Abstract
Objectives:
Stand-alone surgical ablation(SA) of atrial fibrillation(AF) is indicated in patients with refractory AF who have failed medical and/or catheter-based ablation. Few reports of late outcomes following stand-alone SA exist using comprehensive follow-up with strict definitions of success. This study examined our late outcomes of the stand-alone Cox-Maze IV(CMP-IV) procedure.
Methods:
Between January 2003 and December 2019, 236 patients underwent a stand-alone CMP-IV for refractory AF. Freedom from atrial tachyarrhythmias(ATAs) was assessed by electrocardiography, Holter, and/or pacemaker interrogation for up to 10 years, with a mean follow-up of 4.8±3.5years. Rhythm outcomes were compared in multiple subgroups. Factors associated with recurrence were determined using Fine-Gray regression, allowing for death as the competing risk.
Results:
The majority of patients (176/236,75%) had non-paroxysmal AF. Median duration of preoperative AF was 6.2 years(IQR[3,11]). Fifty-nine percent of patients(140/236) failed ≥ 1 prior catheter-based ablation. Thirteen patients(6%) experienced a major complication. There was no 30-day mortality. Freedom from ATAs was 94%(187/199), 89%(81/91), and 77%(24/31) at 1, 5, and 10 years, respectively. There was no difference in freedom from ATAs between patients with paroxysmal AF versus non-paroxysmal AF(P>0.05), or those undergoing sternotomy versus a minimally-invasive approach(P>0.05). Increased left atrial size and number of catheter ablations were associated with late AF recurrence. For patients who experienced any ATAs recurrence, the median number of recurrences was 1.5[1.0,3.0].
Conclusions:
The stand-alone CMP-IV had excellent late efficacy at maintaining SR in patients with symptomatic, refractory AF, with low morbidity and no mortality. The CMP-IV, in contrast to catheter-based ablation, was equally effective in patients with paroxysmal and non-paroxysmal AF.
Keywords: Surgical Ablation, Cox Maze Procedure, Atrial Fibrillation, Long-term Outcomes
Category: Adult, Acquired Cardiovascular Disease
Background
Atrial fibrillation(AF) is the most common sustained cardiac arrhythmia, and carries significant morbidity and mortality due to the risk of hemodynamic compromise and stroke.[1,2] Treatment with anti-arrhythmic drugs remains disappointing due to poor efficacy and adverse side effects, necessitating referral for interventional treatment.
The Cox-Maze procedure(CMP), developed by Dr. Cox and colleagues in 1987, is the most effective surgical treatment for AF.[3] The initial “cut-and-sew” technique, the Cox-Maze III procedure(CMP-III), employed biatrial incisions to interrupt the reentrant circuits felt to be responsible for AF while allowing myocardial electrical impulses to progress from the sinoatrial to atrioventricular(AV) node.[4] Despite the procedure’s excellent success, the operation was not widely used due to its technical complexity and prolonged cardiopulmonary bypass(CPB) time.[5–6]
Over the past two decades, the CMP-III surgical incisions were replaced by ablations made by different energy sources, including radiofrequency(RF) energy and cryoablation.[7] The Cox-Maze IV procedure(CMP-IV), introduced by Damiano and colleagues in 2002, utilizes RF and cryoablation technology to reliably create transmural lesions in a simpler, faster, and less invasive manner.[8,9] Our group and others showed the CMP-IV has excellent efficacy in maintaining sinus rhythm(SR) at short and long-term follow-up, equivalent to that seen with the CMP-III with decreased morbidity and CPB times.[10–13]
These advances in technology and surgical techniques led to increased numbers of stand-alone SA procedures performed annually.[14,15] Unfortunately, the majority of SA procedures have employed epicardial ablations limited to the LA on the beating heart, which have been shown to be less effective than a complete biatrial CMP – the preferred approach by our institution.[16,17] Our group previously showed the stand-alone CMP-IV was equally effective in restoring SR in patients with paroxysmal and non-paroxysmal AF at early follow-up.[18] However, few studies are available with long-term follow-up using strict definitions of success following stand-alone SA in a sizable patient cohort.[19–21] This study examined our late outcomes of the stand-alone CMP-IV in lone AF patients.
Methods
This study was approved by the Washington University School of Medicine Institutional Review Board. Informed consent and permission for release of information were obtained from all patients. The data were prospectively entered into a longitudinal database maintained at our institution, including demographic data, operative details, and perioperative results using STS definitions for complications. Rhythm and other follow-up data were prospectively entered into our institutional AF outcomes database. Missing data were ascertained through chart review, contact with patients, and from referring physicians when needed.
Patient Population
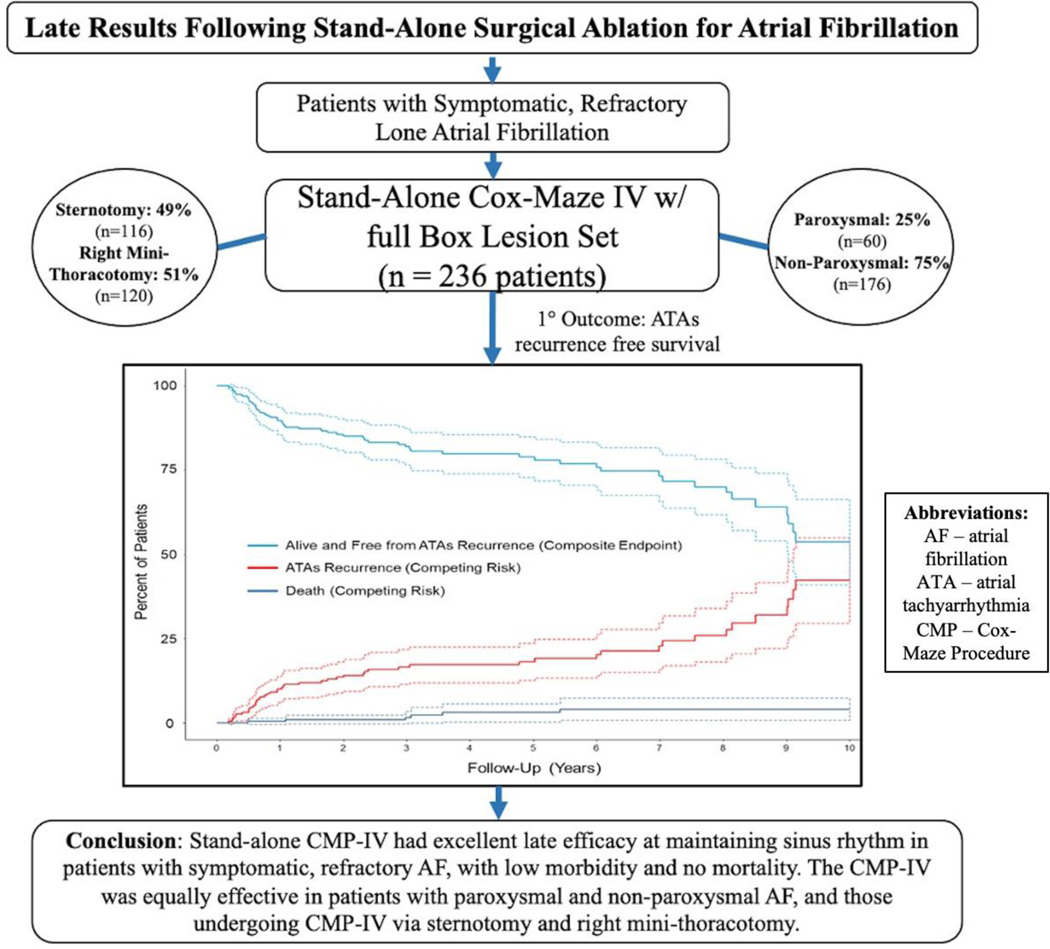
From January 2001 to December 2019, 259 patients with lone AF underwent stand-alone SA. Patients who underwent a CMP-III or other SA techniques, or did not have a complete CMP-IV (i.e. incomplete posterior LA wall isolation), were excluded(n=23). Of the initial patients screened, 236 patients underwent biatrial CMP-IV. The patients were subsequently divided into two cohorts based on AF type: paroxysmal(n=60) and non-paroxysmal AF(n=176). The operative techniques of the CMP-IV through either a median sternotomy or a right mini-thoracotomy(RMT), our preferred approach, have been previously described.[22,23]
Follow-Up and Postoperative Care
Patient follow-up was performed at 3, 6, and 12 months, and annually thereafter. Patients underwent history and physical evaluations and electrocardiograms(ECGs) at each visit. Routine prolonged monitoring was initiated in 2006 and included 24-hr Holter monitoring, pacemaker interrogation, or implantable loop recording(ILR). During the study, 89%(210/236) of patients underwent prolonged monitoring during their follow-up. Fifty-seven percent(52/91) and 52%(16/31) of patients underwent prolonged monitoring at 5 and 10-year follow-up, respectively. Based on the Heart Rhythm Society(HRS) 2017 consensus statement, recurrence was defined as any episode of AF, atrial flutter, or atrial tachyarrhythmia(ATA) longer than 30 seconds.[24]
The study examined freedom from recurrence using two methods. First, ATAs recurrence was considered a permanent failure and evaluated as time to first recurrence, with death as a competing risk. This was the strictest definition of recurrence. Patients with documented ATAs recurrences were historically followed more closely than those that remained in sinus rhythm. Use of this methodology minimized bias in follow-up when evaluating predictors of ATAs recurrence. Second, ATA recurrence was examined at discrete follow-up time points, such as 1, 5, and 10 years. In this preferred method, a patient could have recurred at early follow-up, but remained free from ATAs on consecutive Holter monitoring recordings at late follow-up. This is an important method of determining recurrence since the definition of failure is strict (>30 seconds of ATAs), For instance, patients could have a single 45 second episode of AF at 6 months and then no further episodes for the next 5–10 years, and by the first method would be considered a permanent “failure,” rather than an isolated recurrence at 6 months. In fact, 49% of patients who recurred had only a single recurrence.
