Abstract
Background
Healthier lifestyles in early pregnancy are associated with lower rates of pregnancy complications, childhood adiposity, and maternal and child cardiovascular risks. However, it is not known whether lifestyle coaching initiated prior to pregnancy can affect behavior and attitudes during pregnancy.
Methods
Three hundred and twenty six women planning pregnancy within 2 years with BMI ≥27 kg/m2 were randomized to a behavioral weight loss intervention or to usual care. Analyses reported here examined the intervention’s impact on mid‐pregnancy diet quality and activity levels; program acceptability; and effects of pregnancy on intervention engagement.
Results
One hundred and sixty eight participants experienced pregnancy during the study (intervention: 91; usual care: 77). From randomization to mid‐pregnancy, participants who received the intervention had larger increases in fruit intake than usual care participants (+0.67 vs. +0.06 cups; p = 0.02) and engaged in more vigorous‐intensity activity (3.9 [5.5] vs. 1.2 [3.0] Met‐hr/week p = 0.002) and sports/exercise (17.0 [14.1] vs. 11.0 [9.5] Met‐hr/week; p = 0.03); the groups also differed in changes in sedentary time (−4.9 [15.0] vs. +0.5 [7.6] Met‐hr/week; p = 0.02). Intervention satisfaction was high (>80%), and experiencing pregnancy during the intervention was associated with higher engagement.
Conclusion
A coaching‐based intervention beginning in pre‐pregnancy successfully helped women attain healthier diet and exercise habits in mid‐pregnancy.
Clinical trials registration
Registered with ClinicalTrials.gov, NCT02346162, first registered on January 26, 2015, before date of initial participant enrollment (May 2015), https://clinicaltrials.gov/ct2/show/NCT02346162.
Keywords: antenatal lifestyle, diet, exercise, lifestyle intervention acceptability, pre‐pregnancy behavioral lifestyle intervention
1. INTRODUCTION
Overweight or obesity affects over 50% of reproductive‐aged women. 1 This is of particular concern because maternal body mass index (BMI), diet, and physical activity at pregnancy onset have a strong influence on the metabolic environment in which the fetus starts its development. Maternal obesity during early pregnancy has consistently been linked to adverse pregnancy complications, offspring birthweight, and offspring risk of obesity later in life. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 Women who have overweight and obesity are also at high risk of excess early‐pregnancy weight gain, 26 which may be a critical risk factor for adverse pregnancy and offspring outcomes. 2 , 27 , 34
The National Academy of Medicine (NAM; formerly the Institute of Medicine) recommended in 2009 that women begin pregnancy with a normal BMI, and made recommendations for healthy gestational weight gain (GWG). 35 Since then, there have been over 60 trials that have examined lifestyle interventions initiated during pregnancy (usually in the late first or early second trimester) to limit GWG. However, these interventions may be too late to affect the first‐trimester metabolic environment, which has been shown to be critical to long‐term offspring outcomes. 2 , 27 , 34 To address this shortcoming, the Prepare randomized clinical trial examined the impact of a weight loss initiated prior to pregnancy on maternal weight over the course of pregnancy. 36 , 37 , 38
The periconceptional period may be a “teachable moment” when women are motivated to adopt risk‐reducing health behaviors to improve their likelihood of pregnancy, reduce risk of complications during pregnancy, and protect their baby's health. 35 Accordingly, women planning for pregnancy may be particularly open to messages about the value of healthy eating and exercise, resulting in high levels of satisfaction and engagement with lifestyle interventions, potentially resulting in lifestyle changes that could be maintained during pregnancy.
Detailed descriptions of the Prepare trial design 36 and primary outcomes have been published previously. Although participants in the intervention arm successfully lost weight prior to conception, the intervention was associated with greater weight gain in late pregnancy. 37 This paper explores the question of how the intervention affected participant behaviors and attitudes during early pregnancy through secondary analyses examining acceptability of the intervention, effects of the intervention on women's diet quality and physical activity levels at mid‐pregnancy, and effects of pregnancy on engagement with the intervention.
2. METHODS
Participants were recruited from Kaiser Permanente Northwest (KPNW), a nonprofit integrated health care system serving Oregon and Southwest Washington. All female enrollees in the KPNW health plan ages 18–40 years with BMIs ≥27 kg/m2 were contacted via letters, emails, and text messages, encouraging them to visit the Prepare Study website. A BMI threshold of 27 kg/m2 was selected because by the end of pregnancy, these women were likely to be in the obese category. The Prepare website allowed women to self‐screen for study eligibility. To be eligible, women had to be planning pregnancy in the next 2 years, not currently pregnant, and not have conditions or take medications that would affect weight. If likely eligible, women could sign up for an information session. Following the information session, women were scheduled for a screening visit where eligibility was confirmed through questionnaires and interviews with research staff. 36
2.1. Baseline visit and initial session
At the baseline visit, participant's height and weight were recorded in light indoor clothing with their shoes removed; weight was measured with a regularly calibrated electronic scale (Tronix Inc, Model 5022). Participants were then randomized to the intervention or control arm at a 1:1 ratio via a computerized randomization process created by the study statistician. Randomization was stratified by age (<30, ≥30), BMI (27–30, 31–35, ≥36 kg/m2) and parity (0, ≥1). Allocation was concealed until the randomization button was pushed. 36
Participants assigned to the intervention arm (N = 164) immediately attended an in‐person introductory session reviewing the study goals and website (∼30–40 min). They were given a binder containing handouts for each module; a pedometer; and the CalorieKing Calorie, Fat & Carbohydrate Counter book 39 and companion Food and Exercise Journal, which could be used to track calories and exercise. 40 Women assigned to the usual care control arm were given approximately 5–10 min of information on general nutrition, physical activity, safe fish intake, and folic acid intake during pregnancy; participants who received the intervention received this information in later sessions. 36 All participants received routine prenatal care through their obstetrical provider.
2.2. Intervention
The intervention was started before pregnancy and continued, regardless of participant pregnancy status for 24 months, or until delivery. It consisted of individualized 20‐ to 30‐min telephone counseling sessions with a trained behavioral interventionist (health coach) and access to a personalized intervention website. Participants worked with the same health coach throughout the intervention.
Participants were asked to track their weight, minutes of exercise, and number of steps walked daily and to enter this information into the study website; they were also encouraged to keep a food diary, and to report to the website whether they had completed food records for the day. The website then displayed their weight trajectory as well as information on how often they met their exercise goals and kept food records. Participants could use any scale available to them to measure weight, and any method they chose to track diet and activity (coaches suggested options such as the MyFitnessPal and LoseIt website/phone apps, the CalorieKing Food and Exercise Journal, and the notes section of their phone). Coaching sessions occurred weekly for 6 months and then monthly for 18 months or until pregnancy end (mean number of sessions = 42).
Participants were encouraged to lose weight before pregnancy (0.2–0.4 kg per week) by following the DASH dietary pattern without sodium restriction 41 at a customized caloric target set using the Harris‐Benedict equation. 42 This DASH dietary pattern is nutrient dense, varied, and balanced, and is consistent with current USDA healthy diet guidelines, and thus with ACOG diet guidelines. 43 , 44
Women were also encouraged to exercise, working toward two daily goals: 60 min of moderate‐intensity physical activity and walking at least 10,000 steps per day, tracked using the study‐provided pedometer or their own method (such as FitBit or smartphone). The physical activity goal was intended to encourage participants to maintain and/or increase exercise at a moderate intensity level; the steps goal was intended to help participants decrease the amount of time spent on sedentary behaviors. On each intervention telephone call, coaches assessed participants’ goal progress and set new goals, gradually increasing number of steps and exercise frequency, intensity, and duration until goal levels were reached. Participants who reported becoming pregnant continued participating in the intervention with the weight goal modified to keeping GWG within NAM guidelines.
Calls were oriented toward behavior change and applied principles of social cognitive theory 45 , 46 and the techniques of behavioral self‐management. 46 , 47 , 48 The FRAMES model (Feedback, Responsibility, Advice, Menu of options, Empathy, and Self‐Efficacy) provided a conceptual structure that coaches used to tailor intervention goals to accommodate varying degrees of readiness to change. 49 , 50 , 51 At the start of each call, the health coach and participant discussed the participant's current diet and physical activity and the coach elicited participant feedback about successes and challenges from the week. The health coach then worked collaboratively with the participant to set specific goals, guiding the participant in identifying social‐environmental supports and personal/family barriers to achieving their goals, and developing personalized problem‐solving strategies.
