Abstract
Regulatory T cells (Treg) are vital to the maintenance of immune homeostasis. The genetic background of an inbred mouse strain can have a profound effect on the immune response in the animal, including Treg responses. Most Treg studies focus on animals created on the C57BL/6 or BALB/c background. Recent studies have demonstrated a difference in the phenotype and behavior of C57BL/6 and BALB/c Tregs. In this study, we have investigated the function of FVB/N Tregs compared to C57BL/6 and BALB/c. We observed that while FVB/N Tregs appear to suppress normally in a cell contact‐dependent system, FVB/N Tregs are less capable of suppressing when regulation depends on the secretion of a soluble factor. FVB/N Tregs produce IL‐10; however, TGF‐β was not detected in any culture from C57BL/6 or FVB/N. C57BL/6 Foxp3+ Tregs expressed more of the TGF‐β‐related proteins glycoprotein‐A repetitions predominant (GARP) and latency‐associated peptide (LAP) on the cell surface than both FVB/N and BALB/c, but C57BL/6 Tregs expressed significantly less Ctse (Cathepsin E) mRNA. Each strain displayed different abilities of thymic Tregs (tTreg) to maintain Foxp3 expression and had a varying generation of induced Tregs (iTregs). In vitro generated FVB/N iTregs expressed significantly less GARP and LAP. These results suggest Tregs of different strains have varying phenotypes and dominant mechanisms of action for the suppression of an immune response. This information should be taken into consideration when Tregs are examined in future studies, particularly for therapeutic purposes in a genetically diverse population.
Keywords: cell differentiation, FVB, immunosuppression, regulatory, T Cell, T cellautoimmunity
Abbreviations
- APC
antigen‐presenting cell
- CTSE
cathepsin E
- GARP
glycoprotein‐A repetitions predominant
- GITR
glucocorticoid‐induced TNFR‐related protein
- IL
Interleukin
- IPEX
immunodysregulation polyendrocrinopathy, X‐linked
- iTreg
induced regulatory T cell
- LAP
latency‐associated peptide
- MHC
Major Histocompatibility Complex
- NOD
non‐obese diabetic
- pTreg
periphery‐induced regulatory T cell
- T1D
type 1 diabetes
- TCR
T cell receptor
- Teff
effector T cell
- TGF‐β
Transforming Growth Factor β
- TRAIL
TNF‐related apoptosis‐inducing ligand
- Treg
regulatory T cell
- tTreg
thymic regulatory T cell
1. INTRODUCTION
Regulatory T cells (Treg) have been shown to be vital to the maintenance of immune homeostasis and are important in preventing an overactive immune response. This is most evident in immunodysregulation polyendocrinopathy, X‐linked (IPEX) syndrome. IPEX is identified by a lack of Tregs, which results in T cell‐dependent systemic autoimmunity. 1 Tregs are identified by the expression of the transcription factor Foxp3 and the T cell receptor co‐receptor CD4. 2 , 3 CD25, the α‐chain of the IL‐2 receptor been reported to be highly expressed on Tregs. Tregs exist as two main subclasses, naturally arising thymic Tregs (tTreg) and periphery‐induced Tregs (pTreg). Tregs can also be induced from naïve CD4+ T cells in vitro (iTregs). 4 , 5 tTregs develop in the thymus through high TCR interaction with self‐peptide/MHC. pTregs are generated in the periphery under the influence of TGF‐β and IL‐2. 6 , 7 , 8 , 9 , 10 tTregs and pTregs/iTregs may be distinguished by the expression of Helios, a transcription factor thought to be expressed in tTregs, but not in pTregs/iTregs. 11 , 12 Helios has been demonstrated to stabilize Foxp3 expression in tTregs, thus maintaining a Treg phenotype, and has been associated with a higher suppressive ability. 5 , 13 , 14 CD101 is another molecule shown to be present on Tregs with increased suppressive action, perhaps by stabilizing IL‐10 production. 15 , 16
While Tregs may function by suppressing antigen‐presenting cell activity, direct Treg suppression of effector cells has also been demonstrated. Tregs can utilize several mechanisms to exercise their regulatory function on effector cells including the secretion of immunosuppressive cytokines, such as interleukin (IL) ‐10, IL‐35, and TGF‐β. 17 , 18 , 19 , 20 Tregs can also act in a cell contact‐dependent manner on effector cells by inducing effector cell apoptosis or cell cycle arrest. 21
It is known that the genetic background of an inbred mouse strain may have a profound impact on the immune response in the animal. BALB/c and C57BL/6 mice display different lung immune cell infiltrate and cytokine levels after the asthmatic challenge. BALB/c mice also show increased levels and diversity of IgA compared to C57BL/6, and dendritic cells show different levels of toll‐like receptor expression, leading to changes in dendritic cell reactivity. 22 , 23 Sex has also been shown to impact Treg marker expression. 24
These strains also vary in T cell responses. C57BL/6 animals have been shown to predominately generate an IFN‐γ‐driven Th1 response, while BALB/c mice tend to favor an IL‐4/13‐driven Th2 response. 25 Interestingly, recent studies have also demonstrated that similar differences may occur in the Treg populations as well. Studies between C57BL/6 and BALB/c Tregs have shown different mechanisms of action between strains. C57BL/6 Tregs appear to be more dependent on the use of IL‐35 for suppression, while BALB/c Tregs are more dependent on initiating target cell apoptosis. 26 BALB/c Teff cells have also been shown to be more responsive to the suppression from Tregs than Teff from C57BL/6 mice, and differences have been described in thymic Tregs between the BALB/c and C57BL/6 strains. 27 , 28 Different Treg behaviors have also been demonstrated in autoimmune models, including the Type 1 diabetes (T1D) prone Non‐Obese Diabetic (NOD) mouse. 29 C57BL/6 animals with the same MHC haplotype as the NOD (C57BL/6.H2 g7 ) are resistant to T1D. 30 Compared to C57BL/6.H2 g7 iTregs, NOD mice have a reduced capacity to induce in vitro iTregs, and these iTregs are functionally impaired in vitro, as they are unable to effectively suppress CD4+CD25− cell proliferation. Recent studies have also demonstrated variation in regulatory T cell counts, phenotype, and suppressive function between C57BL/6, NOD, and BALB/c mouse strains. 31 In addition, NOD iTregs express lower levels of ecto‐5′‐nucleotidase (CD73), IL‐2Rβ (CD122), and glycoprotein‐A repetitions predominant (GARP), molecules shown to be important to Treg function. 32 These studies implicate genetic background as a major influence on Treg function, and it is also important to note that most modern Treg studies have been done on C57BL/6 or BALB/c mice (Table 1).
TABLE 1.
PubMed search results for Treg terms and strain
| Search Term | C57BL/6 Results | BALB/c Results | FVB Results |
|---|---|---|---|
| “Regulatory T cell” | 418 | 1254 | 4 |
| “Treg” | 315 | 833 | 5 |
| “Foxp3” | 280 | 731 | 3 |
| Total Results | 1013 | 2818 | 12 |
Note: Results of PubMed search for Treg‐related terms and an inbred mouse strain. The search was limited to results from January 1, 2016 to August 31, 2021.
In this study, we have attempted to further our understanding of Treg phenotype and function between various inbred mouse strains by analyzing splenic Tregs from FVB/N in comparison with the commonly used BALB/c and C57BL/6. FVB/N mice have been found to be useful for the generation of transgenic mouse strains due to large eggs for ease of microinjection, and large litter sizes. 33 Biologically relevant differences have also been described between the C57BL/6 and the FVB/N in the development of diabetes and obesity. 34 , 35 Recently, immunological differences have been described in the function of C3 convertase between the C57BL/6 and FVB/N strains. 36
Numerous differences were observed between the strains, including the expression of several common Treg markers, as well as a functional difference between FVB/N and C57BL/6 mice. Understanding the differences in the immune responses of various mouse strains has become increasingly important as Tregs are now the target of therapeutics in a much more genetically diverse human population. 37 This study demonstrates that there are specific genetic differences between inbred mouse strains commonly used in immunologic research that directly affect Treg phenotype and function.