Postoperative antiarrhythmic and anticoagulation drugs were administered to all patients unless contraindicated.[11] Patients who experienced postoperative ATAs unresponsive to antiarrhythmic drugs(AADs) were cardioverted prior to discharge unless contraindicated. Contraindications to cardioversion included a documented LA clot, or a contraindication to post-cardioversion anticoagulation. Sinus node recovery was allowed for 5–7 days in patients with persistent bradycardia due to junctional rhythm, after which a dual chamber pacemaker was inserted if patients remained symptomatic. AADs were discontinued for patients in SR at 2–3 months postoperatively. Anticoagulation was discontinued 3–6 months postoperatively if the patient was both free from ATAs on prolonged Holter monitoring, and had no evidence of atrial stasis or thrombus on transthoracic echocardiograms, irrespective of the patients’ CHA2DS2-VASc score.[25] The average follow-up time was 4.8±3.5 years (median 4.0 years,[2.0,7.3]). At 1, 5, and 10 years, 88%(199/227), 71%(91/129) and 52%(31/60) of patients available for follow-up had documented rhythm data.
Study Design
Forty demographic and perioperative variables were compared among the multiple subgroups. Freedom from ATAs on or off AADs was compared between paroxysmal(n=60) and non-paroxysmal(n=176) AF groups, as well as between those who underwent either a sternotomy(n=116) or RMT(n=120) approach at 1–10 years postoperatively.
Statistical Analysis
Continuous variables were expressed as mean±standard deviation or as a median with interquartile range, as appropriate. Student’s t-test compared means of normally distributed continuous variables, while Mann-Whitney U test was used for skewed distributions. Categorical variables were compared using either χ2 analysis or Fisher’s Exact test. Normality was tested using Kolmogorov-Smirnov or Shapiro-Wilk tests (when appropriate). A P-value <0.05 was considered statistically significant. Composite endpoint survival (freedom from first ATAs recurrence and death) was reported as a Kaplan-Meier estimate. The probability of being both alive and ATAs-recurrence free is equivalent to the probability of experiencing neither of the competing risks, as described below.[26] Competing risk methodology evaluated ATAs recurrence, with death during follow-up period as the competing risk.[27] Gray’s test compared the incidence of first ATAs recurrence between groups (paroxysmal vs non-paroxysmal, sternotomy vs RMT). Sixteen clinically-relevant variables with a minimum of 10 events per variable were evaluated using adjusted multivariable Fine-Gray regression to identify factors associated with ATAs recurrence. Data analysis was performed using SPSS version 25 (SPSS Inc., Chicago, IL, USA) and R3.6.1 using the cmprsk package (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Demographics
The overall mean age of patients at the time of stand-alone CMP-IV was 59.0±10.4 years. In the entire cohort, 25%(60/236) had paroxysmal AF and 75%(176/236) had non-paroxysmal AF, of which 91%(161/176) had longstanding persistent AF. Compared to the paroxysmal AF patients, non-paroxysmal AF patients were older (60.1±10.1years vs 55.9±10.7,P=0.007) and more likely to be male (127/176(72%) vs 32/60(63%),P=0.010; Table 1). No statistically significant differences existed between the groups in regard to BMI, hypertension, diabetes, or incidence of prior stroke (27/176(15%) vs 4/40(7%),P=0.120). In addition, the non-paroxysmal and paroxysmal AF groups had similar proportion of patients with CHA2DS2-VASc score ≥2 (129/176(73%) vs 42/60(70%),P=0.244).
Table 1.
Patient Demographics
| Variable | Paroxysmal AF (n=60) | Non-Paroxysmal AF (n=176) | P value |
|---|---|---|---|
| Age (years), mean±std | 55.9±10.7 | 60.1±10.1 | 0.007 |
| BMI (kg/m2), mean±std | 32.2±7.1 | 32.0±6.9 | 0.831 |
| Male gender, n(%) | 32(63.0) | 127(72.2) | 0.010 |
| Caucasian, n(%) | 58(96.7) | 176(100) | 0.064 |
| NYHA class III or IV, n(%) | 27(45.0) | 73(41.5) | 0.652 |
| Prior Myocardial Infarction, n(%) | 6(10.0) | 8(4.5) | 0.201 |
| Smoker, n(%) | 17(28.3) | 63(35.8) | 0.345 |
| Chronic lung disease, n(%) | 5(8.3) | 18(10.2) | 0.183 |
| Diabetes, n(%) | 6(10.0) | 23(13.1) | 0.652 |
| Hypertension, n(%) | 34(56.7) | 117(66.5) | 0.213 |
| Hyperlipidemia, n(%) | 26(43.3) | 95(54.0) | 0.179 |
| Peripheral vascular disease, n(%) | 2(3.3) | 9(5.1) | 0.734 |
| Cerebrovascular disease, n(%) | 4(6.7) | 27(15.3) | 0.120 |
| Pre-operative creatinine, mean±std | 0.94±0.17 | 0.99±0.20 | 0.080 |
| Dialysis, n(%) | 0(0.0) | 0(0.0) | 1.000 |
| CHA2DS2-VASc Score ≥ 2, n(%) | 42(70.0) | 129(73.3) | 0.244 |
BMI=body mass index; NYHA=New York Heart Association; STD=standard deviation
Atrial Fibrillation and Hemodynamic Characteristics
Patients with non-paroxysmal AF had larger LA diameters (4.9±1.0cm vs 4.4±1.0,P=0.004) and lower left ventricular ejection fractions (54.0±13.2% vs 58.3±9.4%,P=0.018; Table 2 and Figure E5). There was no statistically significant difference in preoperative length of time in AF (median 7.0[3.0,11.0] years vs 5.0[2.5,8.3],P=0.533). A higher proportion of non-paroxysmal AF patients had a prior catheter ablation [117/176(67%) vs 23/60(38%),P<0.001], with a median of 2.0[1.0,3.0] ablations.
