Abstract
Green nanotechnology facilitates the blooming of zinc oxide (ZnO) and silver (Ag) nanoparticles (NPs) with distinct flowerlike and spherical morphologies, respectively. The well-characterized NPs with an average size of 35 nm (ZnO) and 25 nm (Ag) were functionalized on the cresty plates for antibacterial inhibition against Staphylococcus aureus and Pseudomonas aeruginosa, with the flowerlike ZnONPs exhibiting 90.9% inhibition and AgNPs exhibiting 100% inhibition. Further, the in vivo underwater troughs for hematological, immunological, and serological analysis in Labeo rohita exhibited 102 > 575 > 104 and 206 > 109 > 81% at concentrations of 1, 2, and 3 mg/L with 4-day and 15-day treatment, respectively, over ZnONPs. However, AgNPs exhibited 257 > 408 > 124 and 86 > 202 > 43% with 4-day and 15-day treatment, respectively, at the same concentrations. The classical ZnNPs and AgNPs exhibited excellent inhibition potential and significant transfiguration of hematological, enzymological, and protein parameters as safe nanomedicine, but ZnONPs were found to be 58, 69, 29 and 34, 51, 70% more active than AgNPs with 4-day and 15-day treatment, respectively. Therefore, the onset of ROX and antioxidant arena favors beneficial cellular drifting of NPs.
1. Introduction
Nanotechnological advancements have approached a more ecofriendly and biocompatible green method for the formulation of NPs by collecting phytochemicals and secondary metabolites as reducing and capping agents.1,2 This green arena has replaced hazardous chemicals and considered natural chemical agents with higher intrinsic potentials that govern higher efficacies.3,4 The more efficient and biocompatible green-synthesized NPs have shown ground-breaking medical studies against Gram-positive and Gram-negative bacteria,5,6 anticancer and antitumor activities,7,8 and other screenings like infection, inflammation, growth, and catalysis due to the targeted penetration through the intact physiological barriers.9,10 Correspondingly, Withania coagulans plays an excellent role in the synthesis and surface enhancement of NPs with a distinct morphology.11,12 The biointrinsic potential of the diverse biological molecules enhances the functionality of synthesized particles.13 Moreover, ZnNPs and AgNPs are considered as promising candidates for nanomedicine as these NPs constitute a major proportion in human medicine, aquaculture, and agriculture,14,15 where they serve as antimicrobial, anti-inflammatory, anticancer, and wound-healing agents as well as growth enhancers in aquaculture and agricultural potential.16 But the major concerns to the economical world is the microbial resistance, growth, and productivity. Therefore, to overcome such crucial factors, emulsion-based nanoparticles are designed that could prevent diseases, prevent contamination, and enhance water purification and nutritional values using NPs.17 Primarily, the current landscape of microbial resistance of various strains is being controlled by ZnNPs categorized as “Generally Recognistanceized as Safe” (GRAS) and AgNPs successfully,18 where the NP-based ROX production amplifies the amplitude crest of oxidative stress, leading to bacterial apoptosis.19 In a similar way, the aquaculture trough of ZnONP- and AgNP-assisted nanomedicines and feeds have promoted growth and metabolism by means of feed utilization, antioxidation potential, and nanocatalysis.20 Further, deep insight screening has blueprinted closely related catalytic pathways mediated by ZnO and AgNPs that connect hematological, enzymological, and protein alterations when used as nanomedicine,21,22 where the nanoparticles are used as feed additives, nanomedicine, sensors for mediating growth, and toxicity evaluation.23,24 The Rohu (Labeo rohita) is one of the Indian major carps fish commonly found and widely consumed in South Asia, India, Pakistan, Bangladesh, Myanmar, and Nepal. It is a major source of protein and major nutrients like vitamin B12, vitamin D, iodine, and selenium. But aquaculture production of L. rohita is greatly threatened by aquatic pollution. Thereby, proficient green nanotools with inhibitory and antioxidant catalysis provide significant outcomes for cellular modulations.25,26
In this communication, we synthesized ZnONPs and AgNPs using W. coagulans by one-pot synthesis. This plant is a member of the family Solanaceae and is a traditional plant found in the east of the Mediterranean region expanding to South Asia, commonly being used for the synthesis of NPs. It is known for its ethnopharmacological application, with diverse biological activities, such as antioxidant, antidiabetic, anti-inflammatory, antihyperglycemic,27,28 hemopoietin, antitumor, anticancer, antimicrobial, immunosuppressive, hepatoprotective, and antidepressant activities.29 In addition, W. coagulans serves as a reducing agent for nanoparticle synthesis. The well-characterized flower-shaped ZnONPs and spherical AgNPs with a size of 35 and 25 nm, respectively, were used against Staphylococcus aureus and Pseudomonas aeruginosa for in vitro analysis at different concentrations, exhibiting 90.9 and 100% inhibition with ZnONPs and AgNPs with respect to the control. The in vivo experiment was conducted on L. rohita, where an adequate amount and optimized (1, 2, 3 mg/L) concentration were added to investigate on the hematological, enzymological, and protein parameters of L. rohita after 4 days and 15 days of exposure. On average, ZnONPs were found to be 58, 69, 29% on day 4 and 34, 51, and 70% on day 15, being more active than AgNPs. The significant ROX and antioxidant concentrations highlighted the mechanism of NPs for in vitro and in vivo succession.
