Abstract
Cerebrovascular effects of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) inactivation have not been systematically studied. In the present study we employed cultured human brain microvascular endothelial cells (BMECs), BACE1-knockout (BACE1−/−) mice and conditional (tamoxifen-induced) endothelium-specific BACE1-knockout (eBACE1−/−) mice to determine effect of BACE1 inhibition on expression and function of endothelial nitric oxide synthase (eNOS). Deletion of BACE1 caused upregulation of eNOS and glypican-1 (GPC1) in human BMECs treated with BACE1-siRNA, and cerebral microvessels of male BACE1−/− mice and male eBACE1−/− mice. In addition, BACE1siRNA treatment increased NO production in human BMECs. These effects appeared to be independent of amyloid β-peptide production. Furthermore, adenoviral-mediated overexpression of BACE1 in human BMECs down-regulated GPC1 and eNOS. Treatment of human BMECs with GPC1siRNA suppressed mRNA and protein levels of eNOS. In basilar arteries of male eBACE1−/− mice, endothelium-dependent relaxations to acetylcholine and endothelium-independent relaxations to NO donor, DEA-NONOate, were not affected, consistent with unchanged expression of eNOS and phosphorylation of eNOS at Ser1177 in large cerebral arteries. In aggregate, our findings suggest that under physiological conditions, inactivation of endothelial BACE1 increases expression of eNOS in cerebral microvessels but not in large brain arteries. This effect appears to be mediated by increased GPC1 expression.
Keywords: BACE1, eNOS, endothelial cells, glypican-1, Alzheimer’s disease
In the cerebral circulation, endothelial nitric oxide synthase (eNOS) is an essential enzyme responsible for production of vascular protective molecule nitric oxide (NO).1,2 Prior studies have established that loss of endothelial NO promotes development of Alzheimer’s disease (AD) pathology. 3 Indeed, impaired production of NO causes dysregulation of amyloid precursor protein (APP) metabolism manifested by increased expression of endothelial β-site APP cleaving enzyme 1 (BACE1) and production of amyloid β (Aβ) peptides in the cerebral blood vessels and neuronal tissue.4–6 In addition, endothelial NO deficiency in the cerebral circulation activates microglia, promotes neuronal Tau phosphorylation, and causes impairment of spatial memory.3,7,8 These observations support the concept that chronic loss of cerebrovascular endothelial NO is an important contributor to the development of AD pathology.
Previous studies demonstrated that BACE1 is expressed in the brain endothelium6,9–11 however, the exact function of BACE1 in endothelial cells is incompletely understood. This gap in knowledge is critically important because BACE1 inhibitors are still considered potentially important therapeutic approach to prevention and treatment of AD. 12 In this study we examined the effects of BACE1 suppression on vascular endothelial function with particular focus on the effects of genetic inactivation of endothelial BACE1 on expression and function of eNOS.
Glypicans (GPCs), are membrane heparan sulfate proteoglycans anchored to the extracellular surface, and they play important roles in regulating cell behavior. 13 GPC1 is known to have beneficial effects on endothelial cell survival and preservation of endothelial integrity.13,14 Indeed, GPC1 has been shown to protect endothelium from dysfunction induced by aging. 15 Most importantly, several prior studies suggest that GPC1 may participate in control of eNOS function including shear stress-induced activation of eNOS.14,16–23 Our interest in GPC1 was stimulated by the observation demonstrating that genetic deletion of BACE1 causes accumulation of GPC1-like protein (Zgc:63947) in the brain of BACE1-knockout zebrafish. 24 However, the role of GPC1 in BACE1-regulated eNOS expression has not been studied. Our results support the concept that GPC1 mediates increase in eNOS expression induced by inhibition of endothelial BACE1.
Material and methods
Mice
All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Mayo Clinic. Experimental protocols complied with the National Institute of Health and the ARRIVE 2.0 guidelines. 25
Homozygous BACE1-deficient (BACE1−/−) mice (B6.129-Bace11mPcw/J) on C57BL/6 background (Model #004714) and C57BL/6 mice (Model #000664) were obtained from the Jackson Laboratory (Bar Harbor, ME) and were bred to generate heterozygous BACE1+/− mice, which then were used to generate wild type (WT) littermates and BACE1−/− off springs. All mice were maintained on 12 h/12 h light/dark cycle, with free access to drinking water and on standard chow. PCR was performed to identify the genotype by using wild-type and BACE1 primers designed by the Jackson Laboratory and purchased from Integrated DNA Technologies, Inc. (Coralville, Iowa). Male mice (3–5 months old) were used in the experiments. We also performed several experiments on female BACE1−/− mice (3–6 months old; please see supplemental material).
Breeding of tamoxifen-inducible endothelium-specific BACE1−/− (eBACE1−/−) mice: the heterozygous BACE1flox/+ mice on C57BL/6 background (Model #8263) were purchased from Taconic Biosciences Inc. (Rensselaer, NY) and were bred to generate floxed BACE1 (BACE1flox/flox) mice in our laboratory. These mice were genotyped using primers provided by Taconic. Female BACE1flox/flox mice were crossed with male Cdh5(PAC)-CreERT2 (Cdh5-Cre) mice (Model #13073, Taconic) to generate BACE1flox/+;Cdh5-Cre− and BACE1flox/+; Cdh5-Cre+ mice. The latter were used to breed with female BACE1flox/flox mice to generate BACE1flox/flox; Cdh5-Cre− (WT littermates) mice and BACE1flox/flox; Cdh5-Cre+ (eBACE1−/−) mice. The genotyping was performed by using Cdh5-Cre primers provided by Taconic. Male eBACE1−/− (5−12 weeks old) were fed with tamoxifen contained chow diet (400 mg tamoxifen citrate per kg diet [TD.130859], Envigo, Indianapolis, IN) for 8−10 weeks. Age-matched male WT littermates were used as controls. Mice were 3−5.5 months old when blood samples and vasculature samples were collected. In order to confirm endothelium-specific deletion of BACE1, we isolated total RNA from endothelial cells of mouse aorta, and reverse-transcribed RNA to cDNA, as described in previous study. 26 Reverse transcription PCR was performed using primers spanning the deletion site (exon 2). Primer sequences for BACE1 (NCBI accession number NM_001145947.2) were: forward 5′-TTTGTGGAGATGGTGGACAA-3′, and reverse 5′-GGACAGCTGCCTCTGGTAGT-3′. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, NCBI access number NM_001289726.1) primers (forward: 5′-AGAACATCATCCCTGCATCC-3′ and reverse: 5′-GGTCCTCAGTGTAGCCCAAG-3′) were used as reference controls. The following PCR conditions were used: 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min (30 cycles).
Mice were trained for blood pressure measurement as described. 27 Systolic blood pressure and mean blood pressure were recorded in non-anesthetized quiescent mice by a tail-cuff method (Harvard Apparatus, Kent, England).
Glucose and lipid profile
Mice were anaesthetized with overdoses of pentobarbital (200–250 mg/kg BW, intraperitoneal). Blood samples were collected by right ventricle puncture and transferred to a tube containing EDTA. Blood glucose levels were measured immediately with ACCU-CHEK (Roche Diagnostics, Indianapolis, IN). After blood samples were centrifuged at 2000 rpm at 4°C for 10 min, the supernatants were collected and stored at −80°C. Plasma levels of cholesterol, HDL, and triglyceride were measured on the Hitachi 912 chemistry analyzer (Roche Diagnostics).
