Abstract
Objectives
We aimed to investigate the outcomes of pulmonary vein isolation in athletes.
Methods
We retrospectively identified endurance athletes who underwent catheter ablation at our institution (2004–2018). Endurance athletes were defined as participating in competitive athletics for at least 1500 lifetime hours in sports at the IB or IIA Bethesda classification or higher. Primary endpoints were freedom from atrial arrhythmias at 12, 24, and 36 months after the procedure. Secondary endpoints were defined as qualitative improvement in symptoms allowing athletes to return to their previous level of activity. Athletes were compared with a control group of nonathletes in a 3‐to‐1 matched analysis by age and sex.
Results
A total of 39 endurance athletes who underwent catheter ablation were identified during the study period. At 12 months, there was no difference in treatment outcomes for athletes versus nonathletes (relative risk [RR], 1.06; 95% CI, 0.92–1.22; p = .40). Freedom from atrial arrhythmias was 35% less likely in athletes than nonathletes at 24 months (RR, 0.65; 95% CI, 0.50–0.83; p < .001) and 42% less likely at 36 months (RR, 0.58; 95% CI, 0.41–0.79; p < .001). Overall, 77% of the athletes were able to return to their previous level of activity following catheter ablation.
Conclusion
Endurance athletes with atrial fibrillation appear to have higher rates of atrial arrhythmia recurrence than nonathletes after catheter ablation, with higher rates of atypical flutter. The majority of athletes were able to return to their previous level of activity after ablation.
Keywords: athlete, atrial fibrillation, catheter ablation, outcomes, pulmonary vein isolation
Endurance athletes with atrial fibrillation have high rates of atrial arrhythmia recurrence than nonathletes after catheter ablation. Regardless, 77% of athletes were able to return to their previous level of activity after ablation.
1. INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia in clinical practice, and an increasingly recognized risk factor for AF is participation in high‐endurance sports. Whereas moderate exercise appears to be protective against AF, at extreme levels of exercise the risk is increased, which results in a U‐shaped curve. 1
In high‐endurance athletes with AF, management is challenging and recommendations are largely based on anecdotal evidence and expert opinion. Guidelines on AF in athletes include the 36th Bethesda Conference Eligibility Recommendations for Competitive Athletes with Cardiovascular Abnormalities 2 and The European Society of Cardiology Guidelines for the management of AF. 3 Deconditioning, in which athletes are recommended to decrease their intensity and duration of exercise, is a common first step with some evidence for benefit. 4 , 5 , 6 , 7
However, deconditioning is sometimes not a practical or desired option because it can lead to decreased quality of life and affect the continued participation of athletes in their respective competitive sports. Rate control is often not tolerated because of high vagal tone and resting bradycardia. Furthermore, β‐blockers are prohibited in many sports by the World Anti‐Doping Agency. 8 Although rhythm control with antiarrhythmic medications can be tried, rhythm control with medications fails for many athletes, and pulmonary vein isolation is required.
The current study aimed to determine the outcomes of catheter ablation for AF in high‐endurance athletes compared with nonathletes.
2. METHODS
2.1. Study population
This study was approved by the Mayo Clinic Institutional Review Board. We performed a retrospective cohort study of patients undergoing first‐time catheter ablation using cryoablation or radiofrequency technology for AF at Mayo Clinic from January 1, 2004 to December 31, 2018. The study included patients at the three academic campuses in Rochester, Minnesota; Phoenix, Arizona; and Jacksonville, Florida. Patients were retrospectively identified from our patient database by using Current Procedural Terminology codes for catheter ablation procedures. Those who had a sports activity history were identified with the keywords “athlete” and “sports.” Additional chart review was then performed to identify potential endurance athletes. Potential athletes were contacted by telephone, and only high‐endurance athletes were enrolled. A group of controls who were not identified as being high‐endurance athletes and who underwent catheter ablation for AF were matched to athletes in a 3‐to‐1 ratio on the basis of age, sex, and closest date of AF catheter ablation procedure.
2.2. Definitions
High‐endurance athletes were defined as those who have participated in competitive athletics for at least 1500 lifetime hours, as similarly defined by Koopman et al. 9 Only athletes who competed in sports at the IB or IIA Bethesda classification or higher were considered. Recreational activity such as light jogging or exercising was not considered.
Patients identified as potential high‐endurance athletes were contacted via telephone to document the number of lifetime hours of exercise as part of a questionnaire. Other questions were about type of sport, their AF, and their ability to return to competition after ablation. Patients were excluded from the athlete group if they could not be contacted, if they did not meet the athlete definition, or if the initial ablation was a maze procedure.
2.3. Data collection
Baseline characteristics were obtained for all patients from the health records: age, sex, body mass index (BMI), echocardiographic characteristics (left atrial volume index, left ventricular [LV] end‐diastolic diameter, LV end‐systolic diameter, and LV ejection fraction), and comorbid conditions (LV hypertrophy, coronary artery disease, obstructive sleep apnea, and valvular heart disease). The presence of atrial flutter, type of AF (paroxysmal vs. persistent) at time of ablation, and duration of AF were also obtained.
2.4. Outcomes
The primary study endpoint was freedom from atrial arrhythmias at 12, 24, and 36 months after the procedure. Freedom from atrial arrhythmias was defined as freedom from AF, atrial flutter, or atrial tachycardia that did not require repeated ablation, cardioversion, or antiarrhythmic therapy after a 3‐month blanking period. Follow‐up was at the discretion of the provider, which typically includes Holter monitoring and clinical follow‐up of symptoms at 3 months and 1 year. Data on qualitative improvement in symptoms of athletes were also obtained. Secondary endpoints were defined as qualitative improvement in symptoms allowing athletes to return to their previous level of activity and number of hours of training able to be performed after ablation.
2.5. Statistical analysis
Patient characteristics and variables related to athletic history were summarized as frequency and percentage for categorical variables and mean or median for continuous variables. Differences in demographic and patient history between athletes and nonathletes were assessed with Fisher exact tests for categorical variables and t tests for continuous variables. Fisher exact tests and t tests were also used to assess associations between patient characteristics and freedom from atrial arrhythmias. Differences in rates of freedom from atrial arrhythmias between athletes and nonathletes were assessed with log‐binomial regression models. The difference in the total number of ablations between these two groups was assessed with Poisson regression models. All models included inverse probability weighting to adjust for age, sex, BMI, type of AF, cardiovascular history (atrial flutter, hypertension, LV hypertrophy, coronary artery disease, obstructive sleep apnea, and valvular heart disease), and AF duration. Kaplan–Meier analysis was performed for the primary endpoint, where survival probability was defined as freedom from atrial arrhythmias. p < .05 were considered statistically significant. All analyses were performed with R Statistical Software (version 3.6; R Foundation for Statistical Computing).
