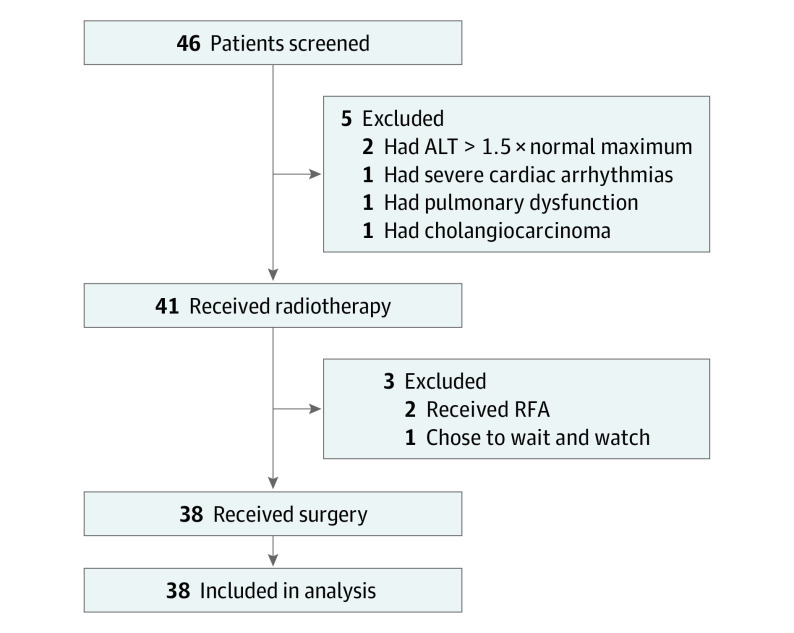
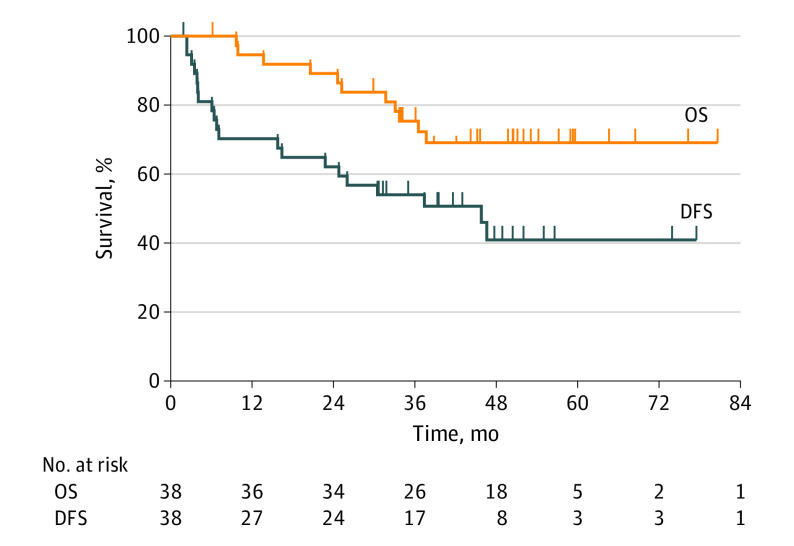
This nonrandomized controlled trial evaluates the overall and disease-free survival rates as well as adverse events associated with combined neoadjuvant intensity-modulated radiotherapy and hepatectomy.
Key Points
Question
Is neoadjuvant intensity-modulated radiotherapy (IMRT) plus surgery effective and safe in patients with centrally located hepatocellular carcinoma?
Findings
In this nonrandomized controlled trial of 38 patients who underwent neoadjuvant IMRT and surgery, the 5-year overall survival rate was higher than in patients who underwent surgery alone (69.1% vs 37.2%). Treatment-related adverse events were generally mild to moderate.
Meaning
Findings of this study suggest that patients with centrally located hepatocellular carcinoma could benefit from neoadjuvant IMRT plus surgery, a promising treatment regimen that warrants further exploration in a randomized clinical trial.
Abstract
Importance
Centrally located hepatocellular carcinoma (HCC) is a special type of HCC whose outcome is unsatisfactory when treated with surgery alone. No standard adjuvant or neoadjuvant treatment for this disease has been established that improves clinical outcomes.
Objective
To evaluate the effectiveness and safety of adding neoadjuvant intensity-modulated radiotherapy (IMRT) before surgery in patients with centrally located HCC.
Design, Setting, and Participants
This phase 2, single-center, single-group prospective nonrandomized controlled trial was conducted between December 16, 2014, and January 29, 2019, at the Cancer Institute and Hospital of the Chinese Academy of Medical Sciences in Beijing, China. The last follow-up was on July 30, 2021. Patients with centrally located HCC who underwent neoadjuvant IMRT and surgery were included in the analysis.
Interventions
Neoadjuvant IMRT followed by hepatectomy.
Main Outcomes and Measures
The primary end point was 5-year overall survival (OS). The secondary end points were tumor response to IMRT, 5-year disease-free survival (DFS), and treatment-related adverse events.
