Abstract
There is evidence that men are more likely to undergo deep brain stimulation (DBS) for Parkinson’s disease (PD), suggesting that women are relatively undertreated. 121 consecutive PD patients undergoing awake DBS with microelectrode recording and intraoperative clinical testing (30 patients, 5 women) or asleep MRI-guided and CT-verified (91 patients, 38 women) bilateral subthalamic nucleus DBS were included in this study. The results showed an increase in the proportion of female patients from 16.7% to 41.8% after changing our operative technique (OR = 5.61; 95% CI: 1.52–20.78; p = 0.010) from awake to asleep, suggesting that women are more likely to undergo DBS when operated asleep.
Keywords: Deep brain stimulation, gender, asleep, awake, MRI-guided, microelectrode recording
INTRODUCTION
Parkinson’s disease (PD) affects men and women differently in various ways. For example, PD is more common in men [1, 2], and the disease presentation and healthcare behavior show considerable differences between genders. A recent review showed that men are more likely than women to undergo deep brain stimulation (DBS) of the subthalamic nucleus (STN), and this difference is greater than what can be expected based on prevalence alone [3]. It could therefore be that women with PD are relatively undertreated. After we changed the operative method at our center from an awake surgical procedure with microelectrode recording (MER) and intraoperative clinical testing (ICT) to an asleep MRI-guided and CT-verified approach, we noticed a change in gender distribution.
METHODS
121 consecutive patients, who underwent awake (30 patients, 5 women) or asleep (91 patients, 38 women) bilateral STN-DBS between 20 March 2018 and 21 September 2021, were included. Baseline data included age, gender, disease duration, Unified Parkinson’s Disease Rating Scale part I-IV (part III ON and OFF medication), PDQ-39, Parkinson Anxiety Scale total score, Beck Depression Inventory, Montreal Cognitive Assessment, Questionnaire for Impulse Control Disorders in PD, Disease Cognition List total score, and Epworth Sleepiness Scale. On 1 July 2019 we changed our operative technique from awake MER-guided surgery to asleep MRI-guided and CT-verified surgery. There was no change in the way patients were recruited, screened, or considered suitable for surgery. All personnel and all preoperative procedures remained identical. Multinomial logistic regression was used to assess the relationship between type of surgery (asleep vs. awake, dependent variable) and gender (men vs. women, factor), with the other studied measures as covariates (Table 1). Statistical significance was set at p < 0.05.
Table 1.
Multinomial logistic regression with gender as factor, type of surgery as dependent variable and covariates listed in the table below. The means and standard deviations (SD) of the mean, as well as the odds ratio, the lower and upper boundary of the 95% confidence interval (CI) and the p-value are given. UPDRS, Unified Parkinson’s Disease Rating Scale; PDQ-39, Parkinson’s DiseaseQuestionnaire 39; PAS, Parkinson Anxiety Scale; BDI, Beck Depression Inventory;MoCA, Montreal Cognitive Assessment; QUIP, Questionnaire for Impulse Control Disorders in PD; ZCL, Disease Cognition List; ESS, Epworth Sleepiness Scale