2. MATERIALS AND METHODS
2.1. Animals
C57BL/6, FVB/N, and BALB/c mice were purchased from Taconic Farms. All animals used in this study were males, 8–10 weeks of age. Animals were bred and maintained under specific pathogen‐free (SPF) conditions in Thoren Isolator racks under positive pressure. The Institutional Care and Use Committee of the University of Alabama at Birmingham (UAB) approved all experiments. SPF conditions at UAB include an absence of the following organisms, as determined by serological screening: mouse parvoviruses, including MPV‐1, MPV‐2, and minute virus of mice; mouse hepatitis virus, murine norovirus, Theiler's murine encephalomyelitis virus; mouse rotavirus (epizootic diarrhea of infant mice), Sendai virus; pneumonia virus of mice; reovirus; Mycoplasma pulmonis; lymphocytic choriomeningitis virus; mouse adenovirus; ectromelia (mousepox) virus; K polyomavirus; and mouse polyomavirus. Testing and other methods were as described at http://main.uab.edu/Sites/ComparativePathology/surveillance/.
2.2. Cell isolation
Spleens were removed and cells were isolated immediately via single‐cell suspension by mechanical disruption. Cells were then passed through a 100 μm nytex filter and washed in HBSS supplemented with 1% FBS (HyClone), penicillin/streptomycin (Mediatech), and HEPES (Mediatech). For flow cytometry experiments, red blood cells were lysed using a 140 mM NH4Cl buffer for 5 min at room temperature. Red cell lysis was not performed on samples for cell culture. Cells were counted with a hemocytometer and compound microscope. The average yields per spleen were approximately 1 × 108 lymphocytes.
CD4+CD25− and CD4+CD25+ cells were further isolated by magnetic bead separation using the MACS CD4+CD25+ Cell Isolation Kit (Miltenyi Biotech) and following the manufacturer's protocol. Briefly, CD4+ cells were first isolated by negative selection. CD25+ cells were then selected by positive selection, allowing separation of the CD25− and CD25+ fractions. After isolation, the CD4+CD25+ fraction contained approximately 80% CD4+Foxp3+ cells in both C57BL/6 and FVB/N strains.
2.3. Cell proliferation assays
For the measurement of cell contact‐dependent cell proliferation, CD4+CD25− and CD4+CD25+ cells freshly isolated via MACS as described above, were cultured individually or co‐cultured together at 2x106 cells/ml in 200 μl RPMI supplemented with 10% FBS, penicillin/streptomycin (Mediatech), L‐glutamine (Mediatech), and 2‐mercaptoethanol (Sigma–Aldrich) (R10) in a round bottom 96‐well plate coated with 1 μg/mL anti‐CD3 (BD Biosciences) and 0.5 μg/mL anti‐CD28 (BD Biosciences) for 96 h total. Supernatants for ELISA were collected after 48 or 96 h of culture and media replaced. 1 μCi 3H‐Thymidine (Perkin‐Elmer, Waltham, MA) was added for the final 24 h of culture.
To measure the suppressive potential of a secreted factor in the supernatant of the CD4+CD25+ Treg cultures, CD4+CD25− and CD4+CD25+ cells freshly isolated via MACS as described above, were cultured alone at 2 × 106 cells/ml in 200 μl R10 in a round bottom 96 well plate coated with 1 μg/ml anti‐CD3 and 0.5 μg/ml anti‐CD28 for 72 h. Hundred microliters of the cell supernatant were then placed onto freshly isolated CD4+CD25− Teff cells with 100 μl fresh media in a round‐bottom 96‐well plate coated with 1 μg/ml anti‐CD3 and 0.5 μg/ml anti‐CD28. The responder CD4+CD25− cells were then cultured for an additional 48 h, and 1 μCi 3H‐Thymindine (Perkin‐Elmer, Waltham, MA) was added for the final 24 h of culture.
For both cell contact and cell supernatant suppression assays, 3H‐Thymidine incorporation was quantitated by harvesting cells on a Perkin‐Elmer Filtermate Unifilter‐96 Harvester and analyzing on a Perkin‐Elmer TopCount NXT scintillation counter using TopCount NXT software.
2.4. Flow cytometry
Cells were stained for both extracellular and intracellular molecules using the eBioscience Foxp3 Staining Buffer Set (eBioscience). In summary, Fc receptors on target cells were blocked by incubating with anti‐CD16/CD32 (1 μg/sample) (BD Biosciences) for 15 min at 4°C. Cells were washed and stained with all extracellular antibodies for 30 min at 4°C. Cells were washed and incubated with 100 μl of eBioscience Permeabilization/Fixation Solution for 30 min at 4°C, then cells were washed and stained with intracellular antibodies for 30 min at 4°C. Cells were then washed and resuspended in 1% paraformaldehyde solution in 0.15 M NaCl2 until analysis. Antibodies purchased from BD Biosciences included: Fluorescein isothiocyanate (FITC)‐conjugated anti‐CD4 (clone L3T4), phycoerythrin (PE)‐conjugated anti‐CD25 (clonePC61), PE‐conjugated anti‐CD103 (clone M290). Antibodies purchased from eBioscience included: allophycocyanin (APC)‐conjugated anti‐Foxp3 (clone FKJ‐16 s), PE‐conjugated anti‐CD101 (clone Moushi101), PE‐conjugated anti‐GITR (clone DTA‐1), and PE‐conjugated anti‐GARP (clone YGIC86). Antibodies purchased from BioLegend included: PE‐conjugated anti‐Helios (clone 22F6) and PE‐conjugated anti‐LAP (clone TW7‐16B4). All flow cytometry was done on a Becton Dickinson FACSCalibur (BD Biosciences) and events were collected using CellQuest software. All analysis was done utilizing FlowJo software (Tree Star, Inc.,).
2.5. Cytokine measurements
ELISAs were performed to measure levels of IL‐10. Briefly, Immunlon 96 well plates were coated with capture antibody (4.0 μg/ml anti‐IL‐10: clone JES5‐2A5), overnight at 4°C. Plates were then washed and blocked with PBS containing 10% newborn calf serum. Plates were again washed and culture supernatant or known standard was added for 2 h at room temperature. Standards consisted of 7 dilutions and a blank, run in triplicate. Dilutions started at 5 μg/ml IL‐10 and were diluted at 1:2. Samples were washed off and the biotinylated detection antibody (0.75 μg/ml anti‐IL‐10: clone JES5‐16E3) was added for 1 hour at room temperature. Plates were washed again, and streptavidin‐horseradish peroxidase (Jackson ImmunoResearch) was added for 30 min. Plates were washed for a final time, and 3,3′,5,5′‐tetramethylbenzidine (Thermo Scientific) was added and developed for 30 min IL‐10. The reaction was stopped by adding 0.5 M H2SO4 and the plates were read on a VERSAmax microplate reader (Molecular Devices) at 450 nm using SoftMax Pro software (Molecular Devices). All capture and detection antibodies were purchased from BioLegend.
TGF‐β levels were measured using Transfected TGF‐βR mink lung epithelial cells (TMLEC). Briefly, 2 × 105 TMLECs were plated in DMEM with 4.5 g/dl glucose, supplemented with 10% FBS and 4 ml/L neomycin. TMLECs were incubated for 4 h at 37°C. Cells were then rinsed twice with DMEM. Supernatants from the CD4+CD25− or CD4+CD25+ cells were then added to the TMLECs and BSA was then added at a final concentration of 0.5%. TMLECs were then incubated for 16 h at 37°C. TMLECs were then lysed with lysis buffer (Promega, Madison, WI) for 30 min at room temperature on a rocker. After lysis, 20 μl of lysate was added to an opaque 96 well plate and 100 μl of luciferase substrate was added to each well. Known TGF‐β standards were used from 0pM to 32pM were used to generate a standard curve. The luminescence was then read.
2.6. Real‐time PCR
RNA was obtained from fresh MACS isolated cells using the QIAgen RNeasy RNA extraction kit (QIAGEN Inc.,). cDNA was synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche). Quantitative real‐time reverse‐transcriptase polymerase chain reaction (RT‐PCR) was performed using TaqMan Universal PCR Mix (Invitrogen) in combination with Applied Biosystems TaqMan Gene Expression Assay primer‐probe sets (Applied Biosystems) for Il2 (Applied Biosystems ID Mm00445259_m1), Il10 (Mm00439616_m1), Il12a (Mm00434165_m1), Ebi3 (Mm00469294_m1), Tfgb1 (Mm00441724_m1), Nt5e (Mm00501910_m1), Lrrc32 (Mm01273954_m1), Ctse (Mm00456010). The RNA expression level was calculated using the crossing threshold of detectable fluorescence level as determined by the RT cycler MX3000P (Stratagene). Crossing thresholds were then averaged to get a gene‐specific value that was then normalized to the average expression of the 18S housekeeping gene for each strain and experimental condition studied.