Results
Thirty-eight patients (mean [SD] age, 55.6 [9.3] years; 35 male [92.1%] individuals) completed the prescribed neoadjuvant IMRT without interruption. Radiographic tumor response to IMRT before surgery included partial response (16 [42.1%]) and stable disease (22 [57.9%]). Thirteen patients (34.2%) achieved major pathological response, of which 5 (13.2%) achieved pathologic complete response. With a median follow-up of 45.8 months, the median OS was not reached, and the OS rates were 94.6% at 1 year, 75.4% at 3 years, and 69.1% at 5 years. The median DFS was 45.8 months, and DFS rates were 70.3% at 1 year, 54.1% at 3 years, and 41.0% at 5 years. Radiotherapy-related grade 3 adverse events were observed in 3 patients (7.9%). Nineteen operative complications developed in 13 patients (34.2%), including grade I to II complications in 12 patients (31.6%) and grade IIIa complication in 1 patient (2.6%). No grade IIIb or higher operative complications were observed.
Conclusions and Relevance
Results of this trial suggest that neoadjuvant IMRT plus surgery is effective and well-tolerated in patients with centrally located HCC. These data may inform a future randomized clinical trial of this new treatment strategy.
Trial Registration
ClinicalTrials.gov Identifier: NCT02580929
Introduction
Liver cancer is one of the most common and deadly malignant neoplasms, causing more than 780 000 deaths per year worldwide.1 Hepatocellular carcinoma (HCC) is the dominant pathological type, accounting for approximately 80% of all liver cancers.1 Centrally located HCC represents a special subgroup of HCC, which grows mainly from the Couinaud hepatic segments IV, V, or VIII of the liver, according to its traditional definition.2 Centrally located HCC is defined as “a carcinoma adjoined hepatic portals, less than 1 cm from major vascular structures (including the main portal branches, the main trunks of the hepatic veins as well as the inferior vena cava) which are usually located in Couinaud segments I, IV, V, VIII, or at the junction of the central segments.”3(p382)4(p628) A revised definition of centrally located HCC has been proposed that incorporates its spatial relationship with major vessels.3,4 The survival of patients with centrally located HCC is unsatisfactory, with the 5-year overall survival (OS) being approximately 40%.3,5,6,7
Surgical treatment is the primary curative option for patients with centrally located HCC.4 However, there is a high risk of incomplete resection or narrow surgical margins (<1 cm) with surgery alone, which may result in a high recurrence rate and low OS rate.8,9 Thus, it is necessary to develop new treatment approaches to reduce the risk of positive margins and recurrence and to potentially improve survival.
Radiotherapy (RT) has been tried in combination with surgery for HCC. Previous studies have found that adjuvant RT compared with surgery alone could be a factor in increased disease-free survival (DFS) or OS in patients with HCC, especially those undergoing narrow-margin resections.10,11 Given that HCC is relatively RT-sensitive, we hypothesized a decreased tumor burden and improved outcome by applying intensity-modulated radiotherapy (IMRT) before surgery.12 To our knowledge, only 1 prospective study has suggested that neoadjuvant 3-dimensional conformal RT is associated with improved OS and DFS in patients with resectable HCC and portal vein tumor thrombosis.13 Two other retrospective studies have reported on the potential of neoadjuvant RT to improve OS in patients with HCC and main portal vein tumor thrombosis.14,15 These studies focused on the outcome of neoadjuvant RT for patients with concurrent HCC and portal vein tumor thrombosis. However, the value of neoadjuvant IMRT for centrally located HCC has not been reported. In this study, we aimed to evaluate the effectiveness and safety of adding neoadjuvant IMRT before surgery in patients with centrally located HCC.
Methods
Study Design and Participants
This single-center, single-group, prospective phase 2 nonrandomized controlled trial was conducted at the Cancer Institute and Hospital of the Chinese Academy of Medical Sciences in Beijing, China. The trial protocol (Supplement 1) was approved by the ethical committee of the Cancer Institute and Hospital of the Chinese Academy of Medical Sciences. Written informed consent was obtained from all of the patients before participation. We followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline.16
From December 16, 2014, until January 29, 2019, 46 patients were screened at the study site. Key inclusion criteria were age 18 to 75 years, Eastern Cooperative Oncology Group performance status of 0 to 1, clinical diagnosis of HCC17,18 or pathological diagnosis of HCC through percutaneous liver biopsy, at least 1 measurable centrally located lesion, Child-Pugh class A (class range: A-C, with A indicating a score of 5-6 points) liver function, bilirubin level of less than 1.65 mg/dL (to convert to micromole per liter, multiply by 17.104), alanine aminotransferase and aspartate aminotransferase levels of less than 100 U/L (to convert to microkatal per liter, multiply by 0.0167), normal creatinine level of 0.56 to 1.47 mg/dL (to convert to micromole per liter, multiply by 88.4) and urea nitrogen level of 7.0 to 21.0 mg/dL (to convert to millimoles per liter, multiply by 0.357), baseline white blood cell count greater than 4000/μL (to convert to 109/L, multiply by 0.001), hemoglobin level greater than 11.0 g/dL (to convert to grams per liter, multiply by 10.0), and platelet count greater than 90.0 × 103/μL (to convert to ×109/L, multiply by 1.0). Race and ethnicity data were not collected because they were not considered as inclusion criteria for this study.
