Abstract
Objectives
Multimorbidity, also referred to as multiple chronic conditions (MCCs), is the concurrent presence of 2 or more chronic health conditions. Increasing multimorbidity represents a substantial threat to the health of aging populations. Recent trends suggest greater risk of poor health and mortality among later-born cohorts, yet we are unaware of work examining cohort differences in multimorbidity among aging U.S. adults.
Methods
We examine intercohort variation in MCC burden in adults aged 51 years and older using 20 years (n = 33,598; 1998–2018) of repeated assessment drawn from the Health and Retirement Study. The index of MCCs included 9 chronic conditions (heart disease, hypertension, stroke, diabetes, arthritis, lung disease, cancer excluding skin cancer, high depressive symptoms, and cognitive impairment). We used linear mixed models with various approaches to estimate age/period/cohort effects to model intercohort patterns in MCC burden. We also explored variation in the specific conditions driving cohort differences in multimorbidity.
Results
More recent cohorts had greater MCC burden and developed multimorbidity at earlier ages than those born to prior generations. The burden of chronic conditions was patterned by life-course sociodemographic factors and childhood health for all cohorts. Among adults with multimorbidity, arthritis and hypertension were the most prevalent conditions for all cohorts, and there was evidence that high depressive symptoms and diabetes contributed to the observed cohort differences in multimorbidity risk.
Discussion
Our results suggest increasing multimorbidity burden among more recently born cohorts of aging U.S. adults and should inform policy to address diminishing health in aging populations.
Keywords: Baby Boom cohort, Health and Retirement Study, Life course, Multimorbidity, Population aging
Multimorbidity, also known as multiple chronic conditions (MCCs), is defined as having two or more concurrent chronic conditions, and affects between 55% and 98% of the U.S. population aged 65 and older (Salive, 2013; Vetrano et al., 2018). Management of multimorbidity requires a complex network of in-patient, out-patient, home health services, and pharmaceutical treatment (Lehnert et al., 2011), often resulting in high patient burden (Rosbach & Andersen, 2017), inadequate care (Kastner et al., 2019), and adverse effects of polypharmacy (Cadogan et al., 2016). The high prevalence and difficulty of managing multimorbidity foreshadow a coming public health crisis when framed against population aging. With the number of U.S. adults aged 65+ projected to grow by more than 50% by 2050 (Vespa et al., 2020), multimorbidity will place increasing strain on older adults, their families, and the health care system (Bloom et al., 2015).
Compounding the risks posed by multimorbidity is the slowing or reversal of improving health and well-being observed over the 20th century. This shift was first identified as an increased risk of significant physical disability among adults aged 40–59 from 1997 to 2006 (Martin et al., 2009), aged 50–64 from 1997 to 2007 (Martin et al., 2010), and those aged 60–69 from 1988 to 2004 (Seeman et al., 2010). Later work described increased mortality rates from 1999 to 2013 among non-Hispanic White adults at midlife, mirrored by decreases in self-reported health, psychological well-being, and rising disability (Case & Deaton, 2015). Similar trends have been reported in multimorbidity among aging Canadians, with later-born cohorts having greater risk of multimorbidity than age-matched peers in prior cohorts (Canizares et al., 2018). The scope of this trend has expanded to include reduced cognitive function among Early (born 1948–1953) and Mid Baby Boomers (born 1954–1959) measured from 1996 to 2014 (Zheng, 2020), and increased physiological dysregulation among Baby Boomers, Generation X (born 1973–1980), and Generation Y (born 1981–1999; Zheng & Echave, 2021). Greater prevalence of chronic disease among the Baby Boom cohort may contribute to an increase in health care expenditures and adversely affect the financial solvency of Medicare and Social Security (Meara & Skinner, 2015), yet we are unaware of work examining cohort patterns in MCC burden among aging Americans.
Identifying intercohort trends in multimorbidity requires careful study design and selection of measures capturing heterogeneity in age-related exposures to risk and protective factors. The confounding of age, period, and cohort (APC) effects coupled with statistical methods that incorrectly address the linear dependence of these measures likely result in biased statistical estimates of cohort differences in age-related health outcomes (Bell, 2020; Luo, 2013; Yang & Land, 2013). Age effects represent variation related to chronological and biological aging, period effects are produced by historical events and changing social conditions that influence all age groups simultaneously (e.g., secular trends in economic development, technology, etc.), while cohort effects reflect shared exposure to sociohistorical context that accrues over the life course as experienced by those born to specific years or eras. Conceptualizing cohort effects as the product of life-course processes is helpful as those born to specific cohorts share cultural and environmental exposures at critical periods of life that may pattern later health outcomes, and experience similar sociohistorical exposures over the life span that shape accumulation of risk and subsequent disparities (Kuh et al., 2003; Lynch & Smith, 2005). Individual characteristics, including racial/ethnic minority background (Quiñones et al., 2011, 2019, 2021), lower education (Pathirana & Jackson, 2018), fewer financial resources (Schäfer et al., 2012), and low childhood socioeconomic status (SES) have been linked to greater risk of multimorbidity (Tucker-Seeley et al., 2011), and the differential health effects of life-course SES and childhood health across aging cohorts are a growing area of interest (Haas et al., 2017). Behavioral risks, including smoking and obesity, vary across cohorts (Piontek et al., 2010; Robinson et al., 2012), are linked to multimorbidity (Agborsangaya et al., 2013; Lebenbaum et al., 2018), and likely contribute to cohort differences in MCCs.
Recent commentary on the state of multimorbidity research in aging populations emphasizes that most studies rely on cross-sectional designs (Quiñones et al., 2020; Vetrano et al., 2018), limiting understanding of temporal trends in MCC burden. Also, most longitudinal work on multimorbidity has been conducted outside of the United States (Agborsangaya et al., 2015; Canizares et al., 2018; Hsu, 2015), underscoring the need to address age- and cohort-related changes in MCC burden in the U.S. population. To contribute to the study of multimorbidity trends in the United States, we estimated intercohort variation in MCC burden using 20 years of representative longitudinal assessment of MCCs, and examined heterogeneity related to sociodemographic characteristics, childhood socioeconomic adversity and health, and behavioral risk factors. We utilized inverse probability weight (IPW)-adjusted models to assess the influence of mortality and study attrition on our primary analyses, and finally investigated the conditions driving cohort patterns in multimorbidity.
Method
Data Source
Observations were drawn from the Health and Retirement Study (HRS), a representative biennial panel study sponsored by the National Institute on Aging. The study includes respondents who were born into any of the seven cohort groups currently represented in the HRS who completed at least one interview between 1998 and 2018. The HRS is well-suited for the examination of cohort patterns in health given the inclusion of additional cohorts of adults aged 51–56 every 6 years. Self-reported doctor-diagnosed conditions and information on condition treatment and diagnosis were taken from core HRS data. We also utilized cleaned files distributed by the RAND Corporation containing imputed cognitive scores, depressive symptoms, imputed wealth and income measures, sociodemographic indicators, and childhood SES and health.
The initial sample included 35,010 individuals aged 51 or older with at least one complete measurement on all nine health conditions included in the index of MCC burden. Observations with missing data on covariates used in either the primary models estimating cohort differences in MCC burden, or covariates used to estimate IPWs were removed (n = 1,267), as were participants with missing or zero values for survey weights (n = 145), resulting in an analytic sample of 33,598 respondents contributing 175,162 person-observations. A description of respondent characteristics based on sample inclusion/exclusion is presented in the Supplementary Appendix and Supplementary Table 1 provides descriptive statistics for respondent characteristics by sample inclusion status.
Measures
Multiple chronic conditions
We follow guidance on MCC inclusion from the U.S. Department of Health and Human Services (HHS) Office of the Assistant Secretary of Health (OASH) MCC working group which used a deliberative process with experts in clinical medicine, epidemiology, and public health to define chronic conditions as “conditions that last a year or more and require ongoing medical attention and/or limit activities of daily living” (Goodman et al., 2013; U.S. Department of Health and Human Services, 2010), emphasizing prevalent, persistent, and incurable conditions that are potentially amenable to public health or clinical interventions (Goodman et al., 2013). Taking direction from the HHS strategic framework on MCCs (U.S. Department of Health and Human Services, 2010) and the Goodman et al. (2013) conceptual model for standardizing analysis of health data for specific chronic conditions, we operationalized MCCs as a count of nine chronic conditions available in the HRS that map to the 20 conditions identified by the OASH MCC working group. Our goal was to examine variation in MCCs by cohort using the HRS and this operationalization reflects a purpose-driven selection of data sources and instrumentation (Suls et al., 2021). To capture the persistent nature of chronic conditions and address conflicts in condition identification across interviews, we used diagnoses from previous waves, additional information on treatment and diagnosis date available in the HRS, and established adjudication criteria (Cigolle et al., 2016) to amend responses to reflect having ever been diagnosed or identified with the given condition. The sum of chronic conditions we use as an index of MCC burden is highly correlated with more complex weighted multimorbidity indices and has been identified as a suitable measure of multimorbidity (Quiñones et al., 2011).