Aβ1–40 and Aβ1–42 measurement
Plasma levels of Aβ1-40 or Aβ1-42 were measured using a mouse Aβ1-40 ELISA kit or a mouse Aβ1-42 ELISA kit, respectively (Invitrogen, Camarillo, CA), according to the manufacturer’s protocols.
Isolation and collection of cerebral blood vessels
Cerebral microvessels were isolated as described in previous studies. 28 Briefly, after mouse brain was collected and placed in the cold (4°C) modified Krebs–Ringer bicarbonate solution (in mmol/L: NaCl 118.6; KCl 4.7; CaCl2 2.5; MgSO4 1.2; KH2PO4 1.2; NaHCO3 25.1; glucose 10.1; EDTA 0.026), anterior cerebral, posterior cerebral, middle cerebral and basilar arteries were removed. Cerebral microvessels were then isolated from whole brain by 15% Dextran (Sigma-Aldrich, St. Louis, MO) centrifugation, as previously described. 28 In some experiments, microvessels of eBACE1−/− mice were isolated from the same mice from which large cerebral arteries or basilar arteries were obtained.
For studies of vascular reactivity, basilar arteries were isolated and dissected free from surrounding tissues in cold modified Krebs–Ringer bicarbonate solution under a microscope.
For Western Blot analysis of large cerebral arteries, middle cerebral, anterior cerebral, posterior cerebral arteries, and basilar arteries were removed from the brain and dissected free from surrounding tissue in cold modified Krebs–Ringer bicarbonate solution under a microscope. Isolated arteries obtained from one mouse were pooled into one sample.
Vasomotor reactivity studies of basilar arteries
Basilar artery was dissected free from surrounding tissues and was transferred to a small vessel chamber filled with 37°C modified Krebs–Ringer bicarbonate solution. Transmural pressure was set at 30 mmHg, and basilar arteries were equilibrated for 45 minutes, as described in previous study. 29 Vascular reactivity was studied ex-vivo using a video dimension analyzer system (Living Systems Instrumentation, Burlington, VT) as described in the previous study. 29 Endothelium-dependent relaxations to acetylcholine (10−9–10−5 mol/L; Sigma, St. Louis, MO) and endothelium-independent relaxations to the NO donor diethylammonium (Z)-1-(N,N-diethylamino) diazen-1-ium-1,2-diolate (DEA-NONOate; 10−9–10−5 mol/L; Cayman Chemical, Ann Arbor, MI) were obtained during submaximal contractions to 9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α [U46619, a synthetic analog of prostaglandin H2 (PGH2) analogue and a stable thromboxane A2 receptor agonist, 3 × 10−8–3 × 10−7 mol/L; Cayman Chemical]. Between each protocol, the system was washed out with Krebs solution and then equilibrated for 30 minutes. Concentration-dependent contractions to U46619 (10−9–10−6 mol/L; Cayman Chemical) and endothelin-1 (ET-1, 10−11–10−7 mol/L; EMD Millipore, Billerica, MA) were also obtained.
Cell culture
Primary human brain microvascular endothelial cells (BMECs, from a single donor (24 years old, sex unknown) purchased from Applied Cell Biology Research Institute (Kirkland, WA), were grown in endothelial growth medium 2 (EGM2; Lonza, Allendale, NJ) containing endothelial basal medium 2 (EBM2; Lonza, Allendale, NJ) supplemented with 2% fetal bovine serum, fibroblast growth factor, vascular endothelial growth factor, insulin-like growth factor, epidermal growth factor, ascorbic acid, hydrocortisone, bac-off and heparin. Passages 4–6 were used. In some experiments, human BMECs were treated with β-secretase inhibitor IV (BACE inhibitor IV, CAS 797035-11-1, Calbiochem, EMD Millipore Corp., Billerica, MA) at concentration of 0.1 or 0.2 µM, for 24 hours. Then, the cell samples were collected for Western blot. BACE inhibitor IV has selectivity for BACE1 versus BACE2 (IC50 = 0.015 µM for BACE1; IC50 = 0.23 µM for BACE2). 30
Knockdown of BACE1 and GPC1 by small interfering RNA (siRNA)
Experiments were performed by use of Lipofectamine 2000 (Invitrogen) as described in the previous study. 28 BACE1siRNA targeting human BACE1 mRNA (ON-TARGETplus #9), GPC1siRNA targeting human GPC1 mRNA (ON-TARGETplus #19), and Control-siRNA (ON-TARGETplus Non-targeting siRNA #1) were obtained from Dharmacon, Horizon (Lafayette, CO). Human BMECs were treated with siRNA 30 nM for 40 or 48 hours. RNA samples were isolated for PCR experiments, or protein samples were then collected for Western blot.
Overexpression of BACE1
Human BMECs were incubated with replication-deficient adenoviral constructs containing human BACE1 (Ad-BACE1, Vector Biolabs, Malvem, PA) at 2.5 multiplicities of infection (MOI) in 1.5 mL of EBM-2 for 8 hours. Cells were then recovered in EGM-2 for 2 days. For controls, cells were transduced with 2.5 MOI Adeno CMV Null adenovirus (Ad-Null, Vector Biolabs, Malvem, PA).
Western blot analysis
Western blot analysis was conducted as described in previous studies.28,29,31 Human BMECs, isolated cerebral microvessels, or large cerebral arteries were homogenized in lysis buffer (containing 50 mmol/L NaCl, 50 mmol/L NaF, 50 mmol/L sodium pyrophosphate, 5 mmol/L EDTA, 5 mmol/L EGTA, 0.1 mmol/L Na3VO4, 1% Triton X-100, 10 mmol/L HEPES, pH 7.4) and protease inhibitor cocktail (cat# P8340, Sigma-Aldrich, St. Louis, MO). The protein samples were then subjected to Western blot. Rabbit antibody against BACE1 was obtained from Cell Signaling Technology (Danvers, MA, cat# 5606, dilution 1:250 or 500). Mouse anti-glypican-1 (GPC1) was obtained from EMD Millipore Corporation (Temecula, CA, cat# MAB2600, dilution 1:250). PC-12 cell lysate (cat# 611454, a positive control for GPC1 antibody, recommended by EMD Millipore), mouse anti-eNOS (cat# 610297, dilution 1:250), mouse anti-phosphorylated eNOS at Ser1177 [p-eNOS(S1177), cat# 612393, dilution 1:250], mouse anti-phosphorylated eNOS at Thr495 [p-eNOS(T495), cat# 612706, dilution 1:250], and were purchased from BD Transduction Laboratories (San Jose, CA). Blots probed with mouse anti-β-actin (Sigma-Aldrich, cat# A5316, dilution 1:500 or 1:20000 for some mouse samples) were used as loading controls. Protein expression was normalized to β-actin. The ratio of p-eNOS(S1177)/eNOS of each sample was calculated after p-eNOS(S1177) or eNOS was normalized to β-actin. The ratio of p-eNOS(T495)/eNOS of each sample was also calculated after p-eNOS(T495) or eNOS was normalized to β-actin. The blots were imaged with LI-COR digital imaging analysis system (Odyssey Fc, Model 2800) and analyzed using Image Studio Software Version 5.0) (LI-COR, Inc., Lincoln, NE).