The key exclusion criteria were any previous antitumor treatment of HCC, pathological type other than HCC, tumor distance to major vessels that could not be determined, tumor thrombosis in the main trunk of the portal vein or inferior vena cava, lymph node or remote metastasis, severe cirrhosis complications, a history of other malignant neoplasms, and inability to reach required IMRT doses. Ultimately, 38 patients were enrolled in the study.
Procedures
Liver-directed neoadjuvant IMRT was performed in all patients. Gross tumor volume (GTV), including primary tumor GTV and tumor thrombosis GTV, was delineated on planning computed tomography (CT) scan, referring to pretreatment multiphasic contrast magnetic resonance imaging through an image fusion approach. Clinical target volume included the primary GTV plus a 0.5-cm margin in all directions19 and tumor thrombosis GTV without a margin. The planning target volume included the clinical target volume plus a 0.5-cm margin in the anterior-posterior and left-right directions and a 1.0-cm margin in the cranial-caudal direction.20 The prescription dose to 95% of the planning target volume was 50 to 60 Gy in 25 to 30 fractions over 5 to 6 weeks, depending on the dose constraints of organs at risk. Other details about RT, including dose-volume constraints to organs at risk, respiratory motion management, and cone-beam CT scan for position verification, are described in a previous study.11
A multidisciplinary team discussion was held before surgery for all patients. Surgery was usually performed 4 to 12 weeks after IMRT. The same surgical team completed all operations to standardize operative quality and safety. The surgical procedure has been described in previous studies.3,10 Briefly, the resection was designed on the basis of tumor location, tumor size, tumor spatial relation to the major vascular structures, and degree of hepatic cirrhosis. The procedure included left or right hepatectomy, mesohepatectomy, combined segmental hepatectomy, and nonanatomical hepatectomy. Intraoperative ultrasonography was used to assess the spatial relationship between tumors and major vascular structures. In cases in which the tumor was adherent to the major vascular structures, surgeons carefully dissected and peeled the lesions away from the vascular surface using a cavitron ultrasonic surgical aspirator to avoid cutting the major vessels.
Pathological results were reported by pathologists with expertise in gastrointestinal pathology, according to College of American Pathologists protocols for examining specimens from patients with hepatocellular carcinoma. A negative margin was defined as the absence of tumor cells at the edge of the specimen.
All of the patients were followed up every 3 months during the first 2 years after hepatectomy, every 6 months during the next 3 years, and every year thereafter. Follow-up tests included α-fetoprotein, liver function, chest CT, liver magnetic resonance imaging, and abdominal CT. Recurrence was diagnosed on the basis of typical imaging findings or continually high serum α-fetoprotein levels. Marginal recurrence was defined as recurrence within 2 cm from the resection plane.10,21 Patients were followed up until death or the last follow-up date of July 30, 2021 (data cutoff date), whichever occurred first.
Outcomes
The primary outcome was 5-year OS. The secondary outcomes were tumor response to IMRT, 5-year DFS, and treatment-related adverse events. Overall survival was calculated from the date of the first RT to death from any cause or the last follow-up date. Disease-free survival was defined as the time interval between the date of surgery and the date of the first detection of recurrence, death from any cause, or the last follow-up.
Radiographic tumor response was evaluated according to the modified Response Evaluation Criteria in Solid Tumors guidelines.22 Major pathological response was defined as the presence of 10% or fewer viable tumor cells in the primary tumor. Major pathological response included pathologic complete response, defined as no residual viable tumor cells in the resected liver specimen.
Radiotherapy-related toxic effect was evaluated weekly during RT and monthly after RT and was graded according to Common Terminology Criteria for Adverse Events, version 4.03 (National Cancer Institute).23 Acute toxic effect was defined as events occurring during or within the first 3 months after RT. Late toxic effect was assessed beyond 3 months after RT. Operative complications were graded according to the Clavien-Dindo classification system.24
Statistical Analysis
A previous prospective randomized study estimated that the 5-year OS rate was 37.2% in patients with centrally located HCC who received narrow-margin hepatectomy alone.3 It was assumed that after neoadjuvant IMRT followed by surgery, the 5-year OS rate in patients with centrally located HCC would be close to the rate associated with Barcelona Clinic Liver Cancer staging classification of 0 to A disease (class range: 0-D, with 0 indicating performance status 0, Child-Pugh A, and single lesion <2 cm, and A indicating performance status 0, Child-Pugh A-B, and single tumor >2 cm or 2-3 lesions, each <3 cm), which was approximately 67% at the study site.25,26 The enrollment period was 4 years. All patients were followed up for at least 2.5 years. The minimum sample size was 35 (with 2-sided α = .05 as the threshold of significance; β = 0.10; power of 90%), which was estimated using the Power Analysis and Sample Size software, version 15.0.5 (NCSS LLC). The dropout rate was assumed to be 5%.
Continuous variables were expressed as median (range) or mean (SD), as appropriate. Categorical variables were expressed as number (proportion). The albumin-bilirubin grade was calculated in accordance with Johnson et al.27 The Kaplan-Meier estimate was performed to calculate the median survival time and survival rates (DFS and OS). All statistical analyses were performed using SPSS Statistics, version 24.0 (IBM Corp), and R, version 4.1.0 (R Foundation for Statistical Computing).
