This cross-sectional study investigates the differences in concentration of high-sensitivity cardiac troponin and N-terminal pro–brain natriuretic peptide in healthy transgender men and women compared with that of cisgender men and women.
Key Points
Question
What are the concentration differences in high-sensitivity cardiac troponin (hs-cTn) and N-terminal pro–brain natriuretic peptide (NT-proBNP) between healthy transgender men and women?
Findings
In this cross-sectional study of 79 transgender men and 93 transgender women, the observed differences in cardiac biomarkers were similar to what is seen in cisgender men and women.
Meaning
These findings suggest that sex hormone concentration influences the systemic concentrations of hs-cTn and NT-proBNP in healthy people.
Abstract
Importance
Sex-specific differences in the commonly used cardiac biomarkers high-sensitivity cardiac troponin (hs-cTn) and N-terminal pro–brain natriuretic peptide (NT-proBNP) are apparent. There is an absence of medical literature delineating the concentration differences for these biomarkers in transgender individuals without cardiac disease.
Objective
To determine the distribution of hs-cTn and NT-proBNP in healthy transgender people.
Design, Setting, and Participants
In this cross-sectional prospective study, healthy transgender individuals prescribed testosterone or estradiol for 12 months or more were recruited from internal medicine and primary care clinics that specialize in transgender medical care between November 1, 2017, and July 1, 2018.
Exposures
Testosterone or estradiol for 12 months.
Main Outcomes and Measures
Concentrations for hs-cTnI (troponin I), hs-cTnT (troponin T), and NT-proBNP were measured.
Results
Transgender people prescribed testosterone (n = 79; mean [SD] age, 28.8 [7.8] years) or estrogen (n = 93; mean [SD] age, 35.1 [11.7] years) were recruited. The concentration of hs-cTn was significantly higher in transgender men relative to transgender women. For Abbott hs-cTnI levels, the median (IQR) concentration observed in transgender men and women was 0.9 (0.6-1.7) ng/L and 0.6 (0.3-1.0) ng/L, respectively. Results were similar across 2 additional hs-cTn assays. In contrast, NT-proBNP level was higher in transgender women. The median (IQR) NT-proBNP concentration was significantly higher in transgender women ( 49 [32-86] ng/L) than in transgender men (17 [13-27] ng/L).
Conclusions and Relevance
Findings of this cross-sectional study suggest that the differences in concentration for hs-cTn and NT-proBNP between transgender men and women were similar to what is observed between cisgender men and women. Sex hormones, rather than sex assigned at birth, may be a stronger driver of the observed concentration differences between healthy men and women for biomarkers of cardiac disease.
Introduction
Cardiovascular guidelines recommend that the high-sensitivity cardiac troponin (hs-cTn) 99th percentile of a healthy population should be reported to delineate and facilitate diagnosis of acute myocardial infarction, individual risk of non-ST myocardial infarction, and prediction of outcomes.1 Sex-specific differences in hs-cTn 99th percentile upper reference limits (URLs) are apparent, with female individuals having a lower 99th percentile URL relative to male individuals, but the clinical significance of these differences remains controversial.2
N-terminal pro–brain natriuretic peptide (NT-proBNP) concentrations are higher in female individuals than in male individuals across all ages after puberty, with concentrations rising with age.3 These differences occur in individuals with or without cardiovascular disease.4
Transgender people have a gender that is different from their sex assigned at birth.5 They are commonly prescribed gender-affirming hormones, including testosterone for transgender men and estradiol for transgender women. Scientific literature evaluating and defining cardiac biomarker concentrations in transgender people is, to our knowledge, currently absent. In the current study, we evaluated the concentration distribution of hs-cTn and NT-proBNP in a cohort of healthy transgender adults.
Methods
Patient Recruitment, Questionnaires, and Sample Collection
The Western Institutional Review Board and institutional review board of the University of Iowa approved the study protocol. All participants provided written informed consent. Healthy transgender adults were prospectively recruited from lesbian, gay, bisexual, transgender, and queer-oriented primary care and internal medicine clinics in Seattle, Washington, and Iowa City, Iowa, between November 1, 2017, and July 1, 2018, as previously described.5 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Sample Analysis
The hs-cTnI (troponin I) level was measured with the ARCHITECT STAT High-Sensitivity Troponin I assay (Abbott Diagnostics) and ACCESS High-Sensitivity Troponin I assay (Beckman Coulter Inc). Concentration of hs-cTnT (troponin T) was measured using the Elecsys Troponin T Gen5 STAT (Roche Diagnostics). NT-proBNP concentrations were measured using the Elecsys ProBNP II Immunoassay (Roche Diagnostics). Details of other laboratory measurements have been previously published.5,6 Owing to specimen availability issues, sample size differs between publications by less than 5 individuals.