Real time-quantitative RCR (RT-qPCR)
RT-qPCR was performed as described in previous study. 28 After total RNA was isolated using RNeasy Plus Mini kit (Qiagen, Redwood City, CA), SuperScript III First-Strand Synthesis System kit (Invitrogen) was used to reverse transcribe RNA to cDNA. For quantification of human eNOS and GPC1 mRNA using Bio-Rad CFX Connect Real-Time System, PrimPCR SYBR Green Assay human eNOS, GPC1 and GAPDH (Bio-Rad, Coralville, IA) were used, according to manufacturer’s instruction.
Measurement of nitric oxide production
Human BMECs were treated with BACE1siRNA for 2 days, then conditioned media were collected after 24 hours incubation. Nitric oxide production by human BMECs were measured in conditioned media as total nitrite and nitrate (NO2 + NO3), using a commercially available fluorometric nitrite/nitrate assay kit according to manufacturer’s instructions (Cayman Chemical Co.).
Statistical analysis
Data are presented as mean ± standard deviation, “n” denotes number of independent experiments in cell culture, and the number of mice from which tissue samples were collected (n value indicates number of single values per animal). Differences between mean values of two groups were compared using unpaired Student t-test. Data sets were tested for normality using Shapiro-Wilk normality test and QQ plot analysis. Data sets (Western blot data, GPC1siRNA treatment, and Table 1 Aβ1-40) that were not normally distributed were analyzed by the Mann-Whitney U-test for non-parametric data (GraphPad Prism 9 software). For non-parametric data analysis of multiple groups, Kruskal-Wallis test followed by Mann-Whitney U-test were performed. For analysis of vasomotor reactivity of basilar arteries, efficacy (maximal responses) as well as the potency of the drugs (expressed as negative logarithm of the concentration that caused half-maximal response [pEC50 value]) was determined by non-linear regression analysis (GraphPad Prism 9 software). 32 P < 0.05 was considered statistically significant. Formal prior power calculations were not performed. Sample size determination was based on previous publications.28,29 The investigators were not blinded to genetic characteristics of mice. Investigators knew the group allocation during the experiment and assessing outcome.
Table 1.
Characteristics of male wild-type littermates and eBACE1−/− mice.
| Parameters | Cdh5-Cre−;BACE1flox/flox (WT) | Cdh5-Cre+;BACE1flox/flox (eBACE1−/−) |
|---|---|---|
| Tamoxifen (mg/kg BW/day) | 44 ± 8 (23) | 42 ± 7 (25) |
| BW (g) | 22 ± 2 (16) | 23 ± 2 (16) |
| SBP (mmHg) | 121 ± 4 (7) | 117 ± 8 (7) |
| MBP (mmHg) | 94 ± 3 (7) | 90 ± 6 (7) |
| DBP (mmHg) | 80 ± 4 (7) | 76 ± 5 (7) |
| Glucose (mg/dL) | 170 ± 24 (8) | 179 ± 17 (8) |
| Aβ1–40 (pg/mL) | 226 ± 45 (10) | 194 ± 29 (10)* |
| Aβ1–42 (pg/mL) | 57 ± 7 (12) | 50 ± 6 (12)** |
BW: body weight; SBP: systolic blood pressure; MBP: mean blood pressure; DBP: diastolic blood pressure; Aβ: amyloid-β; WT: wild-type. Data are means ± SD and the numbers of mice are indicated in the parentheses.
*P < 0.05 VS. WT littermates (Mann-Whitney U-test for non-parametric data).
**P < 0.05 vs. WT littermates (unpaired t-test).
Results
Stimulatory effect of BACE1-inhibtion on eNOS expression and NO production in human BMECs
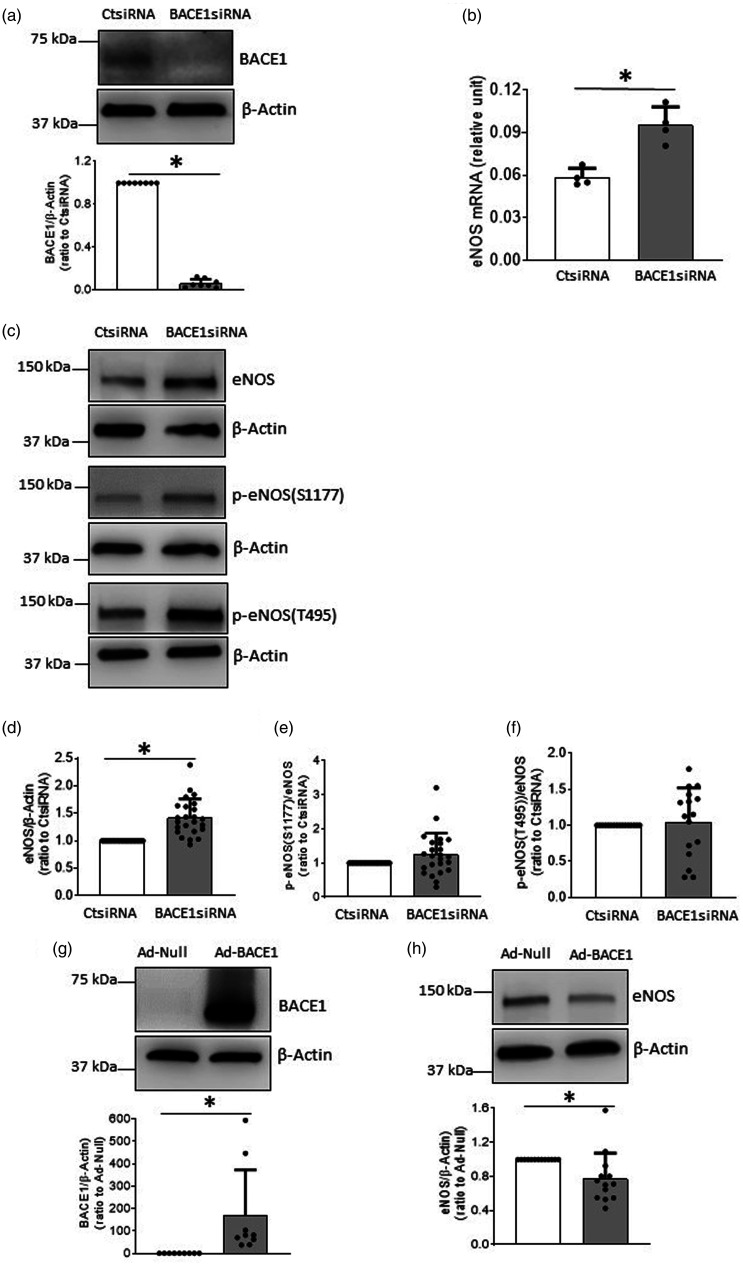
We first examined the effects of genetic inhibition of BACE1 in cultured human BMECs. During cell culturing, endothelial cells maintained their normal cobblestone appearance under microscope. We did not observe any apparent morphological changes in BACE1siRNA-treated cells, compared to CtsiRNA-treated cells. As expected, BACE1 protein expression was significantly reduced in BMECs treated with BACE1siRNA, (Figure 1(a)). In contrast, mRNA and protein levels of eNOS were significantly increased (Figure 1(b) to (d)). However, levels of p-eNOS[(S1177) [up-regulating eNOS activity, calculated as p-eNOS(S1177)/eNOS] and p-eNOS(T495) [down-regulating eNOS activity, calculated as p-eNOS(T495)/eNOS] were not affected (Figure 1(c), (e) and (f)). Next, we overexpressed BACE1 in human BMECs. Transduction of cells with Ad-BACE1 significantly suppressed eNOS protein levels (Figure 1(g) and (h)). Furthermore, we used BACE inhibitor IV to treat human BMECs with concentrations selective for BACE1 inhibition (and shown to reduce amyloid peptide production under cell culture conditions).30,33 Our results demonstrated that 0.2 µM BACE inhibitor IV significantly increased eNOS protein expression (Figure 1(i)).
