Abstract
Morphine is frequently applied in cancer patients for pain management. However, its effects on cancer are not well understood but observed to be specific to certain cancer types. We previously revealed the stimulatory properties of morphine in esophageal carcinoma. This work addressed the effects of morphine and its underlying mechanisms in cervical cancer. Proliferation, apoptosis, and migration assays were performed to examine the effects of morphine alone and its combinatory effects with chemotherapeutic drugs. Immunoblotting and biochemical analysis were performed to determine the underlying mechanisms of morphine's action. Morphine promoted proliferation in opioid receptor‐dependent manner and stimulated migration in opioid receptor‐independent manner. However, morphine did not affect cervical cancer cell survival. Morphine also interfered with all tested chemotherapeutic drugs (e.g., cisplatin, 5‐FU, and paclitaxel) and alleviates their efficacy. Mechanistically, morphine‐stimulated growth via activating EGFR‐mediated signaling pathways and is opioid‐receptor‐dependent; morphine‐stimulated migration via activating RhoA‐mediated signaling pathways and this is opioid receptor‐independent. Our work suggests a strong correlation of this opioid receptor on growth factor signaling to stimulate growth and opioid receptor‐independent activation of RhoA and consequent migration. Our findings have the potential to guide the clinical use of morphine for patients with cervical cancer.
Keywords: cervical cancer, EGFR, morphine, opioid receptor, RhoA
Our work clearlydemonstrates the stimulatory effects of morphine in cervical cancer by opioidreceptor‐dependent EGFR activation and opioid receptor‐independent RhoAactivation. Our preclinical findings may potentially guide clinical use ofmorphine for managing cervical cancer or other cancers with EGFR overexpression.

1. INTRODUCTION
Cervical cancer poses a global challenge for women and is a common malignancy. Its incidence and mortality rates have significantly reduced in developed countries since the introduction of human palillo‐mavirus (HPV) screening and vaccination programs. However, approximately half a million women are diagnosed with cervical cancer in developing countries annually, where mortality is ~20 times higher than that in developed countries. 1 Surgical resection and adjuvant concurrent chemoradiotherapy are the treatment options for localized cervical cancer, whereas platinum‐based chemotherapy is often for metastatic or recurrent disease. 2 , 3 Pain is a common symptom in advanced cervical cancer patients and opioids remain the most effective standard of care to relieve cancer‐related pain. 4 , 5 Interestingly, substantial evidence has recently shown the association between cancer recurrences and anesthesia with a direct role of anesthetics in tumor progression. 6 , 7
Among different opioids, morphine is used exclusively in palliative care, especially for severe pain. Morphine acts on neurons via mainly activating μ‐opioid receptor and also other non‐opioid receptors such as N‐methyl‐D‐aspartate (NMDA) receptor. 8 Recent studies from us and others reveal that increased recurrence risk is associated with high‐dose morphine usage in patients with esophageal carcinoma 9 and morphine stimulates migration and growth in pre‐clinical models of human esophageal carcinoma. 10 Preclinical cancer studies associated morphine with progression and overall survival. Morphine activates survival‐promoting signaling and promotes breast tumor growth, 11 enhances renal cell carcinoma aggressiveness, 12 and activates leukemia stem/progenitor cell properties. 13 In contrast, morphine suppresses human hepatocellular carcinoma cell mobility and migratory capacity and inhibits lung cancer cell growth. 14 The direct effects of morphine on tumor cells are complex and seem to be tumor‐type specific. Nevertheless, the effect of morphine on cervical cancer is largely unknown. The effect of morphine on different cancers varies because of the different surrounding microenvironments in different types of cancers.
In this work, we investigate morphine's effects as a single agent and in combination with multiple chemotherapeutic drugs on cervical cancer cells. This addresses the mechanism of morphine's action. Our work demonstrates that morphine stimulates cervical cancer cells and alleviates cytotoxicity of chemotherapeutic drugs via opioid receptor‐dependent and ‐independent mechanisms.