2.7. Generation of induced regulatory T cells
To investigate the maintenance of Foxp3 expression on CD4+CD25+ tTregs and the generation of iTregs from CD4+CD25− cells, splenic CD4+CD25− and CD4+CD25+ cells were isolated and separated as described above. 2.5 × 105 freshly separated cells were then added to a 96‐well flat‐bottom plate coated with anti‐CD3 (1 μg/ml) and anti‐CD28 (0.5 μg/ml) for 48 or 96 h at 37°C with media alone, 20 ng/ml recombinant mouse IL‐2, 5 ng/ml recombinant human TGF‐β, or both IL‐2 and TGF‐β. If cells were cultured for 96 h, fresh media and cytokines were added after 48 h of culture. Cell supernatants were collected after 48 and 96 h for ELISA. All cytokines were purchased from BioLegend.
2.8. Statistical analysis
Statistics on continuous data was performed using the unpaired t‐test in GraphPad Prism software. p values ≤0.05 are shown.
3. RESULTS
3.1. C57BL/6 and FVB/N regulatory T cells suppress effector T cell proliferation similarly in a cell contact environment
To examine functional differences between C57BL/6 and FVB/N, an in vitro cell proliferation assay was performed to investigate cell contact‐dependent regulation. CD4+CD25− effector T cells (Teff) were isolated via MACS and cultured with or without MACS isolated CD4+CD25+ Treg cells for 96 h. The cells were cultured in the presence of plate‐bound anti‐CD3 and anti‐CD28 to stimulate T cell activation and proliferation, and proliferation was measured via 3H‐Thymidine uptake. Both C57BL/6 and FVB/N CD4+CD25+ Treg cells were capable of suppressing CD4+CD25− Teff cell proliferation in a cell contact‐dependent manner when at a 1:1 or 4:1 ratio. (Figure 1A).
FIGURE 1.
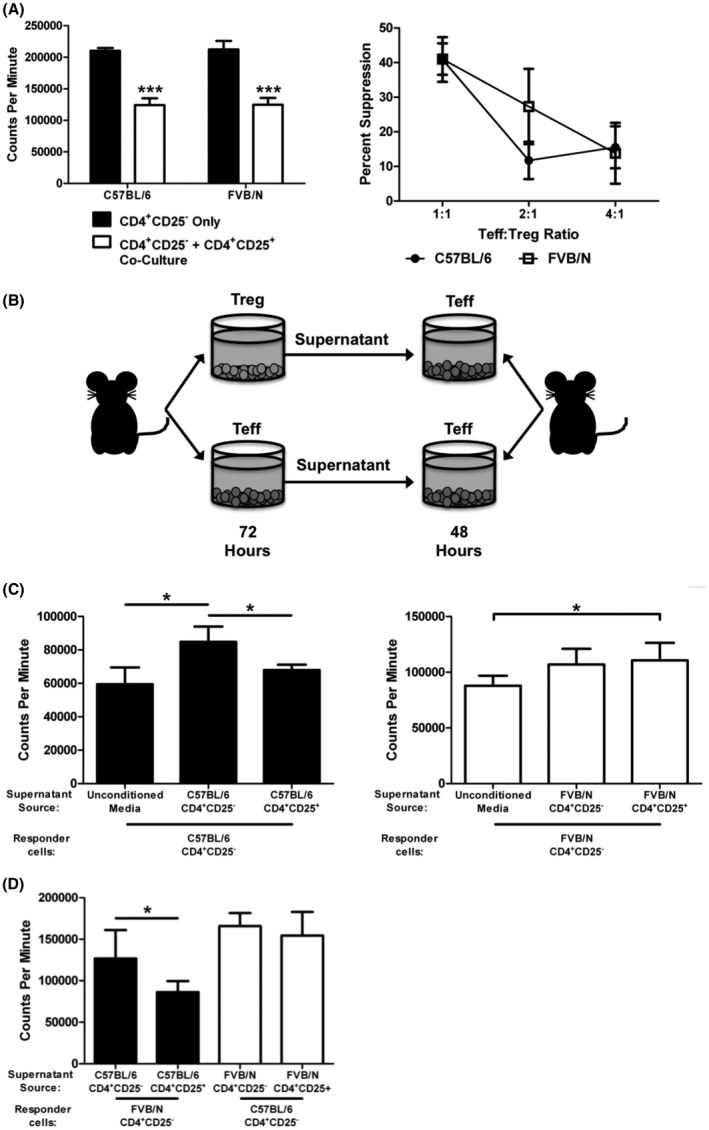
FVB/N Tregs suppress in a cell contact environment, but demonstrate impaired suppression without cell contact. (A) CD4+CD25− Teffs were isolated from C57BL/6 or FVB/N spleens and cultured separately or cocultured with CD4+CD25+ Treg cells at a 1:1 ratio with anti‐CD3 and anti‐CD28 stimulation for 96 h. 3H‐Thymidine was added for the final 24 h of culture. (B) Schematic representing the experimental setup for figures C and D. CD4+CD25− or CD4+CD25+ were cultured with anti‐CD3 and anti‐CD28 stimulation for 72 h. After 72 h, 100 μl of the supernatant +100 μl fresh media was placed onto freshly isolated CD4+CD25− cells. CD4+CD25− responder cells were then cultured for 48 h, with 3H‐Thymidine added for the final 24 h of culture. (C) C57BL/6 and FVB/N supernatant suppression cultures. Experiments for each strain were performed as shown in B. (D) Inter‐strain supernatant suppression cultures. FVB/N CD4+CD25− responder cells were cultured with supernatants generated from C57BL/6 CD4+CD25− or CD4+CD25+ cells (filled bars); and C57BL/6 CD4+CD25− responder cells were cultured with supernatants generated from FVB/N CD4+CD25− or CD4+CD25+ cells (empty bars). *p ≤ 0.05; ***p ≤ 0.001. Data are representative of 3 independent experiments, with 6 samples per group. Mean + standard deviation is shown.
3.2. Supernatants from C57BL/6 Tregs prevent Teff cell proliferation, while FVB/N Treg supernatants do not
Because no difference was observed between the function of C57BL/6 and FVB/N Treg cells in a cell contact‐dependent system, it was then necessary to examine Treg suppression of C57BL/6 and FVB/N Tregs without direct effector cell contact. This was accomplished by culturing either MACS isolated CD4+CD25− Teff cells or CD4+CD25+ Treg cells from either strain for 72 h with anti‐CD3 and anti‐CD28 stimulation. The culture supernatants were then placed onto freshly isolated CD4+CD25− Teff cells. The responding CD4+CD25− Teff cells were cultured for 48 h with the supernatant, and proliferation was measured by 3H‐Thymidine incorporation (Figure 1B). Responding cells were also cultured with fresh media as a control. As shown in Figure 1C, supernatants from both C57BL/6 Treg cells (left panel) and FVB/N Treg cells (right panel) were unable to block baseline CD4+CD25− cell proliferation, as those cells reached proliferation levels similar to CD4+CD25− cells cultured in the supernatant of CD4+CD25− cells.
However, it was observed that when C57BL/6 Teff cells were in the presence of C57BL6 Treg supernatants, the enhanced Teff proliferation seen in the Teff culture combined with Teff supernatant was blocked. Similar inhibition of proliferation was not seen in the FVB cultures. To determine whether the supernatant or a differential Teff response was causing this differential proliferation of Teff cells, the supernatant suppression assay (Figure 1B) was again used. However, different responder cell strains were utilized. C57BL/6 Teff cells or FVB/N Teff (Figure 1D) were cultured with supernatants from CD4+CD25− and CD4+CD25+ cells isolated from both C57BL/6 and FVB/N spleens. The supernatant from the C57BL/6 Treg cells was able to decrease the proliferation of both C57BL/6 (Figure 1C) and FVB/N (Figure 1D) Teff cells compared to the Teff supernatant, however, the FVB/N Treg supernatants were unable to generate the same decrease.