Figure 1.
BACE1 regulated eNOS expression and NO production in human BMECs. A-F: Cells were treated with BACE1siRNA or CtsiRNA (30 nM) for 48 hours (a and c–f) or 40 hours (b), Western blot or real-time PCR were performed, respectively. a, BACE1 protein was knocked down using BACE1siRNA, n = 8 *P < 0.05. b, levels of eNOS mRNA were increased in cells treated with BACE1siRNA, n = 4, *P < 0.05. c–f, protein expressions of eNOS, p-eNOS(S1177)/eNOS, and p-eNOS(T495)/eNOS) in human BMECs treated BACE1siRNA or CtsiRNA, n = 15-24, *P < 0.05. g and h: Human BMECs were transduced with Ad-BACE1 or Ad-Null (2.5 MOI, 2 days). g, n = 9, H, n = 13; *P < 0.05. i: Human BMECs were treated with BACE inhibitor IV for 24 hours (n = 5, *P < 0.05). J: After endothelial cells were treated with 30 nM BACE1siRNA for 2 days, cells were incubated with EGM2 without serum for 24 hours. The supernatants were collected for NO2+NO3 assay (n = 8, *P < 0.05).
As shown in Figure 1(j), concentrations of NO2 + NO3 in conditioned media (reflecting NO production by BMECs) were significantly augmented after treatment with BACE1siRNA, as compared to the cells treated with CtsiRNA. However, BACE1siRNA transfection did not significantly affect intracellular levels of superoxide anions (suppl. Fig. 1A), nitrotyrosine levels (suppl. Fig. 1B), and antioxidant enzyme levels (MnSOD, CuZnSOD, and catalase) (suppl. Fig. 1C–F and suppl. Fig. 2), suggesting that inhibition of BACE1 does not cause uncoupling of eNOS.
Previous study has demonstrated that in conditioned medium derived from human BMECs Aβ1-40 levels are low, and Aβ1-42 is almost undetectable. 28 To rule out the effect of Aβ peptides on eNOS expression in human BMECs, we incubated the cells with Aβ1-40 or Aβ1-42 (10−12–10−5 M; for 24 hours). As shown in suppl. Fig. 3, protein levels of eNOS were not affected after either treatment. These observations suggest that the stimulatory effect of BACE1 inhibition on expression of eNOS may be independent of inhibition of APP β-processing and production of Aβ peptides.
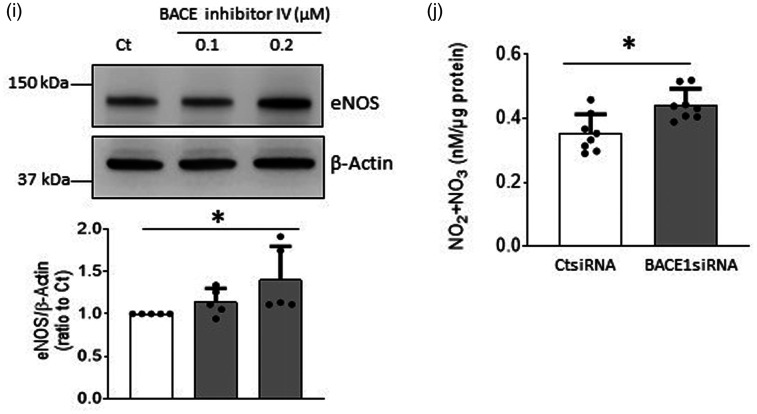
GPC1 mediates BACE1-induced upregulation of eNOS expression
It has been reported that GPC1, a major component of glycocalyx on the plasma membrane, accumulates in the brain of BACE1−/− zebrafish 24 thereby leading to speculation that GPC1 is cleaved by BACE1. Since GPC1 functions as a coreceptor mediating signaling of several growth factors that regulate eNOS gene expression,14,16,17 we examined whether GPC1 plays a role in the up-regulation of eNOS expression induced by BACE1 inhibition. Interestingly, BACE1siRNA significantly increased levels of protein and mRNA of GPC1 in human BMECs (Figure 2(a) and (b)). In contrast, overexpression of BACE1 with Ad-BACE1 reduced GPC1 protein expression (Figure 2(c)). Furthermore, genetic inactivation of GPC1 significantly suppressed mRNA and protein expression of eNOS (Figure 2(d) to (f)). These results suggest that GPC1 is involved in upregulation of eNOS induced by inhibition of BACE1.
Figure 2.
GPC1 mediated effect of BACE1 on eNOS expression in human BMECs. a and b: Cells were treated with 30 nM BACE1siRNA or CtsiRNA for 48 hours (a) or 40 hours (b), the protein or mRNA levels of GPC1 were measured, respectively. (a) n = 12; (b) n = 4; *P < 0.05. PC-12 cell lysate was used as a GPC1 positive control. (c) Cells were transduced with Ad-BACE1 or Ad-Null (2.5 MOI, 2 days). n = 8, *P < 0.05. (d–f) Cells were treated with 30 nM GPC1siRNA or CtsiRNA for 2 days. mRNA expressions of GPC1 (d) and eNOS (e), as well as eNOS protein levels (f) were measured. D, n = 6; E, n = 7, F, n = 4; *P < 0.05.