2.3. Intervention fidelity
The investigators met with coaches monthly to discuss participants who were not meeting study goals. Participants were identified for review based on absence of weight loss, presence of weight gain, and/or low phone call completion rates. The team discussed approaches and strategies for assisting these participants. The PI and/or Co‐I also observed phone coaching sessions quarterly, providing feedback to the coaches.
2.4. Diet and exercise at mid‐pregnancy
Diet and exercise information was self‐reported by questionnaire, and outreach to encourage participants to complete the questionnaires was done by research staff not involved in administering the intervention. To minimize participant burden, diet and activity were assessed using self‐report at two time points: just before randomization (in person) and at 20 weeks of gestation (remotely), after nausea has subsided for most pregnant women. Reported mean energy intake has been shown to be relatively stable from the first to second trimester. 52
Diet information was collected via 24‐h recalls at baseline and mid‐pregnancy (mean = 21.3 weeks gestation, SD = 3.1 weeks), with a target of at least two recalls (1 weekday and 1 weekend) at each time point. Recalls were collected remotely using either the 2014 or 2016 version of the Automated Self‐Administered 24‐h (ASA24®) Dietary Assessment Tool, developed by the National Cancer Institute. The validity of the ASA24 is comparable to the USDA's Automated Multi‐Pass Method, capturing ∼80% of observed foods consumed. 53 The ASA24 software calculated quantities consumed per day from each of the USDA's Food Patterns components 54 using the dietary intake data. ASA24‐2014 analytic files were harmonized with ASA24‐2016 files using SAS code provided by NCI to reflect the most recent USDA Food Patterns Equivalents Database.
Summary measures were generated using ASA24 data, 55 including energy density (for food and beverages), 56 , 57 , 58 , 59 , 60 the Healthy Eating Index (HEI), 61 , 62 and consumption of specific types of foods (fruits, vegetables, and dairy) and food components (whole and refined grains, fiber, added sugars, and fats). Recalls with extreme calorie values (kcal < 500 or kcal > 6000, N = 2) were excluded; in these cases, participants had two other recalls completed at the same time point that were included in analyses.
Self‐reported activity level was measured in person at baseline and remotely at mid‐pregnancy (mean 18.0 weeks gestation, SD = 1.8 weeks) using the Pregnancy Physical Activity Questionnaire (PPAQ), 63 a semi‐quantitative questionnaire that asked respondents to self‐report the time spent participating in 33 activities including household and caregiving activities (13 activities), occupational activities, 5 sports/exercise activities (7 activities + 2 open‐ended spaces), transportation activities, 3 and inactivity (3 categories). An open‐ended section in the sports/exercise portion of the questionnaire allowed participants to list up to two additional activities that were not already listed. For each activity, respondents selected the amount of time spent in that activity per day or per week during their current trimester of pregnancy (options were provided in 30‐min to 1‐h increments). The PPAQ has previously been validated in pregnant women. 63
Average weekly energy expenditure (MET‐h wk/activity) was calculated using PPAQ data by multiplying the time spent in each activity by its metabolic equivalents (METs); activities added in the open‐ended section of the survey were assigned appropriate MET values based on the 2011 Compendium of Physical Activities. In addition, each activity was classified by intensity: sedentary (<1.5 METs), light (1.5–2.9 METs), moderate (3.0–6.0 METs) or vigorous (>6.0 METs) and the average number of MET‐hours per week expended within each intensity level was calculated. Activities were also classified by type (household/caregiving, occupational, and sports/exercise) and the average number of MET hours per week spent in each activity type was calculated.
2.5. Intervention engagement
Health coaches recorded the date and time that each call was conducted and the length of the call. From those data, weekly and monthly call rates and mean duration of weekly and monthly calls were calculated. Website engagement was calculated by measuring the number of days that a participant recorded any data on the site.
2.6. Intervention acceptability
Participants were asked to complete an end‐of‐intervention online questionnaire after delivery (for those who became pregnant during the intervention), or 24 months after their baseline visit (for those who did not become pregnant during the intervention). The questionnaire, which contained 14 questions assessing satisfaction with the intervention, was developed by the study team in order to assess which specific parts of the Prepare program were most helpful to participants. Health coaches were not involved in contacting participants to collect this information.
2.7. Facilitators and barriers
A trained interviewer, who was not involved in the intervention, conducted qualitative interviews with a randomly selected subsample of participants who had completed the intervention and delivered a child within the past 30 months. Of the 28 participants invited for the interviews, 16 completed the interview. Interviews were recorded with the consent of participants and transcribed for analysis. For the analysis, responses were summarized by question to identify facilitators and barriers to behavior change for participants who received the intervention.
2.8. Statistical analyses
Sample size was determined for the primary study outcome of GWG as previously described. 37 For the prespecified secondary outcomes of diet quality and physical activity in pregnancy, women who completed the baseline and mid‐pregnancy ASA‐24 and/or PPAQ were included (Figure 1). Generalized linear models that adjusted for baseline total caloric intake were used to compare change scores in diet quality measures from baseline to mid‐pregnancy between intervention and usual care arms. Independent t‐tests that adjusted for baseline activity level were used to compare the change in activity level from randomization to mid‐pregnancy between intervention and usual care arms, and to assess differences between the arms at each time point. Because exercise and diet measures were both secondary outcomes, the study was not powered to detect minimum important differences in them, and no correction for multiple comparisons was applied.
FIGURE 1.
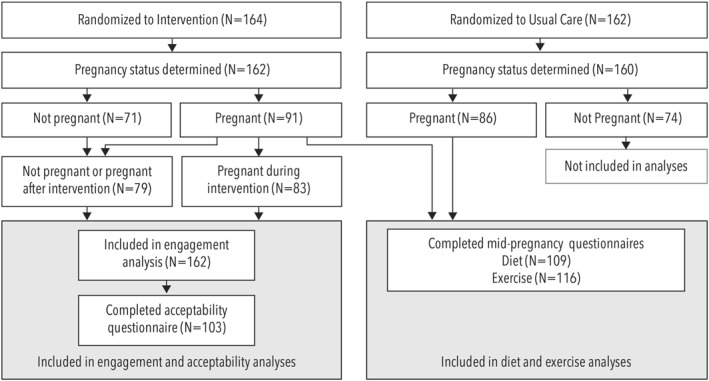
Prepare consort diagram
The analysis of the prespecified secondary outcome of intervention acceptability included all women who completed a post‐intervention survey (Figure 1). The percentage of participants who responded that the intervention and its components were “Very helpful” or “Moderately helpful,” rather than “Slightly helpful” or “Not helpful,” was calculated.
Exploratory analyses compared engagement between participants in the intervention arm who became pregnant with a pregnancy lasting at least 14 weeks during the intervention time period (N = 83) to those who did not (either because they became pregnant after intervention sessions ended or did not become pregnant during the study period [N = 79]). Independent t‐tests were used to assess differences in percentage of calls completed (weekly and monthly phases combined); mean call duration; and mean number of days per week that participants logged weight, food record completion, exercise, and/or steps into the intervention web page. Additionally, paired t‐tests were used to compare these metrics between the weekly phase and the monthly phase for each arm. Using a Fisher's exact test, intervention drop‐out rates were compared between arms. All analyses were performed using SAS software, version 9.4 (SAS Institute, Inc. Cary, NC).
The study was conducted and reported in accordance with a previously published protocol 36 that was approved by the KPNW Institutional Review Board. A Data and Safety Monitoring Board provided independent study monitoring.
3. RESULTS
3.1. Participant characteristics
Three hundred and twenty six participants were randomized to the intervention (N = 164) or the control arm (N = 162). Demographics at randomization did not differ between arms (Table 1). The majority of participants were non‐Hispanic White (77%), over 30 years old (71%), and nulliparous (67%). The average BMI at randomization was 36.7 (±7.3) kg/m2 and participants tended to be well‐educated (76% had a college degree or higher). Overall, 188 participants experienced pregnancy lasting at least 14 weeks (91 in the intervention arm vs. 86 in usual care; Figure 1). Among those in the intervention, 83 experienced a pregnancy during the intervention and 79 were never pregnant or became pregnant after the intervention had ended (Figure 1).
