ABSTRACT
Background
African American (AA) adults have about twice the risk of developing dementia compared with white adults. However, evidence on dietary modification in preventing cognitive decline from diverse populations focusing on AA adults is minimal.
Objectives
We aimed to evaluate the association between a plant-based diet and the rate of cognitive decline in a population-based sample of AA and white adults.
Methods
This study consisted of 3337 participants from the Chicago Health and Aging Project (60% AA participants, 64% female). Plant-based diet quality was evaluated by the overall plant-based diet index (PDI), the healthful PDI (hPDI), and the unhealthful PDI (uPDI). Global cognition was assessed using a composite score of 4 individual tests of cognition. We used mixed models to examine the associations of PDI, hPDI, and uPDI with the rates of decline in global cognition, perceptual speed, and episodic memory. Models were adjusted for age, sex, presence of apoE e4 allele, lifestyle factors including education, cognitive activities, smoking status, calorie intake, risk factors for cardiovascular disease, time, and the interaction terms of time × each covariate.
Results
AA and white participants had various dietary patterns. Higher hPDI was associated with a slower rate of decline in global cognition, perceptual speed, and episodic memory in AA participants but not white participants. AA study participants in the highest quintile of hPDI had significantly slower rates of global cognitive decline (β: 0.0183 ± 0.0086; P = 0.032), perceptual speed (β: 0.0179 ± 0.0088; P = 0.04), and episodic memory (β: 0.0163 ± 0.0118; P = 0.04) than individuals in the lowest quintile of hPDI. There were no associations of either PDI or uPDI with the rate of cognitive decline in either racial group.
Conclusions
A healthy plant-based diet was associated with a slower rate of decline in global cognition, perceptual speed, and episodic memory in AA adults.
Keywords: diet, plant-based diet, dietary pattern, cognition, cognitive decline, episodic memory, perceptual speed, biracial, longitudinal cohort, African Americans
Introduction
By 2050, 83.7 million people in the United States are projected to be 65 y old and older (1). The concurrent increase in the prevalence of age-related cognitive decline paralleled with an increase in Alzheimer dementia (AD) poses a significant burden on health care and social-economic and public health. African American (AA) adults have a disproportionally higher risk of developing AD and other dementia (2). Because persons from minoritized populations are predicted to comprise a more significant segment of the older adult population in the coming decades, understanding the underlying factors affecting cognitive risk is critical, especially at the population level (3).
The limited therapeutic options for AD make prevention through modifiable lifestyle factors, including dietary factors, a high public health priority. Cumulative evidence has reported that healthy dietary patterns, including the Mediterranean diet (MedDiet), Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet (4), and specific foods such as berries (5), vegetables (6, 7), and fish (8), are associated with slower cognitive decline. Current evidence on diet, dietary pattern, and cognitive function is built predominantly from Caucasians (9, 10); AA adults are underrepresented in this work with few characterizations of how their dietary patterns relate with cognitive performance in older adulthood (9, 10). Dietary quality and patterns vary by race and ethnicity (11), socioeconomic status (12), and other social factors (13). Racial disparities in dementia might be attributable to dietary intakes and nutrients closely linked to cognitive health. However, there is a significant knowledge gap regarding how diet influences cognitive function in individuals of different races (14).
Plant-based diets are recommended for their benefits in preventing cardiometabolic diseases, including type 2 diabetes (15), obesity (16, 17), and coronary artery disease (18), and lowering mortality risk (19). The shared common pathophysiologic mechanisms (i.e., oxidative stress, inflammation, and vascular damage) between cardiometabolic diseases and neurodegenerative diseases like AD suggest that a plant-based diet might ameliorate age-related cognitive decline, the hallmark feature of AD. Therefore, in the present study, we aimed to first characterize dietary patterns among AA and white participants of a biracial population-based study, and then investigate the associations of different qualities of plant-based diets using 3 plant-based diet indices with cognitive decline.
Methods
Study population
The Chicago Health and Aging Project (CHAP) is a longitudinal, biracial, population-based study that began in 1993 with a census of individuals aged 65 y or older in neighborhoods on the south side of Chicago, IL (20). The baseline in-home interview was conducted from 1993 to 1997 and repeated every 3 y with ≤6 data collection cycles. From October 1993 to April 1997, of those identified, 6158 (79%) participated in an in-home interview. In the following cycles, newly age-eligible participants (65 y or older) were recruited and received an in-home interview. The interviews included demographic variables, health outcomes, current functioning, and physical and cognitive performance tests. In total, the CHAP study enrolled 10,802 participants with 62% AA adults and 38% white adults, and achieved a follow-up assessment of ∼89% of surviving participants. In the present study, 1228 participants had a date of death before returning FFQs, and 2563 did not return an FFQ. We included participants who responded to a dietary questionnaire. Of the 7011 individuals with dietary data, 5050 participants had ≥2 cognitive assessments. We excluded participants with extreme BMI (in kg/m2; <14 or >55) and implausible caloric intakes (<500 kcal or >3800 kcal for women, <800 kcal or >4200 kcal for men), or who left an entire page or >50% of items unanswered (21). Because apoE genotype has significant influence on cognitive function, a total of 3337 participants with apoE genotype measurements were included in this study (Figure 1). The Rush University Medical Center Institutional Review Board approved the study.
FIGURE 1.
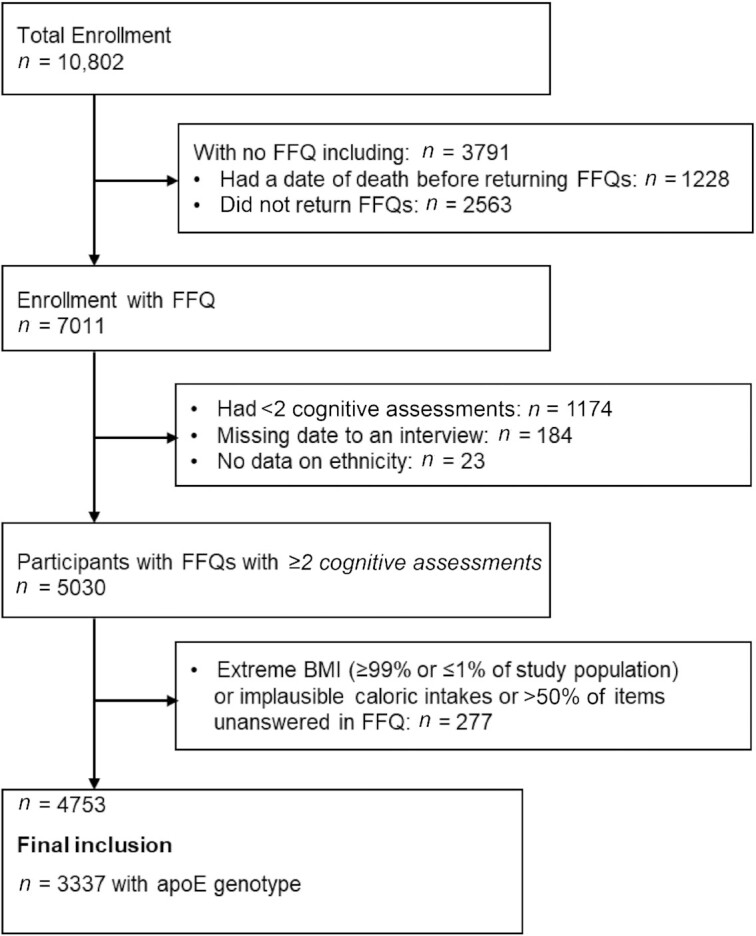
Flow diagram of participant selection.