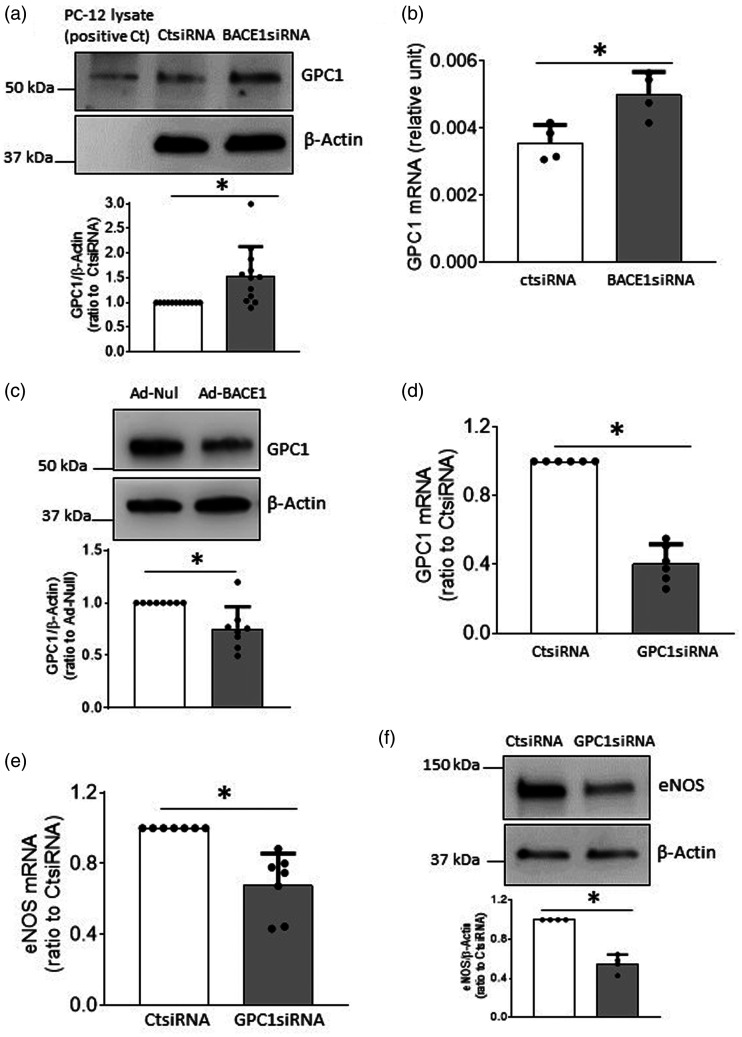
Stimulatory effect of BACE1 deficiency on eNOS expression in mouse brain microvessels
Unless specifically stated, all in vivo experiments were performed on male mice. To confirm and further validate our in vitro observations, we examined eNOS expression in isolated brain microvessels derived from BACE1−/− mice. Compared to WT littermates, BACE1−/− mice had lower body weight, but similar levels of blood pressure, blood glucose and lipid profiles (suppl. Table 1), thus ruling out the possibility that alterations in blood pressure or circulating levels of glucose or lipids may influence vascular function of BACE1−/− mice. As expected, Aβ1-40 in plasma was significantly reduced in BACE1−/−mice (suppl. Table 1). The remaining low levels of Aβ1-40 present in plasma of BACE1−/− mice may be the result of the compensatory β-processing of APP by BACE2. Western blot analysis demonstrated that while BACE1 protein in brain microvessel was deleted (Figure 3(a)), protein levels of eNOS and GPC1 were significantly up-regulated (Figure 3(b) and (d)), consistent with our in vitro findings. However, in cerebral microvessels derived from female BACE1−/− mice, BACE1 expression was abolished but eNOS protein levels were not altered (Suppl. Fig. 4A and B). Of note, female BACE1−/− mice had lower body weight as compared to WT mice. In addition, blood glucose levels and lipid profile were not affected by genetic inactivation of BACE1 (Suppl. Table 2).
Figure 3.
Expressions of eNOS and GPC1 in brain microvessels of BACE1−/− mice were increased. a–d: Cerebral microvessels were isolated from BACE1−/− mice and wild type littermates and subjected to Western blot analysis. n = 8, *P < 0.05.
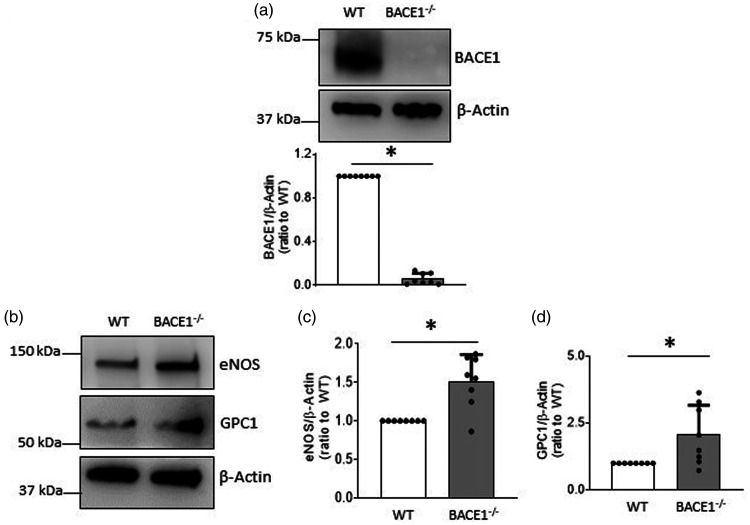
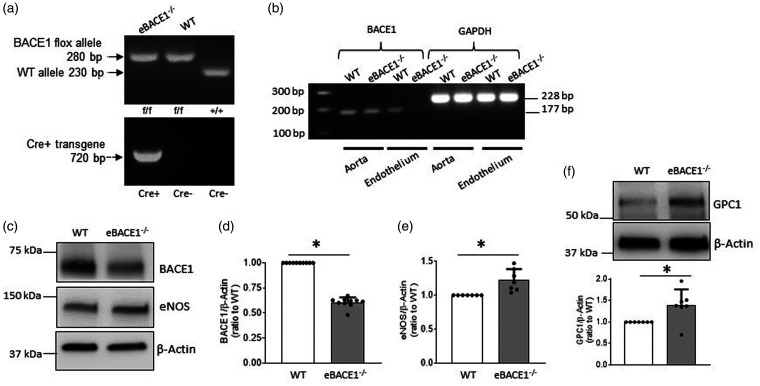
To further verify our observations in cultured BMECs and BACE−/− mice, we created the first endothelium-specific BACE1 knockout mice (eBACE1−/− mice) (Figure 4(a)). Deletion of BACE1 mRNA in endothelial cells isolated from aorta of eBACE1−/− mouse was confirmed by demonstrating successful tamoxifen-induced deletion of endothelial BACE1 gene (Figure 4(b)). Body weight, blood glucose, and blood pressure were not significantly changed in eBACE1−/− mice as compared to WT mice (Table 1). Plasma levels of Aβ1-40 and Aβ1-42 were slightly but significantly decreased in eBACE1−/− mice (Table 1).
Figure 4.
Expressions of eNOS and GPC1 in brain microvessels of eBACE1−/− mice were augmented. (a) Representative genotyping analysis of eBACE1−/− mouse (BACE1flox/flox;Cdh5-Cre+) and WT littermate (BACE1flox/flox;Cdh5-Cre−). (b) PCR analysis of BACE1 in mouse aorta and endothelium isolated from mouse aorta. Notice that BACE1 was deleted from aortic endothelium in eBACE1−/− mice. (c–f): Protein levels of BACE1, eNOS, and GPC1 in brain microvessels of eBACE1−/− mice and WT mice. n = 7–10, *P < 0.05.
In isolated brain microvessels, BACE1 protein levels were significantly reduced (Figure 4(c) and (d)). The incomplete deletion of BACE1 most likely reflected the presence of BACE1 protein in smooth muscle cells of the cerebral microvessels. In agreement with our observations obtained in cultured BMECs and BACE1−/− mice, protein expressions of eNOS and GPC1 in eBACE1−/− microvessels were significantly increased (Figure 4(c), (e) and (f)). These findings support the concept that GPC1 mediates up-regulation of eNOS induced by inhibition of BACE1.