2. MATERIALS AND METHODS
2.1. Cell culture
Human cervical cancer cell lines C‐33A and CaSki were obtained from Cell Bank of Type Culture Collection of Chinese Academy of Sciences. These cancer cell lines were authenticated by performing short tandem repeat profiling (Precision Genomics Biotechnology). Cells were cultured as monolayers in complete Eagle's Minimum Essential Medium supplemented with 10% fetal bovine serum (Invitrogen), 2 mM L‐glutamine, and 1% penicillin–streptomycin (Sigma). Cultures were incubated at 37°C, in humidified atmospheric air with 5% CO2. Cells in the exponential growth phase were examined for mycoplasma using a MycoAlert Mycoplasma Detection kit (Lonza) prior to each experiment.
2.2. Drug treatment
Morphine hydrochloride was purchased from Sigma and diluted in cell media to 0.25, 0.5, 1, 2, and 4 μM prior to the experiment. Cisplatin, Fluorouracil (5‐FU), paclitaxel, and naloxone were obtained from Selleck. Cisplatin was reconstituted in PBS, and the rest of drugs were reconstituted in dimethyl sulfoxide (DMSO). Cells were exposed to drugs (see figure legends for specific concentrations) for 24 h and then were used in proliferation and apoptosis experiments, and western blot analysis.
2.3. 5‐bromo‐2′‐deoxyuridine (BrdU) proliferation and apoptosis assays
Cells at a density of 104 cells (for proliferation assay) or 106 (for apoptosis assay) per well were plated onto 96‐well plate or 6‐well plate and incubated for 24 h in a complete culture medium at 37°C to allow attachment. On the following day, cells were starved using serum‐free medium for 12 h, followed by 24 h of treatment with drugs. Different concentrations or combinations of drugs were used. Cell proliferation and apoptosis were determined by using BrdU Cell Proliferation Assay Kit (Abcam) and Cell Death ELISA kit (Roche), respectively, as per manufacturer's protocol. Proliferating cells were labeled by BrdU. Apoptosis was quantified by measuring cytosolic oligonucleosome‐bound DNA. Both were quantified by reading absorbance at 450 or 405 nm on a microplate reader.
2.4. Migration assays
Cell Migration was assessed with Boyden Chambers that contained polyethylene membranes (8.0 μm pore size; BD Falcon) as described in our previous study. 10 Cell migration was assessed as the number of cells in the lower chamber (migrated through the 8 μm pore‐size membrane). Briefly, cells were starved using serum‐free medium for 12 h. They were resuspended in complete media and placed on the upper chamber. Different concentrations of Morphine were placed in the lower chamber, followed by 8 h of incubation at 37°C. Cells from the upper chamber (unmigrated cells) were gently removed with a cotton swab. Migrated cells were fixed with 2% paraformaldehyde, counter‐stained with crystal violet (Sigma), and counted.
2.5. Denaturing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and Western blot (WB) analyses
Cells at a density of 107 were plated onto Petri dish, incubated for 24 h in complete culture medium to allow attachment. The next day, cells were starved using serum‐free medium for 12 h, followed by 24 h of treatment of drugs at different concentrations or combinations. Cells were then homogenized in radioimmunoprecipitation assay (RIPA) lysis buffer (Invitrogen) supplemented with protease and phosphatase inhibitors (Roche). Protein concentration was measured using BCA protein assay (Pierce). An equal amount of proteins were separated by SDS‐PAGE and then processed for Western blot analysis using standard protocol. 15 Primary antibodies were in 1:1000 dilution and secondary conjugated antibodies were in 1: 5000 dilution. Except anti‐β‐actin antibody (Santa Cruz Biotechnology), all antibodies were obtained from Cell Signaling Technology.
2.6. Statistical analyses
Each result was calculated from at least three independent data points, with each data point generated from a total of three repeats. All data points are expressed as mean ± SD. Paired group analyses were performed by Student's t‐test. An Analysis of Variance (ANOVA) was applied to ascertain differences within each experimental condition relative to its corresponding control experiment. A p‐value <.05 was considered statistically significant.