Statistical Analysis
The 95% CIs for reference limits were calculated using bootstrapping with 10 000 replications. The Wilcoxon rank sum test was used to test for statistical differences between distributions. Data were analyzed using Stata software, version 17 (StataCorp). All P values were 2-sided, and P < .05 was considered statistically significant.
Results
Study Participants
Transgender people prescribed testosterone (n = 79; mean [SD] age, 28.8 [7.8] years) or estrogen (n = 93; mean [SD] age, 35.1 [11.7] years) for more than 1 year were recruited for this analysis (Table). The mean (SD) duration of hormone therapy was 4.8 (4.2) years for transgender men and 3.5 (3.7) years for transgender women. All participants had a hemoglobin A1c (HbA1c) level less than 6.0% (to convert HbA1c level to proportion of total hemoglobin, multiply by 0.01) and an estimated glomerular filtration rate (eGFR) greater than 60 mL/min /1.73m2, regardless of whether male or female constants were applied to the eGFR calculation. Sex hormone, lipid concentrations, and mode of hormone administration are detailed in the Table.
Table. Demographics of Healthy Transgender Cohortsa .
| Characteristic | No. (%) | |
|---|---|---|
| Transgender men (n = 79) | Transgender women (n = 93) | |
| Age, mean (SD) [range], y | 28.8 (7.8) [19-55] | 35.1 (11.7) [18-69] |
| Duration of hormone therapy, mean (SD) [range], y | 4.8 (4.2) [1.0-20.5] | 3.5 (3.7) [1.0-26.0] |
| Mode of hormone administration | ||
| Injection | 73 (92.4) | 29 (31.2) |
| Oral | NA | 9 (9.7) |
| Topical | 5 (6.3) | NA |
| Progesterone (oral) | NA | 12 (12.9) |
| Spironolactone (oral) | NA | 34 (36.6) |
| Both | 1 (1.3) | NA |
| Hormone dose, mean (SD), mg/wk [No./total No.] | ||
| Injection | 73 (13.5) [73/79] | 7.4 (4.1) [29/93] |
| Oral | NA | 5.3 (1.3) [55/93] |
| Topical | 66.7 (28.9) [5/79] | 0.4 (0) [9/93] |
| Estradiol concentration, median (IQR), pg/mL | 51 (37-63) | 207 (114-265) |
| Estradiol level | ||
| Detectable | 47 (59.5) | 91 (97.8) |
| Undetectable | 32 (40.5) | 2 (2.2) |
| Testosterone concentration, median (IQR), ng/mL | 4.6 (3.2-6.2) | 0.4 (0.4-0.7) |
| Testosterone level | ||
| Detectable | 78 (98.7) | 69 (74.2) |
| Undetectable | 1 (1.3) | 24 (25.8) |
| eGFR, mL/min /1.73m2 >60 | ||
| Female | 76 (96.2) | 90 (96.8) |
| Male | 76 (96.2) | 90 (96.8) |
| HbA1c level, median (IQR), % [No./total No.] | 5.0 (4.9-5.3) [78/79] | 5.0 (4.7-5.2) [91/93] |
| Total cholesterol level, median (IQR), mg/dL [No./total No.] | 174.5 (158.0-207.5) [78/79] | 172.0 (147-193) [90/93] |
| LDL cholesterol level, median (IQR), mg/dL [No./total No.] | 110.0 (87.0-127.5) [75/79] | 92.5 (67.3-109.0) [90/93] |
| HDL cholesterol level, median (IQR), mg/dL [No./total No.] | 47.0 (40.0-52.0) [77/79] | 53.5 (48.0-63.0) [90/93] |
| Triglyceride level, median (IQR), mg/dL [No./total No.] | 114.0 (72.0-151.0) [75/79] | 100.5 (70.0-144.3) [90/93] |
Abbreviations: eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NA, not applicable.