Results
A total of 38 patients (mean [SD] age, 55.6 [9.3] years; 35 male [92.1%] and 3 female [7.9%] individuals) completed treatment per study protocol and were analyzed in this study (Figure 1). Clinical and pathological characteristics are summarized in Table 1. Thirty-one patients (81.6%) were infected with hepatitis B virus. The median (range) maximal tumor diameter was 6.1 (3.0-15.0) cm, and 25 patients (65.8%) had tumors larger than 5 cm in diameter. Tumors that were attached (≤1 mm) and adjacent (1-10 mm) to major vessels were identified in 23 patients (60.5%) and 15 patients (39.5%), respectively. Most patients (29 [76.3%]) had Barcelona Clinic Liver Cancer stage A disease. Four patients (10.5%) showed tumor thrombosis (Cheng type I or II). Most patients (33 [86.8%]) had 1 lesion, and the other 5 patients (13.2%) had 2 lesions. Eighteen patients (47.4%) had a baseline α-fetoprotein level of 400 ng/mL or greater (to convert to micrograms per liter, multiply by 1).
Figure 1. Trial Profile.
ALT indicates alanine aminotransferase; RFA, radiofrequency ablation.
Table 1. Clinical and Pathological Characteristics.
| Characteristic | Patients, No. (%) (N = 38) |
|---|---|
| Age, mean (SD), y | 55.6 (9.3) |
| Male sex | 35 (92.1) |
| Female sex | 3 (7.9) |
| Underlying hepatitis B | 31 (81.6) |
| Underlying hepatitis C | 1 (2.6) |
| No underlying hepatitis | 6 (15.8) |
| Liver cirrhosis | 30 (78.9) |
| Child-Pugh class Aa | 38 (100) |
| Maximal size of the lesion, median (range), cm | 6.1 (3.0-15.0) |
| >5 | 25 (65.8) |
| ≤5 | 13 (34.2) |
| BCLC stageb | |
| A | 29 (76.3) |
| B | 5 (13.2) |
| C | 4 (10.5) |
| AJCC stagec | |
| IB | 19 (50.0) |
| II | 12 (31.6) |
| IIIA | 3 (7.9) |
| IIIB | 4 (10.5) |
| No. of primary tumors | |
| 1 | 33 (86.8) |
| 2 | 5 (13.2) |
| Pretreatment distance to major vessels, mm | |
| <1 | 23 (60.5) |
| 1-10 | 15 (39.5) |
| Vascular adhesion | |
| PV | 8 (21.1) |
| HV | 7 (18.4) |
| PV + HV | 5 (13.2) |
| IVC + HV | 3 (7.9) |
| None | 15 (39.5) |
| Tumor differentiation | |
| Well | 1 (2.6) |
| Moderate | 25 (65.8) |
| Poor | 6 (15.8) |
| Not specified | 6 (15.8) |
| Microscopic vascular invasion | 12 (31.6) |
| Satellite nodules | 4 (10.5) |
| Resection margin, median (range), cm | 1.0 (0-1.9) |
| Invasion of liver surface | |
| Yes | 14 (36.8) |
| No | 20 (52.6) |
| Not specified | 4 (10.5) |
| Baseline AFP, ng/mL | |
| >400 | 18 (47.4) |
| 7-400 | 13 (34.2) |
| <7 | 7 (18.4) |
| Baseline ALBI grade | |
| 1 | 35 (92.1) |
| 2 | 3 (7.9) |
Abbreviations: AFP, α-fetoprotein; AJCC, American Joint Committee on Cancer staging system eighth edition; ALBI, albumin-bilirubin; BCLC, Barcelona Clinic Liver Cancer staging classification; HV, hepatic vein; IVC, inferior vena cava; PV, portal vein.
SI conversion factor: To convert AFP to micrograms per liter, multiply by 1.
Child-Pugh classification ranges from class A to class C, with A indicating score of 5-6 points, B indicating score of 7-9 points, and C indicating score of 10-15 points.
BCLC staging classification ranges from 0 to D, with 0 indicating performance status 0, Child-Pugh A, single lesion smaller than 2 cm (all criteria should be fulfilled); A indicating performance status 0, Child-Pugh A-B, single tumor larger than 2 cm or 2-3 lesions smaller than 3 cm (all criteria should be fulfilled); B indicating performance status 0, Child-Pugh A-B, more than 1 lesion with at least 1 larger than 3 cm or more than 3 lesions, regardless of their size (all criteria should be fulfilled); C indicating performance status 1-2, Child-Pugh A-B, vascular invasion and/or nodal disease, and/or metastatic disease (at least 1 criterion should be fulfilled); and D indicating performance status 3-4, Child-Pugh C (at least 1 criterion should be fulfilled).
