Abstract
Background:
The identification of risk factors for SARS-CoV-2 infection and mortality in patients with dementia is a key aspect to support clinical decisions and public health interventions.
Objective:
To assess the incidence of SARS-CoV-2 infection and COVID-19 related death in a cohort of patients with dementia residing in the Lazio region and to investigate predicting factors for both infection and mortality.
Methods:
This population-based study used information from administrative databases and the SARS-CoV-2 infection surveillance system. Patients with dementia (age ≥65) were enrolled as of December 31, 2019 and followed-up until February 28, 2021. Cumulative risk of infection and death within 60 days of infection onset, and age-standardized incidence (SIR) and mortality (SMR) ratios were calculated. Logistic regression models were applied to identify factors associated with infection and mortality.
Results:
Among 37,729 dementia patients, 2,548 had a diagnosis of SARS-CoV-2 infection. The crude risk of infection was 6.7%. An increase in risk of infection was observed both in women (SIR 1.72; 95% CI 1.64–1.80) and men (SIR 1.43; 95% CI 1.33–1.54). Pneumonia, cerebrovascular and blood diseases, femur fracture, anxiety, antipsychotic and antithrombotic use were associated with an increased risk of infection. The crude risk of death was 31.0%, the SMRs 2.32 (95% CI 2.05–2.65) for men, and 2.82 (95% CI 2.55–3.11) for women. Factors associated with mortality included: male gender, age ≥85, symptoms at the diagnosis, antipsychotic and systemic antibiotics treatment.
Conclusion:
These findings emphasize the need of close and tailored monitoring of dementia patients to reduce the impact of COVID-19 on this fragile population.
Keywords: Administrative databases, Alzheimer’s disease, cohort study, COVID-19, dementia, prognostic factors, SARS-CoV-2 infection
INTRODUCTION
The novel coronavirus SARS-CoV-2, identified in China on December 31, 2019, has spread worldwide in a short span of time, producing over 5 million deaths and causing dramatic public health issues. Italy is one of the oldest nations in the world, and one of countries most violently affected by the COVID-19 pandemic [1, 2]. A first pandemic wave was observed between February and May 2020, mainly affecting the Northern regions and a second one from October to December with a more homogeneous impact throughout the country [1, 3]. It quickly became apparent that older adults represent a population group particularly vulnerable to serious illness and complications, especially those living in long-term care facilities [4–6]. The cognitive impairment due to dementia exposes elderly subjects to a greater risk of becoming infected and to suffer worse outcomes as demonstrated by several studies that have identified dementia as an important risk factor for SARS-CoV-2 infection and COVID-19 mortality [7–11].
The worldwide prevalence of dementia has increased dramatically over the past three decades and its burden is expected to increase in the near future to the point that the World Health Organization defined dementia as a “global pandemic” [12]. In Italy, more than 1 million people are currently living with dementia, with a particularly higher prevalence of patients in long-term care facilities [13]. Available evidence suggests that the number of deaths for COVID-19 among those living in nursing and care homes was extremely high during the first year of the pandemic, due to infection quick spread and the age-related high lethality [14].
Strong risk factors for cognitive decline and dementia include cardiovascular diseases, diabetes, obesity, and hypertension [15]. Many of these common comorbidities in patients with dementia are also demonstrated as risk factors for COVID-19 and are associated with worse clinical outcomes [11, 16–18]. Several pandemic-related circumstances correlate with the severity of the impact of COVID-19 on these patients. Lockdowns and social distancing measures have made the social support and healthcare systems less accessible and even unavailable for people with dementia [19]. Moreover, patients with dementia may be unable to follow the recommendations from public health authorities to reduce the transmission of COVID-19: hand hygiene; covering one’s mouth and nose when coughing; maintaining physical distance from others and self-isolating by remaining alone at home [20]. The restrictive measures, necessary to avoid or limit the spreading of the infection, also resulted in an increase in the occurrence of psychological and behavioral disorders in patients with dementia during the pandemic period [21, 22]. The identification of the contributing factors to both the infection and mortality is a very important issue to enable risk stratification and guide clinical and social interventions as well as public health recommendations. Actually, there is still little knowledge regarding the risk factors for SARS-CoV-2 infection and outcomes in individuals with dementia. The purposes of this study were to assess incidence of SARS-CoV-2 infection and 60-days mortality and to investigate predicting factors for infection and death in a population-based cohort of patients with dementia.
METHODS
Study design and sources of data
In this retrospective population-based cohort study, we considered older adults with dementia residing in the Lazio region (central Italy, including Rome, and with about 6,000,000 inhabitants). The study was conducted using health administrative databases, providing a comprehensive regional coverage and including high quality data. We used the Regional Health Assistance file, which includes all individuals registered with a general practitioner or who have ever had a contact with the regional health system; the Hospital Discharge Registry (HDR), which collects demographic and clinical data on all inpatients and day-case admissions in regional hospitals; the Drug Claims Registry (PHARM), including individual records for each drug prescription dispensed from public and private pharmacies in the outpatient setting, and reimbursed by the healthcare system; and, the Ticket Exemption Registry, including all residents who are entitled to co-pay fee exemptions for chronic diseases, low income, or old age. We also accessed the Integrated Surveillance System of SARS-CoV-2 infections, established in the Lazio Region during the pandemic period, which collects individual data on all patients that received a test for SARS-CoV-2 in public services. Information was available on socio-demographic characteristics, date of SARS-CoV-2 infection diagnosis, date of symptoms onset, clinical status at the diagnosis, COVID-19-related outcomes. Mortality was retrieved from the Regional Mortality Registry, which uses the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) to code causes of death. Record linkage procedures were based on a unique anonymous patient identifier.
Study population and outcomes
The cohort was defined as all individuals with dementia residing in the Lazio Region as of December 31, 2019 (enrolment date), aged 65 years or over. Patients were identified using a validated algorithm based on data from the regional health administrative databases [23, 24]. We excluded individuals who were not residing or not covered by the Regional Health Service at the enrolment date. Dementia patients were linked with the database of SARS-CoV-2 infection in order to identify a positive reverse transcription (RT)-PCR test in nasopharyngeal swab during the follow-up period. Among those infected by the end of December 2020, we estimated the mortality rates within 60 days from the date of infection.
Follow-up period
Each patient was followed-up in the period between January 1, 2020 and February 28, 2021 to assess both the incidence of SARS-CoV-2 infection and mortality. SARS-CoV-2 infection was considered as the exposure variable to estimate the mortality rate at 60 days from the date of testing.