SI conversion factor: To convert estradiol to picomoles per liter, multiply by 3.671; to convert testosterone to nanomoles per liter, multiply by 0.0347; to convert HbA1c to proportion of total hemoglobin multiply by 0.01; to convert total cholesterol to millimoles per liter, multiply by 0.0259; to convert LDL cholesterol to millimoles per liter, multiply by 0.0259; to convert HDL cholesterol to millimoles per liter, multiply by 0.0259; to convert triglycerides to millimoles per liter, multiply by 0.0113.
All participants had a body mass index less than 30, with no history of cardiovascular disease, clotting disorders, diabetes, or HIV infection. Sample sizes for each laboratory measurement are listed; sample volume limited measuring all tests in all participants. The 2021 Chronic Kidney Disease Epidemiology Collaboration equation was used, with both the male or female variables, as noted. Undetectable indicates that the sex-hormone concentration was below the assay limit of quantitation.
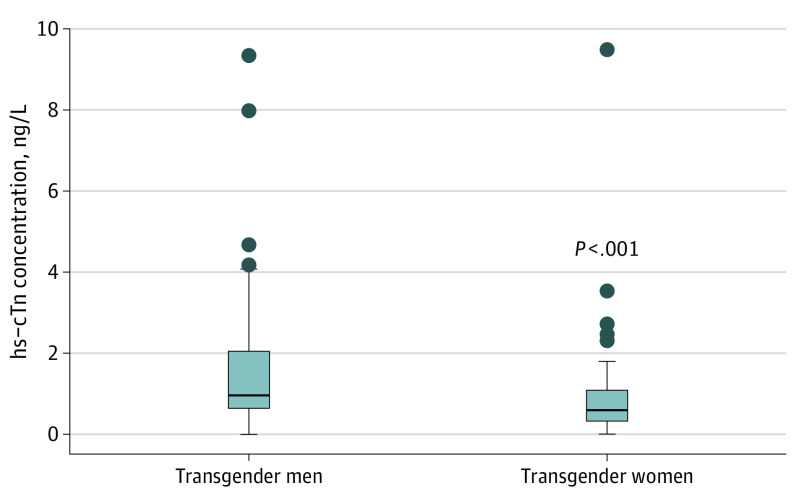
Concentrations of hs-cTnI and hs-cTnT
Concentrations of hs-cTn were higher in transgender men than in transgender women (Figure 1). For Abbott hs-cTnI, the median (IQR) concentration observed in transgender men and women was 0.9 (0.6-1.7) ng/L and 0.6 (0.3-1.0) ng/L, respectively (to convert hs-cTnI level to micrograms per liter, multiply by 0.001). Results were consistent across 2 additional hs-cTn assays (eFigure in the Supplement).
Figure 1. Distribution of High-Sensitivity Cardiac Troponin (hs-cTn) in Transgender Men and Women Using the Abbott ARCHITECT STAT Assay.
The limit of the blank, limit of detection, and limit of quantitation, respectively, for this assay are as follows: 0.7 to 1.3 ng/L, 1.1 to 1.9 ng/L, and 1.3 ng/L. The 99th upper reference limits for the overall, female, and male healthy populations are 26.2 ng/L, 15.6 ng/L, and 34.2 ng/L.
NT-proBNP Concentrations
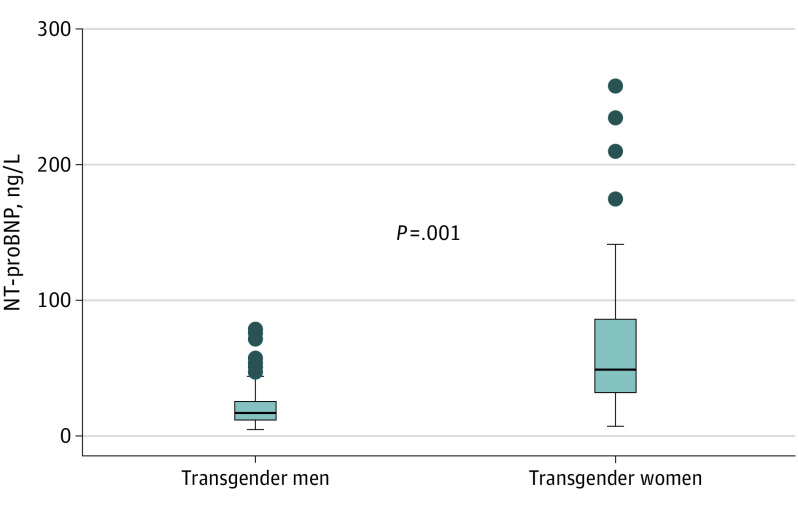
The median NT-proBNP concentration was significantly higher in transgender women (median [IQR], 49 [32-86] ng/L) than in transgender men (median [IQR], 17 [13-27] ng/L; to convert NT-proBNP level to micrograms per liter, multiply by 0.001) (Figure 2).