2.6. Patient and public involvement
Patients were individually contacted via telephone for their consent to participate in the study at the beginning of data collection. They were made aware of the research study's question and that their medical chart would be accessed by the research team staff. In the event that they opted not to participate, they were not included. All suggestions or concerns were addressed during that initial telephone conversation.
3. RESULTS
3.1. Study participants
Among 1427 patients at Mayo Clinic sites in Arizona, Florida, and Minnesota who underwent catheter ablation procedures during the study period, 174 were identified as potential high‐endurance athletes. After these 174 patients were contacted for the athletic questionnaire, 76 met the definition of high‐endurance athlete, and 39 completed the written consent form and were included as part of the analysis. Three nonathletes were matched to each athlete, resulting in 117 patients in the nonathlete control group.
Comparison of characteristics of athletes versus nonathletes showed a lower percentage of BMIs in the obese range and a higher prevalence of hypertension and atrial flutter in the athlete group (Table 1). Athletes had a higher incidence of atrial flutter than nonathletes (67% vs. 44%; p = .01). In addition, the total number of ablations was higher for athletes than nonathletes (relative risk [RR], 1.59; 95% CI, 1.12–2.27; p = .01), meaning that athletes have 1.59 times the total number of ablations as nonathletes. There were no significant differences in other characteristics or cardiac measurements between the groups. Among athletes, 46.2% had at least two ablations compared to 29.9% in nonathletes.
TABLE 1.
Characteristics of athletes versus nonathletes at baseline, and ablation variables a
| Characteristic | Athletes (n = 39) | Nonathletes (n = 117) | p value |
|---|---|---|---|
| Baseline | |||
| Age, year | 62.5 (8.1) | 62.4 (8.0) | .99 |
| Women | 2 (5) | 6 (5.1) | >.99 |
| BMI | .001 | ||
| Normal weight | 11 (28) | 8 (6.8) | |
| Overweight | 18 (46) | 42 (35.9) | |
| Obese | 10 (26) | 67 (57.3) | |
| Persistent AF | 8 (21) | 19 (16.2) | .54 |
| Hypertension | 20 (51) | 37 (31.6) | .03 |
| Obstructive sleep apnea | 14 (36) | 50 (42.7) | .45 |
| Left ventricular hypertrophy | 3 (8) | 3 (2.6) | .15 |
| Coronary artery disease | 10 (26) | 27 (23.1) | .74 |
| Valvular disease | 8 (21) | 12 (10.3) | .10 |
| AF duration, months | 35 (11–96) | 24 (8–60) | .10 |
| Cardiac measurements | |||
| LA, ml/m2 b | 40.2 (9.4) | 37.6 (11.2) | .21 |
| LVEDD, mm | 51.1 (5.4) | 51.3 (5.3) | .84 |
| LVESD, mm | 34.4 (5.5) | 33.7 (6.2) | .56 |
| EF, % | 58.5 (9.0) | 59.4 (9.0) | .56 |
| Ablation | |||
| Type of ablation | .76 | ||
| Cryoablation | 7 (18) | 23 (19.7) | |
| RFA | 27 (69) | 86 (73.5) | |
| Both | 4 (10) | 8 (6.8) | |
| Unknown | 1 (3) | 0 (0) | |
| Atrial flutter | 26 (67) | 51 (43.6) | .01 |
| Total ablations | 1.74 (0.99) | 1.39 (0.71) | .01 |
Note: Abbreviations: AF, atrial fibrillation; BMI, body mass index; EF, ejection fraction; LA, left atrium; LVEDD, left ventricular end‐diastolic diameter; LVESD, left ventricular end‐systolic diameter; RFA, radiofrequency ablation.
Values are mean (SD), no. of patients (%), or median (interquartile range).
Biplane volume index by method of discs.
We have included an analysis of whether baseline characteristics were predictors of freedom from atrial arrhythmias in the Appendix A. At 12 months, independent predictors of freedom from atrial arrhythmias include no history of valvular disease (p = .031), shorter AF duration (p = .025), and a smaller left atrium (p = .011). At 24 months, independent predictors of freedom from atrial arrhythmias include no history of atrial flutter (p = .022), no history of valvular disease (p = .001), shorter AF duration (p = .041), and a smaller left atrium (p = .034). At 36 months, independent predictors of freedom from atrial arrhythmias include no history of valvular disease (p = .003), a smaller left atrium (p = .084), a history of coronary artery disease (p = .037), and higher LVEF (p = .012). At greater than 36 months, independent predictors of freedom from atrial arrhythmias are a smaller left atrium (p = .003).
3.2. Outcomes in athletes versus nonathletes
At 12 months, there was no difference in treatment outcomes, but freedom from atrial arrhythmias was 35% less likely in athletes compared with nonathletes at 24 months (RR, 0.65; 95% CI, 0.50–0.83; p < .001) and 42% less likely at 36 months (RR, 0.58; 95% CI, 0.41–0.79; p < .001) (Table 2; Figure 1). The difference in primary outcomes was due to a higher incidence of atrial flutter ablation in athletes versus nonathletes (RR, 4.15; 95% CI, 2.42–7.73; p < .001) (Table 3). In particular, atypical flutter was seven times more likely in athletes during subsequent ablation (RR, 7.26; 95% CI, 3.13–21.3; p < .001) (Table 4). There were four athletes excluded in the analysis at 36 months and six athletes excluded after 36 months due to insufficient length of follow‐up.
TABLE 2.
Freedom from atrial arrhythmias in athletes versus nonathletes
| Freedom from atrial arrhythmias | Athletes a | Nonathletes a | Relative risk (95% CI) b | p value |
|---|---|---|---|---|
| At 12 months | 26/39 (67) | 86/117 (73.5) | 1.06 (0.91–1.22) | .41 |
| At 24 months | 19/39 (49) | 75/117 (64.1) | 0.65 (0.50–0.83) | <.001 |
| At 36 months | 14/35 (40) | 62/113 (54.9) | 0.58 (0.41–0.79) | <.001 |
| After 36 months | 8/33 (24) | 43/108 (39.8) | 0.45 (0.27–0.69) | <.001 |
Values are no. of patients/no. of patients with data available (%).
