Three molecules of the title compound are present in the asymmetric unit, exhibiting different conformations relative to the ethoxy group.
Keywords: crystal structure, ester, ether, conformational flexibility
Abstract
The title compound, C17H18O3, crystallizes with three molecules in the asymmetric unit. The molecules differ in the conformation related to the ethoxy group and in the orientation of the two phenyl rings, one of which has the ethoxy group disordered over two positions with refined occupancies of 0.735:0.265 (9). In the crystal packing, the molecules are connected by weak C—H⋯π interactions.
1. Chemical context
Alkylbenzoates are an important class of compounds with interesting physical properties and applications in industry. For example, 4-hydroxybenzoic acid and its esters are widely used as preservatives in cosmetic and pharmaceutical products known as parabens, for which the physical properties and crystal structures have been widely described (Giordano et al., 1999 ▸; Yang et al., 2014 ▸).
Alkylbenzoates of different properties have been designed, amongst other things, with the aim of preparing liquid crystalline compounds (Abser et al., 1993 ▸), functionalized poly(benzyl ether) dendrimers with methyl ester decorations as efficient organogelators (Feng et al., 2009 ▸), or non-linear optical materials (Perumal et al., 2002 ▸). Moreover, the ester bond has a prominent position in cell biology and medicinal chemistry (Lavis, 2008 ▸), and carbohydrazones can be obtained by reacting corresponding esters with suitable hydrazine derivatives.
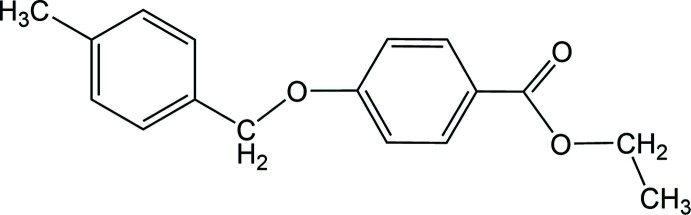
We report here the synthesis and crystal structure of another example of a derivatized alkylbenzoate with an ether group.
2. Structural commentary
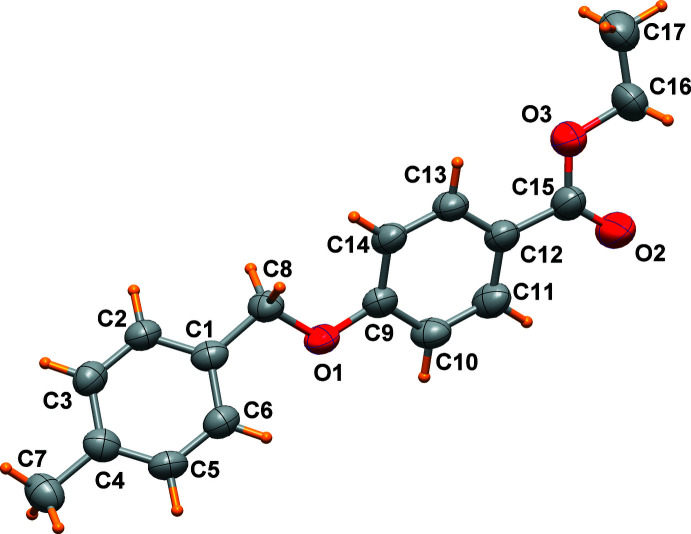
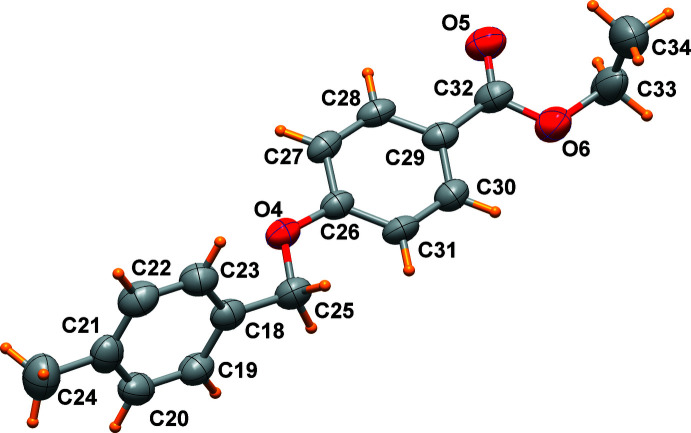
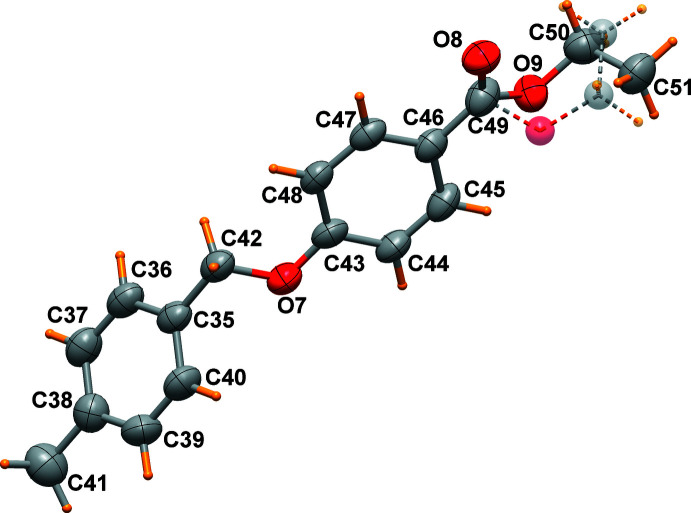
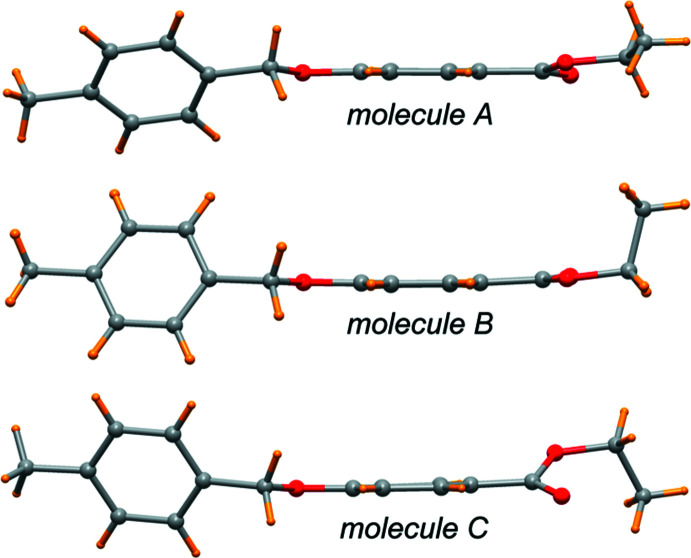
Three molecules, which slightly differ in their conformations, are present in the asymmetric unit of the title compound (Figs. 1 ▸–3 ▸ ▸). The main conformational differences of molecules A, B and C are related to the ethoxy group with C—O—CH2—CH3 torsion angles of 174.0 (6), 82.6 (6) and 89.6 (7)°, and in the orientation of the two phenyl rings that form a dihedral angle of 46.4 (1), 70.3 (1), and 62.2 (1)°, respectively. A side view of the molecules displayed in Fig. 4 ▸ highlights these differences. All these features are indicative of the conformational freedom of this molecule. Nevertheless, all bond lengths and angles in the three molecules relating to the ether and the ester groups are similar within their standard uncertainties. In general, bond lengths (Allen et al., 1987 ▸) and angles are within normal ranges. In molecule C, the ethoxy group O9/C5/C51 is disordered over two sets of sites (Fig. 3 ▸).
Figure 1.
Molecule A of the title compound, drawn with displacement ellipsoids at the 50% probability level.
Figure 2.
Molecule B of the title compound, drawn with displacement ellipsoids at the 50% probability level.
Figure 3.