AJCC staging classification ranges from IA to IVB, with IA indicating lesion smaller than 2 cm in diameter, no regional lymph node metastasis, and no distant metastasis; IB indicating lesion larger than 2 cm without vascular invasion, no regional lymph node metastasis, and no distant metastasis; II indicating lesion larger than 2 cm with vascular invasion or multifocal lesions smaller than 5 cm, no regional lymph node metastasis, and no distant metastasis; IIIA indicating multifocal lesions with at least 1 larger than 5 cm, no regional lymph node metastasis, and no distant metastasis; IIIB indicating lesion(s) involving a major branch of the portal vein or hepatic vein or lesion(s) with direct invasion of adjacent organs other than the gallbladder or with perforation of visceral peritoneum, no regional lymph node metastasis, and no distant metastasis; IVA indicating regional lymph node metastasis regardless of lesions within the liver, and no distant metastasis; and IVB indicating distant metastasis regardless of lesions within the liver and regional lymph nodes.
Treatments and Outcomes
The median (range) prescribed RT dose was 50 Gy in 25 fractions (50-60 Gy in 25-30 fractions), and all patients completed the prescribed RT without interruption. The median (range) interval between surgery and last RT was 8.2 (4.6-12.0) weeks. According to radiographic evaluation, 16 of 38 patients (42.1%) had partial response and the other 22 patients (57.9%) had stable disease (Table 2). None of the patients experienced progressive disease before surgery. Most patients (37 [97.4%]) had negative surgical margins. The median (range) surgical margin was 1.0 (0-1.9) cm. Major pathological response was found in 13 patients (34.2%), including pathologic complete response in 5 patients (13.2%) (Table 2).
Table 2. Radiographic and Pathological Response to Neoadjuvant Radiotherapy.
| Patients, No. (%) (N = 38) | |||
|---|---|---|---|
| Radiographic responsea | pCR | MPRb | |
| Partial response | 16 (42.1) | 3 (7.9) | 7 (18.4) |
| Stable disease | 22 (57.9) | 2 (5.3) | 6 (15.8) |
| Progressive disease | 0 | NA | NA |
| Complete response | 0 | NA | NA |
Abbreviations: MPR, major pathological response; NA, not applicable; pCR, pathologic complete response.
According to modified Response Evaluation Criteria in Solid Tumors guidelines.
Including patients with pCR.
With a median (range) follow-up of 45.8 (6.2-80.2) months, the median OS was not reached. The OS rates were 94.6% at 1 year, 75.4% at 3 years, and 69.1% at 5 years. The median (range) DFS was 45.8 (1.9-77.5) months. The DFS rates were 70.3% at 1 year, 54.1% at 3 years, and 41.0% at 5 years (Figure 2).
Figure 2. Kaplan-Meier Plots for Overall Survival and Disease-Free Survival .
DFS indicates disease-free survival; OS, overall survival.
Pattern of Recurrence
At the last follow-up, 20 of 38 patients (52.6%) experienced tumor recurrence (eTable 1 in Supplement 2). Among these patients, 14 (36.8%) had an early recurrence (within 2 years after surgery). Intrahepatic and extrahepatic recurrences occurred in 15 patients (39.5%) and 9 patients (23.7%), respectively. Marginal recurrence was detected in 2 patients (5.3%). The most frequent metastatic site was lung (7 [18.4%]). Among the 20 patients with recurrent disease, 15 received salvage treatments, primarily including transarterial chemoembolization (n = 7), sorafenib (n = 3), and radiofrequency ablation (n = 2). The remaining 5 patients received supportive care according to poor performance status or personal will.
Treatment-Related Toxic Effect
Acute RT-related adverse events were observed in 33 of 38 patients (86.8%) (eTable 2 in Supplement 2). Among these, grade 3 adverse events occurred in 3 patients (7.9%), including 1 thrombocytopenia, 1 increased alanine aminotransferase level, and 1 increased aspartate aminotransferase level. No grade 4 or late toxic effect of RT was observed.
The operative variables and complications are summarized in Table 3. The mean (SD) hospital stay was 9.4 (3.6) days. No postoperative bile leakage occurred, and no patient died within 30 days after resection. A total of 19 complications occurred in 13 of 38 patients (34.2%). Morbidities included ascites (n = 5), fever (n = 4), abnormal liver function (n = 3), pleural effusion (n = 2), pneumonia (n = 1), intra-abdominal infection (n = 1), increased creatinine level (n = 1), coagulopathy (n = 1), and gastric retention (n = 1). According to the Clavien-Dindo classification, most patients (12 [31.6%]) suffered from grade I or II operative complications, whereas only 1 patient (2.6%) had grade IIIa operative complication, which was massive pleural effusion and was treated with thoracentesis. No grade IIIb or higher operative complications occurred (Table 3).
Table 3. Operative Variables and Complications.
| Variable | Patients, No. (%) (N = 38) |
|---|---|
| Surgical interval, median (range), wka | 8.2 (4.6-12.0) |
| Hospital stay, mean (SD), d | 9.4 (3.6) |
| Operation time, mean (SD), min | 286.7 (81.3) |
| Type of resection | |
| Anatomic | 11 (28.9) |
| Nonanatomic | 27 (71.1) |
| ICG-R15, mean (SD), % | 8.2 (4.5) |
| Blood loss, mean (SD), mL | 544.6 (454.3) |
| RBC transfusion | 13 (34.2) |
| Plasma transfusion | 18 (47.4) |
| Postoperative bile leakage | 0 |
| 30-d Operative mortality | 0 |
| No. of patients with postoperative complicationsb | 13 (34.2) |
| Grade I | 5 (13.2) |
| Grade II | 7 (18.4) |
| Grade IIIa | 1 (2.6) |
| Grade IIIb-V | 0 |
Abbreviations: ICG, indocyanine green retention; ICG-R15, ICG retention test after 15 minutes; RBC, red blood cell.