Table 2.
Patient Atrial Fibrillation Characteristics
| Variable | Paroxysmal AF (n=60) | Non-Paroxysmal AF (n=176) | P value |
|---|---|---|---|
| Length of time in AF (years), median[IQR] | 5.0[2.5,8.3] | 7.0[3.0,11.0] | 0.533 |
|
| |||
| LVEF (%), mean±std | 58.3±9.4 | 54.0±13.2 | 0.018 |
|
| |||
| Preoperative PM, n(%) | 2(3.3) | 11(6.3) | 0.525 |
|
| |||
| LA diameter (cm), mean±std | 4.40±0.97 | 4.85±1.0 | 0.004 |
|
| |||
| Prior Catheter Ablation, n(%) | 23(38.3) | 117(66.5) | <0.001 |
| Number of Ablations, median[IQR] | 2.0[1.0,2.3] | 2.0[1.0,3.0] | 0.588 |
AF=atrial fibrillation; IQR=interquartile range; LA=left atrium; LVEF=left ventricular ejection fraction; PM=pacemaker; STD=standard deviation
Perioperative Results
All patients in the study underwent a biatrial lesion set with LAA excision or exclusion. Fifty-two percent (91/176) of non-paroxysmal AF patients underwent median sternotomy, compared to 42%(25/60) of paroxysmal AF patients(P=0.232; Table 3). Overall, 49%(116/236) of patients underwent stand-alone CMP-IV by a minimally-invasive RMT. There was no difference in post-operative pacemaker rates [13/176(7%) vs 2/60(3%),P=0.367] or overall major complication rates [11/176(6%) vs 2/60(3%),P=0.525] between the two groups. Major complications included pneumonia, mediastinitis, cerebrovascular accident, renal failure requiring dialysis, intra-aortic balloon pump, and reoperation from bleeding. Of the 13 patients(6%) who endured a major complication, six (46%) experienced postoperative pneumonia. Overall, three patients(1%) experienced post-operative stroke, none of whom suffered permanent neurologic deficits. New pacemaker implantation for sinus node dysfunction occurred in 6%(15/236) of patients. There was no 30-day mortality.
Table 3.
Perioperative Outcomes
| Variable | Paroxysmal AF (n=60) | Non-Paroxysmal AF (n=176) | P value |
|---|---|---|---|
| Sternotomy, n(%) | 25(41.7) | 91(51.7) | 0.232 |
|
| |||
| Hospital length of stay (days), median[IQR] | 9.0[7.0,11.3] | 9.0[8.0,12.0] | 0.475 |
|
| |||
| ICU length of stay (hours), median[IQR] | 50.9[24.5,94.8] | 47.8[25.8,92.4] | 0.820 |
|
| |||
| CPB time (min), mean±std | 146.0±31.8 | 147.0±36.5 | 0.854 |
|
| |||
| Crossclamp time (min), mean±std | 56.7±21.8 | 53.3±19.1 | 0.264 |
|
| |||
| Post-operative creatinine, mean±std | 1.45±0.72 | 1.42±0.70 | 0.080 |
|
| |||
| Overall major complications, n(%) | 2(3.3) | 11(6.3) | 0.525 |
| Cerebrovascular accident, n(%) | 0(0.0) | 3(1.7) | |
| Pneumonia, n(%) | 1(1.7) | 5(2.8) | |
| Mediastinitis, n(%) | 0(0.0) | 1(0.6) | |
| Renal failure requiring dialysis, n(%) | 1(1.7) | 1(0.6) | |
| Intra-aortic balloon pump, n(%) | 0(0.0) | 1(0.6) | |
| Reoperation for bleeding, n(%) | 0(0.0) | 1(0.6) | |
|
| |||
| Post-operative PM, n(%) | 2(3.3) | 13(7.4) | 0.367 |
