Abstract
Widespread SARS-CoV-2 infection among pregnant individuals has led to a generation of fetuses exposed in utero, but the long-term impact of such exposure remains unknown. Though fetal infection is rare, children born to mothers with SARS-CoV-2 infection may be at increased risk for adverse neurodevelopmental and cardiometabolic outcomes. Fetal programming effects are likely to be mediated at least in part by maternal immune activation (MIA). Here we review recent evidence regarding the effects of prenatal SARS-CoV-2 infection on the maternal, placental, and fetal immune response, and the implications for long-term health of offspring. Extrapolating from what is known about the impact of MIA in other contexts (e.g. obesity, HIV, influenza), we review the potential for neurodevelopmental and cardiometabolic morbidity in offspring. Based on available data suggesting potential increased neurodevelopmental risk, we highlight the importance of establishing large cohorts to monitor offspring born to SARS-CoV-2-positive mothers for neurodevelopmental and cardiometabolic sequelae.
The potential consequences of the SARS-CoV-2 pandemic on the next generation
In the U.S. alone, over 211,000 documented SARS-CoV-2 infections have occurred in pregnancy, resulting in more than 32,000 hospitalizations and nearly 300 maternal deaths (1). The number of pregnant individuals infected with SARS-CoV-2 is likely underestimated, as it has become evident that clinically milder infections may go unrecognized or unreported with the increasing use of home antigen tests (2). In fact, as of late February, the seroprevalence of infection-induced antibodies against SARS-CoV-2 was over 60% in individuals age 18-49 (3). This proportion will likely continue to rise as the highly transmissible Omicron subvariants continue to circulate in the population in the setting of significant escape of vaccine-induced neutralization (4, 5).
Pregnant women with COVID-19 are known to have worse clinical outcomes compared to nonpregnant women of similar age, including higher rates of intensive care unit admission and mechanical ventilation (6, 7). Maternal COVID-19 is also associated with obstetric complications impacting both mother and fetus including gestational hypertension and preeclampsia (8), preterm birth (9, 10), low birth weight (10), and stillbirth (11). Although some viral infections acquired during pregnancy can have a devastating impact on the developing fetus through transplacental transmission and direct fetal infection, such as cytomegalovirus (CMV), Zika virus (ZIKV), and Rubella (12), to date, no characteristic congenital syndrome has emerged after prenatal SARS-CoV-2 exposure (13–16), and vertical transmission of SARS-CoV-2 is rare (17–19). Consistent with these observations, prenatal neuroimaging of pregnancies affected by SARS-CoV-2 infection has not identified fetal intracranial pathology characteristic of prenatal SARS-CoV-2 exposure (20, 21). Although viral transmission and fetal infection is rare, placental infection has been reported, and in some cases may be accompanied by a destructive COVID placentitis that may contribute to stillbirth, fetal distress, or as yet uncharacterized impacts on the developing fetus and offspring outcomes (22–25).
Despite growing knowledge of the short-term consequences of COVID-19 infection in pregnancy on maternal and pregnancy outcomes, and the observation that most individuals infected with SARS-CoV-2 in pregnancy have normal obstetric outcomes, several key questions have emerged: (1) Are there implications for the long-term health and development of children born to mothers infected with SARS-CoV-2? (2) If so, what organ systems are most vulnerable to malprogramming in this context and how will this manifest in childhood/adolescence? (3) What is the potential public health impact if hundreds of thousands to millions of pregnancies have been exposed to SARS-CoV-2, with more infections occurring daily as highly transmissible subvariants continue to circulate in the population, even in communities with high vaccine uptake?