Adjusted for baseline characteristics.
FIGURE 1.
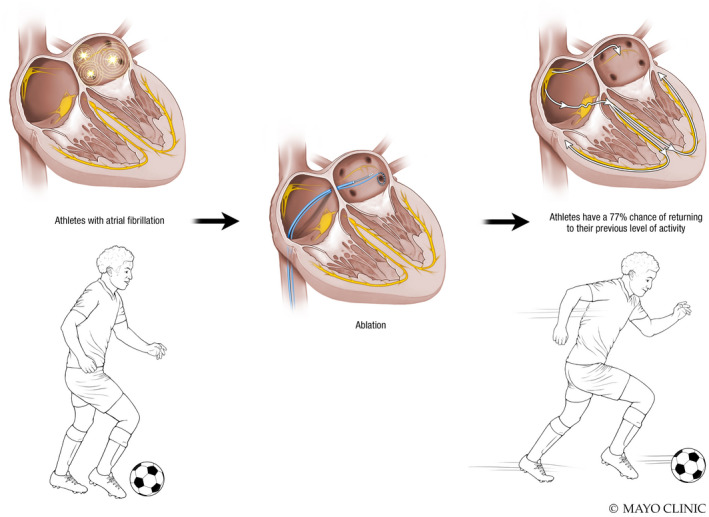
Central Illustration: Athletes have a 77% chance of returning to their previous level of activity after catheter ablation
TABLE 3.
Primary end points in athletes and nonathletes
| Recurrence type | Athletes (n = 39) a | Nonathletes (n = 117) a | Relative risk (95% CI) | p value |
|---|---|---|---|---|
| Antiarrhythmic medication | 14 (36) | 43 (36.8) | 0.78 (0.54–1.11) | .17 |
| Cardioversion | 10 (26) | 29 (24.8) | 1.22 (0.82–1.81) | .33 |
| Atrial fibrillation ablation | 12 (31) | 35 (29.9) | 1.10 (0.76–1.59) | .61 |
| Atrial flutter ablation | 12 (31) | 9 (7.7) | 4.15 (2.42–7.73) | <.001 |
| Atrial tachycardia ablation | 0 (0) | 1 (0.9) | b | |
| Atrioventricular node ablation | 1 (3) | 1 (0.9) | b |
Values are no. of patients (%).
Frequencies were too low to model.
TABLE 4.
Ablation of typical versus atypical flutter in athletes and nonathletes
| Characteristic | Athletes a (n = 39) | Nonathletes a (n = 117) | Relative risk (95% CI) | p value |
|---|---|---|---|---|
| Atypical flutter at initial ablation | 1 (3) | 1 (0.9) | b | |
| Atypical flutter at subsequent ablation | 11 (28) | 4 (3.4) | 7.26 (3.13–21.32) | <.001 |
| Typical flutter at initial ablation | 7 (18) | 1 (0.9) | 17.9 (5.5–110.5) | <.001 |
| Typical flutter at subsequent ablation | 1 (3) | 6 (5.1) | 2.15 (0.98–5.06) | .06 |
Values are no. of patients (%).
Frequencies were too low to model.
3.3. Athlete characteristics and effect of ablation on resumption of activity
Sports practiced by athletes and their training characteristics are shown in Table 5. Overall, 44% of athletes had to discontinue athletic activity because of their AF, but 77% of athletes were able to return to their previous level of activity after ablation (Table 5, Central Illustration). Activity level after the ablation stayed the same in 74% of athletes and decreased in 23%.
TABLE 5.
Questionnaire responses and secondary end points in athletes
| Characteristic/question | Value a (n = 39) |
|---|---|
| Sport b | |
| Running (middle distance) | 9 (23) |
| Cycling | 8 (21) |
| Football | 7 (18) |
| Tennis | 6 (15) |
| Running (long distance) | 6 (15) |
| Basketball | 5 (13) |
| Swimming | 5 (13) |
| Baseball | 3 (8) |
| Triathlon | 3 (8) |
| Skiing | 3 (8) |
| Mountain biking | 3 (8) |
| Weightlifting | 2 (5) |
| Hockey, Ironman, kiteboarding, marathon ski racing, military athletic competition team, racquetball, road biking, rugby, running (sprint), sailing, windsurfing, wrestling (1 each) | 1 (3) |
| Time spent on sport per week, h | 11.8 (5.7) |
| Time spent training, year | 27.0 (16.6) |
| Lifetime hours of exercise | 16,487.3 (13,990.1) |
| Bethesda classification | |
| IB | 0 (0) |
| IC | 12 (31) |
| IIA | 0 (0) |
| IIB | 7 (18) |
| IIC | 20 (51) |
| IIIA | 4 (10) |
| IIIB | 4 (10) |
| IIIC | 16 (41) |
| Was the sport/training in the distant past only, past + current, or current only? | |
| Past only | 21 (54) |
| Past + current | 18 (46) |
| If intense sport/training was only the past, do you participate in activities to stay active today? | (n = 21) |
| Yes | 20 (95) |
| No | 1 (5) |
| Were you practicing to improve performance or to compete in any sports up to 1 year before your ablation? | |
| Yes | 30 (77) |
| No | 9 (23) |
| Did AF significantly decrease your ability to participate in this sport? | |
| Yes | 10 (26) |
| No | 29 (74) |
| Did the AF stop you from playing sports, or did you stop playing years earlier and then AF developed? | |
| AF was responsible | 17 (44) |
| Discontinued sports prior | 12 (31) |
| Not significantly affected by AF | 10 (26) |
| Were you able to successfully return to your previous level of activity after the ablation? | |
| Yes | 30 (77) |
| No | 9 (23) |
| Did your activity level decrease, stay the same, or increase from before to after ablation? | |
| Decreased | 9 (23) |
| Same | 29 (74) |
| Increased | 1 (3) |
Note: Abbreviation: AF, atrial fibrillation.
Respondents could choose more than one sport.
Values are no. of patients (%) or mean (SD).
4. DISCUSSION
Our analysis of athletes with AF who underwent catheter ablation showed that 1 athletes have lower rates of freedom of atrial arrhythmias after catheter ablation compared with nonathletes at 24 months and beyond, 2 athletes have higher rates of atypical flutter after initial AF ablation, and 3 77% of athletes were able to return to their previous level of activity after ablation.
The mechanisms of AF in athletes are not known but are hypothesized to involve morphologic adaptation, autonomic alteration, and chronic systemic inflammation. 10 Lower rates of freedom of atrial arrhythmias after catheter ablation in athletes could be due to worse underlying substrate for AF, different triggers for AF, and the potential role of the autonomic nervous system.
