Abstract
Evidence suggests that hearing loss (HL), even at mild levels, increases the long-term risk of cognitive decline and incident dementia. Hearing loss is one of the modifiable risk factors for dementia, with approximately 4 million of the 50 million cases of dementia worldwide possibly attributed to untreated HL. This paper describes four possible mechanisms that have been suggested for the relationship between age-related hearing loss (ARHL) and Alzheimer’s disease (AD), which is the most common form of dementia. The first mechanism suggests mitochondrial dysfunction and altered signal pathways due to aging as a possible link between ARHL and AD. The second mechanism proposes that sensory degradation in hearing impaired people could explain the relationship between ARHL and AD. The occupation of cognitive resource (third) mechanism indicates that the association between ARHL and AD is a result of increased cognitive processing that is required to compensate for the degraded sensory input. The fourth mechanism is an expansion of the third mechanism, i.e., the function and structure interaction involves both cognitive resource occupation (neural activity) and AD pathology as the link between ARHL and AD. Exploring the specific mechanisms that provide the link between ARHL and AD has the potential to lead to innovative ideas for the diagnosis, prevention, and/or treatment of AD. This paper also provides insight into the current evidence for the use of hearing treatments as a possible treatment/prevention for AD, and if auditory assessments could provide an avenue for early detection of cognitive impairment associated with AD.
Keywords: Age-related hearing loss, Alzheimer’s disease, cognitive function, hearing loss
INTRODUCTION
With a rapidly aging population, the prevalence of dementia has increased and is proposed to continue to increase [1]. Currently, it is estimated that 57 million people worldwide are diagnosed with dementia [2]. The number of dementia cases worldwide is expected to double every 20 years if there is no medical breakthrough, resulting in an estimated 152 million cases by 2050 [1, 2]. Hearing loss is suggested to be one of the modifiable risk factors for dementia contributing to the population attributable fraction of dementia cases worldwide. Hearing loss is estimated to account for 8% of dementia cases, that is, approximately 4 million of the 50 million cases of dementia worldwide may be attributed to untreated hearing loss [3]. Evidence indicates that hearing loss, even at mild levels, increases the long-term risk of cognitive decline and incident dementia [3–6]. Understanding the relationship between hearing loss and dementia has attracted greater public health attention. Increasing this understanding can provide valuable insights for the development of early detection tools and effective treatment interventions.
DEMENTIA AND ALZHEIMER’S DISEASE
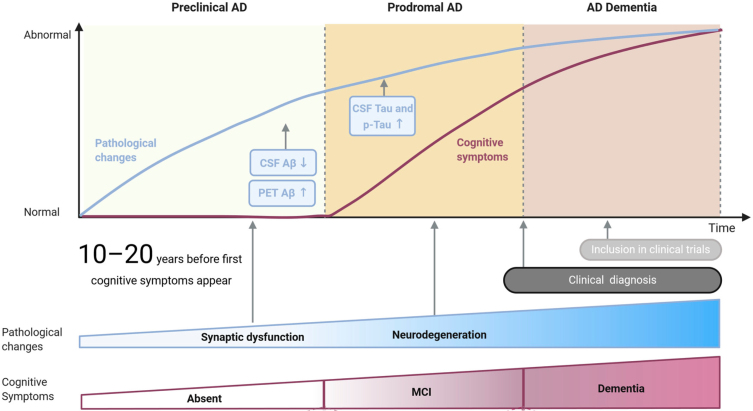
The majority of dementia cases are due to Alzheimer’s disease (AD) neuropathology, which accounts for 60–80% of all dementia cases [7] and is defined by loss of episodic memory and impaired cognitive function combined with the presence of biomarkers of AD [8]. Neurodegenerative changes, which lead to AD, are suggested to start to accumulate 10 to 20 years before the appearance of clinical symptoms (see Fig. 1) [9, 10]. In addition to neuronal and synaptic loss, AD is defined by other neuropathological features, namely, extracellular amyloid-β (Aβ) deposition found in senile plaques, and hyperphosphorylated tau protein which causes the formation of neurofibrillary tangles in neural cell bodies [11–13]. Aβ is a product of the amyloid-β protein precursor (AβPP) after the sequential cleavage by the enzymes β-secretase and γ-secretase. Improper folding of the cleaved AβPP by β-secretase is suggested to result in overproduction of misfolded proteins, which in turn accumulate into senile plaques [14, 15]. Aβ build-up is closely linked to synaptic and neuronal damage, which in turn result in gradual neuronal death and the deterioration of cortical and subcortical structures, i.e., brain atrophy [11].
Fig. 1.
Three subsequent stages of Alzheimer’s disease: preclinical AD, prodromal AD, and AD dementia. Pathological changes are indicated by a blue line on the graph and at the bottom of the figure. Cognitive symptoms are indicated by a purple line and at the bottom of the figure. Aβ and tau are the pathological biomarkers currently used for AD. Adapted from [19].
AD neuropathology also features abnormal concentrations of total and phosphorylated tau protein in the brain that can be accurately reflected in the cerebrospinal fluid (Fig. 1) When tau protein is hyperphosphorylated, microtubules in the cytoskeleton of neurons begin to break down and this causes the development of insoluble neurofibrillary tangles inside the neural cell bodies [12, 15]. It is suggested that the formation of these tangles progresses from brain structures such as the transentorhinal cortex to structures such as the hippocampus and the neocortex [16]. The appearance of tau pathology in the neocortex coincides with cognitive impairment [17]. These neurodegenerative changes are characterized by synaptic loss, neural damage, imbalances in neurotransmitters, and finally, manifest into the clinical cognitive symptoms of AD [11, 18].
The National Institute on Aging-Alzheimer’s Association (NIA-AA) group has suggested a concept that subdivides the course of AD into three subsequent stages. The first stage, known as the preclinical stage of AD, is characterized by having no impairment in cognition based on standard cognitive assessments and having some biomarker evidence of AD (see Fig. 1). This stage is often referred to as subjective cognitive decline (SCD). SCD refers to self-reported experience of decline in cognitive function without objective impairment on cognitive assessments or daily functioning [20]. The prevalence of SCD has been suggested to range between 25% and 50% among older adults (65 years and above) [21]. Although the exact conversion rate of SCD individuals to AD remains unknown, SCD individuals have a higher risk of developing subsequent objective cognitive impairment which in turn leads to the progression to other stages of AD, and it is therefore proposed that SCD with positive AD-related biomarkers is the first symptomatic expression of preclinical AD [22–26].
The second stage is mild cognitive impairment (MCI) due to AD, which is described by impairment in cognition particularly in the memory domain and the presence of biomarker evidence for AD. MCI is regarded as the prodromal stage between normal cognitive changes accompanied with aging and the early clinical symptoms of dementia (see Fig. 1) [27, 28]. MCI is typically defined as performance below 1.5 standard deviations on cognitive assessments, matched to age, gender, and education normative data [20]. It is proposed that approximately 16% of adults over the age of 70 years have MCI [29]. The annual conversion rates from MCI to AD dementia are suggested to range between 4% and 23% in community-based samples and 10–30% in a clinic-based sample [30–33]. The third stage is dementia of the AD type, which is characterized by the presence of dementia syndrome, and is based on objective cognitive impairment and the presence of AD biomarkers (see Fig. 1) [13, 34–36]. The duration and progression of each of these stages is not the same in each individual and it has been proposed that factors such as age of onset, genetic risk factors, and gender play a significant role in the duration of each stage [37]. The stages before dementia due to AD, i.e., SCD and MCI, may present a target population for AD intervention as treatments at the dementia stage of the disease have shown no promise in altering the disease progression, as the is already substantial neuronal injury and cognitive decline [36]. Objectively identifying this target population may be possible by investigating and understanding the connection between AD and associated health conditions.
HEARING LOSS (AGE-RELATED HEARING LOSS)
Age-related hearing loss (ARHL), also known as presbycusis, is the most prevalent health condition that affects older adults worldwide [1, 38]. It is estimated that close to a third of people over the age of 65 experience disabling hearing loss [1]. ARHL is characterized by bilateral, progressive, high-frequency deterioration of hearing sensitivity that is associated with aging [39]. ARHL is also characterized by impaired sound localization, reduced central auditory processing as well as reduced perception and understanding of speech in noisy environments [40]. The development of ARHL is suggested to be multifactorial, influenced by factors such as cochlear aging [38, 41], noise exposure [42, 43], gender [44], race [45, 46], genetic predisposition [47], environmental variables [48, 49], hypertension [50], and other health comorbidities, such as type 2 diabetes [51]. The main pathologies of ARHL are of cochlear origin, including loss of sensory (hair) cells, stria vascularis atrophy, auditory nerve degeneration, and loss of spiral ganglion neurons, and often coupled with changes in central auditory pathways [40, 52].
ARHL has been classified into six categories: sensory, neural, strial, cochlear conductive, mixed, and indeterminate [53]. Sensory ARHL is associated with the degeneration of the basal end of the organ of Corti, in particular loss of outer hair cells, which causes hearing loss in high frequencies [38, 43]. Neural ARHL, which is characterized by diminished speech discrimination, is suggested to be a result of spiral ganglion cell and nerve fiber loss [53]. It is suggested that loss of approximately 50% of the afferent nerves, due to cell death, results in reduced speech discrimination; however, measurable threshold changes may not be observed until over 90% of nerve fibers are lost [54, 55]. The atrophy of the stria vascularis, the secretory tissue in the lateral wall of the cochlear duct which regulates the amount of potassium and sodium ions in the endolymph, results in reduced pure-tone thresholds at all frequencies and this is referred to as strial ARHL [38, 43, 56]. Cochlear conductive ARHL is suggested to be a result of degenerative changes that cause increased stiffness of the basilar membrane. It is often characterized by a gradual down-sloping audiogram, with low-frequency hearing loss [38]. Mixed ARHL is believed to be a result of a combination of the histopathology of other ARHL categories, while indeterminate ARHL is characterized by high-frequency hearing loss without the presence of a consistent histological appearance [38, 48].
Central brain changes due to ARHL
Neurochemical and metabolic changes in the central nervous system associated with ARHL have been observed using magnetic resonance spectroscopy (MRS). One MRS study demonstrated reduced excitatory neurotransmitter levels such as glutamate and N-acetylaspartate concentrations with increased lactate in the auditory cortex of older adults with ARHL [57]. In contrast Gao et al. [58] found that inhibitory neurotransmitter, gamma-aminobutyric acid (GABA), concentrations were decreased in central auditory regions in ARHL participants when compared to age-matched normal hearing participants. Lower GABA concentrations have also been demonstrated in animal models of ARHL, with decreased GABA levels found in both the auditory cortex [59, 60] and the inferior colliculus [59, 61, 62].
Although pathological changes, including reduced gray matter [63–65] and white matter [66, 67] volumes, can accompany healthy aging in the central brain regions, a more pronounced decrease in gray and white matter volume in the auditory cortex has been reported in ARHL patients in comparison with healthy age-matched controls [49, 68–70]. In relation to ARHL, reduced gray matter volume has also been observed in multiple brain regions, including the temporal lobe [44], particularly the superior and middle temporal gyri [68], medial and superior frontal gyri [68], hypothalamus [71], and occipital lobe [71]. Changes in these brain regions and neural networks are associated with impaired cognitive functions including episodic visuospatial memory and learning memory [72, 73], working memory and executive functions [74], attention switching [75], and verbal recognition memory [73]. Additionally, the cingulo-opercular cortex has been shown to be atrophied in individuals with ARHL as well as in those with impaired episodic memory [45]. Furthermore, one MRI study revealed that whole brain volume is reduced in participants with ARHL in comparison to healthy age-matched controls [76]. Collectively, these findings suggest that cochlear dysfunction is linked with cortical changes in regions involved in cognitive functions [45, 47].