The intrauterine environment is a critical determinant of long-term neurologic and cardiometabolic health of offspring (26, 27). There is an increasing recognition that maternal immune activation (MIA) resulting from diverse stimuli ranging from infection in pregnancy, to chronic inflammatory conditions such as obesity, to maternal environmental exposures or stress, can impact placental and fetal immune programming (28–31). Fetoplacental programming resulting from MIA, in turn, can influence offspring risk for neurodevelopmental, psychiatric, and metabolic disease (32–36). Various mechanisms have been posited to underlie these sequelae, including perturbations to fetal organ development (e.g., reduced islet cell mass, cardiovascular changes leading to early-onset coronary artery disease), placental function, and epigenetic programming (37). While the recency of SARS-CoV-2 limits our knowledge of its impact across generations, extrapolating from other contexts of maternal immune activation (MIA) suggests the potential for adverse neurologic and cardiometabolic consequences in offspring that will require careful monitoring (Figure) (38, 39).
Figure. Maternal SARS-CoV-2 infection may drive maternal and fetoplacental immune activation, with subsequent potential for adverse offspring health outcomes.
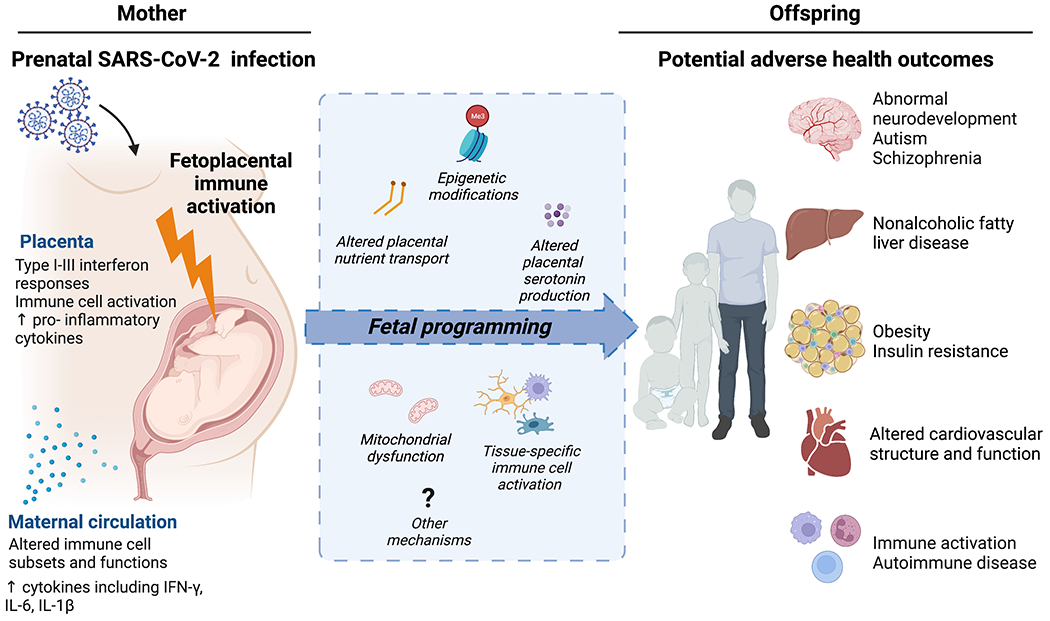
Recent studies demonstrate maternal and fetoplacental immune activation in response to maternal SARS-CoV-2 infection. Selected impacts of SARS-CoV-2 infection on maternal and placental immune activation are detailed on the left panel. Mechanisms implicated in fetal brain and organ programming in response to infectious and non-infectious immune-activating prenatal exposures – which may parallel maternal immune activation in SARS-CoV-2 - are illustrated in the central blue box and include alterations in: placental nutrient (including fatty acid) transport, epigenetic modifications in fetal organs (e.g. brain, liver, white adipose tissue, skeletal muscle), placental serotonin/neurotransmitter production, tissue mitochondrial dysfunction, and tissue-specific macrophage reactivity (e.g., fetal brain microglia, placental macrophages, cardiac and liver macrophages, blood). Although longer-term outcomes of infants and young children born to pregnant individuals with COVID-19 infection have not yet been described, some early reports suggest immune dysregulation and increased neurodevelopmental risk. Maternal-fetoplacental immune activation in other non-SARS-CoV-2 contexts (e.g. obesity, other viral infections) has been linked to multiple other adverse health outcomes in offspring such as those depicted in the right panel. Figure created with BioRender.