Cognitive function assessment
Four performance measures of cognitive function were conducted during in-home interviews at each cycle. We conducted 2 measures of episodic memory: immediate and delayed recall of 12 ideas contained in the East Boston Story (0–12 points) (22, 23). We conducted 1 measure of perceptual speed: the oral form of the Symbol Digit Modalities Test, in which persons match as many digit–symbol pairs as possible in 90 s (24–27). The fourth test was the Mini-Mental State Examination, a widely used 30-item measure of global cognition (28).
For each test, we transformed the raw scores to z-scores using the baseline raw scores as the SD for each of the tests. We then created the global cognitive score by averaging the z-scores from all 4 tests (composite z-score) (29). With this approach, the cognitive function is scaled in standard units with positive scores indicating higher performance (30). As regards individual cognitive functions, the episodic memory score was the average of the z-scores from the 2 components of the East Boston Test; the perceptual speed score was the z-score from the Symbol Digit Modalities Test.
Dietary assessment
Diet was assessed using a 144-item semiquantitative FFQ modified from the Harvard FFQ (31). The FFQ was collected at each cycle. Participants were asked how often, on average, they consumed specific foods or beverages with prespecified portion sizes during the preceding year (31). The modified FFQ was validated for use in older Chicago residents (31). Nutrient intake was calculated based on the USDA and Harvard University food composition databases, updated over time to include new food items and to reflect changes in food composition. Frequency of consumption of foods was multiplied by either the natural portion size (i.e., 1 apple) or serving sizes based on sex-specific mean portion sizes derived from a national survey for older adults (31). A trained research assistant conducted screening on the quality of the FFQs. Those with >50% of pages left unanswered were documented.
Calculation of plant-based diet indices
We calculated 3 plant-based diet indices: the overall plant-based diet index (PDI), a healthful plant-based diet index (hPDI), and an unhealthful plant-based diet index (uPDI) (18). First, 18 food groups were defined based on nutrient and culinary similarities. The frequencies of food consumption were converted into servings consumed per day. Then we summed the number of servings of foods that belonged to each of the 18 food groups. We then categorized these food groups into animal foods and healthy and less healthy plant foods. The healthy plant food groups included whole grains, fruits, vegetables, nuts, legumes, vegetable oils, tea, and coffee. In contrast, the less healthy plant food groups included fruit juices, refined grains, potatoes, sugar-sweetened beverages, and sweets and desserts. The animal food groups included animal fat, egg, fish or seafood, meat (red meat, poultry meat), and various animal-based foods (i.e., hamburger, meat taco fillings, hot dogs or sausages, etc.).
First, the food groups were divided into quintiles of consumption and assigned a score from 1 to 5. For the PDI, participants received a score of 5 if their consumption was within the highest quintile for each plant food group (healthy or less healthy), a score of 4 if their consumption was within the second-highest quintile of each plant food group, and so on, with a score of 1 for consumption within the lowest quintile. In contrast, a reverse score was given for the animal food groups. Participants received a score of 1 when their consumption was within the highest quintile of an animal food group, a score of 2 when their consumption was within the second-highest quintile, and so on. For the hPDI, positive scores were given only to the healthy plant food groups. Reverse scores were given to the less healthy plant and animal food groups. For the uPDI, positive scores were given only to less healthy plant food groups, whereas both healthy plant and animal food groups received reverse scores. For each individual, scores from the 18 food groups were summed to obtain the indices. The range of each index was from 18 (the lowest possible score) to 90 (the highest possible score).
Assessment of covariates
Social and demographic characteristics including age (y), sex (male/female), education (number of years in school), and smoking status (never smoker compared with current or former smoker) were collected during participants’ baseline in-home interview using the 1990 US Census questionnaire (29, 32). Race and ethnicity were self-identified at baseline based on the categories of race and ethnicity from the 1990 US Census questionnaire (32). Cognitive activities were assessed by the type and time of participation in cognitive activities (i.e., viewing television; listening to the radio; reading newspapers; reading magazines; reading books; playing games such as cards, checkers, crosswords, or other puzzles; and going to museums). The mean frequency of each activity was converted to a composite score (29). Weight (kg) and height (m) were measured during the in-home interview and used to compute BMI. History of diabetes, hypertension, heart disease (self-reported history of myocardial infarction or digitalis use), stroke, and medication use were self-reported (20). The moderate correlations between PDI, hPDI, uPDI, the MedDiet score (33), and the MIND diet score (4) in our study (range: −0.51 to 0.49, data not shown) suggested that these 3 plant-based diet indices captured additional components from diets.
The MedDiet score was based on 11 food groups including nonrefined cereals, potatoes, fruit and vegetables, legumes, nuts and beans, fish, olive oil, red meat and meat products, poultry, full-fat cheese and other dairy, and alcohol, as previously described (34). Consumption of items from these food groups was assigned a score of 5 for at least daily consumption, scores <5 were assigned to lesser consumption proportionally. For items reported as being rarely consumed, a score of 0 was assigned. The scoring of MedDiet for the CHAP population has been published previously (33). The MedDiet score ranges from 0 to 55, where higher scores correspond to greater adherence to the MedDiet. The MIND diet score has 15 dietary components including 10 food groups (green leafy vegetables, other vegetables, nuts, berries, beans, whole grains, fish, poultry, olive oil, and wine) related to brain health and 5 unhealthy food groups (red meats, butter and stick margarine, cheese, pastries and sweets, and fried/fast food) (35). For all other diet score components except olive oil, a score of 0, 0.5, or 1 was assigned to the frequency of consumption of 1 portion of each food item. For olive oil, if participants identified olive oil as the primary oil usually used at home, they received a score of 1 and otherwise 0. The total MIND diet score was computed by summing all 15 of the component scores (35).
Statistics
The primary outcome variable was annual rate of decline in global cognition. The secondary outcome variables were rates of decline in perceptual speed and episodic memory. Data were presented as means ± SDs for continuous variables and n (%) for categoric variables. Differences between AA participants and white participants at baseline were compared using Student's t-test for continuous variables and the chi-square test for categoric variables.
We used linear mixed models to examine the associations between plant-based diet indices and the annual rates of change in global cognitive function, episodic memory, and perceptual speed. The random-effects model allowed for within-individual variation in initial cognitive scores and the rate of change in cognitive scores. Each random-effects model included terms of time, the main effect term for each covariate, and interaction terms of time × each covariate. The overall PDI, hPDI, and uPDI were modeled as categoric variables in quintiles, with the lowest quintile as the referent category. Median values of each quintile were used to calculate the P value for the trend. We assessed the correlation of each PDI with 2 other dietary scores—the MedDiet score and the MIND diet score—using Spearman rank correlation coefficients.