3.3. FVB/N Tregs secrete IL‐10 at higher levels than C57BL/6 Tregs
The inability of FVB/N Treg supernatants to prevent the proliferation of Teff cells leads us to test for the presence of the immunoregulatory cytokines. Two immunosuppressive cytokines that Tregs are known to secrete are IL‐10 and TGF‐β. Interestingly, FVB/N Tregs appear to secrete more IL‐10 than their C57BL/6 counterparts, indicating that the lack of IL‐10 does not explain our results (Figure 2B). To evaluate the production of active TGF‐β, we utilized TGF‐βR transfected mink lung epithelial cells (tMLEC). However, this assay did not detect any active TGF‐β in either the C57BL/6 or the FVB/N Treg cultures, ruling out soluble TGF‐β as a potential regulatory molecule in our system (Figure 2C).
FIGURE 2.
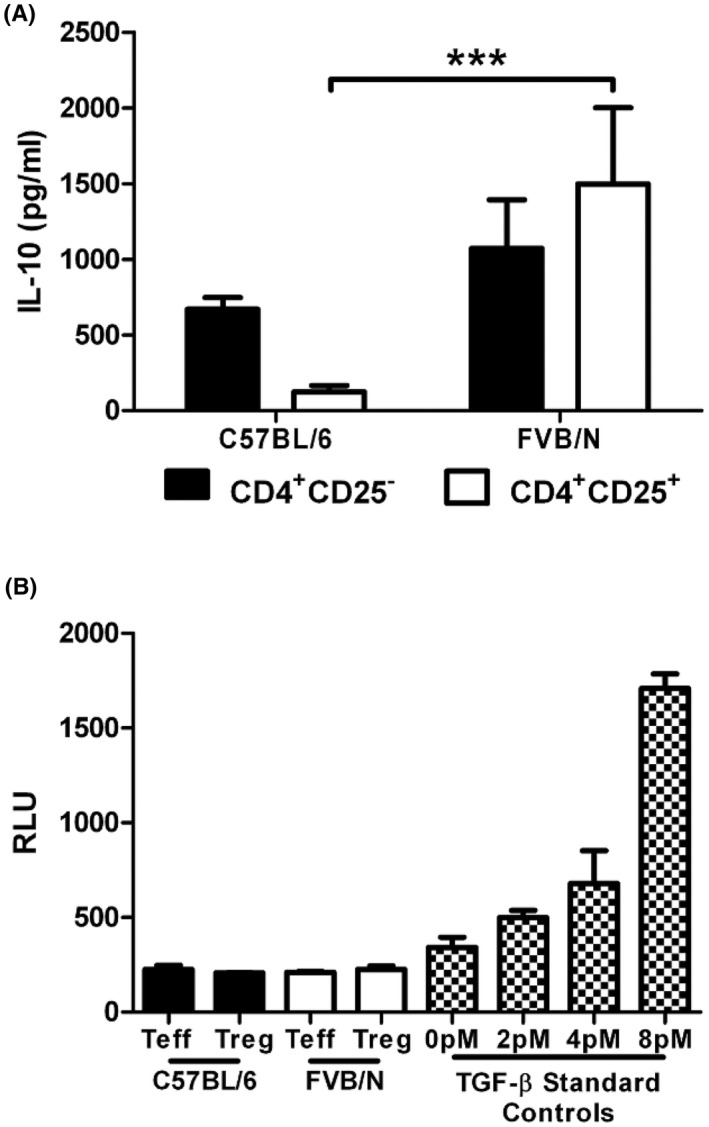
Normal IL‐10 secretion in FVB/N Tregs. CD4+CD25− and CD4+CD25+ cells were cultured separately or together for 72 h and the supernatants were harvested for ELISA. (A) IL‐10 levels were measured by ELISA from the supernatants of CD4+CD25− and CD4+CD25+ cells cultured for 72 h. Data are representative of 3 independent experiments, with 6 samples per group. (B) TGF‐β levels of supernatants from cultured CD4+CD25− and CD4+CD25+ cells. TGB‐β was measured using cultured TMLECs and a luciferase reporter. Data are representative of 2 independent experiments, with 6 samples per group. ***p ≤ 0.001. Mean + standard deviation (A) or standard error of the mean is shown (B).
3.4. C57BL/6, FVB/N, and BALB/c splenic Foxp3+ cells display different cell surface phenotype
To further explore the differences between Tregs from C57BL/6 and FVB/N animals, the expression of common Treg markers was investigated. In addition, several experiments were also carried out utilizing another common inbred mouse strain, the BALB/c mouse. BALB/c mice are frequently used to study Th2 responses, while C57BL/6 mice are commonly used for Th1. 25 , 38 As FVB/N mice have also been described to have a dominant Th2 response, we performed this additional comparison to BALB/c in order to explore if the differences seen between C57BL/6 and FVB/N Treg phenotypes would align with this known difference in effector T cell responses. 38 , 39 CD4+ splenocytes from C57BL/6 and BALB/c mice expressed similar levels of the master Treg transcription factor, Foxp3. Fewer FVB/N CD4+ splenocytes expressed Foxp3 compared to both the C57BL/6 and the BALB/c (Figure 3A).
FIGURE 3.
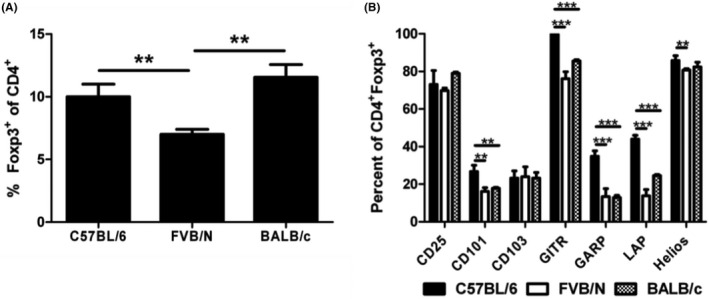
Phenotypic differences of C57BL/6, FVB/N, and BALB/c Tregs. (A) Foxp3 expression of splenic CD4+ cells of C57BL/6, FVB/N, and BALB/c mice. Freshly isolated splenocytes were stained and analyzed. Expression was analyzed using flow cytometry. Lymphocytes were gated based on forward scatter/side scatter and then subsequently gated on CD4+ cells. (B) Expression of various Treg markers of CD4+Foxp3+ cells. Expression was analyzed using flow cytometry. Lymphocytes were gated based on forward scatter/side scatter and then subsequently gated on CD4+Foxp3+ cells. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001. Data are representative of 3 independent experiments, with 4 samples per group. Mean + standard deviation is shown.
The expression of several common T cell markers was also analyzed via flow cytometry on freshly isolated CD4+Foxp3+ splenocytes. No changes were detected in CD25 (the IL‐2Rα chain) or in CD103 (αE integrin), a common intestinal homing marker known to be expressed on Tregs. However, differences were seen in several other markers (Figure 3B). The expression of these markers was significantly higher in C57BL/6 CD4+Foxp3+ cells in all cases. CD101 was found to be decreased to similar levels in both the FVB/N and BALB/c Tregs relative to C57BL/6 Tregs. Similar results were also seen for glucocorticoid‐induced TNFR‐related protein (GITR), a cell surface molecule that is involved in antigen‐presenting cell (APC) activation of Teff cells. 40 , 41 Significant decreases were also seen in the FVB/N and BALB/c Treg expression of glycoprotein‐A repetitions predominant (GARP) and the TGF‐β1 coupling protein latency‐associated peptide (LAP). GARP and LAP are both involved in the expression of cell surface TGF‐β. 42 , 43 , 44 Lastly, a small, but statistically significant, decrease in the transcription factor Helios, shown to stabilize Foxp3 expression in tTregs, was observed in FVB/N Tregs compared to C57BL/6 Tregs. 13 , 14 Several differences in the expression of these markers were also observed within the CD4+Foxp3− population, although not to a statistically significant level (Figure S1A). However, Helios was found to be more highly expressed in C57BL/6 CD4+Foxp3− cells than in CD4+Foxp3− cells from FVB/N or BALB/c mice.