Socio-demographic and clinical factors
Socio-demographic characteristics (gender, age, place of residence) and information on coexisting conditions were obtained from the HDR, while details on drug consumption was retrieved from the PHARM Registry. All patients were characterized at the enrolment date with respect to age, and place of residence. A broad spectrum of diseases, identified through hospitalization episodes and medication use, were considered to measure the health status of the study population and improve the assessment of the level of frailty at the individual level [25]. We included in the analysis the following cardiovascular comorbidities during the two-year period before the enrolment date: disorders of lipid metabolism, hypertension, heart failure, ischemic heart disease (including previous cardiac revascularization), arrhythmia, atrial fibrillation, cerebrovascular diseases (including cerebrovascular revascularization), and peripheral vascular diseases. In the same time span, we retrieved information on hospital admission for the following diseases and conditions: diabetes, obesity, chronic obstructive pulmonary disease (COPD), asthma, pneumonia, neoplasms, electrolytes and base-acid balance disorders, chronic kidney diseases, gastric, duodenal, peptic, and gastrojejunal ulcer, Parkinson’s disease, disease of the blood and blood-forming organs, chronic liver, pancreas and intestine diseases, fractur of neck femur, anxiety, alcohol use disorders, and depression (details on ICD-9-CM codes for cardiovascular comorbidities and other comorbidities are provided in Supplementary Table 1). Number of hospitalizations or visits to emergency departments within 6 months before the enrolment date were considered. The following categories of drugs were taken into account in a 1-year interval before the enrolment date: cardiac, antihypertensives, lipid modifying agents, statins, antiplatelet, proton pump inhibitors, insulin and analogues, blood glucose lowering drugs, antidepressants, antipsychotics, anxiolytics, antiepileptics, hypnotics and sedatives, antithrombotic agents, antibacterial therapies for systemic use, endocrine therapies, analgesic and immunostimulants drugs. In the same period, we investigated use of antidementia drugs, distinguishing use of memantine by acetylcholinesterase inhibitors (details on ATC codes are provided in the Supplementary Table 2). Moreover, in the same time span, we considered the number of neurologic and psychiatric outpatient visits, and those of other specialist clinics including general surgery, endocrinology, gastroenterology, physical medicine and rehabilitation, nephrology, orthopedics and traumatology, pulmonology, and urology. We also analyzed the risk of infection and 60-day death in the first (February-July 2020) and second pandemic phase (August-December 2020), defining a phase as a period characterized by a rising number of infections with a well-defined peak, and followed by a decrease.
Statistical analysis
Demographic and clinical characteristics were described separately for non-infected and infected subjects, and according to vital status. Chi-square test statistics were used to assess between-group differences (p < 0.05). Univariate and multivariate binomial regression models were performed to estimate, crude, and gender and age adjusted risk of SARS-CoV-2 infection and 60-day mortality, and their 95% CI. We estimated age-standardized incidence (SIRs) and mortality (SMRs) ratios, using age-specific infection and fatality rates of the whole population registered in the regional SARS-CoV-2 Surveillance System database. Multivariable logistic regression models, with a stepwise selection for the variables associated at univariate level with SARS-CoV-2 infection and death, were fitted to identify the potential determinants of infection status and 60-day mortality (p to enter = 0.11 and p to remove = 0.05). All analyses were performed using SAS Version 9.4.
Data availability
Anonymized data used for this study are available upon reasonable request.
Standard protocol approvals, registrations, and patient consents
Ethics committee or institutional review board approval and informed consent were not necessary. The Department of Epidemiology of the Lazio Regional Health Service is the regional referral center for epidemiological research, and it has full access to anonymized data from health information systems.
Approval from institutional or licensing committee was not necessary for this study.
The authors used data already collected at the beginning of the study and the data were analyzed anonymously according to the national privacy law (national legislative decree on privacy policy n. 196/30 June 2003). Individuals cannot be identified directly or through identifiers and results are shown in aggregate form.
RESULTS
Characteristics of the study population by infection status
Overall, 37,729 individuals with dementia were identified by the algorithm based on health administrative databases. Between January 1, 2020 and February 28, 2021, 2,548 (6.8%) patients were diagnosed with SARS-CoV-2 infection. Demographic and clinical characteristics of dementia patients according to infection status are shown in Table 1. Infected and non-infected subjects differed both in gender (females: 70% versus 65%) and age distribution (people aged ≥85 years: 43% versus 38%). Compared to uninfected patients, those with SARS-CoV-2 infection showed a higher prevalence of a number of cardiovascular comorbidities (in particular, hypertension and cerebrovascular diseases) and other comorbidities (including diabetes, COPD, pneumonia, and chronic renal diseases). Moreover, SARS-CoV-2 positive patients had more hospitalization episodes and emergency room visits compared with those not infected (≥2:5.6% versus 3.8% and 8.3% versus 6.5%, respectively). A greater consumption of several medications, such as antihypertensives, antiplatelet drugs, insulin, psychotropic drugs (including antipsychotics), and antithrombotic agents was observed among patients with SARS-CoV-2 infection. On the contrary, the proportion of infected patients who received memantine or acetylcholinesterase inhibitors and outpatient neurological visits was significantly lower than in SARS-CoV-2 negative cases (52.6% versus 60.0% and 24.6% versus 28.4%, respectively).