We first analyzed the associations in a basic multivariable model adjusted for age (y), sex (male, female), presence of apoE e4 allele, time, and interaction terms of time × each covariate. We then used a multivariable model to simultaneously control for factors potentially related to change in cognitive function. In a multivariate model (model 2), we further adjusted for dietary and lifestyle factors including calorie intake (kcal), smoking status (never compared with current or former), cognitive activity, and education (number of years in school), time, and the interaction of time × each covariate. Model 3 further adjusted for cardiovascular comorbidities (history of hypertension, diabetes, myocardial infarction, stroke), time, and the interaction of time × each condition. As sensitivity analyses, we further adjusted for a MedDiet score and a MIND score in the model and we conducted the analyses again excluding participants in the lowest 5% of cognitive scores at baseline. We also examined whether the rate of cognitive decline by diet was modified by cardiovascular comorbidities, and excluded participants who had a history of stroke. We presented the results stratified by race given that there are distinct dietary patterns between AA and white participants. Based on previous evidence from others and our group, we therefore predetermined the analytical approach in investigating the association of diet and cognition by race. SAS version 9.4 (SAS Institute Inc.) was used for data analysis with a type 1 error rate for significance at 0.05, and all tests were 2-sided.
Results
Population characteristics
Table 1 presents the participants’ demographics and clinical characteristics. Among the 3337 participants, the mean age was 73.7 ± 5.7 y. Women made up 64% of the participants, and 60% were AA adults. White participants had more years of education (14.5 ± 3.20 compared with 11.9 ± 3.16; P < 0.0001), higher cognitive activity (3.50 ± 0.57 compared with 3.10 ± 0.65; P < 0.0001), and higher global cognitive score (0.64 ± 0.50 compared with 0.25 ± 0.62; P < 0.0001) than AA study participants. AA participants had a higher prevalence of cardiovascular disease (CVD) risk factors.
TABLE 1.
Demographics and clinical characteristics of analyzed CHAP (Chicago Health and Aging Project) participants according to racial groups1
| All (n = 3337) | African American participants (n = 2012) | White participants (n = 1325) | P values | |
|---|---|---|---|---|
| Age, y | 73.7 ± 5.72 | 72.9 ± 5.08 | 74.8 ± 6.41 | <0.0001 |
| BMI, kg/m2 | 28.0 ± 5.52 | 28.8 ± 5.67 | 26.7 ± 5.02 | <0.0001 |
| Education, y | 12.9 ± 3.41 | 11.9 ± 3.16 | 14.5 ± 3.20 | <0.0001 |
| Calories, kcal | 1705 ± 586 | 1655 ± 608 | 1781 ± 543 | <0.0001 |
| Sex, women | 2137 (64.0) | 1307 (65.0) | 830 (62.6) | 0.17 |
| ApoE e4 allele, carrier | 1108 (33.1) | 756 (37.5) | 352 (26.4) | <0.0001 |
| Smoking status, current | 357 (10.7) | 248 (12.3) | 109 (8.2) | 0.0002 |
| Hypertension | 2326 (69.7) | 1520 (75.6) | 806 (60.8) | <0.0001 |
| Stroke | 246 (7.4) | 161 (8.0) | 85 (6.4) | 0.09 |
| Diabetes | 638 (19.1) | 480 (23.9) | 158 (11.9) | <0.0001 |
| Myocardial infarction | 426 (12.8) | 258 (12.8) | 168 (12.7) | 0.90 |
| Cognitive activity | 3.26 ± 0.65 | 3.10 ± 0.65 | 3.50 ± 0.57 | <0.0001 |
| Global cognition, score | 0.41 ± 0.61 | 0.25 ± 0.62 | 0.64 ± 0.50 | <0.0001 |
| Episodic memory, score | 0.40 ± 0.76 | 0.29 ± 0.79 | 0.57 ± 0.67 | <0.0001 |
| Perceptual speed, score | 0.48 ± 0.86 | 0.19 ± 0.80 | 0.91 ± 0.76 | <0.0001 |
| Plant-based diet indices, score | ||||
| Overall plant-based diet index | 53.3 ± 6.31 | 53.0 ± 6.45 | 53.7 ± 6.07 | 0.003 |
| Healthful plant-based diet index | 51.4 ± 6.78 | 51.2 ± 6.61 | 50.2 ± 6.88 | <0.0001 |
| Unhealthful plant-based diet index | 54.9 ± 6.72 | 55.4 ± 6.56 | 54.2 ± 6.90 | <0.0001 |
Values are means ± SDs and n (%) of the study population. P values were calculated using Student's t-test for continuous variables and chi-square test for categoric variables.
Dietary characteristics of AAs and EAs
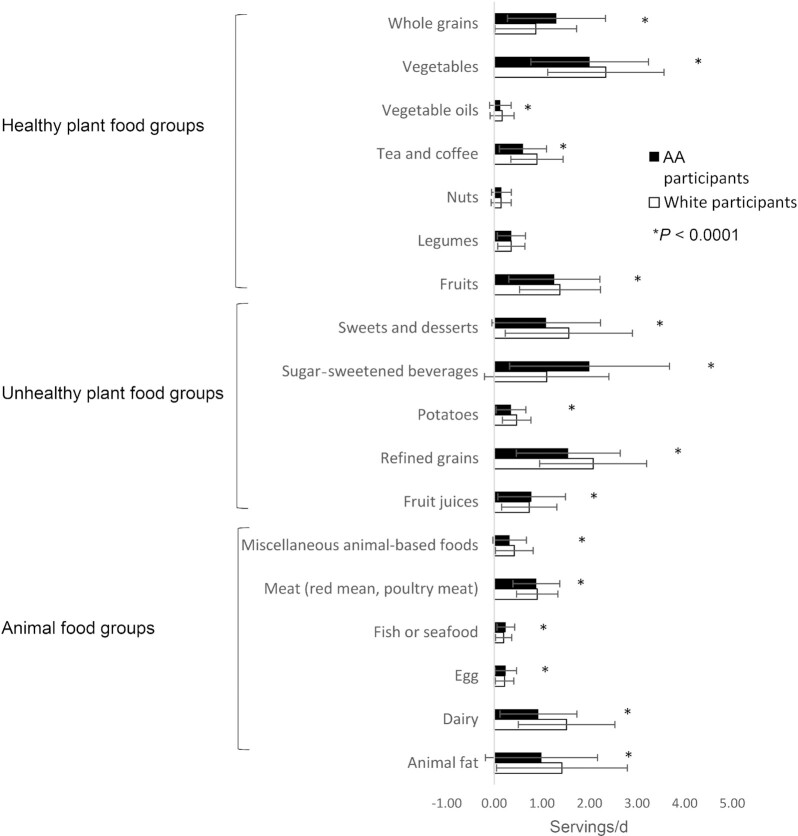
White study participants, compared with AA study participants, had higher calorie intakes (1782 ± 547 kcal compared with 1676 ± 624 kcal; P < 0.0001), higher PDI (53.7 ± 6.07 compared with 53.0 ± 6.45; P = 0.003), and lower hPDI (50.2 ± 6.88 compared with 51.2 ± 6.56; P < 0.0001) and uPDI (54.2 ± 6.90 compared with 55.4 ± 6.56; P < 0.0001). Between AA and white participants, there were differences in their dietary patterns (Figure 2). Compared with AA study participants, white study participants had significantly higher consumption of foods from animal food groups except for eggs, and fish or seafood (P < 0.0001 for all food groups). White study participants also had higher consumption of foods from unhealthy plant-based food groups except for sugar-sweetened beverages and fruit juices (P < 0.001 for all food groups). AA participants had higher intakes of whole grains from healthy plant-based foods (P < 0.0001) than white participants. It is worth noting that when we categorized food intakes according to quintiles of hPDI (higher numbers mean greater intakes of healthful plant-based foods), among those in the lowest quintile of hPDI, AA study participants had twice the consumption of whole grains (0.8 compared with 0.4 servings/d) as compared with white participants (Supplemental Table 1).