Molecule C of the title compound, drawn with displacement ellipsoids at the 50% probability level. The ethoxy group O9/C5/C51 is disordered over two sets of sites.
Figure 4.
Side view of the three independent molecules displaying the different conformations.
In the parent methyl 4-(benzyloxy)-3-methoxybenzoate compound, which is an important organic intermediate for the synthesis of the antineoplastic drug Cediranib (Wang et al., 2013 ▸), the two aromatic rings are almost normal to each other forming a dihedral angle of 85.81 (10)° and bond lengths are close comparable with those determined here.
3. Supramolecular features
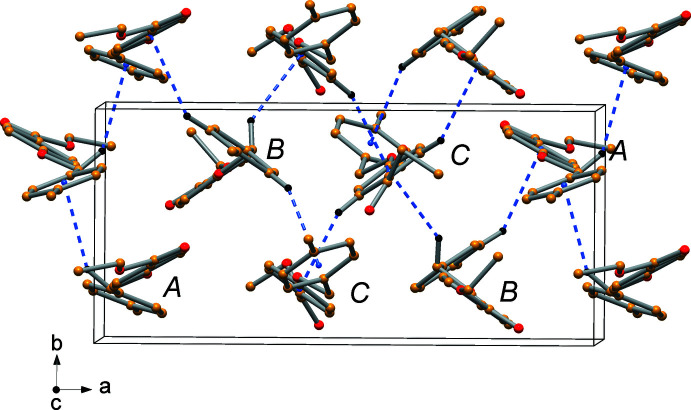
Despite the number of phenyl rings, the aromatic rings have rather distant centroid-to-centroid distances of between 4.727 (3) and 4.946 (3) Å, but with unsuitable orientations for efficient π-stacking interactions. On the other hand, the crystal packing indicates a series of C—H⋯π ring interactions in the range 2.65–2.94 Å (Table 1 ▸), as derived with PLATON (Spek, 2020 ▸). A view of the unit cell is displayed in Fig. 5 ▸, showing these kinds of interactions. In addition, non-conventional C—H⋯O hydrogen bonds are observed in the crystal packing (Table 2 ▸).
Table 1. Analysis of C—H⋯Cg(π-ring) interactions (Å, °).
C—H⋯π = angle of the X—H bond with the π-plane (perpendicular = 90°, parallel = 0°). Ring Cg1 = C35–C40; Cg2 = C43–C48; Cg3 = C1–C6; Cg4 = C9–C14; Cg6 = C26–C31
| C—H | Cg(J) | Symmetry code (J) | H⋯Cg | C—H⋯Cg | C⋯Cg | C—H⋯π |
|---|---|---|---|---|---|---|
| C8—H8A | Cg3 | -x,
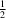 + y, −z
+ y, −z
|
2.90 | 144 | 3.749 (5) | 59 |
| C20—H20 | Cg1 | x, y, z | 2.81 | 147 | 3.653 (6) | 62 |
| C22—H22 | Cg4 | x, 1 + y, 1 + z | 2.93 | 137 | 3.688 (6) | 50 |
| C25—H25A | Cg2 | x, 1 + y, z | 2.81 | 143 | 3.654 (6) | 50 |
| C41—H41B | Cg1 | 1 − x,
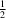 + y, 3 − z
+ y, 3 − z
|
2.67 | 156 | 3.590 (5) | 75 |
| C45—H45 | Cg6 | x, y, z | 2.82 | 147 | 3.654 (4) | 51 |
| C47—H47 | Cg2 | 1 − x, −
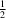 + y, 2 − z
+ y, 2 − z
|
2.77 | 148 | 3.615 (4) | 65 |
Figure 5.
Perspective view of the crystal packing of the title compound down the c axis with indication of the C—H⋯π-ring interactions. H atoms not involved in the interactions were omitted for clarity.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C42—H42A⋯O8i | 0.99 | 2.65 | 3.269 (5) | 121 |
| C44—H44⋯O4 | 0.95 | 2.66 | 3.374 (5) | 133 |
Symmetry code: (i)
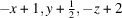 .
.
4. Database survey
The conformations of the three independent molecules present in the crystal structure of the title compound agree with previous structurally characterized species containing the (benzyloxy)phenyl fragment, where the two aromatic rings form dihedral angles of 64.5 (2)° (mean value of two independent molecules; Bats & Canenbley, 1984 ▸) and 69.19 (6)° (Qin et al., 2019 ▸). However, a few structures exhibit almost coplanar orientations of the phenyl rings (Jasinski et al., 2008 ▸; Feng et al., 2009 ▸), or small dihedral angles such as the 4.1 (2) and 10.9 (4)° reported for 3,5-bis(benzyloxy)benzoic acid (Moreno-Fuquen et al. 2012 ▸). The latter conformations favour electron delocalization between the two rings, but packing requirements also play a role.
5. Synthesis and crystallization
A mixture of ethyl-4-hydroxybenzoate (8.75 g, 52.65 mmol) and 4-methylbenzylbromide (9.75 g, 52.68 mmol) in acetone (100 ml) was refluxed for 14 h over anhydrous potassium carbonate (20 g). The solvent was removed in vacuo, and the remaining solid was dissolved in water and extracted with dichloromethane. Left overnight, colourless needle-shaped crystals were formed, filtered off, washed, and dried over silica gel in a desiccator. Yield: 12.58 g, 88% Melting point: 323 −324 K. FT–IR: 1706 ν (C=O), 1258, 1276 ν (C—Oester), 1106, 1102 ν (C—Oether). 1H NMR (CDCl3,600 MHz): δ = 1.37 (t, 3H, CH3CH2-, J = 10.5 Hz), 2.36 (s, 3H, C6H4–CH3),4.35 (q, 2H, CH3–CH2, J = 10.5 Hz), 5.07 (s, 2H, C6H4–CH2–), 6.98 (d, 2H, H-5,6, J =7.8 Hz), 7.20 (d, 2H, H-10,11, J = 11.4 Hz), 7.31 (d, 2H, H-8,9, J = 12 Hz), 7.99 (d, 2H, H-3,4, J = 6.6 Hz), ppm. 13C NMR (CDCl3, 600 MHz): 14.4 (C11), 21.3 (C7), 60.7 (C10), 70.1 (C8), 114.4 (C-3,5), 123.15 (C1), 127.73 (C-2′,6′), 129.1 (C-3′,5′), 131.6 (C-2,6), 133.31 (C1′),138.13 (C4), 162.5 (C4′), 166.4 (C9), ppm. LC–MS (ESI) m/z: [M + H]+. Calculated for C17H18O3 271.13; found 271.13.
6. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 3 ▸. The structure was refined as a two-component inversion twin. The –OCH2CH3 moiety of molecule C was found to be disordered over two sets of sites with refined occupancies of 0.735 (9):0.265 (9). For modelling the minor disordered part, all atoms were refined with isotropic displacement parameters, and C—C and C—O bond lengths were restrained by using DFIX commands.