Seven conditions in this index were based on respondent self-reports of “having ever been told by a doctor” that they had the following diseases: heart disease (including myocardial infarction, coronary heart disease, angina, congestive heart failure, or other heart problems), hypertension, stroke (excluding transient ischemic attack), diabetes, arthritis, lung disease (such as chronic bronchitis or emphysema and excluding asthma), and cancer (any malignant tumor excluding skin cancer). Depressive symptoms were identified using the Center for Epidemiological Studies—Depression scale adapted in the HRS with respondents asked to report for the week prior to interview whether they felt depressed, were happy, felt lonely, enjoyed life, felt sad, could not get going, that everything was an effort, or sleep was restless. Affirmative responses to four or more symptoms indicate high depressive symptoms (Steffick, 2000). Finally, we include an indicator of dementia following the Langa–Weir approach that sums immediate and delayed word recall scores, a backwards counting test, and a serial sevens test with a range from 0 to 27 and dementia identified for those with scores of <7 (Crimmins et al., 2011; Langa et al., 2010). Description of each component of the cognitive impairment index is presented in the Supplementary Appendix.
Age, period, and cohort
Age was calculated as the difference between the respondent’s birth date and interview date at each wave. Period was measured as year of interview. Individual birth years were grouped together into seven unique cohorts: Greatest Generation (born 1923 or earlier), Early Children of Depression (born 1924–1930), Late Children of Depression (born 1931–1941), War Babies (born 1942–1947), Early Baby Boomers (born 1948–1953), Mid Baby Boomers (born 1954–1959), and Late Baby Boomers (born 1960–1965). The Late Children of Depression cohort was used as the reference group in all analyses as they represent the largest cohort in the HRS and have the widest age range of all cohorts under study.
Sociodemographic and behavioral covariates
Covariates were selected to adjust models for background characteristics and explore disparities in MCC burden while minimizing missing data. Demographic indicators included respondents’ sex/gender, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic/Latino, or Other race/ethnicity [including American Indian, Alaskan Native, Asian, Native Hawaiian, Pacific Islander, and other racial/ethnic subgroups]), partnership status (separated, divorced, widowed, or never married; partnered/married with spouse present or absent), and nativity status (born in the United States, not born in the United States). Measures of SES included education (< high school degree, high school degree, > high school degree), household income measured as the combined household income of the respondent and their spouse (including individual earnings, income from Social Security, government transfers, unemployment or workers compensation, and other sources of income). Household net worth was measured as the net value of the respondent’s financial and nonfinancial assets (e.g., residence, real estate savings and retirement accounts, etc.) minus debts owed on mortgages, home loans, and other debt. Both household income and household net worth were examined as quartiles to address potential nonlinear association with MCC burden. Childhood socioeconomic adversity was measured as an additive index of mother’s education (<8 years), father’s education (<8 years), reporting childhood financial status as poor, receiving financial help from relatives, never living with father, and reporting father as having a blue-collar occupation. As few respondents reported all six adversities, those reporting 5–6 adversities were collapsed, resulting in a range of 0–5 possible adversities (Montez & Hayward, 2014). Childhood health was based on respondent self-report of heath before the age of 16, which was dichotomized to indicate poor childhood health (excellent, very good, good; fair, poor). Measures of health-related behaviors included body mass index (BMI; underweight [<18.5 kg/m2], normal weight [18.5–24.9 kg/m2; reference], overweight [25–29.9 kg/m2], or obese [≥30 kg/m2]), current smoking status, and alcohol consumption (nondrinkers, moderate drinkers [men: 1–14 drinks/week; females: 1–7 drinks/week], and heavy drinkers [men: >14 drinks/week; females: >7 drinks/week]). Description of the selection and coding of predictors used to create IPWs is provided in the Supplementary Appendix.
Analytic Methods
To generate substantive estimates of cohort patterns in multimorbidity robust to model specification, we applied both descriptive and inferential techniques to assess cohort patterns in MCC burden. Initially we examined weighted means of MCC burden by cohort and age group (Table 1), then plotted weighted MCC trajectories by cohort and age (Figure 1, Panel A). To estimate cohort dynamics in MCC burden adjusted for repeated measurement and respondent characteristics, we used weighted linear mixed models (LMMs). A Gaussian response distribution was selected based on an adequately normal distribution of residuals identified when predicting MCC burden conditional on all predictor variables. As our aim was to estimate cohort differences in MCC burden net of age and period effects, we estimated three sequential LMMs to assess whether findings were dependent on model specification. First, we estimated an age/cohort model, or a LMM including fixed effects for cohort, linear age, and quadratic age while excluding a random period effect (Model 1). Next, we estimated a LMM including cohort, linear age, and quadratic age as fixed effects, with wave (period) incorporated as a random effect with a first-order autoregressive residual covariance structure (Model 2). To identify whether age was differentially associated with MCC burden across cohorts, Model 3 added cohort by age interaction terms to all parameters included in Model 2. These LMM specifications attempt to address the identification problem in APC analyses as cohort and age are not assumed to have a linear relationship (Models 1–3), and cohort and age are modeled as fixed effects while period is modeled as a random effect (Models 2–3; Bell, 2014; Yang & Land, 2013). Age was centered on the weighted grand mean age across measurements (M = 65.28). To account for the complex design of the HRS, person-level survey weights taken from each respondent’s first observation were included when estimating Models 1–3. Survey weights from 2016 were used to impute missing survey weights for 2018 as survey weights for 2018 were not available at the time of analysis.
Table 1.
Weighted Descriptive Statistics for Multiple Chronic Condition Burden by Cohort and Age Group, HRS 1998–2018
| Greatest Generation (1903–1923) | Early Children of Depression (1924–1930) | Late Children of Depression (1931–1941) | War Babies (1942–1947) | Early Baby Boomers (1948–1953) | Mid Baby Boomers (1954–1959) | Late Baby Boomers (1960–1965) | |
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| 51–54 | 1.19 (1.25) | 1.30 (1.34) | 1.38 (1.40) | 1.44 (1.43) | |||
| 55–59 | 1.58 (1.39) | 1.62 (1.42) | 1.70 (1.51) | 1.71 (1.53) | 1.52 (1.49) | ||
| 60–64 | 1.86 (1.45) | 2.14 (1.54) | 2.22 (1.63) | 2.00 (1.64) | |||
| 65–69 | 1.93 (1.37) | 2.24 (1.52) | 2.60 (1.60) | 2.51 (1.62) | 3.63a (1.99) | ||
| 70–74 | 2.02 (1.43) | 2.18 (1.45) | 2.69 (1.55) | 2.84 (1.61) | 2.89 (1.63) | ||
| 75–79 | 2.43 (1.48) | 2.72 (1.51) | 3.02 (1.53) | 3.02 (1.53) | |||
| 80–84 | 2.75 (1.49) | 3.13 (1.51) | 3.25 (1.53) | ||||
| 85+ | 3.11 (1.52) | 3.33 (1.49) | 3.31 (1.56) | ||||
| Overall | 2.83 (1.52) | 2.68 (1.55) | 2.52 (1.59) | 2.18 (1.61) | 2.06 (1.6) | 1.87 (1.55) | 1.68 (1.46) |
| Number of observations | 16,892 | 21,456 | 61,051 | 25,766 | 24,395 | 18,350 | 7,252 |
| Number of respondents | 4,567 | 3,676 | 8,527 | 3,396 | 4,492 | 4,744 | 4,196 |
Notes: Survey weights from first available interview used to adjust means. HRS = Health and Retirement Study; SD = standard deviation.
aOnly 11 observations included in group, so interpret with caution.