Figure 2. Distribution of N-Terminal Pro–Brain Natriuretic Peptide (NT-proBNP) in Transgender Men and Women.
Optimal diagnostic cutoffs of 450, 900, and 1800 ng/L for age categories of 50 years or younger, 50 to 75 years, and 75 years or older, respectively, are applied for the identification of acute heart failure, along with an age-independent cutoff of 300 ng/L to exclude acute heart failure.
Discussion
Results of this cross-sectional study suggest that, like healthy cisgender people, transgender men have relatively higher concentrations of hs-cTn and lower concentrations of NT-proBNP compared with transgender women. The magnitude of difference for hs-cTn concentration between the transgender cohorts is consistent with that of the general population, including approximately 20 000 individuals from the Generation Scotland Study.7 Previous publications have illustrated that transgender people may have a slightly higher risk for some cardiovascular diseases.8,9 Although an important observation, the etiology is unclear and hard to differentiate from social determinants of health affecting the transgender population. The observed differences in hs-cTn concentration are likely physiological and not pathological, as concentrations of hs-cTn between healthy cisgender people are also apparent and, as population-based observations, are not thought to portend adverse events. Similarly, teasing out the clinical implications of sex-specific hs-cTn URLs for ruling in acute myocardial infarction is complicated by biological and social factors that both contribute to the poorer outcomes observed in women.2,7 This is despite the observed lower baseline hs-cTn concentrations in women. Ultimately, the psychosocial benefits of gender-affirming hormones are substantial, and informed consent is likely the ideal method to balance the undetermined risks.
Systematic reviews indicate that the hs-cTn concentration differences observed between the sexes lead to differing 99th percentile URLs.2 Some clinical guidelines stress the importance of using sex-specific decision points. Our data suggest that when sex-specific 99th percentiles are used, the numeric value associated with affirmed gender, rather than the sex assigned at birth, may be the appropriate URL and that serial measurements are likely critical for diagnostic efficacy. This study was not powered to accurately calculate 99th percentile URLs, highlighting the need for large population-based studies in transgender persons to guide appropriate cutoffs.
NT-proBNP level is less susceptible to diagnostic differences based on sex, age, or other biological differences. However, in healthy individuals, older age, female sex, and lower body mass index correlate with higher NT-proBNP concentrations.10 NT-proBNP concentrations are consistently higher after puberty in cisgender women than in cisgender men. The mechanism is not completely elucidated, although studies have demonstrated an inverse relationship between serum testosterone level and NT-proBNP concentration after puberty; estradiol concentrations show marginal or no association.3,10 Our data suggest that transgender women have a distribution of NT-proBNP concentrations that is similar to that of cisgender women and that transgender men trend similarly to cisgender men, consistent with a significant hormonal effect on natriuretic peptide concentrations. These differences do not lead to distinct sex-specific NT-proBNP diagnostic thresholds owing to the significant concentration elevations in overt heart failure and cardiovascular disease but likely signify the importance of sex hormone concentrations in cardiac metabolism.
Limitations
As a pilot study, our investigation was not powered to accurately calculate gender-specific hs-cTn 99th percentiles or determine comprehensive reference intervals for NT-proBNP across decades of life for the transgender population. Our study was also limited to evaluating hs-cTn and NT-proBNP concentrations at a single time point for individuals, as opposed to longitudinal changes over time; the duration of gender-affirming therapy needed to show changes in hs-cTn or NT-proBNP concentrations remains unknown.
