Abstract
As the SARS-CoV-2 pandemic remains uncontrolled owing to the continuous emergence of variants of concern, there is an immediate need to implement the most effective antiviral treatment strategies, especially for risk groups. Here, we evaluated the therapeutic potency of nirmatrelvir, remdesivir and molnupiravir, and their combinations in SARS-CoV-2 infected K18-hACE2 transgenic mice. Systemic treatment of mice with each drug (20 mg/kg) resulted in slightly enhanced antiviral efficacy and yielded an increased life expectancy of only about 20–40% survival. However, combination therapy with nirmatrelvir (20 mg/kg) and molnupiravir (20 mg/kg) in lethally infected mice showed profound inhibition of SARS-CoV-2 replication in both the lung and brain and synergistically improved survival rates up to 80% compared to those with nirmatrelvir (36%, P < 0.001) and molnupiravir (43%, P < 0.001) administered alone. This combination therapy effectively reduced clinical severity score, virus-induced tissue damage, and viral distribution compared to those in animals treated with these monotherapies. Furthermore, all these assessments associated with this combination were also significantly higher than that of mice receiving remdesivir monotherapy (P < 0.001) and the nirmatrelvir (20 mg/kg) and remdesivir (20 mg/kg) combination (P < 0.001), underscored the clinical significance of this combination. By contrast, the nirmatrelvir and remdesivir combination showed less antiviral efficacy, with lower survival compared to nirmatrelvir monotherapy due to the insufficient plasma exposure of the remdesivir, demonstrating the inefficient therapeutic effect of this combination in the mouse model. The combination therapy with nirmatrelvir and molnupiravir contributes to alleviated morbidity and mortality, which can serve as a basis for the design of clinical studies of this combination in the treatment of COVID-19 patients.
Keywords: SARS-CoV-2, Nirmatrelvir, Remdesivir, Molnupiravir, In vivo, Combination therapy
1. Introduction
Since severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was first identified in China in December 2019, the virus has spread rapidly worldwide, causing 538,321,874 confirmed cases, including 6,320,599 deaths (Organization, 2022). Fortunately, unprecedented efforts have led to the development of an effective vaccine as fast as the disease spread, saving the lives of millions and billions of people.
Despite the availability of vaccines, it is imperative to develop effective drugs that have broad-spectrum effects on current and future potential coronavirus infections. Direct-acting antiviral agents target different viral proteins and are categorized into three classes. Monoclonal antibodies (mAbs) are prescribed as drugs that target the spike protein, and direct-acting small molecules interfere with the viral replication machinery. Usually, mAbs target only the spike protein and are not sufficiently effective against SARS-CoV-2 variants (VanBlargan et al., 2022; Wilhelm et al., 2021). The direct-acting small molecules can be divided into two classes: those targeting RNA-dependent RNA polymerase (RdRp) and those targeting viral proteases, such as the main protease (Mpro, also known as 3CLpro) and papain-like protease (PLpro). Remdesivir (RDV) and Molnupiravir (MPV) target the RdRp and exhibit improved inhibition of several RNA viruses, including SARS-CoV-2 (Toots et al., 2019; Wahl et al., 2021; Wang et al., 2021). Nirmatrelvir (NTV) and ritonavir are co-administered oral antiviral drugs with the trade name Paxlovid. This drug acts as an irreversible inhibitor of the Mpro of SARS-CoV-2 (Mody et al., 2021).
Combination therapy with two distinct drugs has led to different results, such as antagonistic, synergistic, and additive effects (Bobrowski et al., 2021). In general, the synergistic and additive effects of combination therapy are significant and are facilitated by targeting multiple pathways of the virus replication machinery. Viral diseases caused by human immunodeficiency virus (HIV), hepatitis C virus (HCV), and influenza are classic examples, for which combination therapy has become the benchmark treatment (Akanbi et al., 2012; Cohen et al., 2020; Kohli et al., 2015; Stanciu et al., 2021; Wang et al., 2020). Although combination therapy does not always end in a cure, it can significantly improve quality of life and prolong life because of increased therapeutic efficacy and prevention of the development of drug resistance. In addition to historical experience in the treatment of viral diseases, there have been some recent studies on combination therapy synergistically reducing the SARS-CoV-2 load (Bobrowski et al., 2021; Li et al., 2022). Combination therapy has been suggested as potent and effective when it targets different mechanisms and processes of the virus life cycle, such as the proteases (Mpro) and polymerases (RdRp) of SARS-CoV-2 (Wang et al., 2020). Furthermore, although there are several ongoing clinical studies with monotherapies, very few clinical studies have used combination therapies. Moreover, in vivo information in the context of SARS-CoV-2 infection is essential before considering combination therapy for clinical studies.
Screening FDA-approved antiviral drugs for efficacy against SARS-CoV-2 will help reduce the global impact of the current pandemic. Additionally, such an approach strongly emphasizes the practical and clinical need to develop appropriate combination therapies and increase access to COVID-19 patients, especially for high-risk groups. Therefore, we performed an in vivo study using a SARS-CoV-2 infected hACE2 transgenic mouse model and evaluated the potential efficacy of the drugs NTV, RDV and MPV and their combinations, NTV-RDV and NTV-MPV. We summarized our therapeutic insights into these drug combinations, which are of clinical significance, and we provided an alternative prescription for a more effective drug regimen for treating SARS-CoV-2 patients.