Diffusion tensor imaging (DTI) studies in people with hearing loss have revealed changes in the white matter tracks in different regions of the auditory pathway [68, 77]. DTI is used to investigate white matter tracts in vivo and to quantify the directionality of water diffusion in order to yield an index of microstructural integrity [78]. Fractional anisotropy values, a measure of connectivity derived from DTI, have been shown to be reduced in the lateral lemniscus, inferior colliculus, the superior olivary complex, and the auditory cortex [77]. Husain et al. [68] found that the orientation of white matter tracts leading in and out of the frontal cortex and temporal cortex differed significantly between a mild-to-moderate hearing loss group in comparison to a normal hearing group. Alterations to the white matter tracts may be attributed to sensory deprivation resulting in axonal loss or demyelination resulting in damage to white matter tracts or the extension of other fibers into the regions forming disordered white matter tracts [68]. Hence, these findings from DTI studies indicate that ARHL causes secondary changes in the central auditory pathway as well as brain regions that do not play a direct role in auditory processing.
MECHANISMS FOR THE RELATIONSHIP BETWEEN ARHL AND AD
A number of potential mechanisms have been suggested for the relationship between ARHL and AD. These mechanisms include: 1) common cause, 2) sensory deprivation, 3) occupation of cognitive resources, and 4) function and structure interaction.
1) Common cause mechanism
Common factors that may influence hearing function and cognitive function could explain the correlation between ARHL and AD. The common cause mechanisms suggest a third variable that results in impairment in both hearing loss and cognitive impairment, these include: aging, mitochondrial dysfunction, microvascular factors, and inflammation. Age-related neurodegeneration is a common feature of cognitive aging [79], which is characterized by atrophy of multiple brain regions [80] and changes in cognitive performance [81]. Similarly, age-related changes can be seen along the auditory pathway [38, 40, 42, 52], as discussed above. Microvascular complications are common feature in both ARHL and dementia. Research suggests that diabetes mellitus, which is characterized by many microvascular complication, is a common risk factor for both hearing loss [82, 83] and dementia [3, 84].
Inflammatory pathologies have also been suggested to be another common cause for dementia and hearing loss. It has been proposed that inflammatory factors that are influenced by lifestyle factors (e.g., obesity, sleep quality, and physical activity) could be mediators in the development of AD dementia [85, 86]. Inflammatory mediators have been proposed to have harmful effects on small vessels which can result in atrophy of cortical structures critical for cognitive functions [87, 88]. Inflammatory responses that contribute to age-related pathologies have also been observes in the inner ear. While the influence of inflammatory factors on ARHL are not fully understood, there is evidence to suggest that there is an inflammatory response in the inner ear of aging mice which is induced by oxidative stress [89, 90]. Mitochondrial dysfunction is a mediating factor is oxidative stress and there are some theories that suggest ARHL and AD may be a result of mitochondrial dysfunction and changes in signal pathways.
ROS/VEGF pathway
The mitochondria play a key role in oxidative metabolism and is the primary production site for reactive oxygen species (ROS), reactive radical and non-radical derivatives of oxygen [91]. High concentrations of ROS can have toxic effects on cellular mechanisms that lead to accumulation of oxidative stress/damage, ultimately resulting in cellular dysfunction [92]. There is often an increase in ROS activity and decrease in ROS clearance with aging [93–95]. Buildup of ROS in cells has been suggested to result in mtDNA mutations and mitochondrial dysfunction which decreases the quantity of vascular endothelial growth factor (VEGF), a signaling protein, in the brain [96].
Interestingly, VEGF has been suggested to be a common feature in the molecular mechanisms of ARHL and AD. In AD patients, VEGF expression is lower in hippocampal, superior temporal and brainstem regions [96]. Due to the involvement of VEGF in vascular remodeling, endothelial maintenance, and angiogenesis, the reduction of its levels in AD could be an indication of altered capillary function, which can modify Aβ efflux [96–98]. VEGF has also been shown to bind to amyloid plaques with high affinity, resulting in reduced availability of VEGF under hypo-perfusion conditions, in turn contributing to vascular dysfunction and neurodegeneration in AD [99].
Although there is limited information on the specific function of VEGF in ARHL, one animal study has demonstrated that cochlear VEGF expression is reduced in aged mice with ARHL, indicating that vascular abnormalities may be an influencing factor in ARHL [100]. Acoustic trauma, one of the risk factors of ARHL, has been reported to result in increased vascular permeability, changes in cochlear blood flow as well as vasoconstriction [101, 102]. In addition to this, VEGF was shown to be upregulated in spiral ganglion neurons, spiral ligament and stria vascularis following acoustic trauma [103].
SIRT1/PGC-1a pathway
Peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1α), the primary regulator of mitochondrial biogenesis, is thought to play a role in impeding neurodegeneration [67]. PGC-1α has been shown to avert the neurodegenerative effects of 1-methyl-4-phenyl-1, 2, 3, 6- tetrahydropyridine (MPTP) as well as increase ROS detoxification [72]. In cultured hippocampal neurons, PGC-1α has been found to support synaptogenesis and spinogenesis [78]. SIRT1 (silent information regulator 2 homolog 1), a nicotinamide adenine dinucleotide-dependent histone deacetylase, induces adaptive response to metabolic stress and can deacetylate PGC-1α [72, 75]. Deacetylation of PGC-1α results in an increase in its transcriptional function, which is essential for the activation of mitochondrial fatty acid oxidation genes [75, 77].
Age-related reduction in SIRT1-PGC-1α expression has been suggested to cause hair cell apoptosis and neural degeneration due to impaired mitochondrial respiratory function, leading to ARHL. An animal study demonstrated reduced SIRT1-PGC-1α expression in the cochlea of aged mice [38]. Additionally, an in vitro experiment demonstrated that when SIRT1 expression was increased, PGC-1α expression was increased in cochlear hair cells which inhibited cell apoptosis and promoted cell proliferation [38]. Therefore, the SIRT1-PGC-1α pathway is thought to play a major role in the pathogenesis of ARHL. Concurrently, impaired function of the SIRT1-PGC-1α pathway in the brain has also been found to alter the expression of β-secretase 1 (BACE1), which in turn alters AβPP cleaving resulting in Aβ production. Wang et al. [39] demonstrated, using both in vivo and in vitro experiments, that reduced expression of PGC-1α caused altered expression of BACE1, while overexpression also reduced BACE1 expression. Altered expression of BACE1 contributes to the production of Aβ in the brain and to incidence of AD. Although more research is needed, the SIRT1-PGC-1α pathway has been suggested as a potential treatment target to prevent individuals with ARHL from developing AD [104].
2) Sensory Deprivation Mechanism
Sensory degradation in hearing impaired people has been suggested as another mechanism for the relationship between ARHL and AD. Due to hearing loss, there is degradation and loss of input to the cortex [105]. Progressively, this degradation of sensory information results in changes to the function and structure of auditory and cognitive systems in the brain [106–108]. Prolonged sensory deprivation due to peripheral ARHL may lead to decreased cortical volume similar to that seen in cognitive decline [19, 76]. A number of brain regions, including the superior temporal lobe [68, 109], frontal lobe [68, 110], and hippocampus [111], show reduced gray matter density with ARHL. These brain regions play an important role in semantic memory and are involved in advancement along the dementia continuum [68]. Degraded auditory sensory information has also been linked to a number of functional changes, including decreased memory and comprehension of spoken language, impaired speech-in-noise performance, as well as cognitive impairment [105, 107, 112]. Functional changes, such as impaired speech-in-noise processing, have also been associated with reduced social interaction leading to social withdrawal and isolation [113, 114], which are also suggested to be a risk factors in dementia [115, 116] (see Kuiper et al. [117] for a review).
There is also evidence to suggest that ARHL is associated with decreased white matter volume and microstructural integrity in brain regions essential for cognitive function, such as the temporal lobe [70, 118, 119]. Additionally, cross modal reorganization, which is the recruitment of cortical resources from the deprived modality (i.e., auditory) by un-impacted modalities, due to ARHL can be seen in those with early stages of cognitive impairment [120, 121]. In other words, the structural and functional changes seen in ARHL result in additional restriction of cortical resources accessible for cognitive processes [122, 123]. However, the length of sensory deprivation, critical age of HL onset and degree of cortical reorganization that is necessary to induce structural brain changes, brain atrophy and impaired cognitive function remains unclear.
3) Occupation of Cognitive Resources Mechanism
The occupation of cognitive resources mechanism suggests that the association between ARHL and AD is a result of higher cognitive processing that is necessary to compensate for the degraded sensory input. A greater amount of neural resources, i.e., working memory, language processing and attention, must be allocated to auditory processing when auditory input is degraded [122, 124]. This results in less resources available for higher cognitive processing, including memory retention and retrieval [122, 123]. Imaging studies demonstrated that brain activation patterns are altered when processing challenging tasks, which would mean that as auditory processing and listening become more challenging, brain activation patterns change [122, 125, 126]. Furthermore, individuals with hearing loss have decreased gray matter density in the primary auditory cortex and reorganization of processing systems when sensory auditory stimulation is reduced [127].
Dual-task paradigms have been adopted to examine whether difficulty in listening results in decreased cognitive resources available for other tasks [128]. These dual-task paradigms are attentionally demanding, signifying an attentional basis by which the occupation of cognitive resources of listening may impact memory function in AD [122]. It has been demonstrated that listening to speech in noise results in decreased performance in secondary non-auditory cognitive tasks [128]. In addition to this, replicating the challenges of listeners with hearing loss by degrading or masking speech results in impairment in subsequent cognitive task performance [128]. Functional imaging studies demonstrated that the auditory cortex, left inferior frontal lobe, and hippocampus are involved in speech perception in challenging listening conditions [129, 130]. Behavioral data provide evidence for the use of greater cognitive resources in the presence of sensory degradation [128] and imaging studies indicate the involvement of a wide network of brain regions in difficult listening conditions [130–132]. Therefore, cognitive function may decrease in hearing impaired adults due to the occupation of cognitive resources when processing degraded sensory information [133].
4) Function and Structure Interaction Mechanism
Expanding on the previously outlined mechanism, another mechanism involving both cognitive resource occupation (neural activity) and AD pathology has been proposed for the relationship between ARHL and AD. This mechanism suggests that hearing loss modifies cortical activity in the medial temporal lobe (MTL), which can be interrelated to AD pathology in the same region. The MTL plays an important role in memory processing and episodic memory [134, 135]. It is comprised of several sub-regions including the hippocampus, the parahippocampal cortex in the posterior parahippocampal gyrus, and the perirhinal and entorhinal cortices in the anterior parahippocampal gyrus [134, 136–138].
Neurofibrillary changes, as a result of tau protein hyperphosphorylation, have been suggested to best correlate with the cognitive symptoms of AD [139]. The earliest AD related neurofibrillary changes have been found in MTL regions [140, 141]. It is suggested that hearing impairment can cause altered neuronal activity in MTL structures, which in turn can cause or increase AD neuropathology [122]. Studies have demonstrated that the hippocampus, a sub-region of the MTL, plays a critical role in the memory of sound in trace conditioning tasks [142]. The hippocampus has also been shown to be active during other types of sound processing, including pattern recognition from a random sequence [143], statistical learning of tone streams [144], and auditory working memory during investigation of acoustic patterns that change over time [145]. In addition to this, there is research to support the role of the hippocampus in the processing of degraded speech [130, 131].
