Abstract
Background:
Isolated rapid eye movement sleep behavior disorder (iRBD) is prodromal for Parkinson’s disease (PD) and dementia with Lewy bodies (DLB).
Objective:
We investigated the use of cardiac [123I]meta-iodo-benzyl-guanidine scintigraphy ([123I]MIBG) and olfactory testing— in comparison to [123I]N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)nortropane single photon emission computed tomography ([123I]FP-CIT-SPECT)— for identifying iRBD patients as prodromal phenotype of PD/DLB.
Methods:
37 RBD subjects underwent cardiac [123I]MIBG and brain [123I]FP-CIT-SPECT at baseline. Olfactory (Sniffin’ Sticks), cognitive and motor functions were tested annually for ∼4 years.
Results:
29/37 (78.4%) subjects had a pathological [123I]MIBG, of whom 86.2% (25/29) presented at least a moderate hyposmia at baseline (threshold/discrimination/identification-(TDI-)score ≤25). 20/37 (54.1%) subjects had a pathological [123I]FP-CIT-SPECT, always combined with a pathological [123I]MIBG. In subjects with pathological [123I]MIBG, olfactory function worsened (mainly due to threshold and discrimination subscores) from baseline to follow-up (p = 0.005). Olfaction was more impaired in subjects with pathological [123I]MIBG compared to those with normal [123I]MIBG at baseline (p = 0.001) and follow-up (p < 0.001). UPDRS-III scores increased in subjects with both pathological [123I]MIBG and [123I]FP-CIT-SPECT. In this group, seven subjects phenoconverted to PD, all— except for one— presented with at least moderate hyposmia at baseline.
Conclusion:
A combination of the biomarkers “pathological [123I]MIBG” and “hyposmia” likely identifies iRBD patients in an early prodromal stage of PD/DLB, i.e., before nigrostriatal degeneration is visualized. One-third of the subjects with pathological [123I]MIBG had a normal [123I]FP-CIT-SPECT. Noteworthy, in iRBD subjects with pathological [123I]MIBG, olfactory impairment is progressive independent of the [123I]FP-CIT-SPECT status.
Keywords: Biomarker, cardiac [123I]MIBG scintigraphy, [123I]FP-CIT-SPECT, hyposmia, isolated rapid eye movement sleep behavior disorder, prodromal progression marker
INTRODUCTION
Patients with isolated REM sleep behavior disorder (iRBD) are at high risk to develop an alpha-synucleinopathy (aSYN), like Parkinson’s disease (PD), dementia with Lewy bodies (DLB), and rarely multiple system atrophy (MSA) [1–4]. iRBD is considered to be a specific prodromal stage of aSYNs with a strong peripheral component [5, 6]. Thus, iRBD patients represent a suitable population to study disease-modifying therapies, aiming to slow down or even prevent the conversion to manifest aSYNs.
The crucial prerequisite of such studies is to identify a cohort of iRBD patients as homogeneous as possible. The diagnosis of RBD is based on a clinical history of dream enactment during REM-sleep and the demonstration of REM-sleep without atonia in the mandatory video-assisted polysomnography [7]. iRBD has to be distinguished from secondary RBD which is associated with manifest neurodegenerative disorders, narcolepsy type-1, or develops as a drug adverse effect (e.g., antidepressants) [8]. In order to reliably identify iRBD, early biomarkers for prodromal aSYN are needed.
We therefore selected three accepted biomarkers for prodromal PD/DLB [9]: reduced cardiac [123I]meta-iodo-benzyl-guanidine scintigraphy ([123I]MIBG), hyposmia as tested by the full range Sniffin’ Sticks test (threshold, discrimination, identification (TDI)-score) and reduced brain dopamine transporter ligand (DAT)-binding with [123I]N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) single photon emission computed tomography ([123I]FP-CIT-SPECT) for a three-tiered phenotyping strategy.
[123I]MIBG visualizes postganglionary sympathetic cardiac innervation. Several studies showed reduced [123I]MIBG uptake in iRBD compared to healthy controls [6, 10]. Furthermore, it has been reported to differentiate between iRBD and secondary RBD, as the latter group presents with a normal [123I]MIBG[11, 12].
Hyposmia as demonstrated with the odor identification test is common in iRBD [2, 13, 14] and is observed > 20 years before conversion to aSYN [15]. Both pathological [123I]MIBG and hyposmia seem to occur early in the course of iRBD (Braak stage 1 and 2), and accordingly, pathological [123I]MIBG has been reported to precede nigrostriatal dopaminergic deficits [6, 16].
[123I]FP-CIT-SPECT is a biomarker of the dopaminergic nigrostriatal tract. Reduced striatal [123I]FP-CIT-binding is associated with subclinical neurodegeneration of this tract in Braak stage 3 and during the transition from Braak stage 3 to 4 [17].
By correlating the results of the three-tiered phenotyping ([123I]MIBG, olfactory testing, [123I]FP-CIT-SPECT) at baseline with annual 4-year clinical follow-up data, we wanted to know if a pathological [123I]MIBG and hyposmia (TDI-score ≤25) are suitable screening tools to identify iRBD patients early in the prodromal stage of PD/DLB even if [123I]FP-CIT-SPECT is (still) normal. Furthermore, we wanted to know whether both markers can select those who will not phenoconvert. Finally, we investigated whether the olfactory testing with the full range Sniffin’ sticks reveals a progressive impairment of olfactory function in RBD.
MATERIALS AND METHODS
This prospective cohort study included RBD patients between 2013/01/01 to 2017/04/30 who underwent annual follow-up as part of the RBD registry study at the Philipps-University Marburg (UMR), Germany. The study protocol was approved by the institutional review board. Voluntary informed consent was obtained from each subject at baseline after verbal and written explanation of the study, in accordance with the Declaration of Helsinki.
Participants
Thirty-seven subjects fulfilled inclusion criteria with video-polysomnography confirmed RBD [7]. On purpose, RBD subjects with a history of or still ongoing antidepressant pharmacological therapy (ADT) were not excluded. [123I]MIBG and [123 I]FP-CIT-SPECT were performed within the routine diagnostic scheme. Subjects with diseases (heart/kidney failure, myocardial infarction≤five years, diabetes, amyloid or other neuropathy, pheochromocytoma) and/or intake of certain medications (reserpine, opioids, labetalol, phenylpropanolamine, phenylephrine) which may affect [123I]MIBG results were excluded. Starting at baseline, medical history, clinical testing and neurological examination were assessed annually. In six RBD subjects, the baseline visit was more than six months after the time of diagnosis (range 9–96 months). Four RBD subjects received the [123I]MIBG scintigraphy more than six months (15, 22, 60, and 64 months later) after the [123 I]FP-CIT-SPECT and the baseline visit. See Table 1.
Table 1.
