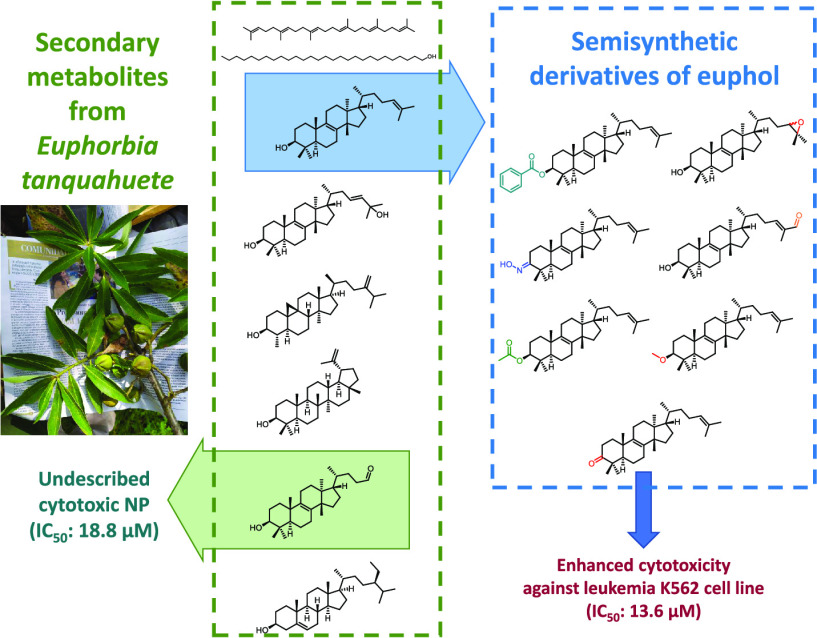
Abstract
The natural compound 25,26,27-trisnor-3β-hydroxy-euphan-24-al (1) was isolated for the first time from the bioactive extract of the leaves of Euphorbia tanquahuete, together with the known compounds euphol, eupha-8,23-dien-3β,25-diol, lupeol, cycloeucalenol, β-sitosterol, squalene, and 1-octacosanol. The structure of the new compound was elucidated based on extensive analysis of spectroscopic data and by semisynthesis from euphol. The chemical modification of the alcohol at C3 and the side chain of euphol afforded seven derivatives (6–12). The cytotoxic activity of the natural and semisynthetic compounds evaluated against a panel of human cancer cell lines showed selectivity for certain cell lines and indicated that natural compound 1 and semisynthetic 8 were the most active against leukemia (K562) cell line.
Introduction
The genus Euphorbia (family Euphorbiaceae, subfamily Euphorbioideae) is one of the largest groups of the angiosperms comprising ∼2000 species that are distributed worldwide.1 Its recognized morphological variety is reflected in the great diversity of secondary metabolites, including terpenoids, steroids, glycerols, acetophenones, and flavonoids, inter alia, displaying a wide array of biological activities2−4 considered relevant in human health.5Euphorbia is also recognized as one of the most diverse genera of Mexican vascular plants.6,7 Following our research on the bioactive constituents of the spurge family,8,9 here we report (i) the chemical constituents of the bioactive extract of the aerial parts of Euphorbia tanquahuete Sessé & Moc. (Euphorbiaceae), a tree found in the central-southern region of Mexico that is used in traditional medicine to treat bone fractures10,11 from which we identify the cytotoxic compounds and (ii) the preparation and preliminary cytotoxic evaluation of a series of derivatives of euphol,12,13 the major bioactive metabolite of this plant, which led to the discovery of selectivity and enhanced cytotoxicity of the derivatives.
Results and Discussion
Structural Elucidation of Isolated Compounds
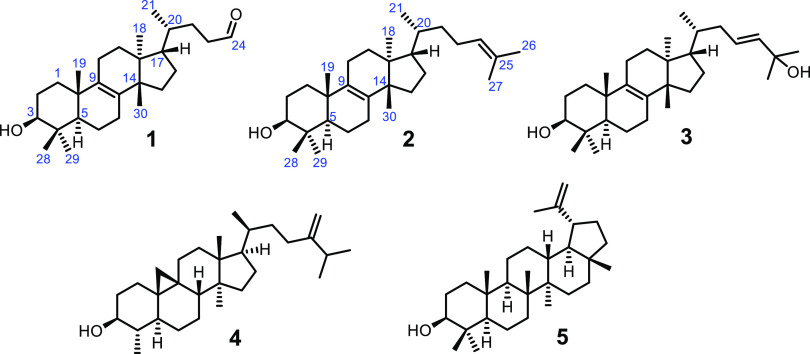
The methylene chloride/methanol extract of the aerial parts of E. tanquahuete exhibited activity against a panel of human cancer cells (see Table 2). This extract was subjected to successive chromatographic procedures affording an undescribed trisnor triterpene (1) and seven known compounds eupha-8,24-dien-3β-ol (euphol, 2),14,15 eupha-8,23-dien-3β,25-diol (3),16,17 lupeol (4),18 cycloeucalenol (5),19 β-sitosterol,20 squalene,21 and 1-octacosanol,22,23 whose structures were confirmed by comparison of spectroscopic data with those reported in the literature (Figure 1).