In this review, we aim to: (1) summarize key observations regarding immune activation that occurs in the mother, placenta, and fetus as the result of SARS-CoV-2 infection during pregnancy; (2) review the connections between viral (influenza, HIV) and non-viral (example case of maternal obesity) pro-inflammatory exposures, and adverse offspring neurodevelopmental or cardiometabolic outcomes due to malprogramming of the fetal brain and other organs; and (3) highlight preliminary observations of the outcomes of children born to women infected with SARS-CoV-2 during pregnancy. We conclude by suggesting methods and modalities to investigate offspring health outcomes in the setting of the now highly-prevalent exposure of SARS-CoV-2 infection in pregnancy. We discuss the importance of multidisciplinary efforts to establish robust longitudinal cohorts as well as the importance of cellular or biological models that can identify the imprints of immune activation and offspring most at risk.
Immune activation at the maternal-fetal interface in prenatal SARS-CoV-2
Maternal immune activation is a signature of SARS-CoV-2 infection in pregnancy regardless of disease severity
Infection with SARS-CoV-2 generates anti-viral immune responses including signaling through the Type I-III interferon pathways, with disruptions in the temporality and balance of these responses implicated in COVID-19 disease severity and progression (40–43). Early studies of pregnant women with COVID-19 disease have demonstrated a pro-inflammatory milieu, with increases in IFN-γ, IL-6, and IL-1β in the maternal circulation that correlate with disease severity (44, 45). A recent study of systemic cytokines in pregnant women with acute COVID-19 infection showed similarly increased cytokine levels, with IL-8, IL-10, and IL-15 elevated compared to controls in both clinically mild and severe cases (46). Data from a small cohort of women with first or second trimester infection versus third trimester infection identified elevated levels of maternal plasma IL-6 and IP-10, an interferon-stimulated chemokine, at the time of delivery in both early and late SARS-CoV-2 infection, compared to SARS-CoV-2 negative controls (47). Although recovery from illness occurs distant from delivery in most SARS-CoV-2 infections occurring in the first and second trimesters of pregnancy, these data suggest that cytokine/chemokine elevations may persist until delivery (47). These findings parallel data from non-pregnant individuals demonstrating elevations in systemic pro-inflammatory cytokines including IFN-γ, CXCL10, and IL-8 in asymptomatic individuals as many as 8 months following symptomatic SARS-CoV-2 infection in comparison to both healthy controls, and to individuals infected with common coronaviruses (48). Taken together, data from these studies point to persistent, systemic maternal immune activation in response to SARS-CoV-2 infection, even in cases of mild clinical disease and sometimes in cases of remote infection. Additional studies in larger cohorts are warranted to confirm the degree and extent to which SARS-CoV-2 infection in pregnancy might lead to lasting immune perturbations.
Comprehensive profiling of the response of peripheral and placental immune cells to SARS-CoV-2 challenge in vitro have offered insights into the unique immune imprinting that occurs in pregnancy (49, 50). Although the oxidative “burst” response of neutrophils and monocytes appears unaffected by pregnancy, pregnancy itself modulates specific cellular cytokine responses, with an overall diminished release of IFN-γ and IL-8 observed in SARS-CoV-2-exposed PBMCs from pregnant donors (46). Placental cell lines have been used to model how the placenta might respond to exposure to Spike protein in maternal blood, and how this placental response could in turn manifest in the maternal circulation. In vitro stimulation with even low levels of recombinant Spike protein appear to generate a cell-type-specific response, with notable induction of IL-6, IL-1β and select chemokines (CCL2, CCL5, CXCL9 and CXCL10), that is augmented by treatment with recombinant human IFN-γ (50). The authors suggest that the interaction of placental trophoblast and endothelial cells with Spike protein might be partly responsible for observed systemic cytokine/chemokine elevations in the maternal circulation during COVID-19 infection. The impact of the unique biological and immune state of pregnancy on responses to SARS-CoV-2, and how the placenta and fetus engage in crosstalk with the maternal immune system in the setting of infection, is complex and multifactorial (51, 52).