Overview of the demographic and clinical data
| [123I]MIBG category | [123I]FP-CIT-SPECT category | No. | Gender | Age at diagnosis (y) | Duration of symptoms at diagnosis (mo) | Diagnosis to last fu or PC (mo) | TDI (bl) | TDI (fu) | UPDRS-III (bl) | UPDRS-III (fu) | MoCA (bl) | MoCA (fu) | Obstipation (bl or fu) | ADT/beginning of ADT in relation to start of RBD symptoms |
| Normal | normal | 1 | f | 49 | 12 | 65 | 36.00 | 32.00 | 1 | 3 | 27 | 27 | No | (venlafaxine/in the past) |
| 2# | m | 57 | 44 | 69 | 33.75 | 28.50 | 9 | 2 | 25 | 28 | No | venlafaxine+agomelatine /after | ||
| 3 | f | 68 | 99 | 74 | 33.50 | 35.25 | 2 | 3 | 23 | 29 | No | fluoxetine/before | ||
| 4# | m | 58 | 74 | 77 | 29.50 | 33.50 | 4 | 9 | 26 | 28 | No | |||
| 5 | m | 60 | 14 | 18 | 27.75 | 28.50 | 0 | 0 | 30 | 30 | No | duloxetine+trimipramine/before | ||
| 6 | m | 62 | 118 | 13 | 23.50 | 27.50 | 5 | 2 | 24 | 22 | No | |||
| 7 | m | 57 | 9 | 44 | 20.50 | 31.75 | 6 | 3 | 27 | 29 | Yes | citalopram+trimipramine/before | ||
| 8 | m | 66 | 257 | 53 | 19.50 | 21.75 | 0 | 1 | 29 | 29 | Yes | citalopram/n.a. | ||
| Abnormal | 9 | m | 57 | 120 | 67 | 29.00 | 18.50 | 2 | 0 | 27 | 29 | Yes | ||
| 10 | m | 63 | 21 | 68 | 24.25 | 9.00 | 2 | 2 | 26 | 28 | Yes | |||
| 11 | m | 58 | 39 | 27 | 19.50 | 14.00 | 0 | 3 | 26 | 28 | Yes | duloxetine/after | ||
| 12 | m | 74 | 93 | 41 | 16.00 | 13.50 | 3 | 2 | 29 | 27 | Yes | |||
| 13# | f | 70 | 20 | 91 | 16.00 | 8.00 | 2 | 3 | 24 | 30 | Yes | |||
| 14 | m | 68 | 171 | 54 | 12.25 | 12.00 | 7 | 3 | 24 | 26 | No | citalopram+quetiapine/after | ||
| 15 | m | 57 | 56 | 9 | 9.00 | 12.75 | 0 | 0 | 30 | 30 | Yes | |||
| 16 | m | 73 | 182 | 58 | 2.00 | 2.00 | 3 | 2 | 24 | 29 | Yes | |||
| 17 | m | 66 | 40 | 39 | 0.00 | 4.00 | 3 | 3 | – | 29 | Yes | |||
| abnormal | 18* | m | 60 | 62 | 115 | 27.50 | 18.25 | 4 | 3 | 28 | 28 | Yes | ||
| 19 | f | 79 | 62 | 36 | 27.50 | 25.00 | 2 | 7 | 28 | 28 | Yes | duloxetin/n.a. | ||
| 20* | m | 66 | 15 | 52 | 24.75 | 23.50 | 0 | 4 | 28 | 29 | No | duloxetine/after | ||
| 21 | m | 68 | 27 | 49 | 20.50 | 16.00 | 1 | 3 | 27 | 30 | Yes | venlafaxine/before | ||
| 22 | m | 67 | 59 | – | 18.25 | – | 0 | – | 25 | – | Yes | |||
| 23 | m | 65 | 44 | – | 17.75 | 23.70 | 0 | – | 29 | – | Yes | paroxetine/after | ||
| 24 | m | 69 | 363 | 36 | 16.50 | 14.00 | 1 | 1 | 29 | 30 | No | |||
| 25 | m | 65 | 64 | 68 | 13.00 | 11.00 | 6 | 3 | 27 | 27 | No | |||
| 26 | m | 66 | 72 | 47 | 13.00 | 8.00 | 3 | 8 | 27 | 27 | Yes | |||
| 27 | m | 61 | 47 | 62 | 12.00 | 15.50 | 0 | 0 | 26 | 30 | Yes | |||
| 28* | f | 63 | 24 | 132 | 10.00 | 11.50 | 0 | 2 | 26 | 20 | No | |||
| 29# | m | 72 | 14 | 58 | 0.00 | – | 5 | 4 | 22 | 28 | Yes | |||
| 30* | m | 71 | 3 | 36 | 0.00 | 0.00 | 4 | 4 | 27 | 28 | Yes | |||
| 31 | m | 62 | 60 | 50 | 31.50 | 26.50 | 1 | 6 | 25 | 27 | No | citalopram/after | ||
| 32 | m | 72 | 69 | 35 | 22.75 | 20.50 | 2 | 9 | 30 | 29 | Yes | |||
| 33 * | m | 56 | 14 | 128 | 21.00 | 13.50 | 2 | 1 | 29 | 30 | No | |||
| 34 * | m | 64 | 104 | 64 | 19.50 | 14.00 | 0 | 9 | 30 | 28 | Yes | |||
| 35 | m | 72 | 123 | 47 | 18.50 | 12.00 | 5 | 13 | 27 | 29 | yes | |||
| 36 | m | 49 | 240 | 61 | 2.00 | 6.00 | 1 | 7 | 24 | 25 | No | |||
| 37 * | m | 61 | 12 | 90 | 0.00 | 0.00 | 1 | 8 | 28 | 28 | No |
Results are ordered according to [123I]MIBG and [123I]FP-CIT-SPECT results and threshold/discrimination/identification (TDI) score. Bold denotes the seven phenoconverted subjects. y, year; mo, months; bl, baseline; fu, follow-up; ADT, antidepressant therapy; PC, phenoconversion; UPDRS-III, Unified PD rating scale; MoCA, Montreal Cognitive Assessment. *These subjects underwent [123I]FP-CIT-SPECT 16–95 months after PSG-confirmed RBD diagnosis. #These subjects underwent [123I]MIBG scintigraphy more than six months after the [123 I]FP-CIT-SPECT and the baseline visit (15–64 months later).
Clinical testing
All subjects were evaluated with the motor part of the Unified PD rating scale (UPDRS-III) [18]; the Montreal Cognitive Assessment (MoCA) [19]; the PD Nonmotor Symptoms Questionnaire (PDNMS) [20] and the Sniffin’ Sticks [21] at baseline and annual follow-up for ∼4 years. Phenoconversion to aSYN (PD, DLB, MSA) was diagnosed by a neurologist (AJ) according to the published diagnostic criteria [22–24]. The diagnosis of manifest aSYN had to be confirmed by a second neurological examination after 3 months.
[123I]MIBG
At baseline, all subjects underwent cardiac [123I]MIBG scintigraphy (performed according to the standard operating procedures of the Department of Nuclear Medicine, UMR, Germany): After blocking of the gastric and thyroid sodium iodide symporters, images were acquired 4 h after injection of 185 MBq (±10%) [123I]MIBG (AdreView Iobenguane, GE Healthcare, Braunschweig, Germany) using a dual-head gamma camera with a low energy high resolution collimator (Siemens, Symbia, Erlangen, Germany) at a window setting of 159 keV (±10%). Regions of interest (ROI) were manually placed on planar anterior images. A rectangular ROI was used for the mediastinum and a circular one for the left ventricle of the heart. According to the in-house code, the heart-to-mediastinum ratio of [123I]MIBG-binding of < 1.5 was considered to be pathological.
Olfaction
The olfactory function was assessed with the full range Sniffin’ Sticks consisting of threshold (T), discrimination (D), and identification (I) of odors [21, 25]. Threshold subtest: after learning the odor n-butanol by presenting the pen with the highest concentration, the subjects were repeatedly presented three pens: two blanks and one with the odor n-butanol in 16 different concentrations (1 = highest concentration, 16 = lowest concentration). During the test, the so-called turning point was determined seven times, this corresponds to the highest odor dilution that was correctly detected by the patient twice in one run. The threshold score was the average value of the dilution steps of the last four turning points (range 1–16). Discrimination subtest: three pens— two with the same and one with a different odor— were presented. The subject always had to identify the one different smelling pen. This task was repeated 16 times in 30 s time intervals. The (D) score was the sum of correct answers ranging from 0–16. Identification subtest: subjects were presented 16 different odors and were asked to identify these from a given choice of four possibilities. The number of correct answers resulted in the identification score (range 0–16). During the threshold and discrimination tests, subjects were blindfolded. The sum of the threshold, discrimination and identification subtests results give a TDI-score that was categorized into: anosmia (≤15 points), severe hyposmia (16–20 points), moderate hyposmia (21–25 points), mild hyposmia (26–30 points) and normosmia (≥31 points) [26].
[123I]FP-CIT-SPECT
All subjects underwent a [123I]FP-CIT-SPECT at baseline. Details on scanning, reconstruction, and analysis methods and protocols have been previously published [27].
Statistical analysis
All values are given as median and interquartile range (IQR). Due to the small subject number, non-parametric tests were used: the Mann-Whitney-U-test to analyze changes between the subgroups at baseline and at last follow-up; a one-sample Wilcoxon signed-rank test for changes within subgroups from baseline to last follow-up. Values were considered significant if p < 0.05. To adjust for multiple comparisons the Bonferroni’s method was performed in the comparisons between the three subgroups at baseline and follow-up (adjusted p value p < 0.017).
For statistical analysis IBM® SPSS® statistics Version 27.0 was used.
RESULTS
Overview
Altogether, 37 RBD subjects were included in the study at baseline. Out of these, 35 subjects were followed over 49.1±18.6 months (see Fig. 1, Table 1) whereas two had no follow-up visits: one died of cardiac disease, the second one self-reported no cognitive or motor impairment five years after diagnosis. Another two subjects had only one follow-up visit: the first had a one-year-follow-up without phenoconversion and subsequently died of cardiac disease, the second one had a 9-months follow-up. For the demographic and clinical data of the whole group see Supplementary Table 1.
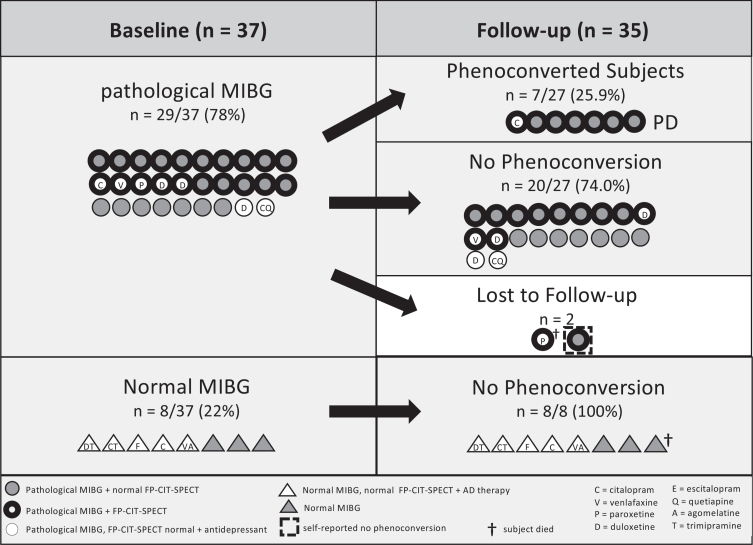
Fig. 1.