Table 1.
Demographic and clinical characteristics of dementia patients by infection status
| Total | Non-infected | Infected | p χ2 | ||||
| N | % | N | % | N | % | ||
| Total | 37,729 | 35,181 | 93.2 | 2,548 | 6.8 | ||
| Gender | <0.001 | ||||||
| Male | 13,004 | 34.5 | 12,237 | 34.8 | 764 | 30.1 | |
| Female | 24,725 | 65.5 | 22,944 | 65.2 | 1,784 | 69.9 | |
| Age | < 0.001 | ||||||
| 65–74 | 5,239 | 13.9 | 4,917 | 14.0 | 322 | 12.7 | |
| 75–84 | 17,987 | 47.7 | 16,852 | 47.9 | 1,135 | 44.7 | |
| ≥85 | 14,503 | 38.4 | 13,412 | 38.1 | 1,091 | 42.9 | |
| Residence | 0.375 | ||||||
| City of Rome | 19,919 | 52.8 | 18,552 | 52.7 | 1,367 | 53.8 | |
| Province of Rome (excl. city of Rome) | 8,014 | 21.2 | 7,466 | 21.2 | 548 | 21.6 | |
| Other provinces of the Lazio region | 9,796 | 26.0 | 9,163 | 26.0 | 633 | 24.9 | |
| Cardiovascular comorbiditiesa | |||||||
| Disorders of lipid metabolism | 769 | 2.0 | 712 | 2.0 | 57 | 2.2 | 0.448 |
| Hypertension | 5,285 | 14.0 | 4,874 | 13.9 | 411 | 16.2 | 0.002 |
| Heart failure | 1,442 | 3.8 | 1,312 | 3.7 | 130 | 5.1 | 0.005 |
| Ischemic heart disease | 1,742 | 4.6 | 1,592 | 4.5 | 150 | 5.9 | 0.002 |
| Arrhythmia | 2,271 | 6.0 | 2,101 | 6.0 | 170 | 6.7 | 0.151 |
| Atrial fibrillation | 1,822 | 4.8 | 1,680 | 4.8 | 142 | 5.6 | 0.070 |
| Cerebrovascular diseases | 4,919 | 13.0 | 4,510 | 12.8 | 409 | 16.1 | < 0.001 |
| Peripheral vascular diseases | 489 | 1.3 | 449 | 1.3 | 40 | 1.6 | 0.206 |
| Other comorbiditiesa | |||||||
| Diabetes | 2,196 | 5.8 | 2,008 | 5.7 | 188 | 7.4 | 0.000 |
| Obesity | 139 | 0.4 | 125 | 0.4 | 14 | 0.6 | 0.118 |
| COPD | 1,777 | 4.7 | 1,624 | 4.6 | 153 | 6.0 | 0.001 |
| Asthma | 29 | 0.1 | 25 | 0.1 | 4 | 0.2 | 0.131 |
| Pneumonia | 1,340 | 3.6 | 1,212 | 3.4 | 128 | 5.0 | < 0.001 |
| Neoplasm | 1,051 | 2.8 | 974 | 2.8 | 77 | 3.0 | 0.452 |
| Electrolytes and base-acid balance disorders | 1,237 | 3.3 | 1,146 | 3.3 | 91 | 3.6 | 0.390 |
| Chronic kidney diseases | 1,410 | 3.7 | 1,283 | 3.6 | 127 | 5.0 | 0.000 |
| Gastric, duodenal, peptic, gastrojejunal ulcer | 108 | 0.3 | 97 | 0.3 | 11 | 0.4 | 0.152 |
| Parkinson’s disease | 764 | 2.0 | 714 | 2.0 | 50 | 2.0 | 0.832 |
| Disease of the blood and blood-forming organs | 1,916 | 5.1 | 1,731 | 4.9 | 185 | 7.3 | < 0.001 |
| Chronic liver, pancreas, and intestine disease | 210 | 0.6 | 196 | 0.6 | 14 | 0.6 | 0.968 |
| Fracture of neck femur | 1,251 | 3.3 | 1,123 | 3.2 | 128 | 5.0 | < 0.001 |
| Anxiety | 314 | 0.8 | 275 | 0.8 | 39 | 1.5 | < 0.001 |
| Alcohol use disorders | 10 | 0.0 | 9 | 0.0 | 1 | 0.0 | 0.680 |
| Depression | 115 | 0.3 | 104 | 0.3 | 11 | 0.4 | 0.225 |
| Hospitalization episodesb | < 0.001 | ||||||
| None | 31,534 | 83.6 | 29,467 | 83.7 | 2,067 | 81.3 | |
| 1 | 4,732 | 12.5 | 4,395 | 12.5 | 337 | 13.3 | |
| 2 | 1,084 | 2.9 | 979 | 2.8 | 105 | 4.1 | |
| ≥3 | 379 | 1.0 | 340 | 1.0 | 39 | 1.5 | |
| Emergency room visitb | 0.001 | ||||||
| None | 29,854 | 79.1 | 27,895 | 79.3 | 1,959 | 77.1 | |
| 1 | 5,392 | 14.3 | 5,014 | 14.2 | 378 | 14.9 | |
| 2 | 1,643 | 4.4 | 1,498 | 4.3 | 145 | 5.7 | |
| ≥3 | 840 | 2.2 | 774 | 2.2 | 66 | 2.6 | |
| Outpatient visits, neurological branchc | < 0.001 | ||||||
| None | 27,118 | 71.9 | 25,196 | 71.6 | 1,922 | 75.6 | |
| 1 | 4,160 | 11.0 | 3,888 | 11.0 | 272 | 10.7 | |
| ≥2 | 6,451 | 17.1 | 6,097 | 17.3 | 354 | 13.9 | |
| Outpatient visits, psychiatric branchc | 0.253 | ||||||
| None | 35,663 | 94.5 | 33,238 | 94.5 | 2,425 | 95.4 | |
| 1 | 618 | 1.6 | 585 | 1.7 | 33 | 1.3 | |
| ≥2 | 1,448 | 3.8 | 1,358 | 3.9 | 90 | 3.5 | |
| Outpatient visits, other clinical specialtiesc | 0.022 | ||||||
| None | 26,009 | 68.9 | 24,214 | 68.8 | 1,795 | 70.6 | |
| 1 | 4,978 | 13.2 | 4,629 | 13.2 | 349 | 13.7 | |
| ≥2 | 6,742 | 17.9 | 6,338 | 18.0 | 404 | 15.9 | |
| Number of drugsc | 0.197 | ||||||
| None | 1,681 | 4.5 | 1,586 | 4.5 | 95 | 3.7 | |
| 1–5 | 9,732 | 25.8 | 9,061 | 25.8 | 671 | 26.4 | |
| 6–10 | 12,411 | 32.9 | 11,592 | 32.9 | 819 | 32.2 | |
| ≥11 | 13,905 | 36.9 | 12,942 | 36.8 | 963 | 37.9 | |
| Drugs prescriptionc | |||||||
| Cardiac | 5,441 | 14.4 | 5,064 | 14.4 | 377 | 14.8 | 0.577 |
| Antihypertensive | 29,168 | 77.3 | 27,139 | 77.1 | 2,029 | 79.9 | 0.004 |
| Lipid modifying agents | 15,002 | 39.8 | 14,060 | 40.0 | 942 | 37.1 | 0.003 |
| Statins | 13,135 | 34.8 | 12,288 | 34.9 | 847 | 33.3 | 0.096 |
| Antiplatelet | 23,226 | 61.6 | 21,604 | 61.4 | 1,622 | 63.8 | 0.023 |
| Proton pump inhibitors | 23,278 | 61.7 | 21,634 | 61.5 | 1,644 | 64.7 | 0.003 |
| Insulin and analogues | 2,506 | 6.6 | 2,306 | 6.6 | 200 | 7.9 | 0.016 |
| Blood glucose lowering drugs | 6,508 | 17.2 | 6,088 | 17.3 | 420 | 16.5 | 0.320 |
| Antidepressants | 15,976 | 42.3 | 14,892 | 42.3 | 1,084 | 42.7 | 0.999 |
| Antipsychotics | 11,923 | 31.6 | 10,806 | 30.7 | 1,117 | 44.0 | < 0.001 |
| Anxiolytics | 69 | 0.2 | 61 | 0.2 | 8 | 0.3 | 0.107 |
| Antiepilectis | 8,457 | 22.4 | 7,796 | 22.2 | 661 | 26.0 | < 0.001 |
| Hypnotics and sedatives | 71 | 0.2 | 66 | 0.2 | 5 | 0.2 | 0.918 |
| Antithrombotic agents | 27,014 | 71.6 | 25,120 | 71.4 | 1,894 | 74.5 | 0.001 |
| Antibacterial for systemic use | 22,109 | 58.6 | 205,58 | 58.4 | 1,551 | 61.0 | 0.014 |
| Endocrine therapy | 757 | 2.0 | 711 | 2.0 | 46 | 1.8 | 0.465 |
| Analgesic drugs | 3,879 | 10.3 | 3,620 | 10.3 | 259 | 10.2 | 0.879 |
| Immunostimulants drugs | 95 | 0.3 | 92 | 0.3 | 3 | 0.1 | 0.164 |
| Memantine | 9,534 | 25.3 | 8,961 | 25.5 | 573 | 22.6 | 0.000 |
| Acetylcholinesterase inhibitors | 12,926 | 34.3 | 12,163 | 34.6 | 763 | 30.0 | < 0.001 |
aHospital admission in the 2-year period before the enrollment, bIn the 6-month period before the enrollment; cIn the 1-year period before the enrollment.
