Abstract
Accumulating evidence suggests that microglia and peripheral immune cells may play determinant roles in the pathogenesis of Parkinson’s disease (PD). Consequently, there is a need to take advantage of immune-related models of PD to study the potential contribution of microglia and peripheral immune cells to the degeneration of the nigrostriatal system and help develop potential therapies for PD. In this review, we have summarised the main PD immune models. From a historical perspective, we highlight first the main features of intranigral injections of different pro-inflammogens, including lipopolysaccharide (LPS), thrombin, neuromelanin, etc. The use of adenoviral vectors to promote microglia-specific overexpression of different molecules in the ventral mesencephalon, including α-synuclein, IL-1β, and TNF, are also presented and briefly discussed. Finally, we summarise different models associated with peripheral inflammation whose contribution to the pathogenesis of neurodegenerative diseases is now an outstanding question. Illustrative examples included systemic LPS administration and dextran sulfate sodium-induced colitis in rodents.
Keywords: Parkinson’s disease, animal models, microglia, inflammation, substantia nigra, lipopolysaccharide, thrombin, dextran sulfate sodium, adenovirus
INTRODUCTION
Parkinson’s disease (PD) is characterized by a significant loss of dopaminergic neurons in the substantia nigra (SN) along with immunopositive intracellular neuronal inclusions for α-synuclein (α-syn) in the midbrain [1]. Different mechanisms have been suggested to play an essential role in the pathogenesis of PD, including impaired mitochondrial function, autophagy, loss of trophic support, protein homeostasis dysfunction, and neuroinflammation. Since the original observation by McGeer et al. in 1988 showing reactive microglia in the SN of human postmortem PD brain tissue [2], evidence supporting an important role of microglia and inflammation in driving neurodegenerative events is overwhelming. For instance, at the genetic level, it has been shown that PD risk alleles likely alter the functioning of microglia-specific enhancers in the loci LRRK2 and FCGR2A, specifically through disrupting a SPIB-binding motif in the latter [3]. Mutations in GBA1, the gene encoding the lysosomal enzyme glucocerebrosidase, are considered the most significant risk factors for PD, which is believed to create toxic species of α-syn aggregates through defective lysosomal function [4]. A recent mouse brain cell atlas supports that Gba1 is mainly expressed by microglia and not by neurons [5]. A role for T cells is also implied by identifying specific major histocompatibility complex (MHC) haplotypes and non-coding SNPs in MHC genes as risk factors for PD [6]. Different animal models have consistently shown that early microglia activation may precede the death of dopaminergic neurons, suggesting that brain immune cells may play a leading role in the pathogenesis of PD. Pattern recognition receptors (PRRs) sense the environment by recognizing pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns molecules (DAMPs) [7]. Illustrative examples of PRRs are toll-like receptors (TLRs), nod-like receptors (NLRs), and triggering receptors expressed on myeloid cells-2 (TREM2) [7], whose selective activation is thought to generate either a proinflammatory or a disease-associated microglia (DAM) phenotype [8]. With the advent of single-cell RNAsequencing (scRNA-seq) of microglia under disease conditions, it has become evident that microglia may acquire an array of activation phenotypes much larger than originally believed including potentially protective microglia phenotypes (DAM) [8], deleterious microglial neurodegenerative phenotype (MGnD) [9] or yet to be defined (such as white matter-associated microglia (WAM) [10]). scRNA-seq from the murine midbrain identified a microglia subtype exhibiting typical pro-inflammatory features including enrichment of TLR signalling pathways [11]. scRNA-seq performed from ventral mesencephalic tissue obtained from postmortem PD patients and age-matched controls identified a disease-specific upregulation of microglia [12]. Interestingly, the authors identified a significant PD risk variant enrichment in microglia, showing the strongest association with the PD gene LRRK2 along with enrichment of NLRP3 inflammasome pathways [12]. In addition, a significant upregulation of GPNMB was found, which was associated with amoeboid microglia [12]. Of note, GPNMB is one of the most upregulated genes in DAM [13] and MGnD [9] phenotypes, and increased brain expression of GPNMB is associated with genome wide significant risk for PD [14].
A sustained and complex systemic activation of the immune system in PD is supported by increases of different cytokines (pro-inflammatory and anti-inflammatory) and immune-related molecules in CSF and serum of PD patients [15, 16]. From the different cytokines, TNF-α deserves special consideration as blocking TNF-α has been found neuroprotective in PD models [17] and usage of TNF-α antibodies has been found to lower PD incidence among patients with inflammatory bowel disease [18]. Increasing evidence supporting an important role of the peripheral immune system in PD pathology is evident and most of the peripheral contributors to PD-related neuroinflammation have been recently described by Romero-Ramos and colleagues [15]. Among them, monocytes/macrophages have been involved in PD pathogenesis since it is known that these cells infiltrate the brain during PD through the CCL2-CCR2 axis [19]. Furthermore, genetic profiling analysis has identified a distinct transcriptomic signature in monocytes from early PD patients [20]. Some researchers have also shown that monocytes from PD patients cannot produce a healthy and balanced response to different stimuli, such as α-syn for example [21]. The involvement of T cells in the pathogenesis of PD has been also demonstrated, since CD4+ and CD8 + T cells surrounding neuromelanin+ neurons have been detected in postmortem PD patients [22]. These observations pinpoint the interactions between brain innate and adaptive immune systems. The contribution of MHC II to PD pathology is inferred by studies demonstrating that MHC null mice are resistant to dopaminergic degeneration under conditions of α-syn overexpression [23]. Confirming these observations, genetic association with PD in the HLA region has been found, including HLA-DRA, HLA-DQA2, HLA-DQB1, HLA-DRB1, and HLA-DRB5 [24–27]. Importantly, a set of peptides derived from α-syn have been found to act as antigenic epitopes to further drive CD4+ and CD8+ cell responses in PD patients [28], thus linking preclinical studies and GWAS studies across the HLA regions. Finally, even though infiltrating B cells have not been detected in the brains of PD [22], a recent scRNA and BCR sequencing for B cells in PD patients and aged-matched controls identified increased memory B cells and increased IgG and IgA isotypes and more frequent class switch recombination events in PD patients [29]. All these findings have contributed to the redefinition of PD as a multisystemic disease that should be managed in a more integrative manner instead of the brain-focused classical approach. More research of this integrated network of communication that exists between peripheral immune cells and glial cells is necessary to improve our understanding of disease pathogenesis and hence provide more effective therapeutic approaches. All this information implies immune-associated models of PD as relevant tools to study the potential contribution of microglia to the degeneration of the nigrostriatal system and help in developing potential therapies for PD.
PD MODELS USING INTRACEREBRAL INJECTION
Lipopolysaccharide
Lipopolysaccharide (LPS; also known as endotoxin), a powerful pro-inflammogen, is the main component of the outer membrane of Gram-negative bacteria. The physiological response to LPS is mediated by the TLR4 in association with other proteins as the LPS binding protein (LBP), the monocyte antigen CD14, and the myeloid differentiation factor (MD)-2 [30]. Injection of LPS into different brain structures such as the cerebral cortex, striatum, choroid plexus-cerebral ventricles, or hippocampus triggers the activation of astroglia and microglia [31–34].
Since the death of dopaminergic neurons in SN is a key feature of PD, it was worth investigating whether injection of LPS into SN could induce a glial reaction and subsequent loss of dopaminergic neurons. A single intranigral injection of 2μg of LPS induces microglial activation and loss of astrocytes on the injection side, studied from two days after injection [35]. Using a single injection of 5μg of LPS into the SN, microglial activation has been described as early as 0.2 h after injection [36]. Importantly, the number of TH positive neurons on the SN is reduced on the ipsilateral side of injection [37]. Similarly, dopamine (DA) levels, its metabolites, and TH activity (a key enzyme in the synthesis of DA) decreased in both the SN and the striatum. The long-term analysis demonstrates that damage to the dopaminergic system is permanent, as seen one year after injection [35]. Other neuronal phenotypes, such as GABAergic or serotoninergic, are not affected, strongly suggesting that injection of LPS into the SN is a specific inflammatory animal model of PD [35]. Other authors have modified this model, using increasing amounts of LPS into the SN, as 5μg [36], 10μg [38], or up to 30μg [39]. Intrastriatal injections have also been used, employing either a low dose (0.05–5μg) [40] or a high dose (from 16 to 60μg) [41, 42]. Intrapallidal injection has also been reported (10μg) [43]. Interestingly, SN is always identified among the brain structures more prone to neuroinflammation. The causes of this situation have not yet been determined, although local differences in the number of microglia [44] and of the inflammation-related factors produced by these cells have been suggested [45]. In this sense, even systemic administration of LPS (which will be discussed in more detail below) has a specific effect on SN, increasing phagoptosis of nigral neurons through a mechanism dependent on the P2Y6 receptor [46].
Injection of LPS at a dose of 2μg did not affect the dopaminergic system when injected into the striatum or the medial forebrain bundle (MFB, the primary neural connection between these two structures) [35, 47]. However, it has been reported that the intrastriatal injection of 10μg of LPS in Sprague Dawley rats produces an inflammatory response, oxidative stress, and activation of the TLR/NF-KB (Nuclear factor-kappa B) pathway, with motor alterations [48]. Other authors administered even higher amounts of LPS (up to 60μg), inducing degeneration of the dopaminergic nigrostriatal system, motor impairment, and α-syn accumulation in nigral dopaminergic neurons [41, 42], with mitochondria affected before dopaminergic neuronal degeneration. Furthermore, a study using the injection of 10μg of LPS into the globus pallidus reported changes in SN iron levels, which could increase stress and subsequent vulnerability of nigral dopaminergic neurons [43]. It has been suggested that microglia could be responsible for differential susceptibility to LPS in brain structures [45]. On the other hand, models based on high LPS doses could deviate from physiological conditions not representing a ‘realistic’ disease model.
The effect of LPS on neuronal and glial cells is prevented by compounds with anti-inflammatory properties, such as dexamethasone (a potent and widely used anti-inflammatory drug) [49], minocycline (a tetracycline antibiotic) [50], simvastatin (a lipid-lowering agent) [51], or naloxone (an opioid receptor antagonist) [52]. It is striking that the LPS-induced neurotoxic effects in SN appear to be DA dependent since the inhibition of TH with α-methyl-p-tyrosine prevents microglial activation and LPS-induced damage to dopaminergic neurons [53]. Synergistic interaction of DA with other compounds to produce a toxic effect has previously been shown; of particular interest is the interaction with α-syn, which changes its aggregation pattern in vivo in contact with DA [54]. On the other hand, the contribution made by DA metabolism through monoamine oxidase (MAO, which produces H2O2) to oxidative stress should not be ruled out.
Stress reinforces the deleterious effect of LPS on SN. Therefore, the number of activated microglial cells in the SN of rats treated with LPS and the loss of astrocytes is almost doubled in stressed animals. The reinforcement by stress of the effect induced by LPS is similar (or even greater) on the expression levels of key proinflammatory molecules, including tumor necrosis factor (TNF), interleukin (IL)-1β, IL-6, and inducible NO synthase (iNOS), and the combined effect of stress and LPS results in a huge expression of monocyte chemoattractant protein 1 (MCP-1) mRNA. The number of TH positive neurons in the SN, which is halved in the animals treated with LPS, decreases to 25% of the control value when LPS is injected into the SN of stressed animals. RU486, a glucocorticoid receptor antagonist, prevents all these effects [55]. These data point to the potential role of stress in the initiation/development of the neurodegenerative process that leads to PD.
