This nonrandomized controlled trial investigates the overall response rate associated with apremilast as an add-on therapy among patients with recalcitrant cutaneous dermatomyositis.
Key Points
Question
What are the safety and efficacy of apremilast as an add-on therapy in patients with recalcitrant cutaneous dermatomyositis?
Findings
In this nonrandomized controlled trial that included 8 patients with recalcitrant cutaneous dermatomyositis, the overall response rate was 87.5% at 3 months after treatment with apremilast, and apremilast was well tolerated, with no grade 3 or 4 adverse events.
Meaning
These findings suggest that in patients with recalcitrant cutaneous dermatomyositis, apremilast may be a safe and efficacious treatment option.
Abstract
Importance
Cutaneous disease in dermatomyositis has no standardized treatment approach and so presents a challenging task for patients and clinicians.
Objective
To study the efficacy and safety of apremilast as an add-on therapy in patients with recalcitrant cutaneous dermatomyositis.
Design, Setting, and Participants
This phase 2a, open-label, single-arm nonrandomized controlled trial was conducted at a single center from June 2018 to June 2021. Participants were 8 patients with recalcitrant cutaneous dermatomyositis, defined by a cutaneous disease activity severity index (CDASI) score greater than 5 despite treatment with steroids, steroid-sparing agents, or both. Data were analyzed from June 2018 to June 2021.
Interventions
Apremilast 30 mg orally twice daily was added to ongoing treatment regimens.
Main Outcomes and Measures
The primary outcome was the overall response rate (ORR) at 3 months. Key secondary outcomes were the safety and toxicity of apremilast and the durability of response at 6 months. The CDASI, muscle score, dermatology life quality index (DLQI), and depression assessments were performed at baseline and regularly until month 7. Skin biopsies were performed at baseline and 3 months after apremilast (defined as 3 months into active apremilast therapy) and tested for gene expression profiling and immunohistochemical stains. Adverse events were assessed using the Common Terminology Criteria for Adverse Events version 5.0.
Results
Among 8 patients with recalcitrant cutaneous dermatomyositis (all women; mean [SD] age, 54 [15.9] years), a response was found at 3 months after apremilast among 7 patients (ORR, 87.5%). The mean (SD) decrease in CDASI was 12.9 (6.3) points at 3 months (P < .001). Apremilast was well tolerated, with no grade 3 or higher adverse events. Sequencing of RNA was performed on skin biopsies taken from 7 patients at baseline and at 3 months after therapy. Appropriate negative (ie, no primary antibody) and positive (ie, tonsil and spleen) controls were stained in parallel with each set of slides studied. Of 39 076 expressed genes, there were 195 whose expression changed 2-fold or more at P < .01 (123 downregulated and 72 upregulated genes). Gene set enrichment analysis identified 13 pathways in which apremilast was associated with downregulated expression, notably signal transducers and activators of transcription 1 (STAT1), STAT3, interleukin 4 (IL-4), IL-6, IL-12, IL-23, interferon γ (IFNγ), and tumor necrosis factor α (TNFα) pathways. In immunohistochemical staining, there was a mean (SD) decrease in phosphorylation levels STAT1 (22.3% [28.3%] positive cells) and STAT3 (13.4% [11.6%] positive cells) at the protein level, a downstream signaling pathway for the downregulated cytokines.
Conclusions and Relevance
These findings suggest that apremilast was a safe and efficacious add-on treatment in recalcitrant dermatomyositis, with an overall response rate of 87.5% and associations with downregulation of multiple inflammatory pathways.
Trial Registration
ClinicalTrials.gov Identifier: NCT03529955
Introduction
Dermatomyositis (DM) is an idiopathic inflammatory myopathy that affects the skin, proximal muscles, and other internal organs.1 Cutaneous DM can present with or without muscle disease, and its course is often more persistent.2 Recalcitrant cutaneous DM significantly impacts patient quality of life, with no standardized treatment approach.2 DM is treated primarily with systemic steroids and steroid-sparing agents, namely hydroxychloroquine, methotrexate, mycophenolate mofetil, intravenous immunoglobulin, rituximab, calcineurin inhibitors, cyclophosphamide, and azathioprine.3 Other emerging therapies include Janus kinase inhibitors and interleukin 6 (IL-6) inhibitors.3
Apremilast is a phosphodiesterase 4 (PDE-4) inhibitor approved for the treatment of plaque psoriasis, psoriatic arthritis, and oral ulcers associated with Behçet disease.4,5,6 Off-label use of apremilast in hidradenitis suppurativa, atopic dermatitis, lupus erythematosus, and alopecia areata has been reported in small studies.7,8,9,10 We previously described the use of apremilast in recalcitrant DM in a case series of 3 patients, finding 85% improvement in cutaneous disease activity severity index (CDASI) score.11 Subsequently, a case report using apremilast in recalcitrant cutaneous DM documented substantial reduction in scalp pruritus and other DM cutaneous signs.12
We present the results of a phase 2a, open-label, single-arm nonrandomized controlled trial studying the efficacy and safety of apremilast as an add-on treatment in recalcitrant cutaneous DM. In addition, we investigated the mechanism by which apremilast was associated with outcomes in DM using pretherapy and posttherapy skin biopsies.
Methods
This nonrandomized controlled trial was approved by Tulane University’s institutional review board and performed in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonization guidelines for Good Clinical Practice. All patients provided written informed consent. The Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) statement was used as a guideline for data reporting.
Study Population and Inclusion and Exclusion Criteria
Among 47 patients with DM who were seen in our clinic and screened, 8 patients enrolled after fulfilling inclusion criteria (eFigure 1 in Supplement 2). Demographic information (age, sex, race, and ethnicity) was collected. Participant race and ethnicity were investigator observed and were assessed because DM is associated with a worse outcome in minority racial groups.13 Race and ethnicity categories were defined by the Office of Management and Budget 1997 revision. Categories for race were American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or other Pacific Islander, and White. The minimum categories for ethnicity were Hispanic or Latino and not Hispanic or Latino. Eligible patients included adults aged 18 years and older with a diagnosis of DM based on characteristic cutaneous findings proposed by Sontheimer,14 a skin biopsy consistent with DM, or both. Recalcitrant DM was defined as cutaneous disease activity of at least 5 on the CDASI scale (range, 0-100, with higher scores indicating higher disease activity) despite adequate treatment with systemic steroids for at least 1 month, steroid-sparing agents for at least 3 months, or both.15,16,17 A CDASI score of 5 is the lowest score associated with clinically significant disease that affects quality of life.18 At the time of study entry, patients had to be on stable dose of steroids, steroid-sparing agents, or both for at least 4 weeks. All enrolled patients had age-appropriate cancer screening. Patients with clinically significant comorbidities and acute DM onset with important systemic involvement were excluded. In addition, pregnant and lactating women and patients with an active malignant neoplasm or a history of malignant neoplasm within the past 4 years were excluded.