3. RESULTS
3.1. Morphine promotes cervical cancer growth and migration in opioid receptor‐dependent and ‐independent manner
To demonstrate biological activities of morphine on cervical cancer, C‐33A, and CaSki cell lines were selected and performed proliferation, apoptosis, and migration assays. Both are representative human cervical cancer cell models and are tumorigenic in mice. 16 C33A is HPV‐negative, whereas CasKi is positive for HPV type 16. To correlate with clinical significance, the concentrations of morphine used in our study are analogous to routine clinical intravenous administration. 17 As assessed by BrdU, we found that morphine significantly increased proliferation in a dose‐dependent manner by 1.5‐fold in C‐33A and 2.4‐fold in CaSki cells, and the effective concentration started from 0.25 μM (Figure 1A). In contrast, up to 4 μM morphine did not affect cervical cancer apoptosis (Figure 1B). Morphine also significantly increased migration by 1.8‐fold in C‐33A and 1.5‐fold in CaSki cells, and the effective concentration started from 1 μM (Figure 1C). Naloxone is a known competitive opioid antagonist with a higher affinity for the μ receptor. 18 We found that morphine was ineffective in increasing cervical cancer proliferation in the presence of naloxone (Figure 1A), suggesting that morphine promotes cervical cancer cell proliferation in opioid receptor‐dependent manner. However, naloxone addition did not affect the pro‐migratory effect of morphine (Figure 1C), suggesting that morphine promotes cervical cancer cell migration in opioid receptor‐independent manner. Cell survival was not affected after treatment of morphine or naloxone alone, or the combination of both (Figure 1B), suggesting that opioid receptor is not involved in cervical cancer cell survival.
FIGURE 1.

Morphine stimulates growth in opioid receptor‐dependent manner and promotes migration in opioid receptor‐independent manner in cervical cancer cells. (A) Morphine significantly increases proliferation in a dose‐dependent manner in C‐33A and CaSki cervical cancer cells. The addition of naloxone reverses increased proliferation induced by morphine. (B) Morphine does not affect cervical cancer cell apoptosis. (C) Morphine significantly increases cervical cancer cell migration in a dose‐dependent manner. Migrated cell number of five random fields were quantified. Naloxone at 10 μM was used. Morphine at 0,25, 0.5, 1, 2, and 4 μM was used. The results were shown as relative to control. The data were derived from three independent experiments. *p < .05, compared to control. ANOVA analysis showed significant differences among control and morphine‐treated groups.
3.2. Morphine alleviates cytotoxicity of multiple chemotherapeutic drugs in cervical cancer via opioid receptor‐dependent manner
To simulate the clinical settings, we addressed if morphine interferes with chemotherapeutic drugs' efficacies in cervical cancer. Cisplatin, 5‐FU, and paclitaxel which are common chemotherapeutic drugs for cervical cancer were tested. We performed proliferation and apoptosis analysis of cervical cells after treatment with morphine or chemotherapeutic drugs alone or combination of both. We found that all chemotherapeutic drugs inhibited the proliferation of cervical cancer cells by 60%–80% (Figure 2A). Morphine's addition significantly alleviated all chemotherapeutic drug‐induced growth inhibition by ~50%. Similarly, morphine significantly alleviated all chemotherapeutic drug‐induced apoptosis (Figure 2B). These clearly demonstrate the protective effect of morphine in cervical cancer in response to chemotherapy. To understand if this is via opioid receptor‐dependent pathway, we performed rescue experiments by adding naloxone to cells together with morphine and chemotherapeutic drugs. We found that the addition of naloxone completely abolished the ability of morphine alleviates cytotoxicity of multiple chemotherapeutic drugs in cervical cancer (Figure 2).
FIGURE 2.

Morphine alleviates chemo drugs' efficacy in cervical cancer cells. Morphine significantly reverses the effects of cisplatin, 5‐FU, and paclitaxel in inhibiting proliferation (A), inducing apoptosis (B), and decreasing migration (C) in C‐33A and CaSki cervical cancer cells. The addition of Naloxone significantly reverses the inhibitory effects of cisplatin, 5‐FU, and paclitaxel on proliferation and survival but not migration. Naloxone at 10 μM, morphine at 4 μM, cisplatin at 1 nM, 5‐FU at 50 nM, and paclitaxel at 20 nM were used. The results were shown as relative to control. The data were derived from three independent experiments. *p < .05, compared to 5‐FU, cisplatin, or paclitaxel alone. # p < .05, compared to 5‐FU + morphine, cisplatin + morphine, or paclitaxel + morphine alone.