Table 3. Experimental details.
| Crystal data | |
| Chemical formula | C17H18O3 |
| M r | 270.31 |
| Crystal system, space group | Monoclinic, P21 |
| Temperature (K) | 173 |
| a, b, c (Å) | 16.1906 (10), 7.5752 (4), 17.7591 (9) |
| β (°) | 95.360 (7) |
| V (Å3) | 2168.6 (2) |
| Z | 6 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.08 |
| Crystal size (mm) | 0.30 × 0.20 × 0.05 |
| Data collection | |
| Diffractometer | Rigaku R-AXIS RAPID |
| Absorption correction | Multi-scan (ABSCOR; Higashi, 1995 ▸) |
| T min, T max | 0.533, 0.996 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 16200, 7609, 5301 |
| R int | 0.042 |
| (sin θ/λ)max (Å−1) | 0.595 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.051, 0.125, 0.97 |
| No. of reflections | 7609 |
| No. of parameters | 560 |
| No. of restraints | 5 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.16, −0.17 |
| Absolute structure | Refined as an inversion twin |
| Absolute structure parameter | 0.6 (14) |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989022009380/wm5660sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989022009380/wm5660Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989022009380/wm5660Isup3.cml
CCDC reference: 2174691
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors are grateful to the Department of Chemistry, University of Rajshahi for laboratory facilities. MCS thanks the Department of Applied Chemistry, Faculty of Engineering, University of Toyama for analytical facilities.
supplementary crystallographic information
Crystal data
| C17H18O3 | F(000) = 864 |
| Mr = 270.31 | Dx = 1.242 Mg m−3 |
| Monoclinic, P21 | Mo Kα radiation, λ = 0.71075 Å |
| a = 16.1906 (10) Å | Cell parameters from 13948 reflections |
| b = 7.5752 (4) Å | θ = 2.3–27.5° |
| c = 17.7591 (9) Å | µ = 0.08 mm−1 |
| β = 95.360 (7)° | T = 173 K |
| V = 2168.6 (2) Å3 | Prism, colorless |
| Z = 6 | 0.30 × 0.20 × 0.05 mm |
Data collection
| Rigaku R-AXIS RAPID diffractometer | 5301 reflections with I > 2σ(I) |
| Detector resolution: 10.000 pixels mm-1 | Rint = 0.042 |
| ω scans | θmax = 25.0°, θmin = 2.5° |
| Absorption correction: multi-scan (ABSCOR; Higashi, 1995) | h = −19→19 |
| Tmin = 0.533, Tmax = 0.996 | k = −9→9 |
| 16200 measured reflections | l = −21→21 |
| 7609 independent reflections |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.051 | w = 1/[σ2(Fo2) + (0.0667P)2] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.125 | (Δ/σ)max = 0.001 |
| S = 0.97 | Δρmax = 0.16 e Å−3 |
| 7609 reflections | Δρmin = −0.17 e Å−3 |
| 560 parameters | Absolute structure: Refined as an inversion twin |
| 5 restraints | Absolute structure parameter: 0.6 (14) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refined as a 2-component inversion twin. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| O1 | 0.10822 (15) | 0.3014 (4) | 0.12187 (14) | 0.0520 (7) | |
| O2 | 0.19050 (18) | 0.4249 (6) | 0.47508 (17) | 0.0864 (12) | |
| O3 | 0.06054 (17) | 0.3271 (5) | 0.47163 (14) | 0.0648 (8) | |
| O4 | 0.24935 (14) | 0.7272 (4) | 1.05090 (15) | 0.0496 (7) | |
| O5 | 0.14434 (17) | 0.5821 (4) | 0.70340 (16) | 0.0648 (8) | |
| O6 | 0.26005 (16) | 0.7442 (4) | 0.69644 (15) | 0.0625 (8) | |
| O7 | 0.40986 (15) | 0.2922 (3) | 1.18050 (15) | 0.0510 (7) | |
| O8 | 0.44677 (16) | 0.0780 (4) | 0.83691 (16) | 0.0599 (8) | |
| C1 | 0.0495 (2) | 0.2152 (5) | −0.0013 (2) | 0.0443 (9) | |
| C2 | −0.0147 (2) | 0.2495 (5) | −0.0573 (2) | 0.0489 (10) | |
| H2 | −0.066049 | 0.293542 | −0.043362 | 0.059* | |
| C3 | −0.0046 (2) | 0.2202 (6) | −0.1331 (2) | 0.0543 (11) | |
| H3 | −0.049429 | 0.242504 | −0.170299 | 0.065* | |
| C4 | 0.0702 (2) | 0.1589 (6) | −0.1552 (2) | 0.0497 (10) | |
| C5 | 0.1338 (2) | 0.1273 (6) | −0.0996 (2) | 0.0527 (10) | |
| H5 | 0.185817 | 0.087533 | −0.113626 | 0.063* | |
| C6 | 0.1236 (2) | 0.1522 (6) | −0.0237 (2) | 0.0510 (10) | |
| H6 | 0.167989 | 0.125624 | 0.013392 | 0.061* | |
| C7 | 0.0823 (3) | 0.1340 (7) | −0.2379 (2) | 0.0659 (13) | |
| H7A | 0.029618 | 0.098181 | −0.265425 | 0.079* | |
| H7B | 0.124086 | 0.042428 | −0.243164 | 0.079* | |
| H7C | 0.100868 | 0.245290 | −0.258874 | 0.079* | |
| C8 | 0.0345 (2) | 0.2349 (6) | 0.0806 (2) | 0.0482 (10) | |
| H8A | −0.012131 | 0.317425 | 0.085443 | 0.058* | |
| H8B | 0.019711 | 0.119154 | 0.101448 | 0.058* | |
| C9 | 0.1080 (2) | 0.3143 (6) | 0.1992 (2) | 0.0461 (9) | |
| C10 | 0.1767 (2) | 0.3941 (6) | 0.2365 (2) | 0.0558 (11) | |
| H10 | 0.219776 | 0.438259 | 0.208893 | 0.067* | |
| C11 | 0.1824 (2) | 0.4095 (6) | 0.3143 (2) | 0.0578 (11) | |
| H11 | 0.229986 | 0.463783 | 0.339851 | 0.069* | |
| C12 | 0.1200 (2) | 0.3471 (6) | 0.3559 (2) | 0.0498 (10) | |
| C13 | 0.0515 (2) | 0.2667 (5) | 0.3178 (2) | 0.0497 (10) | |