Speech-in-noise perception has been associated to cognitive skills, including phonological working memory and auditory working memory [146]. It is suggested that auditory information with similar properties can be connected by working memory to enable the filtering of central information of interest during speech-in-noise listening [122]. Degraded auditory signals caused by hearing loss can impact speech-in-noise performance, which in turn causes auditory pattern analysis and auditory working memory mechanisms to work harder as separation of speech from noise becomes more difficult [130, 131, 146]. This eventually results in elevated neural activity in the MTL [122]. Furthermore, gradual hearing loss has also been suggested to result in widespread reorganization of plasticity-related neurotransmitter expression. Cortical and hippocampal expression of glutamate subunits of the NMDA receptor have been shown to increase in adult mice that have hearing loss in comparison to control mice that lack auditory deficits [147]. Significantly impaired hippocampal synaptic plasticity and compromised function are also suggested to be accompanied by memory impairment [147].
Similar to hearing loss, pathological markers of AD, tau and Aβ, have been suggested to induce alterations in glutamatergic [148–150] and GABAergic [151] function, resulting in altered synaptic activity and disrupted neural connectivity in neurocognitive networks. Human studies have shown the co-localization of elevated neural activity and tau deposition in AD, particularly in parietal and occipital brain regions [152, 153]. In addition, an animal study has suggested a direct relationship between higher neural activity and AD pathology, including Aβ. Bero et al. [154] provided evidence that suggests neuronal activity regulates regional concentrations of interstitial fluid Aβ which in turn drives local Aβ accumulation. Therefore, some brain regions, in particular those with increased neuronal activity, can be more vulnerable to Aβ deposition in AD [154]. The insight that neuronal activity may play a contributing role in the development of AD may have implications for early diagnosis and treatment strategies.
OTHER MEDIATING FACTORS IN THE RELATIONSHIP BETWEEN ARHL AND AD
The link between hearing impairment and cognitive dysfunction could also be partly mediated by other risk factors for dementia, which include social isolation and depression. Studies have found that hearing loss was linked to increased loneliness and social isolation with both being negatively correlated with cognitive function [113, 155]. Furthermore, individuals with ARHL have been suggested to have an increased the risk developing depression later in life [156]. Depression is considered to be an autonomous risk factor or prodromal symptom of dementia and AD [3], with the co-occurrence of ARHL and depression further increasing the possibility of developing cognitive impairment associated with dementia [156]. Although the influence of these mediating factors on the above-mentioned mechanisms is yet to be determined, there is potential for the co-occurrence of multiple risk factors of dementia to be a greater indicator of those at increased risk of developing dementia.
FUTURE DIRECTIONS
Hearing loss intervention as a possible treatment or prevention for AD
The possibility of hearing restoration altering the risk of developing AD or stopping/reversing cognitive decline depends on which, if any, of the postulated mechanisms are correct. In the case of the association between ARHL and AD being due to common cause (mechanism 1), restoration of hearing would have no effect on the progression of dementia or result in any improvement of cognitive function. If the sensory deprivation mechanism is true, restoration of hearing would reduce the risk of dementia, however, would not reverse cognitive impairment. On the other hand, if the association between ARHL and AD is caused by the cognitive resource mechanism (mechanism 3), the increased load of cognitive resources could be reversed by treating ARHL. This should, in theory, reduce the risk of developing AD and restore a degree of cognitive function regardless of the stage of the disease [122]. Finally, if the fourth (function and structure interaction) mechanism is true, early identification and treatment of hearing impairment could delay or prevent neurodegenerative changes caused by altered sensory input and slow progression of structural changes at later stages of cognitive decline. The risk of further cognitive decline would rely upon the duration and severity of hearing loss prior to treatment. That is, if the duration between initial hearing loss and the treatment of hearing impairment is too long, the chain of structural changes may have already begun and could continue, although at a slower rate, to cause future cortical degeneration that would lead to AD [122]. Looking into some of the previous research on the impact of treating ARHL on cognitive function, could provide some insight into which mechanism is more likely.
It has been suggested that the rehabilitation of hearing loss, using hearing aids (HA) or cochlear implants (CI), can have a positive impact on cognitive dysfunction. However, research on the use of HA and CI as a possible treatment or prevention for cognitive decline has yielded mixed results. Previous research comparing cognitive function over a 12-month period between CI recipients and CI candidates showed that hearing rehabilitation improved a variety of cognitive functions, including working memory and strategy use [157]. Mosnier et al. [5] also demonstrated improved cognitive function in patients 12 months following cochlear implantation in addition to improvements in speech perception and quality of life. On the other hand, another study investigating the impact of CI on cognitive ability in older adults with profound hearing loss found no significant improvements in cognitive function 18 months after receiving a CI [6]. However, this study did demonstrate that ARHL and age were correlated with significantly decreased executive function and visual attention before receiving a CI [6].
A recent pilot study on participants with hearing loss has found that HA users had significantly better performance when compared to non-HA users on a working memory task (delayed matching-to-sample test) [158]. Additionally, a population-based longitudinal cohort study, investigating episodic memory function in 2,040 individuals before and after HA use, revealed that HA use resulted in a reduction in the rate of episodic memory decline [4]. These results indicate that ARHL treatment could have a positive impact on age-related cognitive decline. Similarly, Bucholc et al. [159] found in a longitudinal population based study of 2,114 MCI participants with HL that used HA had a significantly lower risk of developing all-cause dementias in comparison those who were non-HA users.
However, in contrast to this, a pilot study on participants with ARHL and primary dementia indicated that HA use did not improve cognitive function or psychiatric symptoms [160]. This suggests that hearing rehabilitation at more severe stages of cognitive decline may be ineffective in improving cognitive function; however, it is noteworthy that this pilot study was conducted over a short 24-week period and had no comparison group, limiting the validity of the conclusions [160]. Similarly, a randomized controlled trail investigating the efficacy of HA use on cognitive function in individuals diagnosed with AD found no significant impact on cognitive decline following 6 month of HA use [161]. Additionally, van Hooren et al. [162] investigated the impact of HA use on cognitive functions in participants without neurological diseases. They found that cognitive functions, including attention and memory, did not improve in HA users in comparison to non-HA users after a 12-month period.
Interestingly, a recent study by Cuoco et al. [163] found that 6 months of HA use has positive implications on cognitive function in participants with mild-HL but not at more severe-HL. The authors suggest that hearing rehabilitation in those with mild-HL may be more effective at delaying cognitive decline; however, a longer follow-up period and further research would be needed to fully understand the effect HA use can have in those with more severe-HL [163]. Additionally, treating HL using HA or CI have been found to have positive impacts on mental health and result in substantial improvement in quality of life in adults with HL [164–166]. Collectively, the research above indicates that treating ARHL may not reverse cognitive decline; however, the rate of decline and the risk of dementia may be decrease and the quality of life may be improved. For this reason, it is suggested that the fourth mechanism, function and structure interaction, is the more plausible theory for the relationship between ARHL and AD.
HEARING ASSESSMENTS AS A POSSIBLE DIAGNOSTIC/SCREENING TOOL FOR AD
In view of the link between ARHL and cognitive decline, the question has been raised whether auditory tests could be used to detect changes in cognitive function.
Behavioral auditory assessments
Central auditory processing (CAP) refers to the integrated neural processing of auditory signals in the central auditory nervous system [167]. Behavioral CAP assessments are used to measure the functional abilities of the auditory system; these tests include auditory discrimination, auditory temporal processing, dichotic listening, monaural low-redundancy speech, and binaural interaction tests, described in more detail by the American Academy of Audiology [167]. Multiple studies have examined the use of behavioral CAP tests as a potential tool to identify cognitive dysfunction associated with AD [168–173].
Age-related CAP dysfunction has been suggested as an indicator for increased risk of cognitive impairment and AD [174, 175]. Dichotic listening assessments, which assess the ability to separate or integrate different auditory stimuli presented simultaneously to each ear, have been proposed to involve cognitive processes, including memory and attention, as neuroimaging studies have shown activation in the frontal, parietal, and temporal lobes in individuals during performance of these tasks [176, 177]. Indeed, studies evaluating central auditory function in cognitively impaired older adults using Dichotic digits test (DDT) [173, 178] and dichotic sentence identification test (DSI) [168, 173, 178] have shown poorer performance in these tests in the cognitively impaired group in comparison to healthy age-matched controls. This could be related to the fact that individuals with AD frequently have impaired executive functions, causing an inability to respond correctly in the dichotic listening task when instructed to maintain attention to a particular ear [176, 179]. Poorer performance in the DSI test has also been proposed to be a predictor of reduced cortical thickness in the middle frontal gyrus, a region associated with cognitive process such as episodic and working memory [180].
Temporal auditory processing, the ability to analyze acoustic events over time, has also been suggested to be impaired in people with cognitive decline [168, 181]. Temporal auditory processing assessments have been proposed to involve and reflect cognitive functions, such as attentive executive function and memory, as they involve information integration from both hemispheres across the corpus callosum in order to identify and process sequences of auditory patterns [168, 181, 182]. Additionally, diminished speech perception in noise, as tested using synthetic sentence identification-ipsilateral competing message (SSI-ICM) and speech perception in noise test (SPIN), has also been associated with cognitive impairment [170, 173, 183]. Performance on the SSI-ICM test has been proposed to be influenced by the cortical thickness of the parahippocampal gyrus and entorhinal region and interestingly both regions are susceptible to atrophy in pre-symptomatic phases of AD [9]. Furthermore, cortical thickness in the inferior parietal lobe and primary auditory cortex has also been suggested to influence SSI-ICM performance [9, 184]. Reduced performance in the SSI-ICM test has been observed in people with MCI and AD, indicating that sensory integration and auditory processing are impaired in those with AD related cognitive decline [168, 173, 181]. Interestingly, SSI-ICM test performance has also been shown to be significantly poorer in people with SCD in comparison to those without SCD [185]. While more research is needed to fully establish the reliability and accuracy of SSI-ICM in differentiating between SCD and non-SCD participants, there is evidence to suggest that this CAP test could have the potential to identify probable pre-clinical AD.
Although impairment in central auditory function is seen in those with cognitive impairment, the use of subjective or behavioral central auditory tests as a possible screening tool for AD has to be carefully considered due to a number of reasons. Firstly, there are a limited number of studies that have investigated CAP in cognitively impaired individuals using behavioral testing. This could be due to the complexity of these behavioral tests and difficulty to conduct in people with cognitive dysfunction. Behavioral CAP tests require substantial attention, understanding, intricate responses and active participation from the individuals, which limits their applicability and reliability in those with moderate to severe cognitive impairment. Additionally, other health related factors could influence the performance of an individual on these behavioral auditory tests, such as visual, physical, mental, and psychological factors [167, 186, 187]. Importantly, hearing loss can also influence the results of behavioral CAP testing and hence these tests cannot be used for older adults with moderately-severe or profound hearing loss [187, 188]. Finally, as many of the behavioral CAP tests cannot be conducted in free field settings, it would not be possible to perform these tests on individuals with hearing aids or cochlear implants [167].
