., Analla M., Aksissou M. Face masks related to COVID-19 in the beaches of the Moroccan Mediterranean: an emerging source of plastic pollution. Mar. Pollut. Bull. 2022;174 doi: 10.1016/j.marpolbul.2021.113181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neo E.R.K., Yeo Z., Low J.S.C., Goodship V., Debattista K. A review on chemometric techniques with infrared, Raman and laser-induced breakdown spectroscopy for sorting plastic waste in the recycling industry. Resour. Conserv. Recycl. 2022;180 doi: 10.1016/j.resconrec.2022.106217. [DOI] [Google Scholar]
- Ni C., Bu X., Xia W., Peng Y., Xie G. Effect of slimes on the flotation recovery and kinetics of coal particles. Fuel. 2018;220:159–166. doi: 10.1016/j.fuel.2018.02.003. [DOI] [Google Scholar]
- O'Brien S., Okoffo E.D., O'Brien J.W., Ribeiro F., Wang X., Wright S.L., Samanipour S., Rauert C., Toapanta T.Y.A., Albarracin R., Thomas K.V. Airborne emissions of microplastic fibres from domestic laundry dryers. Sci. Total Environ. 2020;747 doi: 10.1016/j.scitotenv.2020.141175. [DOI] [PubMed] [Google Scholar]
- Rong H., Li M., He L., Zhang M., Hsieh L., Wang S., Han P., Tong M. Transport and deposition behaviors of microplastics in porous media: Co-impacts of N fertilizers and humic acid. J. Hazard. Mater. 2022;426 doi: 10.1016/j.jhazmat.2021.127787. [DOI] [PubMed] [Google Scholar]
- Shabbir S., Faheem M., Dar A.A., Ali N., Kerr P.G., Yu Z.-G., Li Y., Frei S., Albasher G., Gilfedder B.S. Enhanced periphyton biodegradation of endocrine disrupting hormones and microplastic: intrinsic reaction mechanism, influential humic acid and microbial community structure elucidation. Chemosphere. 2022;293 doi: 10.1016/j.chemosphere.2022.133515. [DOI] [PubMed] [Google Scholar]
- Shen M., Huang W., Chen M., Song B., Zeng G., Zhang Y. (Micro)plastic crisis: un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean. Prod. 2020;254 doi: 10.1016/j.jclepro.2020.120138. [DOI] [Google Scholar]
- Shen M., Zeng Z., Song B., Yi H., Hu T., Zhang Y., Zeng G., Xiao R. Neglected microplastics pollution in global COVID-19: disposable surgical masks. Sci. Total Environ. 2021;790 doi: 10.1016/j.scitotenv.2021.148130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M.C., Song B., Zeng G.M., Zhang Y.X., Teng F.Y., Zhou C.Y. Surfactant changes lead adsorption behaviors and mechanisms on microplastics. Chem. Eng. J. 2021;405:12. doi: 10.1016/j.cej.2020.126989. [DOI] [Google Scholar]
- Shreepannaga Vijaya, Kini M., Pai D. The ageing effect on static and dynamic mechanical properties of fibre reinforced polymer composites under marine environment- A review. Mater. Today Proc. 2022;52:689–696. doi: 10.1016/j.matpr.2021.10.084. [DOI] [Google Scholar]
- Sun J., Yang S., Zhou G.-J., Zhang K., Lu Y., Jin Q., Lam P.K.S., Leung K.M.Y., He Y. Release of microplastics from discarded surgical masks and their adverse impacts on the marine copepod Tigriopus japonicus. Environ. Sci. Technol. Lett. 2021;8:1065–1070. doi: 10.1021/acs.estlett.1c00748. [DOI] [Google Scholar]
- Tabatabaei M., Hosseinzadeh-Bandbafha H., Yang Y., Aghbashlo M., Lam S.S., Montgomery H., Peng W. Exergy intensity and environmental consequences of the medical face masks curtailing the COVID-19 pandemic: malign bodyguard? J. Clean. Prod. 2021;313 doi: 10.1016/j.jclepro.2021.127880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackie-Otoo B.N., Ayoub Mohammed M.A. Experimental investigation of the behaviour of a novel amino acid-based surfactant relevant to EOR application. J. Mol. Liq. 2020;316 doi: 10.1016/j.molliq.2020.113848. [DOI] [Google Scholar]