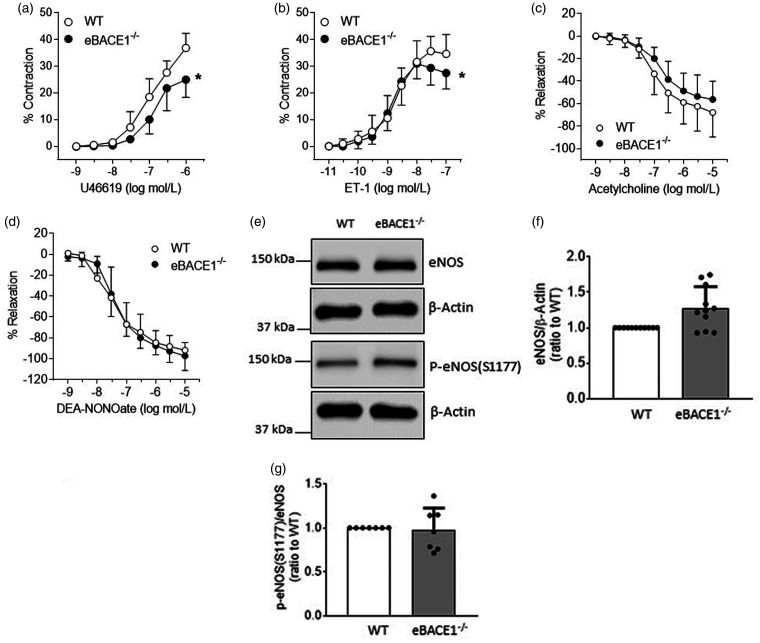
Vasomotor function of basilar arteries of male eBACE1−/− mice
The structure (internal diameter, external diameter, and media thickness) of basilar arteries of eBACE−/− mice was not altered as compared to structure of basilar artery of WT littermates (Suppl. Fig. 5). The contractile responses to U46619 and ET-1 (pEC50) were equipotent among studies groups of mice (Figure 5(a) and (b)), though efficacy of U46619 and ET-1 contractile responses were decreased in basilar arteries of eBACE−/− mice as compared to their WT littermates (Figure 5(a) and (b)). Endothelium-dependent relaxations to acetylcholine and endothelium-independent relaxations to the NO donor DEA-NONOate were not significantly different between WT and eBACE1 mice (Figure 5(c) and (d)).
Figure 5.
Concentration-dependent contractions in basilar arteries to U46619 (a, n = 6, *P < 0.05) and ET-1 (b, n = 5–6, *P < 0.05). Endothelium-dependent relaxations in basilar arteries to acetylcholine (c, n = 6, P > 0.05) and DEA-NONOate (d, n = 6, P > 0.05). e–g: Protein expressions of eNOS and p-eNOS(s1177) in large cerebral arteries were not significantly changed. Quantification of expressions of eNOS (f, n = 11, P > 0.05) and ratio of p-eNOS(1177)/eNOS (g, n = 7, P > 0.05).
To gain additional insights into the mechanisms underlying changes in vasomotor function of basilar artery, we examined expression of eNOS in large cerebral arteries. In contrast to findings obtained on human BMECs as well as brain microvessels of BACE1−/− and eBACE1−/− mice, eNOS protein expression was not significantly increased in isolated large cerebral arteries of eBACE1−/− mice as compared to arteries of WT mice (Figure 5(e) and (f)). In addition, the levels of p-eNOS(S1177) were also not affected (Figure 5(e) and (g)).
Discussion
The present study provides several new insights into the function of BACE1 in the cerebrovascular endothelium. Our findings demonstrate that genetic and pharmacological inactivation of endothelial BACE1 enhances expression of eNOS thereby suggesting that BACE1 exerts inhibitory effect on expression and function of eNOS. This effect appears to be independent of BACE1-induced cleavage of APP and formation of Aβ-peptides. Furthermore, we provide evidence that increased expression of GPC-1 mediates increased expression of eNOS induced by genetic deletion of BACE1. Importantly, we validated our findings in cultured human endothelium with studies in brain microvessels derived from male BACE1-deficient mice. Indeed, both global and endothelium-specific genetic inactivation of BACE1 resulted in significant upregulation of eNOS and GPC-1, thus demonstrating previously unrecognized ability of BACE1 inhibition to upregulate eNOS in cerebral endothelium. However, in female BACE1−/− mice, microvascular expression of eNOS was not changed. Furthermore, in large arteries of male eBACE1−/− mice, endothelial BACE1 deficiency did not significantly affect eNOS expression. Consistent with this observation, endothelium-dependent relaxations were not affected in basilar arteries derived from male eBACE1−/− mice. These are the first findings to demonstrate that under physiological conditions, selective inactivation of endothelial BACE1 does not affect vasodilator function of large brain arteries.
Upregulation of eNOS expression and signaling are generally considered vascular protective effects. 34 Indeed, studies in humans suggest that subjects with genetic variants of eNOS causing enhanced endothelial production of NO have significantly reduced risk of cerebrovascular disease. 34 However, in blood vessels exposed to vascular risk factors including hypertension, hyperlipidemia, diabetes, smoking, and aging, upregulation of eNOS protein expression may result in eNOS uncoupling manifested by elevated production of superoxide anion and peroxynitrite thereby imposing oxidative stress and vascular injury.18,35 In current study, our measurements demonstrated that inhibition of BACE1 did not affect intracellular levels of superoxide anion in cultured brain endothelium. In addition, nitrotyrosine levels were not changed by genetic inactivation of BACE1. These results indicated that the BACE1 inhibition did not cause uncoupling of eNOS.
Our results agree with previous study demonstrating that BACE1 negatively regulates expression of GPC1-like protein (Zgc:63947) in the brain of zebrafish. 24 In addition, prior studies established that GPC1 enhances binding efficiency of vascular endothelial growth factor-A and fibroblast growth factor-2 to their receptors 14 thus exerting beneficial effects on endothelial cell survival and preservation of endothelial integrity.14,15 Moreover, these growth factors up-regulate eNOS gene expression.16,17 Therefore, it is possible that GPC1 may augment eNOS expression by enhancing signaling of these growth factors. In the present study, examination of GPC1 in BACE1-deficient human and murine endothelium revealed that inactivation of BACE1 significantly up-regulated expression of GPC1. In contrast, overexpression of BACE1 significantly reduced protein expression of GPC1. Further analysis indicated that GPC1 is an important regulator of eNOS expression in the brain endothelium. Importantly, genetic deletion of GPC1 in human BMECs resulted in down-regulation of eNOS mRNA and protein expression. Thus, it appears that inactivation of BACE1 increases mRNA and protein expression of GPC1 which in turn lead to elevation of eNOS. Of note, our findings cannot rule out the possibility that GPC1 is a substrate for proteolytic cleavage by BACE1. This question remains to be addressed in the future studies.
Several studies have reported that in systemic vasculature, GPC1 mediates phosphorylation of eNOS at S1177 induced by shear stress.19–23 In the present study, increased expression of GPC1 did not increase phosphorylation of eNOS at S1177. Lack of sheer stress in endothelial cell culture conditions may explain this observation. However, in large cerebral arteries of eBACE1−/− mice, levels of eNOS phosphorylated at S1177 were also not increased. The exact reasons for apparent discrepancy between the results obtained in the systemic blood vessels (previous studies19–23) and the results obtained in the cerebral blood vessels lacking BACE1 (present study) are unknown and remain to be determined.