Thrombin
This multifunctional serine protease, well known for its participation in the blood coagulation cascade, has harmful effects on the CNS. When injected into the SN of Wistar rats, thrombin induces the expression of iNOS and proinflammatory cytokines (TNF, IL-1α, IL-1β) in both the SN and the striatum, increases microglial proliferation and activation, and induces the disappearance of astroglial cells around injection into the SN. Intranigral injection of thrombin also reduces the number of dopaminergic neurons in this structure without affecting other neuronal phenotypes such as GABAergic neurons. Similar results were described in Sprague Dawley rats [56], including the activation of apoptosis and the c-Jun N-terminal kinase (JNK) and p53 signaling pathways [57]. When injected into the striatum, thrombin induces a retrograde loss of dopaminergic neurons in the SN, also affecting the fibers immunopositive for TH that connect both structures and inducing the formation of deposits of α-syn in the SN, a hallmark of PD [58]. Blocking PAR4, a thrombin receptor, prevents these effects suggesting that thrombin could be involved in eliminating presynaptic elements in the striatum, leading to synaptic loss [59].
Anti-inflammatory compounds prevent the effect of compounds that trigger an inflammatory response. For example, minocycline-induced suppression of reactive oxygen species (ROS) derived from NADPH oxidase and expression of proinflammatory cytokines prevented the death of thrombin-induced dopaminergic neurons induced by thrombin [60]. However, in the intranigral thrombin model, systemic administration of dexamethasone, a widely used anti-inflammatory drug, does not only fail to prevent microglial activation but increases dopaminergic neuron damage. In fact, dexamethasone does not decrease the number of apoptotic cells, nor reduces thrombin-induced α-syn deposits, but reduces the amount of P-Akt. Interestingly, these effects appeared to be mediated by increases induced by thrombin in MAO, which was prevented by the MAO inhibitor tranylcypromine [61]. This suggests that in cases in which the integrity of the blood-brain barrier (BBB) has been compromised and thrombin is in contact with the cerebral parenchyma (as occurs in processes that affect the cerebral vasculature, such as stroke), the administration of dexamethasone as an anti-inflammatory therapy would be counterproductive.
α-Synuclein fibrils injections
α-syn protein injection has been widely used in the last decade to promote PD-like features focused on α-syn aggregation. In particular, striatal injection of α-syn pre-formed fibrils (PFF) demonstrated to cause Lewy body-like inclusion and dopaminergic degeneration in mice [62]. The relevance of neuroinflammation in this model was recently investigated in mice by Earls et al. [63] demonstrating upregulation of MHC-II as a signal of microglia activation while also describing astrogliosis and lymphocyte infiltration while similar results were obtained in rats [64]. Interestingly, T-lymphocytes have been proposed to limit phosphorylation of α-syn [65]. Injection of PFFs in transgenic models also demonstrated the importance of neuroinflammation. For instance, injection of PFF in A30P transgenic mice also resulted in increased microgliosis [66]. Similarly, injection of PFF in A53T transgenic mice led to microglia activation and neurodegeneration. However, genetic deletion of TLR2 or pharmacological inhibition achieved to decrease microgliosis and cytokine release while also protecting the dopaminergic system [67]. Overall, injection of PFF is, nowadays, one of the most used models for the study of α-syn aggregation and transmission presenting interesting similarities with the progression of PD in humans. However, the inflammatory component of this model is very relevant and should be considered as one of the best choices for determining the role of neuroinflammation in PD progression.
Other models using intracerebral injection
The tissue-type plasminogen activator (tPA, another serine protease) was the first drug approved (1995) by the Food and Drug Administration to treat acute ischemic stroke. However, beyond its beneficial abilities as a clot-dissolving agent, injection of tPA into Wistar rats’ SN produces microglial activation and loss of astrocytes, degeneration of dopaminergic neurons without affecting GABAergic, disruption of BBB, α-syn deposits, increased expression of the brain-derived neurotrophic factor (BDNF), nNOS and iNOS, and alteration of phosphorylation levels in the proteins JNK, p38, extracellular signal-regulated kinases (ERK), Akt, glycogen synthase kinase (GSK)-3β and cAMP responsive element-binding protein (CREB) [68].
Trisialoganglioside (GT1b; a glycosphingolipid containing sialic acid) is a surface molecule of mammalian cells with endogenous effects on the CNS. Injection of GT1b into the SN of female Sprague-Dawley rats induced the loss of NeuN and TH positive neurons in this structure in a dose-dependent manner. GT1b induced microglial activation and expression of iNOS in microglia (as soon as 4 h after injection). Inhibition of NOS by L-NG-nitroarginine methyl ester (L-NAME) partially prevented the deleterious effect of GT1b [69].
Finally, neuromelanin is a pigment found in human catecholaminergic neurons; however, extracellular neuromelanin has been suggested to activate microglial cells [70]. Zecca et al. [71] published a new model of microglial activation and 50% of dopaminergic degeneration after intranigral injection of human neuromelanin in rats.
Virus-mediated overexpression of proteins
Overexpression of α-syn has been used in the last decade as a model of parkinsonism focused on the aggregation capacities of α-syn inside the dopaminergic neurons [72]. Different adenovirus-associated vector (AAV) serotypes have been used to induce the expression of wild-type or mutant human α-syn. This overexpression is frequently associated with a neuronal promoter and locally injected in the midbrain region to investigate the effect on dopaminergic neurons (see [73]). Inflammation has been closely related to this model, for instance, Sanchez-Guajardo et al. [74] first described early microglia activation in rats midbrain after AAV2/5 serotypes injections along with lymphocytes infiltration. Same serotype was demonstrated to induce microglia activation independently of neurodegeneration in monkeys [75] and mice [76]. Neuroinflammation appeared in the striatum even before than in the SN, including increased levels of several cytokines like IL-1β and TNF-α [77]. Furthermore, inhibition of microglia activation has been demonstrated to protect dopaminergic integrity after AAV9 serotype injection [78] and AAV2 serotype where MHC-II genetic deletion resulted in absence of neurodegeneration [23]. Conversely, further activation of microglia through LPS injection promoted cell-to-cell transmission of α-syn [79]. Interestingly, combination of this model with injection of PFF has also shown to increase microglia activation, microgliosis and dopaminergic degeneration [80].
However, Bido et al. [81] have recently published a novel variant of this model focusing on the effect of α-syn on microglial cells. In their study, they used a novel lentiviral FLEX system of conditional gene expression to provoke microglia-specific overexpression of mutant A53T α-syn associated with the expression of CX3CR1 receptor. The authors achieved high cell specificity with this method and discovered that microglial A53T α-syn overexpression promoted microglial activation and dopaminergic degeneration. Surprisingly, no intraneuronal α-syn accumulation was found, but rather microglia presented signs of α-syn accumulation like phosphorylation in serine S129. Microglia has been proposed as the cell responsible for pathological α-syn degradation. In fact, Heneka and colleagues have recently demonstrated that microglial cells can transport pathological α-syn from microglia to microglia through tunneling nanotubes for cooperative degradation [82]. However, under these conditions, α-syn fibrils induced the production of ROS, resulting in a compromised plasma membrane and mitochondrial network disintegration [82]. Both studies highlight the ability of microglia to isolate and degrade pathological α-syn. However, it is important to keep in mind that levels of pathological α-syn rely on the perfect balance of three independent processes associated with α-syn homeostasis: formation, aggregation rate, and clearance [83]. This view is exemplified in the model used by Bido et al. associated with overexpression of α-syn that provoked microglial exhaustion, inefficient degradation, microglia activation, and neuronal degeneration. Importantly, under these conditions, microglia showed a transcriptomic profile with upregulation of main proinflammatory cytokines like Il1b and Tnfa as well as several chemokines. Additionally, authors discovered upregulation of some of the genes related to DAM phenotypes discovered in other neurodegenerative diseases models like Apoe or Itgax [8, 9]. Consequently, any disturbance of such a balance may make microglia prone to produce high levels of neurotoxic ROS and proinflammatory factors, ultimately leading to cell death. Similarly, Zhang and colleagues also promoted α-syn overexpression in microglia primary cultures and microglial cell lines [84], promoting a robust inflammatory response and cytokine release that could be impaired by the mGluR5 receptor activation.
While the recent study from Bido et al. has shed light on the implication of α-syn in microglia activation and consequent dopaminergic degeneration, other studies had previously used viral-mediated overexpression to promote microglial activation. For instance, in 2006, Ferrari and colleagues [85] proved that chronic overexpression of IL-1β in the SN leads to progressive neurodegeneration in rats associated with microglial activation. Similarly, overexpression of TNF led to mild but progressive neurodegeneration as soon as 14 days [86]. Notably, both studies suggested independent effects of both cytokines, as levels of IL-1β after TNF overexpression remained low and vice versa.
Interestingly, the combination of adenoviral expression with classical PD models could become a novel strategy for studying different immunomodulatory proteins and deciphering PD-specific microglial phenotype. For instance, Ren et al. have studied the role of TREM2 overexpression in a model of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). TREM2 is thought to be a master regulator of microglia phenotype. Upon activation, TREM2 promotes phagocytosis, ameliorated inflammatory response and neuroprotection, but is also a key receptor for neurodegenerative microglia [9] and is needed for complete inflammatory response (reviewed in [87]). In the context of dopaminergic degeneration, overexpression of TREM2 led to decreased neuroinflammation and dopaminergic protection [88], which goes in line with later studies focused on TREM2 knockout mice and supports the view that the DAM phenotype is neuroprotective [89].
Altogether, adenoviral overexpression offers a broad panel of possibilities for studying microglial activation as a PD model and represents a novel tool that should highly impact future PD research.
The experimental animal models of PD described so far, in one way or another, end up activating this inflammatory response, indicating that the different models of this disease show common mechanisms to some extent.
As shown above, LPS is a direct inflammatory model. On the contrary, substances such as 6-OHDA MPTP/MPP+, paraquat, or rotenone are classic toxic models that produce the specific death of dopaminergic neurons. Interestingly, its administration eventually triggers a harmful inflammatory reaction. In recent years, the idea that neuronal death produced by these toxic substances was followed by activation of the immune response has changed towards a scenario in which the latter is activated before (or even in the absence of) neuronal death, so that microgliosis and not neurotoxicity would be the determining factor in neuronal death. For example, MPTP administration not only induced microglial activation but also induced T lymphocyte (CD4+ and CD8+) infiltration into the brain of non-human primates [90]. Thus, both peripheral and cerebral immune responses are involved in the mechanism of death induced by MPTP.
6-OHDA induces a strong production of free radicals that was soon identified [91, 92], in addition to inhibition of complex I of the mitochondrial respiratory chain, a mechanism shared by substances such as MPTP/MPP+and pesticides such as rotenone and paraquat. In the 6-OHDA model, the loss of astrocytes and the alteration of the BBB, the death of dopaminergic neurons, and the activation of microglia observed in the SN and the striatum was accompanied by the infiltration of peripheral immune cells [93]. Interestingly, these effects are prevented in female TLR4 KO animals (suggesting a gender-dependent mechanism) [94], and the treatment with urocortin [95] or P2Y6R KO [46] exert protection. Since these substances also exert protection in the LPS model, the inflammatory response arises as a common mechanism to different molecular challenges showing that the inflammatory response induced by LPS and 6-OHDA share common pathways.