Stratification of patients solely based on cardiac [123I]MIBG scintigraphy. Number of RBD subjects with pathological and normal [123I]MIBG uptake at baseline (n = 37) and phenoconversion rates at follow-up (n = 35) are shown. Marked is also how many subjects with abnormal cardiac [123I]MIBG uptake had an abnormal [123I]FP-CIT SPECT scan, and how many subjects were on antidepressants (and type of antidepressant). Of note, only subjects with both a pathological [123I]MIBG scan and [123I]FP-CIT-SPECT phenoconverted to PD (n = 7).
Stratification according to [123I]MIBG
Subjects were stratified according to [123I]MIBG results: At baseline, 29/37 (78.4%) subjects had pathological [123I]MIBG (RBDpMIBG), while 8/37 (21.6%) subjects presented with normal [123I]MIBG (RBDnMIBG). The RBDpMIBG group was older than the RBDnMIBG group (RBDpMIBG: 66.0 (62.5–71.5), RBDnMIBG: 58.5 (53.8–65.0); p = 0.014). There was no difference in the duration of RBD symptoms at the time of diagnosis and of the follow-up time. (see Table 2)
Table 2.
A) Overview of the demographic data at baseline and follow-up of the MIBGP and the MIBGN group. B) Results of [123I]MIBG scintigraphy and [123I]FP-CIT-SPECT
| Baseline | Follow-up | Statistical Analysis | ||||||
| p < 0.05 | ||||||||
| MIBGP | MIBGN | MIBGP | MIBGN | Bl vs. Fu | MIBGP vs. | |||
| n = 29 | n = 8 | n = 27 | n = 8 | MIBGN | ||||
| MIBGP | MIBGN | Bl | Fu | |||||
| p | p | p | p | |||||
| A: Demographic data | ||||||||
| Male (%) | 26 (89.7) | 6 (75.0) | 24 (88.9) | 6 (75.0) | ||||
| Age at diagnosis (y) | 66.0 (61.0–70.5) | 58.5 (53.8–65.0) | 0.032 | |||||
| Age (y) | 66.0 (62.5–71.5) | 58.5 (53.8–65.0) | 70.0 (66.0–74.0) | 61.5 (60.3–68.3) | 0.014 | 0.020 | ||
| Fu time (mo) | 48.0 (36.0–62.0) | 46.5 (22.5–72.8) | n.s. | |||||
| Duration of RBD at diagnosis (mo) | 59.0 (25.5–102.5) | 57.0 (12.5–113.3) | n.s. | |||||
| ADT (%) | 7 (24.1) | 5 (62.5) | 7 (24.1) | 5 (62.5) | ||||
| UPDRS-III | 2 (0–3) | 3 (0–6) | 4 (2–7) | 3 (1–3) | 0.002 | n.s.* | n.s. | n.s. |
| TDI | 16.3 (9.5–21.9) | 28.6 (21.3–33.7) | 13.5 (8.0–18.3) | 30.1 (27.8–32.4) | 0.005 * | n.s.* | < 0.001 | < 0.001 |
| (T) score | 2.0 (0.0.–4.9) | 6.0 (3.8–7.4) | 0.0 (0.0–2.0) | 5.5 (4.5–7.3) | 0.002 * | n.s.* | 0.006 | < 0.001 |
| (D) score | 9.0 (6.0–10.0) | 11.0 (8.0–13.5) | 7.0 (4.0–9.0) | 11.5 (10.0–13.0) | 0.009 * | n.s.* | 0.029 | < 0.001 |
| (I) score | 5.0 (3.0–8.5) | 11.5 (9.5–13.0) | 5.0 (3.0–8.0) | 13.0 (12.3–13.8) | n.s.* | n.s.* | < 0.001 | < 0.001 |
| MOCA | 27.0 (25.3–28.8) | 26.5 (24.3–28.5) | 28.0 (27.0–29.0) | 28.5 (27.0–29.8) | 0.076 | n.s. | n.s | n.s |
| PDNMS | 7.0 (4.0–8.0) | 10.0 (6.0–13.8) | 8.0 (5.0–11.0) | 8.5 (4.8–15.5) | 0.004 | n.s. | 0.019 | 0.637 |
| PD Converter (%) | 7 (26) | 0 (0) | ||||||
| B: Results of [123I]MIBG scintigraphy and [123I]FP-CIT-SPECT | ||||||||
| MIBG value (n = 28) | 1.15 (1.10–1.26) | 1.7 (1.6–1.9) | < 0.001 | |||||
| Caudate nucleus right | 2.2 (1.7–2.5) | 2.9 (2.7–2.9) | 0.001 | |||||
| Caudate nucleus left | 2.2 (1.8–2.4) | 2.8 (2.5–3.0) | < 0.001 | |||||
| Putamen right | 1.9 (1.4–2.1) | 2.5 (2.2–2.6) | 0.001 | |||||
| Putamen left | 1.8 (1.4–2.0) | 2.5 (2.2–2.5) | < 0.0001 | |||||
Values are shown as median (IQR). Non-parametric Mann-Whitney-U-test was used to compare both subgroups. Wilcoxon-test was used to compare baseline and follow-up results within each group. *If the question was one-sided, the medians were compared. If the observed tendency corresponded to the null hypothesis, the test was evaluated as not significant.
Olfaction
In the RBDpMIBG group, 25/29 (86.2%) subjects had a TDI ≤25 at baseline. The olfactory function worsened from baseline to follow-up (BL: 16.3 (9.5–21.9), FU: 13.5 (8.0–18.3); p = 0.005). The threshold and discrimination subscores deteriorated (T: BL: 2.0 (0.0–4.9), FU: 0.0 (0.0–2.0), p = 0.002; D: BL 9.0 (6.0–10.0), FU: 7.0 (4.0–9.0), p = 0.009) while the impaired identification score did not decline further (I: BL: 5.0 (3.0–8.5; FU: 5.0 (3.0–8.0). In the RBDnMIBG group, the olfaction was unimpaired at baseline and remained unchanged throughout the study. The TDI-score was significantly lower in the RBDpMIBG group at baseline (RBDpMIBG: 16.3 (9.5–21.9), RBDnMIBG: 28.6 (21.3–33.7), p < 0.001) and at follow-up (RBDpMIBG: 13.5 (8.0–18.3), RBDnMIBG: 30.1 (27.8–32.4), p < 0.001) compared to the RBDnMIBG group. The same was observed for the three odor subscores (T: BL: RBDpMIBG: 2.0 (0.0–4.9), RBDnMIBG: 6.0 (3.8–7.4), p = 0.006; FU: RBDpMIBG: 0.0 (0.0–2.0), RBDnMIBG: 5.5 (4.5–7.3), p < 0.001; D BL: RBDpMIBG: 9.0 (6.0–10.0), RBDnMIBG: 11.0 (8.0–13.5), p = 0.029; FU: RBDpMIBG: 7.0 (4.0–9.0), RBDnMIBG: 11.5 (10.0–13.0), p < 0.001; I: BL: RBDpMIBG: 5.0 (3.0–8.5), RBDnMIBG: 11.5 (9.5–13.0), p < 0.001; FU: RBDpMIBG: 5.0 (3.0–8.0), RBDnMIBG: 13.0 (12.3–13.8); p < 0.001).
[123I]FP-CIT-SPECT
Putaminal and caudatal DAT-binding showed lower values in the RBDpMIBG group compared to the RBDnMIBG group (putamen right: RBDpMIBG: 1.9 (1.4–2.1), RBDnMIBG: 2.5 (2.2–2.6), p = 0.001; left: RBDpMIBG: 1.8 (1.4–2.0), RBDnMIBG: 2.5 (2.2–2.5), p < 0.0001; caudate nucleus right: RBDpMIBG: 2.2 (1.7–2.5), RBDnMIBG: 2.9 (2.7–2.9), p = 0.001; left: RBDpMIBG: 2.2 (1.8–2.4), RBDnMIBG: 2.8 (2.5–3.0), p < 0.001) (see Table 2).
Motor, nonmotor, and cognitive function
At baseline and follow-up, there was no difference in the motor and cognitive functions between the two groups. In the RBDpMIBG group, the motor function worsened at follow-up compared to baseline (BL: 2 (0–3), FU: 4 (2–7); p = 0.002) whereas the cognitive function tended to improve (BL: 27.0 (25.3–28.8); FU: 28.0 (27.0–29.0), p = 0.076). In the RBDnMIBG group, motor and cognitive functions remained unchanged (see Table 2). Only at baseline, the score of the PDNMS was higher in the RBDnMIBG group compared to the RBDpMIBG group (RBDnMIBG group: 10.0 (6.0–13.8), RBDpMIBG group: 7.0 (4.0–8.0), p = 0.019). The nonmotor symptoms based on the PDNMS increased in the RBDpMIBG group from baseline to follow-up (BL: 7.0 (4.0–8.0), FU: 8.0 (5.0–11.0), p = 0.004). For the separate description of motor function (UPDRS-III) of phenoconverted and nonconverted subjects in the RBDpMIBG group, see below.