Table 2. Cytotoxic Activities (% of Inhibition) of the Extract, Natural Products, and Derivativesa.
| sample | U251 | PC-3 | K562 | HCT-15 | MCF-7 | SKLU-1 | COS7 |
|---|---|---|---|---|---|---|---|
| CH2Cl2/CH3OH 1:1 (leaves extract) | 52.31 | 43.6 | 74.63 | 34.5 | 62.45 | 58.35 | NP |
| (1) | 59.8 | 87.2 | 100 | 74.6 | 61.4 | 88.4 | 22.0 |
| euphol (2) | NA | NA | 26.9 | 2.18 | 36.62 | 4.9 | NA |
| lupeol (5) | 27.3 | 50.7 | 48.8 | 10.4 | 22.3 | 13.0 | NP |
| (6) | NA | NA | 33.1 | 15.6 | 1.3 | 10.9 | NA |
| (7) | NA | NA | 39.8 | 39.4 | 20.1 | 40.0 | NA |
| (8) | NA | NA | 95.0 | 12.2 | 1.5 | 31.3 | 17.2 |
| (10) | NA | NA | 57.8 | 4.3 | 4.3 | 21.6 | NA |
| (12) | NA | NA | 34.1 | 16.4 | NA | 4.6 | NA |
| etoposideb | 91.1c | 51.4d | 60.2d | 80.8d | 56.8d | 81.7d | NP |
NA: no activity; ND: not determined. Human tumor cell lines: U251 (glioblastoma), PC-3 (prostate), K562 (leukemia), HCT-15 (colon), MCF-7 (breast), and SKLU-1 (lung). COS7: noncancerous cell line of monkey kidney.
Concentrations: 50 μg/mL for the extract, 50 μM for pure compounds, DMSO vehicle.
Positive control.
Concentration at 10 μM.
Concentration at 31 μM.
Figure 1.
Chemical structures of natural compounds 1–5.
The undescribed natural compound 1 was obtained as colorless needles (n-hexane). Its molecular formula was determined as C27H44O2 by HRESIMS, which showed a pseudo-molecular ion peak at m/z 401.34118 [M + H]+ (calcd. for C27H45O2 401.34195), indicating six unsaturations. The IR spectrum indicated absorption bands for hydroxyl (3613 cm–1) and carbonyl (1709 cm–1) groups. A total of 27 carbon signals were observed in the 13C NMR spectrum (Table 1), consistent with the found molecular formula; based on DEPT-90 and DEPT-135 experiments, these carbons were classified as six methyls, ten methylenes, five methines, and six quaternary carbons including two vinylic carbons, which indicated the presence of a tetrasubstituted olefin. The 13C spectrum also showed a carbonyl signal at δC 203.30, justifying the absorption band observed in the IR spectrum, and the signal at δH 9.78 established the presence of an aldehyde. Therefore, this compound was determined as a tetracyclic compound with a tetrasubstituted olefin and an aldehyde, in agreement with the number of unsaturations. The 1H NMR spectrum (Table 1) showed five methyl singlets at δH 0.77, 0.80, 0.88, 0.95, and 1.00 (each 3H), a secondary methyl signal at δH 0.85 (3H, d, J = 6.4 Hz), and an oxy-methine proton at δH 3.24 (1H, dd, J = 11.3, 4.4 Hz), which could be assigned, according to the coupling constants, to a hydrogen geminal to a β-oriented hydroxyl group at C3 of the tetracyclic triterpenes. Taken together, this information suggested that compound 1 was a euphane- or tirucallane-like triterpenoid with three missing carbons. Comparison of 1H and 13C NMR data of compound 1 with our sample of eupha-8,24-dien-3β-ol (2) showed very similar chemical shifts with a remarkable absence of the vinylic methyl singlets in 1, indicating the loss of C25, C26, and C27, and that the aldehyde group is located at C24.24,25 HMBC cross-peaks of H3 (δH 3.24) with C2/C4/C5/C28/C29, of H3-19 (δH 0.95) with C1/C5/C10/C9, of H3-18 (δH 0.77) with C13/C12/C17/C14, and of H3-20 (δH 1.52) with C21/C22/C17/C13 confirmed the molecular connectivity for compound 1.