Objective auditory assessments
Objective methods of measuring central auditory function have been proposed to overcome the clinical limitations associated with behavioral CAP tests outlined above. Auditory electrophysiology provides an objective measure of auditory function with little to no participation required from the listener. It is the measure of auditory event-related potentials (AERP), which reflect the variations in electrical brain activity in response to a particular task/or stimulus [189]. Distinct peaks within the AERPs, presenting at different latencies, represent neural activity from various anatomical locations throughout the auditory pathway and associated brain structures [190]. Some AERPs have been shown to reflect cognitive functions such as auditory memory [191], attention [192, 193], working memory [191, 194], language comprehension [195], discrimination [196] as well as decision-making [197].
AERPs can be divided into early latency, middle latency, and late latency responses, described in more detail by Niedermeyer et al. [198]. Some studies indicate that auditory brainstem responses (ABR), which are early latency responses, are prolonged in participants with AD with normal hearing thresholds, which suggests brainstem and midbrain abnormalities in individuals with AD related cognitive impairment [199, 200]. However, other studies report no differences in ABR measures in people with AD [201, 202] or MCI [203] in comparison to healthy age-matched controls. Disease severity and the duration of the disease have been suggested to contribute to the conflicting results seen across ABR studies, which could limit the reliability of ABR measures to differentiate between healthy and cognitively impaired older adults, particularly at early stages [199].
A recent meta-analysis reported that higher processing of sensory information, as reflected by late latency AERPs N100 and P200, are impaired in those with AD when compared to healthy controls [204]. The N100 and P200 have been proposed to be produced by the primary and secondary auditory cortex, hence, changes in the appearance of these responses are thought to reflect measurable impairments in processing and perceiving variations in an auditory information [205, 206]. Similar to results observed in behavioral CAP tests, particularly SSI-ICM, objective measures of discrimination, perception, and classification of auditory signals using N200 latency have also been shown to be delayed in those with cognitive impairment associated with AD [196, 204, 207]. The neural generators of the N200 peak include the auditory cortex and thalamic region [208, 209] and significant delays in the N200 latency have been observed in participants with MCI and those with AD [204, 207].
The P300, another late latency cortical AERP, is the most widely studied AERP for cognitive function. Research has suggested that this response is altered with the development of neurodegenerative diseases, showing as reduced P300 amplitude and increased P300 latency in those with cognitive impairment associated with AD compared to healthy controls [204, 210, 211]. Multiple brain regions have been associated to the generation of the P300 response and these include the temporal parietal junction, medial temporal lobe, frontal lobe, and parietal cortex [212, 213]. These brain regions are well known to play a role in sensory processing, cognitive function, memory storage, and executive functions. Therefore, P300 is proposed to reflect cortical activity as it is dependent on functions including discrimination, attention, and memory in order to be elicited. A meta-analysis revealed that those with MCI and AD have significantly longer P300 latencies in comparison to healthy age-matched controls [204], indicating dysfunctional attention-driven discrimination processing and reduced classification speed in task processing in those with cognitive decline associated with AD [193, 194]. Although multiple meta-analyses indicate that P300 latency measures do not significantly differ between MCI and AD participants [204, 214], a longitudinal study found that MCI participants that developed probable AD after a 5-year period had significantly longer baseline P300 latency in comparison to MCI participants that did not go on to develop AD [215]. These findings imply that P300 latency measures could be beneficial in the objective detection of people at prodromal stages of AD. Unfortunately, to date, no study has examined AERP measures in those with SCD, which highlights the need for further research in order to evaluate the reliability and usefulness of these measures as an early biomarker of AD. Investigating AERP measures in those with SCD could aid in identifying people at higher risk of developing dementia, years prior the appearance of clinical features of AD [20].
CONCLUSION
Impaired auditory function may be a valuable marker of preclinical stages of cognitive impairment. Multiple potential mechanisms have been suggested for the association between impaired auditory function and cognitive impairment. All four mechanisms mentioned in this paper demonstrate the intricate relationship between auditory and cognitive function. We suggest that the fourth mechanism, function and structure interaction, is the more plausible theory for the association between ARHL and AD. The insight that altered neuronal activity due to ARHL, as suggested by the fourth mechanism, may play a causal role in AD can be beneficial for early detection and treatment strategies. There is evidence to suggest that early identification and treatment of auditory impairment could delay or prevent neurodegenerative changes due to altered sensory input and slow progression of structural changes at later stages of cognitive decline. While this review provides interesting insights into the relationship between ARHL and AD, it is important to note that considerably more research is needed for a cause effect to be established. Nevertheless, investigating the role of auditory markers for identifying cognitive related brain changes in individuals at high risk of developing AD would help improve understanding of the underlying mechanisms for the link between ARHL and AD hopefully enabling the development of effective interventions.
ACKNOWLEDGMENTS
The authors have no acknowledgements to report.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
- [1]. Patterson C (2018) World Alzheimer Report 2018. The State of the Art of Dementia Research: New Frontiers. Alzheimer’s Disease International, London.
- [2]. Nichols E, Steinmetz JD, Vollset SE, Abd-Allah F, Abdoli A, Abu-Gharbieh E, Alipour V, Almustanyir S, Amu H, Arabloo J, Ayano G, Ayuso-Mateos JL, Baune BT, Béjot Y, Bezabhe WMM, Bijani A, Burkart K, Burns RA, Chavan PP, Cherbuin N, Couto RAS, Dandona R, Dela Cruz-Góngora V, Dias da Silva D, Douiri A, Edvardsson D, Ekholuenetale M, Fares J, Farooque U, Filip I, Fillit H, Galluzzo L, Ghashghaee A, Ghith N, Gilani SA, Gupta R, Hall BJ, Hartono RK, Hasaballah AI, Hasan MT, Hay SI, Herteliu C, Iacoviello L, Iavicoli I, Ilesanmi OS, Ilic IM, Ilic MD, Irvani SSN, Iwagami M, Jabbarinejad R, Jha RP, Kalani R, Kandel A, Khan MAB, Kim MS, Kisa S, Koroshetz WJ, Koyanagi A, Kumar GA, Kumar M, Lak HM, Leonardi M, Li B, Lim SS, Logroscino G, Lucchetti G, Lutzky Saute R, Mondello S, Moni MAA, Nayak VC, Pasovic M, Patel UK, Peres MFP, Phillips MR, Pinheiro M, Pond CD, Potashman MH, Rawaf S, Rezaei N, Roshandel G, Sahraian MA, Sattin D, Sawhney M, Schiavolin S, Sha F, Shigematsu M, Shin JI, Skryabin VY, Soshnikov S, Timalsina B, Tran BX, Tsegaye GW, Valadan Tahbaz S, Valdez PR, Vlassov V, Vu LG, Wimo A, Zadey S, Zastrozhin MS, Murray CJL (2022) Estimation of the global prevalenceof dementia in 2019 and forecasted prevalence in 2050: An analysisfor the Global Burden of Disease Study 2019.. Lancet PublicHealth 7, e105–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbaek G, Teri L, Mukadam N (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Maharani A, Dawes P, Nazroo J, Tampubolon G (2018) Longitudinal relationship between hearing aid use and cognitive function in older Americans. J Am Geriatr Soc 66, 1130–1136. [DOI] [PubMed] [Google Scholar]
- [5]. Mosnier I, Bebear JP, Marx M, Fraysse B, Truy E, Lina-Granade G, Mondain M, Sterkers-Artières F, Bordure P, Robier A, Godey B, Meyer B, Frachet B, Poncet-Wallet C, Bouccara D, Sterkers O (2015) Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol Head Neck Surg 141, 442–450. [DOI] [PubMed] [Google Scholar]
- [6]. Sarant J, Harris D, Busby P, Maruff P, Schembri A, Dowell R, Briggs R (2019) The effect of cochlear implants on cognitive function in older adults: Initial baseline and 18-month follow up results for a prospective international longitudinal study. Front Neurosci 13, 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Alzheimer’s Association (2017) 2017 Alzheimer’s disease facts and figures. Alzheimers Dement 13, 325–373. [Google Scholar]
- [8]. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Jack Clifford R, Holtzman David M (2013) Biomarker modeling of Alzheimer’s disease. Neuron 80, 1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Villemagne VLD, Burnham SP, Bourgeat PP, Brown BP, Ellis KAP, Salvado OP, Szoeke CM, Macaulay SLP, Martins RP, Maruff PP, Ames DP, Rowe CCP, Masters CLP (2013) Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol 12, 357–367. [DOI] [PubMed] [Google Scholar]
- [11]. Magalingam KB, Radhakrishnan A, Ping NS, Haleagrahara N (2018) Current concepts of neurodegenerative mechanisms in Alzheimer’s disease. Biomed Res Int 2018, 3740461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Iqbal K, Gong C-X, Liu F (2014) Microtubule-associated protein tau as a therapeutic target in Alzheimer’s disease. Expert Opin Ther Targets 18, 307–318. [DOI] [PubMed] [Google Scholar]
- [13]. Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Anand A, Patience AA, Sharma N, Khurana N (2017) The present and future of pharmacotherapy of Alzheimer’s disease: A comprehensive review. Eur J Pharmacol 815, 364–375. [DOI] [PubMed] [Google Scholar]
- [15]. Shi J, Sabbagh MN, Vellas B (2020) Alzheimer’s disease beyond amyloid: Strategies for future therapeutic interventions. BMJ 371, m3684–m3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259. [DOI] [PubMed] [Google Scholar]
- [17]. Price JL, Morris JC (1999) Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 45, 358–368. [DOI] [PubMed] [Google Scholar]