Antidepressant pharmacological therapy (ADT)
In the RBDnMIBG group, 5/8 (62.5%) subjects had ADT at baseline: 3/5 subjects before and one after the start of RBD symptoms. In the 5th subject, the beginning of ADT remained unclear. One additional subject of the RBDnMIBG group had ADT before the onset of RBD symptoms that had already been stopped before the RBD diagnosis. In the RBDpMIBG group, 7/29 (24.1%) subjects had ADT, one subject started ADT before and 5/7 subjects after the onset of RBD symptoms. In one subject, the beginning of ADT remained unclear. 5/7 subjects with ADT of the RBDpMIBG group had a pathological [123I]MIBG and [123I]FP-CIT-SPECT.
Stratification according to [123I]MIBG and [123I]FP-CIT-SPECT
Stratifying the subjects according to [123I]MIBG and [123I]FP-CIT-SPECT results identified three subgroups: The first group with normal [123I]MIBG and [123I]FP-CIT-SPECT is identical with the above characterized RBDnMIBG group. The second group (n = 9) had abnormal [123I]MIBG but normal [123I]FP-CIT-SPECT, whereas the third group (n = 20) had both pathological [123I]MIBG and [123I]FP-CIT-SPECT. No subject with normal [123I]MIBG showed an abnormal [123I]FP-CIT-SPECT. Subjects with pathological [123I]MIBG and [123I]FP-CIT-SPECT were older than subjects with normal [123I]MIBG and [123I]FP-CIT-SPECT (p = 0.016). The duration of RBD symptoms and the follow-up time was not different in the three groups. 7/20 (35.0%) subjects with pathological [123I]MIBG and [123I]FP-CIT-SPECT at baseline phenoconverted to PD during follow-up time.
Olfaction
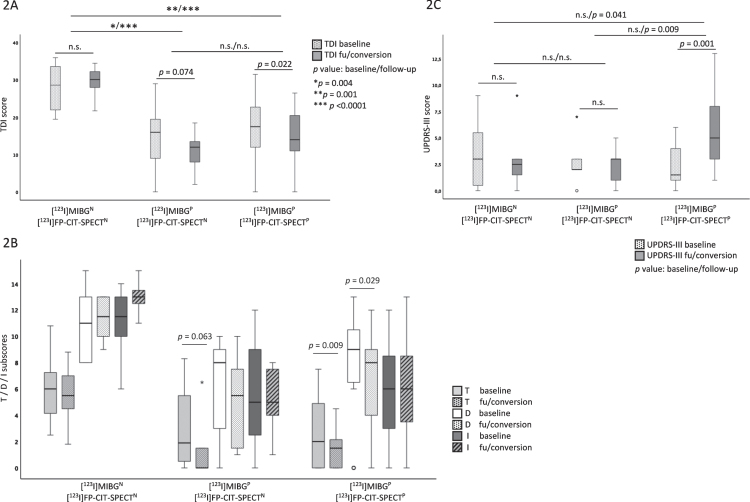
The olfaction scores did not change from baseline to follow-up in the RBDnMIBG group (see above). The second subgroup with abnormal [123I]MIBG and normal [123I]FP-CIT-SPECT worsened in the TDI-score and the threshold subscore, but this failed to reach significance (TDI: BL: 16.0 (5.5–21.9), FU: 12.0 (6.0–13.8), p = 0.074; T: BL: 1.5 (0.0–5.5), FU: 0.0 (0.0–1.5), p = 0.063). In the third group with pathological [123I]MIBG and [123I]FP-CIT-SPECT, the olfactory function and discrimination subscore worsened from baseline to follow-up (TDI: BL: 17.4 (10.5–22.3), FU: 14.0 (10.3–21.3), p = 0.022; D: BL: 9.0 (6.3–10.8), FU: 8.0 (4.0–9.0), p = 0.029) but was not significant after Bonferroni correction for multiple testing. Only the threshold subscore worsened significantly in these subjects (T: BL: 2.4 (0.0–5.0), FU: 1.5 (0.0.–2.3), p = 0.009). When comparing olfactory function between the three subgroups at baseline, subjects with abnormal [123I]MIBG combined either with normal or abnormal [123I]FP-CIT-SPECT had significantly lower TDI-scores and identification subscores compared to those with a normal [123I]MIBG (see Table 3). In subjects with both, an abnormal [123I]MIBG and [123I]FP-CIT-SPECT, the threshold subscore was significantly lower compared to subjects with normal [123I]MIBG. At follow-up, the TDI-score and all subscores were significantly lower in both groups with abnormal [123I]MIBG (combined either with normal or abnormal [123I]FP-CIT-SPECTs) compared to the RBDnMIBG group. There was no statistical difference in the TDI-score and the subscores between the subjects with abnormal [123I]MIBG and either normal or abnormal [123I]FP-CIT-SPECT. (see Table 3, Fig. 2A, B).
Table 3.
Overview of the demographic, clinical and imaging data at baseline and follow-up of the three subgroups
| Demographic data | Baseline (Bl) | Follow-up (Fu) | Statistical Analysis p < 0.017 | |||||||||||||
| 1: | 2: | 3: | 1: | 2: | 3: | Bl vs. Fu | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |||||||
| MIBGN | MIBGP | MIBGP | MIBGN | MIBGP | MIBGP | p | p | p | p | |||||||
| FP-CITN | FP-CITN | FP-CITP | FP-CITN | FP-CITN | FP-CITP | |||||||||||
| n = 8 | n = 9 | n = 20 | n = 8 | n = 9 | n = 18 | |||||||||||
| 1 | 2 | 3 | Bl | Fu | Bl | Fu | Bl | Fu | ||||||||
| Male (%) | 6 (75.0) | 8 (88.9) | 18 (90) | 6 (75.0) | 8 (88.9) | 16 (88.9) | ||||||||||
| Age at diagnosis (y) | 58.5 (53.8–65.0) | 66.0 (57.5–71.0) | 65.0 (61.0–68.5) | 0.078 | 0.047 | n.s. | ||||||||||
| Age (y) | 58.5 (53.8–65.0) | 66.0 (57.5–71.0) | 66.0 (62.3–70.5) | 61.5 (60.3–68.3) | 69.0 (61.0–77.0) | 70.5 (67.8–74.0) | 0.070 | n.s. | 0.016 | 0.012 | n.s. | n.s. | ||||
| Fu time (mo) | 46.5 (22.5–72.8) | 48.0 (33.0–63.0) | 48.5 (36.0–62.3) | n.s. | n.s. | n.s. | ||||||||||
| RBD Duration at diagnosis (mo) | 57.0 (12.5–113.3) | 55.0 (29.5–151.0) | 60.0 (24.8–71.3) | n.s. | n.s. | n.s. | ||||||||||
| ADT (%) | 5 (62.5) | 2 (22.2) | 5 (25.0) | 5 (62.5) | 2 (22.2) | 4 (22.2) | ||||||||||
| UPDRS-III | 3 (0–6) | 2 (1–3) | 1 (0–4) | 3 (1–3) | 3 (1–3) | 5 (2–5) | n.s.* | n.s.* | 0.001* | n.s. | n.s. | n.s. | 0.041 | n.s. | 0.009 | |
| TDI | 28.6 (21.3–33.7) | 16.0 (5.5–21.9) | 17.4 (10.5–22.3) | 30.1 (27.8–32.4) | 12.0 (6.0–13.8) | 14.0 (10.3–21.3) | n.s.* | 0.074* | 0.022* | 0.004 | < 0.0001 | 0.001 | < 0.0001 | n.s. | n.s. | |
| (T) score | 6.0 (3.8–7.4) | 1.5 (0.0–5.5) | 2.4 (0.0–5.0) | 5.5 (4.5 –7.3) | 0.0 (0.0–1.5) | 1.5 (0.0.–2.3) | n.s.* | 0.063* | 0.009* | 0.046 | 0.003 | 0.008 | < 0.0001 | n.s. | n.s. | |
| (D) score | 11.0 (8.0–13.5) | 7.0 (3.0–9.0) | 9.0 (6.3–10.8) | 11.5 (10.0–13.0) | 5.5 (1.3–7.8) | 8.0 (4.0–9.0) | n.s.* | n.s.* | 0.029* | 0.018 | 0.001 | 0.083 | < 0.001 | n.s. | n.s. | |
| (I) score | 11.5 (9.5–13.0) | 4.0 (2.5–9.0) | 5.5 (3.0–8.8) | 13.0 (12.3–13.8) | 5.0 (3.5–7.8) | 6.0 (3.0–9.0) | n.s.* | n.s.* | n.s.* | 0.007 | < 0.001 | 0.001 | < 0.0001 | n.s. | n.s. | |
| MOCA | 26.5 (24.3–28.5) | 26.0 (24.0–28.5) | 27.0 (26.0–28.8) | 28.5 (27.0–29.8) | 28.0 (27.0–29.0) | 28.0 (27.0–29.0) | n.s. | 0.094 | n.s. | n.s. | n.s. | n.s. | 0.685 | n.s. | n.s. | |
| PDNMS | 10.0 (6.0–13.8) | 6.0 (4.0–8.0) | 7.0 (4.0–8.0) | 8.5 (4.8–15.5) | 10.0 (6.0–14.5) | 8.0 (4.8–10.0) | n.s. | 0.047 | 0.035 | n.s. | n.s. | 0.019 | n.s. | n.s. | n.s. | |
| PD conv (%) | 0 (0) | 0 (0) | 7 (38.9) | |||||||||||||
| Results of [123I]MIBG scintigraphy and [123I]FP-CIT-SPECT | ||||||||||||||||
| [123I]MIBG value | 1.7 (1.6–1.9) | 1.2 (1.1–1.3) | 1.1 (1.1–1.2) | < 0.001 | < 0.0001 | 0.077 | ||||||||||
| lowest caudatal value | 2.8 (2.5–2.9) | 2.2 (2.0–2.8) | 1.9 (1.5–2.2) | 0.097 | < 0.0001 | 0.031 | ||||||||||
| lowest putaminal value | 2.4 (2.2–2.5) | 2.0 (1.8–2.4) | 1.5 (1.2–1.8) | 0.145 | < 0.00001 | < 0.001 | ||||||||||
All values are shown as median (IQR). Non-parametric Mann-Whitney-U-test was used to compare subgroups. Wilcoxon-test was used to compare baseline and follow-up results within each group. *If the question was one-sided, the medians were compared. If the observed tendency corresponded to the null hypothesis, the test was evaluated as not significant. ADT, antidepressant pharmacological therapy. (T) threshold. (D) discrimination. (I) identification. Conv, converter.