2. Material and methods
2.1. Ethics statement
The care and maintenance of the animals and animal housing followed the recommendations and guidelines provided by the Ministry of Food and Drug Safety, Republic of Korea. This study was approved by the institutional animal care and use committee of Chungbuk National University, Republic of Korea (CBNUA-1659-22-01).
2.2. Cells and virus
Vero E6 cells (African green monkey kidney cells, ATCC®, cat# CRL-1586™) were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% (w/v) fetal bovine serum (FBS), 4.0 mM L-glutamine, 110 mg/L sodium pyruvate, 4.5 g/L D-glucose and 1% antibiotic-antimycotic at 37°C and 5% CO2.
The virus Beta-CoV/Korea/KCDC03/2020 (NCCP43326) used for this study was obtained from the National Culture Collection for Pathogens, Republic of Korea. It was propagated in Vero E6 cells maintained in DMEM supplemented with 2% FBS and 1% antibiotic-antimycotic at 37°C and 5% CO2. All the infection work related to SARS-CoV-2 was carried out in the biosafety level 3 (BSL3) and animal BSL3 (ABSL3) facilities at Chungbuk National University per the institutional ethical committee standards.
2.3. Compound sources
Nirmatrelvir and molnupiravir were purchased from MedChemExpress (Monmouth Junction, USA). Remdesivir was purchased from Ambeed (Illinois, USA). Stock solutions of all these antiviral drugs were prepared in 2% DMSO and 20% sulfobutylether-β-cyclodextrin (MedChemExpress) in 0.9% saline and stored at −20°C.
2.4. SARS-CoV-2 infection in K18-hACE2 mice and treatment regimen
Eight-week-old female C57BL/6 Cg-Tg (K18-hACE2)2Prlmn/J mice were obtained from Jackson Laboratory, USA. All experimental mice were divided into 7 groups with 14 mice in each group: group 1: Mock-treated, group 2: NTV (20 mg/kg), group 3: RDV (20 mg/kg), group 4: MPV (20 mg/kg), group 5: NTV (20 mg/kg) + RDV (20 mg/kg), group 6: NTV (20 mg/kg) + MPV (20 mg/kg) and group 7: Vehicle. Mice were anesthetized by isoflurane inhalation and inoculated with 5 MLD50 of Beta-CoV/Korea/KCDC03/2020 in 50 μL. NTV and MPV were administered orally for treatment, and RDV was given intraperitoneally, twice daily (b.i.d.) for five days starting at 6 h post-infection.
2.5. Weight, clinical score, and survival
The body weight changes, survival and clinical symptoms of SARS-CoV-2 infected mice were monitored up to 14 days post-infection (DPI). The clinical scoring system categories included body weight, appearance (fur, eye closure), activity and movement, which were evaluated according to standard guidelines with a maximum score of 11 (Winkler et al., 2020). The mice were sacrificed when they lost ≥25% of their initial pre-infection body weight. Lung and brain tissues were collected at 5 DPI (n = 5/group) to determine viral titers, viral RNA copies, histopathology and immunohistochemistry.
2.6. Infectious viral titer estimation
Lung and brain tissues were homogenized with bead disruption and centrifuged at 12,000 rpm for 5 min. The 10-fold serially diluted virus-containing samples were then used to infect 0.2 × 106 Vero E6 cells per well in a 96-well plate and cultured in a 5% CO2 incubator at 37°C for 4 days or until an apparent cytopathic effect (CPE) was detected and further visualized by 0.2% crystal violet staining. The titer was calculated according to the Reed and Muench method and was expressed in log10 50% tissue culture infection dose (TCID50) per mg of tissue (Reed and Muench, 1938).
2.7. Quantitation of SARS-CoV-2 RNA
Isolation of RNA from homogenized lung and brain tissue was performed with a QIAamp Viral RNA Kit (QIAGEN) as per the manufacturer's instructions, with slight modifications. Complementary DNA conversion and RT–qPCR were accomplished by a Bio-Rad Real-Time PCR System using an Allplex™ SARS-CoV-2 RT–qPCR Probe Kit (Seegene Inc.). Viral genome copies of each of the RdRp and N genes of the SARS-CoV-2 viral RNA were counted on the Ct values using the matrix gene-based real-time reverse transcriptase-polymerase chain reaction (Lee et al., 2016).
2.8. Histopathology and immunohistochemistry analysis
Mouse lung and brain tissues collected at 5 DPI were fixed with 10% formalin neutralization buffer (Sigma Aldrich). The paraffin-embedded tissue sections were stained with H&E for evaluation of the histopathological score by a pathologist. The semiquantitative scoring system (0 - normal, 1 - mild, 2 - moderate and 3 - severe) was applied by examining the tissues under digital microscopy as described previously (Winkler et al., 2020; Yang et al., 2022; Zheng et al., 2021). Immunoreactivity in the lung tissues was assessed using a rabbit anti-SARS-CoV-2 Nucleocapsid pAb as the primary antibody (Sinobiological, China) and further processed using a Ventana Discovery Ultra (Roche, USA) system.