Placental immune activation in SARS-CoV-2 and potential for fetal impact
The placental response to maternal SARS-CoV-2 infection is an important target of investigation, as the placenta can not only protect against viral transmission but also plays a critical role in mediating biologic crosstalk between mother and fetus. In cases of significant placental SARS-CoV-2 viral burden and severe maternal disease, massive infiltration of maternal macrophages and T cells has been observed in placental villi in association with a cytokine/chemokine profile akin to what has been observed in COVID-19 lung tissue, and enrichment of genes in pathways associated with viral response to infection, Th1/Th2 responses, and NK cell activation (53). Interestingly, placental explants exposed to SARS-CoV-2 pseudovirus with variant Spike proteins in vitro showed heightened vulnerability to viral entry compared to those exposed to the parental, non-variant Spike (53). Importantly, even in the absence of direct viral infection of placental tissue, several studies have shown the potential for maternal SARS-CoV-2 infection to stimulate a placental immune and inflammatory response (51, 54). Increased density of fetal placental macrophages, i.e. Hofbauer cells, has been observed in uninfected placental samples from women with SARS-CoV-2 infection during pregnancy (51, 55, 56). Transcriptomic analyses have shown upregulation of inflammatory pathways in maternal decidual NK and T cells and in interferon-stimulated genes (ISGs) in the setting of maternal SARS-CoV-2 infection (51, 54), with results implicating male sex as most strongly associated with an upregulated innate immune response (51). Although limited by small sample sizes and primarily limited to cases from unvaccinated pregnancies infected with wild type SARS-CoV-2 virus, these data illustrate the capability of the placenta to generate classical anti-viral responses to a de novo maternal respiratory pathogen, even in the absence of direct viral infection of the placental organ itself. Vaccination is protective against severe COVID-19 disease and provides the infant with protection from SARS-CoV-2 hospitalization for up to 6 months of life, thought to be due primarily to the transplacental transfer of anti-Spike antibodies (57–59). How the placental immune response to maternal SARS-CoV-2 evolves over the course of a pregnancy is an important area of future study, as is the impact of prior vaccination and variant strain, in modulating the maternal or placental immune response to infection.
Lasting immune programming observed in cord blood and fetal cells in response to maternal SARS-CoV-2 infection
Evidence of transplacental transmission of SARS-CoV-2 to the fetus in utero is exceedingly limited, even in cases with evidence of placental infection (17–19). Therefore, umbilical cord blood responses in pregnancies with SARS-CoV-2 infection most often reflect a fetal response to maternal-placental signaling, rather than a response to direct exposure to the virus itself. Several studies investigating the potential fetal response to maternal SARS-CoV-2 infection and resultant immune activation have identified key alterations in cytokine profiles and immune cell makeup in cord blood at delivery (46, 47, 60, 61). Increased cord blood cytokine levels and altered immune cell profiles, including an increased proportion of natural killer cells, Vδ2+ γδ T cells, and regulatory T cells have been observed in the setting of maternal SARS-CoV-2 (46, 60). Transcriptomic profiling of cord blood mononuclear cells from newborns of mothers infected with SARS-CoV-2 in the third trimester demonstrated upregulation of interferon-simulated gene pathways in CD14+ and CD16+ monocytes, consistent with an anti-viral response (61).