3.5. CD4 + CD25 + mRNA expression levels differ between C57BL/6, FVB/N, and BALB/c
In addition to flow cytometry, qRT‐PCR was performed on MACS isolated CD4+CD25− and CD4+CD25+ cells from C57BL/6, FVB/N, and BALB/c splenocytes to detect mRNA levels of the following Treg markers: IL2 (IL‐2), IL10 (IL‐10), IL12a (IL‐12p35), Ebi3 (Ebi3), Tgfb1 (TGF‐β), Nt5e (CD73), Lrrc32 (GARP), and Ctse (Cathepsin E). Ctse was significantly increased in the FVB/N and BALB/c CD4+CD25+ population compared to C57BL/6 mice (Figure 4B). Cathepsin E is expressed by Treg cells and may enhance Treg suppression of Teff proliferation through the cleavage of TNF‐related apoptosis‐inducing ligand (TRAIL). 45 TRAIL can be used by CD4+CD25+ Tregs and function in a cell‐bound fashion, or as a soluble trimer to suppress CD4 and CD8 T cell responses. 46 , 47
FIGURE 4.
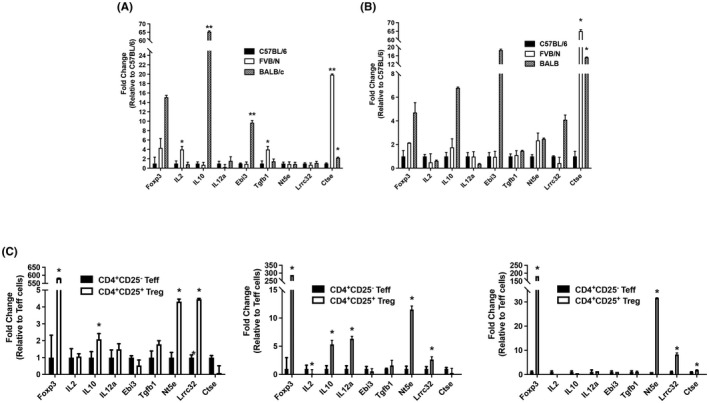
RNA expression of various Treg markers. (A) mRNA expression of Treg markers on CD4+CD25− Teff cells. Cells were cultured for 2 days with anti‐CD3 and anti‐CD28 stimulation to expand cell numbers. RNA was isolated, cDNA generated, and expression analyzed. (B) mRNA expression of Treg markers on CD4+CD25+ Treg cells. Cells were cultured for 2 days to expand cell numbers. RNA was isolated, cDNA generated, and expression analyzed. (C) Comparison of mRNA expression of each strain's Tregs relative to Teff cells. *p ≤ 0.05; **p ≤ 0.01; ***P ≤ 0.001. Data are representative of 2 independent experiments, with 4 samples per group. Mean + standard deviation is shown.
Several anti‐inflammatory genes were upregulated in C57BL/6 Tregs compared to C57BL/6 Teff cells (Il10, Nt5e, and Lrrc32), and the same was true when comparing FVB/N Treg to FVB/N Teff cells (Il10, Il12a, Nt5e, and Lrrc32), and BALB/c Treg to BALB/c Teff (Nt5e, Lrrc32, and Ctse) (Figure 4C). Significant increases were also observed in the FVB/N CD4+CD25− expression of Il2, Tgfb1, and Ctse, while BALB/c CD4+CD25− cells displayed increased Il10, Ebi3, and Ctse (Figure 4A).
3.6. Ability of tTregs to maintain Foxp3 expression differs between strains
Another important aspect of Treg function within a biological system is the ability of the Treg to maintain its Foxp3+ suppressive phenotype. Several studies have shown the expression of Foxp3 can be transient, depending on the cell's current environment. 48 , 49 , 50 , 51 , 52 To test the ability of CD4+CD25+ Tregs to maintain Foxp3 expression, CD4+CD25+ cells were isolated via MACS and cultured for 96 h with the cytokines commonly used to induce Treg cells in vitro, IL‐2, and TGF‐β (Figure 5A). The starting population was approximately 80–85% Foxp3+ at the beginning of culture. Foxp3 expression was significantly decreased when FVB/N Tregs were cultured with IL‐2 only compared to C57BL/6 Tregs. FVB/N and BALB/c Tregs were also more able to maintain Foxp3 expression upon culture with TGF‐β. When both IL‐2 and TGF‐β were used, all three strains were able to maintain a high expression of Foxp3. This indicates that each strain may require different factors to maintain Foxp3 expression.
FIGURE 5.
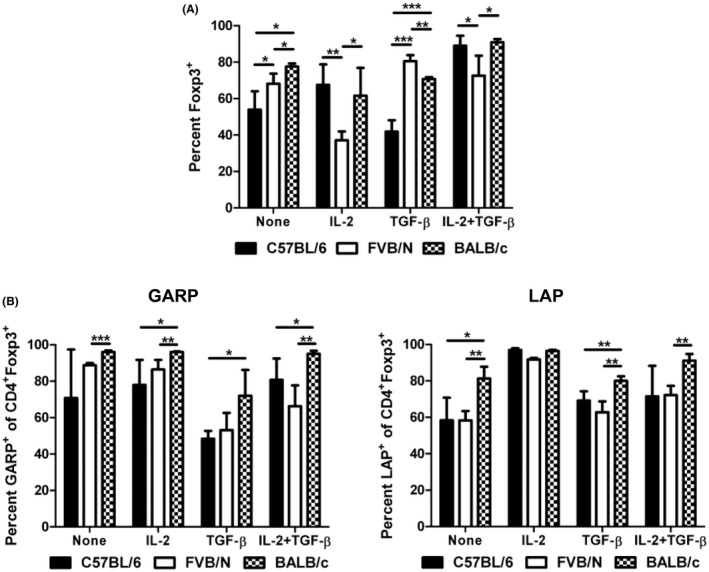
Different expressions of Foxp3, GARP, and LAP in activated Treg cells. (A) CD4+CD25+ Treg cells were isolated from the spleen via MACS and cultured for 4 days with anti‐CD3 and anti‐CD28 stimulation (2 × 106 cells/well in a 96‐well round‐bottom plate). IL‐2, TGF‐β, or both were used to maintain the expression of Foxp3 in these cells. Foxp3 expression was analyzed by flow cytometry. Lymphocytes were gated based on forward scatter/side scatter and then subsequently gated on CD4 + cells. (B) GARP and LAP expression on activated CD4+Foxp3+ tTreg cells. CD4+CD25+ cells were cultured for 2 days with anti‐CD3 and anti‐CD28 stimulation. Il‐2 and TGF‐β were added as indicated. After 2 days of culture, Foxp3, GARP, and LAP expression were analyzed by flow cytometry. Lymphocytes were gated based on forward scatter/side scatter and then subsequently gated on CD4+Foxp3+ cells. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001. Data are representative of 3 independent experiments, with 4 mice per group. Mean + standard deviation is shown.