Study Design and Interventions
This was a phase 2a, open-label, single-arm nonrandomized controlled trial that was conducted from June 2018 to June 2021 in a single tertiary referral center per the study protocol (Supplement 1). Amgen provided the trial drug and paid for correlative analyses. Oral apremilast was added to a stable treatment regimen of steroids, steroid-sparing agents, or both. Apremilast was titrated over 5 days according to the approved titration schedule in the label until the target dose of 30 mg twice daily was reached and continued for up to 6 months. Patients were then followed up for an additional month after discontinuation of therapy. Clinical follow-up visits were performed at 1, 3, 6, and 7 months with laboratory tests and clinical images taken on each visit. CDASI, muscle score using the manual muscle testing in 8 muscles (MMT-8; score range, 0-150, with higher scores indicating full muscle strength), dermatology life quality index (DLQI; score range, 0-30, with higher scores indicating greater change in quality of life), and depression assessment using the Patient Health Questionnaire-2 (PHQ-2; score range, 0-6, with higher scores indicating higher risk of depression) were performed during each visit. At baseline and 3 months after apremilast (defined as 3 months into active apremilast therapy), 5-mm punch biopsies of lesional skin were performed.
Each biopsy was vertically split in 2 pieces, snap frozen on dry ice, and stored at −80 °C for gene expression profiling and immunohistochemical staining. RNA sequencing was performed on skin biopsies taken from 7 patients. Results were subsequently interpreted using Gene Set Enrichment Analysis (GSEA) software version 4.1 (Broad Institute) and immunohistochemical staining. One patient withdrew from the study at 3 months due to disease progression and was not included in the correlative analysis due to lack of specimen.
Primary and Secondary Outcomes
The primary outcome was overall response rate (ORR) of apremilast in patients with cutaneous recalcitrant DM at 3 months. The ORR was measured using CDASI at months 0 and 3. The calculation was performed as the CDASI score at 3 months minus the score at baseline. Complete response was defined as a CDASI score of zero. Partial response was defined by a decrease in CDASI score of at least 4 points, the cutoff point for clinically significant improvement.19 Secondary outcomes were to the safety and toxicity of apremilast, durability of response at 6 months, and biological changes to skin lesions. Adverse events were assessed using the Common Terminology Criteria for Adverse Events version 5.0.
RNA Sequencing
Skin biopsies were mechanically broken down using TissueLyser followed by RNA extraction using the RNeasy extraction Kit (Qiagen). RNA was then subjected to RNA sequencing as previously described.20 Briefly, polyadenylated RNA was enriched by 2 rounds of oligo(dT) selection (Invitrogen), followed by library construction and paired-end sequencing of 100–base pair reads on Illumina HiSeq-2500. We used STAR software version 2.6.1d (Alexander Dobin)21 to map RNA sequencing reads to human genome hg38, Samtools package (DHI Group) to remove duplicate reads and create mpileup files, Strelka software version 2.9.2 (Sangtae Kim, Illumina)22 package for insertion and deletion and single-nucleotide variant calling, and Kallisto software version 0.46.0 (Nicolas L Bray, University of California)23 for expression measurement. RNA sequencing data are deposited in the Gene Expression Omnibus under accession number GSE193276.
Analysis of gene expression profiling was performed using the following programs and software: JMP statistical software package version 16 (SAS Institute), Cluster and Tree View programs version 3.0 (Eisen Laboratory, Stanford University), and GSEA version 4.1 (Broad Institute). Genes that were identified as significantly differentially expressed between the 2 groups with 2-fold or greater change at P < .01 were extracted and displayed in a heatmap. In GSEA analysis, gene sets were selected having a false discovery rate (FDR) of 0.01 or less, a normalized enrichment score (NES) of 1.70 or more, and 10 or more leading-edge genes (ie, genes of a given gene set with the greatest differential expression in the experimental data).
Immunohistochemical Analysis
Immunohistochemical staining was performed on a Dako Autostainer Link 48 (Agilent) in the University of Michigan Rogel Cancer Center Histology core on frozen skin sections obtained from biopsies of lesional skin at baseline and 3 months after apremilast. Deparaffinized sections were labeled with chemokine (C-C motif) ligand 5 (CCL5; Thermo Fisher Scientific), phosphorylated signal transducer and activator of transcription 1 (pSTAT1; Cell Signaling Technology), or pSTAT3 (Cell Signaling Technology) using Envision Flex+ horseradish peroxidase and diaminobenzidine as the chromogen (eTable 1 in Supplement 2). Dako Flex+ Rabbit Linker (Agilent) was used with pSTAT1 and pSTAT3. Appropriate negative (ie, no primary antibody) and positive (ie, tonsil and spleen) controls were stained in parallel with each set of slides studied. Immunostained slides were scanned at 20× magnification on a Vectra Polaris (PerkinElmer). Scoring was performed using the Positive Cell Detection analysis in QuPATH software version 0.3.2 (University of Edinburgh) to quantitate the percentage and the H score of positive inflammatory cells at baseline and 3 months after apremilast.
Statistical Analysis
Descriptive statistics were used to analyze demographics, clinical variables, and ORR. The mean (SD) was calculated for continuous variables. Proportional analysis was used to describe categorical variables. Paired student t test and analysis of variance were used to assess the mean change in CDASI, MMT-8, and DLQI scores and compare the positive cell detection on immunohistochemistry for CCL5, pSTAT1, and pSTAT3 at baseline and 3 months after apremilast. P values were 2-sided, and P values < .05 were considered statistically significant. Analyses were performed using GraphPad Prism version 8 (GraphPad Software). The last observation carried forward approach was used for missing values. Data were analyzed from June 2018 to June 2021.
Results
Demographics
Among 8 patients with recalcitrant DM who were enrolled (7 women and 1 man; mean [SD; range] age, 54 [15.9; 18-72] years; 8 White individuals; 0 Hispanic individuals) (Table), 4 patients had classic DM, 3 patients had amyopathic DM, and 1 patient had juvenile DM. The mean (SD; range) disease duration at the screening visit was 5.5 (3.0; 1-12) years. All study patients were resistant to multiple lines of therapy, including systemic steroids, methotrexate, mycophenolate mofetil, azathioprine, hydroxychloroquine, and rituximab. The median (range) number of prior therapies was 5 (4 to 6) therapies. No patients had active muscle disease at enrollment. One patient had an underlying malignant neoplasm at the time of DM diagnosis and was in remission for more than 4 years at the time of enrollment. Of 8 patients, 6 individuals completed the study, 1 patient discontinued the study due to disease progression, and another patient withdrew due to adverse events. Overall, 2 patients were able to taper their steroid use and 1 patient was able to taper use of a steroid-sparing agent, but the use of apremilast as monotherapy was not achieved.