Table 1. 1H (400 MHz) and 13C NMR (100 MHz) Data, DEPT, and HMBC Correlations of Compound 1 in CDCl3.
| position | δC, type | δH (J in Hz) | HMBC |
|---|---|---|---|
| 1 | 35.37, CH2 | 1.19, 1.75, m | C19, C10, |
| 2 | 28.07, CH2 | 0.82, 1.38, m | C1, C3, C4, C10 |
| 3 | 79.00, CH | 3.24, dd (11.3, 4.4) | C4, C28, C29 |
| 4 | 39.09, C | ||
| 5 | 51.10, CH | 1.25, dd (12.4, 2.0) | C19, C28, C29 |
| 6 | 19.08, CH2 | 1.42, 1.69, m | C4, C5, C10, C8, C7 |
| 7 | 27.80, CH2 | 1.38, 2.13, m | C5, C6, C8, C9, C14 |
| 8 | 133.55, C | ||
| 9 | 134.24, C | ||
| 10 | 37.42, C | ||
| 11 | 21.62, CH2 | 1.95, 2.07, m | C8, C9, C10, C12, C13 |
| 12 | 31.03, CH2 | 1.61–1.75, m | C9, C11, C13, C14, C18 |
| 13 | 44.28, C | ||
| 14 | 50.19, C | ||
| 15 | 29.86, CH2 | 1.22, 1.52, m | C30, C13, C14, C16, C17 |
| 16 | 28.23, CH2 | 0.92–1.09, m | |
| 17 | 49.62, CH | 1.50, m | C13, C18, C20, C21, C16 |
| 18 | 15.68, CH3 | 0.77, s | C12, C13, C14, C17 |
| 19 | 20.29, CH3 | 0.95, s | C1, C5, C9, C10 |
| 20 | 35.61, CH | 1.52, m | C21, C22, C17, C13 |
| 21 | 18.94, CH3 | 0.85, d (6.4) | C17, C20, C22 |
| 22 | 41.10, CH2 | 2.32–2.50, m | C20, C23, C24 |
| 23 | 27.40, CH2 | 1.43, 1.99, m | |
| 24 | 203.30, CH | 9.78, t (2.0) | C22, C23 |
| 28 | 15.80, CH3 | 0.80, s | C4, C5, C3, C29 |
| 29 | 28.20, CH3 | 1.00, s | C3, C4, C5, C28 |
| 30 | 24.59, CH3 | 0.88, s | C8, C13, C14, C15 |
For further identification, compound 1 was semisynthesized from eupha-8,24-dien-3β-ol (2) via oxidative cleavage of the olefin by treatment with mCPBA followed by H5IO6, as described by O’Keeffe et al.26 The properties of semisynthetic 1 were identical to those of the natural compound. Furthermore, the acetylated form of compound 1 was previously reported, and its 1H NMR is in agreement with the expected chemical shift changes of H3 (δH 4.48 for the ester and δH 3.24 for the isolated compound).14
Preparation of Semisynthetic Derivatives of Euphol (6–12)
Taking into account the functional groups of euphol (2), we decided to modify the A ring and the side chain and to identify the changes in the cytotoxicity of the derivatives. Compound 2 was used as starting material for the preparation of the semisynthetic derivatives 6–12 (Figure 2). Ring A modifications consisted in varying the C3 functional group (as in 6–10), and compounds 11 and 12 carried modifications at the side chain. As mentioned previously, the oxidative cleavage of eupha-8,24-dien-3β-ol (2) allowed the chemical correlation to obtain a new natural compound (1).
Figure 2.
