Abstract
Palpitations is one of the most common side effects experienced post‐messenger‐RNA COVID‐19 vaccines. However, some patients experience significant symptoms and further workup needs to be considered. We present an interesting case of inappropriate sinus tachycardia in a fit gentleman who presented with worsening palpitations and elevated heart rate post‐first and ‐second dose of the Pfizer/BioNTech vaccine.
![]()
Keywords: BioNTech BNT162b2, inappropriate sinus tachycardia, palpitations
Palpitations is one of the commonest side effects experienced post‐messenger‐RNA (mRNA) COVID‐19 vaccination. However, in most patients this is self‐limiting. Inappropriate sinus tachycardia (IST) has been documented to occur in patients who continue to experience persistent palpitations post‐recovery from the COVID‐19 infection. 1 It has been postulated that this is a result of immune‐mediated effects on the individual's cardiac autonomic nervous system, resulting in an imbalance between the sympathetic and parasympathetic effects.
We present a case of inappropriate sinus tachycardia post‐mRNA COVID‐19 vaccination in a previously fit and healthy gentleman. He had experienced palpitations, light‐headedness, and a decrease in exercise tolerance 2 days after the first dose and experienced worsening symptoms post‐second dose of the vaccine.
A 53‐year‐old active, healthy male presented to the emergency department with a 2‐month history of worsening palpitations associated with increased fatigue and reduced effort tolerance. He received his first dose of the BNT162b2 COVID‐19 (Pfizer‐BioNTech) vaccine at the end of May 2021 and started noticing palpitations 3 days after the vaccine. The resting heart rate reflected on his wearable fitness tracker showed an increase from a baseline of 70 to 80 bpm and 90 to 100 bpm. The elevated heart rate and palpitations persisted until the second dose of BNT162b2 COVID‐19 (Pfizer‐BioNTech) vaccine was administered 3 weeks later. The patient subsequently experienced worsening palpitations over the next 2 to 3 days with his resting heart rate increasing to a baseline of 100–110 bpm and peaked at 140 bpm with minimal exertion. He denied any illicit drug use and was not on any supplements or regular medications.
Clinical history and examination revealed elevated heart rate with normal blood pressure and temperature. There was no difference in symptoms on change of posture and no documented postural drop in blood pressure. 12‐lead electrocardiograms (ECGs) showed sinus tachycardia (Figure 1). D‐dimer was mildly elevated at 0.85 mg/L (Normal < 0.5 mg/L). The rest of the investigations including serial troponin levels, urine catecholamines and metanephrines screen, thyroid function tests, infective markers, and viral screen were all unremarkable.
FIGURE 1.
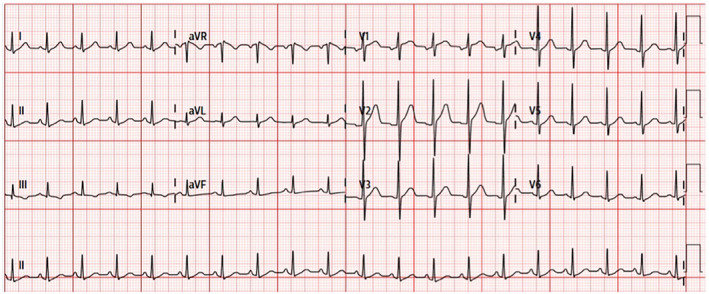
Presenting electrocardiogram (ECG) showing sinus tachycardia.
An enhanced computed tomography of the pulmonary arteries performed in view of the mildly elevated D‐dimer was negative for pulmonary embolism. Transthoracic echocardiography showed normal left ventricular ejection fraction of 57%, normal right ventricular function, morphologically normal valves, and a low probability of pulmonary hypertension. A 24‐h Holter ECG monitoring showed sinus tachycardia with an average heart rate of 107 bpm, with normal diurnal variation but with a slightly elevated nocturnal heart rate of 70–90 bpm (Figures 2 and 3).
FIGURE 2.