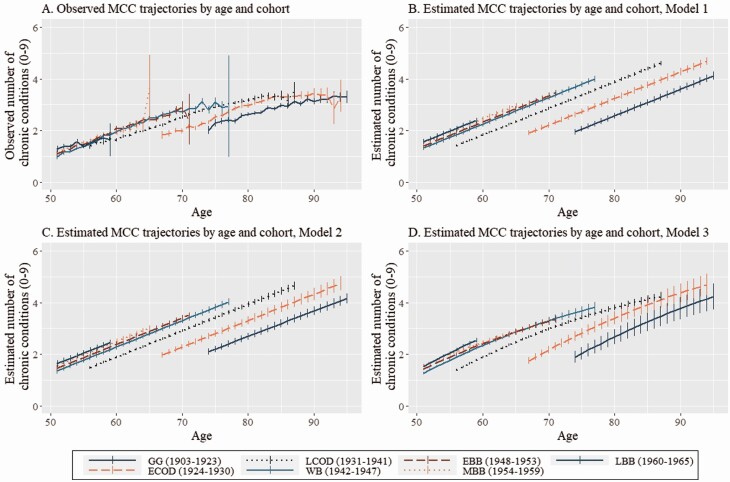
Figure 1.
Observed and estimated trajectories of multiple chronic conditions (MCCs) by age and cohort, HRS 1998–2018. Notes: GG = Greatest Generation (born 1903–1923); ECOD = Early Children of Depression (born 1924–1930); LCOD = Late Children of Depression (born 1931–1941); WB = War Babies (born 1942–1947); EBB = Early Baby Boomers (born 1948–1953); MBB = Mid Baby Boomers (born 1954–1959); LBB = Late Baby Boomers (born 1960–1965). Panel A: Weighted means of MCC burden by cohort and age group. Panel B: Estimates of MCC burden by cohort and age group from Model 1 (fixed effects: cohort, linear age, quadratic age, covariates; no period effect). Panel C: Estimates of MCC burden by cohort and age group from Model 2 (fixed effects: cohort, linear age, quadratic age, covariates; random period effect). Panel D: Estimates of MCC burden by cohort and age group from Model 3 (fixed effects: cohort, linear age, quadratic age, cohort × linear age interaction terms, covariates; random period effect). Survey weights from respondent’s first available interview were used to adjust observed and estimated trajectories. HRS = Health and Retirement Study.
In sensitivity analyses, we used IPWs to adjust estimates of intercohort differences in MCC burden for potential bias due to nonrandom dropout (Weuve et al., 2012). A single IPW model for dropout (mortality or study attrition) was used as IPWs adjusting for death or attrition produce results comparable to weights adjusting for loss to mortality only (Quiñones et al., 2019). After calculation of IPWs, we trimmed respondents with IPWs ≥98th percentile (n = 834; Stürmer et al., 2010), then multiplied the IPW by the person-level HRS survey weights (DuGoff et al., 2014). The trimmed sample and the composite weights were applied to Models 1–3. The Supplementary Appendix describes variable selection and the logit model used to estimate IPWs and presents weighted descriptives for variables used to estimates IPWs (Supplementary Table 3), estimates from the logit model predicting dropout used to calculate IPWs (Supplementary Table 4), and estimates from LMMs adjusted for IPWs (Supplementary Table 5).
To examine how specific chronic conditions contributed to cohort differences in multimorbidity, we estimated the weighted prevalence of each chronic condition among multimorbid respondents. To address repeated within-person measurement, we calculated the prevalence of each condition at last available observation. The prevalence of each condition among those with multimorbidity was estimated by cohort (Supplementary Table 6) and by cohort and age group (Supplementary Table 7). Respondent weights were taken from the first available measurement to align with our primary analyses.
Descriptive and inferential analyses were completed in SAS version 9.4 (PROC GLIMMIX was used to estimate the LMMs) and figures were created in R version 4.0.5 and R Studio version 1.4.1106.
Results
Weighted means of MCC burden by cohort and age group are presented in Table 1. The burden of MCCs generally decreased in each successive cohort when age was not considered, though cohort patterns emerge when comparing within age group. Evaluating MCC burden within age group across cohort indicates that the reported number of chronic conditions was generally greater for later-born cohorts. Among adults aged 80–84, those born to later cohorts reported more chronic conditions than those born to earlier cohorts. In adults aged 60–64, 65–69, 70–74, 75–79, and 85 and older, MCC burden appeared to increase in each successive cohort but tended to level off in later generations. Only 11 respondents were included in the cell used to estimate MCC burden for Mid Baby Boomers aged 65–69, so we do not interpret this value. Among adults aged 55–59, MCC burden was relatively stable across cohort, and among adults aged 51–54, MCC burden appeared to increase in later cohorts.
Figure 1 Panel A displays weighted age trajectories of MCC burden by cohort with 95% confidence intervals (CIs). Over the observed age range, there were several points at which later-born cohorts had greater MCC burden than those born to earlier cohorts at comparable ages. For example, at age 68 the average MCC burden was 1.88 (95% CI: 1.76; 1.99) for the Early Children of Depression cohort, 2.34 (95% CI: 2.28; 2.40) for the Late Children of Depression cohort, and 2.66 (95% CI: 2.57; 2.76) for the War Babies cohort. Though there are several points where CIs overlap or observed cohort trajectories are not comparable due to varying within-cohort age range, this figure provides descriptive evidence that at equivalent ages, later-born cohorts tend to have greater MCC burden than earlier cohorts, and that multimorbidity onset generally occurred at younger ages for later-born cohorts.
Unweighted descriptive statistics for the complete sample used to model cohort dynamics in MCC burden are presented in Supplementary Table 2. Across all available interview waves and without adjustment for survey weights, respondents reported an average of 2.35 chronic conditions (SD = 1.61) and the mean age was 67.58 years (SD = 10.06). To identify variation in background characteristics by cohort, weighted descriptive statistics are presented by cohort in Table 2. Later-born cohorts were generally more likely to have an even sex distribution, be more racially/ethnically diverse, and have a greater likelihood of being born outside the United States. Education and household income appeared to increase in each successive cohort, and earlier born cohorts tended to report greater childhood socioeconomic adversity. The risk of obesity increased in later-born cohorts, as did reporting heavy alcohol consumption. Likelihood of attrition due to mortality or study dropout was greater among earlier born cohorts.
Table 2.