2.9. In vivo pharmacokinetic profiling of NTV, RDV and MPV and their combinations
Mice were administered 20 mg/kg RDV intraperitoneally, and with all other drugs such as 20 mg/kg, NTV and 20 mg/kg MPV administered orally to evaluate the in vivo pharmacokinetic profiles of NTV, RDV, MPV, NTV + RDV and NTV + MPV. Blood was collected from three mice each at 0.25, 0.5, 1, 2, 4, 6 and 12 h after treatment with each mono and combinations, and a 10 μl aliquot of plasma was added to 40 μL of internal standard solution (1 μg/mL) in acetonitrile. The samples were vortexed and centrifuged, and each supernatant was further diluted with 30% acetonitrile containing 0.1% formic acid. An aliquot of each sample was injected into the LC-MS/MS system. The ratio of the peak area of each analyte to the internal standard (chlorpromazine) was determined, and each compound was quantified using an eight-point calibration curve. The intra-batch accuracy and precision of each compound were acceptable according to U.S. Food and Drug Administration guidelines.
2.10. Statistical analysis
All statistical data were analyzed using GraphPad Prism 9 (GraphPad, San Diego, USA) and SPSS 26 (IBM SPSS, New York, USA) software. Statistical significance among different groups was determined using a one-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test. Survival analysis was performed using the Kaplan–Meier method, and differences were calculated using the log-rank Mantel-Cox test. P values of *p < 0.05, **p < 0.01 and ***p < 0.001 were considered to indicate a significant difference.
3. Results
3.1. Therapeutic efficacy of nirmatrelvir, remdesivir and molnupiravir in SARS-CoV-2 infected mice
The suboptimal mouse lethal dose (MLD50) was determined by infecting the mice with various titers of Beta-CoV/Korea/KCDC03/2020 (Figs. S1A and B). We conducted two dose-optimization studies (10 and 20 mg/kg body weight) to determine the low-dose therapeutic efficacy using hACE-2 transgenic mice (Fig. S2). Based on the weight change and survival-related aspects, we determined that a 20 mg/kg body weight dose was optimal for low-dose therapeutic efficacy in vivo (Fig. S2).
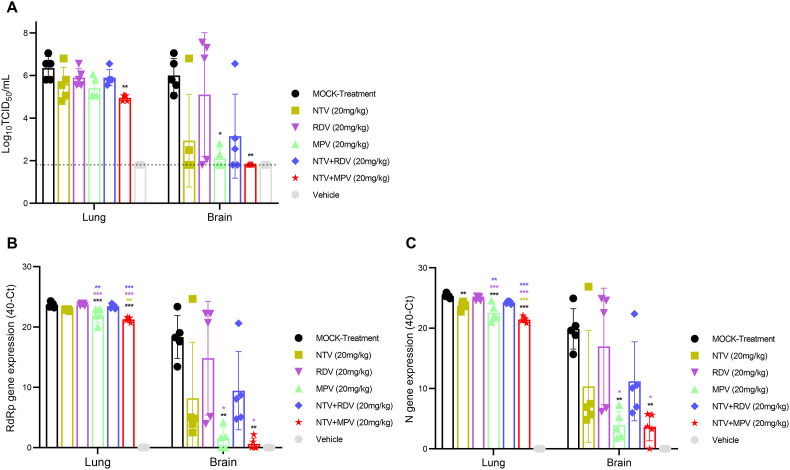
To evaluate the therapeutic efficacy of NTV, RDV and MPV, mice were infected with 5 MLD50 of SARS-CoV-2 followed by administration via oral gavage of 20 mg/kg body weight NTV and MPV and intraperitoneal administration of RDV alone. Six hours after the onset of the infection, treatment was started on the same day and continued twice daily for five consecutive days (Fig. 1 A). No protection against weight loss was observed in RDV treated mice (20.5%) compared to that in mock-treated mice (21.5%) by 7 DPI, whereas approximately 5–6% protection against weight loss was observed in NTV and MPV treated animals (Fig. 1B). Congruent with protection against weight loss, NTV (P < 0.001) and MPV (P < 0.001) produced significantly higher (36% and 43%) survival, respectively, while RDV (P = 0.073) produced a lower (21%) survival rate compared with mock treatment. The survival rates of the NTV (P = 0.024) and MPV treated (P = 0.007) animals were significantly higher compared to the RDV treated animals (Fig. 1C). The NTV and MPV treated mice exhibited higher clinical scores than RDV treated mice from 5 DPI (Fig. 1D). The SARS-CoV-2 mainly replicates in the lung and brain tissues of the K18-hACE2 mice (Fig. S1C) (Bauer et al., 2022; Stanciu et al., 2021); the tissues were collected from both the lung and brain at 5 DPI for viral titer assessment, histopathological and immunohistochemical analysis. We assessed the replication capability of the virus in the lungs and brain via TCID50 and RT–qPCR. Titers reduced by 0.7, 0.4 and 1 log10 TCID50 were observed in the lung tissue of NTV (P = 0.1987), RDV (P = 0.7062) and MPV treated (P = 0.05714) mice, respectively, compared to those in mock-treated mice (Fig. 2 A). The viral titer was significantly reduced in the brain tissue of the MPV treated animals than in the single NTV and RDV treated mice compared to those in mock-treated mice (Fig. 2A). We further performed RT–qPCR on parallel tissues used for TCID50. The MPV treated animals showed significantly higher viral RNA reduction in the lung and brain compared to that observed in mock and RDV treated animals (Fig. 2B and C). We evaluated the viral antigen levels by immunochemistry and histopathological changes determined by H&E staining of the lung (Fig. 3A) and brain (Fig. 3B) tissues at 5 DPI. No potent decrease in the expression levels of the SARS-CoV-2 N protein was observed in the RDV treated animals, whereas a reduction in viral distribution was observed in the NTV and MPV treated animals, with a greater reduction in the MPV treated animals. H&E staining revealed reduced pulmonary and brain damage in MPV treated animals compared to the animals in the other groups at 5 DPI (Fig. 3 ).