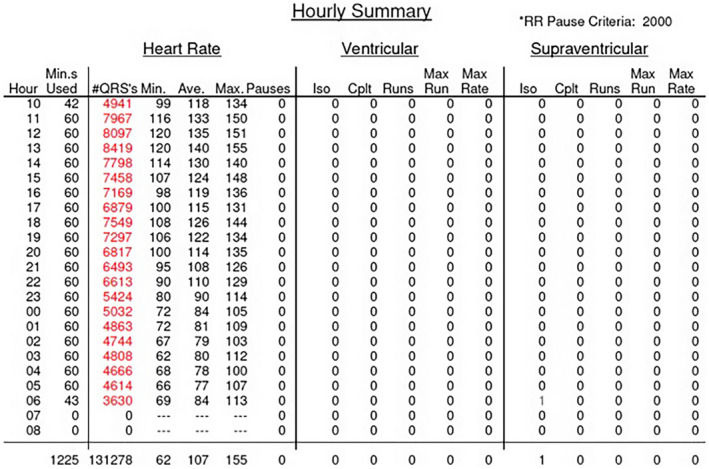
Hourly heart rate summary on initial 24‐h Holter showing elevated average heart rate, especially during awake h.
FIGURE 3.
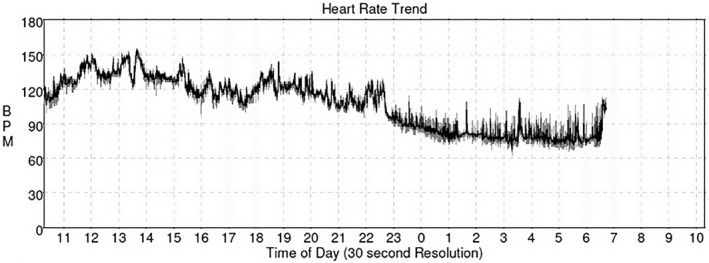
Graph showing heart rate trend on initial 24‐h Holter reflecting peak heart of 155 bpm and average heart rate of 107 bpm.
In view of distressing palpitations affecting his quality of life, he was initiated on Ivabradine 5 mg twice daily. The patient was counseled on beta‐blockers but was not keen because of the potential side effects of fatigue and erectile dysfunction. At a review 2 months later, the patient reported an improvement in both symptoms and effort tolerance. His wearable fitness tracker also showed that the average resting heart rate had improved to an average of 80–90 bpm.
A repeat 24‐h Holter performed 4 months later showed an improvement in the average heart rate from 107 bpm to 89 bpm and the burden of beats in tachycardia had improved from 71% to 33% (Figure 4).
FIGURE 4.
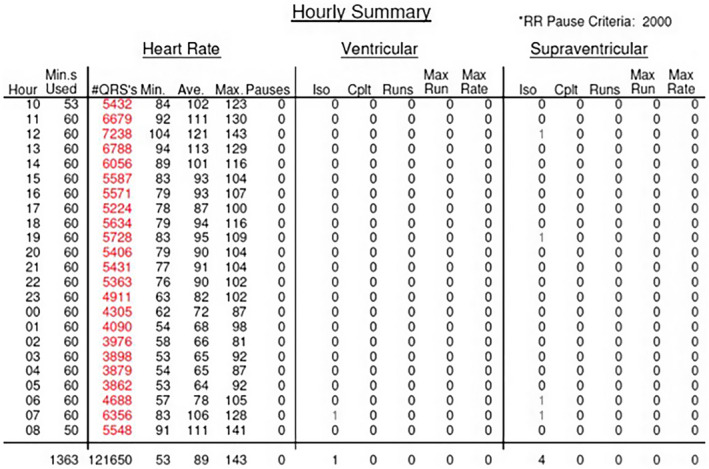
Hourly heart rate summary on follow‐up 24‐h Holter showing improvement in the average heart rate.
Palpitations have been documented to be one of the commonest complaints in patients who have recovered from COVID‐19 infection. A literature search showed that tachycardia post‐COVID‐19 vaccination is common too. 2 , 3 , 4 A study based on the WHO database reported an incidence of 16.4%, 2 whilst a recent US study (DETECT), using a smartphone app‐based research platform, reported elevated resting heart rate in 71% and 76% of vaccinated individuals post‐first and ‐second dose, respectively. 4 Resting heart rate trends documented in the DETECT study showed that the average resting heart rate returned to baseline by days 4–6 in the majority of patients. These changes were attributed to physiological stress response to the vaccine. This case is unique in that it describes symptomatic elevation in resting heart rate that persisted and worsened after the second dose of the COVID‐19 vaccine.