The effect of α-syn overexpression in dopaminergic neurons of the SN produces a down-regulation of TH and an increased sensitivity to MPTP/MPP+ [96]. Interestingly, α-syn can also exert a detrimental effect on mitochondria, altering complex I-dependent respiration [96, 97]. α-syn exerts an unquestionable activating effect on microglia, especially the misfolded forms [98], which extends to monocytes [99, 100]. The Lewy pathology and neuroinflammation can then mutually potentiate in a vicious cycle, facilitating the progression of the pathology and the death of dopaminergic neurons [101, 102].
The involvement of the peripheral immune cells in these PD models has also been described. For instance, peripheral immune components infiltrate the brain following intracranial injection of any of the toxic mediators discussed. Injection of LPS into the SN causes infiltration of peripheral macrophages, which contributes to the observed damage; in fact, the depletion of peripheral macrophages using clodronate not only eliminates their infiltration, but also reduces other harmful effects of LPS injection, such as microglial activation, loss of astrocytes, disruption of the BBB, and death of dopaminergic neurons in the SN [103]. In the 6-OHDA model, the loss of astrocytes and the alteration of the BBB, as well as the activation of microglia and the infiltration of peripheral immune cells observed in the SN and the striatum, decreased when the concentration of DA was depleted by the TH inhibitor α-MPT, suggesting an interaction between endogenous DA and toxins [93].
Any condition affecting the integrity of the BBB can potentially let (or maybe induce) the infiltration of peripheral immune cells. Alteration of the BBB permeability has been shown in several animal models of PD, as for the intranigral/intrastriatal injection of thrombin [57] or tPA [68].
Circulating neutrophils, for example, are important in ischemic stroke [104, 105], where they become the main producers of matrix metalloproteinases (MMP-9), disruptors of the BBB. Peripheral immune cells are arising as interesting therapeutic targets in brain disorders coursing with inflammation; thus, the treatment with L-cysteine (a source of SH2 groups) reduced infiltration of peripheral immune cells in the brain, contributing to a better outcome of neuronal deficits induced by LPS [106]. Overexpression of α-syn in microglial cells induced by lentivirus produces an inflammatory cycle involving infiltrating immune cells [81].
PD MODELS COMBINING PERIPHERAL AND CENTRAL INFLAMMATION
The data discussed above make clear the pivotal role of neuroinflammation in the development of PD. Nevertheless, the increase in understanding of PD has led Brundin and colleagues to redefine its pathogenesis by dividing the course of the disease into three temporal phases mediated by triggers, facilitators, and aggravators [107]. Following this concept, the inflammatory models described so far could be the trigger that initiates the neurodegenerative process. However, in the context of PD, triggers alone may be insufficient for the pathology of PD to develop, requiring facilitators. Consistent with this view, our group was a pioneer in pointing to peripheral inflammation as one of these facilitators. In 2010, Villarán et al. described that peripheral inflammation induced by a model of ulcerative colitis based on the administration of dextran sulfate sodium (DSS) in drinking water exacerbates LPS-induced damage to the nigral dopaminergic system [103]. The contribution of chronic peripheral inflammation to the pathogenesis of neurodegenerative diseases is now an outstanding question. In the past 10 years, several clinical data and animal models have supported this view, suggesting peripheral inflammation as a potential risk factor in neurodegenerative diseases, especially in PD (for a review, see [108]). Sustained activation of the peripheral innate and adaptive immune systems occurs in the context of a wide range of disorders ranging from chronic infectious diseases to autoimmune and metabolic diseases, such as obesity, diabetes mellitus, and atherosclerosis. In addition, it is increasingly recognized that progressive systemic inflammation takes place during aging, a term known as inflammaging. Chronic peripheral inflammation that accompanies these diseases has been proposed to induce the production of proinflammatory cytokines that, following the endocrine route or through the vagus nerve transmission, can enter the brain [109]. In addition, increasing levels of proinflammatory cytokines compromise the permeability of the BBB, allowing many immune blood cells, including monocytes and T cells [110], to cross the altered BBB. This realization arises from multiple clinical studies showing elevated levels of inflammatory mediators in patients with PD, providing strong evidence for the interplay of the innate and adaptive immune system in the CNS and periphery in the context of PD and other synucleinopathies [15]. Some authors have proposed that this humoral immune response could be correlated with the nonmotor symptoms of PD [109]. In this context, we can face two possible scenarios: peripheral inflammation can “prime” microglial cells, which may become over-activated when a second noxious stimulus arrives. On the other hand, peripheral inflammation can transform previously “primed” microglia into an activated state. In both cases, peripheral inflammation can trigger stronger responses and further perpetuate the ongoing neurodegenerative process [111, 112].
Table 2.
Take-home information. All the models described so far share two common features: microglial activation and the death of dopaminergic neurons. This can be achieved by central or peripheral inflammatory challenges while their combination leads to a higher effect
| Model | Substance | Main features | |
| Intranigral injection | LPS, Thrombin, tPA | Loss of astrocytes | |
| Infiltration of peripheral cells | |||
| Dose-dependent TH+ cells death | |||
| Other neuronal phenotypes not affected | |||
| α-Syn PFF | α-Syn aggregation | ||
| GT1b | iNOS upregulation | ||
| Neuromelanin | 50% TH+ cells death | ||
| Virus-mediated overexpression of proteins | α-Syn | Toxic aggregates of α-syn in microglia but not in dopaminergic neurons | |
| IL-1β | Low but progressive TH+ cells death | ||
| TNF | Mild but chronic microglial activation and TH+ cells death | ||
| Peripheral challenge | LPS, DSS | Progressive TH+ cells death | |
| Infiltration of peripheral cells | |||
| Intranigral + Peripheral insult | 6-OHDA | ||
| AdIL-1β | LPS | Higher microglial activation* | |
| Paraquat | DSS | Higher loss of astrocytes* | |
| Lactacystin | AdIL-1β | Higher loss of dopaminergic neurons* | |
| α-syn | Cg | * Compared to single insult | |
| MPTP | Stress | ||
| Rotenone | |||
Fig. 1.
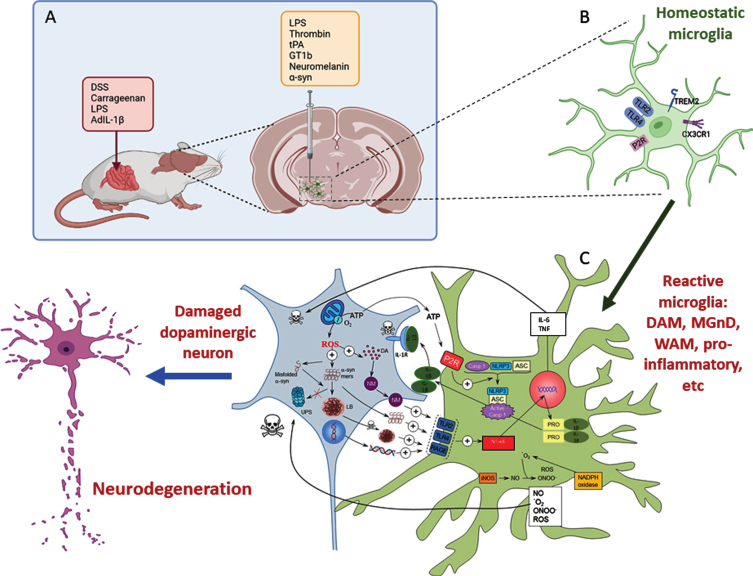
A) Inflammatory models of Parkinson’s disease take advantage of the use of different proinflammatory compounds administered peripherally, intracerebral, or by combining both pathways. B) Whatever compound and route of administration used, homeostatic microglia sense the environment through a set of surface receptors (the pattern recognition receptors, PRRs), including TLR2, TLR4, and RAGE. C) When activated, microglia undergo molecular and morphological changes, becoming reactive microglia. Illustrative examples are the DAM phenotype driven by TREM2 or the proinflammatory phenotype driven by TLR activation. Different microglia phenotypes can coexist under neurodegenerative conditions. Their activation leads to activation of the NF-κB pathway and the transcription of several proinflammatory genes (TNF and IL-6). Assembly of the NLRP3 inflammasome and activation of caspase-1 produce IL-1β and IL-18. Reactive microglia are also a source of ROS and RNS. All these products exert a harmful effect on dopaminergic neurons, which in turn release substances such as ATP, neuromelanin, and different forms of α-syn (either monomers or aggregates) that bind microglial PRRs in a vicious cycle that eventually leads to the death of dopaminergic neurons. Modified from Herrera et al., 2018 [169] using BioRender.
Considering all this information, some authors, including our group, have developed several animal models resulting from the combination of peripheral inflammation and central nigral dopaminergic challenge (reviews in [113, 114]). These models have been summarized in Table 1.
Table 1.
Models based on the combination of central and peripheral inflammation used to study the implications of systemic inflammation in the development of PD
| Central challenge | Peripheral inflammation model | Reference |
| 6-OHDA | LPS (0.4 mg/kg, 1 day) | [115] |
| 6-OHDA | AdIL-1β | [116] |
| AdIL-1β | AdIL-1β | [116] |
| Paraquat | DSS (250 mg/ml, 5 days) | [117] |
| α-syn injection | LPS (1–2.5 mg/kg, 1 day) | [118] |
| Transgenic A53T α-syn model | LPS (1–2.5 mg/kg, 1 day) | [118] |
| Lactacystin | LPS (0.25 mg/kg, 4 days) | [119] |
| Transgenic A53T α-syn model | DSS (0.5%, 12 weeks) | [120] |
| MPTP | DSS (2.5%, 8 days) | [121] |
| DSS (2%, 15 days) | [122] | |
| Iv injections of α-syn | LPS (0.8 mg/kg, 4 days) | [123] |
| Rotenone | Chronic stress-induced intestinal dysfunction | [124] |
| MPTP | LPS (2 mg/kg, 1 day) | [125] |
| LPS | Carrageenan | [126] |
| Rotenone | Carrageenan | [126] |
| α-syn injection | LPS (0.5 mg/kg, 1 day) | [127] |
| LPS | DSS (5%, 7 days) | [103] |
The most popular model of peripheral inflammation used in these combined models is the one based on intraperitoneal injection of LPS at doses ranging from 0.4 to 2.5 mg/kg in just one or several consecutive days. Gut inflammation induced by the ulcerative colitis model based on DSS administration (from 0.5 to 5%) in drinking water is the other most common model of peripheral inflammation. Injection of carrageenan into the paws of rats and injections of adenoviral vector that produce human IL-1β are also useful approaches to achieve peripheral inflammation. These models of peripheral inflammation were combined with a nigral insult induced by several PD models, including the 6-OHDA, MPTP, LPS, rotenone, and paraquat administration models. Inhibition of the proteasome system and injections of α-syn oligomers or transgenic animals that overexpress α-syn are also used as PD models. To note, chronic stress, which is a common condition nowadays, can influence the gut microbiota and alter the complex equilibrium in the intestinal milieu leading to a proinflammatory state that has been shown to accelerate neuronal degeneration and motor deficits in parkinsonism rodent models [124].
All these data reinforce the idea that peripheral inflammation could be a significant risk factor for PD and, therefore, strategies aimed at controlling the systemic inflammatory state arise as potential therapeutic options to control the development of PD. In this sense, Boza-Serrano et al. have shown that modulation of galectin-3, a microglia-related protein recently described as an immunomodulator, plays a significant role in microglia activation induced by α-syn [128]. Indeed, we have demonstrated that this ability of galectin-3 is extensive to emerge as a promising strategy to minimize undesired microglia activation states in PD [129]. The use of anti-inflammatory therapies in PD treatment has also been proposed, although clinical trials do not show significant results [130]. The NLRP3 inflammasome plays a critical role in the pathogenesis of PD, which led some authors to propose liver NLRP3 inhibitors to attenuate systemic inflammation and protect against a model of PD in rodents [131]. Finally, Liu et al. have demonstrated that peripheral immune tolerance mediated by CD200/CD200R signaling can attenuate neuroinflammation and decrease neurodegeneration in the LPS model of PD, suggesting CD200R as a potential therapeutic target to alleviate neuroinflammation in PD [132, 133].