Fig. 1.
Evaluation of the therapeutic efficacy of nirmatrelvir, remdesivir and molnupiravir monotherapies and their combinations, nirmatrelvir-remdesivir and nirmatrelvir-molnupiravir in SARS-CoV-2 infected K18-hACE2 transgenic mice. (A) Schematic illustration of the study design. Mice were infected with 5 MLD50 of SARS-CoV-2 followed by administration of 20 mg/kg drugs for 5 consecutive days. Disease progression and other clinical parameters were monitored until 14 DPI, and the brain and lung were collected at 5 DPI for viral titer estimation and histopathological and immunohistochemical analyses. (B) Body weight was monitored daily for 14 days and is expressed as a percentage relative to the initial body weight on day 0. (C) Kaplan–Meier plot of the survival of all the groups (log-rank Mantel-Cox; **p < 0.01, ***p < 0.001). (D) The clinical score was evaluated by assessing appearance (fur, eye closure), activity and movement from 0 DPI to 14 DPI, and the cumulative clinical score of mice in each group is indicated.
Fig. 2.
Evaluation of the viral load in SARS-CoV-2 infected mice treated with nirmatrelvir, remdesivir and molnupiravir monotherapies, their combinations, nirmatrelvir-remdesivir and nirmatrelvir-molnupiravir. (A) Lungs and brains were collected at 5 DPI, and viral titers were estimated. The viral titer is expressed in log10 PFU/mL, and the limit of detection is shown in a dotted line (one-way ANOVA with Tukey's multiple comparison test; *p < 0.05, **p < 0.01). (B) Viral RdRp gene quantification in both the lungs and brain at 5 DPI was determined via RT–qPCR and results are expressed in 40-Ct (One-way ANOVA with Tukey's multiple comparison test; *p < 0.05, **p < 0.01, ***p < 0.001). (C) Viral N gene quantification in both the lung and brain at 5 DPI via RT–qPCR. The data are expressed in 40-Ct (one-way ANOVA with Tukey's multiple comparison test; *p < 0.05, **p < 0.01, ***p < 0.001).
Fig. 3.
Histopathology and immunohistochemistry analysis of SARS-CoV-2 infected mice treated with 20 mg/kg body weight nirmatrelvir, remdesivir and molnupiravir monotherapy and their combinations, nirmatrelvir-remdesivir and nirmatrelvir-molnupiravir. (A) Pathological changes and virus distribution were measured at 5 DPI in the lung tissue by H&E staining and immunohistochemical staining with an anti-nucleocapsid antibody, respectively. Images are shown at both low (5X) and high (20X) power resolution. (B) Neuronal death and virus distribution were measured at 5 DPI in brain tissue by H&E staining and immunohistochemistry, respectively. Images are shown at low (10X) and high (20X) power resolutions.
3.2. The combination of nirmatrelvir and remdesivir demonstrated less antiviral activity, with a lower survival rate, in an in vivo model
Combination treatment is generally considered more effective than single-drug treatment, so we examined the therapeutic benefits of NTV and RDV combination therapy in SARS-CoV-2 infected mice. Unfortunately, the combination of NTV and RDV did not result in any significant benefits and yielded the same or less antiviral action as NTV monotherapy. Despite the lack of superior protection afforded by this combination regarding weight loss compared to that with a mock treatment and RDV treatment (Fig. 1B), it resulted in approximately 7% lower survival compared to that with NTV monotherapy (Fig. 1C). As shown in Fig. 2, it also failed to significantly inhibit viral replication in both the lungs and brain compared to that mock-treatment (Fig. 2). Although the overall pathological changes in the lungs and brain of the combination-treated mice were similar to those of mock-treated and RDV treated mice at 5 DPI (Fig. 3).