3.3. Morphine stimulates opioid receptor‐dependent EGFR‐mediated pathway in cervical cancer cells
Morphine is reported to induce EGFR activation in cancer cells via μ‐opioid receptor, resulting in growth‐ and survival‐promoting signaling. 19 Since EGFR has an essential role in cervical cancer progression and metastasis, 20 , 21 we examined if morphine stimulates EGFR‐mediated signaling in cervical cancer cells; and if this is through μ‐opioid receptor. We found morphine to significantly increase phosphorylation of EGFR in CaSki cells (Figure 3A). The effective concentration started from 0.25 μM and the stimulatory effect was dose‐dependent (Figure 3B). This correlates well with the effective concentrations of morphine on proliferation. Time course analysis demonstrated that morphine activated EGFR as fast as 15 min of treatment (Figure 3A,C). The addition of naloxone reversed EGFR activation by morphine (Figure 3A,D), indicating that this EGFR activation is dependent on opioid receptor. PI3K/Akt/PTEN/mTOR and the RAS/RAF/MEK/ERK are two primary downstream pathways of EGFR signaling. 22 Consistent with EGFR activation, we observed the increased phosphorylation PI3K, Akt, p70S6K, 90RSK (downstream effector of ERK), and STAT3 in cervical cancer cells after morphine treatment (Figure 3E). Collectively, these demonstrate morphine at the same concentrations that promotes cervical cancer cell proliferation stimulates opioid receptor‐dependent EGFR‐mediated pathway.
FIGURE 3.
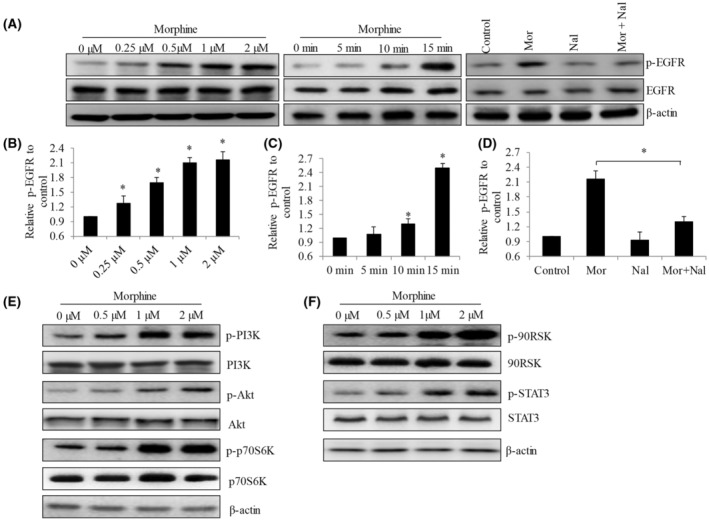
Morphine activates EGFR‐mediated signaling in opioid‐receptor‐dependent manner in cervical cancer cells. (A) Representative western blotting images of p‐EGFR and EGFR in CaSki cells exposed to morphine at various concentrations and time points, and in the presence of naloxone (10 μM). (B–D) Quantification of p‐EGFR level using Image J software free three independent western blot analysis. Morphine significantly increases p‐EGFR level in a dose‐dependent manner. Morphine (4 μM) significantly increases p‐EGFR after 15 mins treatment. Naloxone (10 μM) significantly reverses the increased p‐EGFR level induced by morphine. (E and F) Representative western blot images of CaSki cells treated with morphine at 0.25 to 2 μM for 24 h. Antibodies used in western blot analyses include anti‐p‐PI3K (Tyr199), anti‐p‐Akt (Ser473), anti‐p‐p70S6K (Thr389), anti‐p‐90RSK (Ser380), and anti‐p‐STAT3 (Tyr705). *p < .05, compared to control. ANOVA analysis showed significant differences among control and morphine‐treated groups.