- Voparil I.M., Mayer L.M. Commercially available chemicals that mimic a deposit feeder's (Arenicola marina) digestive solubilization of lipids. Environ. Sci. Technol. 2004;38:4334–4339. doi: 10.1021/es049506y. [DOI] [PubMed] [Google Scholar]
- Wang H., Huang W., Zhang Y., Wang C., Jiang H. Unique metalloid uptake on microplastics: the interaction between boron and microplastics in aquatic environment. Sci. Total Environ. 2021;800 doi: 10.1016/j.scitotenv.2021.149668. [DOI] [PubMed] [Google Scholar]
- Wang K., Zhang Y., Zhong Y., Luo M., Du Y., Wang L., Wang H. Flotation separation of polyethylene terephthalate from waste packaging plastics through ethylene glycol pretreatment assisted by sonication. Waste Manag. 2020;105:309–316. doi: 10.1016/j.wasman.2020.02.021. [DOI] [PubMed] [Google Scholar]
- Wang Z., An C., Chen X., Lee K., Zhang B., Feng Q. Disposable masks release microplastics to the aqueous environment with exacerbation by natural weathering. J. Hazard. Mater. 2021;417 doi: 10.1016/j.jhazmat.2021.126036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Li J., Lu X., Tang Y., Cai Z. Release of tens of thousands of microfibers from discarded face masks under simulated environmental conditions. Sci. Total Environ. 2022;806 doi: 10.1016/j.scitotenv.2021.150458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Yang Z., Niu Y., Xu D., Wang J., Han J., Wang H. Removal of microplastics and attached heavy metals from secondary effluent of wastewater treatment plant using interpenetrating bipolar plate electrocoagulation. Separ. Purif. Technol. 2022;290 doi: 10.1016/j.seppur.2022.120905. [DOI] [Google Scholar]
- Yan M., Yang J., Sun H., Liu C., Wang L. Occurrence and distribution of microplastics in sediments of a man-made lake receiving reclaimed water. Sci. Total Environ. 2022;813 doi: 10.1016/j.scitotenv.2021.152430. [DOI] [PubMed] [Google Scholar]
- Yan Z., Liu Y., Zhang T., Zhang F., Ren H., Zhang Y. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ. Sci. Technol. 2022;56:414–421. doi: 10.1021/acs.est.1c03924. [DOI] [PubMed] [Google Scholar]
- Yao J., Wen J., Li H., Yang Y. Surface functional groups determine adsorption of pharmaceuticals and personal care products on polypropylene microplastics. J. Hazard. Mater. 2022;423 doi: 10.1016/j.jhazmat.2021.127131. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Jiang H., Bian K., Wang H., Wang C. A critical review of control and removal strategies for microplastics from aquatic environments. J. Environ. Chem. Eng. 2021;9 doi: 10.1016/j.jece.2021.105463. [DOI] [Google Scholar]
- Zhang Y., Jiang H., Bian K., Wang H., Wang C. Is froth flotation a potential scheme for microplastics removal? Analysis on flotation kinetics and surface characteristics. Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148345. [DOI] [PubMed] [Google Scholar]
- Zhao H., Liu H., Wei G., Zhang N., Qiao H., Gong Y., Yu X., Zhou J., Wu Y. A review on emergency disposal and management of medical waste during the COVID-19 pandemic in China. Sci. Total Environ. 2022;810 doi: 10.1016/j.scitotenv.2021.152302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.