TABLE 1.
Participant characteristics at baseline
| Variables | Usual care | Intervention | p‐value* |
|---|---|---|---|
| N = 162 | N = 163 | ||
| Age at randomization mean (SD) | 31.6 (3.6) | 31.8 (4.1) | 0.61 |
| Age, N (%) | 0.97 | ||
| <30 years old | 47 (29.0) | 47 (28.8) | |
| ≥30 years old | 115 (71.0) | 116 (71.2) | |
| Weight status, mean (SD) | |||
| BMI | 36.8 (7.3) | 36.7 (7.3) | 0.91 |
| Weight | 221.1 (47.3) | 222.7 (48.6) | 0.76 |
| BMI, N (%) | 0.98 | ||
| 27–29.9 | 30 (18.5) | 29 (17.8) | |
| 30–34.9 | 52 (32.1) | 52 (31.9) | |
| ≥35 | 80 (49.4) | 82 (50.3) | |
| Race, N (%) | N = 158 | N = 159 | 0.31 |
| White | 134 (84.8) | 128 (80.5) | |
| Non‐white | 24 (15.2) | 31 (19.5) | |
| Ethnicity, N (%) | N = 162 | N = 161 | 0.53 |
| Hispanic | 13 (8.0) | 10 (6.2) | |
| Non‐hispanic | 149 (92.0) | 151 (93.8) | |
| Parity, N (%) | 0.75 | ||
| 0 | 109 (67.3) | 107 (65.6) | |
| 1+ | 53 (32.7) | 56 (34.4) | |
| Marital status, N (%) | N = 161 | N = 163 | 0.77 |
| Married | 114 (70.8) | 113 (69.3) | |
| Not married | 47 (29.2) | 50 (30.7) | |
| Education, N (%) | 0.30 | ||
| HS Graduate or GED certificate | 29 (17.9) | 25 (15.3) | |
| Technical school graduate | 8 (4.9) | 15 (9.2) | |
| College graduate or higher | 125 (77.2) | 123 (75.5) | |
| Current smoker, N (%) | N = 160 | N = 162 | 0.98 |
| Yes | 9 (5.6) | 9 (5.6) | |
| No | 151 (94.4) | 153 (94.4) | |
| Alcohol intake, N (%) | 0.58 | ||
| Yes | 121 (74.7) | 126 (77.3) | |
| No | 41 (25.3) | 37 (22.7) | |
Note: The Bold N values in those rows are given to show that the number of women in the analyses was not the same as the total number in the whole cohort (N at top) due to missing data.
*p‐values calculated using independent t‐tests for continuous variables and chi‐square tests for categorical variables.
3.2. Impact on diet and exercise
Dietary data was successfully collected on 62% of participants at both time points. Among these participants, women in the intervention arm increased their total intact fruit intake from randomization to mid‐pregnancy more than women in usual care (0.67 vs. 0.06 cups; p = 0.02; Table 2). Although there were no other significant differences, in nearly every category there was a trend toward healthier diets among those in the intervention arm.
TABLE 2.
Impact of the prepare pre‐pregnancy intervention on diet at mid‐pregnancy
| Diet indicator/food group | Intervention group N = 58 | Usual care N = 51 | p value adjusted for baseline kcal |
|---|---|---|---|
| Energy density, food and beverage (kcal/g) b | |||
| Randomization | 0.71 (0.28) | 0.62 (0.22) | |
| Mid‐pregnancy a | 0.68 (0.31) | 0.69 (0.38) | |
| Mean change | −0.03 (0.37) | 0.07 (0.39) | 0.37 |
| HEI total score c | |||
| Randomization | 52.33 (10.08) | 54.99 (9.08) | |
| Mid‐pregnancy a | 57.63 (10.30) | 58.27 (11.65) | |
| Mean change | 5.30 (11.27) | 3.28 (12.60) | 0.51 |
| Fruit & vegetables (cup eq.) | |||
| Randomization | 3.00 (2.00) | 3.12 (1.67) | |
| Mid‐pregnancy a | 3.81 (1.94) | 3.07 (1.74) | |
| Mean change | 0.81 (2.51) | −0.06 (2.23) | 0.09 |
| Total intact fruit (cup eq.) | |||
| Randomization | 0.95 (0.92) | 1.23 (1.14) | |
| Mid‐pregnancy a | 1.62 (1.15) | 1.29 (1.21) | |
| Mean change | 0.67 (1.16) | 0.06 (1.38) | 0.02 |
| Total vegetables (cup eq.) | |||
| Randomization | 2.04 (1.81) | 1.90 (0.89) | |
| Mid‐pregnancy a | 2.18 (1.35) | 1.78 (1.12) | |
| Mean change | 0.14 (2.24) | −0.12 (1.41) | 0.57 |
| Whole grains (oz eq.) | |||
| Randomization | 0.91 (0.82) | 0.70 (0.80) | |
| Mid‐pregnancy a | 1.32 (1.26) | 1.45 (1.21) | |
| Mean change | 0.41 (1.39) | 0.75 (1.45) | 0.28 |
| Refined grains (oz. eq.) | |||
| Randomization | 5.56 (3.10) | 4.59 (3.20) | |
| Mid‐pregnancy a | 5.09 (2.84) | 4.75 (3.16) | |
| Mean change | −0.48 (4.26) | 0.17 (3.29) | 0.86 |
| Total milk, yogurt, cheese, whey (cup eq.) | |||
| Randomization | 1.79 (1.08) | 1.99 (1.12) | |
| Mid‐pregnancy a | 2.30 (1.50) | 2.19 (1.29) | |
| Mean change | 0.51 (1.83) | 0.20 (1.31) | 0.32 |
| Foods defined as added sugars (tsp. eq.) | |||
| Randomization | 16.11 (12.16) | 11.44 (6.93) | |
| Mid‐pregnancy a | 14.82 (11.98) | 11.53 (6.31) | |
| Mean change | −1.29 (16.46) | 0.09 (7.75) | 0.80 |
| Fiber (g) | |||
| Randomization | 19.85 (7.1) | 17.97 (5.99) | |
| Mid‐pregnancy a | 22.69 (8.46) | 21.88 (9.97) | |
| Mean change | 2.84 (9.70) | 3.91 (10.29) | 0.58 |
| Saturated fat Percentage | |||
| Randomization | 13.14 (3.71) | 13.49 (2.71) | |
| Mid‐pregnancy a | 12.34 (3.06) | 13.62 (4.00) | |
| Mean change | −0.804 (4.238) | 0.124 (3.862) | 0.29 |
| Fats naturally present in nuts, seeds, seafood (g) | |||
| Randomization | 26.53 (15.98) | 25.07 (12.84) | |
| Mid‐pregnancy a | 25.58 (14.89) | 28.41 (17.77) | |
| Mean change | −0.95 (20.99) | 3.34 (20.95) | 0.44 |
| Fats naturally present in meat, poultry, eggs, dairy (lard, tallow, butter) (g) | |||
| Randomization | 48.99 (29.04) | 41.36 (16.61) | |
| Mid‐pregnancy a | 37.77 (17.18) | 40.25 (22.53) | |
| Mean change | −11.22 (32.87) | −1.11 (22.18) | 0.26 |
Diet colleted at mean 21.3 (SD 3.1) weeks gestation.
Energy denity is the amount calories in a particular weight of food; foods with a lower energy density (e.g., fruits and vegetables) provide fewer calories per gram than foods with a higher energy density (e.g., bacon and eggs).
HEI scoresrange from 0 to 100; an HEI score of 100 reflects that the diet fully aligns with the dietary recommendations from the Dietary Guidelines for Americans.
Of the 66% who completed a physical activity questionnaire at both time points, women in the intervention arm reported fewer hours spent in sedentary activity at mid‐pregnancy than randomization (−4.9 [15.0] Met‐hr/week), while those in usual care reported a modest increase in number of hours spent in sedentary activity (+0.5 [7.6] Met‐hr/week) yielding a significant difference in change scores between the two arms (p = 0.02; Table 3). Compared to usual care participants, those in the intervention arm engaged in more vigorous‐intensity activity (3.9 [5.5] vs. 1.2 [3.0] Met‐hr/week; p = 0.002) and more sports/exercise (17.0 [14.1] vs. 11.0 [9.5] Met‐hr/week; p = 0.03) at mid‐pregnancy. There were no significant differences on other activity measures.