To date, four studies have evaluated the efficacy of pulmonary vein isolation in athletes. 9 , 11 , 12 , 13 These prior studies suggested that pulmonary vein isolation by catheter ablation is an effective treatment option for athletes and leads to similar outcomes as in nonathletes. The Bethesda Conference allows for return to sports after 4–6 weeks without recurrence of AF or after an electrophysiologic study has confirmed noninducibility. 2
Our results differ from those of prior studies, although limitations of those studies should be noted. The first study by Furlanello et al 11 involved 20 patients and did not include a control group. Calvo et al 12 reported similar freedom from arrhythmias after a single pulmonary vein isolation procedure, although there was a confounding presence of endurance athletes in the control group. Koopman et al 9 avoided these limitations and reported freedom from AF rates at 3 years of 84.5% in athletes and 87.3% in controls, with no significant difference between the groups. Our study reports a lower rate of freedom from atrial arrhythmias but this could be partly explained by our broader definition of our primary endpoint. We included all atrial arrhythmias, including atrial flutter and atrial tachycardia, whereas Koopman et al 9 focused solely on AF. Furthermore, compared with Koopman et al, 9 our study also included more patients with persistent AF (21.4% vs. 12.8%).
Our study also showed that 77% of athletes were able to return to their former level of activity after ablation. This finding is reassuring as it is often a concern expressed by athletes. This finding is consistent with existing evidence of symptomatic improvement after ablation in prior studies. 9 , 11 Furlanello et al 11 used both subjective (symptoms) and objective (Holter monitoring) methods to document recurrence, and 18 of their 20 patients (90%) did not have recurrence. Calvo et al 12 defined recurrence of AF as a documented AF or atrial flutter episode of longer than 30 s after the 3‐month blanking period and did not include symptomatic improvement as an end point. Koopman et al 9 included symptoms as a relevant end point and defined recurrence as a documented episode of AF, atrial flutter, or intra‐atrial reentrant tachycardia lasting for longer than 1 min, as well as subjective symptoms such as palpitations or those identical to symptoms before the procedure.
Of note, there are no standardized definitions of athlete among the previous studies, which makes it difficult to compare similar populations of interest. Furlanello et al 11 defined athlete as “individuals of young and adult age, either amateur or professional, who are engaged in exercise training on a regular basis and participate in official sports competition.” Calvo et al 12 defined athlete as “those who performed regular endurance sports activity for at least 3 hours per week for at least the ten years immediately preceding the arrhythmia diagnosis.” Koopman et al 9 defined athlete as those participating in the IB, IIA, or higher Bethesda classification of sports for three or more hours per week for 10 years or more, or for a total of 1500 lifetime hours or more of exercise after age 14 years. 14 Decroocq et al 13 used a definition of vigorous athlete in which athletes performed at least 6 hours of exercise per week for at least 7 years, for 2000 lifetime hours of exercise. We chose a similar definition as Koopman et al 9 because some evidence suggests an increased prevalence of AF at more than 1500 lifetime hours of exercise. 15 Unlike Koopman et al, 9 we did not distinguish between high‐endurance athletes and nonhigh‐endurance athletes because 1500 lifetime hours of exercise or more has been shown to be a risk factor for AF. 12 , 15
Our current study had a mix of patients who are active athletes and those who stopped athletic activity. Although prior studies did not make this distinction, it can be inferred from their definitions that prior studies also most likely had a mix of active and nonactive athletes.
Our study found increased rates of recurrent atypical atrial flutter ablations in athletes. The reason for this could be due to development of scar over time or related to more redo ablations in the athlete group. With redo ablations, typically additional ablation lines are performed, which increases the risk of future atypical atrial flutters. 16 Further studies are required to investigate whether high‐level physical activity impacts the maturation of ablation lesions.
In the context of these prior studies, further research should investigate whether pulmonary vein isolation is truly a viable treatment modality for athletes. Because of conflicting outcomes data, we would advise athletes to consider a stepwise approach with a period of detraining to determine whether AF is associated with exercise. If detraining is unsuccessful, then the pros and cons of medical therapy (rate or rhythm control) should be discussed with the patient, as well as its effects on sports performance, versus pursuing first‐line catheter ablation. Catheter ablation may decrease time to first recurrence of AF than antiarrhythmic medications, as previously shown in the CABANA trial, 17 although this comparison in athletes has not been studied.
We advise athletes to steadily return to their previous level of activity after ablation for AF. If the ablation is successful, athletes can proceed with intense training and competition without restrictions. Athletes with AF should be periodically evaluated in the context of a multidisciplinary sports cardiology and electrophysiology practice to ensure proper management of lifestyle and control of risk factors for AF.
4.1. Limitations
The main limitation of our study is that our patient population is small. Our population of athletes may not be representative of the athlete population in general. Furthermore, 1500 lifetime hours of athletic participation may not represent extreme levels of athletics. These patients also tended to be older, which suggests that chronic changes from athletics may have more of a chronic role in cardiac adaptations. The second limitation is retrospective recall lifetime hours of exercise remains an estimation. The third limitation is the asymptomatic arrhythmias between follow‐up visits could be missed, especially in the nonathlete group. It is possible that athletes have closer monitoring of their heart rate and rhythm, or have more symptoms related to AF, and therefore are more aware of recurrences of atrial arrhythmias compared to nonathletes. A fourth limitation is that our control group was not contacted for questionnaire submission, so we could not verify that there were not athletes as we do not know their amount of exercise.
AUTHOR CONTRIBUTIONS
Michael B. Liu, MD was the first author, who was primarily responsible for manuscript submission, study design, IRB submission, and also helped collect data. Justin Z. Lee, MBBS offered guidance for study design and manuscript edits. Lindsay Klooster was primarily responsible for data collection, including calling patients individually for their consent to participate in the study and extracting information from the medical record. Skye A. Buckner Petty was our statistician, who offered statistical support and authored the section on statistical analysis. Luis R. Scott, MD was principal investigator who came up with the initial research question and also helped with manuscript submission. All authors participated in the research and preparation of the manuscript as per the International Committee of Medical Journal Editors (ICMJE).
FUNDING INFORMATION
This study was supported by a micro‐grant from the Cardiovascular Research Center, Mayo Clinic Arizona.
CONFLICT OF INTEREST
All authors have no conflicts of interest to disclose.