Fig. 2.
Olfactory and motor functions of the three subgroups according to [123I]MIBG and [123I]FP-CIT-SPECT status (N = normal, P = pathological) at baseline and follow-up (fu). A) TDI-score at baseline and follow-up. B) Threshold (T), discrimination (D) and identification (I) subscores at baseline and follow-up. C) UPDRS-III score at baseline and follow-up. p values < 0.017 were considered to be significant.
At baseline, 7/9 subjects with abnormal [123I]MIBG and normal [123I]FP-CIT-SPECT were diagnosed with severe hyposmia (3/9 (33.3%)) or anosmia (4/9 (44.4%)). At follow-up, all subjects in this group had severe hyposmia (1/9 (11.1%)) or anosmia (8/9 (88.9%)). In the subjects with pathological [123I]MIBG and [123I]FP-CIT-SPECT, 13/20 had severe hyposmia (5/20 (25.0%)) or anosmia (8/20 (40.0%)) at baseline. At follow-up the number of anosmic subjects increased (severe hyposmia: 3/18 (16.7%); anosmia: 10/18 (55.6%)). In contrast, subjects with normal [123I]MIBG and [123I]FP-CIT-SPECT showed only mild hyposmia or normosmia (baseline: 5/8 (62.5%); follow-up: 7/8 (87.5%)).
Motor and cognitive function
At baseline, no difference of the UPDRS-III between the three subgroups was observed. Throughout the study, the motor function only worsened significantly in the subjects with pathologcial [123I]MIBG and [123I]FP-CIT-SPECT (UPDRS-III: BL: 1 (0–4), FU: 5 (2–5), p = 0.001). At follow-up, the UPDRS-III in this group was higher compared to both other groups (abnormal [123I]MIBG/normal [123I]FP-CIT-SPECT UPDRS-III (FU): 3 (1–3), p = 0.009; RBDnMIBG group UPDRS-III (FU): 3 (1–3), p = 0.041). The UPDRS-III of the seven subjects who converted to PD worsened from 1 (1–2) at baseline to 9 (7–10) at phenoconversion while the non-converters of this group changed from 1 (0–4) to 4 (2–4) throughout the study. At baseline and follow-up, there was no difference in cognitive functioning between the three subgroups. (see Table 3, Fig. 2C).
PD phenoconversion
Throughout the study, 7/35 subjects converted to PD within 4.2 (3.7–5.3) years after RBD diagnosis and 11.8 (8.8–14.8) years after the patient-reported start of RBD symptoms. One of these fulfilled the criteria of mild cognitive impairment (MCI) but not the consensus criteria of DLB and had a family history of PD. At baseline, 6/7 phenoconverted subjects had hyposmia with TDI ≤25 and all had pathological [123I]MIBG and [123I]FP-CIT-SPECT. Annual conversion rates (CRs) according to different stratifying conditions are shown for the diagnosis RBD (i.e., including the seven subjects with ADT before or unknown relation to start of RBD symptoms) and iRBD in Supplementary Table 2. Stratifying (i)RBD according to [123I]FP-CIT-SPECT results leads to the highest annual CR (RBD: 9.7%; iRBD: 10.2%) whereas the CR is 6.5% /year (RBD) and 6.7% /year (iRBD) if stratifying subjects according to [123I]MIBG results— both without missing any phenoconverters.
A too strict stratification according to baseline olfactory dysfunction with TDI < 18 (previously described to be associated with increased risk of phenoconversion within 5 years[28]) would miss 5/7 (71.4%) phenoconverted subjects, whereas the combination of “hyposmia with TDI ≤25” and “iRBD” results in a CR of 6.7% /year but would miss 1/7 phenoconverter.
DISCUSSION
In this study, we investigated the role of three biomarkers of prodromal PD/DLB in RBD: cardiac sympathetic denervation ([123I]MIBG), olfactory impairment and reduced DAT-binding ([123I]FP-CIT-SPECT). A pathological [123I]MIBG was not only associated with hyposmia in 86.2% (25/29) of RBD subjects but, most importantly, with a progression of olfactory dysfunction based on the subscores threshold and discrimination in the full range Sniffin’ Sticks test. Vice versa hyposmia was most frequently associated with an abnormal [123I]MIBG, as 89.3% (25/28) of the hyposmic RBD subjects at baseline had a pathological [123I]MIBG, in contrast to 78.4% (29/37) of all subjects independent of the olfactory function (see also ROC analysis in the Supplementary Material). Additionally, 69.0% (20/29) of the subjects with a pathological [123I]MIBG had an abnormal [123I]FP-CIT-SPECT. Only of these, seven converted to PD during the 4-year follow-up— all except one presenting with hyposmia at baseline.
The single parameter “pathological [123I]FP-CIT-SPECT” provided an annual CR in all, i.e., RBD and iRBD patients of ∼10% and identified all phenoconverters. To select iRBD patients in the late prodromal stage, i.e., with an already affected dopaminergic nigrostriatal pathway, [123I]FP-CIT-SPECT appears to be the best single parameter, which is in agreement with earlier studies [29, 30]. However, it fails to detect iRBD patients at risk with a still intact nigrostriatal pathway, i.e., who are in the prodromal “prenigral” stage. In this stage, pathological [123I]MIBG and hyposmia with TDI ≤25 as single indicators seem to be of similar quality in screening and preselecting iRBD patients at risk. However, combining the biomarker pathological [123I]MIBG with hyposmia provides additional strong support that the particular iRBD patient suffers from aSYN with a peripheral component and at the same time argues against the presence of a prodromal MSA [31, 32].
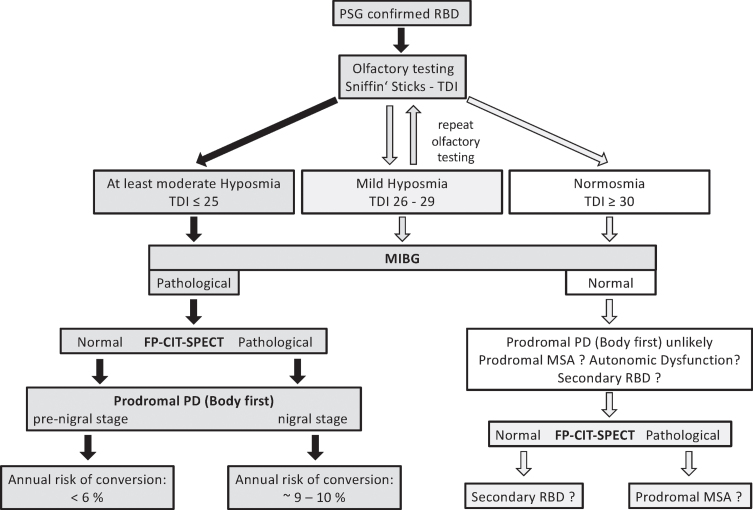
A pathological [123I]MIBG alone identified RBD subjects with a cardiac sympathetic denervation even though the nigrostriatal pathway was still intact and/or the patient belonged to the few cases with a normal or only mildly impaired olfaction. However, a longer follow-up period with serial [123I]FP-CIT-SPECTs in subjects with an abnormal [123I]MIBG and a normal DAT-binding is necessary, to demonstrate the conversion of a normal to an abnormal [123I]FP-CIT-SPECT in time. In contrast, and as important for selecting iRBD subjects for disease-modifying therapy, subjects with normal findings in [123I]MIBG, [123I]FP-CIT-SPECT, and olfactory testing likely are not prodromal to PD/DLB. Thus, for initial screening and phenotyping of a polysomnography-confirmed RBD subject the sequence of the easy to perform olfactory testing followed by [123I]MIBG appears to exclude those who will hardly or not progress or might present the rare case of a prodromal MSA (see Fig. 3). Furthermore, in iRBD subjects with an abnormal [123I]MIBG the repeated assessment of the olfactory function with the full range Sniffin’ Sticks test (TDI-score) establishes a potential progression marker for neuroprotective trials.