Table. Patient Demographic Characteristics.
| Patient number | Sex | Age range, y | Race | Onset of DM, y | Type of DM | History of malignancy | Previous treatments | Concurrent treatments with apremilast |
|---|---|---|---|---|---|---|---|---|
| 1 | Woman | 60-69 | White | 2013 | Classic | No | Azathioprine, MMF, MTX, rituximab | Hydroxychloroquine, IVIG, prednisone 15 mg daily |
| 2 | Man | 50-59 | White | 2011 | Amyopathic | No | Azathioprine, hydroxychloroquine, MTX, prednisone | MMF |
| 3 | Woman | 60-69 | White | 2012 | Classic | Yes, for ovarian and anal cancer | Hydroxychloroquine, IVIG, MTX, prednisone | Hydroxychloroquine, IVIG, MTX, prednisone 3mg every other day |
| 4 | Woman | 70-79 | White | 2006 | Amyopathic | No | Azathioprine, MMF, MTX, prednisone | Hydroxychloroquine |
| 5 | Woman | 60-69 | White | 2012 | Classic | No | Azathioprine, MTX | Hydroxychloroquine, MMF, prednisone 5 mg daily |
| 6 | Woman | 18-19 | White | 2017 | Juvenile | No | Hydroxychloroquine, MMF, MTX, prednisone | Hydroxychloroquine, MMF, MTX |
| 7 | Woman | 50-59 | White | 2015 | Classic | No | MTX, Prednisone, rituximab | Hydroxychloroquine, MMF |
| 8 | Woman | 40-49 | White | 2014 | Amyopathic | No | MMF, Prednisone | Hydroxychloroquine, MTX |
Abbreviations: DM, dermatomyositis; IVIG, intravenous immunoglobulin; MMF, mycophenolate mofetil; MTX, methotrexate.
Efficacy
Cutaneous Disease Activity Severity Index
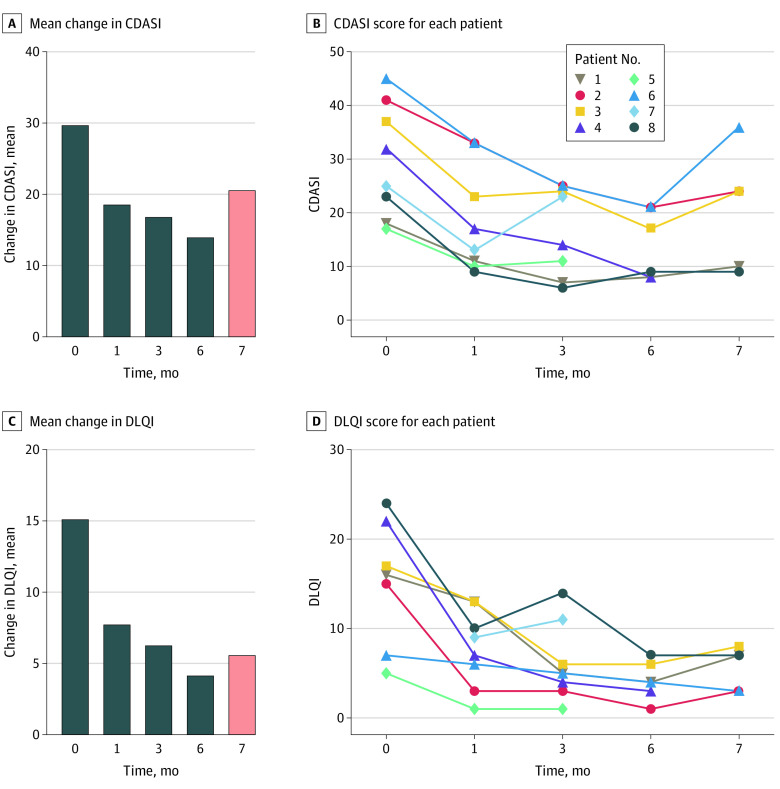
Among all patients, 7 individuals (ORR, 87.5%) achieved the primary outcome of a decrease in CDASI score of at least 4 points at 3 months after apremilast (Figure 1A-D; eFigure 2 in Supplement 2). One patient had an initial favorable response at 1 month but presented with disease progression at 3 months and withdrew from the study. All patients had a baseline CDASI score of moderate to severe, defined as more than 14.19 The mean (SD) baseline CDASI score of 29.8 (10.6) decreased to 16.9 (8.3) at 3 months (P < .001) and 14 (6.4) at the end of 6 months of treatment (P < .001) (Figure 2A-B). One month after apremilast discontinuation, the mean (SD) CDASI score increased to 20.6 (11.3). The mean (SD) decrease in CDASI score at 3 months was 12.9 (6.3) points, a statistically significant 56.7% change from baseline (P < .001). A decrease in CDASI score was noted at 1 month after initiation of apremilast, from a mean (SD) of 22.5 (5.9) to 9.5 (3.2). At 3 months after apremilast, 4 patients (50.0%) had an improvement in their disease score from moderate-severe to a mild disease, with a CDASI score of 14 or less.
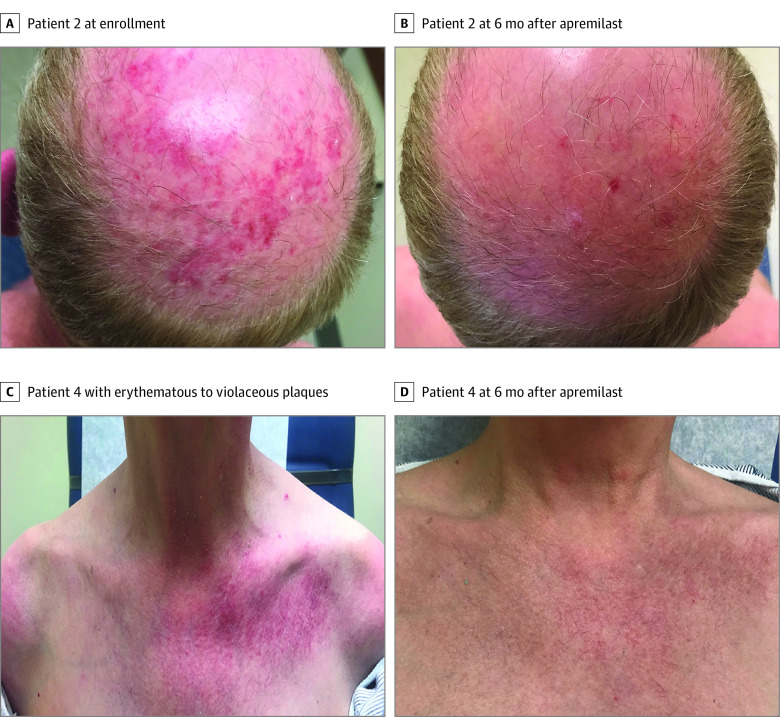
Figure 1. Clinical Photographs.