IST is most often defined as an average heart rate of more than 90 bpm, or a persistent increase in heart rate to more than 100 bpm disproportionate to the level of physical or emotional state of the individual. 5 The diagnosis of IST can be difficult. Patients may be asymptomatic or present with symptoms like palpitations, light‐headedness, or decrease in exercise capacity. Numerous mechanisms have been proposed for IST. This ranges from autonomic dysfunction, neurohormonal dysregulation, intrinsic sinus node hyperactivity, and channelopathies like gain‐of‐function mutation of the pacemaker hyperpolarization‐activated cyclic nucleotide‐gated 4 (HCN4) channel in familial causes. Closely associated with IST is postural orthostatic tachycardia syndrome (POTS), a clinical syndrome which is defined as an increase in heart rate of ≥30 bpm when standing for >30 s without orthostatic hypotension.
Management of IST is challenging, beta‐blockers are often ineffective and high doses may result in debilitating side effects like fatigue. Ivabradine an If blocker has been shown to be effective in reducing the average heart rate and, most importantly, ameliorating symptoms and improving exercise tolerance. 5
Despite the temporal sequence of events, it is also important to consider the possibility that this patient could have coincidentally developed IST during the time of vaccination or have undiagnosed IST.
There is still much to be known about the mRNA COVID‐19 vaccine and this case illustrates that we need to consider a variety of differential diagnoses including IST and postural orthostatic tachycardia syndrome. Other differential diagnoses like pulmonary embolism and even anxiety should also be considered. This will allow for appropriate management and patient counseling.
Palpitations is a commonly experienced side effect of post‐mRNA COVID‐19 vaccination and is often self‐limiting in most patients. However, further evaluation should be considered in patients with prolonged symptoms, especially those who have documented sinus tachycardia. Ivabradine may be considered in patients diagnosed with IST, especially in those who have a contraindication to beta‐blockers or have concerns about potential side effects like fatigue.
FUNDING INFORMATION
No funding was involved in this paper.
CONFLICT OF INTEREST
All authors have no conflicts of interest to declare.
ETHICS STATEMENT
No ethics approval required as this is a clinical case.
DISCLOSURES
All authors have no relationships with industry.
Teo HK, Ho KL, Tan BY, Ching CK, Chong DTT. A racing heart post‐Pfizer/BioNTech BNT162b2 . J Arrhythmia. 2022;38:827–830. 10.1002/joa3.12773
Patient verbal consent was attained and documented prior to the initiation of this paper.
No clinical trial registration required as this is a clinical case.
REFERENCES
- 1. Arano Llach J, Victor Bazan VB, Gemma Llados GL, Raquel Adelino RA, Maria Jesus Dominguez MJ, Marta Massanella MM, et al. Inappropriate sinus tachycardia in post‐COVID‐19 syndrome. Europace. 2021;23(suppl 3):iii126,21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jeet Kaur R, Dutta S, Charan J, Bhardwaj P, Tandon A, Yadav D, et al. Cardiovascular adverse events reported from COVID‐19 vaccines: a study based on WHO database. Int J Gen Med. 2021;14:3909–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tate C, Demashkieh L, Hakmeh W. Isolated tachycardia presenting after Pfizer‐BioNTech COVID‐19 vaccination. Cureus. 2021;13(7):e16706. 10.7759/cureus.16706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quer G, Gadaleta M, Radin JM, Andersen KG, Baca‐Motes K, Ramos E, et al. Inter‐individual variation in objective measure of reactogenicity following COVID‐19 vaccination via smartwatches and fitness bands. npj Digit Med. 2022;5:49. 10.1038/s41746-022-00591-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olshansky B, Sullivan RM. Inappropriate sinus tachycardia. Europace. 2019;21(2):194–207. doi: 10.1093/europace/euy128 [DOI] [PubMed] [Google Scholar]