- [18]. Yiannopoulou KG, Papageorgiou SG (2013) Current and future treatments for Alzheimer’s disease. Ther Adv Neurol Disord 6, 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Siedlecki-Wullich D, Miñano-Molina AJ, Rodríguez-Álvarez J (2021) microRNAs as early biomarkers ofAlzheimer’s disease: A synaptic perspective. Cells 10, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, de Leon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M (2014) A conceptualframework for research on subjective cognitive decline inpreclinical Alzheimer’s disease.. Alzheimers Dement 10, 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Jonker C, Geerlings MI, Schmand B (2000) Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry 15, 983–991. [DOI] [PubMed] [Google Scholar]
- [22]. Donovan NJ, Amariglio RE, Zoller AS, Rudel RK, Gomez-Isla T, Blacker D, Hyman BT, Locascio JJ, Johnson KA, Sperling RA, Marshall GA, Rentz DM (2014) Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry 22, 1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kölsch H, Luck T, Mösch E, van den Bussche H, Wagner M, Wollny A, Zimmermann T, Pentzek M, Riedel-Heller SG, Romberg HP, Weyerer S, Kaduszkiewicz H, Maier W, Bickel H (2010) Prediction of dementia by subjective memory impairment: Effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry 67, 414–422. [DOI] [PubMed] [Google Scholar]
- [24]. Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B (2014) Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr Scand 130, 439–451. [DOI] [PubMed] [Google Scholar]
- [25]. Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W (2010) Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement 6, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. van Oijen M, de Jong FJ, Hofman A, Koudstaal PJ, Breteler MMB (2007) Subjective memory complaints, education, and risk of Alzheimer’s disease. Alzheimers Dement 3, 92–97. [DOI] [PubMed] [Google Scholar]
- [27]. Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues J-F, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Gouw AA, Habert M-O, Holtzman DM, Kivipelto M, Lista S, Molinuevo J-L, O’Bryant SE, Rabinovici GD, Rowe C, Salloway S, Schneider LS, Sperling R, Teichmann M, Carrillo MC, Cummings J, Jack CR (2016) Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement 12, 292–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, Chertkow H, Cummings JL, de Leon M, Feldman H, Ganguli M, Hampel H, Scheltens P, Tierney MC, Whitehouse P, Winblad B (2006) Mild cognitive impairment. Lancet 367, 1262–1270. [DOI] [PubMed] [Google Scholar]
- [29]. Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, Boeve BF, Tangalos EG, Ivnik RJ, Rocca WA (2010) Prevalence of mild cognitive impairment is higher in men: The Mayo Clinic Study of Aging. Neurology 75, 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Bruscoli M, Lovestone S (2004) Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr 16, 129–140. [DOI] [PubMed] [Google Scholar]
- [31]. Luis CA, Loewenstein DA, Acevedo A, Barker WW, Duara R (2003) Mild cognitive impairment: Directions for future research. Neurology 61, 438–444. [DOI] [PubMed] [Google Scholar]
- [32]. Schmidtke K, Hermeneit S (2008) High rate of conversion to Alzheimer’s disease in a cohort of amnestic MCI patients. Int Psychogeriatr 20, 96–108. [DOI] [PubMed] [Google Scholar]
- [33]. Rozzini L, Chilovi BV, Conti M, Bertoletti E, Delrio I, Trabucchi M, Padovani A (2007) Conversion of amnestic mild cognitive impairment to dementia of Alzheimer type is independent to memory deterioration. Int J Geriatr Psychiatry 22, 1217–1222. [DOI] [PubMed] [Google Scholar]
- [34]. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitiveimpairment due to Alzheimer’s disease: Recommendations from theNational Institute on Aging-Alzheimer’s Association workgroups ondiagnostic guidelines for Alzheimer’s disease. AlzheimersDement 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Jack CR, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH (2011) Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Sperling RA, Jack CR Jr, Aisen PS (2011) Testing the right target and right drug at the right stage. Sci Transl Med 3, 111cm133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Vermunt L, Sikkes SAM, van den Hout A, Handels R, Bos I, van der Flier WM, Kern S, Ousset P-J, Maruff P, Skoog I, Verhey FRJ, Freund-Levi Y, Tsolaki M, Wallin ÅK, Olde Rikkert M, Soininen H, Spiru L, Zetterberg H, Blennow K, Scheltens P, Muniz-Terrera G, Visser PJ, Vellas B, Reynish E, Ousset PJ, Andrieu S, Burns A, Pasquier F, Frisoni G, Salmon E, Michel JP, Zekry DS, Boada M, Dartigues JF, Olde-Rikkert MGM, Rigaud AS, Winblad B, Malick A, Sinclair A, Frölich L, Scheltens P, Ribera C, Touchon J, Robert P, Salva A, Waldemar G, Bullock R, Tsolaki M, Rodriguez G, Spiru L, Jones RW, Stiens G, Stoppe G, Eriksdotter Jönhagen M, Cherubini A, Lage PM, Gomez-Isla T, Camus V, Agüera-Morales E, Lopez F, Savy S, Cantet C, Coley N (2019) Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement 15, 888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Xue T, Wei L, Zha D-J, Qiu J-H, Chen F-Q, Qiao L, Qiu Y (2016) MiR-29b overexpression induces cochlear hair cell apoptosis through the regulation of SIRT1/PGC-1 signaling: Implications for age-related hearing loss. Int J Mol Med 38, 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Wang R, Li Jing J, Diao S, Kwak Y-D, Liu L, Zhi L, Büeler H, Bhat Narayan R, Williams Robert W, Park Edwards A, Liao F-F (2013) Metabolic stress modulates Alzheimer’s β-secretase gene transcription via SIRT1-PPARγ-PGC-1 in neurons. Cell Metab 17, 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Martini A, Castiglione A, Bovo R, Vallesi A, Gabelli C (2015) Aging, cognitive load, dementia and hearing loss. Audiol Neurotol 19, 2–5. [DOI] [PubMed] [Google Scholar]
- [41]. Someya S, Prolla TA (2010) Mitochondrial oxidative damage and apoptosis in age-related hearing loss. Mech Ageing Dev 131, 480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. De Maria L, Caputi A, Sardone R, Cannone ESS, Mansi F, Birtolo F, Delfino MC, Cavone D, Vimercati L (2020) Occupational exposure to noise and age-related hearing loss in an elderly population of Southern Italy. Open Public Health J 13, 69–74. [Google Scholar]
- [43]. Fransen E, Topsakal V, Hendrickx J-J, Van Laer L, Huyghe JR, Van Eyken E, Lemkens N, Hannula S, Mäki-Torkko E, Jensen M, Demeester K, Tropitzsch A, Bonaconsa A, Mazzoli M, Espeso A, Verbruggen K, Huyghe J, Huygen PLM, Kunst S, Manninen M, Diaz-Lacava A, Steffens M, Wienker TF, Pyykkö I, Cremers CWRJ, Kremer H, Dhooge I, Stephens D, Orzan E, Pfister M, Bille M, Parving A, Sorri M, Van de Heyning P, Van Camp G (2008) Occupational noise, smoking, and a high body mass index are risk factors for age-related hearing impairment and moderate alcohol consumption is protective: A European population-based multicenter study. J Assoc Res Otolaryngol 9, 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Gopinath B, Rochtchina E, Wang JJ, Schneider J, Leeder SR, Mitchell P (2009) Prevalence of age-related hearing loss in older adults: Blue Mountains Study. Arch Intern Med 169, 415–418. [DOI] [PubMed] [Google Scholar]
- [45]. Lin FR, Maas P, Chien W, Carey JP, Ferrucci L, Thorpe R (2012) Association of skin color, race/ethnicity, and hearing loss among adults in the USA. J Assoc Res Otolaryngol 13, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Agrawal Y, Platz EA, Niparko JK (2008) Prevalence of hearing loss and differences by demographic characteristics among US adults: Data from the National Health and Nutrition Examination Survey, 1999-2004. Arch Intern Med 168, 1522–1530. [DOI] [PubMed] [Google Scholar]
- [47]. Newman DL, Fisher LM, Ohmen J, Parody R, Fong C-T, Frisina ST, Mapes F, Eddins DA, Robert Frisina D, Frisina RD, Friedman RA (2012) GRM7 variants associated with age-related hearing loss based on auditory perception. Hear Res 294, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Campo PP, Morata TCP, Hong OPF (2013) Chemical exposure and hearing loss. Dis Mon 59, 119–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Chang S-J, Chen C-J, Lien C-H, Sung F-C (2006) Hearing loss in workers exposed to toluene and noise. Environ Health Perspect 114, 1283–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Brant LJ, Gordon-Salant S, Pearson JD, Klein LL, Morrell CH, Metter EJ, Fozard JL (1996) Risk factors related to age-associated hearing loss in the speech frequencies. J Am Acad Audiol 7, 152–160. [PubMed] [Google Scholar]
- [51]. Mitchell P, Gopinath B, McMahon CM, Rochtchina E, Wang JJ, Boyages SC, Leeder SR (2009) Relationship of Type 2 diabetes to theprevalence, incidence and progression of age-related hearing loss. Diabet Med 26, 483–488. [DOI] [PubMed] [Google Scholar]
- [52]. Wu PZ, Liberman LD, Bennett K, de Gruttola V, O’Malley JT, Liberman MC (2019) Primary neural degeneration in the human cochlea: Evidence for hidden hearing loss in the aging ear. Neuroscience 407, 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Schuknecht HF, Gacek MR (1993) Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol 102, 1–16. [DOI] [PubMed] [Google Scholar]
- [54]. Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN (2011) Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol 12, 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Schuknecht HF, Woellner RC (1953) Hearing losses following partial section of the cochlear nerve. Laryngoscope 63, 441–465. [DOI] [PubMed] [Google Scholar]
- [56]. Pauler M, Schuknecht HF, White JA (1988) Atrophy of the stria vascularis as a cause of sensorineural hearing loss. Laryngoscope 98, 754–759. [DOI] [PubMed] [Google Scholar]
- [57]. Profant O, Balogová Z, Dezortová M, Wagnerová D, Hájek M, Syka J (2013) Metabolic changes in the auditory cortexin presbycusis demonstrated by MR spectroscopy.. Exp Gerontol 48, 795–800. [DOI] [PubMed] [Google Scholar]
- [58]. Gao F, Wang G, Ma W, Ren F, Li M, Dong Y, Liu C, Liu B, Bai X, Zhao B, Edden RA (2015) Decreased auditory GABA+concentrations in presbycusis demonstrated by edited magnetic resonance spectroscopy. Neuroimage 106, 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Syka J (2010) The Fischer 344 rat as a model of presbycusis. Hear Res 264, 70–78. [DOI] [PubMed] [Google Scholar]
- [60]. Caspary DM, Hughes LF, Ling LL (2013) Age-related GABAA receptor changes in rat auditory cortex. Neurobiol Aging 34, 1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Burianova J, Ouda L, Profant O, Syka J (2009) Age-related changes in GAD levels in the central auditory system of the rat. Exp Gerontol 44, 161–169. [DOI] [PubMed] [Google Scholar]
- [62]. Caspary DM, Milbrandt JC, Helfert RH (1995) Central auditory aging: GABA changes in the inferior colliculus. Exp Gerontol 30, 349–360. [DOI] [PubMed] [Google Scholar]