FIGURE 2.
Dietary patterns of participants from the CHAP (Chicago Health and Aging Project) study according to racial groups (n = 3337). The filled bars and whiskers represent mean ± SD consumption of AAs (n = 2012) and the open bars and whiskers represent mean ± SD consumption of whites (n = 1325), respectively. A t-test was used to compare between AA and white study participants. *P < 0.0001. AA, African American.
Plant-based diet indices and global cognitive function
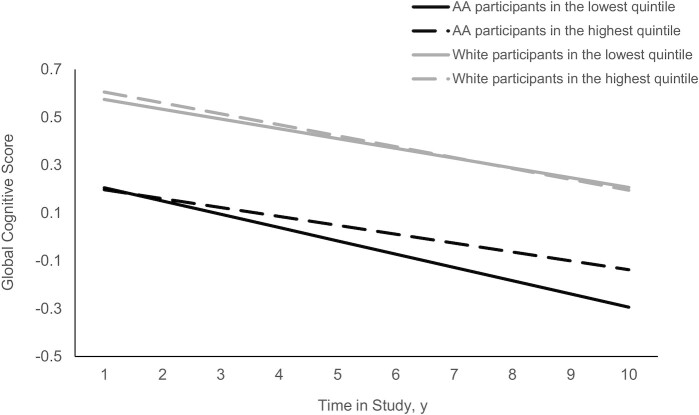
Table 2 presents the associations between plant-based diet indices and global cognition. Among AA participants, those in the highest quintile of hPDI had significantly slower rates of cognitive decline (β: 0.0183 ± 0.0086; P = 0.032) (Table 2) than the individuals in the lowest quintile. There were no associations of either PDI or uPDI with the rate of cognitive decline in either racial group (Table 2). As shown in Figure 3, rates of global decline in the highest quintile were slowed by 28.4% when compared with individuals in the lowest quintile of hPDI in AA participants. We did not see a similar trend in white participants (Figure 3).
TABLE 2.
Associations of plant-based indices with global cognitive decline in AA and white study participants1
| AA participants (n = 2012) | White participants (n = 1325) | |||||
|---|---|---|---|---|---|---|
| n | Quintile median (minimum, maximum) | β ± SE | n | Quintile median (minimum, maximum) | β ± SE | |
| PDI | ||||||
| Q1 | 407 | 45 (30, 47) | Reference | 204 | 45 (37, 47) | Reference |
| Q2 | 320 | 49 (48, 50) | 0.0052 ± 0.0076 | 203 | 49 (48, 50) | 0.0105 ± 0.0099 |
| Q3 | 456 | 52 (51, 54) | 0.0039 ± 0.0068 | 333 | 53 (51, 54) | −0.0005 ± 0.0091 |
| Q4 | 330 | 56 (55, 57) | −0.0014 ± 0.0076 | 243 | 56 (55, 57) | −0.0037 ± 0.0097 |
| Q5 | 499 | 61 (58, 74) | 0.0072 ± 0.0073 | 342 | 60 (58, 73) | 0.0010 ± 0.0095 |
| P-trend | 0.51 | 0.24 | ||||
| hPDI | ||||||
| Q1 | 238 | 42 (29, 44) | Reference | 266 | 42 (30, 44) | Reference |
| Q2 | 350 | 47 (45, 48) | 0.0086 ± 0.0084 | 296 | 47 (45, 48) | −0.0106 ± 0.0084 |
| Q3 | 372 | 50 (49, 51) | 0.0167 ± 0.0084 | 222 | 50 (49, 51) | −0.0053 ± 0.0093 |
| Q4 | 532 | 54 (52, 56) | 0.0175 ± 0.0082 | 308 | 54 (52, 56) | −0.0031 ± 0.0088 |
| Q5 | 520 | 60 (57, 72) | 0.0183 ± 0.0086 | 233 | 60 (57, 76) | −0.0047 ± 0.0098 |
| P-trend | 0.04 | 0.65 | ||||
| uPDI | ||||||
| Q1 | 307 | 46 (34, 48) | Reference | 281 | 46 (35, 48) | Reference |
| Q2 | 467 | 51 (49, 53) | −0.0091 ± 0.0075 | 321 | 51 (49, 53) | −0.0022 ± 0.0083 |
| Q3 | 325 | 55 (54, 56) | −0.0015 ± 0.0084 | 244 | 55 (54, 56) | −0.0041 ± 0.0090 |
| Q4 | 462 | 59 (57, 60) | −0.0097 ± 0.0080 | 236 | 58 (57, 60) | −0.0084 ± 0.0094 |
| Q5 | 451 | 64 (61, 78) | −0.0137 ± 0.0086 | 243 | 63 (61, 77) | −0.0091 ± 0.0096 |
| P-trend | 0.21 | 0.44 | ||||
Model was adjusted by age (y), sex (F/M), apoE e4 allele, education (y), calorie (kcal), cognitive activities, smoking status (current, former), comorbidities (history of hypertension, diabetes, myocardial infarction, stroke), time (y), and their respective interactions with time. AA, African American; hPDI, healthful plant-based diet index; PDI, plant-based diet index; Q, quintile; uPDI, unhealthful plant-based diet index.
FIGURE 3.
hPDI and the rate of change in global cognitive score among AA and white participants of the CHAP (Chicago Health and Aging Project) study (n = 3337). The hPDI was categorized into quintiles, with the lowest quintile as the referent group. The dark solid line and the dark dashed line represent the lowest and highest quintiles of hPDI, respectively, among AA study participants. The gray solid line and the gray dashed line represent the lowest and highest quintiles of hPDI, respectively, among white study participants. A multivariate model was adjusted for age, sex, apoE e4 allele, calorie intake, smoking status, cognitive activity, education, cardiovascular comorbidities, time, and the interaction of time × each condition. AA, African American; hPDI, healthful plant-based diet index.