A, Patient 2, with erythematous plaques with white scale on the scalp at enrollment. B, Patient 2, with resolution of active lesions 6 months after apremilast. C, Patient 4, with erythematous to violaceous plaques with white scale in a characteristic V neck distribution on the chest. D, Patient 4, with resolution of active lesions 6 months after apremilast with remaining faint telangiectasia.
Figure 2. Disease Severity and Quality of Life After Treatment With Apremilast.
A, Paired analysis of the mean change in cutaneous disease activity severity index (CDASI) score at baseline and 1 (P < .001), 3 (P = .002), 6 (P = .004), and 7 (P = .01) months after apremilast. Pink bar at 7 months indicates a relapse with increase in numbers after cessation of therapy. B, Graph showing CDASI score for each patient at baseline and 1, 3, 6, and 7 months after apremilast. C, Paired analysis of the mean change in dermatology life quality index (DLQI) score at baseline and 1 (P = .04), 3 (P = .02), 6 (P = .02), and 7 (P = .02) months after apremilast. Pink bar at 7 months indicates a relapse with increase in numbers after cessation of therapy. D, Graph showing DLQI score for each patient at baseline and 1, 3, 6, and 7 months after apremilast.
Manual Muscle Testing in 8 Muscles
At enrollment, there was no active muscle disease, with a baseline mean (SD) MMT-8 score of 144.5 (6.9). There was no significant change in mean (SD) MMT-8 score at 3 months (143.3 [10.9]) or 6 months (144.5 [8.0]).
Dermatology Life Quality Index
The mean (SD) DLQI at enrollment was 15.1 (7.1), which is considered to be associated with a large change in patient quality of life (defined as score of 11-20).24,25 There was a statistically significant change in mean (SD) DLQI, with a decrease to 6.3 (4.6) at 3 months and 4.2 (2.1) at 6 months (Figure 2C-D). Scores of 2 to 5 and 6 to 10 are considered to be associated with small and moderate changes in quality of life, respectively.25
PHQ-2 and Laboratory Studies
No active depression was observed using the PHQ-2 questionnaire screening tool. No significant abnormalities in tests, including complete blood count, comprehensive metabolic profile, creatine kinase, and aldolase evaluations, were identified during the study.
Safety
Apremilast was well tolerated, without any grade 3 or higher adverse events (eTable 2 in Supplement 2). The most frequent adverse effects included transient headache, nausea, and diarrhea as previously described with apremilast.4,26 Less frequent side effects included acute sinusitis, pneumonia, herpes zoster infection, influenza, hypertension, and high ocular pressure. One patient with grade 2 diarrhea declined symptomatic therapy and withdrew from the study at 4 months.
Association With Inhibition of Inflammatory Signaling Pathways and Disruption of Biological Networks In Vivo
Apremilast inhibits PDE-4–dependent signaling and induces remission in psoriasis.4 However, the biological factors associated with its efficacy in DM remain unknown. To investigate intracellular signaling pathways associated with apremilast, we compared global changes in gene expression by RNA sequencing of skin samples collected from 7 patients before and at 3 months after apremilast initiation. Of 39 076 expressed genes, there were 195 genes whose expression changed 2-fold or more at P < .01 (123 downregulated and 72 upregulated genes).
To investigate biologic factors associated with the differences in gene expression, we chose GSEA as an unsupervised approach. GSEA identified 13 pathways in which apremilast was associated with downregulation at an FDR of 0.01 or less and NES of 1.70 or more. Pathways with the greatest downregulation included STAT1, STAT3, IL-4, IL-6, IL-12, IL-23, interferon α (IFNα), IFNγ, and tumor necrosis factor α (TNFα) (Figure 3) signal through Janus kinase (JAK)/STAT pathways (Figure 3A-C).
Figure 3. Association of Apremilast With Inflammatory Pathways Involved in Dermatomyositis.
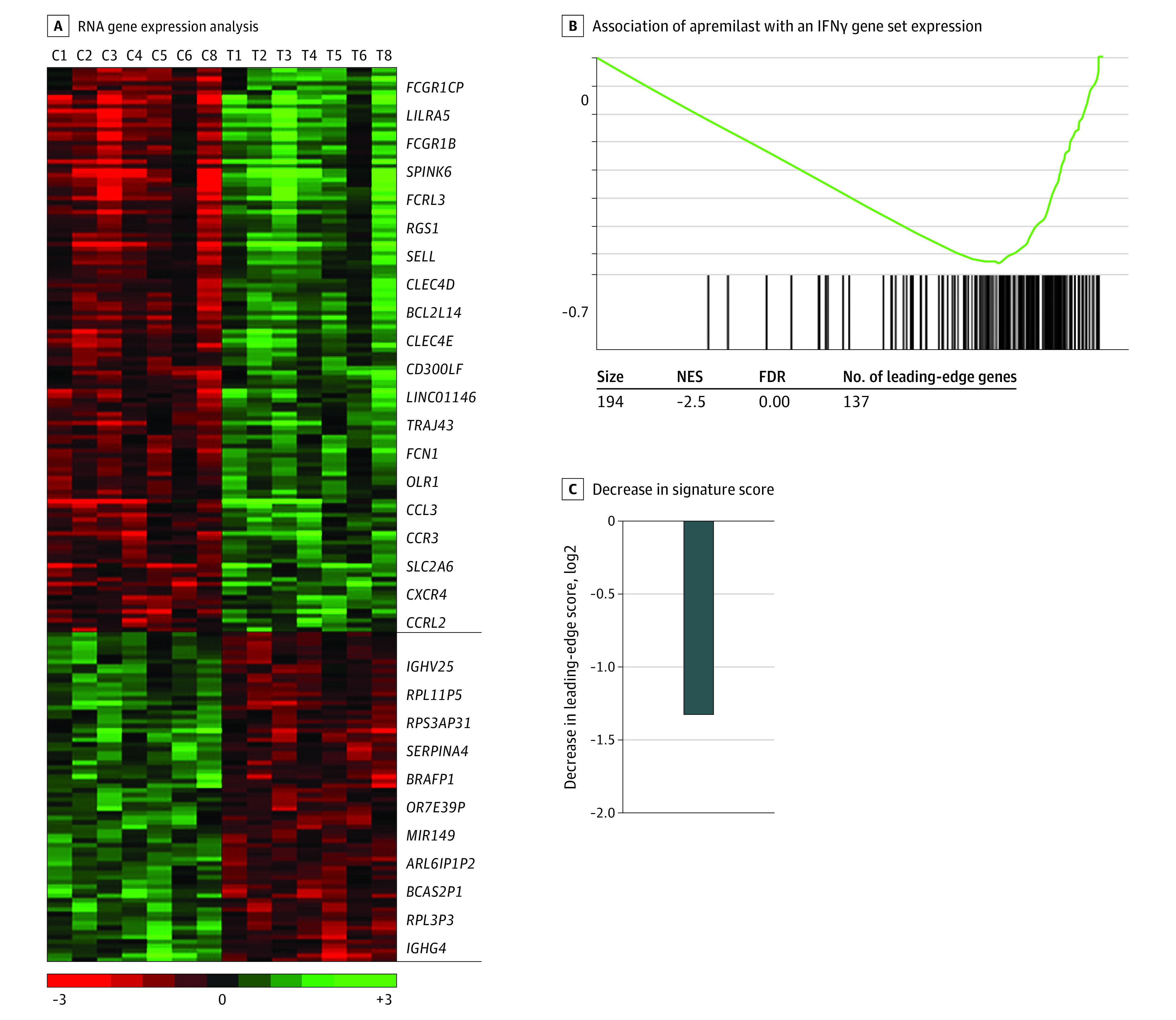
A, RNA gene expression analysis of skin biopsies at baseline and 3 months after apremilast performed by RNA sequencing. The heat map represents 195 genes whose expression changed 2-fold or more at P < .01 (123 downregulated and 72 upregulated genes). Gene expression is median centered and scaled as indicated. Each column represents a sample, and each row represents a gene. Gene symbols highlight select genes. C indicates control; horizontal black lines, ends of downregulated and upregulated gene examples; T, treatment. B, A representative enrichment plot showing the association of apremilast with an inhibitory outcome in an interferon γ (IFNγ) gene set identified by gene set enrichment analysis. NES indicates normalized enrichment score; FDR, false discovery rate. C, Decrease in the signature score of this IFNγ gene set is shown and was computed as the mean of the messenger RNA expression level of the leading-edge genes of apremilast-treated samples minus corresponding control. Leading-edge genes represent genes of this IFNγ gene set with the largest differential expression in the experimental data, as determined by gene set enrichment analysis. P ≤ .05 in the set criteria in the software to analyze genes with significant change.