3.4. Morphine stimulates opioid receptor‐independent RhoA‐mediated pathway in cervical cancer cells
The above findings imply morphine activates growth and migration via differential molecular mechanisms. We previously reported that morphine stimulates esophageal carcinoma cell migration via RhoA‐mediated signaling, 10 we hypothesized that RhoA activation contributed to pro‐migratory effect of morphine in cervical cancer. We observed morphine to increase RhoA‐GTP level significantly (Figure 4A,B), suggesting that morphine stimulates RhoA activity. Phosphorylation of downstream effectors of RhoA, such as myosin phosphatase‐targeting subunit 1 (MYPT1) and myosin light chain (MLC), 23 were also observed in cells exposed to morphine (Figure 4A,B). It is noted that the effective concentrations of morphine on RhoA activation and its downstream effectors correlated well with the ones of morphine on cell migration, suggesting that morphine promotes cervical cancer cell migration via RhoA‐mediated signaling. As expected, the addition of naloxone did not reverse the activation of RhoA‐signaling by morphine (Figure 4C,D), demonstrating that morphine stimulates opioid receptor‐independent RhoA‐mediated pathway.
FIGURE 4.
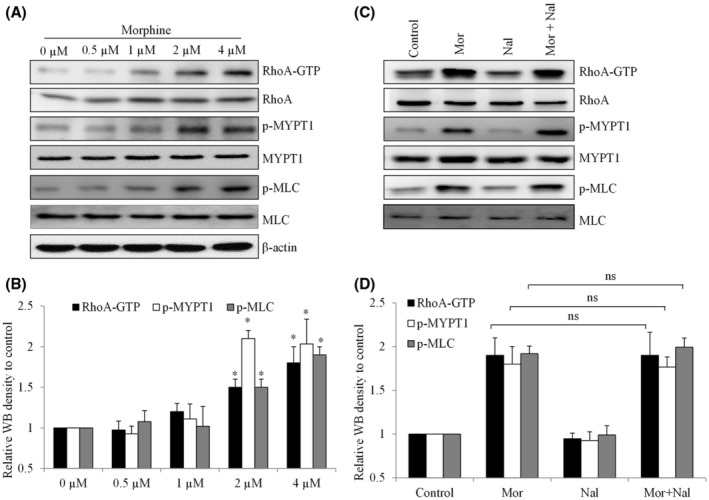
Morphine stimulates RhoA‐mediated signaling in opioid receptor‐independent manner in cervical cancer cells. Representative western blot image (A and C) and quantification (B and D) of CaSki cells treated with morphine at 0.5 to 4 μM for 24 h, and in the presence of naloxone (10 μM). Antibodies used in western blot analyses include anti‐RhoA‐GRP, anti‐p‐MYPT1 (Thr853), and anti‐p‐MLC(Ser19). *p < .05, compared to control. ANOVA analysis showed significant differences among control and morphine‐treated groups.
4. DISCUSSION
Accumulating evidence suggests that postoperative opioid use in cancer patients for pain management could influence the risk of cancer recurrence. This requires high‐quality prospective, randomized controlled clinical trials to derive the association between opioids and longer‐term recurrence, overall survival, or disease‐free survival in cancer patients. Such studies are currently lacking. 24 One retrospective study on 258 samples shows esophageal squamous cell carcinoma patients with high‐dose postoperative morphine use to have an increased risk of disease recurrence. 9 This is consistent with our previous findings obtained from in vitro cell models that morphine stimulates esophageal carcinoma cell biological activities such as migration and proliferation. 10 This also suggests that preclinical studies are important to understand the association between opioids and cancer mortality, particularly given the fact that data from prospective trials may not be available for many years. Consistent with our previous efforts, this work demonstrates the potential effects of morphine in activating cervical cancer cell growth and migration, and alleviating chemotherapy efficacy, via classical and non‐classical opioid receptor signaling.