TABLE 3.
Impact of the prepare pre‐pregnancy intervention on physical activity at mid‐pregnancy
| Randomization | Mid‐pregnancy a | Mean change | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Exercise measurementsMet‐hr/week, Mean (SD) | Intervention N = 62 | Usual care N = 54 | p value d | Intervention N = 62 | Usual care N = 54 | p value d , e | Intervention N = 62 | Usual care N = 54 | p value e |
| Total activity | 222.9 | 210.4 | 0.93 | 206.3 | 196.0 | 0.66 | −16.6 | −14.4 | 0.68 |
| (156.4) | (90.3) | (90.0) | (74.6) | (102.6) | (64.4) | ||||
| Light intensity and above | 208.4 | 200.6 | 0.89 | 196.6 | 185.7 | 0.63 | −11.8 | −14.9 | 0.37 |
| (146.7) | (90.2) | (90.0) | (73.4) | (93.8) | (63.5) | ||||
| Intensity b | |||||||||
| Sedentary | 14.5 | 9.8 | 0.09 | 9.7 | 10.3 | 0.92 | −4.9 | 0.5 | 0.016 |
| (15.3) | (7.3) | (6.5) | (7.1) | (15.0) | (7.6) | ||||
| Light | 130.1 | 129.7 | 0.91 | 128.2 | 119.6 | 0.39 | −1.9 | −10.1 | 0.24 |
| (61.0) | (52.1) | (45.1) | (37.2) | (49.3) | (47.4) | ||||
| Moderate | 72.5 | 66.4 | 0.73 | 64.5 | 64.9 | 0.91 | −8.0 | −1.5 | 0.78 |
| (104.0) | (70.3) | (54.1) | (52.7) | (72.1) | (49.9) | ||||
| Vigorous | 5.7 | 4.5 | 0.86 | 3.9 | 1.2 | 0.0002 | −1.8 | −3.3 | 0.25 |
| (8.1) | (5.8) | (5.5) | (3.0) | (6.8) | (6.33) | ||||
| Types of activity | |||||||||
| Household/caregiving | 85.7 | 73.3 | 0.60 | 73.82 | 74.3 | 0.99 | −11.9 | 1.0 | 0.11 |
| (78.5) | (52.6) | (55.9) | (55.9) | (44.0) | (38.2) | ||||
| Occupational c | 81.2 | 80.9 | 0.36 | 79.3 | 73.9 | 0.41 | −2.2 | −6.5 | 0.38 |
| (72.5) | (46.3) | (41.6) | (33.0) | (50.0) | (41.4) | ||||
| Sports/exercise | 17.7 | 15.5 | 0.90 | 17.0 | 11.0 | 0.03 | −0.7 | −4.5 | 0.11 |
| (15.8) | (12.3) | (14.1) | (9.5) | (13.6) | (13.8) | ||||
| Transportation | 21.7 | 24.5 | 0.56 | 26.2 | 24.7 | 0.50 | 4.5 | 0.2 | 0.17 |
| (14.5) | (20.8) | (18.0) | (20.7) | (15.3) | (21.3) | ||||
| Inactivity | 22.9 | 20.7 | 0.75 | 16.3 | 18.8 | 0.59 | −6.6 | −1.9 | 0.22 |
| (22.7) | (12.5) | (9.7) | (74.6) | (23.2) | (12.0) | ||||
Abbreviation: METs, metabolic equivalents.
Physical activity collected at mean 18.0 (SD 1.8) weeks gestation.
Classified as sedentary if < 1.5 METs, light if 1.5–<3.0 METs, moderate if 3.0–6.0 METs, or vigorous if >6.0 METs.
Missing data on 10 intervention and 7 usual care participants.
p‐values calculated using linear regression on log‐transformed values.
Adjusted for baseline total activity.
3.3. Intervention engagement
All participants who became pregnant during the weekly or monthly portion of the intervention completed the intervention, compared with 92% of those who did not become pregnant during the intervention (p = 0.01, Fisher's exact test). Overall call completion rates were higher for participants who became pregnant during the intervention than those who did not (72% vs. 58%; p = 0.0007; Table 4). Completion in monthly calls was higher than in weekly calls in those who became pregnant (weekly calls: 69%; monthly calls: 87%; p < 0.0001), while the opposite pattern occurred for those who did not become pregnant (weekly calls: 62%; monthly calls: 53%; p = 0.02).
TABLE 4.
Prepare intervention engagement
| Pregnant during intervention | Not pregnant during intervention a | Pregnant versus not pregnant during intervention (overall across weekly and monthly phases) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N = 83 | N = 79 | N = 162 | |||||||
| Weekly phase | Monthly phase | Weekly versus monthly | Weekly phase | Monthly phase | Weekly versus monthly | Pregnant | Not pregnant | Pregnant versus not pregnant | |
| N = 83 | N = 83 | N = 74 b | N = 73 c | N = 83 | N = 73 c | ||||
| Mean (SD) | Mean (SD) | p‐value | Mean (SD) | Mean (SD) | p‐value | Mean (SD) | Mean (SD) | p‐value | |
| Phone calls | |||||||||
| Completion rate, percentage | 68.6 | 87.3 | <0.0001 | 62.0 | 53.0 | 0.02 | 71.61 | 58.39 | 0.0007 |
| (17.6) | (42.3) | (21.5) | (39.1) | (21.45) | (25.57) | ||||
| Average duration, minutes | 21.43 | 20.21 d | 0.03 | 22.24 | 18.52 e | <0.0001 | 21.05 | 21.23 | 0.86 |
| (6.63) | (8.69) | (6.44) | (6.05) | (6.65) | (6.09) | ||||
| Website engagement, days per week | |||||||||
| Weight | 4.53 | 2.49 | <0.0001 | 3.02 | 0.89 | <0.0001 | 3.27 | 1.48 | <0.0001 |
| (2.49) | (2.64) | (2.59) | (1.75) | (2.39) | (1.79) | ||||
| Food record completion | 4.32 | 2.09 | <0.0001 | 3.38 | 1.01 | <0.0001 | 3.00 | 1.67 | <0.0001 |
| (2.23) | (2.48) | (2.44) | (1.86) | (2.24) | (1.83) | ||||
| Exercise | 4.82 | 2.73 | <0.0001 | 3.48 | 1.13 | <0.0001 | 3.54 | 1.78 | <0.0001 |
| (2.35) | (2.81) | (2.60) | (1.95) | (2.45) | (1.95) | ||||
| Number of steps | 4.82 | 2.73 | <0.0001 | 3.65 | 1.28 | <0.0001 | 3.57 | 1.93 | <0.0001 |
| (2.38) | (2.79) | (2.66) | (2.09) | (2.44) | (2.02) | ||||
Either never pregnant or pregnant after intervention ended.
5 dropped out of the intervention during the weekly phase.
1 dropped out of the intervention during the monthly phase.
6 who did not have any monthly calls were not included.
1 who did not have any weekly calls and 11 who did not have any monthly calls were not included.
The duration of calls did not differ by pregnancy status, with calls decreasing in mean duration from the weekly to monthly time points in both arms (pregnancy during intervention: 21.4 versus 20.2 min, p = 0.03; no pregnancy during intervention: 22.2 versus 18.5 min; p < 0.0001). Website engagement (number of days on which data were recorded) was higher for participants who became pregnant during the intervention than those who did not (p < 0.001) and decreased for all participants regardless of pregnancy status from the weekly to the monthly phase (p < 0.0001).
3.4. Intervention acceptability
Of those who completed the post‐intervention surveys, 85% were very or moderately satisfied with the program and 84% reported they would definitely or probably recommend it to a friend (Table 5). The weekly coaching calls and initial in‐person meeting with a health coach received the most positive ratings (87% and 82% of respondents rated these as very or moderately helpful, respectively). The monthly coaching calls, email communication with health coaches, and certain website features (logging of weight, minutes of exercise, steps, and food record completion, as well as being able to see a graph of weight and physical activity changes over time) were also rated as helpful by most users (rates of positive responses ranged from 73% to 77%). The pedometer, CalorieKing calorie guide, and CalorieKing food diary/journal were not consistently rated as helpful (27%, 47%, and 47% positive responses, respectively).