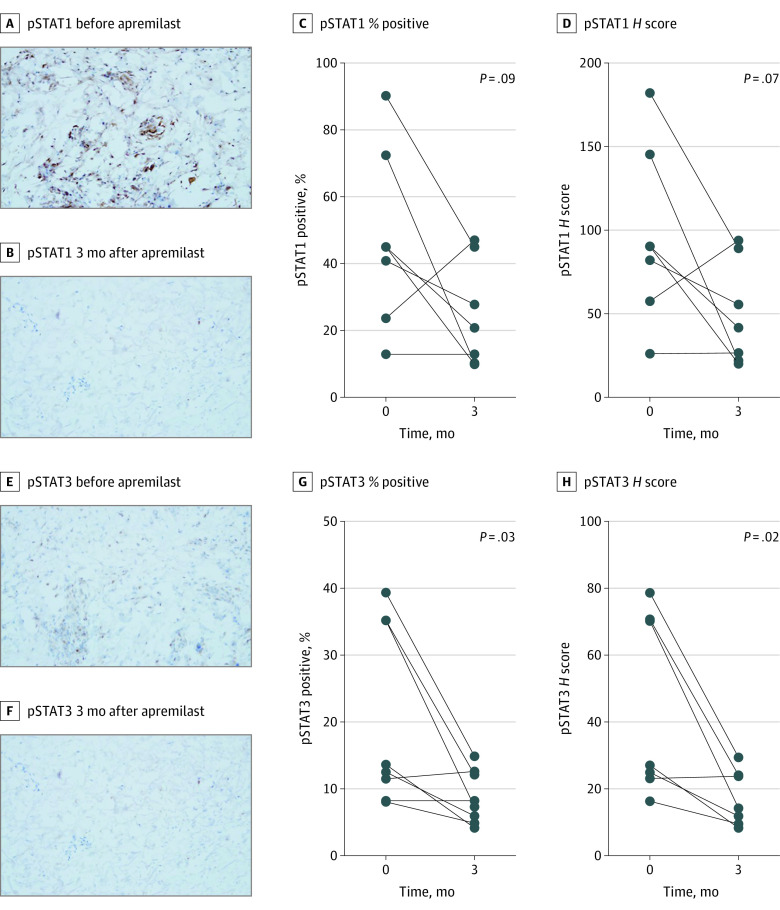
To evaluate the association of apremilast with downstream JAK/STAT signaling, we evaluated changes in levels of activated (phosphorylated) STAT1 and STAT3 before and after therapy using immunohistochemistry. In addition, we evaluated changes in levels of CCL5, a known PDE-4 target and an inflammatory marker in DM.27,28 After therapy, phosphorylation of STAT1 decreased by a mean (SD) of 22.3% (28.3%) positive cells (P = .09), with a mean (SD) H score decrease of 46.1 (54.9) (P = .07) (Figure 4A-D). Similarly, pSTAT3 levels decreased by a mean (SD) of 13.4% (11.6%) positive cells (P = .03), with a mean (SD) H score decrease of 26.9 (22.9) (P = .02) (Figure 4E-H). In contrast, apremilast was not associated with a change in CCL5 levels (eFigure 3 in Supplement 2). Collectively, apremilast was associated with inhibited PDE4 levels, which are associated with increased levels of intracellular cAMP and downregulation of various inflammatory cytokines, including IL12, 23, TNF α, and interferons. The previously listed inflammatory cytokines signal through JAK/STAT in DM with minimal outcomes in CCL5.
Figure 4. Association of Apremilast With Protein Phosphorylation on Immunohistochemistry.
A-B, Decrease in phosphorylated signal transducers and activators of transcription 1 (pSTAT1) immunostaining on a frozen section of patient 2 skin biopsies before and 3 months after apremilast (original magnification ×200). C-D, Paired analysis change in pSTAT1 percent positive cells and H score at baseline and 3 months after apremilast. E-F, Decrease in pSTAT3 immunostaining on frozen section from patient 2 skin biopsies before and 3 months after apremilast (original magnification ×200). G-H, Paired analysis change in pSTAT3 percent positive cells and H score at baseline and 3 months after apremilast.