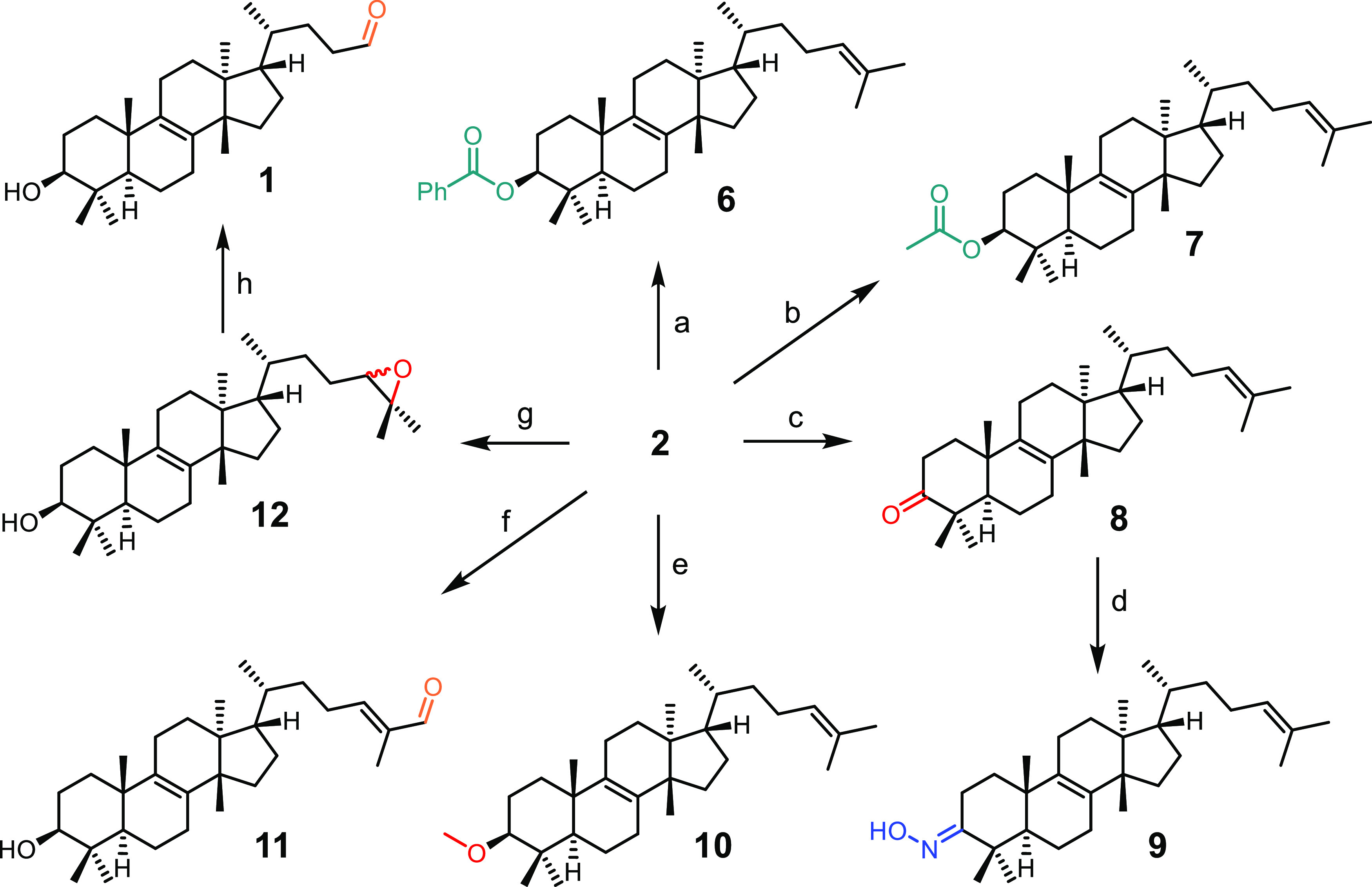
Reaction scheme for the preparation of derivatives of euphol (2). (a) BzCl, py; (b) Ac2O, py; (c) Jones reagent; (d) NH2OH·HCl, NaOAc; (e) CH3I, NaH; (f) SeO2; (g) mCPBA; and (h) H5IO6.
Esters 6 and 7 were synthesized by reacting euphol (2) with benzoyl chloride and acetic anhydride, respectively. Euphone (8) was obtained by reaction of 2 with Jones reagent, and ketone 8 in turn served as the starting material for the preparation of the oxime 9. The preparation of methyl ether 10 was achieved by reaction with methyl iodide in the presence of NaH. Allylic oxidation of euphol (2) with SeO2 afforded α,β-unsaturated aldehyde 11. Derivative 12 was prepared by reaction of compound 2 with mCPBA. Epoxide 12 was used in turn for the preparation of compound 1 through oxidative cleavage with H5IO6. Semisynthetic compounds 6–12 were characterized by their physical and spectroscopic characteristics. It is noteworthy that although derivatives 6–9 were previously prepared, here we report the complete characterization for these compounds. Furthermore, this is the first report for semisynthetic derivatives 10–12.