Exogenous Aβ peptides may affect various signaling pathways in endothelial cells. 36 In this regard, two observations are important to consider. First, in previous studies the Aβ1-42 was almost undetectable in the cell culture medium derived from human BMECs.6,28 Second, in current study, acute treatment (24 hours) of human BMECs with wide range of concentrations of Aβ1-40 or Aβ1-42 peptides did not affect eNOS expression thus suggesting that inhibition of Aβ production may not be the mechanism responsible for increased expression of eNOS. However, we cannot completely rule out contribution of Aβ peptides because we only performed limited analysis in cultured endothelium exposed to Aβ peptides for a short period of time (24 hours). Regarding in vivo studies, it is important to note that in eBACE1−/− mice circulating Aβ1-40 and Aβ1-42 levels were mildly decreased (14% and 12%, respectively). These observations suggest that overall contribution of endothelial cells to circulating levels of Aβ is relatively small. Previous study in wild type mice showed that 4 weeks of infusion of Aβ1-42 peptide did not change eNOS protein levels in aortas, 37 thus further supporting the notion that Aβ1-42 peptide does not affect eNOS protein expression. However, at the present time, we can only speculate that long-term mild reduction of circulating concentrations of Aβ peptides may be insufficient to increased expression of eNOS in cerebral microvessels. In addition, while it has been reported that infusion of Aβ1-42 for 4 weeks decreases phosphorylation of eNOS at S1177 in mouse aortas, 37 we did not detect any changes in levels of p-eNOS(S1177) in large cerebral arteries of eBACE1−/− mice. Mild reduction of circulating Aβ levels as well as possible differences in mechanisms underlying phosphorylation of eNOS in cerebral arteries lacking endothelial BACE1 as opposed to aorta under Aβ1-42 treatment conditions, could be responsible for unchanged eNOS phosphorylation in large cerebral arteries of eBACE1−/− mice.
BACE1−/− mice have normal arterial blood pressure, circulating levels of glucose and lipid profile. We confirmed previously reported observations demonstrating significantly lower body weight of BACE1−/− mice. 38 This reduction of body weight was ascribed to impaired growth caused by low plasma insulin concentrations in global BACE1 knockout mice. 39 In contrast, endothelium-specific inactivation of BACE1 did not affect body weight thus indicating that upregulation of eNOS and GPC-1 in eBACE1−/− mice is phenomenon independent of systemic metabolic changes caused by global deletion of BACE1. In addition, normal circulating levels of glucose and arterial blood pressure in eBACE1−/− reinforced our conclusion that inactivation of BACE1 in endothelium is responsible for upregulation of eNOS and GPC-1.
In basilar arteries of eBACE1−/− mice, endothelium-dependent relaxations to acetylcholine or reactivity of smooth muscle to NO were not changed. Consistent with these findings, experiments on large cerebral arteries demonstrated that protein levels of eNOS and p-eNOS(S1177) were not significantly changed thereby suggesting that inactivation of BACE1 in endothelium does not affect vasodilator function. We also observed modest attenuation of maximal contractions to U46619 and endothelin-1 even though pEC50 were equipotent between WT and eBACE1−/− mice. Previous study has reported that Aβ1-40 enhances contractions to endothelin-1 via cyclooxygenase-2 and p38 activation in human middle cerebral artery. 40 One possibility is that in the present study, detected reduction of circulating Aβ peptides might affect prostaglandins metabolism, thus resulting in modest reduction of maximal contraction.
We also wish to point out that in female BACE1−/− mice, cerebral microvascular eNOS protein levels were not changed. Although current study was not designed to study differences between male and female mice, we speculate that estrogen may play a role in lack of eNOS upregulation in response to BACE1 deletion. Previous studies have demonstrated that male endothelial cells constitutively express lower eNOS mRNA and protein levels as compared to female endothelium. 41 Activation of G protein-coupled estrogen receptor stimulates eNOS expression and phosphorylation at Ser1177 in cultured endothelial cells,42–45 leading to an increased NO production. Indeed, eNOS protein expression in hindlimb vasculatures of female mice is higher than in male mice. 46 It is possible that higher levels of NO production in female mice may reduce sensitivity to interventions designed to increase expression of eNOS.
We conclude that under physiological conditions, inhibition of endothelial BACE1 increases expression of eNOS in the cerebral microvessels. This phenomenon appears to be dependent on upregulation of GPC-1. Whether up-regulation of these vascular protective molecules may contribute to overall therapeutic effects of BACE1 inhibition in prevention of AD remains to be determined in the future studies.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221105683 for Inactivation of BACE1 increases expression of endothelial nitric oxide synthase in cerebrovascular endothelium by Tongrong He, Livius V d’Uscio, Ruohan Sun, Anantha Vijay R Santhanam and Zvonimir S Katusic in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from National Institutes of Health (HL131515), National Institutes on Aging (AG071190) and the Mayo Foundation.
Acknowledgements: We would like to acknowledge The Cancer Research Technology Repository at Taconic as the source and Dr Ralf Adams of the London Research Institute as the creator of mouse line 13073 [(Cdh5(PAC)-CreERT2 mice)].
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: TH contributed to experimental design, performed data collection and analysis, and manuscript writing. LVU contributed to genotyping, experiments on large arteries and data analysis, as well as manuscript writing. RS and AVRS participated in in vitro experiments and manuscript writing. ZSK participated in experimental design, data interpretation, and manuscript writing.
ORCID iD: Livius V d’Uscio https://orcid.org/0000-0001-9577-9480
Supplemental material: Supplemental material for this article is available online.
References
- 1.Santisteban MM, Iadecola C. Hypertension, dietary salt and cognitive impairment. J Cereb Blood Flow Metab 2018; 38: 2112–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faraci FM. Protecting against vascular disease in brain. Am J Physiol Heart Circ Physiol 2011; 300: H1566–H1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austin SA, Santhanam AV, Hinton DJ, et al. Endothelial nitric oxide deficiency promotes Alzheimer's disease pathology. J Neurochem 2013; 127: 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin SA, Katusic ZS. Partial loss of endothelial nitric oxide leads to increased cerebrovascular beta amyloid. J Cereb Blood Flow Metab 2020; 40: 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan XL, Xue YQ, Ma T, et al. Partial eNOS deficiency causes spontaneous thrombotic cerebral infarction, amyloid angiopathy and cognitive impairment. Mol Neurodegener 2015; 10: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin SA, Santhanam AV, Katusic ZS. Endothelial nitric oxide modulates expression and processing of amyloid precursor protein. Circ Res 2010; 107: 1498–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austin SA, Katusic ZS. Loss of endothelial nitric oxide synthase promotes p25 generation and tau phosphorylation in a murine model of Alzheimer's disease. Circ Res 2016; 119: 1128–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katusic ZS, Austin SA. Endothelial nitric oxide: protector of a healthy mind. Eur Heart J 2014; 35: 888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devraj K, Poznanovic S, Spahn C, et al. BACE-1 is expressed in the blood-brain barrier endothelium and is upregulated in a murine model of Alzheimer's disease. J Cereb Blood Flow Metab 2016; 36: 1281–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons ER, Marshall DC, Long HJ, et al. Blood brain barrier endothelial cells express candidate amyloid precursor protein-cleaving secretases. Amyloid 1998; 5: 153–162. [DOI] [PubMed] [Google Scholar]