ETHICS APPROVAL
IRB approval was obtained following standard Institutional research guidelines.
PATIENT CONSENT FOR PUBLICATION
We obtained written consent forms that were stored in a secure location on a Mayo Clinic server. No identifying information is in our manuscript.
ACKNOWLEDGMENTS
This study was made possible through the collaboration of physicians and Mayo Clinic staff.
APPENDIX A.
TABLE A1.
Demographics and patient history versus postablation freedom from atrial arrhythmias at 12 months
| Freedom from atrial arrhythmias (N = 112) | No freedom from atrial arrhythmias (N = 44) | Total (N = 156) | p value | |
|---|---|---|---|---|
| Age | .317 a | |||
| Under 50 | 10 (8.9%) | 2 (4.5%) | 12 (7.7%) | |
| 50–59 | 35 (31.2%) | 9 (20.5%) | 44 (28.2%) | |
| 60–69 | 47 (42.0%) | 21 (47.7%) | 68 (43.6%) | |
| 70+ | 20 (17.9%) | 12 (27.3%) | 32 (20.5%) | |
| Sex | 1.000 a | |||
| Male | 106 (94.6%) | 42 (95.5%) | 148 (94.9%) | |
| Female | 6 (5.4%) | 2 (4.5%) | 8 (5.1%) | |
| BMI | .376 a | |||
| Normal weight | 11 (9.8%) | 8 (18.2%) | 19 (12.2%) | |
| Overweight | 44 (39.3%) | 16 (36.4%) | 60 (38.5%) | |
| Obese | 57 (50.9%) | 20 (45.5%) | 77 (49.4%) | |
| Type of AF | .346 a | |||
| Paroxysmal | 95 (84.8%) | 34 (77.3%) | 129 (82.7%) | |
| Persistent | 17 (15.2%) | 10 (22.7%) | 27 (17.3%) | |
| History of atrial flutter | .075 a | |||
| No | 62 (55.4%) | 17 (38.6%) | 79 (50.6%) | |
| Yes | 50 (44.6%) | 27 (61.4%) | 77 (49.4%) | |
| History of HTN | .274 a | |||
| Yes | 68 (60.7%) | 31 (70.5%) | 99 (63.5%) | |
| No | 44 (39.3%) | 13 (29.5%) | 57 (36.5%) | |
| History of OSA | .857 a | |||
| No | 67 (59.8%) | 25 (56.8%) | 92 (59.0%) | |
| Yes | 45 (40.2%) | 19 (43.2%) | 64 (41.0%) | |
| History of LVH | .351 a | |||
| No | 109 (97.3%) | 41 (93.2%) | 150 (96.2%) | |
| Yes | 3 (2.7%) | 3 (6.8%) | 6 (3.8%) | |
| History of CAD | .209 a | |||
| No | 82 (73.2%) | 37 (84.1%) | 119 (76.3%) | |
| Yes | 30 (26.8%) | 7 (15.9%) | 37 (23.7%) | |
| History of valvular disease | .031 a | |||
| No | 102 (91.1%) | 34 (77.3%) | 136 (87.2%) | |
| Yes | 10 (8.9%) | 10 (22.7%) | 20 (12.8%) | |
| AF duration | .025 b | |||
| Mean (SD) | 46.4 (67.0) | 77.4 (97.4) | 55.1 (77.8) | |
| Median (Q1, Q3) | 24.0 (7.8, 60.0) | 36.0 (17.5, 87.0) | 27.0 (8.8, 61.5) | |
| Range | 2.0–408.0 | 1.0–360.0 | 1.0–408.0 | |
| Type of ablation | .164 a | |||
| N‐Miss | 0 | 1 | 1 | |
| Cryoablation | 18 (16.1%) | 12 (27.9%) | 30 (19.4%) | |
| Radiofrequency ablation | 86 (76.8%) | 27 (62.8%) | 113 (72.9%) | |
| Both | 8 (7.1%) | 4 (9.3%) | 12 (7.7%) | |
| LA (ml/m2) c | .011 b | |||
| Mean (SD) | 36.9 (10.1) | 41.9 (11.7) | 38.3 (10.8) | |
| Median (Q1, Q3) | 36.0 (30.0, 42.0) | 40.0 (35.0, 48.0) | 37.0 (31.0, 44.0) | |
| Range | 16.0–67.0 | 20.0–81.0 | 16.0–81.0 | |
| LVEDD (mm) | .655 b | |||
| Mean (SD) | 51.4 (5.2) | 51.0 (5.6) | 51.3 (5.3) | |
| Median (Q1, Q3) | 51.0 (48.0, 55.0) | 52.0 (47.0, 54.0) | 52.0 (48.0, 55.0) | |
| Range | 38.0–69.0 | 35.0–61.0 | 35.0–69.0 | |
| LVESD (mm) | .993 b | |||
| Mean (SD) | 33.9 (5.9) | 33.9 (6.3) | 33.9 (6.0) | |
| Median (Q1, Q3) | 33.0 (30.8, 36.0) | 33.0 (31.0, 36.5) | 33.0 (31.0, 36.0) | |
| Range | 23.0–58.0 | 21.0–50.0 | 21.0–58.0 | |
| LVEF (%) | .568 b | |||
| Mean (SD) | 59.5 (9.1) | 58.5 (9.0) | 59.2 (9.0) | |
| Median (Q1, Q3) | 62.0 (57.0, 65.0) | 61.0 (53.5, 64.8) | 61.5 (57.0, 65.0) | |
| Range | 22.0–74.0 | 34.0–75.0 | 22.0–75.0 |
Fisher's exact test for count data.
Linear model ANOVA.
Biplane volume index by method of discs.
TABLE A2.