Fig. 3.
Algorithm for selecting patients with prodromal Parkinson’s disease in clinical trials with disease-modifying therapy based on the results of this study. Subjects with video-polysomnography confirmed RBD should be screened with olfactory testing (Sniffin’ Sticks, to determine TDI score) and [123I]MIBG scintigraphy. In the RBD subjects with abnormal [123I]MIBG scintigraphy, only four subjects presented with TDI > 25 at baseline. In all of these, the olfactory function deteriorated from baseline to follow-up to TDI ≤25 –except for one. Two RBD subjects with normal [123I]MIBG scintigraphy had a TDI ≤25 at baseline: one stayed below TDI < 25, one improved to normosmia at follow-up. In case of abnormal [123I]MIBG scintigraphy, this should be followed by [123I]FP-CIT-SPECT. RBD subjects with both, abnormal [123I]MIBG scintigraphy and [123I]FP-CIT-SPECT show an annual conversion rate between ∼9–10%.
Progressive hyposmia and pathological [123I]MIBG
In contrast to subjects with normal [123I]MIBG, subjects with pathological [123I]MIBG showed olfactory dysfunction already at baseline that worsened throughout the study. Further stratifying the subjects with an abnormal [123I]MIBG based on the [123I]FP-CIT-SPECT results demonstrated that olfactory function was similarly impaired in subjects with normal or reduced DAT-binding. Thus, in concordance with the results of other groups [6], impaired olfaction can occur before nigrostriatal degeneration is detectable.
Of note, normal or only mildly impaired olfaction at baseline did not exclude an underlying aSYN as observed before [28]: 4/9 subjects with mild hyposmia/normosmia at baseline had pathological [123I]MIBG, three of them had a reduced striatal DAT-binding, of those, one phenoconverted to PD. Hyposmia is a common and early symptom in iRBD and is the risk factor with the highest hazard ratio in respect to phenoconversion among non-motor symptoms [2, 28, 33], preceding phenoconversion > 20 years [15]. Only slight or no decline of olfactory function was described in iRBD [15, 33, 34]. Therefore, hyposmia was considered a non-progressive, although important prodromal biomarker for iRBD [9]. However, these studies employed the University of Pennsylvania Smell Identification Test or only the odor identification part of the Sniffin’ Sticks test. In contrast, we used the full range odor test (threshold, discrimination, identification) to assess olfactory function at baseline and at annual follow-up visits. Compared to the RBDnMIBG group, in subjects with pathological [123I]MIBG the TDI-score and all three subscores were impaired throughout the study. However, and most relevant, a decline was observed in the subscores “threshold” and “discrimination” in all subjects with abnormal [123I]MIBG during follow-up time, but not in the identification subscore— resulting in a progressive decline of the TDI-score. After the stratification according to DAT-binding only subjects with abnormal [123I]FP-CIT-SPECT had a significant decline in threshold subscore and a trend to a decrease in discrimination subscore and in TDI, whereas in those with normal [123I]FP-CIT-SPECT only a trend was seen for progressive deterioration of TDI and threshold scores. This could be explained by the small number of subjects in this group as well as by the observation that in the latter group 7/9 (77.8%) subjects already presented with at least severe hyposmia at baseline in contrast to 13/20 (65%) subjects with both pathological [123I]MIBG and [123I]FP-CIT-SPECT. Nevertheless, these data demonstrate that the repeated use of the full range Sniffin’ Sticks test over time in iRBD qualifies the biomarker olfactory dysfunction as a progression marker in iRBD. In contrast the identification odor test alone does not appear to be suitable to detect the progression of olfactory dysfunction.
Subjects with normal [123I]MIBG did not deteriorate in motor and cognitive function, whereas subjects with a pathological [123I]MIBG worsened in motor functioning over time. However, significant worsening of UPDRS-III from baseline to follow-up was only observed in subjects with pathological [123I]MIBG and [123I]FP-CIT-SPECT and in particular, “per definition”, in the group of PD converters.
[123I]MIBG: Pros and contras for identifying prodromal PD/DLB
Our results are in agreement with several studies describing pathological [123I]MIBG in iRBD [10, 35, 36]. These studies showed that the majority of iRBD patients already have a pathological [123I]MIBG, even though a nigrostriatal dopaminergic deficit could not yet be detected [6, 16]. Thus, [123I]MIBG could be a suitable marker to identify subjects in the prodromal stage of PD/DLB earlier than [123I]FP-CIT-SPECT.
Additionally, [123I]MIBG can differentiate between secondary RBD and RBD as prodromal stage of PD/DLB [11]. However, false abnormal [123I]MIBG results can be caused by several diseases or medication, including different antidepressants (tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors) and antipsychotic drugs (clozapine, olanzapine, quetiapine) [37]. Depressive symptoms are common in prodromal PD stages [38]. ADT can cause RBD or trigger it in patients who have prodromal aSYN [39, 40]. Thus, in clinical practice, it can be challenging to figure out if RBD preceded ADT or not, and/or if RBD is exclusively caused by ADT. In our study, up to seven RBD patients fulfilled criteria of secondary RBD. Five of these presented with normal [123I]MIBG. The other two had abnormal [123I]MIBG combined with abnormal [123I]FP-CIT-SPECT at baseline. So [123I]MIBG correctly recognized the subjects in prodromal stage of PD/DLB. At time of [123I]MIBG acquisition, eight subjects had ADT possibly influencing [123I]MIBG results: three presented with normal [123I]MIBG and TDI > 25 at baseline and/or follow-up; five subjects with abnormal [123I]MIBG presented with TDI ≤25 either at baseline or follow-up, three of those already had reduced DAT-binding. Thus, we propose that the combination of abnormal [123I]MIBG and impaired olfactory function is able to identify “isolated” RBD in prodromal stages of PD/DLB even in patients with ADT.
Of note, RBD patients with normal [123I]MIBG and normosmia could be in the prodromal stage of MSA. The vast majority of MSA patients have normal [123I]MIBG [31, 32], and most of them have RBD [41]. However, the statistical risk to phenoconvert to MSA is very low in the prospective iRBD cohort studies [2]. We postulate that RBD patients with normal [123I]MIBG and normosmia are not in the prodromal stage of PD/DLB and should be carefully assessed for autonomic dysfunctions in the search for prodromal MSA. However, as recently reported, no pronounced autonomic symptoms were detectable in four MSA-P converters of a large cohort of iRBD subjects before phenoconversion [42].
There is ongoing discussion if a PD subgroup with less autonomic symptoms has normal sympathetic cardiac innervation at diagnosis [5, 43, 44]. One study revealed normal [123I]MIBG in 44/160 de novo PD subjects, and orthostatic hypotension, olfactory dysfunction and probable RBD were associated with abnormal [123I]MIBG [43]. Nomura et al. described reduced [123I]MIBG uptake in PD with RBD compared to PD without RBD, and RBD was associated with reduced [123I]MIBG uptake in PD [44]. Other studies showed reduced [123I]MIBG uptake in iRBD compared to PD [10, 36]. Horsager et al. described a similarly and more severely reduced [123I]MIBG in PD with RBD and iRBD than in PD without RBD [5]. Moreover, PD patients without RBD were less hyposmic than those with RBD [5]. They propose that PD plus RBD reflects a “body-first” PD subtype, of which iRBD is the premotor phenotype [5]. Our findings could further support this statement.
Generally, hyposmia and pathological [123I]MIBG seem to precede nigrostriatal degeneration. However, RBD patients with pathological [123I]FP-CIT-SPECT have the highest risk of conversion to manifest aSYN within 3–5 years [29]. As a disease-modifying therapy could be more effective if started as early as possible, [123I]MIBG is a promising diagnostic tool to identify iRBD patients in prodromal stages of PD/DLB before nigrostriatal pathology has occurred. Because of the expected long conversion time, especially in iRBD in a “prenigral” stage, longitudinal studies are necessary that prove the “conversion” from a normal to a pathological [123I]FP-CIT-SPECT. Of course, there are limitations of using [123I]MIBG that can lead to false abnormal results: Our proposed two-tiered strategy with combined [123I]MIBG and olfactory testing is expected to result in a higher safety: First both biomarkers when reduced, were highly associated with each other, and secondly olfactory dysfunction was already impaired at baseline (even to the level of anosmia) and qualified in the follow-up period as a prodromal progression marker only in the subjects with a pathological [123I]MIBG, independent of a normal or reduced DAT-binding. Still, there is need of additional markers reflecting prodromal disease progression in iRBD: a promising candidate could be the assessment of the “PD related pattern”-expression in [18F]FDG-PET as recently published [45].