Discussion
In this phase 2a investigator-initiated nonrandomized controlled trial that included 8 patients with recalcitrant cutaneous DM, we found an ORR of 87.5% at 3 months after treatment with apremilast. Enrolled patients had long-standing DM with a mean disease duration of 5.5 years. All study patients had a baseline CDASI score of moderate to severe as defined by a CDASI score greater than 14 on steroid and steroid-sparing agents. Significant clinical response was noted as soon as 1 month after initiation of apremilast. The mean change in CDASI score was 12.9, a 56.7% decrease from baseline. For patients with CDASI scores greater than 14, a 40% change in the CDASI score is associated with a meaningful improvement in quality of life.29 Patients with DM have a more severe impairment of their quality of life compared with individuals with other chronic skin conditions.30 In our study, there was a significant decrease in DLQI scores, associated with a large change to a small to moderate change in quality of life. Durability of the response was observed at 6 months, with further improvement of CDASI and DLQI scores. At 1 month after apremilast discontinuation, there was an increase in CDASI score, highlighting the need for a maintenance therapy with apremilast to sustain response. Apremilast was well tolerated, with mainly grade 1 or 2 adverse events. These results were consistent with our previous observation on the efficacy of apremilast as an add-on therapy in the treatment of recalcitrant DM.11 In our previous case series,11 2 patients were able to taper off steroids and nonsteroid agents over a year and continued apremilast as a monotherapy. In our nonrandomized controlled trial of 6 months duration, 2 patients were able to taper their steroid use and 1 patient was able to taper use of a steroid-sparing agent, but the use of apremilast as monotherapy was not achieved. This suggests that a subset of patients could potentially be taken off steroids or use a lower dose, thus minimizing long-term toxic effects associated with steroids. In our study, there was continued improvement of CDASI score at 1, 3, and 6 months. Whether apremilast has the cumulative benefit with longer duration of treatment that is required to achieve monotherapy needs to be determined with further studies.
The pathogenesis of DM is an interplay of environmental triggers and autoimmune reactions in individuals who are genetically susceptible.31,32 Studies from 2002 to 202033,34,35,36,37,38,39,40,41,42,43,44 revealed the role of cytokines and IFN in DM, including IFNγ and STAT pathways, TNF α, IL-4, IL-6, IL-17, IL-18, and IL-23. In addition, response to treatment and improvement in disease activity have been observed with downregulation of the aforementioned interferons and cytokines.45,46 Apremilast is a PDE-4 inhibitor approved for the treatment of plaque psoriasis, psoriatic arthritis, and oral ulcers associated with Behçet disease.4,5,6 However, its usage among patients with dermatomyositis has not been investigated in a controlled trial, to our knowledge. By inhibiting PDE-4, apremilast increases levels of cyclic adenosine monophosphate, leading to downregulation of proinflammatory cytokines, such as TNF α, IL-23, IFN α, IFN γ, and others, while upregulating anti-inflammatory cytokines, such as IL-10.4,47 In psoriasis, apremilast downregulated expression of many cytokines in the T helper (Th)1 pathway, including IL-8, IL-12, IL-17, IL-22, and IL-23.4 In addition, apremilast blocks Th2 response by interfering with the level of IL-6 secreted by type 2 macrophages.48 In our study, we identified 13 pathways in which apremilast was associated with downregulation, notably JAK/STAT, IL-4, IL-6, IL-12, IL-23, IFN α and γ, and TNF α signaling pathways. These downregulated pathways represent key signaling pathways known to be altered in DM.33,34,35,36,37,38,39,40,41,42,43 Autoimmune inflammatory diseases have shared inflammatory pathways, suggesting the possibility of drug repurposing.49 In a 2019 study50 on the role of apremilast in ulcerative colitis, apremilast was associated with decreased phosphorylation of STAT1, STAT3, STAT5, and STAT6 through an increase in suppression of cytokine signaling 3 expression in murine models. JAK/STAT signaling pathways are downstream pathways to many inflammatory cytokines involved in DM, notably IL4, IL12, IL23, IFN α, and IFNγ.51,52 Few studies have investigated the role of JAK/STAT inhibitors in the treatment of recalcitrant DM.17,37,53,54,55,56,57 We suggest that the role of apremilast in the treatment of DM may be associated with disruption of multiple inflammatory cytokines with downstream signaling through JAK/STAT pathways. Further evidence suggesting this hypothesis was found on the translational level, with a significant decrease in the phosphorylation of STAT3 on immunohistochemical analysis of skin biopsies from baseline and 3 months after apremilast. In addition, pSTAT1 levels decreased after therapy, although this change was not statistically significant. CCL5 has proinflammatory and antitumor properties, and its role in dermatomyositis was investigated in few studies.28,58 In our study, apremilast was not associated with CCL5 levels, suggesting the complexity of the inflammatory pathways involved in DM and the need for multiple targeted therapies.
Limitations
This study has several limitations. Our conclusions are limited by the small sample size, lack of a control group, and use of stable concomitant therapy. In addition, our patients were all White and predominantly women, which may limit the generalizability of our results.
Conclusions
The findings of this proof-of-concept nonrandomized controlled trial suggest that apremilast may be a safe and efficacious option in the treatment of recalcitrant DM. To our knowledge, this is the first study to investigate the role of apremilast in DM, suggesting its mechanism of action in recalcitrant DM. Larger clinical trials are needed to study apremilast in the treatment of naïve DM and as a monotherapy.
Trial Protocol
eTable 1. Immunohistochemical Staining Technique
eTable 2. Adverse Events After Apremilast
eFigure 1. Flow Chart
eFigure 2. Absolute Change in Cutaneous Disease Activity Severity Index Score by Patient at 3 mo Into Apremilast Therapy
eFigure 3. Chemokine (C-C Motif) Ligand 5 (CCL5) Level Before and 3 mo After
Apremilast
Data Sharing Statement
References
- 1.Callen JP. Dermatomyositis. Lancet. 2000;355(9197):53-57. doi: 10.1016/S0140-6736(99)05157-0 [DOI] [PubMed] [Google Scholar]