3.3. Nirmatrelvir and molnupiravir combination therapy conferred significantly enhanced survival to SARS-CoV-2 infected mice
We further tested another combination with the same therapeutic targets, NTV and MPV to determine if the result would be beneficial. Hence, we administered NTV together with MPV (20 mg/kg body weight each) to mice after SARS-CoV-2 infection and observed more promising results than those with either of the drugs administered alone. The NTV-MPV combination treatment exhibited protective efficacy against weight loss (15.5%) compared to that mock treatment (21.5%, P = 0.0176) at 7 DPI (Fig. 1B) which is the only group that showed significance (Fig. S3). Remarkably, almost 80% survival probability was observed by NTV-MPV treated group, exhibiting synergetic and significant protection compared to that with NTV (36%, P < 0.001) or MPV (43%, P < 0.001) monotherapy (Fig. 1C). Similarly, the improvement in survival associated with this combination was substantially greater than that associated with RDV monotherapy (P < 0.001) and NTV-RDV combination (P < 0.001) treatment. In addition, the NTV-MPV combination drug-treated animals exhibit the least decline in the clinical score, as did other treated animals (Fig. 1D). The NTV-MPV combination significantly reduced the log10 TCID50 viral titers by 1.4 in the lung (P = 0.0019) and 4.2 in the brain (P = 0.0091) at 5 DPI compared to that with mock treatment (Fig. 2A). Additionally, with the combination therapy, the virus titer was reduced by 0.65, 0.9, 0.45, or 0.95 log10 TCID50 in the lung compared to that with the NTV, RDV and MPV monotherapies and NTV-RDV combination, respectively. The viral RNA replication was significantly inhibited in the NTV-MPV treated group than in the other treatment groups except for MPV treated for both RdRp and N genes in the lung, whereas it was significant with the mock and RDV treated groups in the brain (Fig. 2B and C). Excessive viral titer reduction in the brains of NTV-MPV treated mice compared to other groups revealed that administration of the NTV-MPV combination may be more beneficial than other therapies in reducing the lung virus load and preventing the spread of SARS-CoV-2 beyond the respiratory system. The most effective reduction of bronchiolitis, alveolitis and encephalitis with neutrophils and macrophages was observed in the NTV-MPV combination-treated animals at 5 DPI (Fig. 3). The NTV-MPV combination-treated groups exhibited greater recovery of lung impairment, lung inflammation and other related issues at 14 DPI than the other treated groups (Fig. S4).
3.4. NTV and MPV concentrations are sufficient in mice receiving combination therapy
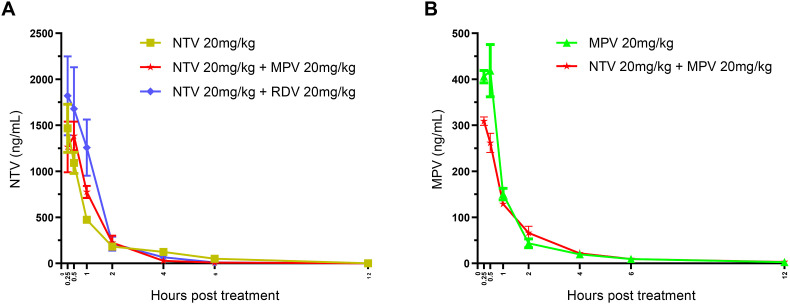
Mice were treated with NTV, MPV, RDV, or their combinations, and the concentrations of NTV, MPV, and RDV in blood were measured. NTV was detected in blood from 0.25 to 6 h, and MPV (EIDD-1931) was detected for up to 12 h, whereas RDV was not present, even at 0.25 h. The NTV concentration peaked at 0.25 h in mice administered NTV alone, NTV-RDV and NTV-MPV combinations and continued to decrease for up to 6 h (Fig. 4 A). The average plasma concentrations of NTV were stable in mice administered NTV alone (484.18 ng/mL) and NTV-MPV (526.10 ng/mL) combination, but increased in mice administered NTV-RDV (720.70 ng/mL) combination. The concentration of the MPV prodrug EIDD-1931 also peaked at 0.25 h, followed by a continuous decrease for up to 12 h (Fig. 4B). Similar to NTV, the average plasma concentrations of MPV were stable in mice administered MPV alone (150.00 ng/mL) and NTV-MPV combination (114.26 ng/mL).
Fig. 4.
In vivo plasma pharmacokinetic analysis of mice treated with NTV, RDV and MPV mono and NTV-RDV and NTV-MPV combinations. (A) Plasma pharmacokinetic profiles of NTV in mice administered NTV alone, NTV-MPV and NTV-RDV combinations at 0.25, 0.5, 1, 2, 4, 6 and 12 h. (B) Plasma concentrations of MPV (ng/mL) in mice administered MPV alone and NTV-MPV combination at 0.25, 0.5, 1, 2, 4, 6 and 12 h.
4. Discussion
Despite the widespread availability of vaccines, the unrestrained SARS-CoV-2 pandemic needs the advancement of effective antiviral therapy, especially for high-risk groups (Fontanet et al., 2021). In October 2020, the FDA approved RDV and recommended the treatment of hospitalized patients with severe COVID-19 in many countries. Later, MPV was recognized for its clinical benefits for patients who have been administered MPV orally (Cox et al., 2021; Jayk Bernal et al., 2022; Rosenke et al., 2021; Wahl et al., 2021). NTV is an essential component of Paxlovid, which impedes the main protease of SARS-CoV-2 and has recently been reported to reduce the risk of hospitalization or death in COVID-19 patients by 89% (Mahase, 2021; Owen et al., 2021). Antiviral therapy with a combination of drugs that can target viral replication and entry machinery components is more efficient in eradicating viruses. As such, we evaluated the therapeutic efficacy of NTV, RDV and MPV and their combinations in SARS-CoV-2 infected K18-hACE2 transgenic mice and demonstrated the superior antiviral activity of the NTV-MPV combination than NTV, MPV, RDV and NTV-RDV.