Interval between surgery and last radiotherapy.
According to the Clavien-Dindo classification. For patients with more than 1 complication, the higher grade was counted. Complications included ascites (n = 5), fever (n = 4), transient liver dysfunction (n = 3), pleural effusion (n = 2), pneumonia (n = 1), intra-abdominal infection (n = 1), creatinine increase (n = 1), coagulopathy (n = 1), and gastric retention (n = 1).
Discussion
In this nonrandomized controlled trial, a new treatment modality consisting of neoadjuvant IMRT and surgery was performed in 38 patients with centrally located HCC. The 5-year OS was superior to that of patients who received surgery alone in the historical control group.3 In addition, RT-related adverse events and surgical complications were generally mild to moderate, suggesting that this combined treatment strategy is feasible.
Clinical and pathological factors, such as tumor size, number of primary tumors, surgical margin, and presence of microvascular invasion, may change the survival of patients with HCC.28,29,30 Most characteristics in this cohort (including age; underlying hepatitis; liver cirrhosis; tumor location; tumor size; number of primary tumors; tumor differentiation; and presence of tumor thrombosis, microvascular invasion, and satellite nodules) were similar to those in the historical control group,3 making the results comparable. The 5-year OS was 69.1% in this study, which was higher than the 37.2% in the historical control group treated with surgery alone,3 suggesting that neoadjuvant IMRT is associated with improved long-term survival in patients with centrally located HCC.
The increase in survival time may be explained by several aspects. First, wide-margin resection (≥1 cm) has been associated with better OS compared with narrow-margin resection (<1 cm).8,9,31 In this trial, with the addition of neoadjuvant IMRT, the median surgical margin increased from 0 to 1.0 cm, making nearly half of the lesions resectable with wide margins. Second, positive resection margins were present in approximately 5% to 12% of all patients with HCC who underwent surgery and were associated with poor outcome.8,32 This rate was even higher (17%) in patients with narrow resection margins.33 However, only 1 patient (2.6%) had a positive surgical margin in this study, suggesting that neoadjuvant IMRT could be associated with improved OS by increasing the rate of R0 resection. Third, neoadjuvant IMRT potentially decreased recurrence rate. The 5-year DFS rate in this trial was 41.0%, higher than the 16.0% in the historical control group,3 suggesting that neoadjuvant IMRT was a factor in increased OS through decreasing recurrence. Among the 20 patients with recurrence, 14 experienced recurrence within 2 years after surgery. This finding suggests that early recurrence was still the dominant recurrence pattern in neoadjuvant IMRT plus surgery. Thus, a frequent follow-up schedule during the first 2 years is proposed. The 5-year OS in this study (69.1%) was also higher than that in the historical adjuvant RT group (48.4%).3 This finding is consistent with the results of 2 retrospective studies using the Surveillance, Epidemiology, and End Results database,34,35 indicating that neoadjuvant IMRT may provide more survival benefits than adjuvant RT. The underlying mechanisms need to be clarified in future studies.
Radiotherapy dose may be a factor in tumor responses. In this trial, the RT doses (50-60 Gy in 25-30 fractions) delivered to HCCs were higher compared with the 18 Gy in 6 fractions in the study by Wei et al.13 As a result, the objective response rate of primary lesions after RT in the Wei et al study13 was 13%. No patient achieved complete response, whereas 7 patients (8.5%) had progressive disease.13 In contrast, 16 of 38 patients (42.1%) in the present study achieved partial response and none experienced progressive disease. Moreover, major pathological response was achieved in 13 patients (34.2%), including pathologic complete response in 5 patients (13.2%). In a prospective, phase 2 trial by Mornex et al,36 when the 3-dimensional conformal RT dose was increased to 66 Gy in 33 fractions, the objective response rate reached 92%, including a complete response rate of 80%, and there was no progressive disease. These results suggested that higher RT doses may have contributed to better tumor responses. In addition, the interval between the RT and surgery was reported to be associated with the tumor response.37,38 The median interval between last RT and surgery was longer in the present study (8.2 weeks) than in the Wei et al13 study (4 weeks). Longer interval may also partially account for the higher tumor response in this study. However, the optimal RT dose for HCC has not been established, and the optimal RT dose and surgical interval have not been established for centrally located HCC.
Radiotherapy-related adverse effects were mainly mild to moderate, although high doses of IMRT were delivered in this trial. Grade 3 RT-related adverse events occurred in only 3 patients (7.9%), and no grade 4 acute or late toxic effect of RT was observed, reflecting the safety of neoadjuvant IMRT. During surgery, the radiated part of the liver became more solid and less elastic than its counterpart, but it did not hamper the surgical procedure. The structure of the liver parenchyma and vessels after RT could be well identified using an ultrasound knife and cavitron ultrasonic surgical aspirator. The overall operative complication rate was close to that in patients who underwent surgery alone.3 Ascites, fever, transient liver dysfunction, and pleural effusion were the main components of grade I to II operative complications. The only grade IIIa complication was massive pleural effusion managed by thoracentesis. No grade IIIb or higher complications were observed. These results suggested that, in this trial, neoadjuvant IMRT was not a factor in the increased incidence of surgical complications. However, considering the small sample size, further observation in a larger cohort is necessary to identify potential adverse events.