2. Materials and Method
2.1. Instruments and Glassware
The following equipment and instruments were used during the experimental research:
Digital electronic balance (Denver Instrument), pH meter (Hanna Instruments), micropipette (1000 μL, Biohit.), 96-well plate, vortex mixer (VELP Scientifica), laminar flow cabinet (Streamline, 5127 chemicals, oxoid), refrigerator (Sanyo Co., Ltd., Japan), weighing balance, thermometer, incubator (VELP Scientifica), spectrophotometer (Epoch, Biotek), falcon tubes (Biologix), reagent bottles, shaker (Heidolph, Unimax 1010), measuring cylinder, autoclave (Hirayama, model HVA-110, Japan), hot plate stirrer, conical flask (Pyrex Iwaki, Asahi Glass), beakers (500, 250, 100 mL Pyrex Boro 3.3), pestle and mortar, funnel, Petri plates, spatula, syringes, yellow cap vacutainer, purple cap vacutainer, and digital analyzer. The materials and chemicals used are provided in detail in the Supporting Information (Table S1).
2.2. Preparation of Extract
Withania coagulans seeds were bought from the local market. W coagulans seeds were first washed with tap water and then finally washed with double-distilled water. Then, the seeds were dried until all of the moisture was lost and then ground with a mortar and pestle into coarse powder. Ten grams of plant powder was added to 200 mL of distilled water. This was boiled on a hot plate stirrer for 45 min and then cooled and filtered with Whatman filter paper; then, the filtered plant extract was stored at 4 °C in a refrigerator for the next process for the formation of NPs (Schematic Figure S1).
2.3. Synthesis of Zinc Oxide and Sliver NPs
Zinc oxide and silver NPs were synthesized using a biological method. The detailed procedure is provided in the Supporting Information (S1 and S2).
2.4. Ultraviolet–Vis Spectrometry
Withania coagulans plant extract as an oxidizing and reducing agent was confirmed by the synthesis of ZnONPs and AgNPs. UV–visible spectrometry was performed using a microplate spectrophotometer (Epoch, BioTek Instruments). This spectrum was recorded after 1 h for different concentrations of samples of ZnO and Ag nanoparticles.
2.5. X-ray Diffraction Analysis (XRD)
To determine the crystalline size and structural properties of ZnONPs and AgNPs, XRD analysis was performed (XRD, Rigaku, Ultima IV, X-ray Diffractometer System). XRD was carried out using Cu Kα radiation, and all diffracted intensities were recorded at 40 kv and 30 mA current in the range of 20 to 80°.
2.6. Transmittance Electron Microscope (TEM)
A transmittance electron microscope was used to measure the size and shape of ZnONPs and AgNPs. Selected areas within the TEM sections were subjected to elemental composition analysis using an energy-dispersive X-ray spectroscopy microanalysis system.
2.7. Antioxidant of Biologically Synthesized Zinc Oxide And Silver NPs
The DPPH method was used to evaluate the radical scavenging potential of the ZnONPs and AgNPs by the spectrophotometric method, with slight modification (S3)
2.8. Biological Properties of Zinc Oxide and Silver NPs
The antibacterial activity of ZnONPs and AgNPs was studied against Gram-negative (P. aeruginosa) and Gram-positive (Staphylococcus aureus) strains (S4). The minimum inhibitory concentration (MIC) was determined to calculate the minimum concentration of synthesized ZnONPs and AgNPs required to constrain the growth of microorganisms (S5). Details on fish collection and acclimatization and experimental design and exposures of NPs (S6), hematological study, biochemical studies, assessment of LFT enzymes, histology of fish, histological examination, section cutting using a microtome (S7), and enzymological parameters of the L. rohita fish on exposure to ZnONPs and AgNPs (S8) are provided in the Supporting Information.
3. Results and Discussion
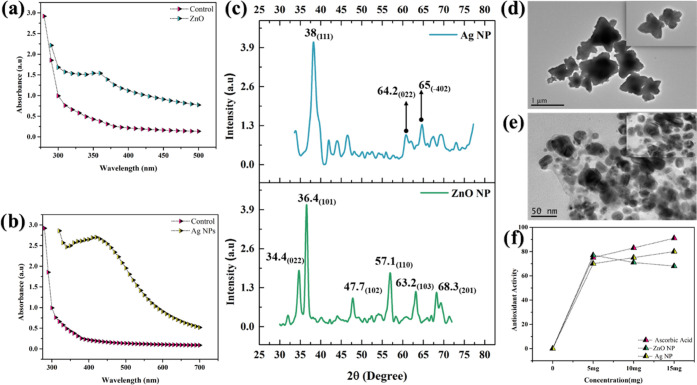
The synthesis of functional nanomaterials is a challenging task for the unique and novel applications.30,31 These nanomaterials can be used for multiple purposes in many research areas.32−34 In this work, a parallel series of synthesis of nanoparticle was confirmed by ultraviolet–vis spectrometry (UV), which provided a sharp peak at 360 nm (Figure 1a) and 430 nm (Figure 1b) for ZnONPs and AgNPs, respectively (S2, S3, ESI). Current results were compared with previously reported work, and it has been observed (UV) that zinc oxide and silver NPs showed better and authentic confirmation.35,36 These particles were further characterized using X-ray diffraction analysis (XRD) in the range of 20–80°. The intense and narrow diffraction peaks of ZnONPs revealed a cubic phase and high purity with distinct diffraction peaks in the 022, 101, 102, 110, 103, and 201 planes,37 whereas AgNPs also revealed a cubic phase and high purity with distinct diffraction peaks at the 111, 022, and −402 planes (Figure 1c). The structures of zinc oxide and silver were matched with already reported work, and it was found that the current structure for silver and zinc showed confirmation and can be used for physical, chemical, and biological applications.38,39 High-resolution microscopy using a transmittance electron microscope (TEM) showed fine flower-shaped structures of ZnONPs measuring 25 nm (Figure 1d) and AgNPs measuring 30 nm with a regular spherical structure (Figure 1e). The well-characterized particles were tested for their antioxidant potential by conducting in vitro and in vivo trials.40 Therefore, the antioxidant potential of synthesized NPs was evaluated against 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals. The electron-donating ability of ZnONPs and AgNPs was measured by the bleaching of a purple-colored ethanolic solution of DPPH. Ten microliters of ZnONPs, AgNPs, and ascorbic acid at 5, 10, and 15 mg/mL were added separately in different wells of a microtiter plate, and then 70 μL of DPPH was added to each well. After 1 h of incubation, the absorbance was measured at 517 nm. The amplitude of signals showed 77% radical scavenging activity with ZnONPs and 80% with AgNPs compared with the standard ascorbic acid, which is 90%. The results showed that the free radical scavenging activity of the green-synthesized NPs on DPPH radicals increases with the increasing concentration of NPs. By comparing our results with previously reported NPs, it has been observed that green-synthesized NPs showed better results as compared to chemically synthesized NPs41 (Figure 1f). The biologically active ZnONPs and AgNPs exhibited strong antioxidant potential that governs antioxidant reactions with higher efficacies. These results were compared with previous reports regarding the characterization and their biomedical application.42,43
Figure 1.