The possibility that maternal SARS-CoV-2 might generate a sustained fetal immune response with onset remote from delivery has been demonstrated by two studies. One study of umbilical cord cytokine and chemokine profiles in pregnancies with early (first and second trimester) and late (third trimester) maternal SARS-CoV-2 infection identified IL-6, IP-10 and IL-8 elevations, with IL-8 levels elevated only in cases of early infection, suggesting the possibility of a sustained fetal inflammatory response (47). A second study identified an immune priming effect of SARS-CoV-2 even with infection remote from delivery, demonstrating significantly increased cytokine production by neonatal cord blood immune cells exposed to maternal infection, whether recent/ongoing or remote from delivery. Production of both IFN-γ and TNF by subpopulations of neonatal cord blood immune cells subject to polyclonal stimulation was significantly increased in pregnancies exposed to maternal SARS-CoV-2 infection in both early and late gestation. While only recent or ongoing maternal infection was associated with increased neonatal CD4+ cells expressing IL-17, IL-17-producing γδ T cells were significantly increased in both remote and recent maternal infection, compared to never-infected mothers (60). Maternal and fetal IL-17 production has been demonstrated to be associated with autism-like phenotypes in offspring, and thus may be an important mechanistic link between maternal immune activation, fetal programming, and adverse neurodevelopmental outcomes in offspring (62). Taken together, these lines of evidence suggest that maternal SARS-CoV-2 infection may leave a lasting immune imprint on the fetus, even when infection occurs remote from delivery and in the absence of transplacental viral transmission. The impacts of SARS-CoV-2 virus strain, maternal vaccination status, fetal sex, gestational age of infection, exposure to anti-viral therapies, host genetics, and other potentially immune-modulating factors, on fetal development in the setting of SARS-CoV-2 infection during pregnancy are not known and will be important to assess in future studies.
The potential consequences of the SARS-CoV-2 immune imprint on infant and child health and development
A growing body of literature has linked immune activation at the maternal-fetal interface – in response to both viral and non-viral exposures – with lasting offspring neurodevelopmental and cardiometabolic consequences. This framework is instructive in understanding the potential impact of SARS-CoV-2 infection on the health and disease liability of the next generation.
Maternal immune activation is linked to adverse neurodevelopment in offspring
Multiple lines of evidence support the concept that MIA itself plays a mechanistic role in the development of neurodevelopmental or behavioral abnormalities in exposed offspring (63–65). Epidemiologic studies have consistently linked maternal bacterial or viral infections in pregnancy, including both seasonal and pandemic H1N1 influenza infections, with an increased risk in offspring for a variety of neurodevelopmental or psychiatric comorbidities, including schizophrenia, autism spectrum disorder, and cognitive delay, substance use disorders, and disordered eating (66–74). Specifically, as an acute and self-limited viral infection that does not cross the placenta but can cause a notable maternal inflammatory response, the 2009 H1N1 influenza pandemic may be an instructive parallel to SARS-CoV-2. Pregnancies affected by influenza also have higher rates of preterm birth and low birth weight, paralleling birth outcomes seen with maternal COVID-19 (75, 76). A cohort study of offspring born to women infected with H1N1 influenza identified modest associations between maternal infection and delayed psychomotor development at 6 months (77).
Human epidemiologic and observational cohort studies are limited in their ability to elucidate mechanisms, and thus animal models have been used to seek insights into the mechanistic pathways by which MIA might drive adverse programming of the fetal brain. As an illustrative example, evidence from a Rhesus macaque model has demonstrated decreased gray and white matter volumes at 1 year in pregnancies exposed to maternal influenza infection, suggesting direct impact of MIA on offspring brain structure (78). In other animal models of MIA, exposing pregnant animals to various infectious mimics (Poly(I:C), mimicking viral infection with dsRNA, and lipopolysaccharide, mimicking gram-negative bacterial infection) and other non-infectious insults (maternal high fat diet, prenatal stress) has also led to changes in offspring brain and behavior (33, 79). Common brain and behavioral abnormalities have been observed in offspring following in utero exposure to LPS, influenza, Poly(I:C), and maternal IL-6 administration, suggesting that maternal immune activation, rather than any specific pathogen stimulus, is evoking the neurodevelopmental phenotypes (35, 80, 81). Aberrant programming of fetal microglia toward a pro-inflammatory phenotype (63), dysregulated placental serotonin and other neurotransmitter signaling pathways (82, 83), fetal brain mitochondrial dysfunction and oxidative stress, and epigenetic modifications (79) have all been implicated as mechanisms whereby MIA directly impacts the developing fetal brain (84).