C‐33A and CaSki are representative in vitro cervical cancer models and cover varying proteomic and genetic profiling, 25 and therefore were selected to validate the biological effects of morphine in cervical cancer. We observed that morphine at nano‐ and low micro‐molar concentrations increased cervical cancer cell proliferation and migration significantly without affecting survival (Figure 1). Of note, morphine at lower concentrations promotes cell proliferation but not migration, suggesting that pro‐proliferative rather than pro‐migratory effect is the predominant effect of morphine in cervical cancer. Using preclinical cancer models, various studies show that morphine possesses pro‐cancer activity, whereas others demonstrate opposite conclusions. 10 , 11 , 12 , 13 , 14 The discrepancies remain unclear but may be attributed to differences in cancer model systems and drug concentrations tested. Consistent with previous findings that morphine protects cancer cells from anti‐cancer drug‐induced cytotoxicity, 10 , 13 , 26 , 27 we also demonstrate that morphine interferes with all tested chemotherapeutic drugs via alleviating their efficacy in inhibiting proliferation and inducing apoptosis in cervical cancer cells (Figure 2). It is worthy of confirming the antagonistic effect of morphine with chemotherapy in mice models and clinical trials. We and others have reported that the stimulated effects of morphine are not limited to tumor cells but to other types of normal cells, such as normal esophageal epithelial cells and normal hematopoietic cells. 10 , 13 In addition, morphine inhibits immune cell functions leading to immunosuppression, whereas stimulates angiogenesis by activating proangiogenic signaling. 28 Our work together with previous findings suggests that morphine plays a positive role in tumor progression.
A significant result from our work that is consistent with previous findings, 10 , 12 , 14 , 29 , 30 shows morphine activates cervical cancer cells via both non‐classical and classical opioid receptor signaling. Rescue experiments using naloxone clearly demonstrate that morphine promotes proliferation and interferes with chemotherapy drugs in opioid receptor‐dependent manner, and increases migration in opioid receptor‐independent manner (Figures 1 and 2). Morphine also binds to N‐methyl‐D‐aspartate (NMDA) receptor which is expressed in human ovarian cancer cells. 31 However, to our best knowledge, it remains unknown whether morphine binds to NMDA in cancer cells. We demonstrate that EGFR activation is the consequence of opioid receptor activation by morphine in cervical cancer cells (Figure 3). CaSki displays much more EGFR expression level than C‐33A, 32 which correlates well with our findings that CaSki is more sensitive to C‐33A to morphine in increasing proliferation (Figure 1A). Other opioid family members such as oxycodone also demonstrate the ability to activate EGFR in opioid receptor‐dependent pathways. 33 Our findings confirm the association/crosstalk between EGFR and opioid receptor. 34 EGFR activation by morphine has the potential to affect cervical cancer patients because it is found that EGFR overexpression is in >70% of cervical cancer patients. The molecular mechanisms of morphine's stimulatory effect on cell migration include induction of epithelial‐mesenchymal transition and P2X4 receptor modulation. 10 , 35 We demonstrate that morphine promotes cervical cancer cell migration via activating RhoA‐mediated signaling, and furthermore that this is opioid receptor‐independent (Figure 4). Interestingly, RhoA activity is also regulated by G‐protein signaling. Morphine might activate RhoA via other G‐protein pathways that are not downstream of opioid receptor signaling.
5. CONCLUSION
Our work clearly demonstrates the stimulatory effects of morphine in cervical cancer by opioid receptor‐dependent EGFR activation and opioid receptor‐independent RhoA activation. Our preclinical findings may potentially guide the clinical use of morphine for managing cervical cancer or other cancers with EGFR overexpression.
AUTHORS' CONTRIBUTIONS
Zhengwen Yu and Shiming Tian performed the experiments and analyzed the results. Zhengwen Yu and Sheng Jin conceived and guided and project, analyzed the results, and revised the manuscript. All authors approved the manuscript.
FUNDING INFORMATION
This work was supported by a research grant provided by the Hubei University of Arts and Science (Grant No. SJ166K).
CONFLICT OF INTEREST
All authors declare no conflict of interest.
ETHICS APPROVAL
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGMENT
Not applicable.
Yu Z, Jin S, Tian S, Wang Z. Morphine stimulates cervical cancer cells and alleviates cytotoxicity of chemotherapeutic drugs via opioid receptor‐dependent and ‐independent mechanisms. Pharmacol Res Perspect. 2022;10:e01016. doi: 10.1002/prp2.1016
Zhengwen Yu and Sheng Jin contributed to this work equally and are co‐first authors.