Risk of SARS-CoV-2 infection among dementia patients and main risk factors
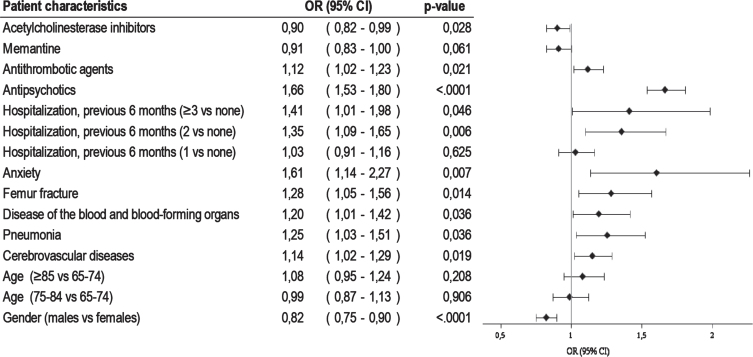
The crude risk of infection was 6.7 per 100 population (95 CI% 6.5–70.0), and 5.9 per 100 (95 CI% 55.5–64.0) after adjusting for gender and age. SIR was 1.43 (95% CI 1.33–1.54) for males and 1.71 (95% CI 1.64–1.80) for females. The multivariable logistic regression model showed that previous hospitalization for cerebrovascular diseases (OR 1.14, 95% CI 1.02–1.29), pneumonia (OR 1.25, 95% CI 1.03–1.51), blood diseases (OR 1.20, 95% CI 1.01–1.42), femur fracture (OR 1.28, 95% CI 1.05–1.56), anxiety (OR 1.61, 95% CI 1.14–2.27), and antipsychotic (OR 1.66, 95% CI 1.53–1.80) and antithrombotic medications use (OR 1.12, 95% CI 1.02–1.23) had a statistically significant association with an increased risk of infection (Fig. 1). In this population of dementia patients, males were less likely to be infected by SARS-CoV-2 than females (OR 0.82, 95% CI 0.75–0.90). Furthermore, patients who had prescribed with acetylcholinesterase inhibitors (OR 0.90, 95% 0.82–0.99) or memantine (OR 0.91, 95% CI 0.83–1.00) showed a lower probability to be infected than those not taking any anti-dementia drug.
Fig. 1.
Factors associated with SARS-CoV-2 infection among dementia patients.
60-day mortality from infection onset and main risk factors
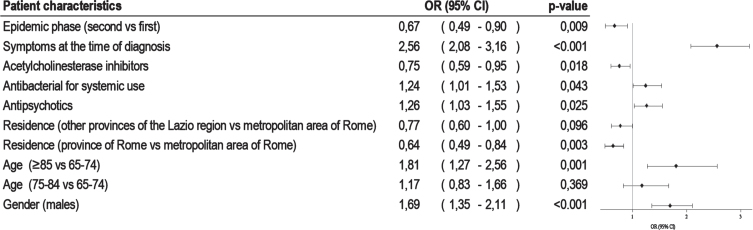
Among SARS-CoV-2 infected patients, 2013 were diagnosed up to December 31, 2020 and 31% (n = 626) of these died by the end of February 2021. The patient characteristics according to vital status are shown in Table 2. Deceased individuals were mainly over the age of 85 years and were living in the metropolitan area of Rome. Compared to 60-day survivors, patients who died showed a higher prevalence of cardiovascular comorbidities, chronic kidney diseases and diseases of the blood and blood-forming organs. Moreover, non-survivors had more hospitalizations (22.7% versus 17.7.%) and emergency room visits (21.8% versus 28.8%), a greater consumption of antipsychotics (51.4% versus 42.7%) and systemic antibacterial agents (67.3% versus 59.3%), and a lower use of any anti-dementia drug (2.1% versus 10.8%). There was a higher prevalence of symptomatic patients among those who died and a higher proportion of patients infected during the first pandemic phase. The crude 60-day risk of death was 31.0 per 100 (95 CI% : 28.8–33.6), growing up to 40.0 per 100 (95 CI% : 35.1–45.6) after adjusting for gender and age. Standardized mortality ratio (SMR) was 2.32 (95% CI 2.05–2.65) and 2.82 (95% CI 2.55–3.11) for males and females, respectively. Males (OR 1.69, 95% CI 1.35–2.11), the oldest old (OR 1.81, 95% CI 1.27–2.56), patients with symptoms at the diagnosis (OR 2.56, 95% CI 2.08–3.16), those with antipsychotic prescriptions (OR 1.26, 95% CI 1.03–1.55), and finally patients treated with systemic antibiotics (OR 1.24, 95% CI 1.01–1.53) were more likely to die within 60 days of the positive SARS-CoV-2 test (Fig. 2). Patients who developed the infection during the second pandemic phase (OR 0.67, 95% CI 0.49–0.90) and those residing outside the metropolitan area of Rome (OR 0.64, 95% CI 0.49–0.84) showed a reduced risk of death.
Table 2.
Characteristics of dementia patients infected with SARS-CoV-2 by vital status within 60 days from the infection
| Total | Alive | Died | p χ2 | ||||
| N | % | N | % | N | % | ||
| Total | 2,013 | 1,387 | 68.9 | 626 | 31.1 | ||
| Gender | < 0.001 | ||||||
| Male | 597 | 30.3 | 366 | 26.4 | 231 | 37.8 | |
| Female | 1,416 | 71.9 | 1,021 | 73.6 | 395 | 64.6 | |
| Age | < 0.001 | ||||||
| 65–74 | 240 | 12.2 | 181 | 13.0 | 59 | 9.7 | |
| 75–84 | 874 | 44.4 | 633 | 45.6 | 241 | 39.4 | |
| ≥85 | 899 | 45.7 | 573 | 41.3 | 326 | 53.4 | |
| Residence | 0.004 | ||||||
| City of Rome | 1109 | 56.3 | 730 | 52.6 | 379 | 62.0 | |
| Province of Rome (excl. city of Rome) | 427 | 21.2 | 312 | 22.5 | 115 | 18.8 | |
| Other provinces of the Lazio region | 477 | 24.2 | 345 | 24.9 | 132 | 21.6 | |
| Cardiovascular comorbiditiesa | |||||||
| Disorders of lipid metabolism | 44 | 2.2 | 33 | 2.4 | 11 | 1.8 | 0.493 |
| Hypertension | 319 | 16.2 | 212 | 15.3 | 107 | 17.5 | 0.297 |
| Heart failure | 103 | 5.2 | 66 | 4.8 | 37 | 6.1 | 0.000 |
| Ischemic heart disease | 112 | 5.7 | 66 | 4.8 | 46 | 7.5 | 0.006 |
| Arrhythmia | 135 | 6.9 | 85 | 6.1 | 50 | 8.2 | 0.133 |
| Atrial fibrillation | 113 | 5.7 | 66 | 4.8 | 47 | 7.7 | 0.014 |