|
| |||
| 30 Day Mortality, n(%) | 0(0.0) | 0(0.0) | 1.000 |
AF=atrial fibrillation; CPB=cardiopulmonary bypass; ICU=intensive care unit; IQR=interquartile range; PM=pacemaker
When comparing outcomes of patients who underwent median sternotomy and RMT, patients who underwent RMT experienced longer cardiopulmonary bypass (168.4±26.8min vs 124.3±28.3,P<0.001) and cross-clamp (66.9±15.1min vs 41.0±14.9,P<0.001) times. There was no difference between groups in hospital and ICU length of stay, post-operative pacemaker rate, or overall major complication rate (Table E3).
Overall Efficacy
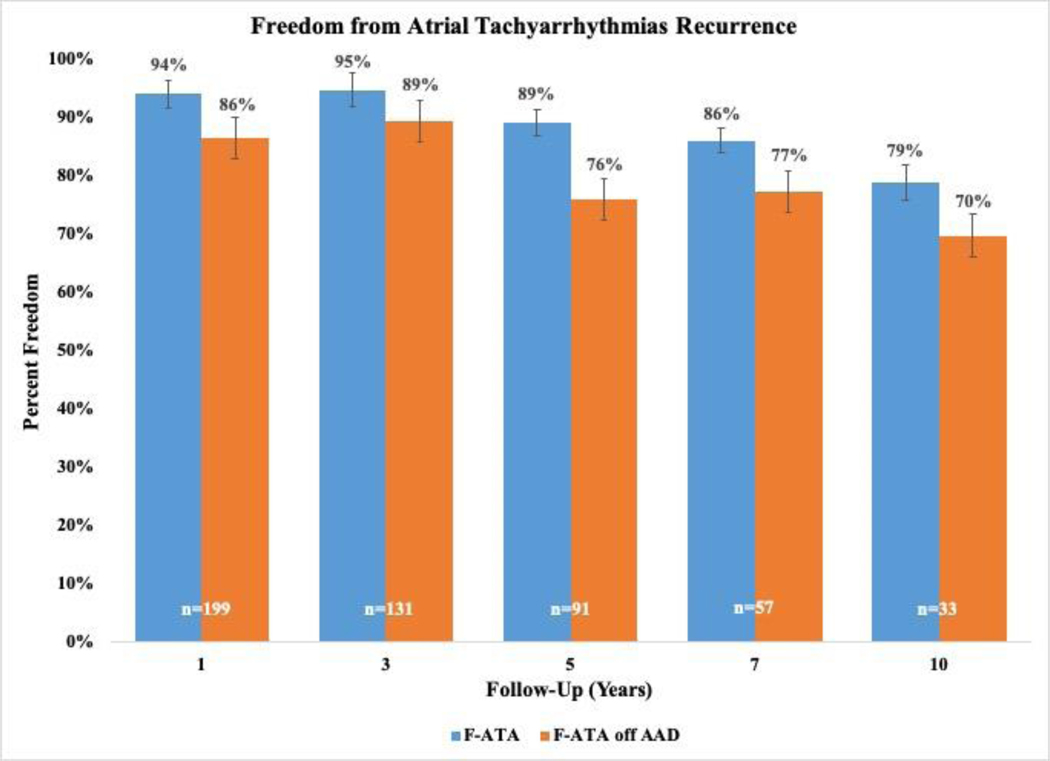
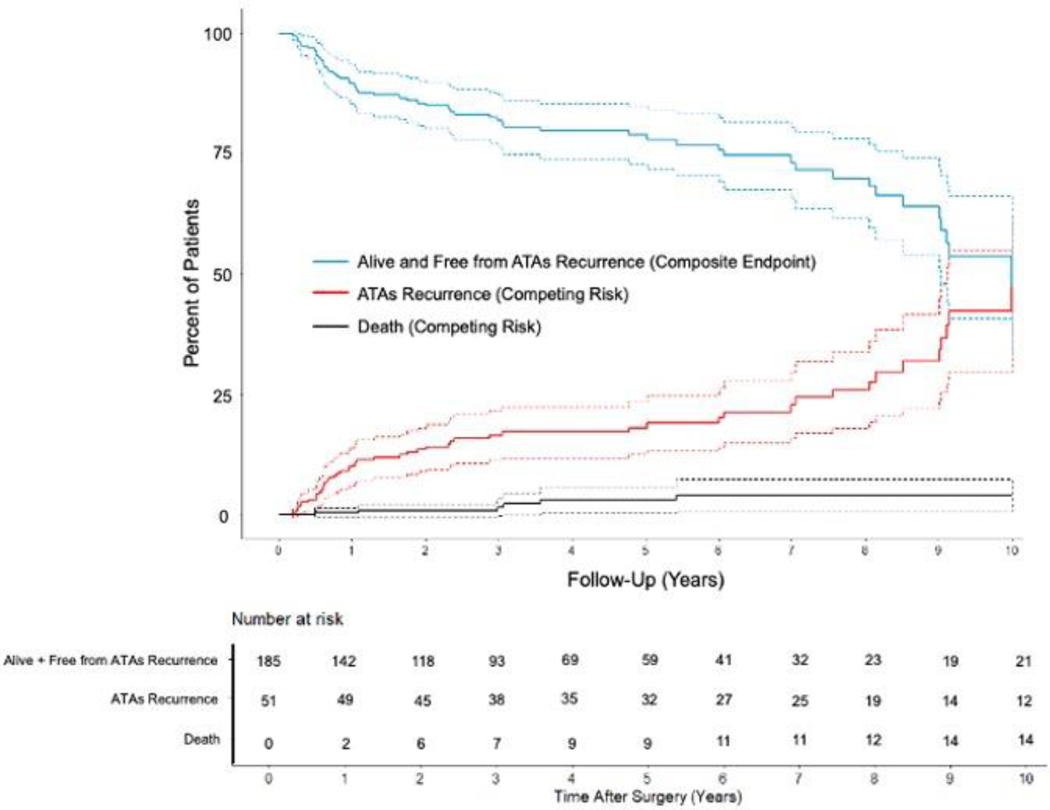
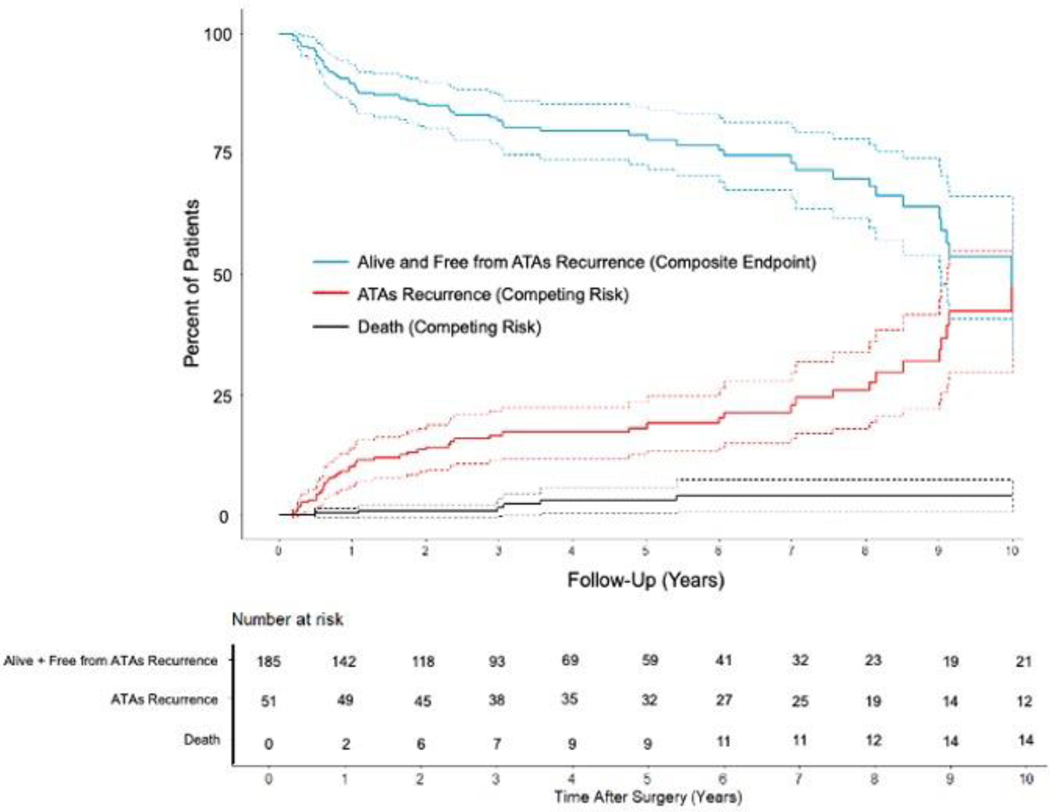
Overall freedom from ATAs recurrence was 94%(187/199), 95%(124/131), 89%(81/91), 86%(49/57), and 79%(26/33) at 1, 3, 5, 7, and 10 years, respectively when evaluating rhythm outcomes at discrete follow-up timepoints (Figure 2 and Figure E1). Freedom from ATAs recurrence off AADs was 86%(172/199), 89%(117/131), 76%(69/91), 77%(44/57), and 70%(23/33) at the same time points. By competing risk analysis using ATAs recurrence as a permanent failure, the probability of remaining alive and free of ATAs recurrence was estimated to be 90%, 82%, 79%, 73%, and 54% at follow-up years 1, 3, 5, 7, and 10, respectively (Figure 1 and Table E4). The estimated incidence of first ATAs recurrence at 1, 3, 5, 7, and 10 years was 10%, 16%, 18%, 23%, and 42%, respectively. Estimated mortality at these time points was 0%, 2%, 3%, 4%, and 4%.
Figure 2.
Ten-year follow-up showing freedom from atrial tachyarrhythmia (ATA) recurrence on and off antiarrhythmic drugs (AAD) with 95% confidence intervals following stand-alone Cox-Maze IV procedure (CMP-IV). Graph displays rhythm outcome results at years 1, 3, 5, 7, and 10 of post-operative follow-up.
Figure 1.
Atrial tachyarrhythmias (ATAs) recurrence-free survival and cumulative incidence function (CIF) curves for competing events (ATAs recurrence and death) following stand-alone Cox-Maze IV procedure (CMP-IV) up to ten-year follow-up. Patients were assumed to be in one of three distinct states: alive and free from ATAs recurrence (composite endpoint) in blue, alive and having experienced first ATAs recurrence (CIF) in red, or dead before ATAs recurrence (CIF) in black.
Of patients with ATAs recurrence, about half (26/53,49%) experienced only one recurrence over the entire follow-up period and only 23% (12/53) experienced a second recurrence. The majority (38/53,72%) were in AF at first ATAs recurrence and the remaining had atrial flutter (13/53,25%). The median number of recurrences was 1.5[1.0,3.0]. Only twelve patients (12/236,5%) experienced symptomatic recurrence, the majority (8/12,67%) of which were mild palpitations.