Contributor Information
Shiming Tian, Email: 349200989@qq.com.
Zhibao Wang, Email: wzb3239@hbuas.edu.cn.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.
REFERENCES
- 1. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393(10167):169‐182. [DOI] [PubMed] [Google Scholar]
- 2. Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol. 2016;27(4):e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar L, Harish P, Malik PS, Khurana S. Chemotherapy and targeted therapy in the management of cervical cancer. Curr Probl Cancer. 2018;42(2):120‐128. [DOI] [PubMed] [Google Scholar]
- 4. Mark J, Argentieri DM, Gutierrez CA, et al. Ultrarestrictive opioid prescription protocol for pain management after gynecologic and abdominal surgery. JAMA Netw Open. 2018;1(8):e185452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ward K, Ramzan A, Sheeder J, Fischer S, Lefkowits C. Persistent opioid use after radiation therapy in opioid‐naive cervical cancer survivors. Int J Gynecol Cancer. 2019;29(7):1105‐1109. [DOI] [PubMed] [Google Scholar]
- 6. Sekandarzad MW, van Zundert AAJ, Lirk PB, Doornebal CW, Hollmann MW. Perioperative anesthesia care and tumor progression. Anesth Analg. 2017;124(5):1697‐1708. [DOI] [PubMed] [Google Scholar]
- 7. Mao L, Lin S, Lin J. The effects of anesthetics on tumor progression. Int J Physiol, Pathophysiol Pharmacol. 2013;5(1):1‐10. [PMC free article] [PubMed] [Google Scholar]
- 8. Kelly E. Efficacy and ligand bias at the mu‐opioid receptor. Br J Pharmacol. 2013;169(7):1430‐1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oh TK, Jeon JH, Lee JM, et al. Association of high‐dose postoperative opioids with recurrence risk in esophageal squamous cell carcinoma: reinterpreting ERAS protocols for long‐term oncologic surgery outcomes. Dis Esophagus. 2017;30(10):1‐8. [DOI] [PubMed] [Google Scholar]
- 10. Zhang J, Yao N, Tian S. Morphine stimulates migration and growth and alleviates the effects of chemo drugs via AMPK‐dependent induction of epithelial‐mesenchymal transition in esophageal carcinoma cells. Biol Pharm Bull. 2020;43(5):774‐781. [DOI] [PubMed] [Google Scholar]
- 11. Gupta K, Kshirsagar S, Chang L, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival‐promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62(15):4491‐4498. [PubMed] [Google Scholar]
- 12. Ma Y, Ren Z, Ma S, et al. Morphine enhances renal cell carcinoma aggressiveness through promotes survivin level. Ren Fail. 2017;39(1):258‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou Z, Liu T, Zhang J. Morphine activates blast‐phase chronic myeloid leukemia cells and alleviates the effects of tyrosine kinase inhibitors. Biochem Biophys Res Commun. 2019;520(3):560‐565. [DOI] [PubMed] [Google Scholar]
- 14. Kim JY, Ahn HJ, Kim JK, Kim J, Lee SH, Chae HB. Morphine suppresses lung cancer cell proliferation through the interaction with opioid growth factor receptor: an in vitro and human lung tissue study. Anesth Analg. 2016;123(6):1429‐1436. [DOI] [PubMed] [Google Scholar]
- 15. Liu ZQ, Mahmood T, Yang PC. Western blot: technique, theory and trouble shooting. N Am J Med Sci. 2014;6(3):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pappa KI, Kontostathi G, Makridakis M, et al. High resolution proteomic analysis of the cervical cancer cell lines Secretome documents deregulation of multiple proteases. Cancer Genomics Proteomics. 2017;14(6):507‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wandel C, Kim R, Wood M, Wood A. Interaction of morphine, fentanyl, sufentanil, alfentanil, and loperamide with the efflux drug transporter P‐glycoprotein. Anesthesiology. 2002;96(4):913‐920. [DOI] [PubMed] [Google Scholar]
- 18. Rozenfeld‐Granot G, Toren A, Amariglio N, et al. MAP kinase activation by mu opioid receptor in cord blood CD34(+)CD38(−) cells. Exp Hematol. 2002;30(5):473‐480. [DOI] [PubMed] [Google Scholar]