Characterization of zinc oxide and silver NPs: (a) UV analysis of zinc oxide NPs and (b) silver NPs. (c) XRD analysis of zinc oxide and silver NPs. (d, e) TEM analysis of zinc oxide and silver NPs. (f) antioxidant activity of zinc oxide and silver NPs.
Further, the in vitro analysis included the strong antibacterial activity against both Gram-positive (S. aureus) and Gram-negative (P. aeruginosa) bacteria by the disk diffusion method (S4, ESI). The green-synthesized ZnONPs and AgNPs exhibited excellent bacterial inhibition with respect to previous studies,44 where ZnONPs exhibited 59.5:76:83.3:100% against S. aureus and at 54:68:81:90.9% against P. aeruginosa (Figure 2a–d) with respect to the control at 5:10:15:20 mg concentration (Figure 2). And AgNPs exhibited 52.3:69:80.9:100% against S. aureus (Figure 2e-h) and at 45.4:63:70.4:100% against P. aeruginosa with respect to control at 5:10:15:20 mg concentration.45 In addition, to determine the minimum value of NPs to kill bacteria, an assay was performed, that is, the minimum inhibitory concentration (MIC) analysis assay (S5, ESI) performed using 100 μL of broth culture and 50 μL of synthesized NPs on serial dilution and 24 h incubation provided significant results. The results showed that ZnONPs exhibited an MIC value of 2.5 for P. aeruginosa and 1.25 for S. aureus. And in the case of AgNPs, the values were 1.26 and 0.625 for P. aeruginosa and S. aureus, respectively. These MIC values elucidate that a low concentration of AgNPs was required for bacterial inhibition as compared to ZnONPs, which is consistent with previous studies (Figure 2i). The underlying mechanism is ROS production and green surface facilitation,46 indicating that a high concentration of ROS mediates and catalyzes bacterial inhibition mark up. The cascade of hydroxyl, superoxide, and hydrogen peroxide lightens up the apoptotic pathway with an amplified magnitude and leads to bacterial cell death.47 The in vivo study of ZnONPs and AgNPs was conducted in L. rohita, where the NPs were added to the feed in three different concentrations: 1 mg/10 g (T1), 2 mg/10 g (T2), and 3 mg/5 g (T3) for ZnONPs and 1 mg/10 g (T4), 2 mg/10 g (T5), 3 mg/10 g (T6) for AgNPs (S6, ESI). The mentioned treatments were carried out for 4 and 15 days. In vivo trials at different concentrations and days were tested for hematological parameters (S7) (Table S2), enzymological parameters (Table S3), and protein parameters (Table S4). As shown in Figure 2j,k, after the uptake of silver and zinc oxide nanoparticles for 4 days with the food, it was observed that zinc oxide and silver nanoparticles strongly influence the metabolites of the blood. Similarly, after 15 days of absorption, these nanoparticles showed a very strong effect (Figure 2kl).
Figure 2.
Activity of zinc oxide NPs against P. aeruginosa. (a–d) Efficiency of silver NPs against S. aureus. (e–h) MIC of zinc oxide NPs and silver NPs for bacterial strains. (I) Effects of zinc oxide NPs and silver NPs on hematological, enzymes, and proteins parameter of L. rohita after 4 days. (j, k) Effects of zinc oxide NPs and silver NPs on hematological, enzyme, and protein parameters of L. rohita after 15 days (l, m).
Initially, hematological parameters after treatment for 4 days with 1 mg/5 g concentration, ZnONPs were 3% more potent compared to treatment with 1 mg/5 g AgNPs for 4 days. Similarly, as for the average effect of 2 mg/5 g ZnONPs and AgNPs for 4 days, ZnONPs exhibited 90% potency compared with Ag treatment. For 3 mg/5 g concentration, ZnONPs is exhibited 81% potency with 4-day treatment compared with AgNPs, i.e., 1 mg/10 g > 2 mg/10 g > 3 mg/10 g concentration showing an average percentage of 3 > 90 > 81%, respectively.