- [63]. Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C (2003) Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci 23, 3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A (1995) Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiol Aging 16, 591–606. [DOI] [PubMed] [Google Scholar]
- [65]. Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD (2005) Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex 15, 1676–1689. [DOI] [PubMed] [Google Scholar]
- [66]. Silver NC, Barker GJ, MacManus DG, Tofts PS, Miller DH (1997) Magnetisation transfer ratio of normal brain white matter: A normative database spanning four decades of life. J Neurol Neurosurg Psychiatry 62, 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Salat DH, Kaye JA, Janowsky JS (1999) Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol 56, 338–344. [DOI] [PubMed] [Google Scholar]
- [68]. Husain FT, Medina RE, Davis CW, Szymko-Bennett Y, Simonyan K, Pajor NM, Horwitz B (2011) Neuroanatomical changes due to hearing loss and chronic tinnitus: A combined VBM and DTI study. Brain Res 1369, 74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Eckert MA, Cute SL, Vaden KI Jr , Kuchinsky SE, Dubno JR (2012) Auditory cortex signs of age-related hearing loss. J Assoc Res Otolaryngol 13, 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Rigters S, Bos D, Metselaar M, Roshchupkin G, Baatenburg de Jong RJ, Ikram K, Vernooij M, Goedegebure A (2017) Hearing impairment is associated with smaller brain volume in aging. Front Aging Neurosci 9, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Boyen K, Langers DRM, de Kleine E, van Dijk P (2013) Gray matter in the brain:, Differences associated with tinnitus and hearing loss, Hear Res 295, 67–78. . [DOI] [PubMed] [Google Scholar]
- [72]. Chan D, Anderson V, Pijnenburg Y, Whitwell J, Barnes J, Scahill R, Stevens JM, Barkhof F, Scheltens P, Rossor MN, Fox NC (2009) The clinical profile of right temporal lobe atrophy. Brain 132, 1287–1298. [DOI] [PubMed] [Google Scholar]
- [73]. Nilakantan AS, Voss JL, Weintraub S, Mesulam MM, Rogalski EJ (2017) Selective verbal recognition memory impairments are associated with atrophy of the language network in non-semantic variants of primary progressive aphasia. Neuropsychologia 100, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74]. Barbey AK, Colom R, Solomon J, Krueger F, Forbes C, Grafman J (2012) An integrative architecture for general intelligence and executive function revealed by lesion mapping. Brain 135, 1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Makhani A, Akbaryan F, Cernak I (2015) Cognitive performance improvement in Canadian Armed Forces personnel during deployment. J Mil Veteran Fam Health 1, 59–67. [Google Scholar]
- [76]. Lin FR, Ferrucci L, An Y, Goh JO, Doshi J, Metter EJ, Davatzikos C, Kraut MA, Resnick SM (2014) Association of hearing impairment with brain volume changes in older adults. Neuroimage 90, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77]. Chang Y, Lee S-H, Lee Y-J, Hwang M-J, Bae S-J, Kim M-N, Lee J, Woo S, Lee H, Kang D-S (2004) Auditory neural pathway evaluation on sensorineural hearing loss using diffusion tensor imaging. Neuroreport 15, 1699–1703. [DOI] [PubMed] [Google Scholar]
- [78]. Kubicki M, Westin CF, Maier SE, Mamata H, Frumin M, Ersner-Hershfield H, Kikinis R, Jolesz FA, McCarley R, Shenton ME (2002) Diffusion tensor imaging and its application to neuropsychiatric disorders. Harv Rev Psychiatry 10, 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79]. Jagust W (2013) Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron 77, 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. Park DC, McDonough IM (2013) The dynamic aging mind: Revelations from functional neuroimaging research. Perspect Psychol Sci 8, 62–67. [DOI] [PubMed] [Google Scholar]
- [81]. Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L (2012) Memory aging and brain maintenance.. Trends Cogn Sci 16, 292–305. [DOI] [PubMed] [Google Scholar]
- [82]. Felício JS, de Souza d’Albuquerque Silva L, Martins CLeL P, Neto JFA, de Lemos MN, de Souza Resende F, da Silva WM, de Alcântara AL, de Oliveira MCNI, de Souza Neto NJK, de Franco IIF, Zahalan NA, Janaú LC, de Souza ACCB, Santos FM, de Queiroz NNM, Mourão NAL, dos Santos MC, Felício KM, de Melo FTC (2018) Cochleardysfunction and microvascular complications in patients with type 1diabetes mellitus. Diabetol Metab Syndr 10, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83]. Gupta S, Eavey RD, Wang M, Curhan SG, Curhan GC (2019) Type 2 diabetes and the risk of incident hearing loss. Diabetologia 62, 281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84]. Albai O, Frandes M, Timar R, Roman D, Timar B (2019) Risk factors for developing dementia in type 2 diabetes mellitus patients with mild cognitive impairment. Neuropsychiatr Dis Treat 15, 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85]. Newcombe EA, Camats-Perna J, Silva ML, Valmas N, Huat TJ, Medeiros R (2018) Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J Neuroinflammation 15, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86]. Uzoni A, Ciobanu O, Sandu RE, Buga AM, Popa-Wagner A (2015) Life style, perfusion deficits and co-morbidities precipitate inflammation and cerebrovascular disorders in aged subjects. Discoveries (Craiova) 3, e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87]. Ter Telgte A, van Leijsen EMC, Wiegertjes K, Klijn CJM, Tuladhar AM, de Leeuw FE (2018) Cerebral small vessel disease: From a focal to a global perspective. Nat Rev Neurol 14, 387–398. [DOI] [PubMed] [Google Scholar]
- [88]. Hakim AM (2021) A proposed hypothesis on dementia: Inflammation, small vessel disease, and hypoperfusion is the sequence that links all harmful lifestyles to cognitive impairment. Front Aging Neurosci 13, 679837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89]. Shi X, Qiu S, Zhuang W, Yuan N, Wang C, Zhang S, Sun T, Guo W, Gao F, Yang S, Qiao Y (2017) NLRP3-inflammasomes are triggered by age-related hearing loss in the inner ear of mice. Am J Transl Res 9, 5611–5618. [PMC free article] [PubMed] [Google Scholar]
- [90]. Riva C, Donadieu E, Magnan J, Lavieille JP (2007) Age-related hearing loss in CD/1 mice is associated to ROS formation and HIF target proteins up-regulation in the cochlea. Exp Gerontol 42, 327–336. [DOI] [PubMed] [Google Scholar]
- [91]. Giorgi C, Marchi S, Simoes ICM, Ren Z, Morciano G, Perrone M, Patalas-Krawczyk P, Borchard S, Jedrak P, Pierzynowska K, Szymański J, Wang DQ, Portincasa P, Wegrzyn G, Zischka H, Dobrzyn P, Bonora M, Duszynski J, Rimessi A, Karkucinska-Wieckowska A, Dobrzyn A, Szabadkai G, Zavan B, Oliveira PJ, Sardao VA, Pinton P, Wieckowski MR (2018) Mitochondria and reactive oxygen species inaging and age-related diseases.. Int Rev Cell Mol Biol 340, 209–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92]. Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13, 757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93]. Kaur A, Webster MR, Marchbank K, Behera R, Ndoye A, Kugel CH, Dang VM, Appleton J, O’Connell MP, Cheng P, Valiga AA, Morissette R, McDonnell NB, Ferrucci L, Kossenkov AV, Meeth K, Tang H-Y, Yin X, Wood WH, Lehrmann E, Becker KG, Flaherty KT, Frederick DT, Wargo JA, Cooper ZA, Tetzlaff MT, Hudgens C, Aird KM, Zhang R, Xu X, Liu Q, Bartlett E, Karakousis G, Eroglu Z, Lo RS, Chan M, Menzies AM, Long GV, Johnson DB, Sosman J, Schilling B, Schadendorf D, Speicher DW, Bosenberg M, Ribas A, Weeraratna AT (2016) SFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature 532, 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94]. Sohal RS, Ku H-H, Agarwal S, Forster MJ, Lal H (1994) Oxidativedamage, mitochondrial oxidant generation and antioxidant defensesduring aging and in response to food restriction in the mouse. Mech Ageing Dev 74, 121–133. [DOI] [PubMed] [Google Scholar]
- [95]. Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, Baudry M (2003) Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci U S A 100, 8526–8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96]. Provias J, Jeynes B (2014) Reduction in vascular endothelial growth factor expression in the superior temporal, hippocampal, and brainstem regions in Alzheimer’s disease. Curr Neurovasc Res 11, 202–209. [DOI] [PubMed] [Google Scholar]
- [97]. Melincovici CS, Bosca AB, Suşman S, Marginean M, Mihu C, Istrate M, Moldovan I-M, Roman AL, Mihu CM (2018) Vascular endothelial growth factor (VEGF)-key factor in normal and pathological angiogenesis. Rom J Morphol Embryol 59, 455–467. [PubMed] [Google Scholar]
- [98]. Shibuya M (2011) Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2, 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99]. Yang S-P, Bae D-G, Kang HJ, Gwag BJ, Gho YS, Chae C-B (2004) Co-accumulation of vascular endothelial growth factor with β-amyloid in the brain of patients with Alzheimer’s disease. Neurobiol Aging 25, 283–290. [DOI] [PubMed] [Google Scholar]
- [100]. Picciotti A, Torsello I, Wolf F, Paludetti G, Gaetani E, Pola R (2004) Age-dependent modifications of expression level of VEGF and its receptors in the inner ear. Exp Gerontol 39, 1253–1258. [DOI] [PubMed] [Google Scholar]
- [101]. Hawkins JE Jr, Johnsson LG, Preston RE (1972) Cochlear microvasculature in normal and damaged ears. Laryngoscope 82, 1091–1104. [DOI] [PubMed] [Google Scholar]
- [102]. Shi X (2009) Cochlear pericyte responses to acoustic trauma and the involvement of hypoxia-inducible factor-1alpha and vascular endothelial growth factor. Am J Pathol 174, 1692–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103]. Picciotti, Fetoni A, Paludetti G, Wolf F, Torsello A, Troiani D, Ferraresi A, Pola R, Sergi B (2006) Vascular endothelial growth factor (VEGF) expression in noise-induced hearing loss. Hear Res 214, 76–83. [DOI] [PubMed] [Google Scholar]
- [104]. Shen Y, Ye B, Chen P, Wang Q, Fan C, Shu Y, Xiang M (2018) Cognitive decline, dementia, Alzheimer’s disease and presbycusis: Examination of the possible molecular mechanism. Front Neurosci 12, 394–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105]. Pichora-Fuller MK, Schneider BA, Daneman M (1995) How young and old adults listen to and remember speech in noise. J Acoust Soc Am 97, 593–608. [DOI] [PubMed] [Google Scholar]
- [106]. Ryugo D, Ryugo D (2015) Auditory neuroplasticity, hearing loss and cochlear implants. Cell Tissue Res 361, 251–269. [DOI] [PubMed] [Google Scholar]
- [107]. Schneider BA, Pichora-Fuller MK (2000) Implications of perceptual deterioration for cognitive aging research. In The Handbook of Aging and Cognition, 2nd ed. Lawrence Erlbaum Associates Publishers,Mahwah, NJ, US, pp. 155- 219.