3.7. C57BL/6, FVB/N, and BALB/c‐activated Tregs display different levels of GARP and LAP on the cell surface
Due to the altered expression of GARP and LAP seen on freshly isolated splenic Tregs (Figure 3B) and the altered maintenance of Foxp3 expression on CD4+CD25+ (Figures 5A) between C57BL/6, FVB/N, and BALB/c mice, we next investigated the expression of GARP and LAP on activated tTregs. GARP and LAP have both been identified on Foxp3+ Tregs. 42 TGF‐β is produced in a complex with LAP, and LAP must be cleaved for TGF‐β to become active. 43 GARP has been shown to be associated with LAP on the cell surface and GARP is believed to be a downstream effector molecule of Foxp3 expression in humans, although this is less clear in murine systems. 42 , 44 To study the expression of GARP and LAP on activated Tregs, CD4+CD25+ cells were isolated from splenocytes and cultured with or without IL‐2 and/or TGF‐β for 48 h in the presence of anti‐CD3 and anti‐CD28. GARP and LAP expression on CD4+Foxp3+ cells was then analyzed by flow cytometry (Figure 5B). In several cases, the GARP expression on activated BALB/c Foxp3+ cells was increased relative to both the C57BL/6 (IL‐2, TGF‐β, and IL‐2 + TGF‐β cultures) and FVB/N (no cytokine, IL‐2, and IL‐2 + TGF‐β cultures). LAP was increased on BALB/c Foxp3+ cells compared to FVB/N Foxp3+ cells with no cytokine, TGF‐β, and IL‐2 + TGF‐β. The high percentage of GARP+ and LAP+ cells in all activated Treg cultures suggests the importance of surface TGF‐β in the function of tTreg cells. Activated Tregs are known to express higher levels of GARP than naïve Tregs, explaining the discrepancy in GARP expression between freshly isolated splenic cells and cultured tTreg cells. 42
3.8. Differential generation of induced Treg cells between C57BL/6, FVB/N, and BALB/c naïve CD4 + CD25 − cells
Next, the ability of CD4+CD25− cells to become Foxp3+ iTreg cells was analyzed. Again, these cells were MACS isolated and cultured for 96 h in the presence of IL‐2, TGF‐β, or in combination (Figure 6A). Approximately 2%–4% of the CD4+CD25− starting population was Foxp3+. As expected, no Foxp3 induction was observed when these cells were cultured with no cytokines, or with IL‐2. However, upon culture with TGF‐β, C57BL/6 CD4+CD25− cells generated significantly more Foxp3+ cells than BALB/c CD4+CD25− cells. A similar result was observed when both IL‐2 and TGF‐β were used for culture, and the C57BL/6 CD4+CD25− cells generated significantly more Foxp3+ cells than FVB/N CD4+CD25− cells as well.
FIGURE 6.
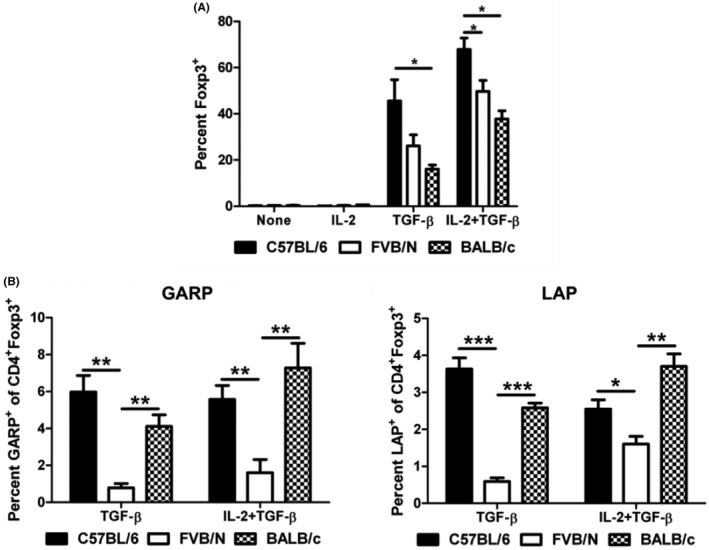
Differential iTreg induction and GARP and LAP expression between C57BL/6, FVB/N, and BALB/c mice. (A) In vivo iTreg generation. Splenic CD4+CD25− naïve T cells were cultured for 4 days with anti‐CD3 and anti‐CD28 stimulation (2 x 106 cells/well in a 96‐well round‐bottom plate). IL‐2 and TGF‐β were used to induce the expression of Foxp3 to generate iTreg cells. Foxp3 expression was analyzed by flow cytometry. Lymphocytes were gated based on forward scatter/side scatter and then subsequently gated on CD4+ cells. (B) GARP and LAP expression on CD4+Foxp3+ iTreg cells. CD4+CD25− cells were cultured for 4 days to induce Foxp3 expression. After 4 days, Foxp3, GARP, and LAP expression were analyzed by flow cytometry. Lymphocytes were gated based on forward scatter/side scatter and then subsequently gated on CD4+Foxp3+ cells. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Data are representative of 3 independent experiments with 4 mice per group, and 3 replicates per mouse. Mean + standard error of the mean shown.
3.9. FVB/N iTregs express significantly less cell surface GARP and LAP
To further investigate the role of GARP and LAP on iTregs, MACS isolated CD4+CD25− were cultured with or without IL‐2 and/or TGF‐β for 96 h in the presence of anti‐CD3 and anti‐CD28. Foxp3, GARP, and LAP expression on CD4+ cells were analyzed by flow cytometry (Figure 6B). Very few Foxp3+ cells were detected in the no cytokine and IL‐2 cultures. When Foxp3+ Treg cells were induced utilizing TGF‐β or TGF‐β and IL‐2, low‐level GARP and LAP expression was detected on C57BL/6 and BALB/c Foxp3+ cells. However, FVB/N cells almost no expression of LAP and GARP after iTreg generation. Although the population of GARP+ and LAP+ populations was small in both the C57BL/6 and BALB/c cultures, this demonstrates a potential role for GARP/LAP‐associated TGF‐β in these strains, which is absent in the FVB/N animals.
4. DISCUSSION
Most past and current murine regulatory T cell studies have been performed on C57BL/6 and BALB/c animals. The first Foxp3‐GFP reporter mice were generated on the C57BL/6 background, providing an excellent tool for investigating the development and function of Treg cells. 53 However, this has also created a bias toward the C57BL/6 in the current literature. Recently, differences between the C57BL/6 and BALB/c animals have been shown. A secreted factor was important for C57BL/6 Treg function, while target cell apoptosis was a more prevalent mechanism in BALB/c Treg suppression. 26 Detailed differences between Tregs from C57BL/6, BALB/c, and Type 1 diabetes‐prone NOD mice have recently been reported, and significant impairment of iTreg function has also been demonstrated in the NOD mouse. 31 , 32 In this study, we have further investigated the differences in Treg cells by examining the Treg phenotype in the FVB/N inbred strain compared to C57BL/6 and BALB/c Tregs.
Splenic FVB/N and C57BL/6 Tregs displayed similar effectiveness in suppressing Teff cell proliferation when co‐cultured, suggesting that both strains' splenic Tregs can successfully suppress Teff cell activity in a cell‐contact dependent manner. However, when supernatants from FVB/N Tregs were cultured with Teff cells from FVB/N or C57BL/6 mice, there was a decreased ability to prevent Teff cell proliferation compared to C57BL/6 Treg supernatant. The inability of the FVB/N Treg supernatant to prevent Teff cell proliferation over baseline suggests that FVB/N Tregs require cell contact for optimal suppression, while C57BL/6 can suppress via secreted factors without cell contact.
Our studies show that the immunosuppressive factor IL‐10 is secreted effectively by FVB/N splenic Tregs, even at higher levels than C57BL/6 Treg cells. This would suggest that IL‐10 is not the critical factor that is produced by C57BL/6 Treg cells. Our studies were not able to identify the soluble factor produced by C57BL/6 Tregs; however, others have identified IL‐35 or TGF‐β as likely candidates. 26
Also interesting was the extremely high expression of the cathepsin E gene, Ctse, in FVB/N splenic Tregs. Cathepsin E is utilized to activate TRAIL and induce target cell apoptosis. TRAIL is effective in both a soluble form and bound to the cell surface. The high expression of Ctse in both FVB/N and BALB/c CD4+CD25+ cells compared to C57BL/6 cells may highlight the requirement of this mechanism in FVB/N and BALB/c Treg cell suppression. The role of TRAIL has yet to be investigated in FVB/N Treg function, but BALB/c Tregs have been shown to be dependent on Cathepsin E/TRAIL for immunoregulation. 26 Further studies are necessary, but if FVB/N Tregs can only utilize cathepsin E/TRAIL present on the cell surface, it would support the hypothesis that FVB/N Tregs require cell contact for effective suppression. While the mechanism of cathepsin E/TRAIL is outside the scope of this study, mRNA levels and protein levels have been closely linked, supporting the role of cathepsin E/TRAIL in FVB/N Treg function. 54
Differences were also detected in the phenotype of splenic FVB/N and C57BL/6 Foxp3+ Treg cells, as well as BALB/c splenic Foxp3+ Treg cells. Interestingly, FVB/N animals displayed a significant decrease in Foxp3+ cells, indicating that they may have fewer tTreg cells circulating. Several differences were also observed in the Foxp3+ cell phenotypes. Most notable was the significant decrease in LAP and GARP expression of BALB/c and FVB/N Treg cells compared to the C57BL/6. Although decreases in CD101 and GITR were also observed, the change in LAP and GARP is particularly interesting, indicating differential utilization of cell surface TGF‐β. Because GARP and LAP have been shown to be associated with both Foxp3 and membrane‐bound TGF‐β expression, decreased levels of these proteins could have significant effects on the function of the Treg cells. 42 , 43 , 44 It is thought that TGF‐β may act primarily via a cell‐surface mechanism, by presenting TGF‐β to effector cells to initiate immunosuppression. 55 Our data would indicate usage in a cell‐contact method by FVB/N. C57BL/6 may use increased LAP/GARP to further enhance immunosuppression via a cell‐contact mechanism, and may also utilize TGF‐β in a secreted form. 56 This difference in usage may impact the ability to modulate LAP/GARP for TGF‐β immunosuppression in potential anti‐tumor or autoimmune treatments. 57
In addition to altered GARP and LAP expression on freshly isolated splenocytes, GARP and LAP levels were significantly decreased on FVB/N iTreg cells generated by culture with TGF‐β or TGF‐β + IL‐2. GARP and LAP levels were generally found to be higher on BALB/c tTregs that had been activated for 2 days with anti‐CD3 and anti‐CD28 stimulation.