X-ray Structure Analysis
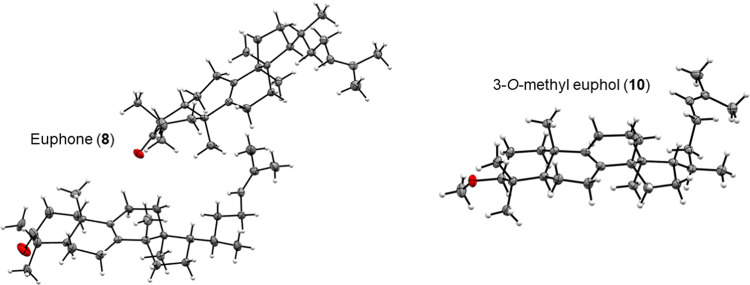
Ketone 8 and methyl ether 10 afforded appropriate crystals for X-ray diffraction by recrystallization from iPrOH and MeOH, respectively. X-ray crystal diffraction analysis confirmed the absolute configuration of these compounds [Flack parameter: 0.09(6) and 0.09(4), respectively], and therefore, the starting material and the other derivatives have the connectivity and stereochemistry of an euphane core. Inspection of the crystalline structure (Figure 3) shows that the asymmetric unit for the crystal of derivative 8 is composed of two stacked molecules, each one with a different orientation and conformation. For both derivatives, the side chain orientation in the crystalline structure is defined by the R configuration of C20, which favors an anti-periplanar arrangement of the hydrogens at the C17–C20 bond. This is consistent with the conformation found in the crystalline structure of acetyl derivative 7 reported in the literature.27 Parameters on the crystallographic information file (CIF) format of compounds 8 and 10 were deposited at the Cambridge Crystallographic Data Centre [CCDC2178145 (8) and CCDC2181277 (10)] (details in the Supporting Information).
Figure 3.
ORTEP drawing of X-ray structure of euphone (8) and 3-O-methyl euphol (10).
Cytotoxic Activity
The cytotoxic activity was evaluated for the extract, natural products 1, 2, and 5, and derivatives 6–8, 10, and 12 as percentages of inhibition of proliferation against the following tumor cell lines (see Table 2): glioblastoma (U251), prostate (PC-3), leukemia (K562), colon (HCT-15), breast (MCF-7), and lung (SKLU-1). The cytotoxic evaluation indicated that compound 1 was the most active among the natural products, in agreement with the observed activity of the extract. Complementarily, the most abundant secondary metabolites of the extract of E. tanquahuete, euphol (2) and lupeol (5), showed activity in some cell lines. The results also indicated remarkable selectivity of euphol (2) and its derivatives since they did not display activity in two cell lines (U251 and PC-3) and in the noncancerous cell line (COS7). The IC50 values are determined for compounds 1 and 8 and are shown in Table 3, indicating that ketone 8 displayed better activity than the natural product 1 in the leukemia cell line.
Table 3. IC50 (μM) for Compounds 1 and 8.
| cancerous
cell lines |
||||
|---|---|---|---|---|
| sample | U251 | K562 | HCT-15 | SKLU-1 |
| (1) | 30.9 ± 1.3 | 18.8 ± 0.5 | 39.0 ± 2.9 | 39.9 ± 1.6 |
| (8) | ND | 13.6 ± 0.7 | ND | ND |
| etoposide | 2.4 ± 0.2 | 2.2 ± 0.7 | 4.8 ± 0.5 | 2.6 ± 0.3 |
ND: not determined. Human tumor cell lines: U251 (glioblastoma), K562 (leukemia), HCT-15 (colon), and SKLU-1 (lung).
Conclusions
Squalene, 1-octacosanol, β-sitosterol, and compounds 1–5 have been reported for the first time from the aerial parts of E. tanquahuete, compound 1 being identified as a new natural product displaying high toxicity against some cancer cell lines. Derivatives 6–12 were prepared from the majoritarian constituent, euphol (2). The cytotoxic evaluation of euphol (2) and its derivatives (6–8, 10, and 12) showed that they were inactive against U251 and PC-3 cell lines. Nevertheless, all of the semisynthetic derivatives showed higher cytotoxicity than the parent natural compound against the K562 and SKLU-1 cell lines, displaying significant selectivity. Derivative 8 showed the best activity in the leukemia cell line (K562). Therefore, compound 1 and the semisynthetic derivatives of the natural compound euphol (2) represent compounds of interest for further investigation as selective antiproliferative agents for certain types of cancer.
Materials and Methods
General Experimental Procedure
Melting points were determined in a Cole–Palmer apparatus and are uncorrected. TLC was performed on Merck aluminum-backed plates coated with 0.2 mm thick silica gel 60 F254. Column chromatography was carried out on silica gel 70–230 or 230–400 mesh from Sigma-Aldrich, eluting with mixtures of increasing polarity of n-hexane/methylene chloride or n-hexane/ethyl acetate. Electronic impact mass spectra (EIMS) were obtained in a JEOL JMS-AX505HA spectrometer with an ionization potential of 70 eV. DART and high-resolution electro-spray ionization mass spectra (HRESIMS) were obtained in an AccuTOF JMS-T100LC spectrometer. IR spectra were recorded using a Bruker Tensor 750 FT-IR spectrophotometer. The specific rotation was determined on a PerkinElmer 343 polarimeter using chloroform as the solvent and sodium D line as the source of light. 1H, 13C, and bidimensional NMR spectra were recorded in Bruker Avance III (400/100 MHz), Bruker Fourier (300/75 MHz), and Jeol Eclipse (300/75 MHz). HPLC was carried out in a Thermo Scientific Ultimate 3000 chromatographer using analytical C18 (5 μm, 100 Å, 15 × 4.6 mm, 5 μm). The HPLC-grade solvents employed (iPrOH, MeOH, MeCN) were from the brand Fermont. The X-ray data were collected on a Bruker APEX II Duo diffractometer.