| Cerebrovascular diseases | 329 | 16.7 | 220 | 15.9 | 109 | 17.8 | 0.287 |
| Peripheral vascular diseases | 32 | 1.6 | 18 | 1.3 | 14 | 2.3 | 0.186 |
| Other comorbiditiesa | |||||||
| Diabetes | 150 | 7.6 | 96 | 6.9 | 54 | 8.8 | 0.237 |
| Obesity | 9 | 0.5 | 6 | 0.4 | 3 | 0.5 | 0.691 |
| COPD | 128 | 6.5 | 87 | 6.3 | 41 | 6.7 | 0.917 |
| Asthma | 3 | 0.2 | 2 | 0.1 | 1 | 0.2 | 0.931 |
| Pneumonia | 104 | 5.3 | 62 | 4.5 | 42 | 6.9 | 0.057 |
| Neoplasm | 56 | 2.8 | 32 | 2.3 | 24 | 3.9 | 0.099 |
| Electrolytes and base-acid balance disorders | 77 | 3.9 | 53 | 3.8 | 24 | 3.9 | 0.689 |
| Chronic kidney diseases | 103 | 5.2 | 57 | 4.1 | 46 | 7.5 | 0.001 |
| Gastric, duodenal, peptic, gastrojejunal ulcer | 8 | 0.4 | 4 | 0.3 | 4 | 0.7 | 0.245 |
| Parkinson’s disease | 38 | 1.9 | 22 | 1.6 | 16 | 2.6 | 0.105 |
| Disease of the blood and blood-forming organs | 149 | 7.6 | 87 | 6.3 | 62 | 10.1 | 0.005 |
| Chronic liver, pancreas and intestine disease | 11 | 0.6 | 9 | 0.6 | 2 | 0.3 | 0.356 |
| Fracture of neck femur | 100 | 5.1 | 69 | 5.0 | 31 | 5.1 | 0.962 |
| Anxiety | 25 | 1.3 | 18 | 1.3 | 7 | 1.1 | 0.741 |
| Alcohol use disorders | 1 | 0.1 | 1 | 0.1 | 0 | 0.0 | 0.502 |
| Depression | 11 | 0.6 | 6 | 0.4 | 5 | 0.8 | 0.300 |
| Hospitalization episodesb | 0.034 | ||||||
| None | 1,628 | 82.7 | 1,141 | 82.3 | 487 | 79.7 | |
| 1 | 268 | 13.6 | 170 | 12.3 | 98 | 16.0 | |
| 2 | 83 | 4.2 | 52 | 3.7 | 31 | 5.1 | |
| ≥3 | 34 | 1.7 | 24 | 1.7 | 10 | 1.6 | |
| Emergency room visitb | 0.007 | ||||||
| None | 1,534 | 77.9 | 1,084 | 78.2 | 450 | 73.6 | |
| 1 | 299 | 15.2 | 187 | 13.5 | 112 | 18.3 | |
| 2 | 119 | 6.0 | 76 | 5.5 | 43 | 7.0 | |
| ≥3 | 61 | 3.1 | 40 | 2.9 | 21 | 3.4 | |
| Outpatient visits, neurological branchc | 0.077 | ||||||
| None | 1,535 | 78.0 | 1,038 | 74.8 | 497 | 81.3 | |
| 1 | 211 | 10.7 | 152 | 11.0 | 59 | 9.7 | |
| ≥2 | 267 | 13.6 | 197 | 14.2 | 70 | 11.5 | |
| Outpatient visits, psychiatric branchc | 0.865 | ||||||
| None | 1,922 | 97.6 | 1,322 | 95.3 | 600 | 98.2 | |
| 1 | 24 | 1.2 | 17 | 1.2 | 7 | 1.1 | |
| ≥2 | 67 | 3.4 | 45 | 3.2 | 22 | 3.6 | |
| Outpatient visits, other clinical specialtiesc | 0.947 | ||||||
| None | 1,435 | 72.9 | 990 | 71.4 | 445 | 72.8 | |
| 1 | 264 | 13.4 | 183 | 13.2 | 81 | 13.3 | |
| ≥2 | 314 | 15.9 | 214 | 15.4 | 100 | 16.4 | |
| Number of drugsc | 0.219 | ||||||
| None | 82 | 4.2 | 62 | 4.5 | 20 | 3.3 | |
| 1–5 | 544 | 27.6 | 375 | 27.0 | 169 | 27.7 | |
| 6–10 | 647 | 32.9 | 457 | 32.9 | 190 | 31.1 | |
| ≥11 | 740 | 37.6 | 493 | 35.5 | 247 | 40.4 | |
| Drugs prescriptionc | |||||||
| Cardiac | 293 | 14.9 | 193 | 13.9 | 100 | 16.4 | 0.225 |
| Antihypertensive | 1,609 | 81.7 | 1,094 | 78.9 | 515 | 84.3 | 0.078 |
| Lipid modifying agents | 733 | 37.2 | 516 | 37.2 | 217 | 35.5 | 0.273 |
| Statins | 660 | 33.5 | 462 | 33.3 | 198 | 32.4 | 0.457 |
| Antiplatelet | 1,284 | 65.2 | 883 | 63.7 | 401 | 65.6 | 0.864 |
| Proton pump inhibitors | 1,304 | 66.2 | 889 | 64.1 | 415 | 67.9 | 0.339 |
| Insulin and analogues | 160 | 8.1 | 109 | 7.9 | 51 | 8.3 | 0.824 |
| Blood glucose lowering drugs | 331 | 17.2 | 226 | 16.3 | 105 | 17.2 | 0.788 |
| Antidepressants | 829 | 42.1 | 575 | 41.5 | 254 | 41.6 | 0.712 |
| Antipsychotics | 906 | 46.0 | 592 | 42.7 | 314 | 51.4 | 0.002 |
| Anxiolytics | 6 | 0.3 | 4 | 0.3 | 2 | 0.3 | 0.905 |
| Antiepilectis | 522 | 26.5 | 354 | 25.5 | 168 | 27.5 | 0.533 |
| Hypnotics and sedatives | 4 | 0.2 | 2 | 0.1 | 2 | 0.3 | 0.413 |
| Antithrombotic agents | 1,494 | 75.9 | 1,020 | 73.5 | 474 | 77.6 | 0.301 |
| Antibacterial for systemic use | 1,234 | 62.7 | 823 | 59.3 | 411 | 67.3 | 0.007 |
| Endocrine therapy | 36 | 1.8 | 22 | 1.6 | 14 | 2.3 | 0.308 |
| Analgesic drugs | 200 | 10.2 | 147 | 10.6 | 53 | 8.7 | 0.139 |
| Immunostimulants drugs | 3 | 0.2 | 2 | 0.1 | 1 | 0.2 | 0.933 |
| Memantine | 422 | 1.1 | 293 | 0.8 | 129 | 5.1 | 0.791 |
| Acetylcholinesterase inhibitors | 580 | 1.5 | 435 | 1.2 | 145 | 5.7 | 0.000 |
| Symptoms at diagnosis | 1,192 | 60.5 | 921 | 66.4 | 271 | 44.4 | < 0.001 |
| Infection periodd | < 0.001 | ||||||
| First epidemic phase | 257 | 13.1 | 146 | 10.5 | 111 | 18.2 | |
| Second epidemic phase | 1,754 | 89.1 | 1,240 | 89.4 | 514 | 84.1 | |
aHospital admission in the 2-year period before the enrollment, bIn the 6-month period before the enrollment; cIn the 1-year period before the enrollment; dmissing data for two individuals.
Fig. 2.
Factors associated with 60-day mortality in dementia patients infected with SARS-CoV-2.