MODELS OF PD BASED ON PERIPHERAL INFLAMMATION
All these studies target systemic inflammation as a possible facilitator of PD. The question is whether peripheral inflammation is able per se and without any other central stimulus to induce a neuroinflammatory environment in the brain that subsequently could induce dopaminergic neurodegeneration. In this sense, two important PD models based on peripheral inflammation are arising: the systemic LPS injection and the gut-brain axis.
Systemic LPS injection
The relevance of systemic inflammation in the integrity of the dopaminergic system was first demonstrated by Qin et al. in 2007 [134] when they administered a single dose of intraperitoneal LPS (5 mg/kg) in adult mice and discovered that the mice suffer from chronic and progressive dopaminergic degeneration at 7 and 10 months after the injection. Interestingly, LPS is not reported to cross the BBB, but authors identified the upregulation of peripheral TNF as the responsible for dopaminergic vulnerability. Indeed, effects of injection include increased microglial activation and reduced TH staining in the first hours post-injection [135]. Notably, the study by Qin et al. stimulated the combination of systemic LPS injection with other parkinsonian models (see Table 1). Later, other studies have observed a more accelerated degeneration with repeated injections of LPS. For instance, Bodea and colleagues [136] proposed that systemic injection of LPS during 4 consecutive days (1 mg/kg per day) led to dopaminergic degeneration 15 days after the last injection, in contrast with the same dose in a single injection that was unable to promote degeneration at that time point. Systemic LPS injection was characterized by an increased initial microglial response with significant cytokine production, particularly TNF and IL-1β; however, this activation returned to basal levels 15 days after the injection. Importantly, systemic LPS can be a valuable model for studying prodromal PD. For instance, systemic LPS models present early α-syn alterations and non-motor symptoms in the gut [137], olfactory impairments, and anxiety-like behavior [138]. Indeed, Song and colleagues [139] demonstrated that systemic LPS promotes sequential degeneration in the brain, resembling the initial phases of the Braak theory [140], starting in the locus coeruleus, followed by the SN, and lastly, the cortex and hippocampus.
An innovative variant of the effect of peripheral LPS on the dopaminergic system is the chronic intranasal administration, which after 5 months of daily administration, promoted microglia activation, moderate (∼50%) dopaminergic degeneration, and, remarkably, α-syn aggregation [141]. A similar but shorter model was also used by Li et al. [142], leading to a 38% of TH neuronal loss after 1 month of treatment, increased α-syn expression, and behavioral impairment. The same approach was used by Niu et al. [143], describing a more intense nigral degeneration after 6 weeks of treatment while also identifying IL-1β signaling as a relevant factor in this model.
Gut-brain axis
Different authors have recently suggested that intestinal inflammation could be a silent driver of PD pathogenesis [144]. The term gut-brain axis has been progressively gaining interest in the last 20 years. This term refers to the bidirectional communication between the CNS and the enteric nervous system and incorporates the fine regulation of immune responses in the gut and brain [144]. It is known that inflammatory processes can enter the CNS through different mechanisms, including the humoral and neuronal pathways (see [113]). Braak and colleagues, based on the appearance of Lewy pathology, already hypothesized the possibility that PD may start in the gastrointestinal tract to spread to the brain via the vagus nerve to further reach the ventral mesencephalon [140]. Indeed, experimental evidence has shown that the gastrointestinal tract is a potential starting point for aggregated α-syn, with the vagus nerve acting as a route by which pathology may be transmitted to the lower brainstem [145]. Therefore, a new model for PD pathogenesis has been recently proposed [144]. In this model, the disorder originates in the intestine to further progress to the ventral mesencephalon in an inflammation-mediated process. Thus, in a susceptible individual, inflammatory triggers, such as bacteria, viruses, or environmental toxins could initiate immune responses in the gut that eventually could deleteriously impact the microbiota, increasing intestinal permeability and inducing increased expression and aggregation of α-syn. Aberrant conformations of α-syn may be transmitted from the gut to the brain via the vagus nerve, while chronic intestinal inflammation promotes systemic inflammation. As mentioned before, this peripheral inflammation can increase BBB permeability, allowing the entrance of cytokines and immune blood cells to the brain parenchyma. Combination of intestinal inflammation, systemic inflammation and α-syn pathology in the brain promote neuroinflammation, which eventually drives the neurodegeneration process that characterizes PD. This model is sustained by epidemiological data showing that patients with inflammatory bowel disease (IBD) have a higher risk of developing PD than non-IBD individuals [146]. Moreover, gene association studies have found a genetic link between PD and IBD [147]. Therefore, it would be interesting to look for parkinsonian signs in animals using models to mimic IBD pathology. In this context, Labandeira-García’s group showed that a subchronic regimen of 2.5% of DSS for three weeks results in early changes in the nigrostriatal dopaminergic homeostasis, dopaminergic neuronal death, and increased levels of nigral proinflammatory mediators [148]. These are intriguing data that deserve further investigation since, if confirmed, this model would greatly contribute to understanding the underlying mechanisms involved in PD.
Anti-inflammatory interventions
All this information has encouraged some authors to deepen their understanding of the effects of peripheral inflammation on neuroinflammation. These studies have revealed that peripheral inflammation, especially gut inflammation, induces neuroinflammation in certain brain structures that is accompanied by several manifestations such as anxiety, depression, chronic pain and memory and cognitive impairments [149, 150]. However, this neuroinflammation and its associated symptoms decrease with some anti-inflammatory interventions in animal models. These treatments include inhibitors of the S-100 protein, TNF inhibitors, and neutrophil depletion [151, 152]. Melatonin, fermented rice brand, and DHA/EPA treatments also improve the symptoms associated to peripheral inflammation-related neuroinflammation [153–155]. In this regard, our group has recently published a study on the peripheral and central anti-inflammatory effects of galectin-3 inhibitors in DSS-induced gut inflammation [129].
There is, therefore, increasing interest in testing these anti-inflammatory treatments in humans, which is why several clinical trials are running (CN-02323358). Considering that intestinal inflammation appears to be the most powerful driver of neuroinflammation, most of these trials focus on the reduction of gut inflammation, modifying the microbiota through probiotics and prebiotics (CN-02355534; NCT04512599; NCT05146921; NCT04032262, NCT05173701, NCT04159727). However, to date, only one trial has been completed [156]. In this study, the authors evaluate the effects of probiotic supplementation on inflammation-related gene expression in PD patients, finding an overall significant improvement on several inflammatory-related genes such as IL-1, IL-8, TNF-α and TGF-β. Further studies need to be completed to gain a better understanding of whether interruption in inflammatory signaling ameliorates inflammation and subsequent neurodegeneration.
Viral parkinsonism models
A viral onset has been long proposed for PD since 1918 influenza led to some encephalitis cases that mimicked some parkinsonism symptoms [157]. It remains however unclear if there is a real implication of viral infections on PD onset [158]. However, viral infection can lead to systemic hyperinflammation known as “cytokine storm” that can penetrate in the brain and promote a potent inflammatory response, oxidative stress and upregulation of α-syn [159, 160]. In Sadasivan et al. [161], authors examined the effect of H1N1 influenza virus in an MPTP model of PD. The authors observed an increased loss of dopaminergic neurons but they failed to attribute it to increased microglia activation, what suggests an implication of peripheral immune system, but also the direct action of the virus in the dopaminergic neurons [162]. However, the best described virus used to induce PD in animal models has been Japanese encephalitis virus [163]. In this model, strong microglia reactivity has been observed through TLRs activation while also compromising dopaminergic system integrity [164]. Similarly, alphaviruses have also been described as an alternative parkinsonism model [165].
The new pandemic caused by coronavirus SARS-CoV-2 has gained a lot of attention from a PD research perspective. Despite the relation of COVID-19 with PD, this is yet not clear as the neurological effects of COVID-19 are still arising [166]. It has been reported that PD patients could develop prolonged post-COVID19 syndrome with worsen motor behavior and poor levodopa response [167]. Several COVID-19 features could lead to worsening PD symptoms; in particular, those related with cytokine storm that increase serum levels of distinct cytokines and neurotoxic components that have been previously related with PD [168]. No model for the impact of SARS-CoV-2 in PD, or vice versa, has been proposed. However, health care systems overload, pandemic-derived psychological stress and lockdown restrictions have had a major (but variable) impact on PD patients’ status that should be carefully addressed before analyzing any physiological effect of COVID-19 on PD etiology.
CONCLUSIONS
This plethora of immune models may help to understand the complex molecular mechanisms associated with PD like the contribution of central and peripheral immune cells in key events of the disease. Among them, we may cite the role in the aggregation and spreading of α-syn and identification of microglia subtypes and their contribution in the disease. Elucidation of signaling pathways behind these events may be critical for identification of preclinical drugs potentially relevant for PD.