Weighted Descriptive Statistics for Multiple Chronic Condition Burden and Background Characteristics by Cohort at First Interview, HRS 1998–2018
| Greatest Generation (1903–1923) | Early Children of Depression (1924–1930) | Late Children of Depression (1931–1941) | War Babies (1942–1947) | Early Baby Boomers (1948–1953) | Mid Baby Boomers (1954–1959) | Late Baby Boomers (1960–1965) | |
|---|---|---|---|---|---|---|---|
| Continuous variables | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Chronic disease burden | 2.43 (1.46) | 1.99 (1.40) | 1.74 (1.40) | 1.23 (1.29) | 1.40 (1.40) | 1.37 (1.39) | 1.39 (1.44) |
| Age | 80.12 (4.75) | 70.90 (2.74) | 61.96 (3.91) | 54.34 (3.98) | 54.60 (3.46) | 53.69 (2.04) | 53.84 (1.74) |
| # waves observed | 3.81 (2.42) | 5.82 (3.20) | 7.20 (3.38) | 7.65 (3.33) | 5.64 (2.24) | 3.86 (1.28) | 1.72 (0.44) |
| Categorical variables | % | % | % | % | % | % | % |
| Female | 61.28 | 56.08 | 53.36 | 51.26 | 51.44 | 52.63 | 51.57 |
| Race/ethnicity | |||||||
| Non-Hispanic White | 88.21 | 84.28 | 79.88 | 79.38 | 70.97 | 72.35 | 63.24 |
| Non-Hispanic Black | 6.80 | 8.31 | 10.25 | 9.97 | 13.85 | 11.65 | 12.14 |
| Hispanic/Latino | 3.98 | 5.14 | 7.63 | 7.82 | 10.92 | 10.80 | 13.15 |
| Other race/ethnicity | 1.01 | 2.26 | 2.24 | 2.83 | 4.26 | 5.19 | 11.47 |
| Partnered/married | 46.13 | 65.51 | 72.43 | 75.68 | 72.03 | 72.20 | 69.14 |
| Not U.S. born | 7.42 | 7.19 | 10.46 | 8.01 | 11.87 | 12.47 | 15.94 |
| Education | |||||||
| <HS degree | 35.62 | 30.11 | 24.35 | 16.47 | 13.26 | 11.97 | 11.82 |
| HS degree | 33.33 | 34.26 | 35.39 | 32.83 | 27.26 | 27.07 | 26.21 |
| >HS degree | 31.05 | 35.62 | 40.25 | 50.70 | 59.48 | 60.96 | 61.97 |
| Household income quartile | |||||||
| 1 | 38.18 | 26.94 | 20.20 | 13.82 | 17.32 | 14.49 | 16.96 |
| 2 | 31.38 | 31.78 | 21.78 | 14.95 | 14.05 | 14.57 | 15.47 |
| 3 | 18.58 | 25.18 | 27.54 | 25.84 | 25.96 | 26.30 | 22.71 |
| 4 | 11.86 | 16.10 | 30.48 | 45.39 | 42.66 | 44.64 | 44.86 |
| Household net worth quartile | |||||||
| 1 | 24.90 | 21.42 | 23.16 | 25.88 | 30.59 | 27.93 | 27.42 |
| 2 | 25.30 | 24.58 | 23.46 | 26.65 | 24.28 | 25.65 | 25.03 |
| 3 | 25.77 | 26.11 | 25.31 | 24.08 | 23.98 | 24.12 | 24.63 |
| 4 | 24.02 | 27.89 | 28.06 | 23.38 | 21.16 | 22.30 | 22.92 |
| Childhood socioeconomic adversity | |||||||
| 0 | 11.02 | 13.66 | 18.29 | 22.84 | 25.46 | 32.55 | 31.30 |
| 1 | 21.47 | 20.81 | 28.56 | 28.38 | 28.03 | 28.12 | 27.77 |
| 2 | 21.38 | 20.11 | 20.06 | 19.21 | 17.36 | 15.83 | 17.75 |
| 3 | 25.37 | 23.78 | 18.42 | 15.18 | 13.22 | 12.42 | 12.08 |
| 4 | 15.22 | 15.23 | 10.01 | 9.22 | 9.58 | 6.98 | 7.25 |
| 5 | 5.54 | 6.41 | 4.67 | 5.16 | 6.34 | 4.09 | 3.85 |
| BMI | |||||||
| Underweight | 3.94 | 1.29 | 1.22 | 1.18 | 1.00 | 0.92 | 0.89 |
| Normal | 46.91 | 35.58 | 31.31 | 29.00 | 26.86 | 24.50 | 21.05 |
| Overweight | 35.82 | 41.72 | 40.45 | 39.68 | 37.91 | 36.51 | 36.07 |
| Obese | 13.33 | 21.41 | 27.03 | 30.14 | 34.22 | 38.07 | 41.99 |
| Poor childhood health | 6.72 | 5.80 | 6.62 | 5.77 | 6.37 | 5.67 | 6.85 |
| Current smoker | 6.33 | 13.94 | 19.14 | 24.83 | 22.51 | 22.89 | 20.71 |
| Alcohol consumption | |||||||
| None | 75.99 | 69.81 | 66.14 | 62.12 | 56.23 | 49.05 | 47.14 |
| Moderate | 21.36 | 25.31 | 27.77 | 30.66 | 35.92 | 41.39 | 41.12 |
| Heavy | 2.65 | 4.88 | 6.09 | 7.21 | 7.84 | 9.55 | 11.74 |
| Study dropout | 95.21 | 75.06 | 46.40 | 27.69 | 16.02 | 5.90 | 0.95 |
Notes: HRS respondent’s first available interview used as data source. Survey weights from first available interview used to adjust means and percentages. BMI = body mass index; HRS = Health and Retirement Study; HS = high school; SD = standard deviation.
Table 3 presents results from the weighted LMMs estimating cohort differences in MCC burden under varying assumptions about APC effects. Estimates from the age/cohort model adjusted for cohort, linear and quadratic age terms, and covariates while excluding adjustment for period effects (Model 1) suggest that compared to adults born to the Late Children of Depression reference cohort, those born to prior cohorts had fewer expected chronic conditions, and those born to later cohorts had greater MCC burden. In Model 2, including adjustment for period effects in addition to effects included in the age/cohort model slightly attenuated estimates of cohort differences in MCC burden, with estimates somewhat decreasing in magnitude, except for the estimate for Late Baby Boomers, which was unchanged. Including cohort by age interaction terms (Model 3) changes the substantive interpretation of the estimates, with the direct-effect cohort parameters estimating MCC burden within cohort at the weighted grand mean age (M = 65.28) relative to the reference cohort. The linear age term (b = 0.11, 95% CI: 0.11; 0.11) represents the expected increase in MCC burden with a one-unit increase in age for the reference cohort, and estimates for each cohort by age interaction term represent deviations from this value. Not all cohorts included members observed at the weighted grand mean age, suggesting comparisons of MCC burden by cohort estimated in Model 3 should be interpreted cautiously, though the estimates generally align with those derived from Model 1 and Model 2.
Table 3.
Restricted Maximum Likelihood Estimates of Multiple Chronic Condition Burden From Linear Mixed Models, HRS 1998–2018
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Est | 95% CI | Est | 95% CI | Est | 95% CI | ||||
| Cohort | |||||||||
| Greatest Generation (1903–1923) | −1.31 | −1.37 | −1.26 | −1.22 | −1.27 | −1.16 | −1.92 | −2.02 | −1.82 |
| Early Children of Depression (1924–1930) | −0.64 | −0.69 | −0.59 | −0.63 | −0.68 | −0.57 | −1.01 | −1.07 | −0.94 |
| Late Children of Depression (1931–1941; ref) | |||||||||
| War Babies (1942–1947) | 0.42 | 0.37 | 0.47 | 0.39 | 0.34 | 0.44 | 0.39 | 0.33 | 0.44 |
| Early Baby Boomers (1948–1953) | 0.50 | 0.45 | 0.55 | 0.49 | 0.44 | 0.54 | 0.41 | 0.36 | 0.46 |
| Mid Baby Boomers (1954–1959) | 0.62 | 0.57 | 0.67 | 0.60 | 0.55 | 0.66 | 0.39 | 0.33 | 0.45 |
| Late Baby Boomers (1960–1965) | 0.66 | 0.61 | 0.72 | 0.66 | 0.61 | 0.72 | 0.72 | 0.61 | 0.83 |
| Cohort × linear age interaction terms | |||||||||
| Greatest Generation (1903–1923) × linear age | 0.05 | 0.05 | 0.06 | ||||||
| Early Children of Depression (1924–1930) × linear age | 0.04 | 0.04 | 0.04 | ||||||
| Late Children of Depression (1931–1941; ref) × linear age | |||||||||
| War Babies (1942–1947) × linear age | −0.01 | −0.02 | −0.01 | ||||||
| Early Baby Boomers (1948–1953) × linear age | −0.02 | −0.03 | −0.02 | ||||||
| Mid Baby Boomers (1954–1959) × linear age | −0.04 | −0.04 | −0.03 | ||||||
| Late Baby Boomers (1960–1965) × linear age | −0.01 | −0.02 | 0.00a | ||||||
| Linear age | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.11 | 0.11 | 0.11 |
| Female | 0.08 | 0.05 | 0.11 | 0.07 | 0.04 | 0.10 | 0.08 | 0.05 | 0.11 |
| Race/ethnicity | |||||||||
| Non-Hispanic White (ref) | |||||||||
| Non-Hispanic Black | 0.24 | 0.20 | 0.28 | 0.28 | 0.23 | 0.32 | 0.28 | 0.24 | 0.33 |
| Hispanic/Latino | −0.01 | −0.07 | 0.05a | 0.01 | −0.05 | 0.07a | 0.01 | −0.05 | 0.07a |