TABLE 5.
Participant feedback about the prepare intervention
| Survey items | Percentage a of participants with positive response b |
|---|---|
| Very or moderately satisfied with the program | 85% |
| Would definitely or probably recommend program to a friend | 84% |
| How helpful was… | |
| Meeting in person with the health coach at the start of the study | 82% |
| Talking with my health coach each week by phone | 87% |
| Talking with my health coach each month by phone | 77% |
| The binder of materials I received | 57% |
| The pedometer I received | 27% |
| The calorie guide c I received | 47% |
| The food diary/journal d I received | 47% |
| Seeing the graph of my weight during the study | 77% |
| Seeing the graph of my physical activity during the study | 75% |
| The informational handouts (available as PDFs) | 52% |
| Sending email message to and receiving them from my health coach | 75% |
| Recording my weight, minutes of exercise, steps, and food intake | 73% |
Percentages refer to those who answered positively to the question among those who answered the question; 103 participants completed at least part of the survey, all questions had at least 99 participants,and 97 completed all survey questions.
Positive response defined as response of “Very helpful” or “Moderately helpful” nonpositive response defined as “Slightly helpful” or “Not helpful”.
CalorieKing Calorie, Fat & Carbohydrate Counter.
CalorieKing Food and Exercise Journal.
3.5. Facilitators and barriers
The central theme that emerged from the 16 post‐intervention qualitative interviews was that participation in the weekly health coach phone calls created effective accountability for weight loss and maintenance. The health coaches were generally described as very helpful, supportive, and critical to the success of the program. A major barrier in the eyes of participants was translating program learnings into their daily routines. In general, the interviewed participants’ expectations of achieving weight loss before pregnancy and maintaining a healthy weight during pregnancy were met.
4. DISCUSSION
A weight loss intervention initiated before pregnancy led to a larger increase in fruit intake, greater reduction in sedentary activity, and more time spent on sports/exercise and vigorous exercise at mid‐pregnancy compared to usual care. These changes aligned with the goals of the intervention, which included increasing the consumption of healthy foods such as fruit and increasing exercise while decreasing sedentary time. The intervention also received high acceptability scores by those who completed the program, and resulted in high levels of participation, particularly among women who became pregnant during the intervention. Taken together, these findings show the promise of interventions beginning before the start of pregnancy in engaging women and promoting positive diet and exercise behaviors during the critical early pregnancy window.
Healthier lifestyles in early pregnancy are associated with lower rates of pregnancy complications, childhood adiposity, and maternal and child cardiovascular risk factors. 64 , 65 , 66 However, pregnancy can be a challenging time to make healthy lifestyle changes, given nausea and fatigue. 67 , 68 Interventions for women planning pregnancy may have more success in impacting metabolic health in early pregnancy. Indeed, several organizations recommend healthy lifestyles in the period leading up to pregnancy. 35 , 69 However, prior to this study, there were no data on how to implement healthy lifestyle changes during this period, or whether changes made before pregnancy persist into the first half of pregnancy. 2 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 These data suggest that women can make healthy lifestyle changes prior to pregnancy and maintain these changes through the early pregnancy period. Long‐term follow‐up of this cohort is ongoing to assess if diet and physical activity differences persist and impact offspring lifestyles.
The program was well‐received by participants, with high acceptability ratings at the end of the intervention (delivery or 24 months after baseline), and becoming pregnant during the intervention was associated with higher levels of engagement. These results are consistent with the hypothesis that the periconceptional period provides a “teachable moment” when women are motivated to adopt risk‐reducing health behaviors, and that motivation to continue these behaviors increased in women who got pregnant during the intervention.
The intervention involved frequent study contacts, which has been identified as a key factor in the success of behavioral weight loss interventions, 70 , 71 , 72 and allowed coaches to tailor the intervention to each participant's needs and schedules. The coaching calls were identified by participants as the most helpful portion of the program, and decreased health coaching contacts, and thus lower levels of accountability, as women moved from weekly to monthly health coach contacts during later pregnancy may have contributed to the previously reported primary finding that GWG was greater in participants who received the intervention than usual care participants in later pregnancy. 37 , 70 , 73 , 74 , 75
The main barrier that participants identified was figuring out how to implement learnings from the intervention into their daily lives. Modules to help women continue to implement the program into their lives as accountability decreases would be helpful to include in future trials. Although the women found that logging their weight, exercise, and food record completion into the Prepare website was helpful, the tools supplied to help with tracking diet and exercise (the pedometer, CalorieKing calorie guide and companion food diary/journal) were not rated as very helpful by participants. Being able to upload their diet and exercise records (e.g., by providing integration with online tracker tools) might have been more helpful to the women in this study.
This study had several strengths, including that it was the first RCT of a behavioral weight loss intervention initiated in pre‐pregnancy. Previous studies had examined the impact of interventions started after pregnancy onset. 38 , 76 Recruitment goals were met, exceeding target pregnancies by 12%, and overall retention for follow‐up of the primary outcome (gestational weight gain) was 98%.
However, because recruitment occurred before pregnancy and not all women experienced pregnancy, measured differences in achieving pregnancy could have affected the comparisons. Also, only 62% and 67% of participants, respectively, had both diet and activity data at the baseline and mid‐pregnancy time points, presumably because the ASA 24 dietary recalls and physical activity questionnaire were somewhat burdensome for the participant population, particularly at the mid‐pregnancy timepoint. This leaves open the possibility that effects of the intervention on these outcomes may have differed among those who did not complete these measures. Similarly, the post‐intervention survey measuring intervention acceptability was only completed by 64% of the study sample, many of whom were in the early postpartum period; it is possible that those who did not respond had different perspectives on the intervention.
To reduce burden on participants, subjective self‐assessments of diet and activity were used. However, self‐report can be subject to recall bias and inaccuracies, which could have impacted the findings. The study population was also limited in that most participants were White and highly educated relative to the overall US population. The phone‐based intervention was designed to allow women to participate even if they had limited ability to attend frequent in‐person sessions, likely making this intervention more widely implementable among many different settings and populations; future research is needed to examine the acceptability and effects of the intervention in wide variety of settings and populations, as the impact of any intervention likely depends on social, environmental, and individual factors. 77
5. CONCLUSION
The Prepare intervention was well‐received by participants, had high participation rates, and led to improvements in diet and exercise at mid‐pregnancy. It can serve as a model for future interventions aimed at helping women modify their lifestyles for a healthier early pregnancy.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Erin S LeBlanc: Conceptualization; Funding acquisition; Methodology; Supervision; Visualization; Writing – original draft. Cassie Boisvert: Investigation; Writing – original draft. Chris Catlin: Investigation; Project administration; Writing – review & editing. Mi H Lee: Data curation; Formal analysis; Methodology; Validation; Visualization; Writing – review & editing. Ning Smith: Formal analysis; Methodology; Validation; Visualization; Writing – review & editing. Kimberly K. Vesco: Conceptualization; Funding acquisition; Supervision; Writing – review & editing. Jennifer Savage: Methodology; Writing – review & editing. Diane C. Mitchell: Methodology; Writing – review & editing. Inga Gruß: Investigation; Writing – review & editing. Victor J Stevens: Conceptualization; Funding acquisition; Supervision; Writing – review & editing.