Plant Material
The aerial parts of E. tanquahuete were collected in October 2014 at the State Park “El Texcal” in the municipality of Jiutepec, Morelos, Mexico. The plant was identified as E. tanquahuete (synonym: E. fulva) by Prof. Clara H. Ramos (Instituto de Biología, UNAM) and a voucher specimen was deposited in the Herbario Nacional de México (MEXU) with registry number 1394140.
Extraction and Isolation
The air-dried powdered leaves (0.65 kg) of E. tanquahuete were extracted by maceration with a mixture of methylene chloride/methanol (DCM/MeOH) 1:1 (r.t., three times, 24 h each), affording a polar extract (328.5 g).
The methylene chloride/methanol extract was fractionated by open-column chromatography using a gradient with a mixture of n-hexane/EtOAc from 100:0 to 0:100 and washing the column with MeOH, affording eight major fractions (A–H). Fraction B (which was eluted with n-hexane/EtOAc 19:1) was further fractionated by column chromatography (CC) with a mixture of n-hexane/CHCl3 of increasing polarity to afford squalene as a colorless oil (175 mg).21 From fraction D precipitated a white solid that after filtration and recrystallization from n-hexane/EtOAc afforded 1-octacosanol (1.15 g).22,23 The mother liquors of fraction D were concentrated and subjected to further CC to yield six subfractions (D1–D6). Subsequent CC of subfraction D2 afforded euphol (2, 1.35 g) as the majoritarian constituent. Subfraction D3 contained a mixture of 2 and a second component that was identified as lupeol (5, 234 mg).18 Subfraction D4 contained a mixture of euphol, lupeol, and a third component that after successive column chromatography was identified as cycloeucalenol (4, 10 mg).19 Subfraction D5 contained two major components that were isolated by preparative TLC using a mixture of n-hexane/DCM/EtOAc/EtOH 70:20:9:1. These compounds were identified as 25,26,27-trisnor-3β-hydroxy-eupha-24-al (1, 7 mg) and eupha-8,23-dien-3β,25-diol (3, 6 mg) according to the extensive spectroscopic analysis and comparison with data reported in the literature.16,17 The purity of the compounds (>96%) was determined by HPLC.
Evaluation of Cytotoxic Activity
The cytotoxicity of the extract and the pure compounds was tested in six human tumor cell lines as percent inhibition of proliferation using the colorimetric method of sulforhodamine B (SRB, protein binding dye).28 Human tumor cell lines tested were central nervous system (U251), prostate (PC-3), leukemia (K562), colon (HCT-15), breast (MCF-7), and lung (SKLU), provided by the National Cancer Institute (NCI). Colored solutions were extracted, and optical densities were read on an Ultra Reader of Microplate (Elx 808, Bio-Tek Instruments, Inc.) at a wavelength of 515 nm.
Single-Crystal X-ray Diffraction Analysis
Crystallographic data for compounds 8 and 10 were collected on a Bruker SMART APEX DUO three-circle diffractometer equipped with an Apex II CCD detector using Cu Kα radiation (λ = 1.54178 Å, Incoatec Iμ microsource and Helios optic monochromator) for the correct estimation of the anomalous dispersion and an adequate determination of the absolute structure parameter due to the nature of the sample (only carbon, oxygen, and hydrogen atoms), at −173 °C. Suitable crystals were coated with Paratone hydrocarbon oil, picked up with a nylon cryoloop, and mounted on the diffractometer.
Acknowledgments
The authors thank Universidad Nacional Autónoma de México for financial support (Dirección General de Asuntos del Personal Académico PAPIIT IG200821) and Consejo Nacional de Ciencia y Tecnología (CONACYT Grant 651375). This work was taken in part from the PhD Thesis submitted by L.J.R.M. to Programa de Maestría y Doctorado en Ciencias Químicas de la UNAM. The authors thank Prof. Clara H. Ramos (Instituto de Biología, UNAM) for the collection and identification of the plant material. The authors also thank María de los Ángeles Peña, Elizabeth Huerta, María Isabel Chávez, Beatriz Quiroz, Rubén Gaviño, Rocío Patiño Maya† (1965-2021), Javier Pérez Flores, and María del Carmen García González (Instituto de Química de la UNAM) for technical assistance.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03963.