ACKNOWLEDGMENTS
This work was supported by grants from the Spanish Ministerio de Ciencia, Innovación y Universidades (RTI 2018-098830-B-I00), from the Consejería de Economía y Conocimiento of Junta de Andalucía (P18-RT-1372 and US-1264806).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
- [1]. Goedert M, Spillantini MG, Del Tredici K, Braak H (2013) 100 years of Lewy pathology. Nat Rev Neurol 9, 13–24. [DOI] [PubMed] [Google Scholar]
- [2]. McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38, 1285–1291. [DOI] [PubMed] [Google Scholar]
- [3]. Schilder BM, Raj T (2022) Fine-mapping of Parkinson’s disease susceptibility loci identifies putative causal variants. Hum Mol Genet 31, 888–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Bartels T, De Schepper S, Hong S (2020) Microglia modulate neurodegeneration in Alzheimer’s and Parkinson’s diseases. Science 370, 66–69. [DOI] [PubMed] [Google Scholar]
- [5]. Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, Bien E, Baum M, Bortolin L, Wang S, Goeva A, Nemesh J, Kamitaki N, Brumbaugh S, Kulp D, McCarroll SA (2018) Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174, 1015–1030.e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. MacMahon Copas AN, McComish SF, Fletcher JM, Caldwell MA (2021) The pathogenesis of Parkinson’s disease: A complex interplay between astrocytes, microglia, and T lymphocytes? Front Neurol 12, 666737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. García-Revilla J, Alonso-Bellido IM, Burguillos MA, Herrera AJ, Espinosa-Oliva AM, Ruiz R, Cruz-Hernández L, García-Domínguez I, Roca-Ceballos MA, Santiago M, Rodríguez-Gómez JA, Soto MS, de Pablos RM, Venero JL (2019) Reformulating pro-oxidant microglia in neurodegeneration. JClin Med 8, 1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I (2017) A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e1217. [DOI] [PubMed] [Google Scholar]
- [9]. Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O’Loughlin E, Xu Y, Fanek Z, Greco DJ, Smith ST, Tweet G, Humulock Z, Zrzavy T, Conde-Sanroman P, Gacias M, Weng Z, Chen H, Tjon E, Mazaheri F, Hartmann K, Madi A, Ulrich JD, Glatzel M, Worthmann A, Heeren J, Budnik B, Lemere C, Ikezu T, Heppner FL, Litvak V, Holtzman DM, Lassmann H, Weiner HL, Ochando J, Haass C, Butovsky O (2017) The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases.. Immunity 47, 566–581.e569. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Safaiyan S, Besson-Girard S, Kaya T, Cantuti-Castelvetri L, Liu L, Ji H, Schifferer M, Gouna G, Usifo F, Kannaiyan N, Fitzner D, Xiang X, Rossner MJ, Brendel M, Gokce O, Simons M (2021) White matter aging drives microglial diversity.1100-1117.e. Neuron 109, 1110. [DOI] [PubMed] [Google Scholar]
- [11]. Uriarte Huarte O, Kyriakis D, Heurtaux T, Pires-Afonso Y, Grzyb K, Halder R, Buttini M, Skupin A, Mittelbronn M, Michelucci A (2021) Single-cell transcriptomics and in situ morphological analyses reveal microglia heterogeneity across the nigrostriatal pathway. Front Immunol 12, 639613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Smajić S, Prada-Medina CA, Landoulsi Z, Ghelfi J, Delcambre S, Dietrich C, Jarazo J, Henck J, Balachandran S, Pachchek S, MorrisCM, Antony P, Timmermann B, Sauer S, Pereira SL, Schwamborn JC, MayP, Grünewald A, Spielmann M (2022) Single-cell sequencing ofhuman midbrain reveals glial activation and a Parkinson-specificneuronal state. Brain 145, 964–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Deczkowska A, Keren-Shaul H, Weiner A, Colonna M, Schwartz M, Amit I (2018) Disease-associated microglia: A universal immune sensor of neurodegeneration. Cell 173, 1073–1081. [DOI] [PubMed] [Google Scholar]
- [14]. Murthy MN, Blauwendraat C, UKBEC Guelfi S, IPDGC Hardy J, Lewis PA, Trabzuni D (2017) Increased brain expression of GPNMB is associated with genome wide significant risk for Parkinson’s disease on chromosome 7p15.3. Neurogenetics 18, 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Harms AS, Ferreira SA, Romero-Ramos M (2021) Periphery and brain, innate and adaptive immunity in Parkinson’s disease. Acta Neuropathol 141, 527–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Qin XY, Zhang SP, Cao C, Loh YP, Cheng Y (2016) Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol 73, 1316–1324. [DOI] [PubMed] [Google Scholar]
- [17]. McCoy MK, Tansey MG (2008) TNF signaling inhibition in the CNS: Implications for normal brain function and neurodegenerative disease. J Neuroinflammation 5, 45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Peter I, Dubinsky M, Bressman S, Park A, Lu C, Chen N, Wang A (2018) Anti-tumor necrosis factor therapy and incidence of Parkinson disease among patients with inflammatory bowel disease. JAMA Neurol 75, 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Gliem M, Schwaninger M, Jander S (2016) Protective features of peripheral monocytes/macrophages in stroke. Biochim Biophys Acta 1862, 329–338. [DOI] [PubMed] [Google Scholar]
- [20]. Grozdanov V, Bliederhaeuser C, Ruf WP, Roth V, Fundel-Clemens K, Zondler L, Brenner D, Martin-Villalba A, Hengerer B, Kassubek J, Ludolph AC, Weishaupt JH, Danzer KM (2014) Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol 128, 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Nissen SK, Shrivastava K, Schulte C, Otzen DE, Goldeck D, Berg D, Møller HJ, Maetzler W, Romero-Ramos M (2019) Alterations in blood monocyte functions in Parkinson’s disease. Mov Disord 34, 1711–1721. [DOI] [PubMed] [Google Scholar]
- [22]. Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S (2009) Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest 119, 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, Cron RQ, Shacka JJ, Raman C, Standaert DG (2013) MHCII is required for α-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci 33, 9592–9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, Payami H (2010) Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet 42, 781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Pandi S, Chinniah R, Sevak V, Ravi PM, Raju M, Vellaiappan NA, Karuppiah B (2021) Association of HLA-DRB1, DQA1 and DQB1 alleles and haplotype in Parkinson’s disease from South India. Neurosci Lett 765, 136296. [DOI] [PubMed] [Google Scholar]
- [26]. Yu E, Ambati A, Andersen MS, Krohn L, Estiar MA, Saini P, Senkevich K, Sosero YL, Sreelatha AAK, Ruskey JA, Asayesh F, Spiegelman D, Toft M, Viken MK, Sharma M, Blauwendraat C, Pihlstrøm L, Mignot E, Gan-Or Z (2021) Fine mapping of the HLA locus in Parkinson’s disease in Europeans. NPJ Parkinsons Dis 7, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Wissemann WT, Hill-Burns EM, Zabetian CP, Factor SA, Patsopoulos N, Hoglund B, Holcomb C, Donahue RJ, Thomson G, Erlich H, Payami H (2013) Association of Parkinson disease with structural and regulatory variants in the HLA region. Am J Hum Genet 93, 984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, Dawson VL, Dawson TM, Oseroff C, Pham J, Sidney J, Dillon MB, Carpenter C, Weiskopf D, Phillips E, Mallal S, Peters B, Frazier A, Lindestam Arlehamn CS, Sette A (2017) T cells from patients with Parkinson’s diseaserecognize α-synuclein peptides. Nature 546, 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Wang P, Luo M, Zhou W, Jin X, Xu Z, Yan S, Li Y, Xu C, Cheng R, Huang Y, Lin X, Yao L, Nie H, Jiang Q (2022) Global characterization of peripheral B cells in Parkinson’s disease by single-cell RNA and BCR sequencing. Front Immunol 13, 814239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Ciesielska A, Matyjek M, Kwiatkowska K (2021) TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci 78, 1233–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Andersson PB, Perry VH, Gordon S (1992) The acute inflammatory response to lipopolysaccharide in CNS parenchyma differs from that in other body tissues. Neuroscience 48, 169–186. [DOI] [PubMed] [Google Scholar]
- [32]. Bourdiol F, Toulmond S, Serrano A, Benavides J, Scatton B (1991) Increase in omega 3 (peripheral type benzodiazepine) binding sites in the rat cortex and striatum after local injection of interleukin-1, tumour necrosis factor-alpha and lipopolysaccharide. Brain Res 543, 194–200. [DOI] [PubMed] [Google Scholar]
- [33]. Montero-Menei CN, Sindji L, Pouplard-Barthelaix A, Jehan F, Denechaud L, Darcy F (1994) Lipopolysaccharide intracerebral administration induces minimal inflammatory reaction in rat brain. Brain Res 653, 101–111. [DOI] [PubMed] [Google Scholar]
- [34]. Szczepanik AM, Fishkin RJ, Rush DK, Wilmot CA (1996) Effects of chronic intrahippocampal infusion of lipopolysaccharide in the rat. Neuroscience 70, 57–65. [DOI] [PubMed] [Google Scholar]
- [35]. Herrera AJ, Castaño A, Venero JL, Cano J, Machado A (2000) The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol Dis 7, 429–447. [DOI] [PubMed] [Google Scholar]
- [36]. Flores-Martinez YM, Fernandez-Parrilla MA, Ayala-Davila J, Reyes-Corona D, Blanco-Alvarez VM, Soto-Rojas LO, Luna-Herrera C, Gonzalez-Barrios JA, Leon-Chavez BA, Gutierrez-Castillo ME, Martínez-Dávila IA, Martinez-Fong D (2018) Acuteneuroinflammatory response in the substantia nigra pars compacta of rats after a local injection of lipopolysaccharide. J ImmunolRes 2018, 1838921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Castaño A, Herrera AJ, Cano J, Machado A (1998) Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem 70, 1584–1592. [DOI] [PubMed] [Google Scholar]
- [38]. Li DW, Zhou FZ, Sun XC, Li SC, Yang JB, Sun HH, Wang AH (2019) Ginsenoside Rb1 protects dopaminergic neurons from inflammatory injury induced by intranigral lipopolysaccharide injection. Neural Regen Res 14, 1814–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Hsieh PF, Chia LG, Ni DR, Cheng LJ, Ho YP, Tzeng SF, Chang MH, Hong JS (2002) Behavior, neurochemistry and histology after intranigral lipopolysaccharide injection. Neuroreport 13, 277–280. [DOI] [PubMed] [Google Scholar]
- [40]. Stern EL, Quan N, Proescholdt MG, Herkenham M (2000) Spatiotemporal induction patterns of cytokine and related immune signal molecule mRNAs in response to intrastriatal injection of lipopolysaccharide. J Neuroimmunol 106, 114–129. [DOI] [PubMed] [Google Scholar]
- [41]. Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG, Bing G (2007) Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem 100, 1375–1386. [DOI] [PubMed] [Google Scholar]
- [42]. Shukuri M, Uchino M, Sakamaki T, Onoe S, Hosoi R, Todoroki K, AranoY, Sakai T, Akizawa H, Inoue O (2021) Ex vivo imaging andanalysis of ROS generation correlated with microglial activation inrat model with acute neuroinflammation induced by intrastriatalinjection of LPS. Biochem Biophys Res Commun 584, 101–106. [DOI] [PubMed] [Google Scholar]
- [43]. Zhang J, Stanton DM, Nguyen XV, Liu M, Zhang Z, Gash D, Bing G (2005) Intrapallidal lipopolysaccharide injection increases iron and ferritin levels in glia of the rat substantia nigra and induces locomotor deficits. Neuroscience 135, 829–838. [DOI] [PubMed] [Google Scholar]
- [44]. Lawson LJ, Perry VH, Dri P, Gordon S (1990) Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39, 151–170. [DOI] [PubMed] [Google Scholar]