The K18-hACE2 mouse is an ideal model with severe pathological changes in both the lung and brain, morbidity and mortality, as previously reported (Bauer et al., 2022; Winkler et al., 2020). Upon SARS-CoV-2 infection, these mice showed a sensitive and rapid response to viral replication, clinical parameters, weight loss by 4 or 5 DPI, and lethality by 7–8 DPI, whereas other models exhibit a mild response (Dong et al., 2022). As described in previous studies (Douglas et al., 2018; Sheahan et al., 2017), all NTV, RDV and MPV mono-treated animals also exhibited similar dynamics in terms of weight loss, viral titer, pulmonary function, lung pathology and virus-induced death. Comparable weight loss was observed between mock and RDV treated animals, whereas NTV and MPV treated animals had lower body weight loss and higher body weight recovery. Furthermore, mice treated with MPV had a lower viral burden and genomic viral RNA level than mice treated with other drugs, which was consistent with a previous report and indicated the ability of MPV to reduce virus replication (Fischer et al., 2021). As viral burden and clinical pathology correlate, our study is also reminiscent of this phenomenon and showed that mice treated with MPV exhibited decreased clinical severity, with improvement in the clinical score, pathophysiological changes and viral distribution compared with those in other single treatment groups. According to several studies, NTV and RDV antivirals exhibited survival rates of 36% and 21%, respectively, while MPV treated animals had approximately 7 and 22% higher survival rates than NTV and RDV treated animals, respectively (Gottlieb et al., 2022; Jayk Bernal et al., 2022; Reis et al., 2022). In support of previous studies (Consortium, 2021, 2022; Ohl et al., 2021; Singh et al., 2021), our in vivo study also clearly illuminates the survival benefits unrelated to RDV monotherapy, which resulted in only 21% survival, although it may be of clinical significance because it has yielded improved weight loss recovery and clinical scores. Collectively, we confirmed that the three antivirals have therapeutic significance against SARS-CoV-2 pathogenesis in the K18-hACE2 mouse model, with lower protection afforded by RDV and higher protection afforded by MPV. RDV was not present in the plasma of mice, even at earlier time points such as 0.25 h. This was due to the presence of the enzyme carboxylesterase 1c (Ces1c), which markedly degrades RDV before it reaches tissues (Sheahan et al., 2017, 2020). Because its exposure in plasma was lower, RDV had reduced antiviral activity when administered alone or combined with NTV.
Patients with SARS-CoV-2 have been treated with combinations of NTV and ritonavir (RTV). The present study evaluated the efficacy of NTV alone against SARS-CoV-2 infected mice. The plasma exposure of NTV was both sustained and sufficient, resulting in significant and effective antiviral activity. Future studies are needed to evaluate the in vivo efficacy of NTV plus RTV in K18-hACE2 transgenic mice, as well as MPV alone and combined with Paxlovid. MPV also showed sufficient plasma concentrations for 12 h after administration. The adequate plasma concentrations of NTV and MPV, both individually and together, were indicative of the improved antiviral activity of the NTV - MPV combination. The in vivo pharmacokinetic profiles of this combination are indicative of therapeutic benefits, suggesting that this combination would be both appropriate and effective in the treatment of SARS-CoV-2 virus infected patients.
The antiviral potency of the drug combinations against SARS-CoV-2 viral infection in vitro has been reported (Li et al., 2022; Rosales et al., 2022); however, there are limited studies available on FDA-approved drugs in vivo models. This study was the first to examine the value of combination therapy with NTV-RDV and NTV-MPV combinations against SARS-CoV-2 infection in an animal model and, more importantly, the ability of combination therapy to extend the treatment window. We reported here that the drug combinations target the same viral proteins; however, NTV-MPV yielded significantly better viral inhibition and clinical scores than NTV-RDV, emphasizing the importance of the NTV-MPV combination in preventing the clinical progression of the disease. Interestingly, the clinical score associated with NTV-RDV was significantly lower than that with NTV and MPV monotherapy, which is a considerable factor when recommending this combination therapy for the treatment of SARS-CoV-2 infection. The favipiravir and MPV combination has recently been reported to reduce the viral load better than monotherapy in SARS-CoV-2 infected mice (Abdelnabi et al., 2021). The reduced viral load with the NTV-MPV combination is superior to that in animals treated with the other drugs at 5 DPI, representing the clinical significance of this combination in the clearance of the virus. Combinations of different drugs, such as ribavirin-favipiravir and oseltamivir-azithromycin in Lassa virus and influenza-infected mice, respectively, substantially extended the survival period and led to a better survival rate compared to those with monotherapy (Ishaqui et al., 2020; Oestereich et al., 2016). Mice treated with the NTV-MPV combination had a significantly reduced viral distribution in the lungs as well as in the brain and ultimately exhibited 80% survival, while mice treated with other drugs exhibited a lower (<45%) survival rate. Moreover, this achieved survival rate is more pronounced than expected from the additive activity of NTV and MPV monotherapies. Together, these data reveal that the administration of the NTV-MPV combination can considerably diminish virus replication in the early onset of the disease, where the predominant viral load is usually expected, limiting deleterious COVID-19 infectious events.
5. Conclusions
Combination therapy has been associated with the acquisition of fewer genomic mutations; however, there is an advantage to examining the genetic mutation of the SARS-CoV-2 virus against this combination therapy. Our study demonstrated that three FDA-approved antiviral drugs had therapeutic potential by diminishing the viral burden, rescuing the lung and brain pathology, and ultimately improving life expectancy by 20–40%. The NTV-MPV combination was highly protective against lethal SARS-CoV-2 infection and yielded a survival rate of approximately 80% by controlling all pathological features associated with morbidity and mortality. Combination therapy with different drugs affects different portions of the viral life cycle and may be a beneficial strategy based on their mechanisms of action against SARS-CoV-2 infection. We achieved such a potential effect while applying a combinatorial approach with NTV-MPV combination therapy and presented in vivo evidence of the potential utility of this combination in treating SARS-CoV-2 patients.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the Korea National Institute of Health, the Korea Disease Control and Prevention Agency (2021-ERG1903-00 to M-.S.S.), and the National Research Foundation of Korea (NRF-2021R1A2C2006961 to M-.S.S. and NRF-2020R1A5A2017476 to M-.S.S.).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.antiviral.2022.105430.