Spectroscopic description of the natural compounds, squalene, 1-octacosanol, β-sitosterol, and compounds 2–5; preparation of compounds 1 and 6–12; 1D and 2D NMR spectra, IR, and HRESIMS of compound 1; NMR spectra of compounds 6–12; and crystal data and structure refinement of euphone (8) and 3-O-methyl euphol (10) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Dorsey B. L.; Haevermans T.; Aubriot X.; Morawetz J. J.; Riina R.; Steinmann V. W.; Berry P. E. Phylogenetics, morphological evolution, and classification of Euphorbia subgenus Euphorbia. Taxon 2013, 62, 291–315. 10.12705/622.1. [DOI] [Google Scholar]
- Shi Q.-W.; Su X.-H.; Kiyota H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. 10.1021/cr078350s. [DOI] [PubMed] [Google Scholar]
- Vasas A.; Hohmann J. Euphorbia diterpenes: isolation, structure, biological activity, and synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. 10.1021/cr400541j. [DOI] [PubMed] [Google Scholar]
- Kemboi D.; Peter X.; Langat M.; Tembu J. A review of the ethnomedicinal uses, biological activities, and triterpenoids of Euphorbia species. Molecules 2020, 25, 4019. 10.3390/molecules25174019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B.; Iriti M.; Vitalini S.; Antolak H.; Pawlikowska E.; Kregiel D.; Sharifi-Rad J.; Oyeleye S. I.; Ademiluyi A. O.; Czopek K.; Staniak M.; Custódio L.; Coy-Barrera E.; Segura-Carretero A.; Cádiz-Gurrea M. L.; Capasso R.; Cho W. C.; Seca A. M. L. Euphorbia-derived natural products with potential for use in health maintenance. Biomolecules 2019, 9, 337. 10.3390/biom9080337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaseñor J. L. Checklist of the native vascular plants of México. Rev. Mex. Biodiv. 2016, 87, 559–902. 10.1016/j.rmb.2016.06.017. [DOI] [Google Scholar]
- Jiménez-Ramírez J.; Martínez-Gordillo M.; Cruz-Durán R.; Juárez-Arriaga E.; García R.; Cervantes A.; Mejía-Hernández R. Los géneros de la familia Euphorbiaceae en México. An. Inst. Biol. UNAM Ser. Bot. 2002, 73, 155–281. [Google Scholar]
- Novillo F.; Velasco-Barrios E.; Nieto-Camacho A.; López-Huerta F. A.; Méndez-Cuesta C. A.; Ramírez-Apan M. T.; Chávez M. I.; Martínez E. M.; Hernández-Delgado T.; Espinosa-García F. J.; Delgado G. 3β-Palmitoyloxy-olean-12-ene analogs from Sapium lateriflorum (Euphorbiaceae): Their cytotoxic and anti-inflammatory properties and docking studies. Fitoterapia 2021, 155, 105067 10.1016/j.fitote.2021.105067. [DOI] [PubMed] [Google Scholar]
- López-Huerta F. A.; Nieto-Camacho A.; Morales-Flores F.; Hernández-Ortega S.; Chávez M. I.; Méndez-Cuesta C. A.; Martínez I.; Espinoza B.; Espinosa-García F. J.; Delgado G. Hopane-type triterpenes from Cnidoscolus spinosus and their bioactivities. Bioorg. Chem. 2020, 100, 103919 10.1016/j.bioorg.2020.103919. [DOI] [PubMed] [Google Scholar]
- Atlas de las Plantas de la Medicina Tradicional Mexicana. 2022, http://www.medicinatradicionalmexicana.unam.mx/apmtm/termino.php?l=3&t=pegahueso (accessed June 23, 2022).