Plant-based diet indices and individual tests of cognitive function: perceptual speed and episodic memory
AA study participants in the highest quintile of hPDI had a slower rate of change in perceptual speed (β: 0.0179 ± 0.0088; P = 0.037) and in episodic memory (β: 0.0163 ± 0.0118; P = 0.041) than those in the lowest quintile (Table 3). Among AA participants, for individuals in the highest quintile, the decline rates for perceptual speed and memory were significantly slower, by 49.3% and 44.2%, respectively, than for those in the lowest quintile (Figure 4). The overall PDI and uPDI were not associated with domain-specific cognitive function in AA or white study participants.
TABLE 3.
Associations of plant-based indices with rates of change in perceptual speed and episodic memory in AA and white study participants1
| AA participants (n = 2012) | White participants (n = 1325) | |
|---|---|---|
| Perceptual speed | ||
| PDI | ||
| Q1 | Referent | Referent |
| Q2 | −0.0002 ± 0.0078 | 0.0222 ± 0.0118 |
| Q3 | 0.0023 ± 0.0070 | 0.0130 ± 0.0108 |
| Q4 | 0.0040 ± 0.0078 | 0.0108 ± 0.0115 |
| Q5 | 0.0113 ± 0.0075 | 0.0054 ± 0.0113 |
| P-trend | 0.10 | 0.80 |
| hPDI | ||
| Q1 | Referent | Referent |
| Q2 | 0.0040 ± 0.0087 | −0.0109 ± 0.0100 |
| Q3 | 0.0146 ± 0.0086 | 0.0072 ± 0.0110 |
| Q4 | 0.0117 ± 0.0084 | −0.0070 ± 0.0104 |
| Q5 | 0.0179 ± 0.0088 | 0.0049 ± 0.0117 |
| P-trend | 0.03 | 0.67 |
| uPDI | ||
| Q1 | Referent | Referent |
| Q2 | −0.0074 ± 0.0078 | 0.0012 ± 0.0099 |
| Q3 | −0.0097 ± 0.0086 | 0.0066 ± 0.0107 |
| Q4 | −0.0075 ± 0.0082 | −0.0002 ± 0.0112 |
| Q5 | −0.0083 ± 0.0088 | 0.0056 ± 0.0114 |
| P-trend | 0.48 | 0.66 |
| Episodic memory | ||
| PDI | ||
| Q1 | Referent | Referent |
| Q2 | 0.0052 ± 0.0076 | 0.0105 ± 0.0099 |
| Q3 | 0.0039 ± 0.0068 | −0.0005 ± 0.0091 |
| Q4 | −0.0014 ± 0.0076 | −0.0037 ± 0.0097 |
| Q5 | 0.0072 ± 0.0073 | 0.0010 ± 0.0095 |
| P-trend | 0.51 | 0.24 |
| hPDI | ||
| Q1 | Referent | Referent |
| Q2 | 0.0014 ± 0.0116 | −0.0106 ± 0.0084 |
| Q3 | 0.0109 ± 0.0116 | −0.0053 ± 0.0093 |
| Q4 | 0.0165 ± 0.0113 | −0.0031 ± 0.0088 |
| Q5 | 0.0163 ± 0.0118 | −0.0047 ± 0.0098 |
| P-trend | 0.04 | 0.66 |
| uPDI | ||
| Q1 | Referent | Referent |
| Q2 | −0.0091 ± 0.0075 | −0.0022 ± 0.0083 |
| Q3 | −0.0015 ± 0.0084 | −0.0041 ± 0.0090 |
| Q4 | −0.0097 ± 0.0080 | −0.0084 ± 0.0094 |
| Q5 | −0.0137 ± 0.0086 | −0.0091 ± 0.0096 |
| P-trend | 0.21 | 0.44 |
Values are β ± SE unless indicated otherwise. Model was adjusted by age (y), sex (F/M), apoE e4 allele, education (y), calorie (kcal), cognitive activities, smoking status (current, former), comorbidities (history of hypertension, diabetes, myocardial infarction, stroke), time (y), and their respective interactions with time. AA, African American; hPDI, healthful plant-based diet index; PDI, plant-based diet index; Q, quintile; uPDI, unhealthful plant-based diet index.
FIGURE 4.
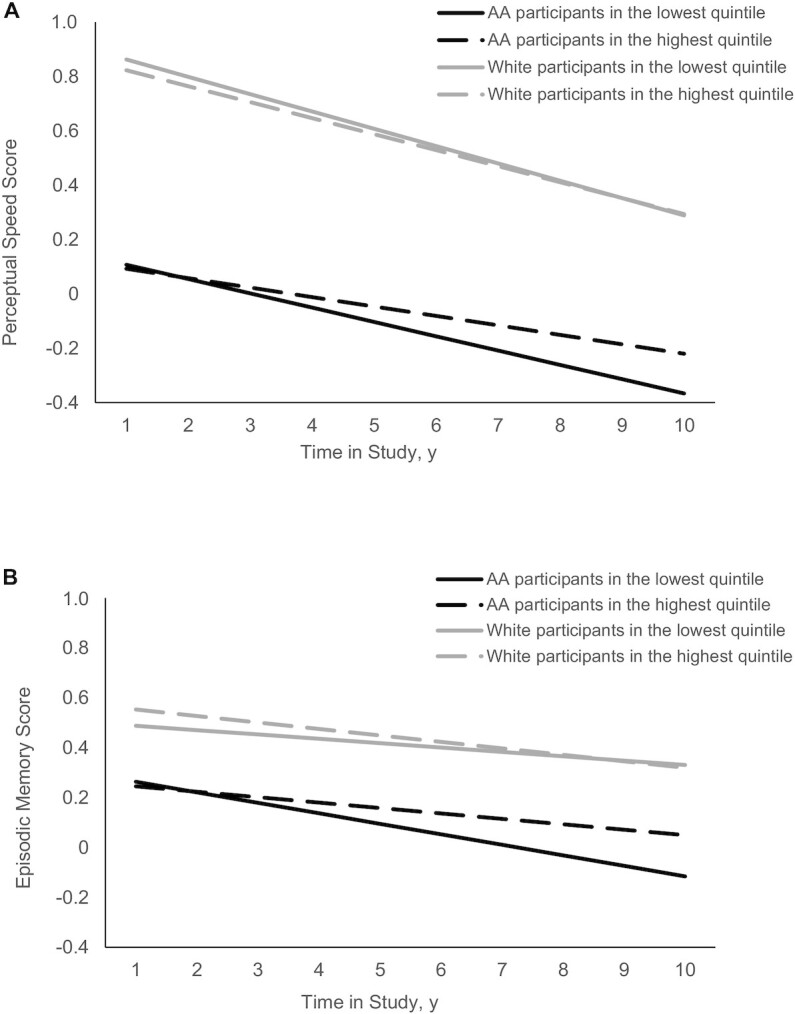
hPDI and the rate of change in perceptual speed score (A) and episodic memory score (B) among AA and white participants of the CHAP (Chicago Health and Aging Project) study (n = 3337). The hPDI was categorized into quintiles, with the lowest quintile as the referent group. The dark solid line and the dark dashed line represent the lowest and highest quintiles of hPDI, respectively, among AA study participants. The gray solid line and the gray dashed line represent the lowest and highest quintiles of hPDI, respectively, among white study participants. A multivariate model was adjusted for age, sex, apoE e4 allele, calorie intake, smoking status, cognitive activity, education, cardiovascular comorbidities, time, and the interaction of time × each condition. AA, African American; hPDI, healthful plant-based diet index.