ACKNOWLEDGMENTS
We thank the participants and staff of the Prepare study for their dedication to study. We also thank the following people at the Kaiser Center for Health Research (paid for by direct and indirect funding from a grant from NIDDK (R01/R56 KD099882): Neon Brooks, PhD, who assisted with editing, and Cassandra Angus, who contributed to paper formatting. Data Sharing Statement: Some or all datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request. Supported by grant from NIDDK (R01/R56 KD099882). The sponsor had no role in any of the following: design or conduct of the study; collection management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
LeBlanc ES, Boisvert C, Catlin C, et al. Prepare randomized clinical trial: acceptability, engagement, and lifestyle effects of a weight loss intervention beginning in pre‐pregnancy. Obes Sci Pract. 2022;8(5):603‐616. 10.1002/osp4.596
REFERENCES
- 1. Deputy NP, Dub B, Sharma AJ. Prevalence and trends in prepregnancy normal weight—48 states, New York City, and District of Columbia, 2011–2015. MMWR Morb Mortal Wkly Rep. 2018;66(51‐52):1402‐1407. 10.15585/mmwr.mm665152a3. Epub 2018/01/05. PubMed PMID: 29300720; PubMed Central PMCID: PMCPMC5758298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Retnakaran R, Wen SW, Tan H, et al. Association of timing of weight gain in pregnancy with infant birth weight. JAMA Pediatr. 2018;172(2):136‐142. 10.1001/jamapediatrics.2017.4016. Epub 2017/12/28. PubMed PMID: 29279903; PubMed Central PMCID: PMCPMC5796742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruager‐Martin R, Hyde MJ, Modi N. Maternal obesity and infant outcomes. Early Hum Dev. 2010;86(11):715‐722. 10.1016/j.earlhumdev.2010.08.007. Epub 2010/09/18. PubMed PMID: 20846795. [DOI] [PubMed] [Google Scholar]
- 4. Sen S, Carpenter AH, Hochstadt J, et al. Nutrition, weight gain and eating behavior in pregnancy: a review of experimental evidence for long‐term effects on the risk of obesity in offspring. Physiol Behav. 2012;107(1):138‐145. 10.1016/j.physbeh.2012.04.014. Epub 2012/05/02. PubMed PMID: 22546810. [DOI] [PubMed] [Google Scholar]
- 5. Hochner H, Friedlander Y, Calderon‐Margalit R, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow‐up Study. Circulation. 2012;125(11):1381‐1389. 10.1161/CIRCULATIONAHA.111.070060. Epub 2012/02/22. PubMed PMID: 22344037; PubMed Central PMCID: PMCPMC3332052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rooney BL, Mathiason MA, Schauberger CW. Predictors of obesity in childhood, adolescence, and adulthood in a birth cohort. Matern Child Health J. 2011;15(8):1166‐1175. 10.1007/s10995-010-0689-1. Epub 2010/10/12. PubMed PMID: 20927643. [DOI] [PubMed] [Google Scholar]
- 7. Jedrychowski W, Maugeri U, Kaim I, et al. Impact of excessive gestational weight gain in non‐smoking mothers on body fatness in infancy and early childhood. Prospective prebirth cohort study in Cracow. J Physiol Pharmacol. 2011;62(1):55‐64. Epub 2011/04/01. PubMed PMID: 21451210. [PubMed] [Google Scholar]
- 8. Kitsantas P, Pawloski LR, Gaffney KF. Maternal prepregnancy body mass index in relation to hispanic preschooler overweight/obesity. Eur J Pediatr. 2010;169(11):1361‐1368. 10.1007/s00431-010-1230-7. Epub 2010/06/10. PubMed PMID: 20532798. [DOI] [PubMed] [Google Scholar]
- 9. Reynolds RM, Osmond C, Phillips DI, Godfrey KM. Maternal BMI, parity, and pregnancy weight gain: influences on offspring adiposity in young adulthood. J Clin Endocrinol Metab. 2010;95(12):5365‐5369. 10.1210/jc.2010-0697. Epub 2010/08/13. PubMed PMID: 20702520. [DOI] [PubMed] [Google Scholar]
- 10. Mesman I, Roseboom TJ, Bonsel GJ, Gemke RJ, van der Wal MF, Vrijkotte TG. Maternal pre‐pregnancy body mass index explains infant's weight and BMI at 14 months: results from a multi‐ethnic birth cohort study. Arch Dis Child. 2009;94(8):587‐595. adc.2008.137737 [pii]; 10.1136/adc.2008.137737 [DOI] [PubMed] [Google Scholar]
- 11. Tequeanes AL, Gigante DP, Assuncao MC, Chica DA, Horta BL. Maternal anthropometry is associated with the body mass index and waist: height ratio of offspring at 23 years of age. J Nutr. 2009;139(4):750‐754. jn.108.100669[pii]; 10.3945/jn.108.100669 [DOI] [PubMed] [Google Scholar]
- 12. Gale CR, Javaid MK, Robinson SM, Law CM, Godfrey KM, Cooper C. Maternal size in pregnancy and body composition in children. J Clin Endocrinol Metab. 2007;92(10):3904‐3911. 10.1210/jc.2007-0088. Epub 2007/08/09. PubMed PMID: 17684051; PubMed Central PMCID: PMCPMC2066182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stamnes Koepp UM, Frost Andersen L, Dahl‐Joergensen K, Stigum H, Nass O, Nystad W. Maternal pre‐pregnant body mass index, maternal weight change and offspring birthweight. Acta Obstet Gynecol Scand. 2012;91(2):243‐249. 10.1111/j.1600-0412.2011.01321.x. Epub 2011/11/15. PubMed PMID: 22077818. [DOI] [PubMed] [Google Scholar]
- 14. Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90(5):1303‐1313. 10.3945/ajcn.2008.27416. Epub 2009/09/18. PubMed PMID: 19759171; PubMed Central PMCID: PMCPMC2762159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shankar K, Zhong Y, Kang P, et al. Maternal obesity promotes a proinflammatory signature in rat uterus and blastocyst. Endocrinology. 2011;152(11):4158‐4170. 10.1210/en.2010-1078. Epub 2011/08/25. PubMed PMID: 21862610; PubMed Central PMCID: PMCPMC3199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hellmuth C, Lindsay KL, Uhl O, et al. Association of maternal prepregnancy BMI with metabolomic profile across gestation. Int J Obes (Lond). 2017;41(1):159‐169. 10.1038/ijo.2016.153. Epub 2016/08/30. PubMed PMID: 27569686. [DOI] [PubMed] [Google Scholar]
- 17. Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1. 10.1136/bmj.j1. Epub 2017/02/10. PubMed PMID: 28179267; PubMed Central PMCID: PMCPMC6888512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Catalano P, deMouzon SH. Maternal obesity and metabolic risk to the offspring: why lifestyle interventions may have not achieved the desired outcomes. Int J Obes (Lond). 2015;39(4):642‐649. 10.1038/ijo.2015.15. Epub 2015/03/18. PubMed PMID: 25777180; PubMed Central PMCID: PMCPMC4700513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moll U, Olsson H, Landin‐Olsson M. Impact of pregestational weight and weight gain during pregnancy on long‐term risk for diseases. PLoS One. 2017;12(1):e0168543. 10.1371/journal.pone.0168543. Epub 2017/01/04. PubMed PMID: 28045917; PubMed Central PMCID: PMCPMC5207749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kalliala I, Markozannes G, Gunter MJ, et al. Obesity and gynaecological and obstetric conditions: umbrella review of the literature. BMJ. 2017;359:j4511. 10.1136/bmj.j4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adane AA, Mishra GD, Tooth LR. Maternal pre‐pregnancy obesity and childhood physical and cognitive development of children: a systematic review. Int J Obes (Lond). 2016;40(11):1608‐1618. 10.1038/ijo.2016.140. Epub 2016/08/17. PubMed PMID: 27528251. [DOI] [PubMed] [Google Scholar]
- 22. Alvarez‐Bueno C, Cavero‐Redondo I, Lucas‐de la Cruz L, Notario‐Pacheco B, Martinez‐Vizcaino V. Association between pre‐pregnancy overweight and obesity and children's neurocognitive development: a systematic review and meta‐analysis of observational studies. Int J Epidemiol. 2017;46(5):1653‐1666. 10.1093/ije/dyx122. Epub 2017/10/19. PubMed PMID: 29040611. [DOI] [PubMed] [Google Scholar]