Efficacy in Paroxysmal versus Non-Paroxysmal AF
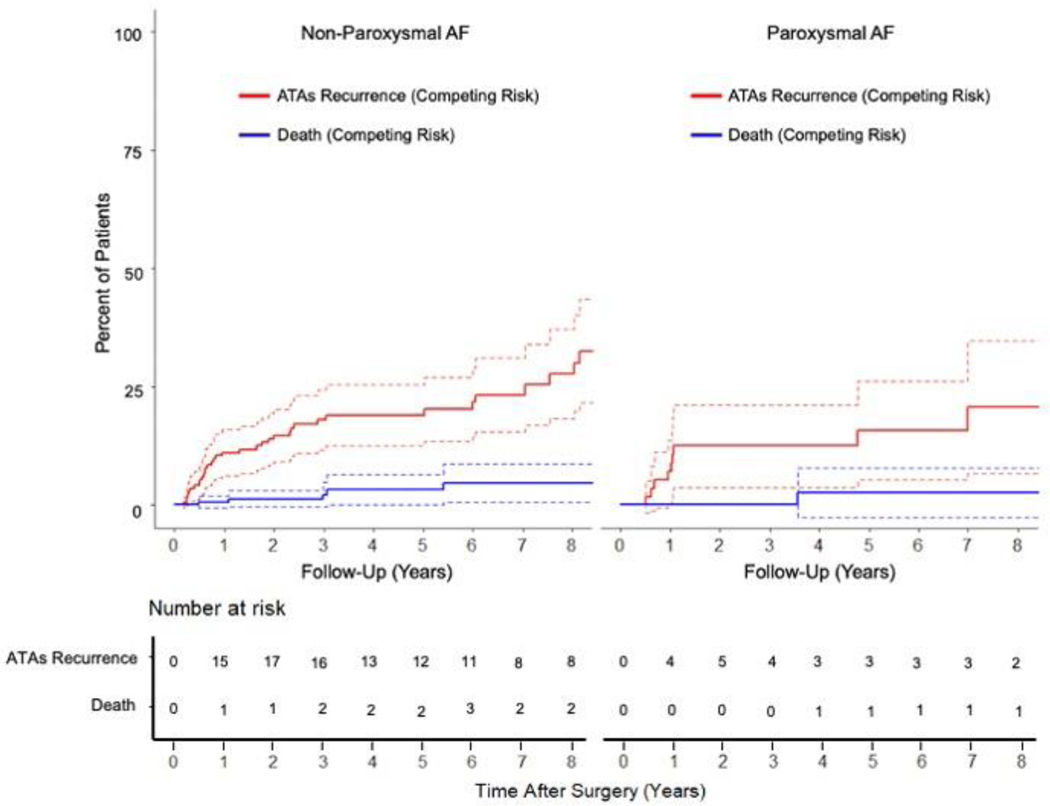
Freedom from ATAs was similar between patients with paroxysmal and non-paroxysmal AF at 1 [93%(50/54) vs 94%(137/145),P=0.738], 5 [96%(24/25) vs 86%(57/66),P=0.274], and 10 years [89%(8/9) vs 83%(20/24),P=1.000] when evaluating rhythm outcomes at discrete follow-up timepoints. Similar results were found when examining freedom of ATAs off AADs in the two groups at the same time points (Figure E3). There was no difference in time to first ATAs recurrence between the paroxysmal and non-paroxysmal AF groups (1.0[0.8,5.9] years vs 1.7[0.6,6.0],P=0.874). By competing risk analysis using ATAs recurrence as a permanent failure, there was no difference in freedom from ATAs between the two cohorts (Gray’s test,P=0.366; Figure 3 and Table E5).
Figure 3.
Cumulative incidence functions showing the competing risks of atrial tachyarrhythmias (ATAs) recurrence (red) and death (blue) for patients with non-paroxysmal and paroxysmal atrial fibrillation (AF) following stand-alone Cox-Maze IV procedure (CMP-IV) up to ten-year follow-up.
Efficacy in Sternotomy versus RMT
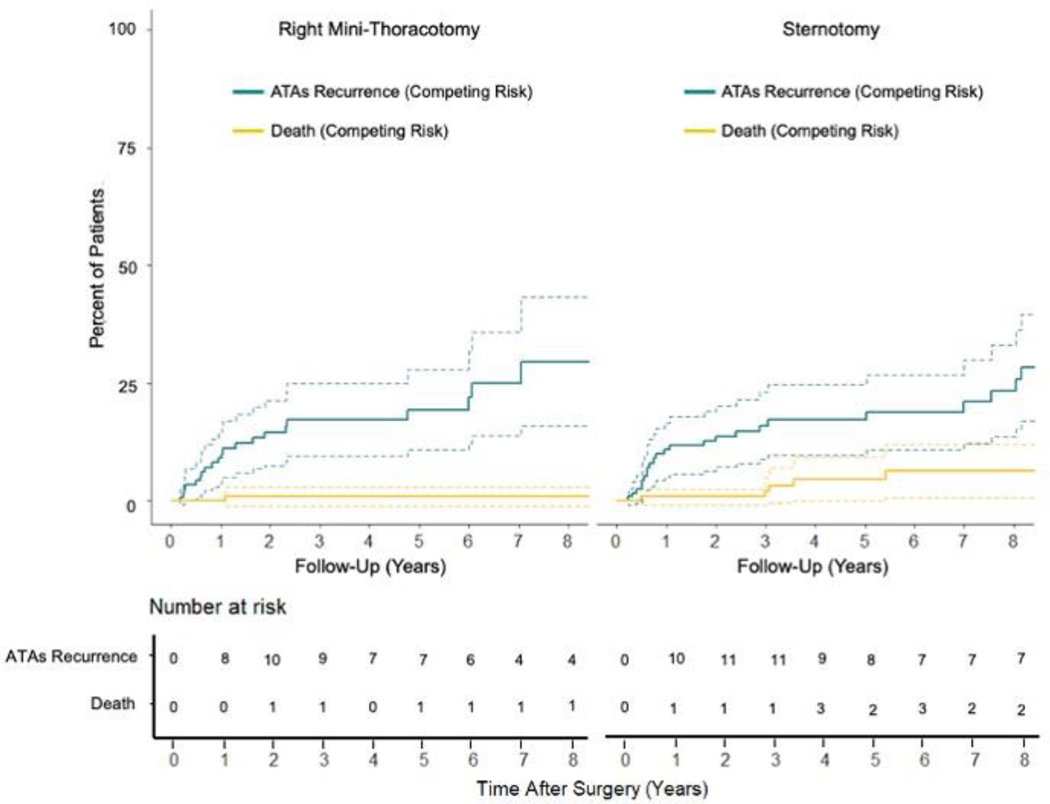
Similarly, there was no difference in freedom from ATAs between patients undergoing median sternotomy and RMT at 1 [96%(97/101) vs 92%(90/98),P=0.246], 5 [86%(42/49) vs 93%(39/42),P=0.331], and 10 years [84%(21/25) vs 88%(7/8),P=1.000] when evaluating rhythm outcomes at discrete follow-up timepoints. Similar results were found when examining freedom of ATAs off AADs in the two groups at the same time points (Figure E4). There was no difference in time to first ATAs recurrence between the sternotomy and RMT groups (1.9[0.6,7.7] years vs 1.0[0.6,3.6],P=0.225). By competing risk analysis using ATAs recurrence as a permanent failure, there was no difference in freedom from ATAs between the two cohorts (Gray’s test, P=0.652; Figure 4 and Table E6).
Figure 4.
Cumulative incidence functions showing competing risks of atrial tachyarrhythmias (ATAs) recurrence (green) and death (yellow) up to ten-year follow-up for patients undergoing stand-alone Cox-Maze IV procedure (CMP-IV) via right mini-thoracotomy and median sternotomy.
Additional Late Follow-Up
There was no difference in freedom from anticoagulation therapy between the paroxysmal and non-paroxysmal groups at 1 [72%(39/54) vs 74%(108/145),P=0.747; Table E1], 5-year [76%(19/25) vs 65%(43/66),P=0.322], and 10-year [67%(6/9) vs 88%(21/24),P=0.167] follow-up. There were no patients with a documented, clinically-significant late stroke (≥30 days post-operatively) in the entire cohort, with a total of 1130 patient-years of follow-up. Of the 53 patients who experienced ATAs recurrence, four (4/53,8%) required a post-Maze catheter ablation due to symptoms. Two patients underwent pulmonary vein isolation(PVI) and were successfully converted to sinus rhythm, while two required AV nodal ablation and remained in AF.