Demographics and patient history versus postablation freedom from atrial arrhythmias at 24 months
| Freedom from atrial arrhythmias (N = 94) | No freedom from atrial arrhythmias (N = 62) | Total (N = 156) | p value | |
|---|---|---|---|---|
| Age | .362 a | |||
| Under 50 | 8 (8.5%) | 4 (6.5%) | 12 (7.7%) | |
| 50–59 | 29 (30.9%) | 15 (24.2%) | 44 (28.2%) | |
| 60–69 | 42 (44.7%) | 26 (41.9%) | 68 (43.6%) | |
| 70+ | 15 (16.0%) | 17 (27.4%) | 32 (20.5%) | |
| Sex | .479 a | |||
| Male | 88 (93.6%) | 60 (96.8%) | 148 (94.9%) | |
| Female | 6 (6.4%) | 2 (3.2%) | 8 (5.1%) | |
| BMI | .720 a | |||
| Normal weight | 10 (10.6%) | 9 (14.5%) | 19 (12.2%) | |
| Overweight | 36 (38.3%) | 24 (38.7%) | 60 (38.5%) | |
| Obese | 48 (51.1%) | 29 (46.8%) | 77 (49.4%) | |
| Type of AF | .084 a | |||
| Paroxysmal | 82 (87.2%) | 47 (75.8%) | 129 (82.7%) | |
| Persistent | 12 (12.8%) | 15 (24.2%) | 27 (17.3%) | |
| History of atrial flutter | .022 a | |||
| No | 55 (58.5%) | 24 (38.7%) | 79 (50.6%) | |
| Yes | 39 (41.5%) | 38 (61.3%) | 77 (49.4%) | |
| History of HTN | 1.000 a | |||
| Yes | 60 (63.8%) | 39 (62.9%) | 99 (63.5%) | |
| No | 34 (36.2%) | 23 (37.1%) | 57 (36.5%) | |
| History of OSA | .740 a | |||
| No | 54 (57.4%) | 38 (61.3%) | 92 (59.0%) | |
| Yes | 40 (42.6%) | 24 (38.7%) | 64 (41.0%) | |
| History of LVH | .682 a | |||
| No | 91 (96.8%) | 59 (95.2%) | 150 (96.2%) | |
| Yes | 3 (3.2%) | 3 (4.8%) | 6 (3.8%) | |
| History of CAD | .340 a | |||
| No | 69 (73.4%) | 50 (80.6%) | 119 (76.3%) | |
| Yes | 25 (26.6%) | 12 (19.4%) | 37 (23.7%) | |
| History of valvular disease | .001 a | |||
| No | 89 (94.7%) | 47 (75.8%) | 136 (87.2%) | |
| Yes | 5 (5.3%) | 15 (24.2%) | 20 (12.8%) | |
| AF duration | .041 b | |||
| Mean (SD) | 44.8 (67.7) | 70.8 (89.3) | 55.1 (77.8) | |
| Median (Q1, Q3) | 19.0 (7.2, 60.0) | 36.0 (13.0, 84.0) | 27.0 (8.8, 61.5) | |
| Range | 2.0–408.0 | 1.0–360.0 | 1.0–408.0 | |
| Type of ablation | .429 a | |||
| N‐Miss | 0 | 1 | 1 | |
| Cryoablation | 16 (17.0%) | 14 (23.0%) | 30 (19.4%) | |
| Radiofrequency ablation | 72 (76.6%) | 41 (67.2%) | 113 (72.9%) | |
| Both | 6 (6.4%) | 6 (9.8%) | 12 (7.7%) | |
| LA (ml/m2) c | .034 b | |||
| Mean (SD) | 36.8 (10.6) | 40.6 (10.8) | 38.3 (10.8) | |
| Median (Q1, Q3) | 36.0 (29.8, 42.0) | 40.0 (35.0, 45.0) | 37.0 (31.0, 44.0) | |
| Range | 16.0–67.0 | 19.0–81.0 | 16.0–81.0 | |
| LVEDD (mm) | .324 b | |||
| Mean (SD) | 50.9 (4.7) | 51.8 (6.1) | 51.3 (5.3) | |
| Median (Q1, Q3) | 51.0 (48.0, 54.0) | 52.5 (48.2, 55.0) | 52.0 (48.0, 55.0) | |
| Range | 38.0–66.0 | 35.0–69.0 | 35.0–69.0 | |
| LVESD (mm) | .134 b | |||
| Mean (SD) | 33.3 (5.0) | 34.8 (7.2) | 33.9 (6.0) | |
| Median (Q1, Q3) | 32.0 (30.5, 35.5) | 33.0 (31.0, 39.0) | 33.0 (31.0, 36.0) | |
| Range | 26.0–58.0 | 21.0–56.0 | 21.0–58.0 | |
| LVEF (%) | .073 b | |||
| Mean (SD) | 60.3 (8.2) | 57.6 (10.0) | 59.2 (9.0) | |
| Median (Q1, Q3) | 62.0 (57.2, 65.0) | 60.5 (52.0, 64.0) | 61.5 (57.0, 65.0) | |
| Range | 22.0–74.0 | 32.0–75.0 | 22.0–75.0 |
Fisher's exact test for count data.
Linear model ANOVA.
Biplane volume index by method of discs.
TABLE A3.
Demographics and patient history versus postablation freedom from atrial arrhythmias at 36 months
| Freedom from atrial arrhythmias (N = 76) | No freedom from atrial arrhythmias (N = 72) | Total (N = 148) | p value | |
|---|---|---|---|---|
| Age | .727 a | |||
| Under 50 | 6 (7.9%) | 6 (8.3%) | 12 (8.1%) | |
| 50–59 | 25 (32.9%) | 18 (25.0%) | 43 (29.1%) | |
| 60–69 | 31 (40.8%) | 31 (43.1%) | 62 (41.9%) | |
| 70+ | 14 (18.4%) | 17 (23.6%) | 31 (20.9%) | |
| Sex | .443 a | |||
| Male | 71 (93.4%) | 70 (97.2%) | 141 (95.3%) | |
| Female | 5 (6.6%) | 2 (2.8%) | 7 (4.7%) | |
| BMI | .547 a | |||
| Normal weight | 8 (10.5%) | 9 (12.5%) | 17 (11.5%) | |
| Overweight | 25 (32.9%) | 29 (40.3%) | 54 (36.5%) | |
| Obese | 43 (56.6%) | 34 (47.2%) | 77 (52.0%) | |
| Type of AF | .195 a | |||
| Paroxysmal | 66 (86.8%) | 56 (77.8%) | 122 (82.4%) | |
| Persistent | 10 (13.2%) | 16 (22.2%) | 26 (17.6%) | |
| History of atrial flutter | .188 a | |||
| No | 43 (56.6%) | 32 (44.4%) | 75 (50.7%) | |
| Yes | 33 (43.4%) | 40 (55.6%) | 73 (49.3%) | |
| History of HTN | .395 a | |||
| Yes | 51 (67.1%) | 43 (59.7%) | 94 (63.5%) | |
| No | 25 (32.9%) | 29 (40.3%) | 54 (36.5%) | |
| History of OSA | .619 a | |||
| No | 43 (56.6%) | 44 (61.1%) | 87 (58.8%) | |
| Yes | 33 (43.4%) | 28 (38.9%) | 61 (41.2%) | |
| History of LVH | .433 a | |||
| No | 74 (97.4%) | 68 (94.4%) | 142 (95.9%) | |
| Yes | 2 (2.6%) | 4 (5.6%) | 6 (4.1%) | |