DISCUSSION
This large population-based cohort study evaluated the impact of SARS-CoV-2 infection among patients with dementia in a large Italian region, during the first year of the COVID-19 pandemic. Several studies reported that people with dementia are more likely to contract SARS-CoV-2 infection than people without and that dementia is in itself a risk factor for COVID-19-related mortality [8, 9, 26, 27]. However, very limited data comes from cohort studies of patients with dementia, analyzing what clinical and demographic characteristics affect the risk of SARS-CoV-2 infection and severity in this fragile population [28, 29].
We found both the risk of infection and mortality to be markedly higher in people with dementia as compared with general elderly population. A high burden of comorbidities and antipsychotic use were identified as risk factors for both infection and 60-day mortality, while severity of symptoms at diagnosis and male gender were specifically associated with an increased risk of COVID-19-related death.
Consistently with previous reports, individuals with dementia enrolled in our cohort were 60% more likely to get SARS-CoV-2 infection than people in the general population. Indeed, a comparable likelihood of SARS-CoV-2 infection was estimated in a cohort of individuals with pre-existing dementia recruited in psychiatric inpatient settings [29]. Additionally, Wang and colleagues showed that people with dementia had a twofold increased risk of contracting COVID-19, with odds varying between 3.2 and 1.8 depending on type of dementia, an excess very close to that estimated in our study [30].
We observed that dementia patients with frequent hospitalization episodes, who have comorbidities such as blood, cerebrovascular and respiratory diseases, anxiety, those using antithrombotic agents and with a history of hip fracture presented a higher risk of infection. This greater susceptibility may reflect a more severe stage of dementia and, as a consequence, an increased frailty and higher level of care from caregivers that may convey the infection [31, 32]. In addition, more severe, non-self-sufficient individuals with dementia may be more likely to reside in long-term care facilities sharing confined and enclosed spaces with other people, enhancing the risk of exposure to COVID-19 and other infectious diseases. This finding highlights that special attention is needed toward these patients to prevent SARS-CoV-2 infection, considering these specific risk factors to guide healthcare workers, family members, and caregivers in implementing appropriate protection measures and monitoring.
After adjusting for age and comorbidities, women enrolled in our study looked less resistant to SARS-CoV-2 infection than men. Current evidence on sex and gender differences in the susceptibility to the infection is not conclusive, with studies reporting conflicting results both for the general population and people with cognitive impairment [9, 32–34]. Indeed, women have a greater resistance to viral infections compared with men, due to a stronger innate immunity, steroid hormones, and factors related to sex chromosomes [35]. Although some evidence is available of a greater resistance against SARS-CoV-2 in women [36], we cannot exclude that our result may be due to a higher prevalence of women residing in long-term care facilities, which are a risk contest for respiratory infections and that were particularly affected in the first pandemic phase [37].
Consistently with other reports, we found a COVID-19-associated crude case fatality rate of 31%, with a more than double risk of dying in dementia patients compared to the general population of the same age and gender [38, 39]. Our analysis revealed a strong association between presence of symptoms at diagnosis and short-term mortality. Recent research showed that in patients with Alzheimer’s disease or other forms of dementia, more frequently had diabetes or cardiovascular disease and presented to the healthcare centers with more critical symptoms such as loss of consciousness, respiratory distress, and lower partial pressure of oxygen in arterial blood [40]. It has also been shown that the onset of COVID-19 disease in individuals with dementia often occurs with non-respiratory symptoms such as delirium or functional decline [41]. Anyway, our result highlights the need for increased attention to both specific and atypical clinical COVID-19 symptoms in this population of patients for early and rapid identification of the disease.
Of particular interest was the association between COVID-19 infection and 60-day mortality in dementia patients using antipsychotic (AP) medications. People with late-stage dementia and those residing in long-term care facilities represent the group most likely to receive a prescription for AP drugs [42]. The COVID-19 pandemic and infection control measures have impacted greatly on routine activities and care of people with dementia, both living in an institutional setting and in the community, causing the onset/worsening of behavioral and psychological symptoms of dementia, with increased personal distress, and a higher risk of infection and mortality. Because of concerns about safety and limited efficacy, the use of APs has been declining in recent years [43], but some evidence is available that during the COVID-19 pandemic there was an increase in the proportion of dementia patients receiving these drugs [44]. A recent review found evidence of a strong association between antipsychotics intake and COVID-19-related death in patients with pre-existing mental disorders, including dementia [45]. The harmful effect of antipsychotics in patients with dementia may be enhanced by concomitant COVID-19, as seem to be confirmed by the excess mortality we observed in patients using AP compared with non-users, even taking into account a number of coexisting risk factors. The results of our study suggest limiting as much as possible AP prescription in persons with dementia, especially during the pandemic period. In contrast, the use of acetylcholinesterase inhibitors and memantine was slightly protective for infection and mortality. This result could be due to a lower severity of cognitive impairment e functional decline in patients treated with anti-dementia drugs and their well-documented protective effect against inflammatory processes [46].
Although men were less likely to get the infection, we observed that they faced higher risk of death compared to women, independent of age. Several COVID-19 studies have shown that men have a higher risk of developing a severe form of the disease compared with women and a higher death rate [47]. This finding is in line with the fact that, in general, respiratory infectious diseases are more severe among males and leads to higher mortality in males than in women [48]. Very interestingly, patients living outside the metropolitan area of Rome were less likely to die for COVID-related causes, while studies carried out in other countries have shown an opposite result, with rural/suburban areas associated with greater mortality burden than urban zones [49, 50]. There are several potential explanations for the observed urban/suburban mortality divide, including socioeconomic disparities in access and use of healthcare services, different availability and/or organization of community healthcare facilities and hospitals, and level of coordination and continuity of care across different care settings, the latter being critical elements of health care delivery for frail older adults. Moreover, the exposure to environmental risk factors (air pollution) may have exacerbated the COVID-19-related mortality risk in vulnerable groups living in the metropolitan area of Rome. Anyway, the observed heterogeneity in mortality deserves further and deeper investigation to target specific interventions for both healthcare organizations and population subgroups.
The current study has some limitations. Firstly, the lack of information on diverse potential determinants such as place of residence (long-term facilities or private homes), socio-economic position, and behavioral risk factors. Secondly, we cannot exclude that during the first phase, when public health emergency was severe and unexpected, the most severely infected patients were not even admitted to hospitals and died at home undiagnosed. Thirdly, to best identify patients’ comorbidities and obtain a more exhaustive control of confounding, we used both hospital discharges and drug prescriptions. Drug prescription information are important complements to the hospital discharge data, as it is shown by the different proportion of patients identified from these two data sources. However, a certain amount of overlap between the two registries cannot be ruled out. Finally, administrative databases do not allow for a detailed description of patients’ clinical characteristics. Consequently, we were unable to distinguish the different types of dementia and had to rely on hospital discharge information and drug prescription claims to define patients’ comorbidities.
The main strengths of this study are the population-level approach and the large sample size. Moreover, to our knowledge, this is the first study analyzing the determinants of SARS CoV-2 infection and death from COVID-19 among dementia patients, using health administrative databases and a validated algorithm to identify the study population. Indeed, the study relies on good quality health administrative data, currently used not only for descriptive but also for analytical purposes. At last, it is noteworthy that the prompt availability of information coming from administrative databases allows to monitor health care delivery in the region, identify critical points in provision of and access to healthcare, in order to implement corrective measures. This aspect is particularly relevant in the current public health emergency due to COVID-19 pandemic.