This prospective cohort study has several limitations. The sample size is small, especially in the subgroups. Because of the relatively short follow-up time, only seven subjects phenoconverted to PD. This leads to a low specificity and positive predictive value of [123I]MIBG (29%, 0.26) as well as olfactory testing (29%, 0.27), although the sensitivity (100% and 86% respectively) and negative predictive value (1 and 0.89 respectively) are high. Increasing the follow-up time will most likely result in a growing number of phenoconverters in the RBDpMIBG group. Furthermore, [123I]FP-CIT-SPECTs were not repeated to evaluate progression of nigrostriatal degeneration. Neither early images were performed nor washout rates determined in [123I]MIBG scintigraphy. For the in-house threshold of “HMR < 1.5 = pathological” no normal data set is available.
In summary, in our study, reduced cardiac [123I]MIBG uptake and hyposmia with TDI ≤25 seem to be suitable diagnostic findings to identify iRBD subjects in prodromal stages of PD/DLB before nigrostriatal degeneration has occurred. iRBD subjects with a pathological [123I]MIBG presented with hyposmia at baseline and showed a progression of the olfactory impairment and an increase in motor symptoms over time mainly due to the manifestation of PD motor symptoms in the subgroup of PD converters. Thus, olfactory function is a prodromal progression marker in iRBD with a pathological [123I]MIBG. This could be independent of whether the [123I]FP-CIT-SPECT is normal or abnormal. Further longitudinal studies are needed to assess conversion time in iRBD subjects in the “prenigral” prodromal stage of aSYN, i.e. who present at baseline a normal [123I]FP-CIT-SPECT, into the “nigral” stage with a pathological [123I]FP-CIT-SPECT.
Supplementary Material
ACKNOWLEDGMENTS
We thank the “ParkinsonFonds Deutschland” for supporting this study. Wolfgang H Oertel was supported by the Charitable Hertie Foundation, Frankfurt/Main, Germany.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-223201
CONFLICT OF INTEREST
Annette Janzen, MD, reports grants from ParkinsonFonds Deutschland outside the submitted work. David Vadasz, MD, reports no competing interests. Jan Booij, MD, PhD, is consultant at GE healthcare (all paid to the institution). Markus Luster, MD, reports no competing interests. Damiano Librizzi, MD, reports no competing interests. Martin T. Henrich, MD, reports grants from the ParkinsonFonds Deutschland and the German Society for Parkinson and Movement Disorders outside the submitted work. Lars Timmermann, MD, received payments as a consultant for Boston Scientific between September 2018 and September 2021 and received honoraria as a speaker on symposia sponsored by UCB, Desitin, Boston Scientific, AbbVie, Novartis, GlaxoSmithKline, and DIAPLAN. The institution of L.T., not L.T. personally, received funding from Boston Scientific, the German Research Foundation, the German Ministry of Education and Research, and the Deutsche Parkinson Vereinigung. Neither Lars Timmermann nor any member of his family holds stocks, stock options, patents, or financial interests in any of the aforementioned companies or their competitors. Mahboubeh Habibi reports no competing interests. Elisabeth Sittig reports no competing interests. Geert Mayer, MD, reports no competing interests. Fanni F. Geibl, MD, PhD, reports grants from ParkinsonFonds Deutschland and P.E. Kempkes outside the submitted work. Wolfgang H. Oertel, MD, PhD, reports grants from ParkinsonFonds Deutschland, grants from Michael J Fox Foundation, grants from Deutsche Forschungsgemeinschaft (DFG), during the conduct of the study; personal fees from Adamas, MODAG, Roche and UCB; outside the submitted work. WHO is Hertie-Senior-Research Professor supported by the Charitable Hertie-Foundation, Frankfurt/Main, Germany.
REFERENCES
- [1]. Fernández-Arcos A, Iranzo A, Serradell M, Gaig C, Santamaria J (2016) The clinical phenotype of idiopathic rapid eye movement sleep behavior disorder at presentation: A study in 203 consecutive patients. Sleep 39, 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Postuma RB, Iranzo A, Hu M, Högl B, Boeve BF, Manni R, Oertel WH, Arnulf I, Ferini-Strambi L, Puligheddu M, Antelmi E, Cochen De Cock V, Arnaldi D, Mollenhauer B, Videnovic A, Sonka K, Jung KY, Kunz D, Dauvilliers Y, Provini F, Lewis SJ, Buskova J, Pavlova M, Heidbreder A, Montplaisir JY, Santamaria J, Barber TR, Stefani A, St Louis EK, Terzaghi M, Janzen A, Leu-Semenescu S, Plazzi G, Nobili F, Sixel-Doering F, Dusek P, Bes F, Cortelli P, Ehgoetz Martens K, Gagnon JF, Gaig C, Zucconi M, Trenkwalder C, Gan-Or Z, Lo C, Rolinski M, Mahlknecht P, Holzknecht E, Boeve AR, Teigen LN, Toscano G, Mayer G, Morbelli S, Dawson B, Pelletier A (2019) Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain 142, 744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Iranzo A, Santamaria J, Tolosa E (2016) Idiopathic rapid eye movement sleep behaviour disorder: Diagnosis, management, and the need for neuroprotective interventions. Lancet Neurol 15, 405–419. [DOI] [PubMed] [Google Scholar]
- [4]. Schenck CH, Mahowald MW (2002) REM sleep behavior disorder: Clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep 25, 120–138. [DOI] [PubMed] [Google Scholar]
- [5]. Horsager J, Andersen KB, Knudsen K, Skjærbæk C, Fedorova TD, Okkels N, Schaeffer E, Bonkat SK, Geday J, Otto M, Sommerauer M, Danielsen EH, Bech E, Kraft J, Munk OL, Hansen SD, Pavese N, Göder R, Brooks DJ, Berg D, Borghammer P (2020) Brain-first versus body-first Parkinson’s disease: A multimodal imaging case-control study. Brain 143, 3077–3088. [DOI] [PubMed] [Google Scholar]
- [6]. Knudsen K, Fedorova TD, Hansen AK, Sommerauer M, Otto M, Svendsen KB, Nahimi A, Stokholm MG, Pavese N, Beier CP, Brooks DJ, Borghammer P (2018) staging of pathology in REM sleep behaviour disorder: A multimodality imaging case-control study. Lancet Neurol 17, 618–628. [DOI] [PubMed] [Google Scholar]
- [7]. American Academy of Sleep Medicine (2014) International classification of sleep disorders, 3rd ed. American Academy of Sleep Medicine, Darien, IL.