| History of CAD | .037 a | |||
| No | 52 (68.4%) | 60 (83.3%) | 112 (75.7%) | |
| Yes | 24 (31.6%) | 12 (16.7%) | 36 (24.3%) | |
| History of valvular disease | .003 a | |||
| No | 72 (94.7%) | 56 (77.8%) | 128 (86.5%) | |
| Yes | 4 (5.3%) | 16 (22.2%) | 20 (13.5%) | |
| AF duration | .056 b | |||
| Mean (SD) | 44.6 (66.0) | 69.4 (89.5) | 56.6 (79.0) | |
| Median (Q1, Q3) | 24.0 (8.0, 60.0) | 36.0 (11.5, 84.0) | 32.0 (9.0, 67.5) | |
| Range | 2.0–408.0 | 1.0–360.0 | 1.0–408.0 | |
| Type of ablation | .439 a | |||
| N‐Miss | 0 | 1 | 1 | |
| Cryoablation | 12 (15.8%) | 17 (23.9%) | 29 (19.7%) | |
| Radiofrequency ablation | 58 (76.3%) | 48 (67.6%) | 106 (72.1%) | |
| Both | 6 (7.9%) | 6 (8.5%) | 12 (8.2%) | |
| LA (ml/m2) c | .084 b | |||
| Mean (SD) | 36.9 (10.1) | 40.0 (11.2) | 38.4 (10.7) | |
| Median (Q1, Q3) | 36.0 (30.0, 42.5) | 40.0 (33.5, 45.0) | 38.0 (31.0, 44.5) | |
| Range | 16.0–61.0 | 19.0–81.0 | 16.0–81.0 | |
| LVEDD (mm) | .237 b | |||
| Mean (SD) | 50.7 (4.8) | 51.8 (5.9) | 51.2 (5.4) | |
| Median (Q1, Q3) | 51.0 (48.0, 54.0) | 52.0 (48.0, 55.5) | 52.0 (48.0, 55.0) | |
| Range | 38.0–66.0 | 35.0–69.0 | 35.0–69.0 | |
| LVESD (mm) | .068 b | |||
| Mean (SD) | 32.8 (4.8) | 34.8 (7.1) | 33.8 (6.1) | |
| Median (Q1, Q3) | 32.0 (30.0, 35.0) | 33.0 (31.0, 39.0) | 33.0 (30.8, 36.0) | |
| Range | 26.0–58.0 | 21.0–56.0 | 21.0–58.0 | |
| LVEF (%) | .012 b | |||
| Mean (SD) | 61.2 (7.4) | 57.4 (10.2) | 59.4 (9.1) | |
| Median (Q1, Q3) | 62.0 (58.0, 65.0) | 61.0 (52.0, 64.0) | 62.0 (57.0, 65.0) | |
| Range | 22.0–74.0 | 32.0–75.0 | 22.0–75.0 |
Fisher's exact test for count data.
Linear model ANOVA.
Biplane volume index by method of discs.
TABLE A4.
Demographics and patient history versus postablation freedom from atrial arrhythmias at beyond 36 months
| Freedom from atrial arrhythmias (N = 51) | No freedom from atrial arrhythmias (N = 90) | Total (N = 141) | p value | |
|---|---|---|---|---|
| Age | .757 a | |||
| Under 50 | 4 (7.8%) | 8 (8.9%) | 12 (8.5%) | |
| 50–59 | 18 (35.3%) | 24 (26.7%) | 42 (29.8%) | |
| 60–69 | 20 (39.2%) | 38 (42.2%) | 58 (41.1%) | |
| 70+ | 9 (17.6%) | 20 (22.2%) | 29 (20.6%) | |
| Sex | .668 a | |||
| Male | 48 (94.1%) | 87 (96.7%) | 135 (95.7%) | |
| Female | 3 (5.9%) | 3 (3.3%) | 6 (4.3%) | |
| BMI | .909 a | |||
| Normal weight | 7 (13.7%) | 10 (11.1%) | 17 (12.1%) | |
| Overweight | 18 (35.3%) | 34 (37.8%) | 52 (36.9%) | |
| Obese | 26 (51.0%) | 46 (51.1%) | 72 (51.1%) | |
| Type of AF | .105 a | |||
| Paroxysmal | 46 (90.2%) | 71 (78.9%) | 117 (83.0%) | |
| Persistent | 5 (9.8%) | 19 (21.1%) | 24 (17.0%) | |
| History of atrial flutter | .161 a | |||
| No | 30 (58.8%) | 41 (45.6%) | 71 (50.4%) | |
| Yes | 21 (41.2%) | 49 (54.4%) | 70 (49.6%) | |
| History of HTN | .720 a | |||
| Yes | 33 (64.7%) | 55 (61.1%) | 88 (62.4%) | |
| No | 18 (35.3%) | 35 (38.9%) | 53 (37.6%) | |
| History of OSA | .593 a | |||
| No | 32 (62.7%) | 51 (56.7%) | 83 (58.9%) | |
| Yes | 19 (37.3%) | 39 (43.3%) | 58 (41.1%) | |
| History of LVH | .418 a | |||
| No | 50 (98.0%) | 85 (94.4%) | 135 (95.7%) | |
| Yes | 1 (2.0%) | 5 (5.6%) | 6 (4.3%) | |
| History of CAD | .224 a | |||
| No | 35 (68.6%) | 71 (78.9%) | 106 (75.2%) | |
| Yes | 16 (31.4%) | 19 (21.1%) | 35 (24.8%) | |
| History of valvular disease | .070 a | |||
| No | 48 (94.1%) | 74 (82.2%) | 122 (86.5%) | |
| Yes | 3 (5.9%) | 16 (17.8%) | 19 (13.5%) | |
| AF duration | .052 b | |||
| Mean (SD) | 40.3 (60.7) | 67.6 (87.9) | 57.7 (80.0) | |
| Median (Q1, Q3) | 24.0 (8.0, 60.0) | 36.0 (12.0, 84.0) | 36.0 (9.0, 72.0) | |
| Range | 2.0–408.0 | 1.0–360.0 | 1.0–408.0 | |
| Type of ablation | .579 a | |||
| N‐Miss | 0 | 1 | 1 | |
| Cryoablation | 8 (15.7%) | 20 (22.5%) | 28 (20.0%) | |
| Radiofrequency ablation | 38 (74.5%) | 63 (70.8%) | 101 (72.1%) | |
| Both | 5 (9.8%) | 6 (6.7%) | 11 (7.9%) | |
| LA (mL/m2) c | .003 b | |||
| Mean (SD) | 34.6 (10.4) | 40.6 (10.8) | 38.4 (11.0) | |
| Median (Q1, Q3) | 32.0 (29.0, 39.5) | 40.0 (34.0, 45.0) | 37.0 (31.0, 45.0) | |
| Range | 16.0–57.0 | 19.0–81.0 | 16.0–81.0 | |
| LVEDD (mm) | .070 b | |||
| Mean (SD) | 50.1 (4.6) | 51.9 (5.8) | 51.3 (5.5) | |
| Median (Q1, Q3) | 50.0 (47.0, 53.0) | 52.0 (48.0, 56.0) | 52.0 (48.0, 55.0) | |
| Range | 42.0–66.0 | 35.0–69.0 | 35.0–69.0 | |
| LVESD (mm) | .051 b | |||
| Mean (SD) | 32.3 (5.0) | 34.6 (6.7) | 33.8 (6.2) | |
| Median (Q1, Q3) | 32.0 (30.0, 35.0) | 33.0 (31.0, 38.0) | 33.0 (30.2, 36.0) | |
| Range | 26.0–58.0 | 21.0–56.0 | 21.0–58.0 | |
| LVEF (%) | .106 b | |||