- 2.Wolstencroft PW, Chung L, Li S, Casciola-Rosen L, Fiorentino DF. Factors associated with clinical remission of skin disease in dermatomyositis. JAMA Dermatol. 2018;154(1):44-51. doi: 10.1001/jamadermatol.2017.3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldman R, DeWane ME, Lu J. Dermatomyositis: diagnosis and treatment. J Am Acad Dermatol. 2020;82(2):283-296. doi: 10.1016/j.jaad.2019.05.105 [DOI] [PubMed] [Google Scholar]
- 4.Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37-49. doi: 10.1016/j.jaad.2015.03.049 [DOI] [PubMed] [Google Scholar]
- 5.Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173(6):1387-1399. doi: 10.1111/bjd.14164 [DOI] [PubMed] [Google Scholar]
- 6.Hatemi G, Mahr A, Ishigatsubo Y, et al. Trial of apremilast for oral ulcers in Behçet’s syndrome. N Engl J Med. 2019;381(20):1918-1928. doi: 10.1056/NEJMoa1816594 [DOI] [PubMed] [Google Scholar]
- 7.Maloney NJ, Zhao J, Tegtmeyer K, Lee EY, Cheng K. Off-label studies on apremilast in dermatology: a review. J Dermatolog Treat. 2020;31(2):131-140. doi: 10.1080/09546634.2019.1589641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Souza A, Strober BE, Merola JF, Oliver S, Franks AG Jr. Apremilast for discoid lupus erythematosus: results of a phase 2, open-label, single-arm, pilot study. J Drugs Dermatol. 2012;11(10):1224-1226. [PubMed] [Google Scholar]
- 9.Nassim D, Alajmi A, Jfri A, Pehr K. Apremilast in dermatology: a review of literature. Dermatol Ther. 2020;33(6):e14261. doi: 10.1111/dth.14261 [DOI] [PubMed] [Google Scholar]
- 10.Sakkas LI, Mavropoulos A, Bogdanos DP. Phosphodiesterase 4 inhibitors in immune-mediated diseases: mode of action, clinical applications, current and future perspectives. Curr Med Chem. 2017;24(28):3054-3067. doi: 10.2174/0929867324666170530093902 [DOI] [PubMed] [Google Scholar]
- 11.Bitar C, Maghfour J, Ho-Pham H, Stumpf B, Boh E. Apremilast as a potential treatment for moderate to severe dermatomyositis: a retrospective study of 3 patients. JAAD Case Rep. 2019;5(2):191-194. doi: 10.1016/j.jdcr.2018.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlton D, Moghadam-Kia S, Smith K, Aggarwal R, English JC III, Oddis CV. Refractory cutaneous dermatomyositis with severe scalp pruritus responsive to apremilast. J Clin Rheumatol. 2019. doi: 10.1097/RHU.0000000000000999 [DOI] [PubMed] [Google Scholar]
- 13.Phillippi K, Hoeltzel M, Byun Robinson A, Kim S; Childhood Arthritis and Rheumatology Research Alliance (CARRA) Legacy Registry Investigators . Race, income, and disease outcomes in juvenile dermatomyositis. J Pediatr. 2017;184:38-44.e1. doi: 10.1016/j.jpeds.2017.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sontheimer RD. Dermatomyositis: an overview of recent progress with emphasis on dermatologic aspects. Dermatol Clin. 2002;20(3):387-408. doi: 10.1016/S0733-8635(02)00021-9 [DOI] [PubMed] [Google Scholar]
- 15.Oddis CV, Reed AM, Aggarwal R, et al. ; RIM Study Group . Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65(2):314-324. doi: 10.1002/art.37754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scarpone R, Meier K, Ghoreschi K, Worm M. Intravenous immunoglobulins in a series of 32 rare and recalcitrant immune dermatoses. Acta Derm Venereol. 2020;100(17):adv00298. doi: 10.2340/00015555-3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paik JJ, Casciola-Rosen L, Shin JY, et al. Study of tofacitinib in refractory dermatomyositis: an open-label pilot study of ten patients. Arthritis Rheumatol. 2021;73(5):858-865. doi: 10.1002/art.41602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed S, Chen KL, Werth VP. The validity and utility of the Cutaneous Disease Area and Severity Index (CDASI) as a clinical outcome instrument in dermatomyositis: a comprehensive review. Semin Arthritis Rheum. 2020;50(3):458-462. doi: 10.1016/j.semarthrit.2020.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anyanwu CO, Fiorentino DF, Chung L, et al. Validation of the Cutaneous Dermatomyositis Disease Area and Severity Index: characterizing disease severity and assessing responsiveness to clinical change. Br J Dermatol. 2015;173(4):969-974. doi: 10.1111/bjd.13915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saba NS, Liu D, Herman SE, et al. Pathogenic role of B-cell receptor signaling and canonical NF-κB activation in mantle cell lymphoma. Blood. 2016;128(1):82-92. doi: 10.1182/blood-2015-11-681460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15-21. doi: 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Scheffler K, Halpern AL, et al. Strelka2: fast and accurate calling of germline and somatic variants. Nat Methods. 2018;15(8):591-594. doi: 10.1038/s41592-018-0051-x [DOI] [PubMed] [Google Scholar]
- 23.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34(5):525-527. doi: 10.1038/nbt.3519 [DOI] [PubMed] [Google Scholar]
- 24.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210-216. doi: 10.1111/j.1365-2230.1994.tb01167.x [DOI] [PubMed] [Google Scholar]
- 25.Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: W\what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125(4):659-664. doi: 10.1111/j.0022-202X.2005.23621.x [DOI] [PubMed] [Google Scholar]
- 26.Lee EB, Amin M, Egeberg A, Wu JJ. Adverse events associated with apremilast use and withdrawal for psoriasis in a real-world setting. J Eur Acad Dermatol Venereol. 2018;32(10):e393-e394. doi: 10.1111/jdv.15061 [DOI] [PubMed] [Google Scholar]
- 27.Salvator H, Buenestado A, Brollo M, et al. Clinical relevance of the anti-inflammatory effects of roflumilast on human bronchus: potentiation by a long-acting beta-2-agonist. Front Pharmacol. 2020;11:598702. doi: 10.3389/fphar.2020.598702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Civatte M, Bartoli C, Schleinitz N, Chetaille B, Pellissier JF, Figarella-Branger D. Expression of the beta chemokines CCL3, CCL4, CCL5 and their receptors in idiopathic inflammatory myopathies. Neuropathol Appl Neurobiol. 2005;31(1):70-79. doi: 10.1111/j.1365-2990.2004.00591.x [DOI] [PubMed] [Google Scholar]
- 29.Ahmed S, Chakka S, Concha J, Krain R, Feng R, Werth VP. Evaluating important change in cutaneous disease activity as an efficacy measure for clinical trials in dermatomyositis. Br J Dermatol. 2020;182(4):949-954. doi: 10.1111/bjd.18223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hundley JL, Carroll CL, Lang W, et al. Cutaneous symptoms of dermatomyositis significantly impact patients’ quality of life. J Am Acad Dermatol. 2006;54(2):217-220. doi: 10.1016/j.jaad.2004.12.015 [DOI] [PubMed] [Google Scholar]
- 31.Bogdanov I, Kazandjieva J, Darlenski R, Tsankov N. Dermatomyositis: current concepts. Clin Dermatol. 2018;36(4):450-458. doi: 10.1016/j.clindermatol.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 32.Thompson C, Piguet V, Choy E. The pathogenesis of dermatomyositis. Br J Dermatol. 2018;179(6):1256-1262. doi: 10.1111/bjd.15607 [DOI] [PubMed] [Google Scholar]