With 15-day treatment with 1 mg/10 g concentration, ZnONPs appear to be 33% more effective with respect to AgNP treatment. With 2 mg/5 g concentration treatment, ZnONPs were 34% more effective than that for AgNP treatment for 15 days, i.e.,1 mg/10 g > 2 mg/10 g. Therefore, the results elaborate that 4D ZnONPs treatment is very effective on the hematological parameters48 (Figure 3a). Subsequently, as for enzyme analysis, treatment with 1 mg/L concentration for 4 days showed that ZnONPs were found to be 84% more active compared with treatment with AgNPs for 4 days. Similarly, in 2 mg/L concentration treatment, ZnONPs were found to be 52% more potent than AgNPs.49 As for 3 mg/L concentration, the average effectiveness of ZnONPs was 71% with respect to AgNPs treatment, i.e., 1 > 2 > 3 mg/L concentrations, showing an average percentage of 84 > 52 > 71%, respectively. As for treatment with 1 mg/L ZnONPs for 15 days, ZnONPs exhibited 56% potency compared with AgNPs. Similarly, at a concentration of 2 mg/L, ZnONPs exhibited 47% potency compared to AgNPs, i.e.,1 > 2 mg/L concentrations showed an average percentage of 56 > 47%, respectively. Therefore, we conclude that after 15 days of treatment, ZnONPs exhibited greater activity for enzyme parameters (Figure 3b). Further, as for protein analysis results for ZnONP treatment for 4 days, the potency level was the same for ZnONPs and AgNPs, i.e., 100%. Upon treatment with 2 mg/L concentration for 4 days, ZnONPs were found to exhibit 75% potency compared with AgNPs. As for treatment with 3 mg/L concentration for 4 days, ZnONPs exhibited 13% potency compared to AgNPs, i.e.,1 > 2 > 3 mg/L concentrations showed an average percentage of 100 > 75 > 13%, respectively. However, upon treatment with 1 mg/L ZnO for 4 days, ZnO exhibited 64% activity compared with AgNPs. Similarly, upon treatment with 2 mg/L concentration, ZnONPs were 71% more effective than AgNPs, i.e. 1 > 2 mg/L, showing an average percentage of 64 > 71%, respectively. Therefore, on evaluating the average result, AgNPs were found exhibit greater activity for protein parameters (Figure 3c). All of these nanomedicinal treatments provided with nontoxic and favorable results, indicating the antioxidant and catalytic potential of NPs.50 ZnONPs are considered significant owing to their special morphological features and enhanced surface chemistry that provides numerous active sites and biomolecular arena for higher functionality.51 The already determined antioxidant potential of particles reduces oxidative stress and at the same time mediates antibacterial properties, thereby directly maintaining health and reducing contamination. Also, the enzymatic antioxidant activity of the particle catalyzes several vital reactions and enzymes involved in metabolic pathways.
Figure 3.
(a) Hematological changes in silver and zinc oxide nanoparticles in L. rohita after 4 and 15 days for different treatments; (b) enzymological parameters; and (c) protein parameters.
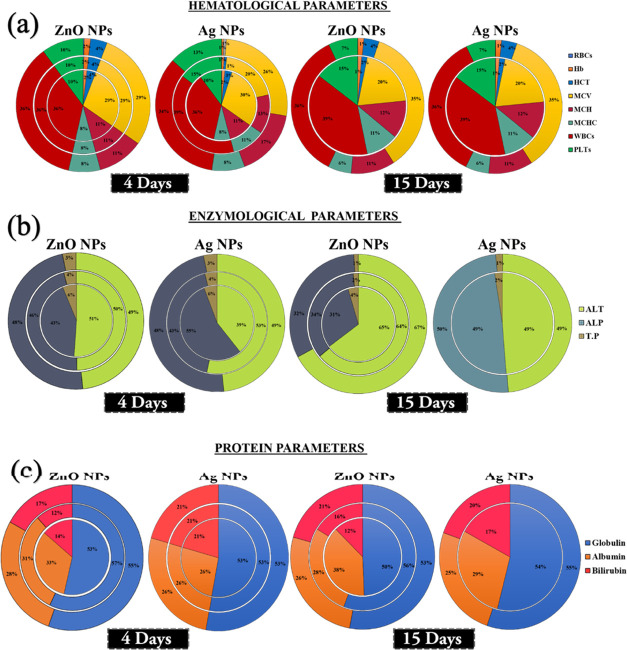
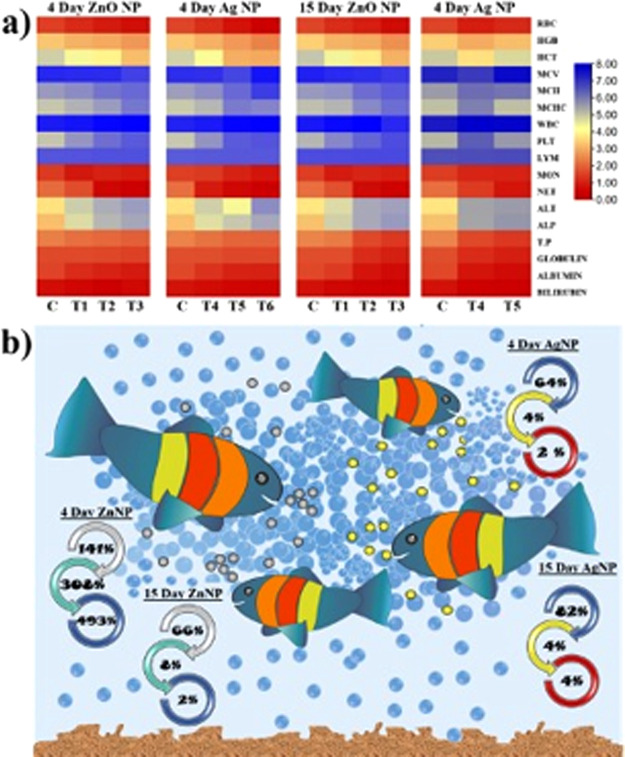
As seen in Figure 4a, all of the values from the experimental results under different treatments were totally different and significant as compared to the control. The values were different in the case of 4-day treatment in both silver and zinc nanoparticles. In the same way, with 15-day treatment, the values were different and showed different distinct colors, which indicates significant data. These results were a lot better as compared to previously reported results regarding the influence of silver and zinc nanoparticles on fish physiology or their metabolite functionality.52Figure 4b shows the percentage value of different parameters like hematology, enzymology, and changes in protein content and showed marked significant difference with 4-day and 15-day treatments. The heatmap shows the entire alteration of all parameters through changes in the color intensity with ZnONPs and AgNPs at all of the set treatments along with trial duration. Thereby, the details are provided in the manuscript. The aligned parameters on the right show specific variations in accordance with the time and treatment using different intensities. As far as the alterations get more significant, the intensity from red shifts to blue, representing the intense change in the parameter. The maximum changes occurred in the hematological parameters, followed by protein and enzyme parameters, as clearly seen through the heatmap.