| Other race/ethnicity | 0.15 | 0.07 | 0.24 | 0.19 | 0.11 | 0.27 | 0.19 | 0.11 | 0.27 |
| Partnered/married | −0.08 | −0.10 | −0.07 | −0.08 | −0.09 | −0.06 | −0.08 | −0.09 | −0.06 |
| Born outside United States | −0.36 | −0.41 | −0.31 | −0.35 | −0.41 | −0.30 | −0.35 | −0.41 | −0.30 |
| Education | |||||||||
| < HS degree | 0.34 | 0.30 | 0.39 | 0.36 | 0.32 | 0.40 | 0.36 | 0.31 | 0.40 |
| HS degree (ref) | |||||||||
| > HS degree | −0.18 | −0.22 | −0.15 | −0.19 | −0.23 | −0.16 | −0.19 | −0.23 | −0.16 |
| Household income quartile | |||||||||
| Q1 (ref) | |||||||||
| Q2 | −0.08 | −0.09 | −0.07 | −0.03 | −0.04 | −0.02 | −0.03 | −0.04 | −0.02 |
| Q3 | −0.11 | −0.13 | −0.10 | −0.05 | −0.06 | −0.04 | −0.04 | −0.05 | −0.03 |
| Q4 | −0.12 | −0.14 | −0.11 | −0.06 | −0.07 | −0.05 | −0.06 | −0.07 | −0.05 |
| Household net worth quartile | |||||||||
| Q1 (ref) | |||||||||
| Q2 | −0.07 | −0.08 | −0.06 | −0.05 | −0.06 | −0.04 | −0.05 | −0.06 | −0.04 |
| Q3 | −0.13 | −0.15 | −0.12 | −0.09 | −0.10 | −0.08 | −0.08 | −0.10 | −0.07 |
| Q4 | −0.19 | −0.21 | −0.17 | −0.12 | −0.13 | −0.10 | −0.11 | −0.13 | −0.10 |
| Childhood socioeconomic adversities | |||||||||
| 0 (ref) | |||||||||
| 1 | 0.09 | 0.04 | 0.13 | 0.10 | 0.06 | 0.15 | 0.10 | 0.06 | 0.15 |
| 2 | 0.19 | 0.14 | 0.23 | 0.21 | 0.16 | 0.26 | 0.21 | 0.16 | 0.26 |
| 3 | 0.23 | 0.18 | 0.28 | 0.26 | 0.20 | 0.31 | 0.26 | 0.20 | 0.31 |
| 4 | 0.37 | 0.31 | 0.43 | 0.40 | 0.34 | 0.46 | 0.40 | 0.34 | 0.46 |
| 5 | 0.50 | 0.43 | 0.58 | 0.54 | 0.47 | 0.62 | 0.54 | 0.47 | 0.62 |
| Childhood health (fair/poor) | 0.55 | 0.49 | 0.61 | 0.58 | 0.52 | 0.64 | 0.58 | 0.52 | 0.64 |
| BMI | |||||||||
| Underweight | 0.07 | 0.03 | 0.10 | 0.05 | 0.02 | 0.08 | 0.05 | 0.02 | 0.07 |
| Normal (ref) | |||||||||
| Overweight | 0.00 | −0.02 | 0.01a | −0.01 | −0.02 | 0.00a | −0.01 | −0.02 | 0.00a |
| Obese | 0.07 | 0.05 | 0.09 | 0.03 | 0.02 | 0.04 | 0.03 | 0.02 | 0.04 |
| Current smoker | −0.17 | −0.19 | −0.15 | −0.11 | −0.12 | −0.09 | −0.11 | −0.12 | −0.09 |
| Alcohol consumption | |||||||||
| None (ref) | |||||||||
| Moderate | −0.10 | −0.11 | −0.09 | −0.05 | −0.06 | −0.05 | −0.05 | −0.06 | −0.04 |
| High | −0.14 | −0.16 | −0.12 | −0.08 | −0.10 | −0.07 | −0.08 | −0.10 | −0.07 |
| Intercept | 2.34 | 2.29 | 2.39 | 2.40 | 2.35 | 2.45 | 2.47 | 2.42 | 2.52 |
| Age squared | 0.00 | 0.00 | 0.00a | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Random effects | |||||||||
| Individual | 1.69 | 1.66 | 1.72 | 1.02 | 0.98 | 1.05 | 1.03 | 0.99 | 1.06 |
| Period | 0.84 | 0.84 | 0.84 | 0.84 | 0.84 | 0.84 | |||
Notes: Survey weights from respondent’s first available interview used. Model 1: Fixed effects: cohort, linear age, quadratic age, covariates; no period effect. Model 2: Fixed effects: cohort, linear age, quadratic age, covariates; random period effect. Model 3: Fixed effects: cohort, linear age, quadratic age, cohort × linear age interaction terms, covariates; random period effect. BMI = body mass index; CI = confidence interval; HRS = Health and Retirement Study; HS = high school.
aIdentifies estimate p > .001.
As we prefer to interpret estimates of cohort patterns in MCC burden net of age and period effects, we focus attention on estimates produced by Model 2. In this model, cohort appeared to have a nonlinear monotonic association with MCC burden. Those cohorts born prior to 1931 had lower expected MCC burden, and those cohorts born after 1941 had greater expected MCC burden. For example, members of the Greatest Generation cohort had an estimated 1.22 fewer chronic conditions (95% CI: −1.27; −1.16), while the Mid Baby Boomers had an estimated 0.60 more chronic conditions (95% CI: 0.55; 0.66), than Late Children of Depression cohort members, respectively. Linear age was positively associated with MCC burden net of cohort and period effects (b = 0.10, 95% CI: 0.10; 0.10). Notable associations between MCC burden and covariates net of age/period/cohort included greater MCC burden among non-Hispanic Black respondents and respondents grouped in the “Other” racial/ethnic category, those born in the United States, and those with less education, compared to their respective reference groups. Greater household net worth was modestly associated with lower MCC burden, and greater childhood socioeconomic adversity and poor childhood health were positively associated with MCC burden. Obese individuals had more chronic conditions than their normal-weight counterparts, although this association appeared modest.
Figure 1 Panels B–D display the estimated MCC trajectories by age and cohort produced by the three LMMs. Trajectories estimated by Model 1 (Panel B) and Model 2 (Panel C) were similar, reflecting the likeness of estimates reported for these models in Table 3. The trajectory of MCC burden by cohort estimated by Model 3 (Panel D) demonstrates the effect of allowing MCC burden to vary by age within cohort. Across models with varying approaches to estimating APC effects, adults born to later cohorts generally reported greater MCC burden than earlier born cohorts at comparable ages. Later-born cohorts also crossed the threshold of multimorbidity at younger ages than those born to prior cohorts. Based on estimates from Model 3 (displayed in Figure 1 Panel D), the age of multimorbidity onset was about 75 years of age for the Greatest Generation, about 69 years of age for Early Children of Depression, about 61 years of age for Late Children of Depression, and between ages 55–57 for members of the War Babies and all Baby Boom cohorts.
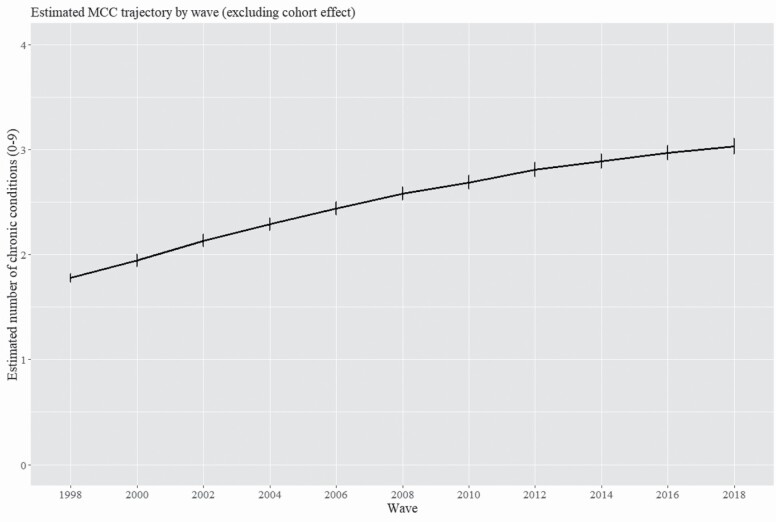
To estimate the magnitude of period effects, or trends in MCC burden as a function of chronological time, Figure 2 presents predicted MCC burden by wave in models adjusted for linear and quadratic age while excluding cohort effects. Covariates included in Models 1–3 and an indicator of study dropout adjusted the model estimating period effects. The plot suggests an increase in MCC burden over the observational period net of age, but this trend should be interpreted cautiously as the statistical model excluded cohort effects to break APC collinearity.
Figure 2.
Estimated trajectory of multiple chronic conditions (MCCs) by interview wave, HRS 1998–2018. Notes: Statistical model used to generate estimates included fixed effects for wave, linear age, quadratic age, and covariates; no cohort effect estimated. Survey weights from respondent’s first available interview were used to adjust estimated trajectory. HRS = Health and Retirement Study.
To examine the impact of mortality and other forms of nonrandom attrition on the observed cohort patterns in MCC burden, we reestimated the primary LMMs adjusted for IPWs. Supplementary Table 3 presents weighted descriptive statistics for the cross-wave sample used to estimate IPWs. Notably, about 31% of the sample dropped out of the study due to mortality or other forms of attrition. Estimates from the IPW-adjusted LMMs, presented in Supplementary Table 5, generally align with those from models unadjusted for IPWs, suggesting the primary results are likely robust to the influence of mortality and loss to follow-up.