- [45]. Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS (2000) Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: Role of microglia. J Neurosci 20, 6309–6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Milde S, van Tartwijk FW, Vilalta A, Hornik TC, Dundee JM, Puigdellívol M, Brown GC (2021) Inflammatory neuronal loss inthe substantia nigra induced by systemic lipopolysaccharide isprevented by knockout of the P2Y. J Neuroinflammation 18, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Hoban DB, Connaughton E, Connaughton C, Hogan G, Thornton C, Mulcahy P, Moloney TC, Dowd E (2013) Further characterisation of the LPS model of Parkinson’s disease: A comparison of intra-nigral and intra-striatal lipopolysaccharide administration on motor function, microgliosis and nigrostriatal neurodegeneration in the rat. Brain Behav Immun 27, 91–100. [DOI] [PubMed] [Google Scholar]
- [48]. Li B, Xu P, Wu S, Jiang Z, Huang Z, Li Q, Chen D (2018) Diosgenin attenuates lipopolysaccharide-induced Parkinson’s disease by inhibiting the TLR/NF-κB pathway. J Alzheimers Dis 64, 943–955. [DOI] [PubMed] [Google Scholar]
- [49]. Castaño A, Herrera AJ, Cano J, Machado A (2002) The degenerative effect of a single intranigral injection of LPS on the dopaminergic system is prevented by dexamethasone, and not mimicked by rh-TNF-alpha, IL-1beta and IFN-gamma. J Neurochem 81, 150–157. [DOI] [PubMed] [Google Scholar]
- [50]. Tomás-Camardiel M, Rite I, Herrera AJ, de Pablos RM, Cano J, Machado A, Venero JL (2004) Minocycline reduces thelipopolysaccharide-induced inflammatory reaction,peroxynitrite-mediated nitration of proteins, disruption of theblood-brain barrier, and damage in the nigral dopaminergic system. Neurobiol Dis 16, 190–201. [DOI] [PubMed] [Google Scholar]
- [51]. Hernández-Romero MC, Argüelles S, Villarán RF, de Pablos RM, Delgado-Cortés MJ, Santiago M, Herrera AJ, Cano J, Machado A (2008) Simvastatin prevents the inflammatory process and thedopaminergic degeneration induced by the intranigral injection oflipopolysaccharide. J Neurochem 105, 445–459. [DOI] [PubMed] [Google Scholar]
- [52]. Lu X, Bing G, Hagg T (2000) Naloxone prevents microglia-induced degeneration of dopaminergic substantia nigra neurons in adult rats. Neuroscience 97, 285–291. [DOI] [PubMed] [Google Scholar]
- [53]. De Pablos RM, Herrera AJ, Villarán RF, Cano J, Machado A (2005) Dopamine-dependent neurotoxicity of lipopolysaccharide in substantianigra. FASEB J 19, 407–409. [DOI] [PubMed] [Google Scholar]
- [54]. Mor DE, Tsika E, Mazzulli JR, Gould NS, Kim H, Daniels MJ, Doshi S, Gupta P, Grossman JL, Tan VX, Kalb RG, Caldwell KA, Caldwell GA, Wolfe JH, Ischiropoulos H (2017) Dopamine induces soluble α-synuclein oligomers and nigrostriatal degeneration. Nat Neurosci 20, 1560–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. de Pablos RM, Herrera AJ, Espinosa-Oliva AM, Sarmiento M, Muñoz MF, Machado A, Venero JL (2014) Chronic stress enhances microgliaactivation and exacerbates death of nigral dopaminergic neuronsunder conditions of inflammation. J Neuroinflammation 11, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Choi SH, Joe EH, Kim SU, Jin BK (2003) Thrombin-induced microglialactivation produces degeneration of nigral dopaminergic neurons invivo. J Neurosci 23, 5877–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Carreño-Müller E, Herrera AJ, de Pablos RM, Tomás-Camardiel M, Venero JL, Cano J, Machado A (2003) Thrombininduces in vivo degeneration of nigral dopaminergic neuronesalong with the activation of microglia. J Neurochem 84, 1201–1214. [DOI] [PubMed] [Google Scholar]
- [58]. Kim D, Paik JH, Shin DW, Kim HS, Park CS, Kang JH (2014) What is the clinical significance of cerebrospinal fluid biomarkers in Parkinson’s disease? Is the significance diagnostic or prognostic? Exp Neurobiol 23, 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Herrera AJ, de Pablos RM, Carreño-Müller E, Villarán RF, Venero JL, Tomás-Camardiel M, Cano J, Machado A (2008) Theintrastriatal injection of thrombin in rat induced a retrogradeapoptotic degeneration of nigral dopaminergic neurons throughsynaptic elimination. J Neurochem 105, 750–762. [DOI] [PubMed] [Google Scholar]
- [60]. Choi SH, Lee DY, Chung ES, Hong YB, Kim SU, Jin BK (2005) Inhibitionof thrombin-induced microglial activation and NADPH oxidase byminocycline protects dopaminergic neurons in the substantia nigra in vivo. J Neurochem 95, 1755–1765. [DOI] [PubMed] [Google Scholar]
- [61]. Argüelles S, Herrera AJ, Carreño-Müller E, de Pablos RM, Villarán RF, Espinosa-Oliva AM, Machado A, Cano J (2010) Degeneration of dopaminergic neurons induced by thrombin injectionin the substantia nigra of the rat is enhanced by dexamethasone:Role of monoamine oxidase enzyme. Neurotoxicology 31, 55–66. [DOI] [PubMed] [Google Scholar]
- [62]. Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM (2012) Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Earls RH, Menees KB, Chung J, Barber J, Gutekunst CA, Hazim MG, Lee JK (2019) Intrastriatal injection of preformed alpha-synuclein fibrils alters central and peripheral immune cell profiles in non-transgenic mice. J Neuroinflammation 16, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Harms AS, Delic V, Thome AD, Bryant N, Liu Z, Chandra S, Jurkuvenaite A, West AB (2017) α-Synuclein fibrils recruit peripheral immune cells in the rat brain prior to neurodegeneration. Acta Neuropathol Commun 5, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. George S, Tyson T, Rey NL, Sheridan R, Peelaerts W, Becker K, Schulz E, Meyerdirk L, Burmeister AR, von Linstow CU, Steiner JA, Galvis MLE, Ma J, Pospisilik JA, Labrie V, Brundin L, Brundin P (2021) T cells limit accumulation of aggregate pathology following intrastriatal injection of α-synuclein fibrils. J Parkinsons Dis 11, 585–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Gentzel RC, Toolan D, Jinn S, Schachter JB, Ma L, Kahle PJ, Smith SM, Marcus JN (2021) Intracranial administration of alpha-synuclein fibrils in A30P-synuclein transgenic mice causes robust synucleinopathy and microglial induction. Neurobiol Aging 106, 12–25. [DOI] [PubMed] [Google Scholar]
- [67]. Dutta D, Jana M, Majumder M, Mondal S, Roy A, Pahan K (2021) Selective targeting of the TLR2/MyD88/NF-κB pathway reducesα-synuclein spreading in vitro and in vivo. Nat Commun 12, 5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Villarán RF, de Pablos RM, Argüelles S, Espinosa-Oliva AM, Tomás-Camardiel M, Herrera AJ, Cano J, Machado A (2009) Theintranigral injection of tissue plasminogen activator inducedblood-brain barrier disruption, inflammatory process anddegeneration of the dopaminergic system of the rat. Neurotoxicology 30, 403–413. [DOI] [PubMed] [Google Scholar]
- [69]. Ryu JK, Shin WH, Kim J, Joe EH, Lee YB, Cho KG, Oh YJ, Kim SU, Jin BK (2002) Trisialoganglioside GT1b induces in vivo degenerationof nigral dopaminergic neurons: Role of microglia. Glia 38, 15–23. [DOI] [PubMed] [Google Scholar]
- [70]. Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, Lucius R (2003) Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: Implications for Parkinson’s disease. FASEB J 17, 500–502. [DOI] [PubMed] [Google Scholar]
- [71]. Zecca L, Wilms H, Geick S, Claasen JH, Brandenburg LO, Holzknecht C, Panizza ML, Zucca FA, Deuschl G, Sievers J, Lucius R (2008) Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: Implications for Parkinson’s disease. Acta Neuropathol 116, 47–55. [DOI] [PubMed] [Google Scholar]
- [72]. Visanji NP, Brotchie JM, Kalia LV, Koprich JB, Tandon A, Watts JC, Lang AE (2016) α-synuclein-based animal models of Parkinson’s disease: Challenges and opportunities in a new era. Trends Neurosci 39, 750–762. [DOI] [PubMed] [Google Scholar]
- [73]. Cenci MA, Björklund A (2020) Animal models for preclinical Parkinson’s research: An update and critical appraisal. Prog Brain Res 252, 27–59. [DOI] [PubMed] [Google Scholar]
- [74]. Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M (2010) Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS One 5, e8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Barkholt P, Sanchez-Guajardo V, Kirik D, Romero-Ramos M (2012) Long-term polarization of microglia upon α-synuclein overexpression in nonhuman primates. Neuroscience 208, 85–96. [DOI] [PubMed] [Google Scholar]
- [76]. Theodore S, Cao S, McLean PJ, Standaert DG (2008) Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J Neuropathol Exp Neurol 67, 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77]. Chung CY, Koprich JB, Siddiqi H, Isacson O (2009) Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci 29, 3365–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78]. Rodriguez-Perez AI, Sucunza D, Pedrosa MA, Garrido-Gil P, Kulisevsky J, Lanciego JL, Labandeira-Garcia JL (2018) Angiotensin type 1 receptor antagonists protect against alpha-synuclein-induced neuroinflammation and dopaminergic neuron death. Neurotherapeutics 15, 1063–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79]. George S, Rey NL, Tyson T, Esquibel C, Meyerdirk L, Schulz E, Pierce S, Burmeister AR, Madaj Z, Steiner JA, Escobar Galvis ML, Brundin L, Brundin P (2019) Microglia affect α-synuclein cell-to-cell transfer in a mouse model of Parkinson’s disease. Mol Neurodegener 14, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. Negrini M, Tomasello G, Davidsson M, Fenyi A, Adant C, Hauser S, Espa E, Gubinelli F, Manfredsson FP, Melki R, Heuer A Sequential or simultaneous injection of preformed fibrils and AAV overexpression of alpha-synuclein are equipotent in producing relevant pathology and behavioral deficits. J Parkinsons Dis, doi: 10.3233/JPD-212555. [DOI] [PMC free article] [PubMed]
- [81]. Bido S, Muggeo S, Massimino L, Marzi MJ, Giannelli SG, Melacini E, Nannoni M, Gambarè D, Bellini E, Ordazzo G, Rossi G, Maffezzini C, Iannelli A, Luoni M, Bacigaluppi M, Gregori S, Nicassio F, Broccoli V (2021) Microglia-specific overexpression of α-synuclein leads to severe dopaminergic neurodegeneration by phagocytic exhaustion and oxidative toxicity. Nat Commun 12, 6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82]. Scheiblich H, Dansokho C, Mercan D, Schmidt SV, Bousset L, Wischhof L, Eikens F, Odainic A, Spitzer J, Griep A, Schwartz S, Bano D, Latz E, Melki R, Heneka MT (2021) Microglia jointly degrade fibrillar alpha-synuclein cargo by distribution through tunneling nanotubes. Cell 184, 5089–5106.e5021.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83]. Fares MB, Jagannath S, Lashuel HA (2021) Reverse engineering Lewy bodies: How far have we come and how far can we go? Nat Rev Neurosci 22, 111–131. [DOI] [PubMed] [Google Scholar]
- [84]. Zhang YN, Fan JK, Gu L, Yang HM, Zhan SQ, Zhang H (2021) Metabotropic glutamate receptor 5 inhibits α-synuclein-induced microglia inflammation to protect from neurotoxicity in Parkinson’s disease. J Neuroinflammation 18, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85]. Ferrari CC, Pott Godoy MC, Tarelli R, Chertoff M, Depino AM, Pitossi FJ (2006) Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1beta in the substantia nigra. Neurobiol Dis 24, 183–193. [DOI] [PubMed] [Google Scholar]