Limitations
This study has several limitations. First, this study had a small sample size, which could lead to inherent biases in patient selection. However, the basic clinical and pathological characteristics of the cohort were similar to those in the historical control group; therefore, the improvement in survival may be reasonably attributed to the addition of neoadjuvant IMRT. Second, the risk factors associated with treatment outcomes were not analyzed mainly because of the small simple size. Thus, further studies are necessary to identify the subgroup population that would more likely benefit from neoadjuvant IMRT. Third, the generalizability of the results may be limited because the epidemiologic characteristics of patients with HCC vary across racial and ethnic groups and regions, and the indications for surgery differ among centers.
Conclusions
Results of this trial suggest that neoadjuvant IMRT plus surgery could be an effective treatment option with acceptable toxic effects for patients with centrally located HCC. We believe that the data are promising for initiating randomized clinical trials to explore the efficacy of this new treatment strategy.
Trial Protocol
eTable 1. Pattern of Recurrence
eTable 2. Acute Radiation-Related Adverse Events
Data Sharing Statement
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Wu C-C, Ho W-L, Chen J-T, et al. Mesohepatectomy for centrally located hepatocellular carcinoma: an appraisal of a rare procedure. J Am Coll Surg. 1999;188(5):508-515. doi: 10.1016/S1072-7515(99)00026-5 [DOI] [PubMed] [Google Scholar]
- 3.Yu W, Wang W, Rong W, et al. Adjuvant radiotherapy in centrally located hepatocellular carcinomas after hepatectomy with narrow margin (<1 cm): a prospective randomized study. J Am Coll Surg. 2014;218(3):381-392. doi: 10.1016/j.jamcollsurg.2013.11.030 [DOI] [PubMed] [Google Scholar]
- 4.Yu WB, Rao A, Vu V, Xu L, Rao JY, Wu JX. Management of centrally located hepatocellular carcinoma: update 2016. World J Hepatol. 2017;9(13):627-634. doi: 10.4254/wjh.v9.i13.627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu J, Chen S, Wu H, Du C. The prognostic value of a classification system for centrally located liver tumors in the setting of hepatocellular carcinoma after mesohepatectomy. Surg Oncol. 2016;25(4):441-447. doi: 10.1016/j.suronc.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Li B, He W, Wei YG, Du ZG, Jiang L. Mesohepatectomy versus extended hemihepatectomy for centrally located hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2014;13(3):264-270. doi: 10.1016/S1499-3872(14)60253-8 [DOI] [PubMed] [Google Scholar]
- 7.Chen XP, Hu DY, Zhang ZW, et al. Role of mesohepatectomy with or without transcatheter arterial chemoembolization for large centrally located hepatocellular carcinoma. Dig Surg. 2007;24(3):208-213. doi: 10.1159/000102901 [DOI] [PubMed] [Google Scholar]
- 8.Aoki T, Kubota K, Hasegawa K, et al. ; Liver Cancer Study Group of Japan . Significance of the surgical hepatic resection margin in patients with a single hepatocellular carcinoma. Br J Surg. 2020;107(1):113-120. doi: 10.1002/bjs.11329 [DOI] [PubMed] [Google Scholar]
- 9.Zhong FP, Zhang YJ, Liu Y, Zou SB. Prognostic impact of surgical margin in patients with hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore). 2017;96(37):e8043. doi: 10.1097/MD.0000000000008043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang WH, Wang Z, Wu JX, et al. Survival benefit with IMRT following narrow-margin hepatectomy in patients with hepatocellular carcinoma close to major vessels. Liver Int. 2015;35(12):2603-2610. doi: 10.1111/liv.12857 [DOI] [PubMed] [Google Scholar]
- 11.Chen B, Wu JX, Cheng SH, et al. Phase 2 study of adjuvant radiotherapy following narrow-margin hepatectomy in patients with HCC. Hepatology. 2021;74:2595-2604. doi: 10.1002/hep.31993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tai A, Erickson B, Khater KA, Li XA. Estimate of radiobiologic parameters from clinical data for biologically based treatment planning for liver irradiation. Int J Radiat Oncol Biol Phys. 2008;70(3):900-907. doi: 10.1016/j.ijrobp.2007.10.037 [DOI] [PubMed] [Google Scholar]
- 13.Wei X, Jiang Y, Zhang X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol. 2019;37(24):2141-2151. doi: 10.1200/JCO.18.02184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li N, Feng S, Xue J, et al. Hepatocellular carcinoma with main portal vein tumor thrombus: a comparative study comparing hepatectomy with or without neoadjuvant radiotherapy. HPB (Oxford). 2016;18(6):549-556. doi: 10.1016/j.hpb.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamiyama T, Nakanishi K, Yokoo H, et al. Efficacy of preoperative radiotherapy to portal vein tumor thrombus in the main trunk or first branch in patients with hepatocellular carcinoma. Int J Clin Oncol. 2007;12(5):363-368. doi: 10.1007/s10147-007-0701-y [DOI] [PubMed] [Google Scholar]