- 19. Fujioka N, Nguyen J, Chen C, et al. Morphine‐induced epidermal growth factor pathway activation in non‐small cell lung cancer. Anesth Analg. 2011;113(6):1353‐1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen Q, Huang Y, Shao L, et al. An EGFR‐amplified cervical squamous cell carcinoma patient with pulmonary metastasis benefits from Afatinib: a case report. Onco Targets Ther. 2020;13:1845‐1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soonthornthum T, Arias‐Pulido H, Joste N, et al. Epidermal growth factor receptor as a biomarker for cervical cancer. Ann Oncol. 2011;22(10):2166‐2178. [DOI] [PubMed] [Google Scholar]
- 22. Liu Q, Yu S, Zhao W, Qin S, Chu Q, Wu K. EGFR‐TKIs resistance via EGFR‐independent signaling pathways. Mol Cancer. 2018;17(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aburima A, Wraith KS, Raslan Z, Law R, Magwenzi S, Naseem KM. cAMP signaling regulates platelet myosin light chain (MLC) phosphorylation and shape change through targeting the RhoA‐rho kinase‐MLC phosphatase signaling pathway. Blood. 2013;122(20):3533‐3545. [DOI] [PubMed] [Google Scholar]
- 24. Amaram‐Davila J, Davis M, Reddy A. Opioids and cancer mortality. Curr Treat Options Oncol. 2020;21(3):22. [DOI] [PubMed] [Google Scholar]
- 25. Higareda‐Almaraz JC, Enriquez‐Gasca Mdel R, Hernandez‐Ortiz M, Resendis‐Antonio O, Encarnacion‐Guevara S. Proteomic patterns of cervical cancer cell lines, a network perspective. BMC Syst Biol. 2011;5:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao LH, Li HT, Lin WQ, et al. Morphine, a potential antagonist of cisplatin cytotoxicity, inhibits cisplatin‐induced apoptosis and suppression of tumor growth in nasopharyngeal carcinoma xenografts. Sci Rep. 2016;6:18706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niu DG, Peng F, Zhang W, et al. Morphine promotes cancer stem cell properties, contributing to chemoresistance in breast cancer. Oncotarget. 2015;6(6):3963‐3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gach K, Wyrebska A, Fichna J, Janecka A. The role of morphine in regulation of cancer cell growth. Naunyn Schmiedebergs Arch Pharmacol. 2011;384(3):221‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ecimovic P, Murray D, Doran P, McDonald J, Lambert DG, Buggy DJ. Direct effect of morphine on breast cancer cell function in vitro: role of the NET1 gene. Br J Anaesth. 2011;107(6):916‐923. [DOI] [PubMed] [Google Scholar]
- 30. Koodie L, Ramakrishnan S, Roy S. Morphine suppresses tumor angiogenesis through a HIF‐1alpha/p38MAPK pathway. Am J Pathol. 2010;177(2):984‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. North WG, Liu F, Tian R, Abbasi H, Akerman B. NMDA receptors are expressed in human ovarian cancer tissues and human ovarian cancer cell lines. Clin Pharmacol. 2015;7:111‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eiblmaier M, Meyer LA, Watson MA, Fracasso PM, Pike LJ, Anderson CJ. Correlating EGFR expression with receptor‐binding properties and internalization of 64Cu‐DOTA‐cetuximab in 5 cervical cancer cell lines. J Nucl Med. 2008;49(9):1472‐1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu Y, Li D, Duan J, et al. The pro‐ and anti‐cancer effects of oxycodone are associated with epithelial growth factor receptor level in cancer cells. Biosci Rep. 2020;40(2):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Araldi D, Ferrari LF, Levine JD. Role of GPCR (mu‐opioid)‐receptor tyrosine kinase (epidermal growth factor) crosstalk in opioid‐induced hyperalgesic priming (type II). Pain. 2018;159(5):864‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Horvath RJ, DeLeo JA. Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J Neurosci. 2009;29(4):998‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