Figure 4.
(a) Heatmap showing the effect of silver and zinc oxide nanoparticles after 4 days and 15 days with different treatments. (b) Uptake of silver and zinc nanoparticles with food and their biochemical effect on fish hematology, enzymology, and protein.
4. Conclusions
In conclusion, the highly purified ZnONPs and AgNPs were synthesized by the green method using the natural plant extract of Withania coagulans, which enhanced inhibitory, antioxidant, and catalytic properties. The synthesized particle enduring green surface chemistry and distinct morphology provides significant outcomes through in vitro and in vivo analysis. Interestingly, in vitro analysis exhibited on-plate bacterial inhibition, with nearly complete inhibition of 91 and 100% using ZnONPs and AgNPs, respectively. In vivo studies provided improved haematological, enzymological and protein parameters in L. rohita that enhanced the survival potential and improved the cellular modulations. Altogether, the in vitro and in vivo cellular drifts of NPs added a vote to the nanomedicinal world that provide safe and significant outcomes.
Acknowledgments
Current work was supported by the Zhongkai Agriculture University and Engineering and Guangzhou Science and Technology Project 202206010035. The authors are thankful to Higher Education Commission (HEC) funded National Research Programme for Universities (NRPU) (9458). They also acknowledge the Islamia University Bahawalpur, Pakistan.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02178.
(Table S1) Materials and chemicals; (Figure S1) schematic sketch of plant extract preparation; (supporting data S1) synthesis of zinc oxide NPs (ZnONPs); (supporting data S2) synthesis of silver NPs (AgNPs); (supporting data S3) antioxidant; (supporting data S4) antibacterial activity; (supporting data S5) minimum inhibitory concentration (MIC); (supporting data S6) fish collection and acclimatization; (supporting data S7) hematological studies; (Table S2) hematological parameters of freshwater fish L. rohita exposed to ZnONPs and AgNPs; (Table S3) enzymological parameters of the L. rohita fish after exposure to ZnONPs and AgNPs; (Table S4) protein parameters of the L. rohita fish after 4-day exposure to ZnONPs and AgNPs (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Zafar A.; Tariq T.; Hasan M.; Nazar M.; Rasheed M. N.; Mahmood N.; Shu X. Green-Maturation of Cobalt-Oxide Nano-Sponges for Reinforced Bacterial Apoptosis. Colloid Interface Sci. Commun. 2021, 45, 100531 10.1016/j.colcom.2021.100531. [DOI] [Google Scholar]
- Hussein M. Z.; Azmin W. H. W. N.; Mustafa M.; Yahaya A. H. Bacillus Cereus as a Biotemplating Agent for the Synthesis of Zinc Oxide with Raspberry- and Plate-like Structures. J. Inorg. Biochem. 2009, 103, 1145–1150. 10.1016/j.jinorgbio.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Munawar T.; Mukhtar F.; Nadeem M. S.; Manzoor S.; Ashiq M. N.; Mahmood K.; Batool S.; Hasan M.; Iqbal F. Fabrication of Dual Z-Scheme TiO2-WO3-CeO2 Heterostructured Nanocomposite with Enhanced Photocatalysis, Antibacterial, and Electrochemical Performance. J. Alloys Compd. 2022, 898, 162779 10.1016/j.jallcom.2021.162779. [DOI] [Google Scholar]
- Luo F.; Wang W.; Chen M.; Zheng Z.; Zeng D.; Hasan M.; Fu Z.; Shu X. Synthesis and Efficacy of the N-Carbamoyl-Methionine Copper on the Growth Performance, Tissue Mineralization, Immunity, and Enzymatic Antioxidant Capacity of Nile Tilapia (Oreochromis Niloticus). ACS Omega 2020, 5, 22578–22586. 10.1021/acsomega.0c03220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam A.; Ahmed A. S.; Oves M.; Khan M. S.; Habib S. S.; Memic A. Antimicrobial Activity of Metal Oxide Nanoparticles against Gram-Positive and Gram-Negative Bacteria: A Comparative Study. Int. J. Nanomed. 2012, 7, 6003–6009. 10.2147/IJN.S35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oves M.; Ahmar Rauf M.; Aslam M.; Qari H. A.; Sonbol H.; Ahmad I.; Sarwar Zaman G.; Saeed M. Green Synthesis of Silver Nanoparticles by Conocarpus Lancifolius Plant Extract and Their Antimicrobial and Anticancer Activities. Saudi J. Biol. Sci. 2022, 29, 460–471. 10.1016/j.sjbs.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oves M.; Aslam M.; Rauf M. A.; Qayyum S.; Qari H. A.; Khan M. S.; Alam M. Z.; Tabrez S.; Pugazhendhi A.; Ismail I. M. I. Antimicrobial and Anticancer Activities of Silver Nanoparticles Synthesized from the Root Hair Extract of Phoenix Dactylifera. Mater. Sci. Eng., C 2018, 89, 429–443. 10.1016/j.msec.2018.03.035. [DOI] [PubMed] [Google Scholar]