In addition to no patients experiencing a 30-day mortality in the study cohort, only 5% had a late mortality. Though most patients remained alive and free from ATAs recurrence in the follow-up period, the survival rate of patients who remained in SR was higher than those who experienced at least one ATA recurrence [94% (95% CI (90%,99%)) vs 75% (95% CI (61%,94%)), Log-rank test,P=0.021; Figure E2].
Factors Associated with ATAs Recurrence
Univariable analysis of sixteen preoperative and perioperative variables was performed to determine potential factors contributing to first ATAs recurrence probability within the ten-year follow-up period (Table E2). On multivariable Fine-Gray regression adjusted for clinically relevant covariates, increased left atrial size (SHR 1.33, 95%CI (1.01,1.76),P=0.040) and increased number of prior catheter ablations (SHR 1.44, 95%CI (1.18,1.76),P<0.001) were associated with increased risk of ATAs recurrence.
Discussion
The CMP remains the most effective surgical treatment of AF with the highest success rate of any interventional procedure.[3–6,8–11,23] The CMP-IV was developed after ablation devices allowed for a technically faster and less-invasive surgical approaches, and has been shown to be equally effective in patients with paroxysmal and non-paroxysmal AF.[10,11] The stand-alone CMP-IV is effective in lone AF patients at early follow-up, though few reports exist investigating late outcomes.[18]
This report of 236 consecutive patients undergoing a stand-alone CMP-IV for lone AF showed excellent freedom from ATAs at early-, mid-, and late follow-up. With strict adherence to recurrence guidelines as established by recent consensus statements and with the majority of patients having prolonged monitoring, the freedom from ATAs off AADs was 70% at 10-years postoperatively (Figure 5). The stand-alone CMP-IV was equally effective in patients with paroxysmal and non-paroxysmal AF, as well as those who underwent median sternotomy and RMT approaches at late follow-up. This supports the recent guidelines from the HRS, the Society of Thoracic Surgeons(STS), and the European Cardiac Arrhythmia Society(ECAS) that recommend stand-alone SA to restore SR in patients refractory to AADs and/or catheter-based ablation (Class IIA, Level B, NR).[2,24,28]
Figure 5.
Graphical Abstract. Overview of study design including total number of study patients undergoing stand-alone Cox-Maze IV procedure with full Box Lesion set for symptomatic atrial fibrillation (n = 236). Patients divided into two cohorts: paroxysmal AF (n = 60) and non-paroxysmal AF (n = 176). Primary outcome was incidence of first ATAs recurrence. Stand-alone CMP-IV had excellent results at late follow-up, with low morbidity and no mortality. By competing risk analysis, there was no difference in the incidence of first ATAs recurrence between patients with paroxysmal and non-paroxysmal AF, and those who underwent median sternotomy and right mini-thoracotomy. AF. = atrial fibrillation; ATA = atrial tachyarrhythmia; CMP = Cox-Maze Procedure.
Our results are favorable when compared to the late results of catheter ablation studies or more limited surgical approaches.[29,30] One study of 255 patients undergoing catheter ablation showed AF-free survival of 32% at 10-years following a single ablation procedure, with 25% AF-free survival in non-paroxysmal AF patients at the same time-point.[29] Scherr and colleagues reported 17% ATAs recurrence free survival at 5-year follow-up after single catheter ablation in patients with persistent AF.[30] In a study of 109 patients who underwent thoracoscopic surgical PVI, Saini and colleagues found 5-year AF-free survival was only 38%(37/98) in patients available for follow-up.[31] Another recent study showed only 29% of non-paroxysmal AF patients who underwent off-pump epicardial ablation were free of AF after a single-procedure at 5-year follow-up. [32] These results are in contrast to our study that showed freedom from ATAs and AADs was 76% and 70% at 5 and 10 years, respectively, with no difference in success rates between type of AF.
The results of this study are consistent with the early- and mid-term results of the stand-alone CMP-IV previously shown by our group and others.[18,20,33] In a study of 59 patients with non-paroxysmal AF who underwent stand-alone CMP-IV, freedom from ATAs on and off AADs at 7-year follow-up was 85% and 74%, respectively.[20] Likewise, a study of 39 patients with non-paroxysmal AF showed 3-year freedom from ATAs recurrence on and off AADs of 93% and 85%, respectively.[33] Our experience with the CMP has better defined the long-term results with this procedure in a larger group of patients. In our study, which included patients with paroxysmal and non-paroxysmal AF, freedom from ATAs on or off AADs at 10 years was 79%.
This study also showed no difference in ATAs recurrence between patients undergoing stand-alone CMP-IV by median sternotomy and RMT. These results are comparable to those published by Ad and colleagues.[19,21] In a study of 104 patients with non-paroxysmal AF undergoing minimally-invasive CMP-IV, 92% and 80% of patients were free of ATAs on and off AADs at 3-year follow-up, respectively.[21] A more recent follow-up study of the same cohort showed 5-year freedom from ATAs on and off AADs of 90% and 79%, respectively, similar to our results.[19] These findings suggest the minimally-invasive CMP-IV can be performed with equal efficacy as a sternotomy approach. In fact, the minimally-invasive RMT was used at a higher frequency throughout the past decade compared to the first decade of stand-alone SAs performed at our institution (Figure E6). While patients undergoing RMT experienced increased cardiopulmonary bypass times compared to those undergoing sternotomy, likely because the operation was performed through a small incision without rib spreading in most patients and with thoracoscopic guidance, there was a similar length of ICU and hospital stay, and a similar complication rate.
The stand-alone CMP-IV was performed with minimal morbidity and no 30-day mortality, regardless of AF type or surgical approach. Conducting the procedure as safely as possible is of critical importance, as these patients have no other cardiac pathology. Only three patients (1%) experienced a post-operative stroke, which is comparable to the rates previously reported for stand-alone SA.[18,19,21] There were no late strokes, despite the fact that 72%(171/236) of patients had CHA2DS2-VASc score ≥2. This is likely attributable both to the high success rate and the excision or exclusion of the LAA in all patients. The post-operative pacemaker rate found in the study (6%) was similar to rates previously reported after stand-alone CMP-IV, and can be partially attributed to uncovering underlying sick sinus syndrome.[18,19] However, there also may be a contribution of the right atrial lesion set. In our opinion, this is an acceptable trade-off for the high degree of restoration of an AV rhythm and the improved late survival in patients undergoing successful ablation both in this study and in previous work from our group and others.[34,35]
Importantly, only 12 patients (5%) experienced symptomatic ATAs recurrence during the entire follow-up period, the majority (67%) of which were mild palpitations. Thus, most patients with recurrence were asymptomatic. However, four required post-Maze catheter-based ablation for symptoms, with only one patient remaining symptomatic from refractory atrial flutter. This low incidence of symptomatic recurrent ATAs is remarkable considering the high percentage of patients with non-paroxysmal AF (75%) and those with prior failed catheter ablations (59%). Furthermore, no patients in the cohort experienced a late stroke during the follow-up period, despite 71% and 87% of patients being off anticoagulation therapy at 5 and 10-year follow-up, respectively. The absence of stroke may be attributed both to the high rate of SR restoration, and to successful exclusion or excision of the LAA. However, patients who remained in SR throughout the follow-up period experienced a survival benefit compared to those with ATA recurrence, which confirmed our group’s previous findings and the importance of restoring SR and not just managing the LAA.[34]
Finally, using multivariable Fine-Gray regression, increased left atrial size and prior number of catheter ablations were the only factors associated with first ATAs recurrence at late follow-up. It was interesting to note that type of AF and preoperative duration of AF were not predictive of failure as has been shown in previous studies with shorter follow-up. However, it is important to note the factors associated with first ATAs recurrence in this study was determined by Fine-Gray regression, using death as a competing risk, which is different than analyzing factors at a specific follow-up time point as previously published by our group and others.[5,6,10] Left atrial size was associated with increased risk of AF recurrence by evaluation at specific timepoints [5,6,10], as well as Fine-Gray regression as we have used here [11,36], lending to consistent reproducibility of these results despite varying statistical methods. Prior number of catheter ablations have not been extensively studied previously with this level of granularity.