- [86]. De Lella Ezcurra AL, Chertoff M, Ferrari C, Graciarena M, Pitossi F (2010) Chronic expression of low levels of tumor necrosis factor-alpha in the substantia nigra elicits progressive neurodegeneration, delayed motor symptoms and microglia/macrophage activation. Neurobiol Dis 37, 630–640. [DOI] [PubMed] [Google Scholar]
- [87]. Jay TR, von Saucken VE, Landreth GE (2017) TREM2 in neurodegenerative diseases. Mol Neurodegener 12, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88]. Ren M, Guo Y, Wei X, Yan S, Qin Y, Zhang X, Jiang F, Lou H (2018) TREM2 overexpression attenuates neuroinflammation and protects dopaminergic neurons in experimental models of Parkinson’s disease. Exp Neurol 302, 205–213. [DOI] [PubMed] [Google Scholar]
- [89]. Guo Y, Wei X, Yan H, Qin Y, Yan S, Liu J, Zhao Y, Jiang F, Lou H (2019) TREM2 deficiency aggravates α-synuclein-induced neurodegeneration and neuroinflammation in Parkinson’s disease models. FASEB J 33, 12164–12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Seo J, Park J, Kim K, Won J, Yeo HG, Jin YB, Koo BS, Lim KS, Jeong KJ, Kang P, Lee HY, Choi WS, Baek SH, Jeon CY, Hong JJ, Huh JW, Kim YH, Park SJ, Kim SU, Lee DS, Lee SR, Lee Y (2020) Chronic infiltration of T lymphocytes into the brain in a non-human primate model of Parkinson’s disease. Neuroscience 431, 73–85. [DOI] [PubMed] [Google Scholar]
- [91]. Ungerstedt U, Ljungberg T, Steg G (1974) Behavioral, physiological, and neurochemical changes after 6-hydroxydopamine-induced degeneration of the nigro-striatal dopamine neurons. Adv Neurol 5, 421–426. [PubMed] [Google Scholar]
- [92]. Cadet JL, Brannock C (1998) Free radicals and the pathobiology of brain dopamine systems. Neurochem Int 32, 117–131. [DOI] [PubMed] [Google Scholar]
- [93]. Espinosa-Oliva AM, de Pablos RM, Sarmiento M, Villarán RF, Carrillo-Jiménez A, Santiago M, Venero JL, Herrera AJ, Cano J, Machado A (2014) Role of dopamine in the recruitment of immune cellsto the nigro-striatal dopaminergic structures. Neurotoxicology 41, 89–101. [DOI] [PubMed] [Google Scholar]
- [94]. Somensi N, Lopes SC, Gasparotto J, Mayer Gonçalves R, Tiefensee-Ribeiro C, Oppermann Peixoto D, Ozorio Brum P, Pinho CM, Agnes JP, Santos L, de Oliveira J, Spiller F, Fonseca Moreira JC, Zanotto-Filho A, Prediger RD, Pens Gelain D (2021) Role of toll-like receptor 4 and sex in 6-hydroxydopamine-induced behavioral impairments and neurodegeneration in mice. Neurochem Int 151, 105215. [DOI] [PubMed] [Google Scholar]
- [95]. Abuirmeileh A, Lever R, Kingsbury AE, Lees AJ, Locke IC, Knight RA, Chowdrey HS, Biggs CS, Whitton PS (2007) The corticotrophin-releasing factor-like peptide urocortin reverses key deficits in two rodent models of Parkinson’s disease. Eur J Neurosci 26, 417–423. [DOI] [PubMed] [Google Scholar]
- [96]. Maries E, Dass B, Collier TJ, Kordower JH, Steece-Collier K (2003) The role of alpha-synuclein in Parkinson’s disease: Insights from animal models. Nat Rev Neurosci 4, 727–738. [DOI] [PubMed] [Google Scholar]
- [97]. Ludtmann MHR, Angelova PR, Horrocks MH, Choi ML, Rodrigues M, Baev AY, Berezhnov AV, Yao Z, Little D, Banushi B, Al-Menhali AS, Ranasinghe RT, Whiten DR, Yapom R, Dolt KS, Devine MJ, Gissen P, Kunath T, Jaganjac M, Pavlov EV, Klenerman D, Abramov AY, Gandhi S (2018) α-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat Commun 9, 2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98]. Hoffmann A, Ettle B, Bruno A, Kulinich A, Hoffmann AC, von Wittgenstein J, Winkler J, Xiang W, Schlachetzki JCM (2016) Alpha-synuclein activates BV2 microglia dependent on its aggregation state. Biochem Biophys Res Commun 479, 881–886. [DOI] [PubMed] [Google Scholar]
- [99]. Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL (2008) Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging 29, 739–752. [DOI] [PubMed] [Google Scholar]
- [100]. Lee SB, Park SM, Ahn KJ, Chung KC, Paik SR, Kim J (2009) Identification of the amino acid sequence motif of alpha-synuclein responsible for macrophage activation. Biochem Biophys Res Commun 381, 39–43. [DOI] [PubMed] [Google Scholar]
- [101]. Jiang T, Li G, Xu J, Gao S, Chen X (2018) The challenge of thepathogenesis of Parkinson’s disease: Is autoimmunity the culprit? Front Immunol 9, 2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102]. Olanow CW, Savolainen M, Chu Y, Halliday GM, Kordower JH (2019) Temporal evolution of microglia and α-synuclein accumulation following foetal grafting in Parkinson’s disease. Brain 142, 1690–1700. [DOI] [PubMed] [Google Scholar]
- [103]. Villarán RF, Espinosa-Oliva AM, Sarmiento M, De Pablos RM, Argüelles S, Delgado-Cortés MJ, Sobrino V, Van Rooijen N, Venero JL, Herrera AJ, Cano J, Machado A (2010) Ulcerative colitisexacerbates lipopolysaccharide-induced damage to the nigraldopaminergic system: Potential risk factor in Parkinson‘s disease. J Neurochem 114, 1687–1700. [DOI] [PubMed] [Google Scholar]
- [104]. Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR (2015) Targeting neutrophils in ischemic stroke: Translational insights from experimental studies. J Cereb Blood Flow Metab 35, 888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105]. Ren X, Hu H, Farooqi I, Simpkins JW (2021) Author Correction: Blood substitution therapy rescues the brain of mice from ischemic damage. Nat Commun 12, 2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106]. Li T, Chu X, Xin D, Ke H, Wang S, Liu D, Chen W, Wang Z (2021) H2S prevents peripheral immune cell invasion, increasing[Ca 2+]i and excessive phagocytosis following hypoxia-ischemiainjury in neonatal mice. Biomed Pharmacother 135, 111207. [DOI] [PubMed] [Google Scholar]
- [107]. Johnson ME, Stecher B, Labrie V, Brundin L, Brundin P (2019) Triggers, facilitators, and aggravators: Redefining Parkinson’s disease pathogenesis. Trends Neurosci 42, 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108]. Sübeta P, Lana AJ, Schlachetzki JCM (2021) Chronicperipheral inflammation: A possible contributor to neurodegenerativediseases. Neural Regen Res 16, 1711–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109]. Wang H, Liu Y, Zhao J, Guo X, Hu M, Chen Y (2021) Possible inflammatory mechanisms and predictors of Parkinson’s disease patients with fatigue (Brief Review). Clin Neurol Neurosurg 208, 106844. [DOI] [PubMed] [Google Scholar]
- [110]. Batchu S (2020) Prefrontal cortex transcriptomic deconvolution implicates monocyte infiltration in Parkinson’s disease. Neurodegener Dis 20, 110–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111]. Lheureux S, Mirza M, Coleman R (2019) The DNA repair pathway as a target for novel drugs in gynecologic cancers. J Clin Oncol 37, 2449–2459. [DOI] [PubMed] [Google Scholar]
- [112]. Kustrimovic N, Marino F, Cosentino M (2019) Peripheral immunity, immunoaging and neuroinflammation in Parkinson’s disease. Curr Med Chem 26, 3719–3753. [DOI] [PubMed] [Google Scholar]
- [113]. Herrera AJ, Espinosa-Oliva AM, Oliva-Martin MJ, Carrillo-Jimenez A, Venero JL, de Pablos RM (2015) Collateral damage: Contribution of peripheral inflammation to neurodegenerative diseases. Curr Top Med Chem 15, 2193–2210. [DOI] [PubMed] [Google Scholar]
- [114]. Machado A, Herrera AJ, Venero JL, Santiago M, De Pablos RM, Villarán RF, Espinosa-Oliva AM, Argüelles S, Sarmiento M, Delgado-Cortés MJ, Mauriño R, Cano J (2011) Peripheralinflammation increases the damage in animal models of nigrostriataldopaminergic neurodegeneration: Possible implication in Parkinson’sdisease incidence. Parkinsons Dis 2011, 393769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115]. Combrinck MI, Perry VH, Cunningham C (2002) Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience 112, 7–11. [DOI] [PubMed] [Google Scholar]
- [116]. Pott Godoy MC, Ferrari CC, Pitossi FJ (2010) Nigral neurodegeneration triggered by striatal AdIL-1 administration can be exacerbated by systemic IL-1 expression. J Neuroimmunol 222, 29–39. [DOI] [PubMed] [Google Scholar]
- [117]. Dwyer Z, Chaiquin M, Landrigan J, Ayoub K, Shail P, Rocha J, Childers CL, Storey KB, Philpott DJ, Sun H, Hayley S (2021) The impact of dextran sodium sulphate and probiotic pre-treatment in a murine model of Parkinson’s disease. J Neuroinflammation 18, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118]. La Vitola P, Balducci C, Baroni M, Artioli L, Santamaria G, Castiglioni M, Cerovic M, Colombo L, Caldinelli L, Pollegioni L, Forloni G (2021) Peripheral inflammation exacerbates α-synuclein toxicity and neuropathology in Parkinson’s models. Neuropathol Appl Neurobiol 47, 43–60. [DOI] [PubMed] [Google Scholar]
- [119]. Deneyer L, Albertini G, Bentea E, Massie A (2019) Systemic LPS-induced neuroinflammation increases the susceptibility for proteasome inhibition-induced degeneration of the nigrostriatal pathway. Parkinsonism Relat Disord 68, 26–32. [DOI] [PubMed] [Google Scholar]
- [120]. Kishimoto Y, Zhu W, Hosoda W, Sen JM, Mattson MP (2019) Chronic mildgut inflammation accelerates brain neuropathology and motordysfunction in α-synuclein mutant mice. NeuromolecularMed 21, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121]. Gil-Martínez AL, Estrada C, Cuenca L, Cano JA, Valiente M, Martínez-Cáceres CM, Fernández-Villalba E, Herrero MT (2019) Local gastrointestinal injury exacerbates inflammation anddopaminergic cell death in parkinsonian mice. Neurotox Res 35, 918–930. [DOI] [PubMed] [Google Scholar]
- [122]. Houser MC, Caudle WM, Chang J, Kannarkat GT, Yang Y, Kelly SD, Oliver D, Joers V, Shannon KM, Keshavarzian A, Tansey MG (2021) Experimental colitis promotes sustained, sex-dependent, T-cell-associated neuroinflammation and parkinsonian neuropathology. Acta Neuropathol Commun 9, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123]. Peralta Ramos JM, Iribarren P, Bousset L, Melki R, Baekelandt V, Van der Perren A (2019) Peripheral inflammation regulates CNS immune surveillance through the recruitment of inflammatory monocytes upon systemic α-synuclein administration. Front Immunol 10, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124]. Dodiya HB, Forsyth CB, Voigt RM, Engen PA, Patel J, Shaikh M, Green SJ, Naqib A, Roy A, Kordower JH, Pahan K, Shannon KM, Keshavarzian A (2020) Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol Dis 135, 104352. [DOI] [PubMed] [Google Scholar]
- [125]. García-Domínguez I, Veselá K, García-Revilla J, Carrillo-Jiménez A, Roca-Ceballos MA, Santiago M, de Pablos RM, Venero JL (2018) peripheral inflammation enhances microglia responseand nigral dopaminergic cell death in an in vivo MPTP model ofParkinson’s disease. Front Cell Neurosci 12, 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126]. Hernández-Romero MC, Delgado-Cortés MJ, Sarmiento M, dePablos RM, Espinosa-Oliva AM, Argüelles S, Bández MJ, Villarán RF, Mauriño R, Santiago M, Venero JL, Herrera AJ, Cano J, Machado A (2012) Peripheral inflammation increases thedeleterious effect of CNS inflammation on the nigrostriataldopaminergic system. Neurotoxicology 33, 347–360. [DOI] [PubMed] [Google Scholar]