- Ahmar Rauf M.; Oves M.; Ur Rehman F.; Rauf Khan A.; Husain N. Bougainvillea Flower Extract Mediated Zinc Oxide’s Nanomaterials for Antimicrobial and Anticancer Activity. Biomed Pharmacother 2019, 116. 10.1016/j.biopha.2019.108983. [DOI] [PubMed] [Google Scholar]
- Hasan M.; Ullah I.; Zulfiqar H.; Naeem K.; Iqbal A.; Gul H.; Ashfaq M.; Mahmood N. Biological Entities as Chemical Reactors for Synthesis of Nanomaterials: Progress, Challenges and Future Perspective. Mater. Today Chem. 2018, 8, 13–28. 10.1016/j.mtchem.2018.02.003. [DOI] [Google Scholar]
- Zulfiqar H.; Zafar A.; Rasheed M. N.; Ali Z.; Mehmood K.; Mazher A.; Hasan M.; Mahmood N. Synthesis of Silver Nanoparticles Using: Fagonia Cretica and Their Antimicrobial Activities. Nanoscale Advances. 2019, 1, 1707–1713. 10.1039/c8na00343b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M.; Altaf M.; Zafar A.; Ali Z.; Munawar T.; Saif M. S.; Iqbal F.; Khan M. W.; Mustafa G.; Mahmood A.; Mahmood N.; Shu X. Bioinspired Synthesis of Zinc Oxide Nano-Flowers: A Surface Enhanced Antibacterial and Harvesting Efficiency. Mater. Sci. Eng., C 2020, 119, 111280 10.1016/j.msec.2020.111280. [DOI] [PubMed] [Google Scholar]
- Qasim S.; Zafar A.; Saif M. S.; Ali Z.; Nazar M.; Waqas M.; Haq A. U.; Tariq T.; Hassan S. G.; Iqbal F.; Shu X. G.; Hasan M. Green Synthesis of Iron Oxide Nanorods Using Withania Coagulans Extract Improved Photocatalytic Degradation and Antimicrobial Activity. J. Photochem. Photobiol., B 2020, 204, 111784 10.1016/j.jphotobiol.2020.111784. [DOI] [PubMed] [Google Scholar]
- Mahmood K.; Amara U.; Siddique S.; Usman M.; Peng Q.; Khalid M.; Hussain A.; Ajmal M.; Ahmad A.; Sumrra S. H.; Liu Z.-P.; Khan W. S.; Ashiq M. N. Green Synthesis of Ag@CdO Nanocomposite and Their Application towards Brilliant Green Dye Degradation from Wastewater. J. Nanostruct. Chem. 2021, 12, 329–341. 10.1007/s40097-021-00418-5. [DOI] [Google Scholar]
- Bawazeer S.; Rauf A.; Shah S. U. A.; Shawky A. M.; Al-Awthan Y. S.; Bahattab O. S.; Uddin G.; Sabir J.; El-Esawi M. A. Green Synthesis of Silver Nanoparticles Using Tropaeolum Majus: Phytochemical Screening and Antibacterial Studies. Green Process. Synth. 2021, 10, 85–94. 10.1515/gps-2021-0003. [DOI] [Google Scholar]
- Milić M.; Cvetić Ž.; Bendelja K.; Vuković B.; Galić E.; Ćurlin M.; Dobrošević B.; Jurak Begonja A.; Vinković Vrček I. Response of Platelets to Silver Nanoparticles Designed with Different Surface Functionalization. J. Inorg. Biochem. 2021, 224, 111565 10.1016/j.jinorgbio.2021.111565. [DOI] [PubMed] [Google Scholar]
- Saif S.; Tahir A.; Asim T.; Chen Y.; Khan M.; Adil S. F. Green Synthesis of ZnO Hierarchical Microstructures by Cordia Myxa and Their Antibacterial Activity. Saudi J. Biol. Sci. 2019, 26, 1364–1371. 10.1016/j.sjbs.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F.; Zeng D.; Chen R.; Zafar A.; Weng L.; Wang W.; Tian Y.; Hasan M.; Shu X. PEGylated Dihydromyricetin-Loaded Nanoliposomes Coated with Tea Saponin Inhibit Bacterial Oxidative Respiration and Energy Metabolism. Food & Function 2021, 12, 9007–9017. 10.1039/d1fo01943k. [DOI] [PubMed] [Google Scholar]
- Naz G.; Shabbir M.; Ramzan M.; Haq B. U.; Arshad M.; Tahir M. B.; Hasan M.; Ahmed R. Synergistic Effect of Cux/Mgx and Zn1–xO for Enhanced Photocatalytic Degradation and Antibacterial Activity. Phys. B 2022, 624, 413396 10.1016/j.physb.2021.413396. [DOI] [Google Scholar]
- Horie M.; Tabei Y. Role of Oxidative Stress in Nanoparticles Toxicity. Free Radical Res. 2021, 55, 331–342. 10.1080/10715762.2020.1859108. [DOI] [PubMed] [Google Scholar]
- Shaw B. J.; Handy R. D. Physiological Effects of Nanoparticles on Fish: A Comparison of Nanometals versus Metal Ions. Environ. Int. 2011, 37, 1083–1097. 10.1016/j.envint.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Kuchur O. A.; Tsymbal S. A.; Shestovskaya M. V.; Serov N. S.; Dukhinova M. S.; Shtil A. A. Metal-Derived Nanoparticles in Tumor Theranostics: Potential and Limitations. J. Inorg Biochem. 2020, 209, 111117 10.1016/j.jinorgbio.2020.111117. [DOI] [PubMed] [Google Scholar]