| H13 | 0.008507 | 0.222475 | 0.345492 | 0.060* | |
| C14 | 0.0448 (2) | 0.2498 (5) | 0.2392 (2) | 0.0487 (10) | |
| H14 | −0.002383 | 0.194827 | 0.213432 | 0.058* | |
| C15 | 0.1293 (2) | 0.3687 (7) | 0.4396 (2) | 0.0584 (12) | |
| C16 | 0.0629 (3) | 0.3580 (9) | 0.5529 (2) | 0.0770 (15) | |
| H16A | 0.079550 | 0.481406 | 0.564682 | 0.092* | |
| H16B | 0.103932 | 0.278330 | 0.580244 | 0.092* | |
| C17 | −0.0198 (3) | 0.3242 (9) | 0.5765 (3) | 0.0857 (16) | |
| H17A | −0.059461 | 0.407351 | 0.551013 | 0.103* | |
| H17B | −0.018733 | 0.339427 | 0.631386 | 0.103* | |
| H17C | −0.036552 | 0.203129 | 0.562945 | 0.103* | |
| C18 | 0.3073 (2) | 0.8300 (5) | 1.1705 (2) | 0.0478 (10) | |
| C19 | 0.3626 (2) | 0.7371 (6) | 1.2195 (2) | 0.0524 (10) | |
| H19 | 0.406274 | 0.672221 | 1.200302 | 0.063* | |
| C20 | 0.3545 (2) | 0.7383 (6) | 1.2969 (2) | 0.0583 (11) | |
| H20 | 0.393475 | 0.675592 | 1.330204 | 0.070* | |
| C21 | 0.2907 (3) | 0.8295 (6) | 1.3260 (2) | 0.0594 (11) | |
| C22 | 0.2347 (3) | 0.9185 (6) | 1.2765 (3) | 0.0628 (12) | |
| H22 | 0.189626 | 0.979302 | 1.295321 | 0.075* | |
| C23 | 0.2433 (2) | 0.9204 (6) | 1.1995 (2) | 0.0572 (11) | |
| H23 | 0.204792 | 0.984611 | 1.166346 | 0.069* | |
| C24 | 0.2827 (3) | 0.8334 (8) | 1.4110 (2) | 0.0822 (16) | |
| H24A | 0.336608 | 0.806262 | 1.438416 | 0.099* | |
| H24B | 0.241827 | 0.745422 | 1.423610 | 0.099* | |
| H24C | 0.264674 | 0.951011 | 1.425594 | 0.099* | |
| C25 | 0.3159 (2) | 0.8310 (6) | 1.0874 (2) | 0.0523 (10) | |
| H25A | 0.312822 | 0.953494 | 1.067897 | 0.063* | |
| H25B | 0.370048 | 0.780204 | 1.077218 | 0.063* | |
| C26 | 0.2446 (2) | 0.7150 (5) | 0.9736 (2) | 0.0432 (9) | |
| C27 | 0.1790 (2) | 0.6128 (5) | 0.9411 (2) | 0.0470 (10) | |
| H27 | 0.142358 | 0.556082 | 0.972200 | 0.056* | |
| C28 | 0.1676 (2) | 0.5945 (5) | 0.8635 (2) | 0.0489 (10) | |
| H28 | 0.122810 | 0.525418 | 0.841335 | 0.059* | |
| C29 | 0.2212 (2) | 0.6763 (5) | 0.8173 (2) | 0.0457 (9) | |
| C30 | 0.2876 (2) | 0.7729 (5) | 0.8507 (2) | 0.0476 (10) | |
| H30 | 0.325151 | 0.826994 | 0.819724 | 0.057* | |
| C31 | 0.3002 (2) | 0.7918 (5) | 0.9286 (2) | 0.0476 (10) | |
| H31 | 0.346440 | 0.856677 | 0.950938 | 0.057* | |
| C32 | 0.2039 (2) | 0.6608 (6) | 0.7346 (2) | 0.0517 (10) | |
| C33 | 0.2436 (3) | 0.7472 (8) | 0.6140 (2) | 0.0743 (15) | |
| H33A | 0.220428 | 0.632046 | 0.596184 | 0.089* | |
| H33B | 0.296151 | 0.766405 | 0.590866 | 0.089* | |
| C34 | 0.1840 (3) | 0.8902 (10) | 0.5902 (3) | 0.0973 (19) | |
| H34A | 0.174983 | 0.892892 | 0.534905 | 0.117* | |
| H34B | 0.206633 | 1.003831 | 0.608704 | 0.117* | |
| H34C | 0.131214 | 0.868111 | 0.611367 | 0.117* | |
| C35 | 0.4655 (2) | 0.2889 (5) | 1.3101 (2) | 0.0491 (10) | |
| C36 | 0.5264 (2) | 0.3902 (6) | 1.3491 (2) | 0.0564 (11) | |
| H36 | 0.574469 | 0.421203 | 1.325178 | 0.068* | |
| C37 | 0.5190 (3) | 0.4475 (6) | 1.4222 (3) | 0.0598 (11) | |
| H37 | 0.562457 | 0.514344 | 1.448032 | 0.072* | |
| C38 | 0.4486 (3) | 0.4081 (6) | 1.4581 (2) | 0.0572 (11) | |
| C39 | 0.3864 (3) | 0.3103 (6) | 1.4183 (2) | 0.0602 (11) | |
| H39 | 0.337265 | 0.283573 | 1.441409 | 0.072* | |
| C40 | 0.3948 (2) | 0.2508 (6) | 1.3455 (2) | 0.0579 (11) | |
| H40 | 0.351576 | 0.183268 | 1.319594 | 0.070* | |
| C41 | 0.4391 (3) | 0.4632 (7) | 1.5379 (2) | 0.0780 (14) | |
| H41A | 0.380484 | 0.456245 | 1.547340 | 0.094* | |
| H41B | 0.458706 | 0.584850 | 1.545643 | 0.094* | |
| H41C | 0.471799 | 0.384621 | 1.572923 | 0.094* | |
| C42 | 0.4754 (2) | 0.2209 (6) | 1.2318 (2) | 0.0536 (10) | |
| H42A | 0.529913 | 0.257174 | 1.216028 | 0.064* | |
| H42B | 0.472692 | 0.090338 | 1.231359 | 0.064* | |
| C43 | 0.4135 (2) | 0.2591 (5) | 1.1059 (2) | 0.0462 (10) | |
| C44 | 0.3508 (2) | 0.3376 (5) | 1.0580 (2) | 0.0493 (10) | |
| H44 | 0.308706 | 0.404410 | 1.078743 | 0.059* | |
| C45 | 0.3497 (2) | 0.3187 (5) | 0.9804 (2) | 0.0531 (10) | |
| H45 | 0.307418 | 0.374312 | 0.948115 | 0.064* | |
| C46 | 0.4106 (2) | 0.2179 (5) | 0.9493 (2) | 0.0476 (10) | |
| C47 | 0.4712 (2) | 0.1361 (5) | 0.9978 (2) | 0.0482 (10) | |
| H47 | 0.511533 | 0.064016 | 0.977267 | 0.058* | |
| C48 | 0.4739 (2) | 0.1577 (5) | 1.0761 (2) | 0.0464 (9) | |
| H48 | 0.516601 | 0.103709 | 1.108483 | 0.056* | |
| C49 | 0.4124 (2) | 0.1963 (6) | 0.8672 (3) | 0.0584 (11) | |
| O9 | 0.3807 (3) | 0.3384 (6) | 0.8254 (2) | 0.0544 (16) | 0.735 (9) |
| C50 | 0.3807 (5) | 0.3379 (13) | 0.7433 (6) | 0.064 (2) | 0.735 (9) |
| H50A | 0.432423 | 0.282505 | 0.729022 | 0.077* | 0.735 (9) |
| H50B | 0.378868 | 0.460869 | 0.724260 | 0.077* | 0.735 (9) |
| C51 | 0.3068 (4) | 0.2372 (9) | 0.7079 (4) | 0.067 (2) | 0.735 (9) |
| H51A | 0.307393 | 0.237694 | 0.652755 | 0.101* | 0.735 (9) |