- [127]. Couch Y, Alvarez-Erviti L, Sibson NR, Wood MJ, Anthony DC (2011) The acute inflammatory response to intranigral α-synuclein differs significantly from intranigral lipopolysaccharide and is exacerbated by peripheral inflammation. J Neuroinflammation 8, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128]. Boza-Serrano A, Reyes JF, Rey NL, Leffler H, Bousset L, Nilsson U, Brundin P, Venero JL, Burguillos MA, Deierborg T (2014) The role of Galectin-3 in α-synuclein-induced microglial activation. Acta Neuropathol Commun 2, 156 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129]. Espinosa-Oliva AM, García-Miranda P, Alonso-Bellido IM, Carvajal AE, González-Rodríguez M, Carrillo-Jiménez A, Temblador AJ, Felices-Navarro M, García-Domínguez I, Roca-Ceballos MA, Vázquez-Carretero MD, García-Revilla J, Santiago M, Peral MJ, Venero JL, de Pablos RM (2021) Galectin-3deletion reduces LPS and acute colitis-induced pro-inflammatorymicroglial activation in the ventral mesencephalon. FrontPharmacol 12, 706439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130]. Gundersen V (2020) Parkinson’s disease: Can targeting inflammation be an effective neuroprotective strategy? Front Neurosci 14, 580311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131]. Qiao C, Zhang Q, Jiang Q, Zhang T, Chen M, Fan Y, Ding J, Lu M, Hu G (2018) Inhibition of the hepatic Nlrp3 protects dopaminergic neurons via attenuating systemic inflammation in a MPTP/p mouse model of Parkinson’s disease. J Neuroinflammation 15, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132]. Liu Y, Xie X, Xia LP, Lv H, Lou F, Ren Y, He ZY, Luo XG (2017) Peripheral immune tolerance alleviates the intracranial lipopolysaccharide injection-induced neuroinflammation and protects the dopaminergic neurons from neuroinflammation-related neurotoxicity. J Neuroinflammation 14, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133]. Xia L, Xie X, Liu Y, Luo X (2017) Peripheral blood monocyte tolerance alleviates intraperitoneal lipopolysaccharides-induced neuroinflammation in rats via upregulating the CD200R expression. Neurochem Res 42, 3019–3032. [DOI] [PubMed] [Google Scholar]
- [134]. Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55, 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135]. Reinert KR, Umphlet CD, Quattlebaum A, Boger HA (2014) Short-term effects of an endotoxin on substantia nigra dopamine neurons. Brain Res 1557, 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136]. Bodea LG, Wang Y, Linnartz-Gerlach B, Kopatz J, Sinkkonen L, Musgrove R, Kaoma T, Muller A, Vallar L, Di Monte DA, Balling R, Neumann H (2014) Neurodegeneration by activation of the microglial complement-phagosome pathway. J Neurosci 34, 8546–8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137]. Kelly LP, Carvey PM, Keshavarzian A, Shannon KM, Shaikh M, Bakay RA, Kordower JH (2014) Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov Disord 29, 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138]. Deng I, Wiese MD, Zhou XF, Bobrovskaya L (2021) The efficacy of systemic administration of lipopolysaccharide in modelling pre-motor Parkinson’s disease in C57BL/6 mice. Neurotoxicology 85, 254–264. [DOI] [PubMed] [Google Scholar]
- [139]. Song S, Jiang L, Oyarzabal EA, Wilson B, Li Z, Shih YI, Wang Q, Hong JS (2019) Loss of brain norepinephrine elicits neuroinflammation-mediated oxidative injury and selective caudo-rostral neurodegeneration. Mol Neurobiol 56, 2653–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140]. Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24, 197–211. [DOI] [PubMed] [Google Scholar]
- [141]. He Q, Yu W, Wu J, Chen C, Lou Z, Zhang Q, Zhao J, Wang J, Xiao B (2013) Intral LPS-mediated Parkinson’s model challenges the pathogenesis of nasal cavity and environmental toxins. PLoS One 8, e78418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142]. Li YH, He Q, Yu JZ, Liu CY, Feng L, Chai Z, Wang Q, Zhang HZ, Zhang GX, Xiao BG, Ma CG (2015) Lipoic acid protects dopaminergic neurons in LPS-induced Parkinson’s disease model. Metab Brain Dis 30, 1217–1226. [DOI] [PubMed] [Google Scholar]
- [143]. Niu H, Wang Q, Zhao W, Liu J, Wang D, Muhammad B, Liu X, Quan N, Zhang H, Zhang F, Wang Y, Li H, Yang R (2020) IL-1β/IL-1R1 signaling induced by intranasal lipopolysaccharide infusion regulates alpha-Synuclein pathology in the olfactory bulb, substantia nigra and striatum. Brain Pathol 30, 1102–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144]. Houser MC, Tansey MG (2017) The gut-brain axis: Is intestinalinflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145]. Breen DP, Halliday GM, Lang AE (2019) Gut-brain axis and the spreadof α-synuclein pathology: Vagal highway or dead end? Mov Disord 34, 307–316. [DOI] [PubMed] [Google Scholar]
- [146]. Zhu Y, Yuan M, Liu Y, Yang F, Chen WZ, Xu ZZ, Xiang ZB, Xu RS (2022) Association between inflammatory bowel diseases and Parkinson’s disease: Systematic review and meta-analysis. Neural Regen Res 17, 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147]. Brudek T (2019) Inflammatory bowel diseases and Parkinson’s disease. J Parkinsons Dis 9, S331–S344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148]. Garrido-Gil P, Rodriguez-Perez AI, Dominguez-Meijide A, Guerra MJ, Labandeira-Garcia JL (2018) Bidirectional neural interaction between central dopaminergic and gut lesions in Parkinson’s disease models. Mol Neurobiol 55, 7297–7316. [DOI] [PubMed] [Google Scholar]
- [149]. Mitchell J, Kim SJ, Howe C, Lee S, Her JY, Patel M, Kim G, Lee J, Im E, Rhee SH (2022) Chronic intestinal inflammation suppresses brain activity by inducing neuroinflammation in mice. Am J Pathol 192, 72–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150]. Matisz CE, Gruber AJ (2022) Neuroinflammatory remodeling of the anterior cingulate cortex as a key driver of mood disorders in gastrointestinal disease and disorders. Neurosci Biobehav Rev 133, 104497. [DOI] [PubMed] [Google Scholar]
- [151]. Talley S, Valiauga R, Anderson L, Cannon AR, Choudhry MA, Campbell EM (2021) DSS-induced inflammation in the colon drives a proinflammatory signature in the brain that is ameliorated by prophylactic treatment with the S100A9 inhibitor paquinimod. J Neuroinflammation 18, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152]. Barnes SE, Zera KA, Ivison GT, Buckwalter MS, Engleman EG (2021) Brain profiling in murine colitis and human epilepsy reveals neutrophils and TNFα as mediators of neuronal hyperexcitability. J Neuroinflammation 18, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153]. Lv WJ, Liu C, Yu LZ, Zhou JH, Li Y, Xiong Y, Guo A, Chao LM, Qu Q, Wei GW, Tang XG, Yin YL, Guo SN (2020) Melatonin alleviates neuroinflammation and metabolic disorder in DSS-induced depression rats. Oxid Med Cell Longev 2020, 1241894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154]. Islam J, Agista AZ, Watanabe K, Nochi T, Aso H, Ohsaki Y, Koseki T, Komai M, Shirakawa H (2022) Fermented rice bran supplementation attenuates chronic colitis-associated extraintestinal manifestations in female C57BL/6N mice. J Nutr Biochem 99, 108855. [DOI] [PubMed] [Google Scholar]
- [155]. Che H, Li H, Song L, Dong X, Yang X, Zhang T, Wang Y, Xie W (2021) Orally administered DHA-enriched phospholipids and DHA-enriched triglyceride relieve oxidative stress, improve intestinal barrier, modulate inflammatory cytokine and gut microbiota, and meliorate inflammatory responses in the brain in dextran sodium sulfate induced colitis in mice. Mol Nutr Food Res 65, e2000986. [DOI] [PubMed] [Google Scholar]
- [156]. Borzabadi S, Oryan S, Eidi A, Aghadavod E, Daneshvar Kakhaki R, Tamtaji OR, Taghizadeh M, Asemi Z (2018) The effects of probiotic supplementation on gene expression related to inflammation, insulin and lipid in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Arch Iran Med 21, 289–295. [PubMed] [Google Scholar]
- [157]. Jang H, Boltz DA, Webster RG, Smeyne RJ (2009) Viral parkinsonism. Biochim Biophys Acta 1792, 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158]. Cocoros NM, Svensson E, Szépligeti SK, Vestergaard SV, Szentkúti P, Thomsen RW, Borghammer P, Sørensen HT, Henderson VW (2021) Long-term Risk of Parkinson Disease Following Influenzaand Other Infections. JAMA Neurol 78, 1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159]. Smeyne RJ, Noyce AJ, Byrne M, Savica R, Marras C (2021) Infection and risk of Parkinson’s disease. J Parkinsons Dis 11, 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160]. Rosen B, Kurtishi A, Vazquez-Jimenez GR, Møller SG (2021) The intersection of Parkinson’s disease, viral infections, and COVID-19. Mol Neurobiol 58, 4477–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161]. Sadasivan S, Sharp B, Schultz-Cherry S, Smeyne RJ (2017) Synergistic effects of influenza and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) can be eliminated by the use of influenza therapeutics: Experimental evidence for the multi-hit hypothesis. NPJ Parkinsons Dis 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162]. Takahashi M, Yamada T, Nakajima S, Nakajima K, Yamamoto T, Okada H (1995) The substantia nigra is a major target for neurovirulent influenza A virus. J Exp Med 181, 2161–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163]. Ogata A, Tashiro K, Nukuzuma S, Nagashima K, Hall WW (1997) A rat model of Parkinson’s disease induced by Japanese encephalitis virus. J Neurovirol 3, 141–147. [DOI] [PubMed] [Google Scholar]
- [164]. Simanjuntak Y, Liang JJ, Lee YL, Lin YL (2017) Japanese encephalitis virus exploits dopamine D2 receptor-phospholipase C to target dopaminergic human neuronal cells. Front Microbiol 8, 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165]. Bantle CM, Phillips AT, Smeyne RJ, Rocha SM, Olson KE, Tjalkens RB (2019) Infection with mosquito-borne alphavirus induces selective loss of dopaminergic neurons, neuroinflammation and widespread protein aggregation. NPJ Parkinsons Dis 5, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166]. Alonso-Bellido IM, Bachiller S, Vázquez G, Cruz-Hernández L, Martínez E, Ruiz-Mateos E, Deierborg T, Venero JL, Real LM, Ruiz R (2021) The other side of SARS-CoV-2 infection: Neurologicalsequelae in patients. Front Aging Neurosci 13, 632673;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167]. Papa SM, Brundin P, Fung VSC, Kang UJ, Burn DJ, Colosimo C, Chiang HL, Alcalay RN, Trenkwalder C, MDS-Scientific Issues Committee (2020) Impact of the COVID-19 pandemic on Parkinson’s disease and movement disorders. Mov Disord 35, 711–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168]. Garcia-Revilla J, Deierborg T, Venero JL, Boza-Serrano A (2020) Hyperinflammation and fibrosis in severe COVID-19 patients: Galectin-3, a target molecule to consider. Front Immunol 11, 2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169]. Herrera AJ, Espinosa-Oliva AM, Carrillo-Jiménez A, Oliva-Martín MJ, García-Revilla J, García-Quintanilla A, de Pablos RM, Venero JL (2015) Relevance of chronic stress andthe two faces of microglia in Parkinson’s disease. Front CellNeurosci 9, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]