Conclusions
Results of this cross-sectional study suggest that differences in hs-cTn and NT-proBNP concentrations between healthy transgender women and transgender men were similar to those seen between healthy cisgender women and men, suggesting that sex hormones—not sex assigned at birth—drive these differences. Larger studies are needed to determine the optimal cutoffs (eg, 99th percentile for hs-cTn) for disease diagnosis in transgender men and women. The observed differences in hs-cTn and NT-proBNP concentrations between cisgender women and transgender men, and cisgender men and transgender women, suggest the possibility of adverse cardiac remodeling from gender-affirming therapy, but the clinical implications of the small differences remain unclear and deserve future study. Larger cardiovascular studies are needed in the transgender population, ideally with outcomes and more in-depth clinical characterization, including baseline echocardiogram and cardiac size. Gender-affirming therapy is essential to the overall well-being and health of transgender persons, and more research is needed to quantify any potential adverse cardiac consequences and develop better strategies to optimize cardiac health in this population.
eFigure. Distribution of hs-cTnI in Transgender Men and Women Using the Beckman ACCESS hs-cTnI and Roche TnT Gen 5 STAT Assay
References
- 1.Wu AHB, Christenson RH, Greene DN, et al. Clinical laboratory practice recommendations for the use of cardiac troponin in acute coronary syndrome: expert opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem. 2018;64(4):645-655. doi: 10.1373/clinchem.2017.277186 [DOI] [PubMed] [Google Scholar]
- 2.Kimenai DM, Janssen EBNJ, Eggers KM, et al. Sex-specific vs overall clinical decision limits for cardiac troponin I and T for the diagnosis of acute myocardial infarction: a systematic review. Clin Chem. 2018;64(7):1034-1043. doi: 10.1373/clinchem.2018.286781 [DOI] [PubMed] [Google Scholar]
- 3.Saenger AK, Dalenberg DA, Bryant SC, Grebe SK, Jaffe AS. Pediatric brain natriuretic peptide concentrations vary with age and sex and appear to be modulated by testosterone. Clin Chem. 2009;55(10):1869-1875. doi: 10.1373/clinchem.2009.123778 [DOI] [PubMed] [Google Scholar]
- 4.Fradley MG, Larson MG, Cheng S, et al. Reference limits for N-terminal pro–brain natriuretic peptide in healthy individuals (from the Framingham Heart Study). Am J Cardiol. 2011;108(9):1341-1345. doi: 10.1016/j.amjcard.2011.06.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene DN, McPherson GW, Rongitsch J, et al. Hematology reference intervals for transgender adults on stable hormone therapy. Clin Chim Acta. 2019;492:84-90. doi: 10.1016/j.cca.2019.02.011 [DOI] [PubMed] [Google Scholar]
- 6.Humble RM, Greene DN, Schmidt RL, et al. Reference intervals for clinical chemistry analytes for transgender men and women on stable hormone therapy. J Appl Lab Med. Published online May 18, 2022. doi: 10.1093/jalm/jfac025 [DOI] [PubMed] [Google Scholar]
- 7.Kimenai DM, Shah ASV, McAllister DA, et al. Sex differences in cardiac troponin I and T and the prediction of cardiovascular events in the general population. Clin Chem. 2021;67(10):1351-1360. doi: 10.1093/clinchem/hvab109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein Z, Streed C, Resiman T, Mukherjee M, Radix A. Cross-sex hormones and acute cardiovascular events in transgender persons. Ann Intern Med. 2019;170(2):142-143. doi: 10.7326/L18-0563 [DOI] [PubMed] [Google Scholar]
- 9.Streed CG Jr, Beach LB, Caceres BA, et al. ; American Heart Association Council on Peripheral Vascular Disease; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; Council on Hypertension; and Stroke Council . Assessing and addressing cardiovascular health in people who are transgender and gender diverse: a scientific statement from the American Heart Association. Circulation. 2021;144(6):e136-e148. doi: 10.1161/CIR.0000000000001003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suthahar N, Meems LMG, Ho JE, de Boer RA. Sex-related differences in contemporary biomarkers for heart failure: a review. Eur J Heart Fail. 2020;22(5):775-788. doi: 10.1002/ejhf.1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Distribution of hs-cTnI in Transgender Men and Women Using the Beckman ACCESS hs-cTnI and Roche TnT Gen 5 STAT Assay