- Bardajee G. R.; Hooshyar Z.; Rezanezhad H. A Novel and Green Biomaterial Based Silver Nanocomposite Hydrogel: Synthesis, Characterization and Antibacterial Effect. J. Inorg. Biochem. 2012, 117, 367–373. 10.1016/j.jinorgbio.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Hasan M.; Mehmood K.; Mustafa G.; Zafar A.; Tariq T.; Hassan S. G.; Loomba S.; Zia M.; Mazher A.; Mahmood N.; Shu X. Phytotoxic Evaluation of Phytosynthesized Silver Nanoparticles on Lettuce. Coatings 2021, 11, 225. 10.3390/coatings11020225. [DOI] [Google Scholar]
- Zafar A.; Hasan M.; Tariq T.; Dai Z. Enhancing Cancer Immunotherapeutic Efficacy with Sonotheranostic Strategies. Bioconjugate Chem. 2022, 33, 1011–1034. 10.1021/acs.bioconjchem.1c00437. [DOI] [PubMed] [Google Scholar]
- Sarkar B.; Mahanty A.; Gupta S. K.; Choudhury A. R.; Daware A.; Bhattacharjee S. Nanotechnology: A next-Generation Tool for Sustainable Aquaculture. Aquaculture 2022, 546, 737330 10.1016/j.aquaculture.2021.737330. [DOI] [Google Scholar]
- Handy R. D.; Clark N. J.; Boyle D.; Vassallo J.; Green C.; Nasser F.; Botha T. L.; Wepener V.; van den Brink N. W.; Svendsen C. The Bioaccumulation Testing Strategy for Nanomaterials: Correlations with Particle Properties and a Meta-Analysis of: In Vitro Fish Alternatives to in Vivo Fish Tests. Environ. Sci.: Nano 2022, 9, 684–701. 10.1039/d1en00694k. [DOI] [Google Scholar]
- Gupta R.; Sonawane T.; Pai S. An Overview on Pharmaceutical Properties and Biotechnological Advancement of Withania Coagulans. Adv. Tradit. Med. 2021, 00558-7 10.1007/s13596-021-00558-7. [DOI] [Google Scholar]
- Maher S.; Choudhary M. I.; Saleem F.; Rasheed S.; Waheed I.; Halim S. A.; Azeem M.; Abdullah I.; bin Froeyen M.; Mirza M. U.; Ahmad S. Isolation of Antidiabetic Withanolides from Withania Coagulans Dunal and Their in Vitro and in Silico Validation. Biology 2020, 9, 197 10.3390/biology9080197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar A.; Aamir M. N.; Shah M. A.; Syed S. K.; Munir R. Development, Characterization and Evaluation of in Vitro Anti-Inflammatory Activity of Withania Coagulans Extract and Extract Loaded Microemulsion. Pak. J. Pharm Sci. 2021, 34, 473–479. 10.36721/PJPS.2021.34.1.SP.473-479.1. [DOI] [PubMed] [Google Scholar]
- Ghafoor S.; Ata S.; Mahmood N.; Arshad S. N. Photosensitization of TiO2 Nanofibers by Ag2S with the Synergistic Effect of Excess Surface Ti3+ States for Enhanced Photocatalytic Activity under Simulated Sunlight. Sci. Rep. 2017, 7, 00366–00367. 10.1038/s41598-017-00366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif H. M. A.; Mahmood A.; Cheng H. Y.; Djellabi R.; Ali J.; Jiang W. L.; Wang S.; sen Haider M. R.; Mahmood N.; Wang A. J. Fe3O4 Nanoparticles Coated with EDTA and Ag Nanoparticles for the Catalytic Reduction of Organic Dyes from Wastewater. ACS Appl. Nano Mater. 2019, 2, 5310–5319. 10.1021/acsanm.9b01250. [DOI] [Google Scholar]
- Jamil R.; Ali R.; Loomba S.; Xian J.; Yousaf M.; Khan K.; Shabbir B.; McConville C. F.; Mahmood A.; Mahmood N. The Role of Nitrogen in Transition-Metal Nitrides in Electrochemical Water Splitting. Chem. Catal. 2021, 1, 802–854. 10.1016/j.checat.2021.06.014. [DOI] [Google Scholar]
- Zhang R.; Zhang Y. C.; Pan L.; Shen G. Q.; Mahmood N.; Ma Y. H.; Shi Y.; Jia W.; Wang L.; Zhang X.; Xu W.; Zou J. J. Engineering Cobalt Defects in Cobalt Oxide for Highly Efficient Electrocatalytic Oxygen Evolution. ACS Catal. 2018, 8, 3803–3811. 10.1021/acscatal.8b01046. [DOI] [Google Scholar]
- Mahmood N.; Khan H.; Tran K.; Kuppe P.; Zavabeti A.; Atkin P.; Ghasemian M. B.; Yang J.; Xu C.; Tawfik S. A.; Spencer M. J. S.; Ou J. Z.; Khoshmanesh K.; McConville C. F.; Li Y.; Kalantar-Zadeh K. Maximum Piezoelectricity in a Few Unit-Cell Thick Planar ZnO – A Liquid Metal-Based Synthesis Approach. Mater. Today 2021, 44, 69–77. 10.1016/j.mattod.2020.11.016. [DOI] [Google Scholar]
- Aryan; Ruby; Mehata M. S. Green Synthesis of Silver Nanoparticles Using Kalanchoe Pinnata Leaves (Life Plant) and Their Antibacterial and Photocatalytic Activities. Chem. Phys. Lett. 2021, 778, 138760 10.1016/j.cplett.2021.138760. [DOI] [Google Scholar]
- Arumugam J.; Thambidurai S.; Suresh S.; Selvapandiyan M.; Kandasamy M.; Pugazhenthiran N.; Karthick Kumar S.; Muneeswaran T.; Quero F. Green Synthesis of Zinc Oxide Nanoparticles Using Ficus Carica Leaf Extract and Their Bactericidal and Photocatalytic Performance Evaluation. Chem. Phys. Lett. 2021, 783, 139040 10.1016/j.cplett.2021.139040. [DOI] [Google Scholar]