As sensitivity analyses, first, we adjusted for cardiovascular conditions. Second, we further adjusted for dietary quality using the MIND diet and MedDiet scores. Third, we conducted the analyses excluding those participants in the lowest 5% of global cognitive scores at baseline. Last, we examined whether the rate of cognitive decline by diet was modified by cardiovascular comorbidities, and excluded participants who had a history of stroke. These associations remained largely unchanged (data not shown).
Discussion
In the present study, we modeled the effect of 3 plant-based diet indices—PDI, hPDI, uPDI—on the rate of cognitive decline in a biracial population-based community cohort study in older adults. We identified distinct dietary patterns between AA participants and white participants. We demonstrated that a healthy plant-based diet was associated with slower global cognitive decline and slower declines in episodic memory and perceptual speed in AA adults but not white adults.
In prior studies, a plant-based diet has often been defined as “a vegetarian diet” which consists of dietary patterns excluding animal foods totally or in part (36–38). Excluding intakes of the entire animal food group might not be practical for all populations because people with diets based on entirely plant foods may be more susceptible to deficiencies in nutrients such as vitamin B-12 that may offset the benefits of a vegetarian diet (39, 40). More importantly, not all plant-based foods are equally healthy. A healthy plant-based diet with fruits, vegetables, and whole grains is different from a plant-based diet with discretionary foods (i.e., sugar-sweetened beverages, desserts, and potato chips that were associated with deleterious health outcomes) (41–44). In the present study, we used a gradient score system that enabled us to capture a broad range of food groups and compare the quality among plant-based diets. Our study distinguished the quality of plant-based diets (healthful compared with unhealthful) and their associations with cognitive function. In addition, our analysis also highlighted the racial differences between dietary patterns among AA and white adults. Results from the present study expand prior research demonstrating an association between a healthy plant-based diet and slower cognitive decline in AA adults.
Emerging evidence brings more attention to the quality of a plant-based diet. The moderate correlation between PDI, hPDI, uPDI, the MedDiet score (33), and the MIND diet score (4) in our study suggested that these 3 plant-based diet indices captured additional components from diets. Whereas the MedDiet and MIND diet feature certain food items, the plant-based diet indices differentiate the quality among plant-based foods. Under the broader umbrella of plant-based diet, an unhealthy plant-based diet including refined grains, sugar-sweetened beverages, and snacks has distinct differences in dietary quality compared with a healthy plant-based diet that highlights the consumption of fruits, vegetables, and whole grains. The quality of a plant-based diet is, therefore, an important factor when considering it as a preventive strategy for cognitive decline.
Mechanistic evidence supports that nutrients from plant-based foods [i.e., green leafy vegetables (6) and berries (6)] have antioxidant capacity (21), are neuroprotective through reducing inflammation and decreasing oxidative stress, and are associated with less brain atrophy (45).
In the present study, we observed that participants in the highest quintile of hPDI had a significantly slower cognitive decline rate than those in the lowest quintile of hPDI in AA but not white participants. This racial-specific association was not unexpected. In line with our findings, Koyama et al. (46) reported that the MedDiet was associated with a slower rate of cognitive decline among AA older adults, but not white older adults, over 8 y. From a cross-sectional analysis, a Southern diet was associated with worse cognitive performance in AA adults but not white adults (47). In our study, when compared with white participants, AA participants had significantly lower calorie intake and lower intake from animal food groups and significantly higher consumption of sugar-sweetened beverages and whole grains. The association between a healthy plant-based diet and a slower rate of cognitive decline in AA participants is, therefore, likely attributable to the lower intake of animal foods and unhealthy plant-based foods, given that the white participants had slightly higher intakes of healthful plant-based foods (i.e., fruit and vegetables and vegetable oils). The dietary pattern of AA adults from the Chicago area appears different from the typical Southern diet consisting of red meat, processed meat, fried food, and eggs (48). A high-fat diet, particularly with high animal fats, was associated with cognitive impairment (49–51). Direct evidence from animal models suggests that a high-fat diet increases neuroinflammation (52) and impairs insulin signaling in the brain (53, 54), which has harmful effects on synaptic integrity (53) and cognitive behavior (55). It is worth noting that AA participants had higher intakes of whole grains. Whole grains are a component of both the DASH and MedDiet. Higher whole grain consumption was positively associated with higher cognitive scores in elderly US adults (56), which could relate to its bioactive compounds, minerals, and a low glycemic index that could improve cardiometabolic health (57), which may contribute to preserving cognitive function. The association between high sugar-sweetened beverages and cognitive impairment (58, 59) remained inconsistent between different racial groups (47), which warrants further investigation. Also, different methods of food preparation (i.e., fried, grilled, boiled, uncooked) between different racial groups can affect vegetable nutritional values. Further research is warranted to delineate the effects of cooking method on vegetable preparation and their nutrimental values on cognition among different ethnic groups. In addition, AA adults have poorer overall cardiovascular health than white adults (60). In the present study, a higher proportion of AA study participants had hypertension, diabetes, and stroke; the racial-specific association observed in the present study, therefore, might partially contribute through healthful plant-based diet that affects cardiovascular-related conditions in AA participants.
The effects of diets on cognition are susceptible to social and demographic factors among older adults. Previous evidence has demonstrated that the relation between diet and cognition depends on socioeconomic status (61, 62) where the effects of diet were robust in individuals with low social-economic status (61, 62). We accounted for these potentially confounding effects and found that diet was protective against cognitive decline only in AA participants and not white participants. The reasons are not fully clear, but it is plausible that greater exposure to education and cognitive activity in the early years may potentially overshadow the impact of diet on cognitive decline later in life among white individuals. In comparison, diet quality may significantly influence cognition later in life in individuals with low social-economic status.
Nonetheless, there are some limitations to be considered in the interpretation of our study. Dietary data were self-reported using FFQs, which could be prone to measurement error, although the validity of FFQs has been demonstrated using objective biochemical markers in older adults (31, 63). In the present study, we examined the association of dietary data and cognitive decline using the first available FFQ; therefore, we were unable to capture causal associations of changes of diet and cognitive function over time. The study participants were older adults from the south side of Chicago; therefore, our findings may not be generalizable to other populations with different socioeconomic status. Because of the use of self-reported data on dietary intake and other lifestyle factors, measurement errors or misclassification might occur. Because apoE genotype has a significant influence on cognitive function and AA individuals are more likely to carry the apoE e4 allele than white individuals, we included those with apoE e4 allele measurements in the final model. Participants included in the final model shared similar characteristics with the full study population, with 60% AA adults and 64% women. The race-specific association between diet and cognitive function could be mediated by the effects of diet on CVD because AA individuals had a higher prevalence of developing CVD, although we did account for CVD comorbidities during follow-up. Although we have adjusted for multiple potential confounders in the present analysis, we cannot completely rule out residual confounding. Because of the observational study design, we must caution against a causal interpretation of findings. Future studies in different racial groups are warranted to validate our findings, including studies with larger sample sizes and studies investigating the causal role of plant-based diet on cognition. Strengths of the present study include the prospective design in a biracial community cohort. Other strengths include the use of a gradient score system for the quality of a plant-based diet. The evaluations of cognitive function were conducted in person.