| Gender | Type of surgery | Mean | SD | Odds ratio | 95% CI | 95% CI | p |
| Lower boundary | Upper boundary | ||||||
| Age | 1.005 | 0.944 | 1.071 | 0.871 | |||
| Women | Asleep | 62.42 | 1.16 | ||||
| Awake | 64.80 | 1.69 | |||||
| Men | Asleep | 62.04 | 1.37 | ||||
| Awake | 61.00 | 3.78 | |||||
| Disease duration | 1.073 | 0.933 | 1.234 | 0.321 | |||
| Women | Asleep | 9.32 | 3.07 | ||||
| Awake | 10.06 | 2.61 | |||||
| Men | Asleep | 9.21 | 3.48 | ||||
| Awake | 9.71 | 4.15 | |||||
| UPDRS I | 0.943 | 0.929 | 1.072 | 0.369 | |||
| Women | Asleep | 12.26 | 5.31 | ||||
| Awake | 11.20 | 2.39 | |||||
| Men | Asleep | 10.38 | 4.32 | ||||
| Awake | 9.20 | 5.32 | |||||
| UPDRS II | 0.976 | 0.829 | 1.072 | 0.592 | |||
| Women | Asleep | 15.39 | 6.41 | ||||
| Awake | 17.20 | 4.15 | |||||
| Men | Asleep | 16.25 | 8.37 | ||||
| Awake | 14.40 | 6.08 | |||||
| UPDRS III OFF | 0.990 | 0.938 | 1.045 | 0.725 | |||
| Women | Asleep | 49.53 | 12.56 | ||||
| Awake | 50.40 | 11.44 | |||||
| Men | Asleep | 51.79 | 13.19 | ||||
| Awake | 49.60 | 13.48 | |||||
| UPDRS III ON | 0.968 | 0.903 | 1.038 | 0.361 | |||
| Women | Asleep | 19.79 | 8.49 | ||||
| Awake | 20.40 | 12.10 | |||||
| Men | Asleep | 20.94 | 10.75 | ||||
| Awake | 19.24 | 9.98 | |||||
| UPDRS IV | 0.861 | 0.737 | 1.005 | 0.058 | |||
| Women | Asleep | 11.37 | 3.12 | ||||
| Awake | 11.00 | 1.41 | |||||
| Men | Asleep | 10.26 | 3.62 | ||||
| Awake | 9.40 | 3.74 | |||||
| PDQ-39 | 0.610 | 1.006 | 0.984 | 1.028 | |||
| Women | Asleep | 57.18 | 16.64 | ||||
| Awake | 62.80 | 20.39 | |||||
| Men | Asleep | 44.64 | 20.82 | ||||
| Awake | 45.88 | 19.19 | |||||
| PAS Total | 0.973 | 0.887 | 1.069 | 0.570 | |||
| Women | Asleep | 13.95 | 6.17 | ||||
| Awake | 16.00 | 7.18 | |||||
| Men | Asleep | 10.45 | 5.76 | ||||
| Awake | 9.52 | 7.04 | |||||
| BDI | 1.011 | 0.911 | 1.120 | 0.845 | |||
| Women | Asleep | 11.47 | 5.73 | ||||
| Awake | 13.20 | 3.42 | |||||
| Men | Asleep | 11.17 | 6.69 | ||||
| Awake | 10.39 | 5.20 | |||||
| MoCA | 1.079 | 0.925 | 1.258 | 0.334 | |||
| Women | Asleep | 25.89 | 3.11 | ||||
| Awake | 25.60 | 2.88 | |||||
| Men | Asleep | 25.36 | 4.52 | ||||
| Awake | 25.95 | 1.88 | |||||
| QUIP | 0.955 | 0.824 | 1.107 | 0.541 | |||
| Women | Asleep | 25.89 | 2.22 | ||||
| Awake | 25.60 | 0.00 | |||||
| Men | Asleep | 25.36 | 4.03 | ||||
| Awake | 25.95 | 3.68 | |||||
| DCL Total | 0.992 | 0.909 | 1.081 | 0.848 | |||
| Women | Asleep | 42.63 | 6.84 | ||||
| Awake | 40.60 | 2.51 | |||||
| Men | Asleep | 44.42 | 6.16 | ||||
| Awake | 44.08 | 5.15 | |||||
| ESS | 0.802 | 0.661 | 0.973 | 0.072 | |||
| Women | Asleep | 5.84 | 3.85 | ||||
| Awake | 3.80 | 2.68 | |||||
| Men | Asleep | 5.50 | 3.32 | ||||
| Awake | 4.16 | 2.39 |
RESULTS
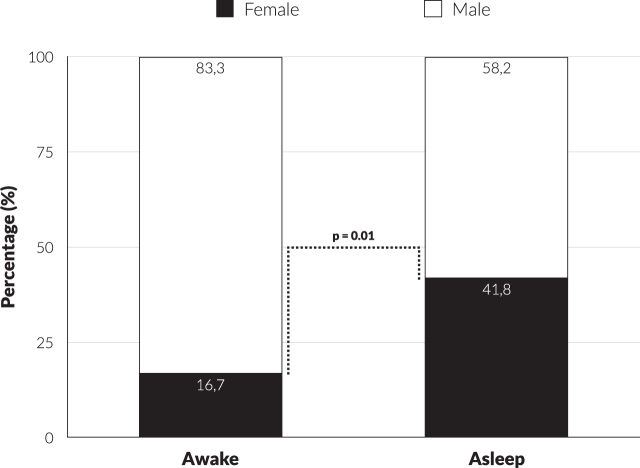
The difference in gender distribution for both types of surgery is shown in Fig. 1. The proportion of female patients increased significantly from 16.7% to 41.8% after the change in operative method (OR = 5.61; 95% CI: 1.52–20.78; p = 0.010). The other investigated factors did not reach statistical significance (Table 1). Nevertheless, there was a trend towards statistical significance for UPDRS part IV in favor of women (OR = 0.861; 95% CI: 0.737–1.005; p = 0.058). There was a similar trend for the Epworth Sleepiness Scale (ESS) (OR = 0.802; 95% CI: 0.661–0.973; p = 0.072).