Appendix ASupplementary data
The following is the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Abdelnabi R., Foo C.S., Kaptein S.J., Zhang X., Do T.N.D., Langendries L., Vangeel L., Breuer J., Pang J., Williams R. The combined treatment of Molnupiravir and Favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model. EBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanbi M.O., Scarsi K., Taiwo B., Murphy R.L. Combination of nucleoside/nucleotide reverse transcriptase inhibitors for treatment of HIV infection. Expet Opin. Pharmacother. 2012;13:65–79. doi: 10.1517/14656566.2012.642865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer L., Laksono B.M., de Vrij F.M., Kushner S.A., Harschnitz O., van Riel D. The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci. 2022 doi: 10.1016/j.tins.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrowski T., Chen L., Eastman R.T., Itkin Z., Shinn P., Chen C.Z., Guo H., Zheng W., Michael S., Simeonov A. Synergistic and antagonistic drug combinations against SARS-CoV-2. Mol. Ther. 2021;29:873–885. doi: 10.1016/j.ymthe.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Beaubrun A., Bashyal R., Huang A., Li J., Baser O. Real-world adherence and persistence for newly-prescribed HIV treatment: single versus multiple tablet regimen comparison among US medicaid beneficiaries. AIDS Res. Ther. 2020;17:1–12. doi: 10.1186/s12981-020-00268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium W.S.T. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N. Engl. J. Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium W.S.T. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet. 2022;399:1941–1953. doi: 10.1016/S0140-6736(22)00519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.M., Wolf J.D., Plemper R.K. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat. Microbiol. 2021;6:11–18. doi: 10.1038/s41564-020-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Mead H., Tian L., Park J.-G., Garcia J.I., Jaramillo S., Barr T., Kollath D.S., Coyne V.K., Stone N.E. The K18-human ACE2 transgenic mouse model recapitulates non-severe and severe COVID-19 in response to an infectious dose of the SARS-CoV-2 virus. J. Virol. 2022;96 doi: 10.1128/JVI.00964-21. e00964-00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M.G., Kocher J.F., Scobey T., Baric R.S., Cockrell A.S. Adaptive evolution influences the infectious dose of MERS-CoV necessary to achieve severe respiratory disease. Virology. 2018;517:98–107. doi: 10.1016/j.virol.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W.A., Eron J.J., Holman W., Cohen M.S., Fang L., Szewczyk L.J., Sheahan T.P., Baric R.S., Mollan K.R., Wolfe C.R. MedRxiv; 2021. Molnupiravir, an Oral Antiviral Treatment for COVID-19. [Google Scholar]
- Fontanet A., Autran B., Lina B., Kieny M.P., Karim S.S.A., Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet. 2021;397:952–954. doi: 10.1016/S0140-6736(21)00370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb R.L., Vaca C.E., Paredes R., Mera J., Webb B.J., Perez G., Oguchi G., Ryan P., Nielsen B.U., Brown M. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N. Engl. J. Med. 2022;386:305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishaqui A.A., Khan A.H., Sulaiman S.A.S., Alsultan M.T., Khan I., Naqvi A.A. Assessment of efficacy of Oseltamivir-Azithromycin combination therapy in prevention of Influenza-A (H1N1) pdm09 infection complications and rapidity of symptoms relief. Expet Rev. Respir. Med. 2020;14:533–541. doi: 10.1080/17476348.2020.1730180. [DOI] [PubMed] [Google Scholar]
- Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., Martín-Quirós A., Caraco Y., Williams-Diaz A., Brown M.L. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N. Engl. J. Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli A., Osinusi A., Sims Z., Nelson A., Meissner E.G., Barrett L.L., Bon D., Marti M.M., Silk R., Kotb C. Virological response after 6 week triple-drug regimens for hepatitis C: a proof-of-concept phase 2A cohort study. Lancet. 2015;385:1107–1113. doi: 10.1016/S0140-6736(14)61228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.-H., Kwon J.-H., Noh J.-Y., Park J.-K., Yuk S.-S., Erdene-Ochir T.-O., Nahm S.-S., Kwon Y.-K., Lee S.-W., Song C.-S. Viscerotropic velogenic Newcastle disease virus replication in feathers of infected chickens. J. Vet. Sci. 2016;17:115–117. doi: 10.4142/jvs.2016.17.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Wang Y., Lavrijsen M., Lamers M.M., de Vries A.C., Rottier R.J., Bruno M.J., Peppelenbosch M.P., Haagmans B.L., Pan Q. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022;32:322–324. doi: 10.1038/s41422-022-00618-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. British Medical Journal Publishing Group; 2021. Covid-19: Pfizer's Paxlovid Is 89% Effective in Patients at Risk of Serious Illness, Company Reports. [DOI] [PubMed] [Google Scholar]