| H51B | 0.309176 | 0.115223 | 0.726315 | 0.101* | 0.735 (9) |
| H51C | 0.255724 | 0.293246 | 0.721562 | 0.101* | 0.735 (9) |
| O9B | 0.3346 (8) | 0.2613 (17) | 0.8386 (6) | 0.056 (4)* | 0.265 (9) |
| C50B | 0.3182 (13) | 0.228 (3) | 0.7576 (11) | 0.078 (6)* | 0.265 (9) |
| H50C | 0.260686 | 0.263008 | 0.740216 | 0.093* | 0.265 (9) |
| H50D | 0.324382 | 0.100218 | 0.747293 | 0.093* | 0.265 (9) |
| C51B | 0.377 (2) | 0.330 (5) | 0.7170 (19) | 0.081 (12)* | 0.265 (9) |
| H51D | 0.366615 | 0.307989 | 0.662536 | 0.122* | 0.265 (9) |
| H51E | 0.370481 | 0.455896 | 0.727113 | 0.122* | 0.265 (9) |
| H51F | 0.433994 | 0.293573 | 0.734169 | 0.122* | 0.265 (9) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0389 (14) | 0.0603 (19) | 0.0572 (17) | −0.0066 (13) | 0.0069 (11) | −0.0051 (15) |
| O2 | 0.0544 (19) | 0.136 (4) | 0.069 (2) | −0.016 (2) | 0.0050 (15) | −0.028 (2) |
| O3 | 0.0547 (17) | 0.085 (2) | 0.0546 (17) | −0.0051 (17) | 0.0072 (13) | −0.0107 (17) |
| O4 | 0.0407 (14) | 0.0462 (16) | 0.0617 (18) | −0.0072 (13) | 0.0043 (12) | 0.0012 (14) |
| O5 | 0.0518 (17) | 0.072 (2) | 0.0698 (19) | −0.0104 (17) | 0.0028 (14) | −0.0147 (17) |
| O6 | 0.0508 (16) | 0.079 (2) | 0.0583 (18) | −0.0110 (16) | 0.0111 (13) | −0.0097 (16) |
| O7 | 0.0411 (14) | 0.0503 (17) | 0.0619 (18) | 0.0051 (13) | 0.0060 (12) | 0.0001 (15) |
| O8 | 0.0538 (16) | 0.0573 (19) | 0.0695 (19) | 0.0051 (15) | 0.0096 (13) | −0.0054 (16) |
| C1 | 0.039 (2) | 0.036 (2) | 0.058 (2) | −0.0038 (18) | 0.0085 (17) | −0.0015 (19) |
| C2 | 0.040 (2) | 0.045 (2) | 0.063 (3) | −0.0003 (18) | 0.0106 (18) | 0.002 (2) |
| C3 | 0.046 (2) | 0.056 (3) | 0.060 (3) | −0.007 (2) | −0.0004 (18) | 0.007 (2) |
| C4 | 0.051 (2) | 0.043 (2) | 0.056 (2) | −0.0052 (19) | 0.0115 (19) | 0.002 (2) |
| C5 | 0.042 (2) | 0.052 (3) | 0.066 (3) | 0.0035 (19) | 0.0130 (19) | −0.002 (2) |
| C6 | 0.041 (2) | 0.052 (2) | 0.061 (3) | 0.0001 (19) | 0.0054 (18) | 0.003 (2) |
| C7 | 0.078 (3) | 0.063 (3) | 0.058 (3) | −0.016 (3) | 0.014 (2) | −0.004 (2) |
| C8 | 0.040 (2) | 0.050 (2) | 0.055 (2) | −0.0056 (19) | 0.0085 (17) | −0.002 (2) |
| C9 | 0.040 (2) | 0.047 (2) | 0.052 (2) | 0.0026 (19) | 0.0081 (16) | −0.004 (2) |
| C10 | 0.044 (2) | 0.060 (3) | 0.066 (3) | −0.008 (2) | 0.0137 (19) | −0.008 (2) |
| C11 | 0.044 (2) | 0.059 (3) | 0.071 (3) | −0.009 (2) | 0.0030 (19) | −0.012 (2) |
| C12 | 0.041 (2) | 0.052 (3) | 0.056 (2) | 0.003 (2) | 0.0038 (17) | −0.006 (2) |
| C13 | 0.040 (2) | 0.050 (3) | 0.059 (3) | −0.0032 (19) | 0.0079 (17) | −0.003 (2) |
| C14 | 0.037 (2) | 0.052 (3) | 0.056 (3) | −0.0034 (19) | 0.0025 (17) | −0.008 (2) |
| C15 | 0.045 (2) | 0.069 (3) | 0.061 (3) | 0.003 (2) | 0.003 (2) | −0.012 (2) |
| C16 | 0.072 (3) | 0.112 (4) | 0.048 (3) | 0.004 (3) | 0.005 (2) | −0.010 (3) |
| C17 | 0.086 (4) | 0.111 (5) | 0.061 (3) | −0.012 (3) | 0.012 (2) | −0.002 (3) |
| C18 | 0.041 (2) | 0.039 (2) | 0.063 (3) | −0.0033 (19) | 0.0035 (18) | 0.001 (2) |
| C19 | 0.042 (2) | 0.049 (3) | 0.066 (3) | 0.004 (2) | 0.0088 (18) | 0.000 (2) |
| C20 | 0.049 (2) | 0.058 (3) | 0.067 (3) | 0.000 (2) | −0.0007 (19) | 0.011 (2) |
| C21 | 0.063 (3) | 0.053 (3) | 0.063 (3) | −0.011 (2) | 0.011 (2) | −0.001 (2) |
| C22 | 0.055 (3) | 0.058 (3) | 0.077 (3) | 0.008 (2) | 0.014 (2) | −0.009 (3) |
| C23 | 0.051 (2) | 0.053 (3) | 0.067 (3) | 0.006 (2) | 0.001 (2) | −0.004 (2) |
| C24 | 0.093 (4) | 0.088 (4) | 0.067 (3) | −0.011 (3) | 0.019 (3) | −0.003 (3) |
| C25 | 0.040 (2) | 0.055 (3) | 0.062 (3) | −0.007 (2) | 0.0046 (17) | −0.007 (2) |
| C26 | 0.0363 (19) | 0.038 (2) | 0.056 (2) | 0.0034 (18) | 0.0050 (16) | −0.0020 (19) |
| C27 | 0.033 (2) | 0.040 (2) | 0.069 (3) | 0.0010 (17) | 0.0104 (18) | 0.000 (2) |
| C28 | 0.0320 (19) | 0.041 (2) | 0.073 (3) | 0.0008 (18) | 0.0020 (18) | −0.005 (2) |
| C29 | 0.0339 (19) | 0.043 (2) | 0.060 (3) | 0.0045 (17) | 0.0030 (17) | −0.005 (2) |
| C30 | 0.039 (2) | 0.043 (2) | 0.062 (3) | −0.0006 (18) | 0.0115 (17) | −0.003 (2) |
| C31 | 0.036 (2) | 0.044 (2) | 0.062 (3) | −0.0045 (17) | 0.0033 (17) | −0.004 (2) |
| C32 | 0.039 (2) | 0.051 (3) | 0.065 (3) | 0.000 (2) | 0.0068 (19) | −0.013 (2) |
| C33 | 0.064 (3) | 0.105 (4) | 0.056 (3) | −0.018 (3) | 0.018 (2) | −0.022 (3) |
| C34 | 0.084 (4) | 0.141 (6) | 0.067 (3) | 0.000 (4) | 0.006 (3) | 0.009 (4) |
| C35 | 0.041 (2) | 0.043 (2) | 0.064 (3) | 0.0045 (18) | 0.0064 (18) | 0.006 (2) |
| C36 | 0.043 (2) | 0.050 (3) | 0.076 (3) | −0.003 (2) | 0.006 (2) | 0.007 (2) |
| C37 | 0.051 (2) | 0.050 (3) | 0.077 (3) | −0.007 (2) | −0.003 (2) | 0.000 (2) |
| C38 | 0.060 (3) | 0.048 (3) | 0.063 (3) | 0.000 (2) | 0.001 (2) | 0.000 (2) |
| C39 | 0.050 (2) | 0.063 (3) | 0.070 (3) | −0.005 (2) | 0.018 (2) | 0.000 (2) |
| C40 | 0.042 (2) | 0.057 (3) | 0.075 (3) | −0.009 (2) | 0.0052 (19) | −0.001 (2) |