In conclusion, AA and white participants have distinct dietary patterns. A healthy plant-based diet was associated with a significantly slower rate of decline in global cognition in AA adults. The results presented herein could be informative in facilitating tailored dietary recommendations for diverse populations.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—XL: conceived and designed the research project, developed the overall analytical plan, and oversaw the study; TB: supported the statistical analysis; KD, LLB, CCT, PA, NA, TMH, and KBR: performed manuscript revision; KBR and DAE: obtained funding; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by National Institute on Aging grants R01AG03154 (to KBR) and R01AG051635 (to KBR) and NIH grants RF1AG057532 (to KBR) and R01AG058679 (to KBR).
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AA, African American; AD, Alzheimer dementia; CHAP, Chicago Health and Aging Project; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; hPDI, healthful plant-based diet index; MedDiet, Mediterranean diet; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; PDI, plant-based diet index; uPDI, unhealthful plant-based diet index.
Contributor Information
Xiaoran Liu, Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL, USA; Department of Internal Medicine, Rush University Medical Center, Chicago, IL, USA.
Klodian Dhana, Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL, USA; Department of Internal Medicine, Rush University Medical Center, Chicago, IL, USA.
Lisa L Barnes, Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, USA; Department of Neurology, Rush University Medical Center, Chicago, IL, USA.
Christy C Tangney, Department of Clinical Nutrition & Preventive Medicine, Rush University Medical Center, Chicago, IL, USA.
Puja Agarwal, Department of Internal Medicine, Rush University Medical Center, Chicago, IL, USA; Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, USA.
Neelum Aggarwal, Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, USA; Department of Neurology, Rush University Medical Center, Chicago, IL, USA.
Thomas M Holland, Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL, USA; Department of Internal Medicine, Rush University Medical Center, Chicago, IL, USA.
Todd Beck, Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL, USA; Department of Internal Medicine, Rush University Medical Center, Chicago, IL, USA.
Denis A Evans, Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL, USA; Department of Internal Medicine, Rush University Medical Center, Chicago, IL, USA.
Kumar B Rajan, Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL, USA; Department of Internal Medicine, Rush University Medical Center, Chicago, IL, USA.
Data Availability
Data, the statistical code, questionnaires, and technical processes are available from the corresponding author. Data used in this study are available through the Rush Institute for Healthy Aging data portal (https://www.riha.rush.edu/dataportal.html) upon request and completion of a DUA. Analytical methods and study materials will be available to other researchers from the corresponding authors on reasonable request for purposes of reproducing the results or replicating the procedure.
References
- 1. United Nations, Department of Economic and Social Affairs, Population Division . World population ageing 2019: highlights. ST/ESA/SER.A/430. New York (NY): UN; 2019. [Google Scholar]
- 2. Weuve J, Barnes LL, Mendes de Leon CF, Rajan KB, Beck T, Aggarwal NTet al. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology. 2018;29(1):151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ortman JM, Victoria AV, Hogan H. An aging nation: the older population in the United States. Current Population Reports. P25-1140. Washington (DC): US Census Bureau; 2014. [Google Scholar]
- 4. Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DAet al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agarwal P, Holland TM, Wang Y, Bennett DA, Morris MC. Association of strawberries and anthocyanidin intake with Alzheimer's dementia risk. Nutrients. 2019;11(12):3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67(8):1370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ye X, Bhupathiraju SN, Tucker KL. Variety in fruit and vegetable intake and cognitive function in middle-aged and older Puerto Rican adults. Br J Nutr. 2013;109(3):503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62(12):1849–53. [DOI] [PubMed] [Google Scholar]
- 9. Scarmeas N, Stern Y, Tang M-X, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol. 2006;59(6):912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawas CH. Diet and the risk for Alzheimer's disease. Ann Neurol. 2006;59(6):877–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hiza HA, Casavale KO, Guenther PM, Davis CA. Diet quality of Americans differs by age, sex, race/ethnicity, income, and education level. J Acad Nutr Diet. 2013;113(2):297–306. [DOI] [PubMed] [Google Scholar]
- 12. McInerney M, Csizmadi I, Friedenreich CM, Uribe FA, Nettel-Aguirre A, McLaren Let al. Associations between the neighbourhood food environment, neighbourhood socioeconomic status, and diet quality: an observational study. BMC Public Health. 2016;16(1):984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bloom I, Edwards M, Jameson KA, Syddall HE, Dennison E, Gale CRet al. Influences on diet quality in older age: the importance of social factors. Age Ageing. 2017;46(2):277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agarwal P, Morris MC, Barnes LL. Racial differences in dietary relations to cognitive decline and Alzheimer's disease risk: do we know enough?. Front Hum Neurosci. 2020;14:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi Let al. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13(6):e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jenkins DJ, Wong JMW, Kendall CW, Esfahani A, Ng VWY, Leong TCKet al. The effect of a plant-based low-carbohydrate (“Eco-Atkins”) diet on body weight and blood lipid concentrations in hyperlipidemic subjects. Arch Intern Med. 2009;169(11):1046–54. [DOI] [PubMed] [Google Scholar]
- 17. Satija A, Malik V, Rimm EB, Sacks F, Willett W, Hu FB. Changes in intake of plant-based diets and weight change: results from 3 prospective cohort studies. Am J Clin Nutr. 2019;110(3):574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett Wet al. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J Am Coll Cardiol. 2017;70(4):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baden MY, Liu G, Satija A, Li Y, Sun Q, Fung TTet al. Changes in plant-based diet quality and total and cause-specific mortality. Circulation. 2019;140(12):979–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP). J Alzheimers Dis. 2003;5(5):349–55. [DOI] [PubMed] [Google Scholar]
- 21. Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS, Aggarwal NTet al. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am J Clin Nutr. 2005;81(2):508–14. [DOI] [PubMed] [Google Scholar]
- 22. Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J Neurosci. 1991;57(3–4):167–78. [DOI] [PubMed] [Google Scholar]
- 23. Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DAet al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17(2):179–93. [PubMed] [Google Scholar]
- 24. Smith A. Symbol Digit Modalities Test (SDMT): manual. Los Angeles (CA): Western Psychological; 1982. [Google Scholar]
- 25. Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology. 2003;61(6):812–6. [DOI] [PubMed] [Google Scholar]
- 26. Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322–6. [DOI] [PubMed] [Google Scholar]
- 27. Weuve J, Rajan KB, Barnes LL, Wilson RS, Evans DA. Secular trends in cognitive performance in older black and white U.S. adults, 1993–2012: findings from the Chicago Health and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2018;73(suppl_1):S73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 29. Wilson RS, Bennett DA, Beckett LA, Morris MC, Gilley DW, Bienias JLet al. Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci. 1999;54B(3):P155–60. [DOI] [PubMed] [Google Scholar]