In conclusion, given the magnitude of impact of COVID-19 pandemic on elderly patients, providing information on the actual rate of SARS-CoV-2 infection on people with dementia and specific determinants of worse outcomes, can support clinicians when considering prevention and treatment among individuals in this vulnerable group. Patients with dementia require close monitoring by both physicians and caregivers to minimize exposure to the virus, recognize timely signs/symptoms of COVID-19, and ensure proper disease management. The pandemic continues to influence our lives and threaten population health, in particular that of frail people. We have to keep in mind that although much has been learned about how to prevent infection and new drugs have been approved by international agencies or are currently under investigation, the most fragile subgroups of the population, such as the elderly with multimorbidity and dementia, need to be adequately monitored to reduce the deadly impact of COVID-19.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Simona Ricci for helpful contribution to graphics.
This study was conducted without additional financial support from any external research bodies.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0369r1).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220369.
REFERENCES
- [1]. Dorrucci M, Minelli G, Boros S, Manno V, Prati S, Battaglini M, Corsetti G, Andrianou X, Riccardo F, Fabiani M, Vescio MF, Spuri M, Urdiales AB, Manso M, Onder G, Pezzotti P, Bella Aon behalf of the Italian Integrated Surveillance COVID-19 group(2021) Excess mortality in Italy during the COVID-19 pandemic: Assessing the differences between the first and the second wave, year 2020. Front Public Health 9, 669209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Odone A, Delmonte D, Gaetti G, Signorelli C (2021) Doubled mortality rate during the COVID-19 pandemic in Italy: Quantifying what is not captured by surveillance. Public Health 190, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Michelozzi P, de’Donato F, Scortichini M, De Sario M, Noccioli F, Rossi P, Davoli M (2020) Mortality impacts of the coronavirus disease (COVID-19) outbreak by sex and age: Rapid mortality surveillance system, Italy, 1 February to 18 April 2020. Eur Surveill 25, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Canevelli M, Palmieri L, Raparelli V, Lo Noce C, Colaizzo E, Tiple D, Vaianella L, Vanacore N, Brusaferro S, Onder G, Italian NationalInstitute of Health COVID-19 Mortality Group (2020) Prevalenceand clinical correlates of dementiaamong COVID-19-related deaths in Italy. Alzheimers Dement(Amst) 12, e12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Amore S, Puppo E, Melara J, Terracciano E, Gentili S, Liotta G, Liotta G (2021) Impact of COVID-19 on older adults and role of long-term care facilities during early stages of pandemic in Italy. Sci Rep 11, 12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Rutten JJS, van Kooten J, van Loon AM, van Buul LW, Joling K, Smalbrugge M, Hertog CMP (2021) Dementia and Parkinson’s disease: Risk factors for 30-day mortality in nursing home residents with COVID-19. J Alzheimers Dis 84, 1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo CL, Kuchel GA, Melzer D (2020) Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank Community Cohort. J Gerontol A Biol Sci Med Sci 75, 2224–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Zhou Y, Yang Q, Chi J, Dong B, Lv W, Shen L, Wang Y (2020) Comorbidities and the risk of severe or fatal outcomes associatedwith coronavirus disease 2019. A systematic review andmeta-analysis. Int J Infect Dis 99, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Wang Q, Davis P, Gurney M, Xu R (2021) COVID-19 and dementia: Analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimers Dement 17, 1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Liu N, Sun J, Wang X, Zhao M, Huang Q, Li H (2020) The impact of dementia on the clinical outcome of COVID-19: A systematic review and meta-analysis. J Alzheimers Dis 78, 1775–1782. [DOI] [PubMed] [Google Scholar]
- [11]. Tahira AC, Verjovski-Almeida S, Ferreira ST (2021) Dementia is an age-independent risk factor for severity and death in COVID-19 inpatients. Alzheimers Dement 17, 1818–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Prince M, Guerchet M, Matthew P (2015) The epidemiology and impact of dementia - current state and future trends. WHO Thematic Briefing, http://www.who.int/mental_health/neurology/dementia/thematic_briefs_dementia/en/.
- [13]. GBD 2019 Dementia Forecasting Collaborators (2022) Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7, e105–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Schultze A, Nightingale E, Evans D, Hulme W, Rosello A, Bates C, Cockburn J, MacKenna B, Curtis HJ, Morton CE, Croker R, Bacon S, McDonald HI, Rentsch CT, Bhaskaran K, Mathur R, Tomlinson LA, Williamson EJ, Forbes H, Tazare J, Grint D, Walker AJ, Inglesby P, DeVito NJ, Mehrkar A, Hickman G, Davy S, Ward T, Fisher L, Green AC, Wing K, Wong AY, McManus R, Parry J, Hester F, Harper S, Evans SJ, Douglas IJ, Smeeth L, Eggo RM, Goldacre B, Leon DA (2022) Mortality among care home residents in England during the first and second waves of the COVID-19 pandemic: An observational study of 4.3 million adults over the age of 65. Lancet Reg Health Eur 14, 100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Anstey KJ, Ee N, Eramudugolla R, Jagger C, Peters R (2019) A systematic review of meta-analyses that evaluate risk factors for dementia to evaluate the quantity, quality, and global representativeness of evidence. J Alzheimers Dis 70(1), S165–S186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Wolff D, Nee S, Hickey NS, Marschollek M (2021) Risk factors for Covid-19 severity and fatality: A structured literature review. Infection 49, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Izcovich A, Ragusa MA, Tortosa F, Lavena Marzio MA, Agnoletti C, Bengolea A, Ceirano A, Espinosa F, Saavedra E, Sanguine V, Tassara A, Candelaira C, Norberto Catalano H, Agarwal A, Foroutan F, Rada G (2020) Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS One 15, e0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Li Y, Ashcroft T, Chung A, Dighero I, Dozier M, Horne M, McSwiggan E, Shamsuddin A, Nair H (2021) Risk factors for poor outcomes in hospitalised COVID-19 patients: A systematic review and meta-analysis. J Glob Health 11, 10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Moretti R, Caruso P, Giuffré M, Tiribelli C (2021) COVID-19 lockdown effect on not institutionalized patients with dementia andcaregivers. Healthcare (Basel) 9, 893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Numbers K, Brodaty H (2021) The effects of the COVID-19 pandemic on people with dementia. Nat Rev Neurol 17, 69–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Keng A, Brown EE, Rostas A, Rajji TK, Pollock BG, Mulsant BH, Kumar S (2020) Effectively caring for individuals with behavioral and psychological symptoms of dementia during the COVID-19 pandemic. Front Psychiatry 11, 573367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Mok VCT, Pendlebury S, Wong A, Alladi S, Au L, Bath PM, Biessels GJ, Chen C, Cordonnier C, Dichgans M, Dominguez J, Gorelick PB, Kim S, Kwok T, Greenberg SM, Jia J, Kalaria R, Kivipelto M, Naegandran K, Lam LCW, Lam BYK, Lee ATC, Markus HS, O’Brien J, Pai MC, Pantoni L, Sachdev P, Skoog I, Smith EE, Srikanth V, Suh GH, Wardlaw J, Ko H, Black SE, Scheltens P (2020) Tackling challenges in care of Alzheimer’s disease and other dementias amid the COVID-19 pandemic, now and in the future . Alzheimers Dement 16, 1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Francesconi P, Gini R, Roti L, Bartolacci S, Corsi A, Buiatti E (2007) The Tuscany experimental registry for Alzheimer’s disease and other dementias: How many demented people does in capture? Aging Clin Exp Res 19, 390–393. [DOI] [PubMed] [Google Scholar]