| C41 | 0.093 (4) | 0.068 (3) | 0.073 (3) | 0.001 (3) | 0.009 (3) | −0.008 (3) |
| C42 | 0.039 (2) | 0.057 (3) | 0.065 (3) | 0.004 (2) | 0.0051 (18) | 0.006 (2) |
| C43 | 0.0341 (19) | 0.040 (2) | 0.066 (3) | −0.0026 (18) | 0.0103 (17) | −0.003 (2) |
| C44 | 0.036 (2) | 0.042 (2) | 0.070 (3) | 0.0046 (18) | 0.0045 (18) | −0.005 (2) |
| C45 | 0.040 (2) | 0.042 (2) | 0.075 (3) | 0.0058 (19) | −0.0065 (18) | −0.006 (2) |
| C46 | 0.039 (2) | 0.041 (2) | 0.061 (3) | −0.0001 (19) | −0.0004 (17) | −0.006 (2) |
| C47 | 0.037 (2) | 0.036 (2) | 0.073 (3) | 0.0008 (18) | 0.0074 (18) | −0.002 (2) |
| C48 | 0.0339 (19) | 0.042 (2) | 0.063 (3) | −0.0001 (18) | 0.0018 (17) | 0.003 (2) |
| C49 | 0.049 (2) | 0.052 (3) | 0.072 (3) | 0.009 (2) | −0.005 (2) | −0.011 (2) |
| O9 | 0.061 (3) | 0.046 (3) | 0.056 (3) | 0.008 (2) | 0.0020 (18) | 0.001 (2) |
| C50 | 0.058 (4) | 0.062 (5) | 0.074 (7) | −0.006 (3) | 0.012 (4) | −0.005 (5) |
| C51 | 0.052 (4) | 0.064 (4) | 0.084 (6) | −0.003 (3) | −0.002 (3) | 0.000 (4) |
Geometric parameters (Å, º)
| O1—C9 | 1.377 (4) | C25—H25B | 0.9900 |
| O1—C8 | 1.432 (4) | C26—C31 | 1.387 (5) |
| O2—C15 | 1.201 (5) | C26—C27 | 1.395 (5) |
| O3—C15 | 1.335 (5) | C27—C28 | 1.380 (5) |
| O3—C16 | 1.459 (4) | C27—H27 | 0.9500 |
| O4—C26 | 1.371 (4) | C28—C29 | 1.393 (5) |
| O4—C25 | 1.438 (4) | C28—H28 | 0.9500 |
| O5—C32 | 1.222 (4) | C29—C30 | 1.387 (5) |
| O6—C32 | 1.341 (5) | C29—C32 | 1.475 (5) |
| O6—C33 | 1.463 (5) | C30—C31 | 1.387 (5) |
| O7—C43 | 1.355 (4) | C30—H30 | 0.9500 |
| O7—C42 | 1.438 (4) | C31—H31 | 0.9500 |
| O8—C49 | 1.207 (5) | C33—C34 | 1.485 (8) |
| C1—C6 | 1.383 (5) | C33—H33A | 0.9900 |
| C1—C2 | 1.393 (5) | C33—H33B | 0.9900 |
| C1—C8 | 1.505 (5) | C34—H34A | 0.9800 |
| C2—C3 | 1.390 (5) | C34—H34B | 0.9800 |
| C2—H2 | 0.9500 | C34—H34C | 0.9800 |
| C3—C4 | 1.387 (5) | C35—C36 | 1.384 (5) |
| C3—H3 | 0.9500 | C35—C40 | 1.387 (5) |
| C4—C5 | 1.379 (5) | C35—C42 | 1.505 (5) |
| C4—C7 | 1.512 (5) | C36—C37 | 1.384 (6) |
| C5—C6 | 1.386 (5) | C36—H36 | 0.9500 |
| C5—H5 | 0.9500 | C37—C38 | 1.389 (6) |
| C6—H6 | 0.9500 | C37—H37 | 0.9500 |
| C7—H7A | 0.9800 | C38—C39 | 1.389 (6) |
| C7—H7B | 0.9800 | C38—C41 | 1.500 (6) |
| C7—H7C | 0.9800 | C39—C40 | 1.388 (6) |
| C8—H8A | 0.9900 | C39—H39 | 0.9500 |
| C8—H8B | 0.9900 | C40—H40 | 0.9500 |
| C9—C10 | 1.380 (5) | C41—H41A | 0.9800 |
| C9—C14 | 1.388 (5) | C41—H41B | 0.9800 |
| C10—C11 | 1.380 (5) | C41—H41C | 0.9800 |
| C10—H10 | 0.9500 | C42—H42A | 0.9900 |
| C11—C12 | 1.389 (5) | C42—H42B | 0.9900 |
| C11—H11 | 0.9500 | C43—C48 | 1.387 (5) |
| C12—C13 | 1.386 (5) | C43—C44 | 1.395 (5) |
| C12—C15 | 1.488 (5) | C44—C45 | 1.384 (5) |
| C13—C14 | 1.395 (5) | C44—H44 | 0.9500 |
| C13—H13 | 0.9500 | C45—C46 | 1.401 (5) |
| C14—H14 | 0.9500 | C45—H45 | 0.9500 |
| C16—C17 | 1.463 (6) | C46—C47 | 1.389 (5) |
| C16—H16A | 0.9900 | C46—C49 | 1.471 (6) |
| C16—H16B | 0.9900 | C47—C48 | 1.397 (5) |
| C17—H17A | 0.9800 | C47—H47 | 0.9500 |
| C17—H17B | 0.9800 | C48—H48 | 0.9500 |
| C17—H17C | 0.9800 | C49—O9 | 1.379 (6) |
| C18—C23 | 1.381 (5) | C49—O9B | 1.403 (12) |
| C18—C19 | 1.382 (5) | O9—C50 | 1.458 (11) |
| C18—C25 | 1.495 (5) | C50—C51 | 1.507 (10) |
| C19—C20 | 1.394 (5) | C50—H50A | 0.9900 |
| C19—H19 | 0.9500 | C50—H50B | 0.9900 |
| C20—C21 | 1.383 (6) | C51—H51A | 0.9800 |
| C20—H20 | 0.9500 | C51—H51B | 0.9800 |
| C21—C22 | 1.379 (6) | C51—H51C | 0.9800 |
| C21—C24 | 1.527 (5) | O9B—C50B | 1.459 (19) |
| C22—C23 | 1.388 (5) | C50B—C51B | 1.47 (2) |
| C22—H22 | 0.9500 | C50B—H50C | 0.9900 |
| C23—H23 | 0.9500 | C50B—H50D | 0.9900 |
| C24—H24A | 0.9800 | C51B—H51D | 0.9800 |
| C24—H24B | 0.9800 | C51B—H51E | 0.9800 |
| C24—H24C | 0.9800 | C51B—H51F | 0.9800 |
| C25—H25A | 0.9900 | ||
| C9—O1—C8 | 117.1 (3) | C27—C28—H28 | 119.6 |
| C15—O3—C16 | 115.9 (3) | C29—C28—H28 | 119.6 |
| C26—O4—C25 | 117.3 (3) | C30—C29—C28 | 118.9 (4) |
| C32—O6—C33 | 116.3 (3) | C30—C29—C32 | 122.4 (3) |
| C43—O7—C42 | 117.0 (3) | C28—C29—C32 | 118.7 (3) |
| C6—C1—C2 | 117.9 (4) | C31—C30—C29 | 121.1 (3) |
| C6—C1—C8 | 122.3 (3) | C31—C30—H30 | 119.4 |
| C2—C1—C8 | 119.6 (3) | C29—C30—H30 | 119.4 |
| C3—C2—C1 | 120.9 (3) | C26—C31—C30 | 119.2 (4) |
| C3—C2—H2 | 119.5 | C26—C31—H31 | 120.4 |
| C1—C2—H2 | 119.5 | C30—C31—H31 | 120.4 |
| C4—C3—C2 | 120.8 (4) | O5—C32—O6 | 123.0 (4) |
| C4—C3—H3 | 119.6 | O5—C32—C29 | 124.0 (4) |
| C2—C3—H3 | 119.6 | O6—C32—C29 | 113.0 (3) |
| C5—C4—C3 | 117.9 (4) | O6—C33—C34 | 110.4 (4) |
| C5—C4—C7 | 121.3 (4) | O6—C33—H33A | 109.6 |
| C3—C4—C7 | 120.8 (4) | C34—C33—H33A | 109.6 |
| C4—C5—C6 | 121.6 (4) | O6—C33—H33B | 109.6 |
| C4—C5—H5 | 119.2 | C34—C33—H33B | 109.6 |
| C6—C5—H5 | 119.2 | H33A—C33—H33B | 108.1 |