- Athar M.; Fiaz M.; Farid M. A.; Tahir M.; Asghar M. A.; Ul Hassan S.; Hasan M. Iron and Manganese Codoped Cobalt Tungstates Co1-(X+ y)Fe XMn YWO4as Efficient Photoelectrocatalysts for Oxygen Evolution Reaction. ACS Omega 2021, 6, 7334–7341. 10.1021/acsomega.0c05412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meenatchi T.; Palanimurugan A.; Dhanalakshmi A.; Maheshkumar V.; Natarajan B. Green Synthesis of Cynodon Dactylon Capped Concentrations on ZnO Nanoparticles for Antibacterial Activity, ROS/ML-DNA Treatment and Compilation of Best Controlling Microbes by Mathematical Comparisons. Chem. Phys. Lett. 2020, 749, 137429 10.1016/j.cplett.2020.137429. [DOI] [Google Scholar]
- Chand K.; Jiao C.; Lakhan M. N.; Shah A. H.; Kumar V.; Fouad D. E.; Chandio M. B.; Ali Maitlo A.; Ahmed M.; Cao D. Green Synthesis, Characterization and Photocatalytic Activity of Silver Nanoparticles Synthesized with Nigella Sativa Seed Extract. Chem. Phys. Lett. 2021, 763, 138218 10.1016/j.cplett.2020.138218. [DOI] [Google Scholar]
- Luo F.; Wang M.; Huang L.; Wu Z.; Wang W.; Zafar A.; Tian Y.; Hasan M.; Shu X. Synthesis of Zinc Oxide Eudragit FS30D Nanohybrids: Structure, Characterization, and Their Application as an Intestinal Drug Delivery System. ACS Omega 2020, 5, 11799–11808. 10.1021/acsomega.0c01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.; Ren Y.; Liang Y.; Huang L.; Yang Y.; Zafar A.; Hasan M.; Yang F.; Shu X. Synthesis, Characterization, Immune Regulation, and Antioxidative Assessment of Yeast-Derived Selenium Nanoparticles in Cyclophosphamide-Induced Rats. ACS Omega 2021, 6, 24585–24594. 10.1021/acsomega.1c03205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.; Gong Y.; Liao W.; Luo Y.; Wu C.; Wang M.; Yang Q. A Review of Cardiovascular Toxicity of TiO 2, ZnO and Ag Nanoparticles (NPs). BioMetals 2018, 31, 457–476. 10.1007/s10534-018-0113-7. [DOI] [PubMed] [Google Scholar]
- Hasan M.; Zafar A.; Yousaf M.; Gulzar H.; Mehmood K.; Hassan S. G.; Saeed A.; Yousaf A.; Mazher A.; Rongji D.; Mahmood N. Synthesis of Loureirin B-Loaded Nanoliposomes for Pharmacokinetics in Rat Plasma. ACS Omega 2019, 4, 6914–6922. 10.1021/acsomega.9b00119. [DOI] [Google Scholar]
- Korkmaz N.; Ceylan Y.; Taslimi P.; Karadağ A.; Bülbül A. S.; Şen F. Biogenic Nano Silver: Synthesis, Characterization, Antibacterial, Antibiofilms, and Enzymatic Activity. Adv. Powder Technol. 2020, 31, 2942–2950. 10.1016/j.apt.2020.05.020. [DOI] [Google Scholar]
- Shah S.; Shah S. A.; Faisal S.; Khan A.; Ullah R.; Ali N.; Bilal M. Engineering Novel Gold Nanoparticles Using Sageretia Thea Leaf Extract and Evaluation of Their Biological Activities. J. Nanostruct. Chem. 2022, 12, 129–140. 10.1007/s40097-021-00407-8. [DOI] [Google Scholar]
- Yousaf H.; Mehmood A.; Ahmad K. S.; Raffi M. Green Synthesis of Silver Nanoparticles and Their Applications as an Alternative Antibacterial and Antioxidant Agents. Mater. Sci. Eng., C 2020, 112, 110901 10.1016/j.msec.2020.110901. [DOI] [PubMed] [Google Scholar]
- Annapoorani B.; Gayathri K.; Sangeetha R.; Orenbemo O. N. O. Comparative Study on the Free Radical Scavenging Potency of Vigna Radiate Sprouts and Its Zinc Oxide Nanoparticle. Int J. Res. Pharm. Sci. 2018, 9, 916–921. 10.26452/ijrps.v9i3.1598. [DOI] [Google Scholar]
- Bruneau A.; Turcotte P.; Pilote M.; Gagné F.; Gagnon C. Fate of Silver Nanoparticles in Wastewater and Immunotoxic Effects on Rainbow Trout. Aquat. Toxicol. 2016, 174, 70–81. 10.1016/j.aquatox.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Leitemperger J.; Menezes C.; de Oliveira V. A.; Fiuza T.; Barcarolli I. F.; Pereira M. E.; Bianchini A.; Loro V. L. The Bioaccumulation of Waterborne Zinc in Tissues of Silver Catfish (Rhamdia Quelen) and Its Effect on Biochemical Parameters. BioMetals 2019, 32, 241–249. 10.1007/s10534-019-00168-6. [DOI] [PubMed] [Google Scholar]
- AnvariFar H.; Amirkolaie A. K.; Jalali A. M.; Miandare H. K.; Sayed A. E. D. H.; Üçüncü S.; Ouraji H.; Ceci M.; Romano N. Environmental Pollution and Toxic Substances: Cellular Apoptosis as a Key Parameter in a Sensible Model like Fish. Aquat. Toxicol. 2018, 204, 144–159. 10.1016/j.aquatox.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Minghetti M.; Schirmer K. Interference of Silver Nanoparticles with Essential Metal Homeostasis in a Novel Enterohepatic Fish: In Vitro System. Environ. Sci.: Nano 2019, 6, 1777–1790. 10.1039/c9en00310j. [DOI] [Google Scholar]
- Lin T. Y.; Chen Y. H.; Liu C. L.; Jeng S. S. Role of High Zinc Levels in the Stress Defense of Common Carp. Fish. Sci 2011, 77, 557–574. 10.1007/s12562-011-0374-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.