- [24]. Bacigalupo I, Lombardo F, Bargagli A, Cascini S, Agabiti N, Davoli M, Scalmana S, Di Palma A, Greco A, Rinaldi M, Imperiale D, Secreto P, Golini N, Gnavi R, Biagini C, Gualdani E, Francesconi P, Magliocchetti N, Di Fiandra T, Vanacore N (2021) Validation of an algorithm based on data of the health information systems (HIS) for the identification of dementia cases. J Neurol Sci 429(Suppl), 115. [Google Scholar]
- [25]. Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS (2018) Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: A prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 3, e323–e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Kim YJ, Jee Y, Park S, HA EH, Jo I, Woon Lee H, Song MS (2021) Mortality risk within 14 days after coronavirus disease 2019 diagnosis in dementia patients: A nationwide analysis. , -. Dement Geriatr Cogn Disord 50, 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Zhang Q, Schultz JL, Aldridge GM, Simmering JE, Kim Y, Ogilvie AC, Narayan NS (2021) COVID-19 case fatality and Alzheimer’s disease. J Alzheimers Dis 84, 1447–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Vrillon A, Mhanna E, Aveneau C, Lebozec M, Grosset L, Nankam D, Albuquerque F, Feroldi RR, Maakaron, Piassareva I, Gherissi DC, Azuar J, Francois V, Hourrègue C, Dumurgier J, Volpe-Gillot L, Paquet C (2021) COVID-19 in adults with dementia: Clinical features and risk factors of mortality-a clinical cohort study on 125 patients. Alzheimers Res Ther 13, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Livingston G, Rostamipour H, Gallagher P, Kalafatis C, Shastri A, Huzzey L, Liu K, Sommerlad A, Marston L (2020) Prevalence,management, and outcomes of SARS-CoV-2 infections in olderpeople and those with dementia in mental healthwards in London, UK: A retrospective observational study. Lancet Psychiatry 12, 1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Wang Y, Yang Y, Ren L, Shao Y, Tao W, Dai XJ (2021) Preexisting mental disorders increase the risk of COVID-19 infection and associated mortality. Front Public Health 9, 684112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Pan AP, Meeks J, Potter T, Masdeu JC, Seshadri S, Lee Smith M, Ory MG, Vahidy FS (2021) SARS-CoV-2 susceptibility and COVID-19 mortality among older adults with cognitive impairment: Cross-sectional analysis from hospital records in a diverse US metropolitan area. Front Neurol 12, 692662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Wang H, Li T, Barbarino P, Gauthier S, Brodaty H, Molinuevo JL, Xie H, Sun Y, Yu E, Tang Y, Weidner W, Yu X (2020) Dementia care during COVID-19. Lancet 395, 1190–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Jin JM, Bai P, He W, Liu XF, Han DM, Liu S Yang JK(2020) Gender differences in patients with COVID-19: Focus on severity and mortality. Front Public Health 8, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Italian National Institute of Statistics. Residential care facilities. Report 2018. Available on https://www.istat.it/it/files/2018/05/Presidi-residenziali_2015.pdf.
- [35]. Jacobsen H, Klein SL (2021) Sex differences in immunity to viral infections. Front Immunol 12, 720952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Rehman S, Ravinayagam V, Nahvi I, Aldossary H, Al-Shammari M, Al Amiri MS, Kishore U, Al Suhaimi E (2021) Immunity, sex hormones, and environmental factors as determinants of COVID-19 disparity in women. Front Immunol 12, 680845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Gugliotta C, Gentili D, Marras S, Dettori M, Muglia PP, Desole MG, Acciaro M, Bellu S, Azara A, Castiglia P (2021) SARS-CoV-2 epidemics in retirement and nursing homes in Italy: A new preparedness assessment model after the first epidemic wave. Int J Environ Res Public Health 18, 5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Saragih ID, Advani S, Saragih IS, Suarilah I, Susanto I, Lin CJ (2021) Frailty as a mortality predictor in older adults with COVID-19: A systematic review and meta-analysis of cohort studies. Geriatr Nurs 42, 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Romagnolo A, Imbalzano G, Artusi CA, Balestrino R, Ledda C, De Rosa FG, Riccardini F, Montanaro E, Bozzali M, Rizzone MG, Zibetti M, Lopiano L (2021) Neurological comorbidities and COVID-19-related case fatality: A cohort study. J Neurol Sci 428, 117610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Fathi M, Taghizadeh F, Mojtahedi H, Jame SZB, Moghaddam NM (2022) The effects of Alzheimer’s and Parkinson’s disease on 28-day mortality of COVID-19. Rev Neurol (Paris) 178, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Wang H (2020) Delirium: A suggestive sign of COVID-19 in dementia. EClinicalMedicine 26, 100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Ralph SJ, Espinet AJ (2018) Increased all-cause mortality by antipsychotic drugs: Updated review and meta-analysis in dementia and general mental health care. J Alzheimers Dis Rep 2, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Kirkham J, Sherman C, Velkers C, Maxwell C, Gill S, Rochon P, Seitz D (2017) Antipsychotic use in dementia. Can J Psychiatry 62, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Howard R, Burns A, Schneider L (2020) Antipsychotic prescribing to people with dementia during COVID-19. Lancet Neurol 19, 892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Vai B, Mazza MG, Delli Colli C, Foiselle M, Allet B, Benedetti F (2021) Mental disorders and risk of COVID-19-related mortality, hospitalisation, and intensive care unit admission: A systematic review and meta-analysis. Lancet Psychiatry 8, 797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Xu H, Garcia-Ptacek S, Jönsson L, Wimo A, Nordström P, Eriksdotter M (2021) Long-term effects of cholinesterase inhibitors on cognitive decline and mortality. Neurology 96, e2220–e2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, Rosser EC, Webb K, Deakin CT (2020) Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 11, 6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Falagas ME, Mourtzoukou EG, Vardakas KZ (2007) Sex differences inthe incidence and severity of respiratory tract infections. Respir Med 101, 1845–1863. [DOI] [PubMed] [Google Scholar]
- [49]. Magallón-Botaya R, Oliván-Blázquez B, Ramírez-Cervantes KL, Méndez-López-de-la-Manzanara F, Aguilar-Palacio I, Closas MC, Andrés-Esteban E (2021) Geographicfactors associated with poorer outcomes in patients diagnosed withCOVID-19 in primary health care. Int J Environ Res PublicHealth 18, 3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Huang Q, Jackson S, Derakhshan S, Lee L, Pham E, Jackson A, Cutter SL (2021) Urban-rural differences in COVID-19 exposures and outcomes in the South: A preliminary analysis of South Carolina. PLoS One 16, e0246548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data used for this study are available upon reasonable request.