| C1—C6—C5 | 120.7 (4) | C33—C34—H34A | 109.5 |
| C1—C6—H6 | 119.6 | C33—C34—H34B | 109.5 |
| C5—C6—H6 | 119.6 | H34A—C34—H34B | 109.5 |
| C4—C7—H7A | 109.5 | C33—C34—H34C | 109.5 |
| C4—C7—H7B | 109.5 | H34A—C34—H34C | 109.5 |
| H7A—C7—H7B | 109.5 | H34B—C34—H34C | 109.5 |
| C4—C7—H7C | 109.5 | C36—C35—C40 | 118.0 (4) |
| H7A—C7—H7C | 109.5 | C36—C35—C42 | 121.3 (3) |
| H7B—C7—H7C | 109.5 | C40—C35—C42 | 120.7 (4) |
| O1—C8—C1 | 109.1 (3) | C35—C36—C37 | 121.5 (4) |
| O1—C8—H8A | 109.9 | C35—C36—H36 | 119.2 |
| C1—C8—H8A | 109.9 | C37—C36—H36 | 119.2 |
| O1—C8—H8B | 109.9 | C36—C37—C38 | 120.7 (4) |
| C1—C8—H8B | 109.9 | C36—C37—H37 | 119.7 |
| H8A—C8—H8B | 108.3 | C38—C37—H37 | 119.7 |
| O1—C9—C10 | 115.7 (3) | C37—C38—C39 | 118.0 (4) |
| O1—C9—C14 | 123.8 (3) | C37—C38—C41 | 122.4 (4) |
| C10—C9—C14 | 120.5 (4) | C39—C38—C41 | 119.6 (4) |
| C9—C10—C11 | 119.6 (4) | C40—C39—C38 | 121.1 (4) |
| C9—C10—H10 | 120.2 | C40—C39—H39 | 119.4 |
| C11—C10—H10 | 120.2 | C38—C39—H39 | 119.4 |
| C10—C11—C12 | 121.3 (4) | C35—C40—C39 | 120.8 (4) |
| C10—C11—H11 | 119.3 | C35—C40—H40 | 119.6 |
| C12—C11—H11 | 119.3 | C39—C40—H40 | 119.6 |
| C13—C12—C11 | 118.6 (4) | C38—C41—H41A | 109.5 |
| C13—C12—C15 | 122.5 (4) | C38—C41—H41B | 109.5 |
| C11—C12—C15 | 118.9 (4) | H41A—C41—H41B | 109.5 |
| C12—C13—C14 | 120.8 (4) | C38—C41—H41C | 109.5 |
| C12—C13—H13 | 119.6 | H41A—C41—H41C | 109.5 |
| C14—C13—H13 | 119.6 | H41B—C41—H41C | 109.5 |
| C9—C14—C13 | 119.2 (3) | O7—C42—C35 | 108.7 (3) |
| C9—C14—H14 | 120.4 | O7—C42—H42A | 109.9 |
| C13—C14—H14 | 120.4 | C35—C42—H42A | 109.9 |
| O2—C15—O3 | 122.7 (4) | O7—C42—H42B | 109.9 |
| O2—C15—C12 | 124.3 (4) | C35—C42—H42B | 109.9 |
| O3—C15—C12 | 112.9 (3) | H42A—C42—H42B | 108.3 |
| O3—C16—C17 | 108.4 (4) | O7—C43—C48 | 124.9 (4) |
| O3—C16—H16A | 110.0 | O7—C43—C44 | 114.9 (3) |
| C17—C16—H16A | 110.0 | C48—C43—C44 | 120.2 (4) |
| O3—C16—H16B | 110.0 | C45—C44—C43 | 120.2 (4) |
| C17—C16—H16B | 110.0 | C45—C44—H44 | 119.9 |
| H16A—C16—H16B | 108.4 | C43—C44—H44 | 119.9 |
| C16—C17—H17A | 109.5 | C44—C45—C46 | 120.4 (4) |
| C16—C17—H17B | 109.5 | C44—C45—H45 | 119.8 |
| H17A—C17—H17B | 109.5 | C46—C45—H45 | 119.8 |
| C16—C17—H17C | 109.5 | C47—C46—C45 | 118.7 (4) |
| H17A—C17—H17C | 109.5 | C47—C46—C49 | 119.1 (4) |
| H17B—C17—H17C | 109.5 | C45—C46—C49 | 122.2 (4) |
| C23—C18—C19 | 118.9 (4) | C46—C47—C48 | 121.3 (3) |
| C23—C18—C25 | 120.5 (4) | C46—C47—H47 | 119.3 |
| C19—C18—C25 | 120.6 (3) | C48—C47—H47 | 119.3 |
| C18—C19—C20 | 120.2 (4) | C43—C48—C47 | 119.1 (4) |
| C18—C19—H19 | 119.9 | C43—C48—H48 | 120.4 |
| C20—C19—H19 | 119.9 | C47—C48—H48 | 120.4 |
| C21—C20—C19 | 120.9 (4) | O8—C49—O9 | 120.3 (4) |
| C21—C20—H20 | 119.5 | O8—C49—O9B | 122.3 (6) |
| C19—C20—H20 | 119.5 | O8—C49—C46 | 125.2 (4) |
| C22—C21—C20 | 118.4 (4) | O9—C49—C46 | 113.9 (4) |
| C22—C21—C24 | 120.7 (4) | O9B—C49—C46 | 102.7 (5) |
| C20—C21—C24 | 120.9 (4) | C49—O9—C50 | 120.1 (5) |
| C21—C22—C23 | 120.9 (4) | O9—C50—C51 | 110.1 (7) |
| C21—C22—H22 | 119.5 | O9—C50—H50A | 109.6 |
| C23—C22—H22 | 119.5 | C51—C50—H50A | 109.6 |
| C18—C23—C22 | 120.7 (4) | O9—C50—H50B | 109.6 |
| C18—C23—H23 | 119.7 | C51—C50—H50B | 109.6 |
| C22—C23—H23 | 119.7 | H50A—C50—H50B | 108.2 |
| C21—C24—H24A | 109.5 | C50—C51—H51A | 109.5 |
| C21—C24—H24B | 109.5 | C50—C51—H51B | 109.5 |
| H24A—C24—H24B | 109.5 | H51A—C51—H51B | 109.5 |
| C21—C24—H24C | 109.5 | C50—C51—H51C | 109.5 |
| H24A—C24—H24C | 109.5 | H51A—C51—H51C | 109.5 |
| H24B—C24—H24C | 109.5 | H51B—C51—H51C | 109.5 |
| O4—C25—C18 | 107.9 (3) | C49—O9B—C50B | 111.7 (11) |
| O4—C25—H25A | 110.1 | O9B—C50B—C51B | 109 (2) |
| C18—C25—H25A | 110.1 | O9B—C50B—H50C | 109.9 |
| O4—C25—H25B | 110.1 | C51B—C50B—H50C | 109.9 |
| C18—C25—H25B | 110.1 | O9B—C50B—H50D | 109.9 |
| H25A—C25—H25B | 108.4 | C51B—C50B—H50D | 109.9 |
| O4—C26—C31 | 124.7 (3) | H50C—C50B—H50D | 108.3 |
| O4—C26—C27 | 114.9 (3) | C50B—C51B—H51D | 109.5 |
| C31—C26—C27 | 120.3 (4) | C50B—C51B—H51E | 109.5 |
| C28—C27—C26 | 119.6 (3) | H51D—C51B—H51E | 109.5 |
| C28—C27—H27 | 120.2 | C50B—C51B—H51F | 109.5 |
| C26—C27—H27 | 120.2 | H51D—C51B—H51F | 109.5 |
| C27—C28—C29 | 120.7 (4) | H51E—C51B—H51F | 109.5 |
| C6—C1—C2—C3 | 0.4 (6) | C27—C28—C29—C32 | −176.7 (3) |
| C8—C1—C2—C3 | −175.3 (4) | C28—C29—C30—C31 | −1.3 (5) |
| C1—C2—C3—C4 | −1.1 (6) | C32—C29—C30—C31 | 177.0 (4) |
| C2—C3—C4—C5 | 0.3 (6) | O4—C26—C31—C30 | −178.7 (3) |
| C2—C3—C4—C7 | −177.6 (4) | C27—C26—C31—C30 | 3.1 (6) |
| C3—C4—C5—C6 | 1.2 (6) | C29—C30—C31—C26 | −1.0 (6) |
| C7—C4—C5—C6 | 179.1 (4) | C33—O6—C32—O5 | 4.1 (6) |
| C2—C1—C6—C5 | 1.2 (6) | C33—O6—C32—C29 | −175.2 (4) |
| C8—C1—C6—C5 | 176.7 (4) | C30—C29—C32—O5 | −177.3 (4) |