- [8]. Dauvilliers Y, Schenck CH, Postuma RB, Iranzo A, Luppi PH, Plazzi G, Montplaisir J, Boeve B (2018) REM sleep behaviour disorder. Nat Rev Dis Primers 4, 19. [DOI] [PubMed] [Google Scholar]
- [9]. Miglis MG, Adler CH, Antelmi E, Arnaldi D, Baldelli L, Boeve BF, Cesari M, Dall’Antonia I, Diederich NJ, Doppler K, Dušek P, Ferri R, Gagnon JF, Gan-Or Z, Hermann W, Högl B, Hu MT, Iranzo A, Janzen A, Kuzkina A, Lee JY, Leenders KL, Lewis SJG, Liguori C, Liu J, Lo C, Ehgoetz Martens KA, Nepozitek J, Plazzi G, Provini F, Puligheddu M, Rolinski M, Rusz J, Stefani A, Summers RLS, Yoo D, Zitser J, Oertel WH (2021) Biomarkers of conversion to α-synucleinopathy in isolated rapid-eye-movement sleep behaviour disorder. Lancet Neurol 20, 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Miyamoto T, Miyamoto M, Suzuki K, Nishibayashi M, Iwanami M, Hirata K (2008) 123I-MIBG cardiac scintigraphy provides clues to the underlying neurodegenerative disorder in idiopathic REM sleep behavior disorder. Sleep 31, 717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Barateau L, Jaussent I, Lopez R, Evangelista E, Chenini S, Benkiran M, Mariano-Goulart D, Dauvilliers Y (2018) Cardiac sympathetic activity differentiates idiopathic and symptomatic rapid eye movement sleep behaviour disorder. Sci Rep 8, 7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Miyamoto T, Miyamoto M, Suzuki K, Ikematsu A, Usui Y, Inoue Y, Hirata K (2009) Comparison of severity of obstructive sleep apnea and degree of accumulation of cardiac 123I-MIBG radioactivity as a diagnostic marker for idiopathic REM sleep behavior disorder. Sleep Med 10, 577–580. [DOI] [PubMed] [Google Scholar]
- [13]. Barber TR, Lawton M, Rolinski M, Evetts S, Baig F, Ruffmann C, Gornall A, Klein JC, Lo C, Dennis G, Bandmann O, Quinnell T, Zaiwalla Z, Ben-Shlomo Y, Hu MT (2017) Prodromal Parkinsonism and neurodegenerative risk stratification in REM sleep behaviour disorder. Sleep 40, zsx071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Dusek P, Ibarburu V, Bezdicek O, Dall’antonia I, Dostalova S, Kovalska P, Krupicka R, Nepozitek J, Nikolai T, Novotny M, Perinova P, Rusz J, Serranova T, Tykalova T, Ulmanova O, Meckova Z, Ptacnik V, Trnka J, Zogala D, Ruzicka E, Sonka K (2019) Relations of non-motor symptoms and dopamine transporter binding in REM sleep behavior disorder. Sci Rep 9, 15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Fereshtehnejad SM, Yao C, Pelletier A, Montplaisir JY, Gagnon JF, Postuma RB (2019) Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: A prospective study. Brain 142, 2051–2067. [DOI] [PubMed] [Google Scholar]
- [16]. Sakakibara R, Tateno F, Aiba Y, Ogata T, Kishi M, Terada H, Inaoka T, Nakatsuka T, Matsuoka K (2019) MIBG myocardial scintigraphy identifies premotor PD/DLB during a negative DAT scan period: Second report. Mov Disord Clin Pract 6, 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24, 197–211. [DOI] [PubMed] [Google Scholar]
- [18]. Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease (2003) The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov Disord 18, 738–750. [DOI] [PubMed] [Google Scholar]
- [19]. Gagnon JF, Postuma RB, Joncas S, Desjardins C, Latreille V (2010) The Montreal Cognitive Assessment: A screening tool for mild cognitive impairment in REM sleep behavior disorder. Mov Disord 25, 936–940. [DOI] [PubMed] [Google Scholar]
- [20]. Chaudhuri KR, Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, Brown RG, Koller W, Barone P, MacPhee G, Kelly L, Rabey M, MacMahon D, Thomas S, Ondo W, Rye D, Forbes A, Tluk S, Dhawan V, Bowron A, Williams AJ, Olanow CW (2006) International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: The NMSQuest study. Mov Disord 21, 916–923. [DOI] [PubMed] [Google Scholar]
- [21]. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G (1997) ‘Sniffin’ sticks’: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22, 39–52. [DOI] [PubMed] [Google Scholar]
- [22]. Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Dürr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71, 670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51, 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Stiasny-Kolster K, Doerr Y, Möller JC, Höffken H, Behr TM, Oertel WH, Mayer G (2005) Combination of ‘idiopathic’ REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain 128, 126–137. [DOI] [PubMed] [Google Scholar]
- [26]. Kobal G, Klimek L, Wolfensberger M, Gudziol H, Temmel A, Owen CM, Seeber H, Pauli E, Hummel T (2000) Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol 257, 205–211. [DOI] [PubMed] [Google Scholar]
- [27]. Meles SK, Vadasz D, Renken RJ, Sittig-Wiegand E, Mayer G, Depboylu C, Reetz K, Overeem S, Pijpers A, Reesink FE, van Laar T, Heinen L, Teune LK, Höffken H, Luster M, Kesper K, Adriaanse SM, Booij J, Leenders KL, Oertel WH (2017) FDG PET, dopamine transporter SPECT, and olfaction: Combining biomarkers in REM sleep behavior disorder. Mov Disord 32, 1482–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Mahlknecht P, Iranzo A, Högl B, Frauscher B, Müller C, Santamaría J, Tolosa E, Serradell M, Mitterling T, Gschliesser V, Goebel G, Brugger F, Scherfler C, Poewe W, Seppi K, Sleep Innsbruck Barcelona Group (2015) Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology 84, 654–658. [DOI] [PubMed] [Google Scholar]
- [29]. Iranzo A, Santamaría J, Valldeoriola F, Serradell M, Salamero M, Gaig C, Niñerola-Baizán A, Sánchez-Valle R, Lladó A, De Marzi R, Stefani A, Seppi K, Pavia J, Högl B, Poewe W, Tolosa E, Lomeña F (2017) Dopamine transporter imaging deficit predicts early transition to synucleinopathy in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol 82, 419–428. [DOI] [PubMed] [Google Scholar]
- [30]. Chahine LM, Brumm MC, Caspell-Garcia C, Oertel W, Mollenhauer B, Amara A, Fernandez-Arcos A, Tolosa E, Simonet C, Hogl B, Videnovic A, Hutten SJ, Tanner C, Weintraub D, Burghardt E, Coffey C, Cho HR, Kieburtz K, Poston KL, Merchant K, Galasko D, Foroud T, Siderowf A, Marek K, Simuni T, Iranzo A (2021) Dopamine transporter imaging predicts clinically-defined α-synucleinopathy in REM sleep behavior disorder. Ann Clin Transl Neurol 8, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Braune S, Reinhardt M, Schnitzer R, Riedel A, Lücking CH (1999) Cardiac uptake of [123I]MIBG separates Parkinson’s disease from multiple system atrophy. Neurology 53, 1020–1025. [DOI] [PubMed] [Google Scholar]
- [32]. King AE, Mintz J, Royall DR (2011) Meta-analysis of 123I-MIBG cardiac scintigraphy for the diagnosis of Lewy body-related disorders. Mov Disord 26, 1218–1224. [DOI] [PubMed] [Google Scholar]
- [33]. Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir JY (2011) Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder. Ann Neurol 69, 811–818. [DOI] [PubMed] [Google Scholar]
- [34]. Iranzo A, Serradell M, Vilaseca I, Valldeoriola F, Salamero M, Molina C, Santamaria J, Tolosa E (2013) Longitudinal assessment of olfactory function in idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord 19, 600–604. [DOI] [PubMed] [Google Scholar]
- [35]. Miyamoto T, Miyamoto M, Inoue Y, Usui Y, Suzuki K, Hirata K (2006) Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Neurology 67, 2236–2238. [DOI] [PubMed] [Google Scholar]
- [36]. Kashihara K, Imamura T, Shinya T (2010) Cardiac 123I-MIBG uptake is reduced more markedly in patients with REM sleep behavior disorder than in those with early stage Parkinson’s disease. Parkinsonism Relat Disord 16, 252–255. [DOI] [PubMed] [Google Scholar]
- [37]. Yamada M, Komatsu J, Nakamura K, Sakai K, Samuraki-Yokohama M, Nakajima K, Yoshita M (2020) Diagnostic criteria for dementia with Lewy bodies: Updates and future directions. J Mov Disord 13, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Reichmann H (2017) Premotor diagnosis of Parkinson’s disease. Neurosci Bull 33, 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. McCarter SJ, St Louis EK, Sandness DJ, Arndt K, Erickson M, Tabatabai G, Boeve BF, Silber MH (2015) Antidepressants increase REM sleep muscle tone in patients with and without REM sleep behavior disorder. Sleep 38, 907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Postuma RB, Gagnon JF, Tuineaig M, Bertrand JA, Latreille V, Desjardins C, Montplaisir JY (2013) Antidepressants and REM sleep behavior disorder: Isolated side effect or neurodegenerative signal? .Sleep 36, 1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Palma JA, Fernandez-Cordon C, Coon EA, Low PA, Miglis MG, Jaradeh S, Bhaumik AK, Dayalu P, Urrestarazu E, Iriarte J, Biaggioni I, Kaufmann H (2015) Prevalence of REM sleep behavior disorder in multiple system atrophy: A multicenter study and meta-analysis. Clin Auton Res 25, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Postuma RB, Pelletier A, Gagnon JF, Montplaisir J (2022) Evolution of prodromal multiple system atrophy from REM sleep behavior disorder: A descriptive study. J Parkinsons Dis 12, 983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Kim JS, Park HE, Park IS, Oh YS, Ryu DW, Song IU, Jung YA, Yoo IR, Choi HS, Lee PH, Lee KS (2017) Normal ‘heart’ in Parkinson’s disease: Is this a distinct clinical phenotype? . Eur J Neurol 24, 349–356. [DOI] [PubMed] [Google Scholar]
- [44]. Nomura T, Inoue Y, Högl B, Uemura Y, Kitayama M, Abe T, Miyoshi H, Nakashima K (2010) Relationship between (123)I-MIBG scintigrams and REM sleep behavior disorder in Parkinson’s disease. Parkinsonism Relat Disord 16, 683–685. [DOI] [PubMed] [Google Scholar]
- [45]. Kogan RV, Janzen A, Meles SK, Sittig E, Renken RJ, Gurvits V, Mayer G, Leenders KL, Oertel WH, Group RW (2021) Four-year follow-up of [18 F]Fluorodeoxyglucose positron emission tomography-based Parkinson’s disease-related pattern expression in 20 patients with isolated rapid eye movement sleep behavior disorder shows prodromal progression. Mov Disord 36, 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.