Taken with the Ctse data, this information suggests the C57BL/6 animals may be more dependent upon surface‐bound TGF‐β, while FVB/N Tregs utilize cathepsin E to effectively suppress immune cells in a cell contact manner, although additional studies are required to investigate the true role of cathepsin E/TRAIL. BALB/c Tregs have already been shown to be more dependent upon cathepsin E/TRAIL than C57BL/6, so it is not surprising that BALB/c Tregs would have different expression of various markers. 26
The maintenance of Foxp3 expression in CD4+CD25+ Treg cells also varied considerably among C57BL/6, FVB/N and BALB/c animals. FVB/N cells lost significant levels of Foxp3 expression after culture with IL‐2. Because Foxp3 expression was maintained relatively well when no cytokine was used in culture, this suggests that IL‐2 alone may cause a downregulation of Foxp3. This could result in these cells becoming Teff cells, and losing the suppressive activity of a Treg cell. This hypothesis is supported by the decreased levels of Helios observed in FVB/N mice, as Helios has been shown to stabilize the expression of Foxp3. 5 , 13 , 14 We cannot rule out the possibility that a rapid expansion of effector T cells may have contaminated the initial culture, leading to reduced expression of Foxp3 in the culture as Foxp3− cells rapidly divided. Contrary to the FVB/N, the C57BL/6 cells lost Foxp3 expression upon TGF‐β only culture but maintained Foxp3 during IL‐2 culture. Although it is known that IL‐2 is required for Treg survival and expansion, this suggests that this dependence may be magnified in C57BL/6 Tregs relative to FVB/N and BALB/c Tregs. 58 , 59 BALB/c Tregs maintained relatively similar levels in all culture environments, suggesting that BALB/c Tregs may be the most stable of the three strains examined.
BALB/c, C57BL/6, and FVB/N also display different levels of Foxp3 induction in vitro, indicating different strategies of Treg sources. BALB/c CD4+CD25− were significantly less likely to express Foxp3 after culture with TGF‐β or culture with IL‐2 + TGF‐β than C57BL/6 CD4+CD25− cells. FVB/N Tregs were also significantly less likely to become iTreg cells when cultured with IL‐2. These experiments imply that C57BL/6 naïve CD4+CD25− cells are more prone to becoming Foxp3+ regulatory T cells. Thus, C57BL/6 animals may rely more on the activity of pTregs/iTregs to maintain immune homeostasis compared to FVB/N and BALB/c mice.
This study continues our understanding of differences in splenic Treg phenotype and function between inbred mouse strains. Although both C57BL/6 and FVB/N splenic Treg cells appear to function similarly in a cell contact‐dependent environment, FVB/N Tregs display an impaired ability to suppress when examining potential secreted factors. In addition, the high expression of TGF‐β‐related molecules GARP and LAP on C57BL/6 Tregs, and high expression of the cathepsin E gene, Ctse, on FVB/N Tregs suggests that these two strains may have different cell contact mechanisms of action. Because C57BL/6 mice have dominated the current Treg literature, it may be necessary to return to many early Treg studies and examine them in light of the differences described in this study. Additional avenues of study include the investigation of phenotype and function of thymic and peripheral‐derived Treg cells between inbred mouse strains.
This information could contribute to the further understanding of human Treg cells as well. The study presented above indicates a strong genetic component in the determination of Treg phenotype and function. Three inbred strains of mice clearly demonstrate different mechanisms of action. Due to the extreme genetic heterogeneity of the human population, it is likely that there are numerous ways in which human Treg acts, varying from person to person. As Tregs develop into potential therapeutic targets, it will be vital to fully understand all the mechanisms of action of human Tregs and how they differ from individual to individual, or group to group, within a population. 37
AUTHOR CONTRIBUTIONS
S. Tanner and R. Lorenz designed research; S. Tanner performed research; S. Tanner and R. Lorenz analyzed data; S. Tanner and R. Lorenz wrote the paper.
CONFLICT OF INTEREST
RGL is a current employee of Genentech, a member of the Roche group, and may hold Roche stock or stock options. SMT declares no competing interests.
Supporting information
Figure S1
ACKNOWLEDGMENTS
The authors would like to thank Kurt Zimmerman and Joanne Murphy‐Ulrich for their assistance in the TMLEC measurement of TGF‐β, and the laboratory of John Mountz for the use of their radiation harvester and β‐counter. We would also like to thank Mason Harris for animal husbandry and members of the Lorenz lab for valuable advice. This study was supported in part by National Institutes of Health (NIH) grants R01 DK059911; P01 DK071176; Crohn's and Colitis Foundation of America Senior Research Award 26971, Juvenile Diabetes Research Foundation Research Grant 36–2008‐930, and the University of Alabama at Birmingham Digestive Diseases Research Development Center grant P30 DK064400. SMT was partially supported by the Howard Hughes Medical Institute Med into Grad Fellowship and the University of Alabama at Birmingham Carmichael Fund. Aspects of this project were conducted in a biomedical research space that was constructed with funds supported in part by National Institutes of Health grant C06RR020136.