To examine cohort differences in the composition of MCCs, Supplementary Table 6 presents the weighted prevalence of each chronic condition for respondents with multimorbidity by cohort. Among multimorbid adults, arthritis and hypertension were the most common chronic conditions, with arthritis the most common condition for respondents born before 1948, and hypertension the most common condition for those born 1948 or later. Prevalence of depressive symptoms and diabetes generally increased in each successive cohort, while heart disease, cancer, stroke, and cognitive impairment decreased among later-born cohorts (though these are likely the result of uncontrolled age differences across cohort). Focusing on condition prevalence across cohorts within age group (Supplementary Table 7), depressive symptoms appeared to consistently increase in later cohorts in all but the oldest age groups, and diabetes prevalence was generally greater in later cohorts for all age groups. Prevalence of heart disease and stroke typically decreased within age group in later cohorts.
Discussion
The present study establishes intercohort patterns in MCC burden in a representative sample of aging U.S. adults. Both descriptive and inferential approaches to identifying cohort effects suggest that later-born cohorts are experiencing greater MCC burden and crossing the threshold of multimorbidity at younger ages than prior cohorts. We identified disparities in MCC burden related to race/ethnicity, childhood socioeconomic adversity, and childhood health, and found that depressive symptomatology and diabetes were likely contributing to the observed cohort multimorbidity patterns. Our results add to evidence of deteriorating health among those entering midlife and late life and represent a potentially significant threat to the health of aging populations and the efficacy of our health care system.
We found that later-born U.S. cohorts experienced greater MCC burden and multimorbidity at earlier ages than cohorts born to prior eras. The identification of increasing MCC burden among later cohorts is alarming yet aligns with several reports of decreasing health and wellness in aging populations (Canizares et al., 2018; Case & Deaton, 2015; Zheng & Echave, 2021). Identifying mechanisms explaining intercohort morbidity patterns is challenging given several plausible causal and artifactual inputs. Differential exposure to risk factors such as obesity may generate greater disease burden at earlier ages (The GBD 2015 Obesity Collaborators, 2017), or declining disease-specific mortality rates may result in populations with expanding morbidity (Crimmins & Beltrán-Sánchez, 2011). Others argue that enhanced disease detection and medicalization explain the recent increase in chronic disease, suggesting that greater chronic disease prevalence is the result of enhanced medical technology, proliferation of diagnostic classifications and criteria, and improved screening and detection (McGrail et al., 2016). Several of our statistical models adjusted for period effects in attempts to account for the influence of secular trends in technology, diagnosis, and screening on the estimates of intercohort patterns in MCC burden, though we recognize that residual confounding due to these factors likely remains. Given our inability to parse the specific contributions of risk exposure, morbidity expansion, and trends in diagnosis/awareness to our findings, continued work to identify drivers of cohort trends in MCC burden is required.
From a methodological perspective, our work must be framed within the ongoing debate surrounding the estimation of APC effects on health outcomes. There are several methodological and theoretical approaches that have generated vigorous discussion on the (in)ability to break APC collinearity (Bell, 2020; Luo, 2013; Yang & Land, 2013), though consensus on preferred methods has not been reached. The inability to derive a point estimate for the association between MCC burden and period net of cohort and age is an example of the constraints on the researcher when attempting to estimate APC effects. As our intention was to identify substantive patterns in MCC burden by cohort net of age and period, and not take a definitive stand on how the APC problem should be resolved, we took several complimentary approaches with evidence of intercohort trends in MCC burden emergent across the descriptive and inferential methods chosen.
Sociodemographic characteristics and childhood health appeared to pattern chronic disease risk for all cohorts. Respondents who were non-Hispanic Black or from racial/ethnic subgroups including American Indians, Alaskan Natives, Asians, Native Hawaiians, and Pacific Islanders had greater MCC burden than White respondents. Racial/ethnic disparities in multimorbidity in older adults are well established (Quiñones et al., 2011, 2019, 2021), though we are unaware of existing evidence of greater multimorbidity among racial/ethnic minorities who are not Black or Hispanic/Latino derived from representative U.S. data. Respondents born outside of the United States had significantly lower MCC burden than native born respondents, potentially reflecting health advantages among immigrant Americans (Elo et al., 2011) and underdiagnosis of chronic disease due to higher rates of uninsured among foreign-born adults. As foreign-born adults are more prevalent in recent cohorts, immigration may be masking even larger adverse shifts in cohort health among U.S. born adults. Socioeconomic disparities in MCC burden were apparent for all cohorts as reporting less than a high school degree, having low household net worth, and experiencing greater socioeconomic adversity in childhood independently increased the likelihood of greater MCC burden. The durable effect of life-course SES on disease risk across cohort reflects the central role of SES in patterning risk exposure and health disparities (Kuh et al., 2003; Lynch & Smith, 2005). The lasting effects of health in childhood, described as the “long arm of childhood health” (Haas, 2007), were apparent as those reporting fair-to-poor childhood health were at risk of greater MCC burden. In comparison to sociodemographic measures, behavioral risk factors including BMI, smoking status, and alcohol consumption were modestly associated with MCC burden.
Regarding the contribution of each condition to cohort differences in multimorbidity, arthritis and hypertension were the most prevalent single conditions among multimorbid older adults, which has been identified elsewhere (Quiñones et al., 2016). The increase in depressive symptoms and diabetes identified in later-born cohorts while accounting for age is more informative of the conditions contributing to cohort patterns in multimorbidity. Increases in depression among adults aged 50 and older were observed from 2005 to 2015 (Weinberger et al., 2017), and more recent cohorts of midlife adults have elevated risk of mental distress (Case & Deaton, 2015) and depressive symptomatology (Abrams & Mehta, 2019). The prevalence of diabetes increased among adults aged 65 and older from 1999 to 2018 (Wang et al., 2021), though we are unaware of work examining cohort variation in diabetes among older adults. As was true of our primary findings, recent trends in depressive symptomatology and diabetes are likely the combined result of differential exposure to factors leading to worse health such as greater wealth inequality and obesity-related chronic disease in later cohorts (Canizares et al., 2018; Zewde, 2021), and surveillance-related increases in diagnoses due to enhanced awareness and medicalization of these conditions over the observational period.
Our work benefits from several strengths including multifaceted assessment of cohort patterns in MCC burden over two decades of observation in a large representative sample of aging Americans, but limitations temper the conclusions that can be drawn from our findings. Common issues in evaluating trends in health and multimorbidity including within-person variability in self-reports of chronic disease, difficulty in operationalizing multimorbidity and disease severity, incomplete population representation, and mortality selection likely influence our results (Quiñones et al., 2020; Weuve et al., 2012). Though we implemented adjudication methodology to address conflicts in condition identification across interviews, our index of multimorbidity relies on self-report of seven of nine conditions, and sufficient information to triangulate diagnosis of depressive symptoms and dementia was unavailable. Also, our MCC index necessarily excluded some conditions as our operationalization of MCC burden was based on conditions available in the HRS that align with the 20 conditions identified by the OASH MCC working group and the Goodman et al. (2013) conceptual model for standardizing analysis of health data for specific chronic conditions. Our use of a standardized conceptual model to define and measure multimorbidity attempts to address the heterogeneity in the assessment of multimorbidity (Diederichs et al., 2011), though we acknowledge our MCC index is one of many operationalizations of the concept of multimorbidity. Lack of detailed information on disease stage and prognosis prevented us from examining whether the severity of conditions varied across cohort, and estimating intercohort variation in unique MCC combinations was beyond the scope of our analyses. Regarding population representation, the lack of specificity on racial/ethnic heritage among Hispanic/Latinos and the multiple racial/ethnic groups included in the masked “Other” race/ethnicity category in the HRS precludes meaningful examination of these groups. Also, adults remaining in the earliest born cohorts are highly select on good health, and adults in later-born cohorts likely live with greater MCC burden due to lower age- and disease-specific mortality rates compared to earlier generations. Though our results did not appear sensitive to dropout, selective mortality prior to HRS enrollment may have contributed to the observed cohort patterns. Finally, the exclusion of respondents with missing data may have underrepresented certain groups, though the relatively small percentage of excluded cases (4.0% of possible cases) suggests this likely did not significantly influence our results.