- 33.Greenberg SA, Sanoudou D, Haslett JN, et al. Molecular profiles of inflammatory myopathies. Neurology. 2002;59(8):1170-1182. doi: 10.1212/WNL.59.8.1170 [DOI] [PubMed] [Google Scholar]
- 34.Greenberg SA, Pinkus JL, Pinkus GS, et al. Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol. 2005;57(5):664-678. doi: 10.1002/ana.20464 [DOI] [PubMed] [Google Scholar]
- 35.Walsh RJ, Kong SW, Yao Y, et al. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum. 2007;56(11):3784-3792. doi: 10.1002/art.22928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rigolet M, Hou C, Baba Am Y, et al. Distinct interferon signatures stratify inflammatory and dysimmune myopathies. RMD Open. 2019;5(1):e000811. doi: 10.1136/rmdopen-2018-000811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ladislau L, Suárez-Calvet X, Toquet S, et al. JAK inhibitor improves type I interferon induced damage: proof of concept in dermatomyositis. Brain. 2018;141(6):1609-1621. doi: 10.1093/brain/awy105 [DOI] [PubMed] [Google Scholar]
- 38.Moneta GM, Pires Marafon D, Marasco E, et al. Muscle Expression of type I and type II interferons is increased in juvenile dermatomyositis and related to clinical and histologic features. Arthritis Rheumatol. 2019;71(6):1011-1021. doi: 10.1002/art.40800 [DOI] [PubMed] [Google Scholar]
- 39.Pinal-Fernandez I, Casal-Dominguez M, Derfoul A, et al. Identification of distinctive interferon gene signatures in different types of myositis. Neurology. 2019;93(12):e1193-e1204. doi: 10.1212/WNL.0000000000008128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim H, Gunter-Rahman F, McGrath JA, et al. Expression of interferon-regulated genes in juvenile dermatomyositis versus mendelian autoinflammatory interferonopathies. Arthritis Res Ther. 2020;22(1):69. doi: 10.1186/s13075-020-02160-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou J, Chen J, Yan Q, Guo Q, Bao C. Serum IL8 and mRNA level of CD11b in circulating neutrophils are increased in clinically amyopathic dermatomyositis with active interstitial lung disease. Clin Rheumatol. 2016;35(1):117-125. doi: 10.1007/s10067-015-3080-1 [DOI] [PubMed] [Google Scholar]
- 42.Umezawa N, Kawahata K, Mizoguchi F, et al. Interleukin-23 as a therapeutic target for inflammatory myopathy. Sci Rep. 2018;8(1):5498. doi: 10.1038/s41598-018-23539-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bilgic H, Ytterberg SR, Amin S, et al. Interleukin-6 and type I interferon-regulated genes and chemokines mark disease activity in dermatomyositis. Arthritis Rheum. 2009;60(11):3436-3446. doi: 10.1002/art.24936 [DOI] [PubMed] [Google Scholar]
- 44.Giriş M, Durmuş H, Yetimler B, Taşli H, Parman Y, Tüzün E. Elevated IL-4 and IFN-γ levels in muscle tissue of patients with dermatomyositis. In Vivo. 2017;31(4):657-660. doi: 10.21873/invivo.11108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reed AM, Peterson E, Bilgic H, et al. Changes in novel biomarkers of disease activity in juvenile and adult dermatomyositis are sensitive biomarkers of disease course. Arthritis Rheum. 2012;64(12):4078-4086. doi: 10.1002/art.34659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin Y, Li F, Shi J, Li S, Cai J, Jiang Y. MiR-146a regulates inflammatory infiltration by macrophages in polymyositis/dermatomyositis by targeting TRAF6 and affecting IL-17/ICAM-1 pathway. Cell Physiol Biochem. 2016;40(3-4):486-498. doi: 10.1159/000452563 [DOI] [PubMed] [Google Scholar]
- 47.Schafer PH, Parton A, Capone L, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26(9):2016-2029. doi: 10.1016/j.cellsig.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 48.Maier C, Ramming A, Bergmann C, et al. Inhibition of phosphodiesterase 4 (PDE4) reduces dermal fibrosis by interfering with the release of interleukin-6 from M2 macrophages. Ann Rheum Dis. 2017;76(6):1133-1141. doi: 10.1136/annrheumdis-2016-210189 [DOI] [PubMed] [Google Scholar]
- 49.Petitdemange A, Blaess J, Sibilia J, Felten R, Arnaud L. Shared development of targeted therapies among autoimmune and inflammatory diseases: a systematic repurposing analysis. Ther Adv Musculoskelet Dis. 2020;12:X20969261. doi: 10.1177/1759720X20969261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, Fan C, Feng C, et al. Inhibition of phosphodiesterase-4 attenuates murine ulcerative colitis through interference with mucosal immunity. Br J Pharmacol. 2019;176(13):2209-2226. doi: 10.1111/bph.14667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villarino AV, Kanno Y, Ferdinand JR, O’Shea JJ. Mechanisms of Jak/STAT signaling in immunity and disease. J Immunol. 2015;194(1):21-27. doi: 10.4049/jimmunol.1401867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salas A, Hernandez-Rocha C, Duijvestein M, et al. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(6):323-337. doi: 10.1038/s41575-020-0273-0 [DOI] [PubMed] [Google Scholar]
- 53.Williams P, McKinney B. Refractory dermatomyositis-systemic lupus erythematosus overlap syndrome and response to tofacitinib. Proc (Bayl Univ Med Cent). 2020;34(1):116-117. doi: 10.1080/08998280.2020.1821589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Navarro-Navarro I, Jiménez-Gallo D, Rodríguez-Mateos ME, Rodríguez-Hernández C, Linares-Barrios M. Treatment of refractory anti-NXP2 and anti-TIF1γ dermatomyositis with tofacitinib. J Dtsch Dermatol Ges. 2021;19(3):443-447. doi: 10.1111/ddg.14276 [DOI] [PubMed] [Google Scholar]
- 55.Moghadam-Kia S, Charlton D, Aggarwal R, Oddis CV. Management of refractory cutaneous dermatomyositis: potential role of Janus kinase inhibition with tofacitinib. Rheumatology (Oxford). 2019;58(6):1011-1015. doi: 10.1093/rheumatology/key366 [DOI] [PubMed] [Google Scholar]
- 56.Yu Z, Wang L, Quan M, Zhang T, Song H. Successful management with Janus kinase inhibitor tofacitinib in refractory juvenile dermatomyositis: a pilot study and literature review. Rheumatology (Oxford). 2021;60(4):1700-1707. doi: 10.1093/rheumatology/keaa558 [DOI] [PubMed] [Google Scholar]
- 57.Kurtzman DJ, Wright NA, Lin J, et al. Tofacitinib citrate for refractory cutaneous dermatomyositis: an alternative treatment. JAMA Dermatol. 2016;152(8):944-945. doi: 10.1001/jamadermatol.2016.0866 [DOI] [PubMed] [Google Scholar]
- 58.Preusse C, Eede P, Heinzeling L, et al. NanoString technology distinguishes anti-TIF-1γ+ from anti-Mi-2+ dermatomyositis patients. Brain Pathol. 2021;31(3):e12957. doi: 10.1111/bpa.12957 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Immunohistochemical Staining Technique
eTable 2. Adverse Events After Apremilast
eFigure 1. Flow Chart
eFigure 2. Absolute Change in Cutaneous Disease Activity Severity Index Score by Patient at 3 mo Into Apremilast Therapy
eFigure 3. Chemokine (C-C Motif) Ligand 5 (CCL5) Level Before and 3 mo After
Apremilast
Data Sharing Statement