| C4—C5—C6—C1 | −2.0 (6) | C28—C29—C32—O5 | 1.0 (6) |
| C9—O1—C8—C1 | −174.9 (3) | C30—C29—C32—O6 | 2.0 (5) |
| C6—C1—C8—O1 | 40.1 (5) | C28—C29—C32—O6 | −179.6 (4) |
| C2—C1—C8—O1 | −144.3 (4) | C32—O6—C33—C34 | 81.8 (5) |
| C8—O1—C9—C10 | −174.1 (4) | C40—C35—C36—C37 | 2.2 (6) |
| C8—O1—C9—C14 | 7.1 (6) | C42—C35—C36—C37 | −177.5 (4) |
| O1—C9—C10—C11 | −178.7 (4) | C35—C36—C37—C38 | −1.6 (7) |
| C14—C9—C10—C11 | 0.1 (6) | C36—C37—C38—C39 | −0.1 (7) |
| C9—C10—C11—C12 | −0.4 (7) | C36—C37—C38—C41 | 178.3 (4) |
| C10—C11—C12—C13 | 0.6 (7) | C37—C38—C39—C40 | 1.1 (7) |
| C10—C11—C12—C15 | −179.5 (4) | C41—C38—C39—C40 | −177.3 (4) |
| C11—C12—C13—C14 | −0.5 (6) | C36—C35—C40—C39 | −1.1 (6) |
| C15—C12—C13—C14 | 179.6 (4) | C42—C35—C40—C39 | 178.6 (4) |
| O1—C9—C14—C13 | 178.8 (4) | C38—C39—C40—C35 | −0.5 (7) |
| C10—C9—C14—C13 | 0.0 (6) | C43—O7—C42—C35 | 173.7 (3) |
| C12—C13—C14—C9 | 0.2 (6) | C36—C35—C42—O7 | −118.9 (4) |
| C16—O3—C15—O2 | 1.5 (7) | C40—C35—C42—O7 | 61.4 (5) |
| C16—O3—C15—C12 | −175.5 (4) | C42—O7—C43—C48 | 2.1 (5) |
| C13—C12—C15—O2 | 173.9 (5) | C42—O7—C43—C44 | −177.2 (3) |
| C11—C12—C15—O2 | −6.0 (7) | O7—C43—C44—C45 | 177.6 (3) |
| C13—C12—C15—O3 | −9.3 (6) | C48—C43—C44—C45 | −1.6 (6) |
| C11—C12—C15—O3 | 170.9 (4) | C43—C44—C45—C46 | 1.2 (6) |
| C15—O3—C16—C17 | 173.7 (5) | C44—C45—C46—C47 | 0.8 (6) |
| C23—C18—C19—C20 | −1.3 (6) | C44—C45—C46—C49 | −179.1 (4) |
| C25—C18—C19—C20 | 179.4 (4) | C45—C46—C47—C48 | −2.2 (6) |
| C18—C19—C20—C21 | 1.0 (7) | C49—C46—C47—C48 | 177.6 (4) |
| C19—C20—C21—C22 | 0.4 (7) | O7—C43—C48—C47 | −179.0 (3) |
| C19—C20—C21—C24 | −178.9 (4) | C44—C43—C48—C47 | 0.2 (5) |
| C20—C21—C22—C23 | −1.6 (7) | C46—C47—C48—C43 | 1.8 (5) |
| C24—C21—C22—C23 | 177.7 (4) | C47—C46—C49—O8 | 21.5 (6) |
| C19—C18—C23—C22 | 0.1 (6) | C45—C46—C49—O8 | −158.6 (4) |
| C25—C18—C23—C22 | 179.4 (4) | C47—C46—C49—O9 | −149.8 (4) |
| C21—C22—C23—C18 | 1.4 (7) | C45—C46—C49—O9 | 30.1 (6) |
| C26—O4—C25—C18 | 178.3 (3) | C47—C46—C49—O9B | 167.6 (7) |
| C23—C18—C25—O4 | −70.6 (5) | C45—C46—C49—O9B | −12.5 (8) |
| C19—C18—C25—O4 | 108.7 (4) | O8—C49—O9—C50 | 6.4 (8) |
| C25—O4—C26—C31 | 1.6 (5) | C46—C49—O9—C50 | 178.2 (5) |
| C25—O4—C26—C27 | 180.0 (3) | C49—O9—C50—C51 | 82.8 (8) |
| O4—C26—C27—C28 | 178.8 (3) | O8—C49—O9B—C50B | −24.0 (16) |
| C31—C26—C27—C28 | −2.7 (6) | C46—C49—O9B—C50B | −171.4 (12) |
| C26—C27—C28—C29 | 0.3 (6) | C49—O9B—C50B—C51B | −66 (2) |
| C27—C28—C29—C30 | 1.7 (6) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C42—H42A···O8i | 0.99 | 2.65 | 3.269 (5) | 121 |
| C44—H44···O4 | 0.95 | 2.66 | 3.374 (5) | 133 |
Symmetry code: (i) −x+1, y+1/2, −z+2.
Funding Statement
Funding for this research was provided by: University Grants Commission Bangladesh.
References
- Abser, M. N., Bellwood, M., Holmes, M. C. & McCabe, R. W. (1993). J. Chem. Soc. Chem. Commun. pp. 1062–1063.
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bats, J. W. & Canenbley, R. (1984). Acta Cryst. C40, 993–995.
- Brandenburg, K. (1999). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Feng, Y., Liu, Z.-T., Liu, J., He, Y.-M., Zheng, Q.-Y. & Fan, Q.-H. (2009). J. Am. Chem. Soc. 131, 7950–7951. [DOI] [PubMed]
- Giordano, F., Bettini, R., Donini, C., Gazzaniga, A., Caira, M. R., Zhang, G. G. Z. & Grant, D. J. W. (1999). J. Pharm. Sci. 88, 1210–1216. [DOI] [PubMed]
- Higashi, T. (1995). ABSCOR. Rigaku Corporation, Tokyo, Japan.
- Jasinski, J. P., Butcher, R. J., Swamy, M. T., Yathirajan, H. S., Mohana, K. N. & Narayana, B. (2008). Anal. Sci. 24, x274.
- Lavis, L. D. (2008). Chem. Biol. 3, 203–206. [DOI] [PubMed]
- Moreno-Fuquen, R., Grande, C., Advincula, R. C., Tenorio, J. C. & Ellena, J. (2012). Acta Cryst. E68, o3247–o3248. [DOI] [PMC free article] [PubMed]
- Perumal, C. K. L., Arulchakkaravarthi, A., Santhanaraghavan, P. & Ramasamy, P. (2002). J. Cryst. Growth, 241, 200–205.
- Qin, Y.-M., Zou, X., Long, D., Ji, C. & Zhao, C.-S. (2019). Z. Kristallogr. New Cryst. Struct. 234, 1295–1296.
- Rigaku (2018). CrystalStructure. Rigaku Corporation, Tokyo, Japan.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Spek, A. L. (2020). Acta Cryst. E76, 1–11. [DOI] [PMC free article] [PubMed]
- Wang, K., Ju, C. F., Xiao, J. & Chen, Q. (2013). Acta Cryst. E69, o1562. [DOI] [PMC free article] [PubMed]
- Yang, H., Svärd, M., Zeglinski, J. & Rasmuson, C. (2014). Cryst. Growth Des. 14, 3890–3902.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989022009380/wm5660sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989022009380/wm5660Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989022009380/wm5660Isup3.cml
CCDC reference: 2174691
Additional supporting information: crystallographic information; 3D view; checkCIF report