Fig. 1.
Difference in distribution gender x type of surgery (awake vs. asleep).
DISCUSSION
Our findings provide a first indication that women are more likely to undergo STN-DBS when an asleep MRI-guided and CT-verified operative method was used. In fact, this change normalized the female-male ratio to closely the ratio as expected in the general population of PD. Similar findings have been reported for other neurosurgical treatment options for PD, such as pallidotomy and thalamotomy [4]. Furthermore, differences in gender distribution have been reported for surgery other than neurosurgery [5]. Prior to this switch, the gender distribution at our center resembled the international experience, with a predominance of men opting for surgery at the time when this was still performed awake. The reason why men were previously more likely to undergo an awake surgical procedure is unclear. A possible explanation is gender referral bias [3]. However, this is unlikely since preoperative recruitment and screening were not changed. Furthermore, women with PD tend to be more anxious than men [1, 6], which might lead to women avoiding surgery [7]. However, our analysis showed no gender differences in anxiety. We also believe that our results were not influenced by any cultural differences, as all except for four (1 female) out of 121 patients had the same cultural background. We cannot rule out that some differences were missed, because of the unequal sample sizes resulting in a wide 95% CI. There was a trend towards statistical significance for UPDRS part IV in favor of women. This would be consistent with the existing literature that women are more likely to develop dyskinesias [1]. Regarding the similar trend in the ESS, both means would classify as normal based on the scale-specific cutoff criteria [8]. Therefore, this trend is not clinically relevant. Further prospective studies should confirm this finding, which may help to alleviate undesirable gender differences in Parkinson’s care.
CONFLICT OF INTEREST
RSV acts as an independent consultant for Boston Scientific. The other authors have nothing to disclose.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- [1]. Georgiev D, Hamberg K, Hariz M, Forsgren L, Hariz GM (2017) Gender differences in Parkinson’s disease: A clinical perspective. Acta Neurol Scand 136, 570–584. [DOI] [PubMed] [Google Scholar]
- [2]. Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA (2016) Time trends in the incidence of Parkinson disease. JAMA Neurol 73, 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Hariz GM, Nakajima T, Limousin P, Foltynie T, Zrinzo L, Jahanshahi M, Hamberg K (2011) Gender distribution of patients with Parkinson’s disease treated with subthalamic deep brain stimulation; a review of the 2000-2009 literature. Parkinsonism Relat Disord 17, 146–149. [DOI] [PubMed] [Google Scholar]
- [4]. Hariz GM, Lindberg M, Hariz MI, Bergenheim AT (2003) Gender differences in disability and health-related quality of life in patients with Parkinson’s disease treated with stereotactic surgery. Acta Neurol Scand 108, 28–37. [DOI] [PubMed] [Google Scholar]
- [5]. Rucker D, Warkentin LM, Huynh H, Khadaroo RG (2019) Sex differences in the treatment and outcome of emergency general surgery. PLoS One 14, e0224278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Picillo M, Amboni M, Erro R, Longo K, Vitale C, Moccia M, Pierro A, Santangelo G, De Rosa A, De Michele G, Santoro L, Orefice G, Barone P, Pellecchia MT (2013) Gender differences in non-motor symptoms in early, drug naive Parkinson’s disease. J Neurol 260, 2849–2855. [DOI] [PubMed] [Google Scholar]
- [7]. Hamberg K, Hariz GM (2014) The decision-making process leading to deep brain stimulation in men and women with parkinson’s disease - an interview study. BMC Neurol 14, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Johns MW (1991) A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 14, 540–545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.