- Monroy Ortiz C.; Castillo España P.. Las plantas medicinales utilizadas en el Estado de Morelos; Centro de Investigaciones Biológicas. UAE Morelos, 2000; p 207. [Google Scholar]
- Passos G. F.; Medeiros R.; Marcon R.; Nascimento A. F. Z.; Calixto J. B.; Pianowski L. F. The role of PKC/ERK1/2 signaling in the anti-inflammatory effect of tetracyclic triterpene euphol on TPA-induced skin inflammation in mice. Eur. J. Pharmcol. 2013, 698, 413–420. 10.1016/j.ejphar.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Dutra R. C.; Bicca M. A.; Segat G. C.; Silva K. A. B. S.; Motta E. M.; Pianowski L. F.; Costa R.; Calixto J. B. The antinociceptive effects of the tetracyclic triterpene euphol in inflammatory and neuropathic pain models: the potential role of PKCε. Neuroscience 2015, 303, 126–137. 10.1016/j.neuroscience.2015.06.051. [DOI] [PubMed] [Google Scholar]
- Bartlett W. R.; Johnson W. S.; Plummer M. S.; Small V. R. Cationic cyclization of a substrate having an internal acetylenic bond. Synthesis of euphol and tirucallol. J. Org. Chem. 1990, 55, 2215–2224. 10.1021/jo00294a043. [DOI] [Google Scholar]
- Knight S. A. Carbon-13 NMR spectra of some tetra- and pentacyclic triterpenoids. Org. Magn. Reson. 1974, 6, 603–611. 10.1002/mrc.1270061112. [DOI] [Google Scholar]
- Guo J.; Zhou L. Y.; He H. P.; Leng Y.; Yang Z.; Hao X. J. Inhibition of 11β-HSD1 by tetracyclic triterpenoids from Euphorbia ansui. Molecules 2012, 17, 11826–11838. 10.3390/molecules171011826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong Y. W.; Harrison L. J. (20R,23E)-Eupha-8,23-diene-3β,25-diol from Tripetalum cymosum. Phytochemistry 1999, 50, 849–857. 10.1016/S0031-9422(98)00565-2. [DOI] [Google Scholar]
- Burns D.; Reynolds W. F.; Buchanan G.; Reese P. B.; Enríquez R. G. Assignment of 1H and 13C spectra and investigation of hindered side-chain rotation in lupeol derivatives. Magn. Reson. Chem. 2000, 38, 488–493. . [DOI] [Google Scholar]
- Kikuchi T.; Kadota S.; Tsubono K. Studies on the constituents of orchidaceus plants. IV. Proton and carbon-13 signal assignments of cycloeucalenol-type triterpenes from Nervilia purpurea Schlechter by two-dimensional nuclear magnetic resonance spectroscopy. Chem. Pharm. Bull. 1986, 34, 2479–2486. 10.1248/cpb.34.2479. [DOI] [Google Scholar]
- Zhang X.; Geoffroy P.; Miesch M.; Julien-David D.; Raul F.; Aoudé-Werner D.; Marchion E. Gram-scale chromatographic purification of β-sitosterol. Synthesis and characterization of β-sitosterol oxides. Steroids 2005, 70, 886–895. 10.1016/j.steroids.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Pogliani L.; Ceruti M.; Ricchiardi G.; Viterbo D. An NMR and molecular mechanics study of squalene and squalene derivatives. Chem. Phys. Lipids 1994, 70, 21–34. 10.1016/0009-3084(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Cravotto G.; Gaudino E. C.; Barge A.; Binello A.; Albertino A.; Aghemo C. Synthesis of 1-octacosanol and GC-C-IRMS discrimination of samples from different origins. Nat. Prod. Res. 2010, 24, 428–439. 10.1080/14786410903194498. [DOI] [PubMed] [Google Scholar]
- Kunkuma V. L.; Kaki S. S.; Rao B. V. S. K.; Prasad R. B. N.; Devi B. L. A. P. A simple and facile method for the synthesis of 1-octacosanol. Eur. J. Lipid Sci. Technol. 2013, 115, 921–927. 10.1002/ejlt.201200195. [DOI] [Google Scholar]
- De Pascual Teresa J.; Urones J. G.; Marcos I. S.; Basabe P.; Sexmero Cuadrado M. J.; Fernández Moro R. Triterpenes from Euphorbia broteri. Phytochemistry 1987, 26, 1767–1776. 10.1016/S0031-9422(00)82286-4. [DOI] [Google Scholar]
- Ponomarenko L. P.; Kalinovsky A. I.; Martyyas E. A.; Doudkin R. V.; Gorovoy P. G.; Stonik V. A. Terpenoid metabolites from the aerial parts of Artemisia lagocephala. Phytochem. Lett. 2012, 5, 118–122. 10.1016/j.phytol.2011.11.006. [DOI] [Google Scholar]
- O’Keeffe R.; Kenny O.; Brunton N. P.; Hossain M. B.; Rai D. K.; Jones P. W.; O’Brien N.; Maguire A. R.; Collins S. G. Synthesis of novel 24-amino-25,26,27-trinorlanost-8-enes: Cytotoxic and apoptotic potential in U937 cells. Bioorg. Med. Chem. 2015, 23, 2270–2280. 10.1016/j.bmc.2015.02.034. [DOI] [PubMed] [Google Scholar]
- Nes W. D.; Wong R. Y.; Benson M.; Landrey J. R.; Nes W. R. Rotational isomerism about the 17(20)-bond of steroids and euphoids as shown by the crystal structures of euphol and tirucallol. Proc. Natl. Acad. Sci. U.S.A. 1984, 81, 5896–5900. 10.1073/pnas.81.18.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichai V.; Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.