Our study contributes to evidence that multimorbidity is increasing in the cohorts just beginning to enter older adulthood. Worsening health among the cohorts driving population aging will likely increase the burden of care on communities, clinicians, and health systems tasked with managing the health of our population. Policies aimed at improving the upstream determinants of health for racial/ethnic minorities and interventions designed to improve equitable access to education, reduce childhood socioeconomic adversity, and enhance childhood health would drive sustained improvements in the health of aging populations. Programs specifically targeting depression and diabetes may be particularly impactful in reducing MCC burden in cohorts beginning to enter older adulthood. Future work on the progression and sequelae of multimorbidity at the individual and population level is crucial to development of policy and interventions able to address the possible cascade of disease resulting from deteriorating health among cohorts beginning to experience age-related health decline.
Supplementary Material
Acknowledgments
The authors would like to thank the peer reviewers for their insightful and supportive comments.
Contributor Information
Nicholas J Bishop, Human Development and Family Sciences, Texas State University, San Marcos, Texas, USA.
Steven A Haas, Department of Sociology and Criminology, The Pennsylvania State University, University Park, Pennsylvania, USA.
Ana R Quiñones, Department of Family Medicine, and OHSU-PSU School of Public Health, Oregon Health & Science University, Portland, Oregon, USA.
Funding
Contributions to this work were supported by the National Institute on Aging at the National Institutes of Health (R01AG055681 to A. R. Quiñones).
Conflict of Interest
A. R. Quiñones serves on the editorial board for The Journals of Gerontology, Series B: Social Sciences.
Author Contributions
N. J. Bishop performed the analyses and wrote the initial draft of the manuscript. S. A. Haas provided statistical advice, contributed to the theoretical background, and revised the manuscript. A. R. Quiñones provided statistical advice, contributed to the theoretical background, and revised the manuscript. All authors contributed to planning the study, interpretation of the data, and revisions to the manuscript.
References
- Abrams, L. R., & Mehta, N. K. (2019). Changes in depressive symptoms over age among older Americans: Differences by gender, race/ethnicity, education, and birth cohort. SSM—Population Health, 7, 100399. doi: 10.1016/J.SSMPH.2019.100399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agborsangaya, C. B., Majumdar, S. R., Sharma, A. M., Gregg, E. W., & Padwal, R. S. (2015). Multimorbidity in a prospective cohort: Prevalence and associations with weight loss and health status in severely obese patients. Obesity, 23(3), 707–712. doi: 10.1002/oby.21008 [DOI] [PubMed] [Google Scholar]
- Agborsangaya, C. B., Ngwakongnwi, E., Lahtinen, M., Cooke, T., & Johnson, J. A. (2013). Multimorbidity prevalence in the general population: The role of obesity in chronic disease clustering. BMC Public Health, 13, 1161. doi: 10.1186/1471-2458-13-1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, A. (2014). Life-course and cohort trajectories of mental health in the UK, 1991–2008—A multilevel age–period–cohort analysis. Social Science & Medicine, 120, 21–30. doi: 10.1016/j.socscimed.2014.09.008 [DOI] [PubMed] [Google Scholar]
- Bell, A. (2020). Age period cohort analysis: A review of what we should and shouldn’t do. Annals of Human Biology, 47(2), 208–217. doi: 10.1080/03014460.2019.1707872 [DOI] [PubMed] [Google Scholar]
- Bloom, D. E., Chatterji, S., Kowal, P., Lloyd-Sherlock, P., Mckee, M., Rechel, B., Rosenberg, L., & Smith, J. P. (2015). Macroeconomic implications of population ageing and selected policy responses. The Lancet, 385(9968), 649–657. doi: 10.1016/S0140-6736(14)61464-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadogan, C. A., Ryan, C., & Hughes, C. M. (2016). Appropriate polypharmacy and medicine safety: When many is not too many. Drug Safety, 39, 109–116. doi: 10.1007/s40264-015-0378-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canizares, M., Hogg-Johnson, S., Gignac, M. A. M., Glazier, R. H., & Badley, E. M. (2018). Increasing trajectories of multimorbidity over time: Birth cohort differences and the role of changes in obesity and income. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73(7), 1303–1314. doi: 10.1093/geronb/gbx004 [DOI] [PubMed] [Google Scholar]
- Case, A., & Deaton, A. (2015). Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proceedings of the National Academy of Sciences of the United States of America, 112(49), 15078–15083. doi: 10.1073/pnas.1518393112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigolle, C. T., Nagel, C. L., Blaum, C. S., Liang, J., & Quiñones, A. R. (2016). Inconsistency in the self-report of chronic diseases in panel surveys: Developing an adjudication method for the Health and Retirement Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73(5), 901–912. doi: 10.1093/geronb/gbw063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins, E. M., & Beltrán-Sánchez, H. (2011). Mortality and morbidity trends: Is there compression of morbidity? The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66(1), 75–86. doi: 10.1093/geronb/gbq088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins, E. M., Kim, J. K., Langa, K. M., & Weir, D. R. (2011). Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66(Suppl. 1), i162–i171. doi: 10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederichs, C., Berger, K., & Bartels, D. B. (2011). The measurement of multiple chronic diseases—A systematic review on existing multimorbidity indices. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 66(3), 301–311. doi: 10.1093/gerona/glq208 [DOI] [PubMed] [Google Scholar]
- DuGoff, E. H., Schuler, M., & Stuart, E. A. (2014). Generalizing observational study results: Applying propensity score methods to complex surveys. Health Services Research, 49(1), 284–303. doi: 10.1111/1475-6773.12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo, I. T., Mehta, N. K., & Huang, C. (2011). Disability among native-born and foreign-born Blacks in the United States. Demography, 48(1), 241–265. doi: 10.1007/s13524-010-0008-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, R. A., Posner, S. F., Huang, E. S., Parekh, A. K., & Koh, H. K. (2013). Defining and measuring chronic conditions: Imperatives for research, policy, program, and practice. Preventing Chronic Disease, 10(4), 120239. doi: 10.5888/pcd10.120239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas, S. A. (2007). The long-term effects of poor childhood health: An assessment and application of retrospective reports. Demography, 44, 113–135. doi: 10.1353/dem.2007.0003 [DOI] [PubMed] [Google Scholar]
- Haas, S. A., Oi, K., & Zhou, Z. (2017). The life course, cohort dynamics, and international differences in aging trajectories. Demography, 54(6), 2043–2071. doi: 10.1007/s13524-017-0624-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, H. C. (2015). Trajectories of multimorbidity and impacts on successful aging. Experimental Gerontology, 66, 32–38. doi: 10.1016/j.exger.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Kastner, M., Hayden, L., Wong, G., Lai, Y., Makarski, J., Treister, V., Chan, J., Lee, J. H., Ivers, N. M., Holroyd-Leduc, J., & Straus, S. E. (2019). Underlying mechanisms of complex interventions addressing the care of older adults with multimorbidity: A realist review. BMJ Open, 9, e025009. doi: 10.1136/bmjopen-2018-025009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh, D., Ben-Shlomo, Y., Lynch, J., Hallqvist, J., & Power, C. (2003). Life course epidemiology. Journal of Epidemiology and Community Health, 57, 778–783. doi: 10.1136/jech.57.10.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa, K. M., Kabeto, M., & Weir, D. (2010). Special report: Race, ethnicity, and AD, in 2010 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 6(2), 158–194. doi: 10.1016/j.jalz.2010.01.009 [DOI] [PubMed] [Google Scholar]
- Lebenbaum, M., Zaric, G. S., Thind, A., & Sarma, S. (2018). Trends in obesity and multimorbidity in Canada. Preventive Medicine, 116, 173–179. doi: 10.1016/J.YPMED.2018.08.025 [DOI] [PubMed] [Google Scholar]
- Lehnert, T., Heider, D., Leicht, H., Heinrich, S., Corrieri, S., Luppa, M., Riedel-Heller, S., & König, H.-H. (2011). Review: Health care utilization and costs of elderly persons with multiple chronic conditions. Medical Care Research and Review, 68(4), 387–420. doi: 10.1177/1077558711399580 [DOI] [PubMed] [Google Scholar]
- Luo, L. (2013). Assessing validity and application scope of the intrinsic estimator approach to the age-period-cohort problem. Demography, 50, 1945–1967. doi: 10.1007/s13524-013-0243-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, J. W., & Smith, G. D. (2005). A life course approach to chronic disease epidemiology. Annual Review of Public Health, 26, 1–35. doi: 10.1146/annurev.publhealth.26.021304.144505 [DOI] [PubMed] [Google Scholar]