- 30. Wilson RS, Beckett LA, Bennett DA, Albert MS, Evans DA. Change in cognitive function in older persons from a community population: relation to age and Alzheimer disease. Arch Neurol. 1999;56(10):1274–9. [DOI] [PubMed] [Google Scholar]
- 31. Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol. 2003;158(12):1213–7. [DOI] [PubMed] [Google Scholar]
- 32. US Census Bureau . 1990 U.S. Census Questionnaire. Suitland (MD): US Census Bureau; 1990. [Google Scholar]
- 33. Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr. 2011;93(3):601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med. 2007;44(4):335–40. [DOI] [PubMed] [Google Scholar]
- 35. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimers Dement. 2015;11(9):1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chan R, Chan D, Woo J. A cross sectional study to examine the association between dietary patterns and cognitive impairment in older Chinese people in Hong Kong. J Nutr Health Aging. 2013;17(9):757–65. [DOI] [PubMed] [Google Scholar]
- 37. Pollakova D, Andreadi A, Pacifici F, Della-Morte D, Lauro D, Tubili C. The impact of vegan diet in the prevention and treatment of type 2 diabetes: a systematic review. Nutrients. 2021;13(6):2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang NC, Bagheri M, Olszewski T, Friese KA, Smith HM, Robles MEet al. New-onset vegetarian diet shows differences in fatty acid metabolites in European American and African American women. Nutr Metab Cardiovasc Dis. 2021;31(8):2436–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rizzo G, Laganà AS, Rapisarda AMC, La Ferrera GMG, Buscema M, Rossetti Pet al. Vitamin B12 among vegetarians: status, assessment and supplementation. Nutrients. 2016;8(12):767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Watanabe F, Yabuta Y, Bito T, Teng F. Vitamin B12-containing plant food sources for vegetarians. Nutrients. 2014;6(5):1861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Drouin-Chartier J-P, Zheng Y, Li Y, Malik V, Pan A, Bhupathiraju SNet al. Changes in consumption of sugary beverages and artificially sweetened beverages and subsequent risk of type 2 diabetes: results from three large prospective U.S. cohorts of women and men. Diabetes Care. 2019;42(12):2181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Malik VS, Hu FB. Sugar-sweetened beverages and cardiometabolic health: an update of the evidence. Nutrients. 2019;11(8):1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malik VS, Li Y, Pan A, De Koning L, Schernhammer E, Willett WCet al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139(18):2113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yin J, Zhu Y, Malik V, Li X, Peng X, Zhang FFet al. Intake of sugar-sweetened and low-calorie sweetened beverages and risk of cardiovascular disease: a meta-analysis and systematic review. Adv Nutr. 2021;12(1):89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mosconi L, Murray J, Tsui WH, Li Y, Davies M, Williams Set al. Mediterranean diet and magnetic resonance imaging-assessed brain atrophy in cognitively normal individuals at risk for Alzheimer's disease. J Prev Alzheimers Dis. 2014;1(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- 46. Koyama A, Houston DK, Simonsick EM, Lee JS, Ayonayon HN, Shahar DRet al. Association between the Mediterranean diet and cognitive decline in a biracial population. J Gerontol A Biol Sci Med Sci. 2015;70(3):354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nutaitis AC, Tharwani SD, Serra MC, Goldstein FC, Zhao L, Sher SSet al. Diet as a risk factor for cognitive decline in African Americans and Caucasians with a parental history of Alzheimer's disease: a cross-sectional pilot study dietary patterns. J Prev Alzheimers Dis. 2019;6(1):50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pearson KE, Wadley VG, McClure LA, Shikany JM, Unverzagt FW, Judd SE. Dietary patterns are associated with cognitive function in the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. J Nutr Sci. 2016;5:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal Net al. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003;60(2):194–200. [DOI] [PubMed] [Google Scholar]
- 50. Morris MC, Evans DA, Tangney CC, Bienias JL, Schneider JA, Wilson RSet al. Dietary copper and high saturated and trans fat intakes associated with cognitive decline. Arch Neurol. 2006;63(8):1085–8. [DOI] [PubMed] [Google Scholar]
- 51. Morris MC, Tangney CC. Dietary fat composition and dementia risk. Neurobiol Aging. 2014;35(Suppl 2):S59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cavaliere G, Trinchese G, Penna E, Cimmino F, Pirozzi C, Lama Aet al. High-fat diet induces neuroinflammation and mitochondrial impairment in mice cerebral cortex and synaptic fraction. Front Cell Neurosci. 2019;13:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arnold SE, Lucki I, Brookshire BR, Carlson GC, Browne CA, Kazi Het al. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis. 2014;67:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Denver P, Gault VA, McClean PL. Sustained high-fat diet modulates inflammation, insulin signalling and cognition in mice and a modified xenin peptide ameliorates neuropathology in a chronic high-fat model. Diabetes Obes Metab. 2018;20(5):1166–75. [DOI] [PubMed] [Google Scholar]
- 55. Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MSet al. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem. 2008;106(1):475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wengreen H, Munger RG, Cutler A, Quach A, Bowles A, Corcoran Cet al. Prospective study of Dietary Approaches to Stop Hypertension– and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County Study on Memory, Health and Aging. Am J Clin Nutr. 2013;98(5):1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zong G, Gao A, Hu FB, Sun Q. Whole grain intake and mortality from all causes, cardiovascular disease, and cancer. Circulation. 2016;133(24):2370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chong CP, Shahar S, Haron H, Din NC. Habitual sugar intake and cognitive impairment among multi-ethnic Malaysian older adults. Clin Interv Aging. 2019;14:1331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ye X, Gao X, Scott T, Tucker KL. Habitual sugar intake and cognitive function among middle-aged and older Puerto Ricans without diabetes. Br J Nutr. 2011;106(9):1423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AGet al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136(21):e393–423. [DOI] [PubMed] [Google Scholar]
- 61. Parrott MD, Shatenstein B, Ferland G, Payette H, Morais JA, Belleville Set al. Relationship between diet quality and cognition depends on socioeconomic position in healthy older adults. J Nutr. 2013;143(11):1767–73. [DOI] [PubMed] [Google Scholar]
- 62. Weng P-H, Chen J-H, Chiou J-M, Tu Y-K, Chen T-F, Chiu M-Jet al. The effect of lifestyle on late-life cognitive change under different socioeconomic status. PLoS One. 2018;13(6):e0197676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tangney CC, Bienias JL, Evans DA, Morris MC. Reasonable estimates of serum vitamin E, vitamin C, and β-cryptoxanthin are obtained with a food frequency questionnaire in older black and white adults. J Nutr. 2004;134(4):927–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data, the statistical code, questionnaires, and technical processes are available from the corresponding author. Data used in this study are available through the Rush Institute for Healthy Aging data portal (https://www.riha.rush.edu/dataportal.html) upon request and completion of a DUA. Analytical methods and study materials will be available to other researchers from the corresponding authors on reasonable request for purposes of reproducing the results or replicating the procedure.