Tanner SM, Lorenz RG. FVB/N mouse strain regulatory T cells differ in phenotype and function from the C57BL/6 and BALB/C strains. FASEB BioAdvances. 2022;4:648‐661. doi: 10.1096/fba.2021-00161
REFERENCES
- 1. Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X‐linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20‐21. [DOI] [PubMed] [Google Scholar]
- 2. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057‐1061. [PubMed] [Google Scholar]
- 3. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330‐336. [DOI] [PubMed] [Google Scholar]
- 4. Sakaguchi S, Wing K, Onishi Y, Prieto‐Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105‐1111. [DOI] [PubMed] [Google Scholar]
- 5. Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014;259:88‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen WJ, Jin W, Hardegen N, et al. Conversion of Peripheral CD4 + CD25 ‐ Naive T Cells to CD4 + CD25 + Regulatory T Cells by TGF‐β Induction of Transcription Factor Foxp3. J Exp Med. 2003;198:1875‐1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF‐β induces a regulatory phenotype in CD4 + CD25 − T cells through Foxp3 induction and down‐regulation of Smad7. J Immunol. 2004;172:5149‐5153. [DOI] [PubMed] [Google Scholar]
- 8. Coombes JL, Siddiqui KRR, Arancibia‐Cárcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF‐β ‐and retinoic acid‐dependent mechanism. J Exp Med. 2007;204:1757‐1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rao PE, Petrone AL, Ponath PD. Differentiation and expansion of T cells with regulatory function from human peripheral lymphocytes by stimulation in the presence of TGF‐β. J Immunol. 2005;174:1446‐1455. [DOI] [PubMed] [Google Scholar]
- 10. Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204(8):1775‐1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic‐derived from peripherally induced Foxp3 + T regulatory cells. J Immunol. 2010;184:3433‐3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3 + regulatory T cells. J Immunol. 2012;188:976‐980. [DOI] [PubMed] [Google Scholar]
- 13. Kim HJ, Barnitz RA, Kreslavsky T, et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science. 2015;350:334‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thornton AM, Lu J, Korty PE, et al. Helios + and Helios − Treg subpopulations are phenotypically and functionally distinct and express dissimilar TCR repertoires. Eur J Immunol. 2019;49:398‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fernandez I, Zeiser R, Karsunky H, et al. CD101 surface expression discriminates potency among murine FoxP3 + regulatory T cells. J Immunol. 2007;179:5605. [DOI] [PubMed] [Google Scholar]
- 16. Schey R, Dornhoff H, Baier JLC, et al. CD101 inhibits the expansion of colitogenic T cells. Mucosal Immunol. 2016;9:1205‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sabat R, Grütz G, Warszawska K, et al. Biology of interleukin‐10. Cytokine Growth Factor Rev. 2010;21:331‐344. [DOI] [PubMed] [Google Scholar]
- 18. Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL‐35 contributes to regulatory T‐cell function. Nature. 2007;450:566‐569. [DOI] [PubMed] [Google Scholar]
- 19. Collison LW, Chaturvedi V, Henderson AL, et al. IL‐35‐mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093‐1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tran DQ. TGF‐β: the sword, the wand, and the shield of FOXP3 + regulatory T cells. J Mol Cell Biol. 2012;4:29‐37. [DOI] [PubMed] [Google Scholar]
- 21. Shevach EM. Mechanisms of Foxp3+ T regulatory cell‐mediated suppression. Immunity. 2009;30:636‐645. [DOI] [PubMed] [Google Scholar]
- 22. Gueders MM, Paulissen G, Crahay C, et al. Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm Res. 2009;58:845‐854. [DOI] [PubMed] [Google Scholar]
- 23. Liu T, Matsuguchi T, Tsuboi N, Yajima T, Yoshikai Y. Differences in expression of Toll‐like receptors and their reactivities in dendritic cells in BALB/c and C57BL/6 mice. Infect Immun. 2002;70(12):6638‐6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh RP, Bischoff DS. Sex hormones and gender influence the expression of markers of regulatory T Cells in SLE patients. Front Immunol. 2021;12:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hsieh CS, Macatonia SE, O'Garra A, Murphy KM. T cell genetic background determines default t helper phenotype development in vitro. J Exp Med. 1995;181:713‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pillai MR, Collison LW, Wang X, et al. The plasticity of regulatory T Cell function. J Immunol. 2011;187:4987‐4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen X, Oppenheim JJ, Howard OMZ. BALB/c mice have more CD4+CD25+ T regulatory cells and show greater susceptibility to suppression of their CD4+CD25‐ responder T cells than C57BL/6 mice. J Leukoc Biol. 2005;78:114‐121. [DOI] [PubMed] [Google Scholar]
- 28. Tellier J, van Meerwijk JPM, Romagnoli P. An MHC‐linked locus modulates thymic differentiation of CD4+ CD25+Foxp3+ regulatory T lymphocytes. Int Immunol. 2006;18:1509‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Serreze DV, Leiter EH. Genes and cellular requirements for autoimmune diabetes susceptibility in nonobese diabetic mice. Curr Dir Autoimmun. 2001;4:31‐67. [DOI] [PubMed] [Google Scholar]
- 30. Gonzalez A, Katz JD, Mattei MG, Kikutani H, Benoist C, Mathis D. Genetic control of diabetes progression. Immunity. 1997;7:873‐883. [DOI] [PubMed] [Google Scholar]
- 31. Godoy GJ, Paira DA, Olivera C, et al. Differences in T regulatory cells between mouse strains frequently used in immunological research: Treg cell quantities and subpopulations in NOD, B6 and BALB/c mice. Immunol Lett. 2020;223:17‐25. [DOI] [PubMed] [Google Scholar]
- 32. D'Alise AM, Ergun A, Hill JA, Mathis D, Benoist C. A cluster of coregulated genes determines TGF‐β ‐ Induced regulatory T‐cell (Treg) dysfunction in NOD mice. Proc Natl Acad Sci U S A. 2011;108:8737‐8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taketo M, Schroeder AC, Mobraaten LE, et al. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci U S A. 1991;88:2065‐2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haluzik M, Colombo C, Gavrilova O, et al. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinol. 2004;145:3258‐3264. [DOI] [PubMed] [Google Scholar]
- 35. Nascimento‐Sales M, Fredo‐da‐Costa I, Borges Mendes ACB, et al. Is the FVB/N mouse strain truly resistant to diet‐induced obesity? Physiol Rep. 2017;5:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park JW, Kim JE, Kang MJ, Choi HJ, Bae SJ, Hwang DY. Compensatory role of C3 convertase on the strain difference for C3 protein expression in FVB/N, C3H/HeN and C57BL/6N mice. Lab Anim Res. 2020;36:4‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Esensten JH, Muller YD, Bluestone JA, Tang Q. Regulatory T‐cell therapy for autoimmune and autoinflammatory diseases: the next frontier. J Allergy Clin Immunol. 2018;142:1710‐1718. [DOI] [PubMed] [Google Scholar]
- 38. Kim EM, Bae YM, Choi MH, Hong ST. Cyst formation, increased anti‐inflammatory cytokines and expression of chemokines support for Clonorchis sinensis infection in FVB mice. Parasitol Int. 2012;61:124‐129. [DOI] [PubMed] [Google Scholar]
- 39. Zhang BB, Yan C, Fang F, et al. Increased hepatic Th2 and Treg subsets are associated with biliary fibrosis in different strains of mice caused by Clonorchis sinensis. PLoS One. 2017;12:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McHugh RS, Whitters MJ, Piccirillo CA, et al. CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid‐induced TNF receptor. Immunity. 2002;16(2):311‐323. [DOI] [PubMed] [Google Scholar]
- 41. Ephrem A, Epstein AL, Stephens GL, Thornton AM, Glass D, Shevach EM. Modulation of Treg cells/T effector function by GITR signaling is context‐dependent. Eur J Immunol. 2013;43(9):2421‐2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13439‐13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF‐β on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13445‐13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oida T, Weiner HL. TGF‐β induces surface LAP expression on murine CD4 T cells independent of Foxp3 induction. PLoS One. 2010;5:5‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kimberley FC, Screaton GR. Following a TRAIL: update on a ligand and its five receptors. Cell Res. 2004;14:359‐372. [DOI] [PubMed] [Google Scholar]
- 46. Wang S, El‐Deiry WS. TRAIL and apoptosis induction by TNF‐family death receptors. Oncogene. 2003;22:8628‐8633. [DOI] [PubMed] [Google Scholar]
- 47. Ren X, Ye F, Jiang Z, Chu Y, Xiong S, Wang Y. Involvement of cellular death in TRAIL/DR5‐dependent suppression induced by CD4+CD25+ regulatory T cells. Cell Death Differ. 2007;14:2076‐2084. [DOI] [PubMed] [Google Scholar]
- 48. Zhou X, Bailey‐Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4+ FoxP3+ T cells. Curr Opin Immunol. 2009;21:281‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou X, Bailey‐Bucktrout SL, Jeker LT, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wohlfert E, Belkaid Y. Plasticity of T reg at infected sites. Mucosal Immunol. 2010;3:213‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Murai M, Krause P, Cheroutre H, Kronenberg M. Regulatory T‐cell stability and plasticity in mucosal and systemic immune systems. Mucosal Immunol. 2010;3:443‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McClymont SA, Putnam AL, Lee MR, et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol. 2011;186:3918‐3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity. 2005;22:329‐341. [DOI] [PubMed] [Google Scholar]
- 54. Gonçalves NP, Moreira J, Martins D, et al. Differential expression of Cathepsin E in transthyretin amyloidosis: from neuropathology to the immune system. J Neuroinflammation. 2017;14:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nakamura K, Kitani A, Strober W. Cell contact‐dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface‐bound transforming growth factor β. J Exp Med. 2001;194(5):629‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Josefowicz SZ, Lu L‐F, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shevach EM. Garp as a therapeutic target for modulation of T regulatory cell function. Expert Opin Ther Targets. 2017;21:191‐200. [DOI] [PubMed] [Google Scholar]
- 58. Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation–mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353‐1362. [DOI] [PubMed] [Google Scholar]
- 59. Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