- [108]. Billings CJ, Tremblay KL, Stecker GC, Tolin WM (2009) Human evoked cortical activity to signal-to-noise ratio and absolute signal level. Hear Res 254, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109]. Armstrong NM, An Y, Doshi J, Erus G, Ferrucci L, Davatzikos C, Deal JA, Lin FR, Resnick SM (2019) Association of midlife hearing impairment with late-life temporal lobe volume loss. JAMA Otolaryngol Head Neck Surg 145, 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110]. Rosemann S, Thiel CM (2020) Neuroanatomical changes associated with age-related hearing loss and listening effort. Brain Struct Function 225, 2689–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111]. Rudner M, Seeto M, Keidser G, Johnson B, Rönnberga J (2019) Poorer speech reception threshold in noise is associated with lower brain volume in auditory and cognitive processing regions. J Speech Lang Hear Res 62, 1117–1130. [DOI] [PubMed] [Google Scholar]
- [112]. Tun PA, Williams VA, Small BJ, Hafter ER (2012) The effects of aging on auditory processing and cognition. Am J Audiol 21, 344–350. [DOI] [PubMed] [Google Scholar]
- [113]. Shukla A, Harper M, Pedersen E, Goman A, Suen JJ, Price C, Applebaum J, Hoyer M, Lin FR, Reed NS (2020) Hearing loss, loneliness, and social isolation: A systematic review. Otolaryngol Head Neck Surg 162, 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114]. Strawbridge WJ, Wallhagen MI, Shema SJ, Kaplan GA (2000) Negative consequences of hearing impairment in old age: A longitudinal analysis. Gerontologist 40, 320–326. [DOI] [PubMed] [Google Scholar]
- [115]. Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS (2006) The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. Lancet Neurol 5, 406–412. [DOI] [PubMed] [Google Scholar]
- [116]. Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA (2004) Social resources and cognitive decline in a population of older African Americans and whites. Neurology 63, 2322–2326. [DOI] [PubMed] [Google Scholar]
- [117]. Kuiper JS, Zuidersma M, Oude Voshaar RC, Zuidema SU, van den Heuvel ER, Stolk RP, Smidt N (2015) Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev 22, 39–57. [DOI] [PubMed] [Google Scholar]
- [118]. Croll PH, Vernooij MW, Reid RI, Goedegebure A, Power MC, Rigters SC, Sharrett AR, Jong RJB, Mosley TH, Groot M, Lin FR, Deal JA (2020) Hearing loss and microstructural integrity of the brain in a dementia-free older population. Alzheimers Dement 16, 1515–1523. [DOI] [PubMed] [Google Scholar]
- [119]. Alfandari D, Vriend C, Heslenfeld DJ, Versfeld NJ, Kramer SE, Zekveld AA (2018) Brain volume differences associated with hearing impairment in adults. Trends Hear 22, 2331216518763689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120]. Glick H, Sharma A (2017) Cross-modal plasticity in developmental and age-related hearing loss: Clinical implications. Hear Res 343, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121]. Campbell J, Sharma A (2014) Cross-modal re-organization in adults with early stage hearing loss. PLoS One 9, e90594–e90594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122]. Griffiths TD, Lad M, Kumar S, Holmes E, McMurray B, Maguire EA, Billig AJ, Sedley W (2020) How can hearing loss cause dementia? . Neuron 108, 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123]. Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM (2011) Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology 25, 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124]. Tun PA, McCoy S, Wingfield A (2009) Aging, Hearing acuity, and the attentional costs of effortful listening. Psychol Aging 24, 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125]. Holtzer R, Rakitin BC, Steffener J, Flynn J, Kumar A, Stern Y (2008) Age effects on load-dependent brain activations in working memory for novel material. Brain Res 1249, 148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126]. Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y (2006) Age-related changes in brain activation during a delayed item recognition task. Neurobiol Aging 28, 784–798. [DOI] [PubMed] [Google Scholar]
- [127]. Peelle JE, Troiani V, Grossman M, Wingfield A (2011) Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci 31, 12638–12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128]. Gagné J-P, Besser J, Lemke U (2017) Behavioral assessment oflistening effort using a dual-task paradigm: A review. TrendsHear 21, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129]. Alain C, Du Y, Bernstein LJ, Barten T, Banai K (2018) Listening under difficult conditions: An activation likelihood estimation meta-analysis. Hum Brain Mapp 39, 2695–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130]. Bishop CW, Miller LM (2009) A multisensory cortical network for understanding speech in noise. J Cogn Neurosci 21, 1790–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131]. Davis MH, Ford MA, Kherif F, Johnsrude IS (2011) Does semantic context benefit speech understanding through “top–down” processes? Evidence from time-resolved sparse fMRI. J Cogn Neurosci 23, 3914–3932. [DOI] [PubMed] [Google Scholar]
- [132]. Eckert MA, Teubner-Rhodes S, Vaden KI (2016) Is listening in noise worth it? The neurobiology of speech recognition in challenging listening conditions. Ear Hear 37(Suppl 1), 101S–110S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133]. Fulton SE, Lister JJ, Bush ALH, Edwards JD, Andel R (2015) Mechanisms of the hearing–cognition relationship. Semin Hear 36, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134]. Eichenbaum H, Yonelinas AP, Ranganath C (2007) The medial temporal lobe and recognition memory. Annu Rev Neurosci 30, 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135]. Raslau FD, Mark IT, Klein AP, Ulmer JL, Mathews V, Mark LP (2015) Memory part 2: The role of the medial temporal lobe. Am J Neuroradiol 36, 846–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136]. Amaral DG (1999) Introduction: What is where in the medial temporal lobe? . Hippocampus 9, 1–6. [DOI] [PubMed] [Google Scholar]
- [137]. Ding SL, Van Hoesen GW (2010) Borders, extent, and topography of human perirhinal cortex as revealed using multiple modern neuroanatomical and pathological markers. Hum Brain Mapp 31, 1359–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138]. van Strien NM, Cappaert NLM, Witter MP (2009) The anatomy of memory: An interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci 10, 272–282. [DOI] [PubMed] [Google Scholar]
- [139]. Jack CR, Wiste HJ, Botha H, Weigand SD, Therneau TM, Knopman DS, Graff-Radford J, Jones DT, Ferman TJ, Boeve BF, Kantarci K, Lowe VJ, Vemuri P, Mielke MM, Fields JA, Machulda MM, Schwarz CG, Senjem ML, Gunter JL, Petersen RC (2019) The bivariate distribution of amyloid-β and tau: Relationship with established neurocognitive clinical syndromes. Brain 142, 3230–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140]. Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, Small SA (2014) Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat Neurosci 17, 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141]. Schöll M, Lockhart Samuel N, Schonhaut Daniel R, O’Neil James P, Janabi M, Ossenkoppele R, Baker Suzanne L, Vogel Jacob W, Faria J, Schwimmer Henry D, Rabinovici Gil D, Jagust William J (2016) PET imaging of tau deposition in the aging human brain. Neuron 89, 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142]. Ahmed MS, Priestley JB, Castro A, Stefanini F, Solis Canales AS, Balough EM, Lavoie E, Mazzucato L, Fusi S, Losonczy A (2020) Hippocampal network reorganization underlies the formation of a temporal association memory. . Neuron 107, 283–291.e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143]. Barascud N, Pearce MT, Griffiths TD, Friston KJ, Chait M (2016) Brain responses in humans reveal ideal observer-like sensitivity to complex acoustic patterns.. Proc Natl Acad Sci U S A 113, E616–E625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144]. Covington NV, Brown-Schmidt S, Duff MC (2018) The necessity of the hippocampus for statistical learning. J Cogn Neurosci 30, 680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145]. Kumar S, Gander PE, Berger JI, Billig AJ, Nourski KV, Oya H, Kawasaki H, Howard MA, Griffiths TD (2021) Oscillatory correlates of auditory working memory examined with human electrocorticography. Neuropsychologia 150, 107691–107691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146]. Lad M, Holmes E, Chu A, Griffiths TD (2020) Speech-in-noise detection is related to auditory working memory precision for frequency. Sci Rep 10, 13997–13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147]. Beckmann D, Feldmann M, Shchyglo O, Manahan-Vaughan D (2020) Hippocampal synaptic plasticity, spatial memory, and neurotransmitter receptor expression are profoundly altered by gradual loss of hearing ability. Cerebr Cortex 30, 4581–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148]. Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R (2006) AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron 52, 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149]. LaFerla FM, Oddo S (2005) Alzheimer’s disease: Aβ, tau and synaptic dysfunction. Trends Mol Med 11, 170–176. [DOI] [PubMed] [Google Scholar]
- [150]. Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL (2007) Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci 27, 2866–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151]. Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y (2009) GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell 5, 634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152]. de Haan W, Mott K, van Straaten ECW, Scheltens P, Stam CJ (2012) Activity dependent degeneration explains hub vulnerability in Alzheimer’s disease. PLoS Comput Biol 8, e1002582–e1002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153]. Kocagoncu E, Quinn A, Firouzian A, Cooper E, Greve A, Gunn R, Green G, Woolrich MW, Henson RN, Lovestone S, Rowe JB (2020) Tau pathology in early Alzheimer’s disease is linked to selective disruptions in neurophysiological network dynamics. Neurobiol Aging 92, 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154]. Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee J-M, Holtzman DM (2011) Neuronal activity regulates the regional vulnerability to amyloid-[beta] deposition. Nat Neurosci 14, 750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155]. Maharani A, Pendleton N, Leroi I (2019) Hearing impairment, loneliness, social isolation, and cognitive function: Longitudinal analysis using English Longitudinal Study on Ageing. Am J Geriatr Psychiatry 27, 1348–1356. [DOI] [PubMed] [Google Scholar]
- [156]. Rutherford BR, Brewster K, Golub JS, Kim AH, Roose SP (2018) Sensation and psychiatry: Linking age-related hearing loss to late-life depression and cognitive decline. Am J Psychiatry 175, 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157]. Jayakody DMP, Friedland PL, Nel E, Martins RN, Atlas MD, Sohrabi HR (2017) Impact of cochlear implantation on cognitive functions of older adults: Pilot test results.. Otol Neurotol 38, e289–e295. [DOI] [PubMed] [Google Scholar]
- [158]. Jayakody DMP, Almeida OP, Ford AH, Atlas MD, Lautenschlager NT, Friedland PL, Robinson S, Makate M, Coetzee L, Liew ASP, Flicker L (2020) Hearing aids to support cognitive functions of older adults at risk of dementia: The Hear Cog trial- clinical protocols. BMC Geriatr 20, 508–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159]. Bucholc M, McClean PL, Bauermeister S, Todd S, Ding X, Ye Q, Wang D, Huang W, Maguire LP (2021) Association of the use of hearing aids with the conversion from mild cognitive impairment to dementia and progression of dementia: A longitudinal retrospective study. Alzheimers Dement (N Y) 7, e12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160]. Allen NH, Burns A, Newton V, Hickson F, Ramsden R, Rogers J, Butler S, Thistlewaite G, Morris J (2003) The effects of improving hearing in dementia. Age Ageing 32, 189–193. [DOI] [PubMed] [Google Scholar]
- [161]. Nguyen M-F, Bonnefoy M, Adrait A, Gueugnon M, Petitot C, Collet L, Roux A, Perrot X (2017) Efficacy of hearing AIDS on the cognitive status of patients with Alzheimer’s disease and hearing loss: A multicenter controlled randomized trial. J Alzheimers Dis 58, 123–137. [DOI] [PubMed] [Google Scholar]
- [162]. van Hooren SAH, Anteunis LJC, Valentijn SAM, Bosma H, Ponds RWHM, Jolles J, van Boxtel MPJ (2005) Does cognitive function in older adults with hearing impairment improve by hearing aid use? . Int J Audiol 44, 265–271. [DOI] [PubMed] [Google Scholar]
- [163]. Cuoco S, Cappiello A, Scarpa A, Troisi D, Autuori M, Ponticorvo S, Cassandro C, Manara R, Esposito F, Santangelo G, Barone P, Cassandro E, Pellecchia MT (2021) Neuropsychological profile of hearing-impaired patients and the effect of hearing aid on cognitive functions: An exploratory study. Sci Rep 11, 9384–9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164]. Contrera KJ, Betz J, Li L, Blake CR, Sung YK, Choi JS, Lin FR (2016) Quality of life after intervention with a cochlear implant or hearing aid. Laryngoscope 126, 2110–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165]. Ciorba A, Bianchini C, Pelucchi S, Pastore A (2012) The impact of hearing loss on the quality of life of elderly adults. Clin Interv Aging 7, 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166]. Said EA (2017) Health-related quality of life in elderly hearing aid users vs. non-users. Egyptian J Ear Nose Throat Allied Sci 18, 271–279. [Google Scholar]
- [167]. AAA, American academy of audiology clinical practice guidelines: Diagnosis, treatment and management of children and adults with central auditory processing disorder., https://audiology-web.s3.amazonaws.com/migrated/CAPD%20Guidelines%208-2010.pdf_539952af956c79.73897613.pdf Last updated August 24 2010, Accessed May 1, 2022.