- 16.Des Jarlais DC, Lyles C, Crepaz N; TREND Group . Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94(3):361-366. doi: 10.2105/AJPH.94.3.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47(1):97-104. doi: 10.1002/hep.21966 [DOI] [PubMed] [Google Scholar]
- 18.European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer . EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908-943. doi: 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Feng X, Zhang T, et al. Prospective evaluation of microscopic extension using whole-mount preparation in patients with hepatocellular carcinoma: definition of clinical target volume for radiotherapy. Radiat Oncol. 2010;5:73. doi: 10.1186/1748-717X-5-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao YT, Liu ZK, Wu QW, et al. Observation of different tumor motion magnitude within liver and estimate of internal motion margins in postoperative patients with hepatocellular carcinoma. Cancer Manag Res. 2017;9:839-848. doi: 10.2147/CMAR.S147185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeng KS, Jeng WJ, Sheen IS, Lin CC, Lin CK. Is less than 5 mm as the narrowest surgical margin width in central resections of hepatocellular carcinoma justified? Am J Surg. 2013;206(1):64-71. doi: 10.1016/j.amjsurg.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 22.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52-60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute . Common Terminology Criteria for Adverse Events v.4.03 (CTCAE). Accessed June 14, 2014. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
- 24.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu F, Wang L, Wu J, Rong W, Tian F, Bi C. Analysis of risk factors of recurrence in patients with BCLC 0-A hepatocellular carcinoma after surgical resection [in Chinese]. Zhonghua Yi Xue Za Zhi. 2015;95(22):1747-1750. [PubMed] [Google Scholar]
- 26.Rong W, Yu W, Wu F, et al. Effect of resection margin and tumor number on survival of patients with small liver cancer [in Chinese]. Zhonghua Zhong Liu Za Zhi. 2015;37(12):928-931. [PubMed] [Google Scholar]
- 27.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550-558. doi: 10.1200/JCO.2014.57.9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang XP, Wang K, Gao YZ, et al. ; Chinese National Research Cooperative Group for Diagnosis and Treatment of Hepatocellular Carcinoma with Tumour Thrombus . Prognostic model for identifying candidates for hepatectomy among patients with hepatocellular carcinoma and hepatic vein invasion. Br J Surg. 2020;107(7):865-877. doi: 10.1002/bjs.11524 [DOI] [PubMed] [Google Scholar]
- 29.Nathan H, Schulick RD, Choti MA, Pawlik TM. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009;249(5):799-805. doi: 10.1097/SLA.0b013e3181a38eb5 [DOI] [PubMed] [Google Scholar]
- 30.Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154(1):128-139. doi: 10.1053/j.gastro.2017.09.025 [DOI] [PubMed] [Google Scholar]
- 31.Yang P, Si A, Yang J, et al. A wide-margin liver resection improves long-term outcomes for patients with HBV-related hepatocellular carcinoma with microvascular invasion. Surgery. 2019;165(4):721-730. doi: 10.1016/j.surg.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 32.Nara S, Shimada K, Sakamoto Y, et al. Prognostic impact of marginal resection for patients with solitary hepatocellular carcinoma: evidence from 570 hepatectomies. Surgery. 2012;151(4):526-536. doi: 10.1016/j.surg.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 33.Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000;231(4):544-551. doi: 10.1097/00000658-200004000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin H, Li X, Liu Y, Hu Y. Neoadjuvant radiotherapy provided survival benefit compared to adjuvant radiotherapy for hepatocellular carcinoma. ANZ J Surg. 2018;88(10):E718-E724. doi: 10.1111/ans.14387 [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Guo X, Chen S, et al. Comparison of the efficacy of pre-surgery and post-surgery radiotherapy in the treatment of hepatocellular carcinoma: a population-based study. Am J Transl Res. 2021;13(1):360-371. [PMC free article] [PubMed] [Google Scholar]
- 36.Mornex F, Girard N, Beziat C, et al. Feasibility and efficacy of high-dose three-dimensional-conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies—mature results of the French phase II RTF-1 trial. Int J Radiat Oncol Biol Phys. 2006;66(4):1152-1158. doi: 10.1016/j.ijrobp.2006.06.015 [DOI] [PubMed] [Google Scholar]
- 37.Sanuki N, Takeda A, Mizuno T, et al. Tumor response on CT following hypofractionated stereotactic ablative body radiotherapy for small hypervascular hepatocellular carcinoma with cirrhosis. AJR Am J Roentgenol. 2013;201(6):W812-20. doi: 10.2214/AJR.12.10169 [DOI] [PubMed] [Google Scholar]
- 38.Price TR, Perkins SM, Sandrasegaran K, et al. Evaluation of response after stereotactic body radiotherapy for hepatocellular carcinoma. Cancer. 2012;118(12):3191-3198. doi: 10.1002/cncr.26404 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Pattern of Recurrence
eTable 2. Acute Radiation-Related Adverse Events
Data Sharing Statement