Limitations
Though this study is one of the largest in the literature of stand-alone surgical AF ablation, with a long mean follow-up of 4.8 years, it is not without limitations. This study was retrospective and non-randomized, thus subject to selection bias. The operations were performed at a single institution, most of which were completed by a single, highly-experienced surgeon. This may prevent the results from being generalizable to other centers. In addition, there were a relatively small number of patients at 10-year follow-up (n=33), but this represented only 72% (33/46) of the patients alive and available for rhythm analysis, which is reasonable considering the length of time and the paucity of studies with even 5-year follow-up in the literature. This was particularly a problem in the paroxysmal AF cohort (n=9), thereby increasing the likelihood of a type II statistical error when comparing the outcomes to the non-paroxysmal AF cohort. Survival data were not available on 10%(24/236) of patients, and could not be found in social security database, medical records, or obituary searches. An inherent limitation in the study design was the lack of continuous monitoring on all patients, which can lead to interval censoring and underestimation of ATAs recurrence. However, Fine-Gray regression does not assume non-informative censoring. Furthermore, Fine-Gray regression with an endpoint of first recurrence was selected over a joint longitudinal mixed model (JLMM) due to the low incidence of ATAs recurrence in our cohort and pattern of missing data relative to JLMM assumptions. Though 90%(212/236) of patients underwent prolonged monitoring at least once during their follow-up, only 57%(52/91) and 55%(18/33) of patients had prolonged monitoring at 5 and 10-year follow-up, respectively. However, this follow-up is one of the most intensive in the literature to date. These limitations must be kept in mind, as a sizeable minority of patients available for late follow-up did not have prolonged rhythm monitoring. However, there was no difference in freedom from ATAs recurrence when comparing outcomes derived from all rhythm monitoring modalities to those from only prolonged monitoring (Table E8). In addition, it should be remembered that due to the high success of the CMP-IV, we have not been able to show any benefit of more intensive monitoring in this patient population.[37] Lastly, though Holter monitoring provides a good measure of ATAs recurrence, it does not address the clinical burden of AF. However, reduction in burden has not been accepted in recent consensus statements on ablation to be a measure of success or failure of ablation procedures.
Conclusion
The stand-alone CMP-IV was excellent at maintaining normal sinus rhythm at late follow-up, and remains the most successful interventional treatment of AF. The stand-alone CMP-IV was equally effective in patients with paroxysmal and non-paroxysmal AF, as well as those undergoing median sternotomy as compared to a minimally-invasive approach. There was a low rate of morbidity and no 30-day mortality following the procedure. There were no late strokes. On Fine-Gray regression, increased left atrial size and number of catheter ablations were associated with failure or first ATAs recurrence at late follow-up. Furthermore, patients who remained in SR at late follow-up had improved long-term survival when compared to those who experienced ATAs recurrence. Based on these findings, it is our recommendation that the stand-alone CMP-IV with complete isolation of the posterior LA should be considered in all patients with symptomatic, refractory AF who have failed or are not good candidates for catheter ablation. The results of the CMP-IV procedure are better than the few published studies on the mid and late-term efficacy of more limited surgical approaches, such as PVI, but more studies documenting the late outcomes of surgical ablation would be beneficial.
Supplementary Material
Central Picture Legend –
ATAs-free survival with competing risks (recurrence, death) following stand-alone CMP-IV
Central Message
The stand-alone CMP-IV procedure had excellent late efficacy at maintaining sinus rhythm in patients with symptomatic, refractory AF, and was performed with low morbidity and no mortality.
Perspective Statement
Limited data exist describing late outcomes of stand-alone surgical ablation using comprehensive follow-up with strict definitions of success. This study examined the long-term efficacy of the stand-alone CMP-IV and showed excellent late results. The procedure was equally effective in patients with paroxysmal and non-paroxysmal AF, and those undergoing sternotomy or minimally invasive surgery.
Acknowledgements
The authors thank Dr. Richard Schuessler for his thoughtful review of his work. The authors would also like to acknowledge the contributions of Dr. Marc Moon, Dr. Ali Khiabani, Dr. Meghan Kelly, and Samuel Perez.
Funding:
This work was supported by the National Institutes of Health RO1-HL032257 to R.J.D., and R.B.S., T32-HL007776 to R.J.D., R.M.M., and M.O.K., and the Barnes-Jewish Foundation.
WUSM IRB:
IRB ID# 201105322, current approval date: 12/18/2018
Abbreviations and Acronyms:
- AAD
Antiarrhythmic drug
- AF
Atrial fibrillation
- ATA
Atrial tachyarrhythmia
- CI
Confidence interval
- CIF
Cumulative incidence function
- CMP
Cox-Maze procedure
- ICU
Intensive care unit
- LA
Left atrium
- LAA
Left atrial appendage
- SA
Surgical ablation
- SHR
Subdistribution hazard ratio
- SR
Sinus rhythm
- STS
Society of Thoracic Surgeons
Footnotes
Conflict of Interest Disclosure:
R.J.D. – Atricure, Inc: Speaker and receives research funding; Medtronic: Consultant. Other authors have nothing to disclose.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics – 2019 Update: A Report from the American Heart Association. Circulation 2019;139:356–e528. [DOI] [PubMed] [Google Scholar]
- 2.Badhwar V, Rankin JS, Damiano RJ Jr, Gillinov AM, Bakaeen FG, Edgerton JR, et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann Thorac Surg 2017;103:329–41. [DOI] [PubMed] [Google Scholar]
- 3.Cox JL, Schuessler RB, D’agostino HJ, Stone CM, Chang BC, Cain ME, et al. The Surgical Treatment of Atrial Fibrillation. III. Development of a Definitive Surgical Procedure. J Thorac Cardiovasc Surg 1991;101(4):569–83. [PubMed] [Google Scholar]
- 4.Cox JL, Schuessler RB, Boineau JP. The Development of the Maze Procedure for the Treatment of Atrial Fibrillation. Semin Thorac Cardiovasc Surg. 2000;12(1):2–14. [DOI] [PubMed] [Google Scholar]
- 5.Prasad SM, Maniar HS, Camillo CJ, Schuessler RB, Boineau JP, Sundt TM, et al. The Cox Maze III Procedure for Atrial Fibrillation: Long-term Efficacy in Patients Undergoing Lone Versus Concomitant Procedures. J Thorac Cardiovasc Surg. 2003;126(6):1822–8 [DOI] [PubMed] [Google Scholar]
- 6.McCarthy PM, Gillinov AM, Castle L, Chung M, Cosgrove D. The Cox-Maze Procedure: The Cleveland Clinic Experience. Semin Thorac Cardiovasc Surg. 2000;12(1):25–9. [DOI] [PubMed] [Google Scholar]
- 7.MacGregor RM, Melby SJ, Schuessler RB, Damiano RJ. Energy Sources for the Surgical Treatment of Atrial Fibrillation. Innovations 2019;14(6):503–8. [DOI] [PubMed] [Google Scholar]