- Mody V., Ho J., Wills S., Mawri A., Lawson L., Ebert M.C., Fortin G.M., Rayalam S., Taval S. Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Commun. Biol. 2021;4:1–10. doi: 10.1038/s42003-020-01577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestereich L., Rieger T., Lüdtke A., Ruibal P., Wurr S., Pallasch E., Bockholt S., Krasemann S., Muñoz-Fontela C., Günther S. Efficacy of favipiravir alone and in combination with ribavirin in a lethal, immunocompetent mouse model of Lassa fever. J. Infectious Dis. 2016;213:934–938. doi: 10.1093/infdis/jiv522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl M.E., Miller D.R., Lund B.C., Kobayashi T., Miell K.R., Beck B.F., Alexander B., Crothers K., Sarrazin M.S.V. Association of remdesivir treatment with survival and length of hospital stay among US veterans hospitalized with COVID-19. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.14741. e2114741-e2114741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization W.H. 2022. WHO Coronavirus (COVID-19) Dashboard. [Google Scholar]
- Owen D.R., Allerton C.M., Anderson A.S., Aschenbrenner L., Avery M., Berritt S., Boras B., Cardin R.D., Carlo A., Coffman K.J. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938;27:493–497. [Google Scholar]
- Reis S., Popp M., Kuehn R., Metzendorf M.-I., Gagyor I., Kranke P., Meybohm P., Skoetz N., Weibel S. Nirmatrelvir combined with ritonavir for preventing and treating COVID-19. Cochrane Database Syst. Rev. 2022 doi: 10.1002/14651858.CD015395.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales R., McGovern B.L., Rodriquez M.L., Rai D.K., Cardin R.D., Anderson A.S., Sordillo E.M., van Bakel H., Simon V., Garcia-Sastre A. Nirmatrelvir, molnupiravir, and remdesivir maintain potent in vitro activity against the SARS-CoV-2 Omicron variant. bioRxiv. 2022 [Google Scholar]
- Rosenke K., Hansen F., Schwarz B., Feldmann F., Haddock E., Rosenke R., Barbian K., Meade-White K., Okumura A., Leventhal S. Orally delivered MK-4482 inhibits SARS-CoV-2 replication in the Syrian hamster model. Nat. Commun. 2021;12:1–8. doi: 10.1038/s41467-021-22580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Khera D., Chugh A., Khera P.S., Chugh V.K. Efficacy and safety of remdesivir in COVID-19 caused by SARS-CoV-2: a systematic review and meta-analysis. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-048416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanciu C., Muzica C.M., Girleanu I., Cojocariu C., Sfarti C., Singeap A.-M., Huiban L., Chiriac S., Cuciureanu T., Trifan A. An update on direct antiviral agents for the treatment of hepatitis C. Expet Opin. Pharmacother. 2021;22:1729–1741. doi: 10.1080/14656566.2021.1921737. [DOI] [PubMed] [Google Scholar]
- Toots M., Yoon J.-J., Cox R.M., Hart M., Sticher Z.M., Makhsous N., Plesker R., Barrena A.H., Reddy P.G., Mitchell D.G. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aax5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBlargan L.A., Errico J.M., Halfmann P.J., Zost S.J., Crowe J.E., Purcell L.A., Kawaoka Y., Corti D., Fremont D.H., Diamond M.S. An infectious SARS-CoV-2 B. 1.1. 529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat. Med. 2022;28:490–495. doi: 10.1038/s41591-021-01678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl A., Gralinski L.E., Johnson C.E., Yao W., Kovarova M., Dinnon K.H., Liu H., Madden V.J., Krzystek H.M., De C. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591:451–457. doi: 10.1038/s41586-021-03312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Fan G., Salam A., Horby P., Hayden F.G., Chen C., Pan J., Zheng J., Lu B., Guo L. Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection. J. Infectious Dis. 2020;221:1688–1698. doi: 10.1093/infdis/jiz656. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li P., Solanki K., Li Y., Ma Z., Peppelenbosch M.P., Baig M.S., Pan Q. Viral polymerase binding and broad-spectrum antiviral activity of molnupiravir against human seasonal coronaviruses. Virology. 2021;564:33–38. doi: 10.1016/j.virol.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm A., Widera M., Grikscheit K., Toptan T., Schenk B., Pallas C., Metzler M., Kohmer N., Hoehl S., Helfritz F.A. Reduced neutralization of SARS-CoV-2 Omicron variant by vaccine sera and monoclonal antibodies. medRxiv. 2021 [Google Scholar]
- Winkler E.S., Bailey A.L., Kafai N.M., Nair S., McCune B.T., Yu J., Fox J.M., Chen R.E., Earnest J.T., Keeler S.P. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 2020;21:1327–1335. doi: 10.1038/s41590-020-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Cao L., Xu W., Xu T., Zheng B., Ji Y., Huang S., Liu L., Du J., Peng H. Comparison of model‐specific histopathology in mouse models of COVID‐19. J. Med. Virol. 2022 doi: 10.1002/jmv.27747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Wong L.-Y.R., Li K., Verma A.K., Ortiz M.E., Wohlford-Lenane C., Leidinger M.R., Knudson C.M., Meyerholz D.K., McCray P.B. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature. 2021;589:603–607. doi: 10.1038/s41586-020-2943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.