| Mean (SD) | 61.1 (7.4) | 58.5 (9.9) | 59.4 (9.2) | |
| Median (Q1, Q3) | 62.0 (59.0, 65.0) | 61.0 (54.0, 65.0) | 62.0 (57.0, 65.0) | |
| Range | 22.0–73.0 | 32.0–75.0 | 22.0–75.0 |
Note: Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; HTN, hypertension; LA, left atrium; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end systolic volume; LVH, left ventricular hypertrophy; OSA, obstructive sleep apnea.
N‐miss: data not available.
SD: standard deviation.
Q1, Q3: interquartile range.
Fisher's exact test for count data.
Linear model ANOVA.
Biplane volume index by method of discs.
Liu MB, Lee JZ, Klooster L, Buckner Petty SA, Scott LR. Influence of endurance sports on atrial fibrillation ablation outcomes. J Arrhythmia. 2022;38:694–709. 10.1002/joa3.12746
Contributor Information
Michael B. Liu, @MichaelLiuMD, @MayoAZ_IMRES.
Luis R. Scott, Email: scott.luis@mayo.edu.
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request. The data are deidentified participant data. They are currently stored on a secure Mayo Clinic server. Please email liu.michael@mayo.edu for requests for the primary data.
REFERENCES
- 1. Mohanty S, Mohanty P, Tamaki M, Natale V, Gianni C, Trivedi C, et al. Differential Association of Exercise Intensity with Risk of atrial fibrillation in men and women: evidence from a meta‐analysis. J Cardiovasc Electrophysiol. 2016;27:1021–9. [DOI] [PubMed] [Google Scholar]
- 2. Pelliccia A, Zipes DP, Maron BJ. Bethesda conference #36 and the European Society of Cardiology Consensus Recommendations revisited a comparison of U.S. and European criteria for eligibility and disqualification of competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol. 2008;52:1990–6. [DOI] [PubMed] [Google Scholar]
- 3. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 4. Guasch E, Benito B, Qi X, Cifelli C, Naud P, Shi Y, et al. Atrial fibrillation promotion by endurance exercise: demonstration and mechanistic exploration in an animal model. J Am Coll Cardiol. 2013;62:68–77. [DOI] [PubMed] [Google Scholar]
- 5. Biffi A, Maron BJ, Verdile L, Fernando F, Spataro A, Marcello G, et al. Impact of physical deconditioning on ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol. 2004;44:1053–8. [DOI] [PubMed] [Google Scholar]
- 6. Furlanello F, Bertoldi A, Dallago M, Galassi A, Fernando F, Biffi A, et al. Atrial fibrillation in elite athletes. J Cardiovasc Electrophysiol. 1998;9:S63–8. [PubMed] [Google Scholar]
- 7. Pelliccia A, Fagard R, Bjørnstad HH, et al. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:1422–45. [DOI] [PubMed] [Google Scholar]
- 8. World Anti‐Doping Agency . Prohibted in particular sports. 2021.
- 9. Koopman P, Nuyens D, Garweg C, la Gerche A, de Buck S, van Casteren L, et al. Efficacy of radiofrequency catheter ablation in athletes with atrial fibrillation. Europace. 2011;13:1386–93. [DOI] [PubMed] [Google Scholar]
- 10. Alexander AM. Atrial fibrillation in the athlete. Curr Sports Med Rep. 2013;12:86–92. [DOI] [PubMed] [Google Scholar]
- 11. Furlanello F, Lupo P, Pittalis M, et al. Radiofrequency catheter ablation of atrial fibrillation in athletes referred for disabling symptoms preventing usual training schedule and sport competition. J Cardiovasc Electrophysiol. 2008;19:457–62. [DOI] [PubMed] [Google Scholar]
- 12. Calvo N, Mont L, Tamborero D, Berruezo A, Viola G, Guasch E, et al. Efficacy of circumferential pulmonary vein ablation of atrial fibrillation in endurance athletes. Europace. 2010;12:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Decroocq M, Ninni S, Klein C, Machuron F, Verbrugge E, Klug D, et al. No impact of sports practice before or after atrial fibrillation ablation on procedure efficacy in athletes: a case‐control study. Europace. 2019;21:1833–42. [DOI] [PubMed] [Google Scholar]
- 14. Mitchell JH, Haskell W, Snell P, Van Camp SP. Task force 8: classification of sports. J Am Coll Cardiol. 2005;45:1364–7. [DOI] [PubMed] [Google Scholar]
- 15. Mont L, Sambola A, Brugada J, Vacca M, Marrugat J, Elosua R, et al. Long‐lasting sport practice and lone atrial fibrillation. Eur Heart J. 2002;23:477–82. [DOI] [PubMed] [Google Scholar]
- 16. Sawhney N, Anousheh R, Chen W, Feld GK. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:243–8. [DOI] [PubMed] [Google Scholar]
- 17. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. The data are deidentified participant data. They are currently stored on a secure Mayo Clinic server. Please email liu.michael@mayo.edu for requests for the primary data.