- 8.Lall SC, Melby SJ, Voeller RK, Zierer A, Bailey MS, Guthrie TJ, et al. The Effect of Ablation Technology on Surgical Outcomes After the Cox-maze Procedure: A Propensity Analysis. J Thorac Cardiovasc Surg. 2007;133(2):389–96. [DOI] [PubMed] [Google Scholar]
- 9.Gaynor SL, Diodato MD, Prasad SM, Ishii Y, Schuessler RB, Bailey MS, et al. A Prospective, Single-Center Clinical Trial of a Modified Cox Maze Procedure with Bipolar Radiofrequency Ablation. J Thorac Cardiovasc Surg. 2004;128(4):535–42. [DOI] [PubMed] [Google Scholar]
- 10.Henn MC, Lancaster TS, Miller JR, Sinn LA, Schuessler RB, Moon MR, et al. Late Outcomes After the Cox Maze IV Procedure for Atrial Fibrillation. J Thorac Cardiovasc Surg. 2015;150(5):1168–76, 1178.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khiabani AJ, MacGregor RM, Bakir NB, Manghelli JL, Sinn LA, Maniar HS, et al. The Long-term Outcomes and Durability of the Cox-Maze IV Procedure for Atrial Fibrillation. J Thorac Cardiovasc Surg. 2020. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ad N, Holmes SD, Massimiano PS, Rongione AJ, Fornaresio LM. Long-term Outcome Following Concomitant Mitral Valve Surgery and Cox Maze Procedure for Atrial Fibrillation. J Thorac Cardiovasc Surg. 2018;155(3):983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CC, Chang JP, Chen MC, Cheng CI, Chung WJ. Long-term Results of Radiofrequency Maze Procedure for Persistent Atrial Fibrillation with Concomitant Mitral surgery. J Thorac Dis. 2017;9(12):5176–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ad N, Suri RM, Gammie JS, Sheng S, O’Brien SM, Henry L. Surgical Ablation of Atrial Fibrillation Trends and Outcomes in North America. J Thorac Cardiovasc Surg. 2012;144:1051–60. [DOI] [PubMed] [Google Scholar]
- 15.Badhwar V, Rankin JS, Ad N, Grau-Sepulveda M, Damiano RJ, Gillinov AM, et al. Surgical Ablation of Atrial Fibrillation in the United States: Trends and Propensity Matched Outcomes. Ann Thorac Surg. 2017;104(2):493–500. [DOI] [PubMed] [Google Scholar]
- 16.Ad N, Holmes S, Roberts H, Rankin JS, Badhwar V. Surgical Treatment for Stand-Alone Atrial Fibrillation in North America. Ann Thorac Surg. 2020;109(3)745–52. [DOI] [PubMed] [Google Scholar]
- 17.Je HG, Shuman DJ, Ad N. A Systematic Review of Minimally Invasive Surgical Treatment for Atrial Fibrillation: A Comparison of the Cox-Maze Procedure, Beating-heart Epicardial Ablation, and the Hybrid Procedure on Safety and Efficacy. Eur J Cardiothorac Surg. 2015;48:531–40. [DOI] [PubMed] [Google Scholar]
- 18.Weimar T, Schena S, Bailey M, Maniar HS, Schuessler RB, Cox JL, et al. The Cox-Maze Procedure for Lone Atrial Fibrillation – A Single-Center Experience Over 2 Decades. Circ Arrhythm Electrophysiol 2012;5:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ad N, Holmes S, Friehling T. Minimally Invasive Stand-Alone Cox Maze Procedure for Persistent and Long-Standing Persistent Atrial Fibrillation – Perioperative Safety and 5-year Outcomes. Circ Arrhythm Electrophysiol 2017; doi: 10:e005352. [DOI] [PubMed] [Google Scholar]
- 20.Lapenna E, De Bonis M, Giambuzzi I, Del Forno B, Ruggeri S, Cireddu M, et al. Long-term Outcomes of Stand-Alone Maze IV for Persistent or Long-standing Persistent Atrial Fibrillation. Ann Thorac Surg 2020;109:124–31. [DOI] [PubMed] [Google Scholar]
- 21.Ad N, Henry L, Friehling T, Wish M, Holmes SD. Minimally Invasive Stand-Alone Cox-Maze Procedure for Patients with Nonparoxysmal Atrial Fibrillation. Ann Thorac Surg 2013;96:792–9. [DOI] [PubMed] [Google Scholar]
- 22.MacGregor RM, Khiabani AJ, Damiano RJ. The Surgical Treatment of Atrial Fibrillation via Median Sternotomy. Operative Techniques in Thoracic and Cardiovascular Surgery 2019;24(1):19–37. [Google Scholar]
- 23.Ruaengsri C, Schill MR, Khiabani AJ, Schuessler RB, Melby SJ, Damiano RJ. The Cox-Maze IV Procedure in its Second Decade: Still the Gold Standard? Eur J Cardiothorac Surg 2018;53:i19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2017;14(10):e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pet M, Robertson JO, Bailey M, Guthrie T, Moon M, Lawton J, et al. The Impact of CHADS2 Score on Late Stroke After the Cox maze Procedure. J Thorac Cardiovasc Surg. 2013;146(1):85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huebner M, Wolkewitz M, Hum S, Enriquez-Sarano M, Schumacher M, Nat D. Competing Risks Need to be Considered in Survival Analysis Models for Cardiovascular Outcomes. J Thorac Cardiovasc Surg. 2017;153(6):1427–31. [DOI] [PubMed] [Google Scholar]
- 28.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071–104. [DOI] [PubMed] [Google Scholar]
- 29.Gaita F, Scaglione M, Battaglia A, Matta M, Gallo C, Galata M, et al. Very Long-term Outcome Following Transcatheter Ablation of Atrial Fibrillation. Are Results Maintained After 10 years of Follow up? Europace. 2018;20(3):443–450. [DOI] [PubMed] [Google Scholar]
- 30.Scherr D, Khairy P, Miyazaki S, Lavignolle VA, Pascale P, Wilton S, et al. Five-Year Outcome of Catheter Ablation of Persistent Atrial Fibrillation Using Termination of Atrial Fibrillation as a Procedural Endpoint. Circ Arrhythm Electrophysiol. 2015;8:18–24. [DOI] [PubMed] [Google Scholar]
- 31.Saini A, Hu Y, Kasirajan V, Han F, Khan M, Wolfe L, et al. Long-term Outcomes of Minimally Invasive Surgical Ablation for Atrial Fibrillation: A Single-center Experience. Heart Rhythm. 2017;14(9):1281–88. [DOI] [PubMed] [Google Scholar]
- 32.Zheng S, Li Y, Han J, Zhang H, Zeng W, Xu C, et al. Long-term Results of a Minimally Invasive Surgical Pulmonary Vein Isolation and Ganglionic Plexi Ablation for Atrial Fibrillation. PLoS One. 2013;8(11):e79755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pozzoli A, Taramasso M, Coppola G, Kamami M, La Canna G, Bella PD, et al. Maze Surgery Normalizes Left Ventricular Function in Patients with Persistent Lone Atrial Fibrillation. European Journal of Cardio-Thoracic Surgery. 2014;46:871–6. [DOI] [PubMed] [Google Scholar]
- 34.Musharbash FN, Schill MR, Sinn LA, Schuessler RB, Maniar HS, Moon MR, et al. Performance of the Cox-maze IV procedure is Associated with Improved Long-term Survival in Patients with Atrial Fibrillation Undergoing Cardiac Surgery. J Thorac Cardiovasc Surg. 2018;155(1):159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakir NH, Khiabani AJ, MacGregor RM, Kelly MO, Sinn LA, Schuessler RB, et al. Concomitant Surgical Ablation for Atrial Fibrillation is Associated with Increased Risk of Acute Kidney Injury but Improved Late Survival. J Thorac Cardiovasc Surg. 2020. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacGregor RM, Khiabani AJ, Bakir NB, Manghelli JL, Sinn LA, Carter DI, et al. Impact of Age on Atrial Fibrillation Recurrence Following Surgical Ablation. J Thorac Cardiovasc Surg. 2020. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Damiano RJ, Lawrence C, Saint L, Henn M, Sinn L, Kruse J, Gleva M, et al. Detection of Atrial Fibrillation After Surgical Ablation: Conventional Versus Continuous Monitoring. Ann Thorac Surg. 2016;101(1):42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.