- 11.Davies TA, Billingslea AM, Long HJ, et al. Brain endothelial cell enzymes cleave platelet-retained amyloid precursor protein. J Lab Clin Med 1998; 132: 341–350. [DOI] [PubMed] [Google Scholar]
- 12.Hampel H, Vassar R, De Strooper B, et al. The β-Secretase BACE1 in Alzheimer's disease. Biol Psychiatry 2021; 89: 745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan J, Ho M. Role of glypican-1 in regulating multiple cellular signaling pathways. Am J Physiol Cell Physiol 2021; 321: C846–C858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whipple CA, Lander AD, Korc M. Discovery of a novel molecule that regulates tumor growth and metastasis. ScientificWorldJournal 2008; 8: 1250–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahmoud M, Mayer M, Cancel LM, et al. The glycocalyx core protein glypican 1 protects vessel wall endothelial cells from stiffness-mediated dysfunction and disease. Cardiovasc Res 2021; 117: 1592–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroll J, Waltenberger J. VEGF-A induces expression of eNOS and iNOS in endothelial cells via VEGF receptor-2 (KDR). Biochem Biophys Res Commun 1998; 252: 743–746. [DOI] [PubMed] [Google Scholar]
- 17.Murphy PR, Limoges M, Dodd F, et al. Fibroblast growth factor-2 stimulates endothelial nitric oxide synthase expression and inhibits apoptosis by a nitric oxide-dependent pathway in Nb2 lymphoma cells. Endocrinology 2001; 142: 81–88. [DOI] [PubMed] [Google Scholar]
- 18.Katusic ZS, d'Uscio LV, Nath KA. Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects. Trends Pharmacol Sci 2009; 30: 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebong EE, Lopez-Quintero SV, Rizzo V, et al. Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1. Integr Biol (Camb) 2014; 6: 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng Y, Liu J. Role of glypican-1 in endothelial NOS activation under various steady shear stress magnitudes. Exp Cell Res 2016; 348: 184–189. [DOI] [PubMed] [Google Scholar]
- 21.Bartosch AMW, Mathews R, Tarbell JM. Endothelial glycocalyx-mediated nitric oxide production in response to selective AFM pulling. Biophys J 2017; 113: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartosch AMW, Mathews R, Mahmoud MM, et al. Heparan sulfate proteoglycan glypican-1 and PECAM-1 cooperate in shear-induced endothelial nitric oxide production. Sci Rep 2021; 11: 11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang H, Sun A, Wu Q, et al. Atherogenic diet-diminished endothelial glycocalyx contributes to impaired vasomotor properties in rat. Am J Physiol Heart Circ Physiol 2020; 319: H814–H823. [DOI] [PubMed] [Google Scholar]
- 24.Hogl S, van Bebber F, Dislich B, et al. Label-free quantitative analysis of the membrane proteome of Bace1 protease knock-out zebrafish brains. Proteomics 2013; 13: 1519–1527. [DOI] [PubMed] [Google Scholar]
- 25.Percie Du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab 2020; 40: 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.d'Uscio LV, He T, Santhanam AV, et al. Mechanisms of vascular dysfunction in mice with endothelium-specific deletion of the PPAR-δ gene. Am J Physiol Heart Circ Physiol 2014; 306: H1001–H1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.d'Uscio LV, Smith LA, Katusic ZS. Erythropoietin increases expression and function of vascular copper- and zinc-containing superoxide dismutase. Hypertension 2010; 55: 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He T, Santhanam AV, Lu T, et al. Role of prostacyclin signaling in endothelial production of soluble amyloid precursor protein-α in cerebral microvessels. J Cereb Blood Flow Metab 2017; 37: 106–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.d'Uscio LV, He T, Santhanam AV, et al. Endothelium-specific amyloid precursor protein deficiency causes endothelial dysfunction in cerebral arteries. J Cereb Blood Flow Metab 2018; 38: 1715–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stachel SJ, Coburn CA, Steele TG, et al. Structure-based design of potent and selective cell-permeable inhibitors of human beta-secretase (BACE-1). J Med Chem 2004; 47: 6447–6450. [DOI] [PubMed] [Google Scholar]
- 31.Santhanam AV, d'Uscio LV, Katusic ZS. Erythropoietin increases bioavailability of tetrahydrobiopterin and protects cerebral microvasculature against oxidative stress induced by eNOS uncoupling. J Neurochem 2014; 131: 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol 1978; 235: E97–102. [DOI] [PubMed] [Google Scholar]
- 33.Satir TM, Agholme L, Karlsson A, et al. Partial reduction of amyloid beta production by beta-secretase inhibitors does not decrease synaptic transmission. Alzheimers Res Ther 2020; 12: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emdin CA, Khera AV, Klarin D, et al. Phenotypic consequences of a genetic predisposition to enhanced nitric oxide signaling. Circulation 2018; 137: 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katusic ZS. Vascular endothelial dysfunction: does tetrahydrobiopterin play a role? Am J Physiol Heart Circ Physiol 2001; 281: H981–H986. [DOI] [PubMed] [Google Scholar]
- 36.d'Uscio LV, He T, Katusic ZS. Expression and processing of amyloid precursor protein in vascular endothelium. Physiology (Bethesda) 2017; 32: 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meakin PJ, Coull BM, Tuharska Z, et al. Elevated circulating amyloid concentrations in obesity and diabetes promote vascular dysfunction. J Clin Invest 2020; 130: 4104–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meakin PJ, Harper AJ, Hamilton DL, et al. Reduction in BACE1 decreases body weight, protects against diet-induced obesity and enhances insulin sensitivity in mice. Biochem J 2012; 441: 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmeister A, Tuennemann J, Sommerer I, et al. Genetic and biochemical evidence for a functional role of BACE1 in the regulation of insulin mRNA expression. Obesity (Silver Spring ) 2013; 21: E626–E633. [DOI] [PubMed] [Google Scholar]
- 40.Paris D, Humphrey J, Quadros A, et al. Vasoactive effects of a beta in isolated human cerebrovessels and in a transgenic mouse model of Alzheimer's disease: role of inflammation. Neurol Res 2003; 25: 642–651. [DOI] [PubMed] [Google Scholar]
- 41.Cattaneo MG, Vanetti C, Decimo I, et al. Sex-specific eNOS activity and function in human endothelial cells. Sci Rep 2017; 7: 9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JS, Lee GH, Jin SW, et al. G protein-coupled estrogen receptor regulates the KLF2-dependent eNOS expression by activating of Ca(2+) and EGFR signaling pathway in human endothelial cells. Biochem Pharmacol 2021; 192: 114721. [DOI] [PubMed] [Google Scholar]
- 43.Fredette NC, Meyer MR, Prossnitz ER. Role of GPER in estrogen-dependent nitric oxide formation and vasodilation. J Steroid Biochem Mol Biol 2018; 176: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hishikawa K, Nakaki T, Marumo T, et al. Up-regulation of nitric oxide synthase by estradiol in human aortic endothelial cells. FEBS Lett 1995; 360: 291–293. [DOI] [PubMed] [Google Scholar]
- 45.Rosenfeld CR, Cox BE, Roy T, et al. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J Clin Invest 1996; 98: 2158–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng X, Wang J, Lassance-Soares RM, et al. Gender differences affect blood flow recovery in a mouse model of hindlimb ischemia. Am J Physiol Heart Circ Physiol 2011; 300: H2027–H2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221105683 for Inactivation of BACE1 increases expression of endothelial nitric oxide synthase in cerebrovascular endothelium by Tongrong He, Livius V d’Uscio, Ruohan Sun, Anantha Vijay R Santhanam and Zvonimir S Katusic in Journal of Cerebral Blood Flow & Metabolism