- [168]. Edwards JD, Lister JJ, Elias MN, Tetlow AM, Sardina AL, Sadeq NA, Brandino AD, Bush ALH (2017) Auditory processing of older adults with probable mild cognitive impairment. J Speech Lang Hear Res 60, 1427–1435. [DOI] [PubMed] [Google Scholar]
- [169]. Rahman TTA, Mohamed ST, Albanouby MH, Bekhet HF (2011) Central auditory processing in elderly with mild cognitive impairment. Geriatr Gerontol Int 11, 304–308. [DOI] [PubMed] [Google Scholar]
- [170]. Ghannoum MT, Shalaby AA, Farghaly M, Hamdy M, Hamdy HS (2018) Central auditory processing findings in a group of cognitively impaired individuals. Hear Balance Commun 16, 145–154. [Google Scholar]
- [171]. Hellström A, Almkvist O (1997) Tone duration discrimination in demented, memory-impaired, and healthy elderly. Dement Geriatr Cogn Disord 8, 49–54. [DOI] [PubMed] [Google Scholar]
- [172]. Lee SJ, Park KW, Kim L-S, Kim H (2018) Association between frontal-executive dysfunction and speech-in-noise perception deficits in mild cognitive impairment. J Clin Neurol 14, 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173]. Gates GA, Anderson ML, Feeney MP, McCurry SM, Larson EB (2008) Central auditory dysfunction in older persons with memory impairment or Alzheimer dementia. Arch Otolaryngol Head Neck Surg 134, 771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174]. Panza F, Quaranta N, Logroscino G (2018) Sensory changes and the hearing loss-cognition link: The cognitive ear. JAMA Otolaryngol Head Neck Surg 144, 127–128. [DOI] [PubMed] [Google Scholar]
- [175]. Yuan J, Sun Y (2018) The risk of cognitive impairment associated with hearing function in older adults: A pooled analysis of data from eleven studies. Sci Rep 8, 2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176]. Gootjes L, Bouma A, Van Strien JW, Scheltens P, Stam CJ (2006) Attention modulates hemispheric differences in functional connectivity: Evidence from MEG recordings. Neuroimage 30, 245–253. [DOI] [PubMed] [Google Scholar]
- [177]. Thomsen T, Specht K, Rimol LM, Hammar A, Nyttingnes J, Ersland L, Hugdahl K (2004) Brain localization of attentional control in different age groups by combining functional and structural MRI. Neuroimage 22, 912–919. [DOI] [PubMed] [Google Scholar]
- [178]. Strouse AL, Hall JW 3rd, Burger MC (1995) Central auditory processing in Alzheimer’s disease. Ear Hear 16, 230–238. [DOI] [PubMed] [Google Scholar]
- [179]. Bouma A, Gootjes L (2011) Effects of attention on dichotic listening in elderly and patients with dementia of the Alzheimer type. Brain Cogn 76, 286–293. [DOI] [PubMed] [Google Scholar]
- [180]. Ranganath C, Johnson MK, D’Esposito M (2003) Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia 41, 378–389. [DOI] [PubMed] [Google Scholar]
- [181]. Fausto BA, Badana ANS, Arnold ML, Lister JJ, Edwards JD (2018) Comparison of subjective and objective measures of hearing, auditory processing, and cognition among older adults with and without mild cognitive impairment. J Speech Lang Hear Res 61, 945–956. [DOI] [PubMed] [Google Scholar]
- [182]. Humes LE, Dubno JR (2010) Factors affecting speech understanding in older adults. In The Aging Auditory System. Springer Handbook of Auditory Research, Gordon-Salant S, Frisina R, Popper A, Fay R, eds. Springer, New York, NY.
- [183]. Jalaei B, Valadbeigi A, Panahi R, Nahrani MH, Arefi HN, Zia M, Ranjbar N (2019) Central auditory processing tests as diagnostic tools for the early identification of elderly individuals with mild cognitive impairment. J Audiol Otol 23, 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184]. Brun A, Englund E (2002) Regional pattern of degeneration in Alzheimer’s disease: Neuronal loss and histopathological grading. A. Brun & E. Englund. Histopathology 1981; 5; 459-564. Histopathology 41, 37–37. [PubMed] [Google Scholar]
- [185]. Jayakody DMP, Menegola HK, Yiannos JM, Goodman-Simpson J, Friedland PL, Taddei K, Laws SM, Weinborn M, Martins RN, Sohrabi HR (2020) The peripheral hearing and central auditory processing skills of individuals with subjective memory complaints. Front Neurosci 14, 888–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186]. Obuchi C, Ogane S, Sato Y, Kaga K (2017) Auditory symptoms and psychological characteristics in adults with auditory processing disorders. J Otol 12, 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187]. Atcherson SR, Nagaraj NK, Kennett SE, Levisee M (2015) Overview of central auditory processing deficits in older adults. Semin Hear 36, 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188]. Humes LE, Dubno JR, Gordon-Salant S, Lister JJ, Cacace AT, Cruickshanks KJ, Gates GA, Wilson RH, Wingfield A (2012) Central presbycusis: A review and evaluation of the evidence. J Am Acad Audiol 23, 635–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189]. Emre Cek M, Ozgoren M, Acar Savaci F (2009) Continuous time wavelet entropy of auditory evoked potentials. Comput Biol Med 40, 90–96. [DOI] [PubMed] [Google Scholar]
- [190]. Sharma A (2008) Basic Auditory evoked potentials: principles and clinical application. Ear Hear 29, 476–477. [Google Scholar]
- [191]. Näätänen R, Kujala T, Escera C, Baldeweg T, Kreegipuu K, Carlson S, Ponton C (2012) The mismatch negativity (MMN)–a unique window to disturbed central auditory processing in ageing and different clinical conditions. Clin Neurophysiol 123, 424–458. [DOI] [PubMed] [Google Scholar]
- [192]. Näätänen R, Paavilainen P, Rinne T, Alho K (2007) The mismatch negativity (MMN) in research of central auditory processing: A basic review. Clin Neurophysiol 118, 2544–2590. [DOI] [PubMed] [Google Scholar]
- [193]. Verleger R, Jaśkowski P, Wascher E (2005) Evidence for anintegrative role of P3b in linking reaction to perception. JPsychophysiol 19, 165–181. [Google Scholar]
- [194]. Chen L, Zhou Y, Liu L, Zhang X, Zhang H, Liu S (2015) Cortical event-related potentials in Alzheimer’s disease and frontotemporal lobar degeneration. J Neurol Sci 359, 88–93. [DOI] [PubMed] [Google Scholar]
- [195]. Brouwer H, Crocker MW (2017) On the proper treatment of the N400 and P600 in language comprehension. Front Psychol 8, 1327–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196]. Duarte JL, Alvarenga Kde F, Banhara MR, Melo AD, Sás RM, CostaFilho OA (2009) P300-long-latency auditory evoked potential innormal hearing subjects: Simultaneous recording value in Fz and Cz. Braz J Otorhinolaryngol 75, 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197]. Bares M, Rektor I, Kanovský P, Streitová H (2003) Cortical and subcortical distribution of middle and long latencyauditory and visual evoked potentials in a cognitive (CNV) paradigm. Clin Neurophysiol 114, 2447–2460. [DOI] [PubMed] [Google Scholar]
- [198]. Niedermeyer E, Schomer DL, Lopes da Silva FH (2011) Niedermeyer’s electroencephalography basic principles, clinical applications, and related fields, Wolters Kluwer/Lippincott Williams & Wilkins Health, Philadelphia.
- [199]. Tachibana H, Takeda M, Okuda B, Kawabata K, Nishimura H, Kodama N, Iwamoto Y, Sugita M (1996) Multimodal evoked potentials in Alzheimer’s disease and Binswanger’s disease. J Geriatr Psychiatry Neurol 9, 7–12. [DOI] [PubMed] [Google Scholar]
- [200]. O’Mahony D, Rowan M, Feely J, Walsh JB, Coakley D (1994) Primary auditory pathway and reticular activating system dysfunction in Alzheimer’s disease. Neurology 44, 2089–2094. [DOI] [PubMed] [Google Scholar]
- [201]. Masanaka T, Hisao T, Keita K, Yasunobu K, Hiroo Y, Bungo O (2005) Multi-modal evoked potentials in corticobasal degeneration, progressive supranuclear palsy and Alzheimer disease. Int Congress Ser 1278, 145–148. [Google Scholar]
- [202]. Kuskowski MA, Morley GK, Malone SM, Okaya AJ (1991) Longitudinal measurements of brainstem auditory evoked potentials in patients with dementia of the Alzheimer type. Int J Neurosci 60, 79–84. [DOI] [PubMed] [Google Scholar]
- [203]. Irimajiri R, Golob EJ, Starr A (2005) Auditory brain-stem, middle- and long-latency evoked potentials in mild cognitive impairment. Clin Neurophysiol 116, 1918–1929. [DOI] [PubMed] [Google Scholar]
- [204]. Tarawneh HY, Mulders WHAM, Sohrabi HR, Martins RN, Jayakody DMP (2021) Investigating auditory electrophysiological measures of participants with mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis of event-related potential studies. J Alzheimers Dis 84, 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205]. Tremblay K, Burkard R (2012) Translational perspectives in auditory neuroscience. Hearing across the life span: Assessment and disorders, Plural Publishing, San Diego, California.
- [206]. Ross B, Tremblay K (2009) Stimulus experience modifies auditory neuromagnetic responses in young and older listeners. Hear Res 248, 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207]. Howe AS (2014) Meta-analysis of the endogenous N200 latency event-related potential subcomponent in patients with Alzheimer’s disease and mild cognitive impairment. Clin Neurophysiol 125, 1145–1151. [DOI] [PubMed] [Google Scholar]
- [208]. Mahajan Y, McArthur G (2012) Maturation of auditory event-related potentials across adolescence. Hear Res 294, 82–94. [DOI] [PubMed] [Google Scholar]
- [209]. Ponton C, Eggermont JJ, Khosla D, Kwong B, Don M (2002) Maturation of human central auditory system activity: Separating auditory evoked potentials by dipole source modeling. Clin Neurophysiol 113, 407–420. [DOI] [PubMed] [Google Scholar]
- [210]. Papadaniil CD, Kosmidou VE, Tsolaki A, Tsolaki M, Kompatsiaris I, Hadjileontiadis LJ (2016) Cognitive MMN and P300 in mild cognitive impairment and Alzheimer’s disease: A high density EEG-3D vector field tomography approach. Brain Res 1648, 425–433. [DOI] [PubMed] [Google Scholar]
- [211]. Tsolaki AC, Kosmidou V, Kompatsiaris I, Papadaniil C, Hadjileontiadis L, Adam A, Tsolaki M (2017) Brain source localization of MMN and P300 ERPs in mild cognitive impairment and Alzheimer’s disease: A high-density EEG approach. Neurobiol Aging 55, 190–201. [DOI] [PubMed] [Google Scholar]
- [212]. Wronka E, Kaiser J, Coenen AM (2012) Neural generators of the auditory evoked potential components P3a and P3b. Acta Neurobiol Exp (Wars) 72, 51–64. [DOI] [PubMed] [Google Scholar]
- [213]. Bledowski C, Prvulovic D, Hoechstetter K, Scherg M, Wibral M, Goebel R, Linden DEJ (2004) Localizing P300 generators in visual target and distractor processing: A combined event-related potential and functional magnetic resonance imaging study. J Neurosci 24, 9353–9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214]. Howe AS, Bani-Fatemi A, De Luca V (2014) The clinical utility of the auditory P300 latency subcomponent event-related potential in preclinical diagnosis of patients with mild cognitive impairment and Alzheimer’s disease. Brain Cogn 86, 64–74. [DOI] [PubMed] [Google Scholar]
- [215]. Golob EJ, Irimajiri R, Starr A (2007) Auditory cortical activity in amnestic mild cognitive impairment: Relationship to subtype and conversion to dementia. Brain 130, 740–752. [DOI] [PubMed] [Google Scholar]