- 23. Bartsch E, Medcalf KE, Park AL, Ray JG. High Risk of Pre‐eclampsia Identification G. Clinical risk factors for pre‐eclampsia determined in early pregnancy: systematic review and meta‐analysis of large cohort studies. BMJ. 2016;353:i1753. 10.1136/bmj.i1753. Epub 2016/04/21. PubMed PMID: 27094586; PubMed Central PMCID: PMCPMC4837230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fuemmeler BF, Wang L, Iversen ES, Maguire R, Murphy SK, Hoyo C. Association between prepregnancy body mass index and gestational weight gain with size, tempo, and velocity of infant growth: analysis of the newborn epigenetic study cohort. Child Obes Print. 2016;12(3):210‐218. 10.1089/chi.2015.0253. Epub 2016/05/03. PubMed PMID: 27135650; PubMed Central PMCID: PMCPMC4876550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev. 2015;16(8):621‐638. 10.1111/obr.12288. Epub 2015/05/29. PubMed PMID: 26016557. [DOI] [PubMed] [Google Scholar]
- 26. Cheney K, Berkemeier S, Sim KA, Gordon A, Black K. Prevalence and predictors of early gestational weight gain associated with obesity risk in a diverse Australian antenatal population: a cross‐sectional study. BMC Pregnancy Childbirth. 2017;17(1):296. 10.1186/s12884-017-1482-6. Epub 2017/09/09. PubMed PMID: 28882122; PubMed Central PMCID: PMCPMC5590236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davenport MH, Ruchat SM, Giroux I, Sopper MM, Mottola MF. Timing of excessive pregnancy‐related weight gain and offspring adiposity at birth. Obstet Gynecol. 2013;122(2 Pt 1):255‐261. 10.1097/AOG.0b013e31829a3b86. Epub 2013/08/24. PubMed PMID: 23969792. [DOI] [PubMed] [Google Scholar]
- 28. Starling AP, Brinton JT, Glueck DH, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr. 2015;101(2):302‐309. 10.3945/ajcn.114.094946. Epub 2015/02/04. PubMed PMID: 25646327; PubMed Central PMCID: PMCPMC4307203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hivert MF, Rifas‐Shiman SL, Gillman MW, Oken E. Greater early and mid‐pregnancy gestational weight gains are associated with excess adiposity in mid‐childhood. Obes (Silver Spring). 2016;24(7):1546‐1553. 10.1002/oby.21511. Epub 2016/06/28. PubMed PMID: 27345963; PubMed Central PMCID: PMCPMC4968400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Margerison‐Zilko CE, Shrimali BP, Eskenazi B, Lahiff M, Lindquist AR, Abrams BF. Trimester of maternal gestational weight gain and offspring body weight at birth and age five. Matern Child Health J. 2012;16(6):1215‐1223. 10.1007/s10995-011-0846-1. Epub 2011/07/08. PubMed PMID: 21735140. [DOI] [PubMed] [Google Scholar]
- 31. Karachaliou M, Georgiou V, Roumeliotaki T, et al. Association of trimester‐specific gestational weight gain with fetal growth, offspring obesity, and cardiometabolic traits in early childhood. Am J Obstet Gynecol. 2015;212(4):502‐14.e1. 10.1016/j.ajog.2014.12.038. Epub 2015/01/06. PubMed PMID: 25557209; PubMed Central PMCID: PMCPMC5081180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gaillard R, Welten M, Oddy WH, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with cardio‐metabolic risk factors in adolescent offspring: a prospective cohort study. BJOG. 2016;123(2):207‐216. 10.1111/1471-0528.13700. Epub 2015/11/04. PubMed PMID: 26525168. [DOI] [PubMed] [Google Scholar]
- 33. Andersen CS, Gamborg M, Sorensen TI, Nohr EA. Weight gain in different periods of pregnancy and offspring's body mass index at 7 years of age. Int J Pediatr Obes. 2011;6(2‐2):e179‐e186. 10.3109/17477166.2010.521560. Epub 2010/10/05. PubMed PMID: 20883124. [DOI] [PubMed] [Google Scholar]
- 34. Josefson JL, Simons H, Zeiss DM, Metzger BE. Excessive gestational weight gain in the first trimester among women with normal glucose tolerance and resulting neonatal adiposity. J Perinatol. 2016;36(12):1034‐1038. 10.1038/jp.2016.145. Epub 2016/09/02. PubMed PMID: 27583397; PubMed Central PMCID: PMCPMC5130601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Institute of Medicine (US) and National Research Council (US) . Committee to Reexamine IOM pregnancy weight guidelines. Setting the stage for revising pregnancy weight guidelines: conceptual framework. In: Rasmussen KM, Yaktine AL, eds. Weight gain during pregnancy: reexamining the guidelines. National Academies Press; 2009:1‐13. [PubMed] [Google Scholar]
- 36. LeBlanc ES, Vesco KK, Funk KL, Karanja N, Smith N, Stevens VJ. Prepare, a randomized trial to promote and evaluate weight loss among overweight and obese women planning pregnancy: study design and rationale. Contemp Clin Trials. 2016;49:174‐180. 10.1016/j.cct.2016.07.002. Epub 2016/07/11. PubMed PMID: 27394386; PubMed Central PMCID: PMCPMC5685182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. LeBlanc ES, Smith NX, Vesco KK, Paul IM, Stevens VJ. Weight loss prior to pregnancy and subsequent gestational weight gain: prepare, a randomized clinical trial. Am J Obstet Gynecol. 2021;224(1):99.e1‐e14. 10.1016/j.ajog.2020.07.027. Epub 2020/07/21. PubMed PMID: 32687819. [DOI] [PubMed] [Google Scholar]
- 38. Cantor AG, Jungbauer RM, McDonagh M, et al. Counseling and behavioral interventions for healthy weight and weight gain in pregnancy: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(20):2094‐2109. 10.1001/jama.2021.4230. Epub 2021/05/26. PubMed PMID: 34032824. [DOI] [PubMed] [Google Scholar]
- 39. Borushek A. The CalorieKing Calorie, Fat & Carbohydrate Counter 2015. Family Health Publications(CA); 2015 September 30th 2014:287. [Google Scholar]
- 40. Borushek A. The CalorieKing Food & Exercise Journal. Family Health Publications; 2006. [Google Scholar]
- 41. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH‐style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713‐720. 10.1001/archinte.168.7.713. Epub 2008/04/17. PubMed PMID: 18413553. [DOI] [PubMed] [Google Scholar]
- 42. Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr. 1984;40(1):168‐182. 10.1093/ajcn/40.1.168. Epub 1984/07/01. PubMed PMID: 6741850. [DOI] [PubMed] [Google Scholar]
- 43. American College of Obstetricians and Gynecologists . Nutrition During Pregnancy FAQ00111/2012. Available from: http://www.acog.org/∼/media/For%20Patients/faq001.pdf?dmc=1&ts=20130628T0140151155 [Google Scholar]
- 44. U.S. Department of Health and Human Services and U.S.Department of Agriculture . Dietery Guidelines for Americans; 2010. Report No. [Google Scholar]
- 45. Stevens VJ, Funk KL, Brantley PJ, et al. Design and implementation of an interactive website to support long‐term maintenance of weight loss. J Med Internet Res. 2008;10(1):e1. 10.2196/jmir.931. Epub 2008/02/05. PubMed PMID: 18244892; PubMed Central PMCID: PMCPMC2483846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Watson DL. Self‐Directed Behavior: Self‐Modification for Personal Adjustment. 5th ed. Brooks/Cole; 1989. [Google Scholar]
- 47. Miltenberger RG. Behavior Modification: Principles and Procedures. 4th ed. Thomson/Wadsworth; 2008. [Google Scholar]
- 48. Prochaska JO, DiClemente CC. Stages and processes of self‐change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390‐395. PubMed PMID: 6863699. [DOI] [PubMed] [Google Scholar]
- 49. Bock BC, Marcus BH, Pinto BM, Forsyth LH. Maintenance of physical activity following an individualized motivationally tailored intervention. Ann Behav Med. 2001;23(2):79‐87. 10.1207/S15324796ABM2302_2. PubMed PMID: 11394558. [DOI] [PubMed] [Google Scholar]
- 50. Miller WR, Stephen R. Motivational Interviewing: Preparing People to Change Addictive Behavior. The Guilford Press; 1991. [Google Scholar]
- 51. Rollnick Pm S, Butler C. Health Behavior Change: A Guide for Practitioners. London Churchill Livingston; 1999. [Google Scholar]