- Martin, L. G., Freedman, V. A., Schoeni, R. F., & Andreski, P. M. (2009). Health and functioning among Baby Boomers approaching 60. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 64(3), 369–377. doi: 10.1093/geronb/gbn040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, L. G., Freedman, V. A., Schoeni, R. F., & Andreski, P. M. (2010). Trends in disability and related chronic conditions among people ages fifty to sixty-four. Health Affairs, 29(4), 725–731. doi: 10.1377/hlthaff.2008.0746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrail, K., Lavergne, R., & Lewis, S. (2016). The chronic disease explosion: Artificial bang or empirical whimper? BMJ, 352, i1312. doi: 10.1136/bmj.i1312 [DOI] [PubMed] [Google Scholar]
- Meara, E., & Skinner, J. (2015). Losing ground at midlife in America. Proceedings of the National Academy of Sciences of the United States of America, 112(49), 15006–15007. doi: 10.1073/pnas.1519763112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montez, J. K., & Hayward, M. D. (2014). Cumulative childhood adversity, educational attainment, and active life expectancy among U.S. adults. Demography, 51(2), 413–435. doi: 10.1007/s13524-013-0261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathirana, T. I., & Jackson, C. A. (2018). Socioeconomic status and multimorbidity: A systematic review and meta-analysis. Australian and New Zealand Journal of Public Health, 42(2), 186–194. doi: 10.1111/1753-6405.12762 [DOI] [PubMed] [Google Scholar]
- Piontek, K., Kraus, L., Müller, S., & Pabst, A. (2010). To what extent do age, period, and cohort patterns account for time trends and social inequalities in smoking? SUCHT, 56(5), 361–371. doi: 10.1024/0939-5911/a000047 [DOI] [Google Scholar]
- Quiñones, A. R., Allore, H. G., Botoseneanu, A., Newsom, J. T., Nagel, C. L., & Dorr, D. A. (2020). Tracking multimorbidity changes in diverse racial/ethnic populations over time: Issues and considerations. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 75(2), 297–300. doi: 10.1093/gerona/glz028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones, A. R., Botoseneanu, A., Markwardt, S., Nagel, C. L., Newsom, J. T., Dorr, D. A., & Allore, H. G. (2019). Racial/ethnic differences in multimorbidity development and chronic disease accumulation for middle-aged adults. PLoS One, 14(6), e0218462. doi: 10.1371/journal.pone.0218462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones, A. R., Liang, J., Bennett, J. M., Xu, X., & Ye, W. (2011). How does the trajectory of multimorbidity vary across Black, White, and Mexican Americans in middle and old age? The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66(6), 739–749. doi: 10.1093/geronb/gbr106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones, A. R., Markwardt, S., & Botoseneanu, A. (2016). Multimorbidity combinations and disability in older adults. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 71(6), 823–830. doi: 10.1093/gerona/glw035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones, A. R., Newsom, J. T., Elman, M. R., Markwardt, S., Nagel, C. L., Dorr, D. A., Allore, H. G., & Botoseneanu, A. (2021). Racial and ethnic differences in multimorbidity changes over time. Medical Care, 59(5), 402–409. doi: 10.1097/MLR.0000000000001527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, W. R., Utz, R. L., Keyes, K. M., Martin, C. L., & Yang, Y. (2012). Birth cohort effects on abdominal obesity in the United States: The Silent Generation, Baby Boomers and Generation X. International Journal of Obesity, 37(8), 1129–1134. doi: 10.1038/ijo.2012.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbach, M., & Andersen, J. S. (2017). Patient-experienced burden of treatment in patients with multimorbidity—A systematic review of qualitative data. PLoS One, 12(6), e0179916. doi: 10.1371/journal.pone.0179916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salive, M. E. (2013). Multimorbidity in older adults. Epidemiologic Reviews, 35, 75–83. doi: 10.1093/epirev/mxs009 [DOI] [PubMed] [Google Scholar]
- Schäfer, I., Hansen, H., Schäön, H., Höfels, S., Altiner, A., Dahlhaus, A., Gensichen, J., Riedel-Heller, S., Weyerer, S., Blank, W. A., König, H.-H., Knesebeck, O. V. D., Wegscheider, K., Scherer, M., Bussche, H. V. D., & Wiese, B. (2012). The influence of age, gender and socio-economic status on multimorbidity patterns in primary care. First results from the Multicare Cohort Study. BMC Health Services Research, 12, 89. doi: 10.1007/s00103-011-1414-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman, T. E., Merkin, S. S., Crimmins, E. M., & Karlamangla, A. S. (2010). Disability trends among older Americans: National Health and Nutrition Examination Surveys, 1988–1994 and 1999–2004. American Journal of Public Health, 100(1), 100–107. doi: 10.2105/AJPH.2008.157388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffick, D. E. (2000). Documentation of affective functioning measures in the health and retirement study (HRS/AHEAD). https://hrs.isr.umich.edu/sites/default/files/biblio/dr-005.pdf
- Stürmer, T., Rothman, K. J., Avorn, J., & Glynn, R. J. (2010). Treatment effects in the presence of unmeasured confounding: Dealing with observations in the tails of the propensity score distribution—A simulation study. American Journal of Epidemiology, 172(7), 843–854. doi: 10.1093/aje/kwq198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suls, J., Bayliss, E. A., Berry, J., Bierman, A. S., Chrischilles, E. A., Farhat, T., Fortin, M., Koroukian, S. M., Quiñones, A., Silber, J. H., Ward, B. W., Wei, M., Young-Hyman, D., & Klabunde, C. N. (2021). Measuring multimorbidity: Selecting the right instrument for the purpose and the data source. Medical Care, 59(8), 743–756. doi: 10.1097/MLR.0000000000001566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The GBD 2015 Obesity Collaborators. (2017). Health effects of overweight and obesity in 195 countries over 25 years. New England Journal of Medicine, 377, 13–27. doi: 10.1056/NEJMOA1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Seeley, R. D., Li, Y., Sorensen, G., & Subramanian, S. V. (2011). Lifecourse socioeconomic circumstances and multimorbidity among older adults. BMC Public Health, 11(1), 1–9. doi: 10.1186/1471-2458-11-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2010). Multiple chronic conditions: A strategic framework. Optimum health and quality of life for individuals with multiple chronic conditions. http://www.pined.info/pdf/framework/6.pdf [DOI] [PubMed]
- Vespa, J., Armstrong, D. M., & Medina, L. (2020). Demographic turning points for the United States: Population projections for 2020 to 2060. https://www.census.gov/content/dam/Census/library/publications/2020/demo/p25-1144.pdf
- Vetrano, D. L., Calderón-Larrañaga, A., Marengoni, A., Onder, G., Bauer, J. M., Cesari, M., Ferrucci, L., & Fratiglioni, L. (2018). An international perspective on chronic multimorbidity: Approaching the elephant in the room. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 73(10), 1350–1356. doi: 10.1093/gerona/glx178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L., Li, X., Wang, Z., Bancks, M. P., Carnethon, M. R., Greenland, P., Feng, Y., Wang, H., & Zhong, V. W. (2021). Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999–2018. Journal of the American Medical Association, 326(8), 704–716. doi: 10.1001/jama.2021.9883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger, A. H., Gbedemah, M., Martinez, A. M., Nash, D., Galea, S., & Goodwin, R. D. (2017). Trends in depression prevalence in the USA from 2005 to 2015: Widening disparities in vulnerable groups. Psychological Medicine, 48, 1308–1315. doi: 10.1017/s0033291717002781 [DOI] [PubMed] [Google Scholar]
- Weuve, J., Tchetgen Tchetgen, E. J., Glymour, M. M., Beck, T. L., Aggarwal, N. T., Wilson, R. S., Evans, D. A., & Mendes De Leon, C. F. (2012). Accounting for bias due to selective attrition: The example of smoking and cognitive decline. Epidemiology, 23(1), 119–128. doi: 10.1097/EDE.0b013e318230e861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., & Land, K. C. (2013). Age-period-cohort analysis: New models, methods, and empirical applications. Taylor & Francis. [Google Scholar]
- Zewde, N. (2021). Impact of the 2008 recession on wealth-adjusted income and inequality for US cohorts. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 77(4), 780–789. doi: 10.1093/geronb/gbab141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, H. (2020). A new look at cohort trend and underlying mechanisms in cognitive functioning. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 76(8), 1652–1663. doi: 10.1093/geronb/gbaa107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, H., & Echave, P. (2021). Are recent cohorts getting worse? Trends in US adult physiological status, mental health, and health behaviors across a century of birth cohorts. American Journal of Epidemiology, 190(11), 2242–2255. doi: 10.1093/aje/kwab076 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.