- 52. Rifas‐Shiman SL, Rich‐Edwards JW, Willett WC, Kleinman KP, Oken E, Gillman MW. Changes in dietary intake from the first to the second trimester of pregnancy. Paediatr Perinat Epidemiol. 2006;20(1):35‐42. 10.1111/j.1365-3016.2006.00691.x. Epub 2006/01/20. PubMed PMID: 16420339; PubMed Central PMCID: PMCPMC1488723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kirkpatrick SI, Subar AF, Douglass D, et al. Performance of the Automated Self‐Administered 24‐hour Recall relative to a measure of true intakes and to an interviewer‐administered 24‐h recall. Am J Clin Nutr. 2014;100(1):233‐240. 10.3945/ajcn.114.083238. Epub 2014/05/03. PubMed PMID: 24787491; PubMed Central PMCID: PMCPMC4144101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. National Cancer Institute Division of Cancer Control & Population Sciences . Automated Self‐Administered 24‐hour. (ASA24®) Dietary Recall System 2016 [updated 0329201606212016]. Available from: http://epi.grants.cancer.gov/asa24/ [Google Scholar]
- 55. National Cancer Institute Division of Cancer Control & Population Sciences Epidemiology and Genomics Research Program . Reviewing & Cleaning ASA24® Data [updated 06/03/202006/29/2020]. Available from: https://epi.grants.cancer.gov/asa24/resources/cleaning.html [Google Scholar]
- 56. Ledikwe JH, Rolls BJ, Smiciklas‐Wright H, et al. Reductions in dietary energy density are associated with weight loss in overweight and obese participants in the PREMIER trial. Am J Clin Nutr. 2007;85(5):1212‐1221. 10.1093/ajcn/85.5.1212. Epub 2007/05/11. PubMed PMID: 17490955. [DOI] [PubMed] [Google Scholar]
- 57. Vernarelli JA, Mitchell DC, Rolls BJ, Hartman TJ. Dietary energy density is associated with obesity and other biomarkers of chronic disease in US adults. Eur J Nutr. 2015;54(1):59‐65. 10.1007/s00394-014-0685-0. Epub 2014/03/26. PubMed PMID: 24664188; PubMed Central PMCID: PMCPMC4176562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vernarelli JA, Mitchell DC, Rolls BJ, Hartman TJ. Methods for calculating dietary energy density in a nationally representative sample. Procedia Food Sci. 2013;2:68‐74. 10.1016/j.profoo.2013.04.011. Epub 2014/01/17. PubMed PMID: 24432201; PubMed Central PMCID: PMCPMC3889114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang J, Luben R, Khaw KT, Bingham S, Wareham NJ, Forouhi NG. Dietary energy density predicts the risk of incident type 2 diabetes: the European Prospective Investigation of Cancer (EPIC)‐Norfolk Study. Diabetes Care. 2008;31(11):2120‐2125. 10.2337/dc08-1085. Epub 2008/08/12. PubMed PMID: 18689693; PubMed Central PMCID: PMCPMC2571060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schwingshackl L, Hoffmann G. Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: a systematic review and meta‐analysis of cohort studies. J Acad Nutr Diet. 2015;115(5):780‐800.e5. 10.1016/j.jand.2014.12.009. Epub 2015/02/15. PubMed PMID: 25680825. [DOI] [PubMed] [Google Scholar]
- 61. Al‐Ibrahim AA, Jackson RT. Healthy eating index versus alternate healthy index in relation to diabetes status and health markers in US adults: NHANES 2007–2010. Nutr J. 2019;18(1):26. 10.1186/s12937-019-0450-6. Epub 2019/04/19. PubMed PMID: 30995902; PubMed Central PMCID: PMCPMC6471947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tande DL, Magel R, Strand BN. Healthy eating index and abdominal obesity. Public Health Nutr. 2010;13(2):208‐214. 10.1017/S1368980009990723. Epub 2009/08/05. PubMed PMID: 19650960. [DOI] [PubMed] [Google Scholar]
- 63. Chasan‐Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a pregnancy physical activity Questionnaire. Med Sci Sports Exerc. 2004;36(10):1750‐1760. 10.1249/01.mss.0000142303.49306.0d. Epub 2004/12/15. PubMed PMID: 15595297. [DOI] [PubMed] [Google Scholar]
- 64. The American College of Obstetricians and Gynecologists . ACOG Committee Opinion No. 804 Physical Activity and Exercise during Pregnancy and the Postpartum Period 2020 [07/20/2020]. Available from: https://www.acog.org/clinical/clinical‐guidance/committee‐opinion/articles/2020/04/physical‐activity‐and‐exercise‐during‐pregnancy‐and‐the‐postpartum‐period [Google Scholar]
- 65. Koletzko B, Godfrey KM, Poston L, et al. EarlyNutrition project systematic review G. Nutrition during pregnancy, lactation and early childhood and its implications for maternal and long‐term child health: the early nutrition project recommendations. Ann Nutr Metab. 2019;74(2):93‐106. 10.1159/000496471. Epub 2019/01/24. PubMed PMID: 30673669; PubMed Central PMCID: PMCPMC6397768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dipietro L, Evenson KR, Bloodgood B, et al. Physical activity guidelines Advisory C. Benefits of physical activity during pregnancy and postpartum: an umbrella review. Med Sci Sports Exerc. 2019;51(6):1292‐1302. 10.1249/MSS.0000000000001941. Epub 2019/05/17. PubMed PMID: 31095086; PubMed Central PMCID: PMCPMC6527310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Morris T, Strommer S, Vogel C, et al. Improving pregnant women's diet and physical activity behaviours: the emergent role of health identity. BMC Pregnancy Childbirth. 2020;20(1):244. 10.1186/s12884-020-02913-z. Epub 2020/04/27. PubMed PMID: 32334540; PubMed Central PMCID: PMCPMC7183631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr. 2010;91(2):373‐380. 10.3945/ajcn.2009.28166. Epub 2009/12/04. PubMed PMID: 19955397. [DOI] [PubMed] [Google Scholar]
- 69. American College of Obstetricians and Gynecologists . ACOG Committee opinion no. 549: obesity in pregnancy. Obstet Gynecol. 2013;121(1):213‐217. http://10.1097/01.AOG.0000425667.10377.60 Epub 2012/12/25. PubMed PMID: 23262963. [DOI] [PubMed] [Google Scholar]
- 70. LeBlanc ELPC, Webber EM, Redmond N, Rushkin M, O’Connor EA. Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity‐Related Morbidity and Mortality in Adults: An Updated Systematic Review for the US Preventive Services Task Force. Evidence Synthesis Rockville, MD; 2018. February. Report No. [DOI] [PubMed] [Google Scholar]
- 71. Hill B, Skouteris H, Fuller‐Tyszkiewicz M. Interventions designed to limit gestational weight gain: a systematic review of theory and meta‐analysis of intervention components. Obes Rev. 2013;14(6):435‐450. 10.1111/obr.12022. Epub 2013/03/29. PubMed PMID: 23534901. [DOI] [PubMed] [Google Scholar]
- 72. Leahey TM, Wing RR. A randomized controlled pilot study testing three types of health coaches for obesity treatment: professional, peer, and mentor. Obesity (Silver Spring). 2013;21(5):928‐934. 10.1002/oby.20271. Epub 2013/06/21. PubMed PMID: 23784896; PubMed Central PMCID: PMCPMC3484232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Svetkey LP, Stevens VJ, Brantley PJ, et al. Weight loss maintenance collaborative research G. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139‐1148. 10.1001/jama.299.10.1139. Epub 2008/03/13. PubMed PMID: 18334689. [DOI] [PubMed] [Google Scholar]
- 74. Ross KM, Qiu P, You L, Wing RR. Characterizing the pattern of weight loss and regain in adults enrolled in a 12‐week internet‐based weight management program. Obesity (Silver Spring). 2018;26(2):318‐323. 10.1002/oby.22083. Epub 2017/12/15. PubMed PMID: 29239141; PubMed Central PMCID: PMCPMC5783775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. MacLean PS, Wing RR, Davidson T, et al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity (Silver Spring). 2015;23(1):7‐15. 10.1002/oby.20967. Epub 2014/12/04. PubMed PMID: 25469998; PubMed Central PMCID: PMCPMC5841916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Peaceman AM, Clifton RG, Phelan S, et al. Lifestyle interventions limit gestational weight gain in women with overweight or obesity: LIFE‐Moms prospective meta‐analysis. Obesity (Silver Spring). 2018;26(9):1396‐1404. 10.1002/oby.22250. Epub 2018/09/20. PubMed PMID: 30230252; PubMed Central PMCID: PMCPmc6148360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. U.S. Preventive Services Task Force , Curry SJ, Krist AH, Owens DK, et al. Behavioral weight loss interventions to prevent obesity‐related morbidity and mortality in adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;320(11):1163‐1171. 10.1001/jama.2018.13022. Epub 2018/10/17. PubMed PMID: 30326502. [DOI] [PubMed] [Google Scholar]