Whether the degree or extent of immune activation observed in maternal SARS-CoV-2 infection is sufficient to meaningfully increase fetal risk is not known, but evidence from models of MIA suggest this possibility. Elevated maternal levels of canonical pro-inflammatory cytokines including IL-6 and IL-1β, which are increased in maternal, placental, and fetal samples in some cases of maternal SARS-CoV-2 infection (44–47, 54), have also been consistently linked to adverse offspring neurodevelopment including early cognitive and behavioral changes as well as overt neuropsychiatric pathology (82, 85–90). Changes in immune cell composition and transcriptional programming in response to maternal SARS-CoV-2 exposure might also drive increased fetal brain vulnerability. For example, MIA models have linked maternal Th17 cells and their effector cytokine, IL-17A, to offspring risk for autism spectrum disorder, and severe COVID-19 infection has been associated with elevations in Th17 cells in peripheral blood as well as significantly increased IL-17A levels (62, 91). Emerging evidence supports the prominent role of tissue-resident, fetal-derived immune cells – macrophages and mast cells, in particular – in mediating lifelong offspring pathology in multiple organ systems, including the brain, in response to prenatal exposures including MIA (92). Maternal obesity, another exposure associated with MIA, has been demonstrated to prime both fetal brain microglia and fetal placental macrophages (Hofbauer cells) toward a highly correlated pro-inflammatory phenotype (93, 94), and multiple lines of evidence link maternal obesity to increased offspring neurodevelopmental and psychiatric risk (95). Given the observed alterations in fetal placental macrophage (Hofbauer cell) density (51, 55, 56), and transcriptomic alterations in maternal macrophage and cord blood immune cell programs observed in response to prenatal exposure to SARS-CoV-2 (54, 61), it is plausible that altered macrophage/monocyte priming in the setting of maternal SARS-CoV-2 confers significant risk for the developing fetal brain.
Maternal immune activation predisposes to cardiometabolic disease in offspring
Mounting evidence indicates that MIA may contribute to the pathogenesis of long-term cardiometabolic sequelae in exposed offspring (38). In an animal model of maternal systemic inflammation, offspring whose mothers were administered the bacterial cell wall product lipopolysaccharide (LPS) during pregnancy to induce an acute non-specific inflammatory response exhibited higher body weight, enhanced adiposity, lower lean mass, and impaired glucose tolerance in adulthood (96, 97). Placentas from LPS-treated animals exhibited impaired trophoblast invasion, deficient maternal spiral artery remodeling, and altered uteroplacental perfusion, which may in part underlie these adverse outcomes (98). In utero exposure to maternal IL-6 – a key cytokine involved in the pathogenesis of severe COVID-19 (99) – similarly has been shown to lead to metabolic and endocrinologic derangements including hypertension and dysregulation of the hypothalamic-pituitary-adrenal axis in animals of both sexes (100).
Epidemiological evidence from prior influenza pandemics suggest an association of prenatal infection with long-term offspring cardiometabolic outcomes, though conclusions are mixed, and these studies are not without significant limitations. Individuals born during the 1918 influenza pandemic were found to have higher rates of self-reported cardiovascular disease at 60 to 82 years old (101). A more recent study from an observational cohort of offspring born to women infected with either seasonal or 2009 H1N1 pandemic influenza revealed an increased likelihood of overweight at 30–80 months of follow-up, suggesting the potential for increased cardiometabolic differences even earlier in life (76), potentially indicative of in utero programming effects.
As a viral infection characterized by persistent immune activation in the absence of placental infection or transplacental transmission (102), human immunodeficiency virus (HIV) infection in pregnancy provides a human model by which to understand the downstream sequelae of MIA on offspring cardiometabolic health. Analogous to COVID-19, rates of vertical transmission of HIV infection are low (103), and thus outcomes can be specifically considered among the predominant subset of offspring with perinatal HIV exposure without infection (perinatal HIV-exposed uninfected, or PHEU). As in the case of COVID-19, preterm birth and low birth weight are more common in those with PHEU as compared to the general population (104). Also similar to COVID-19, blood from infants with PHEU has been shown to display marked elevations in inflammatory cytokines (e.g., IL-8) (46, 105, 106). Whereas data on cardiometabolic outcomes with in utero exposure to COVID-19 are lacking, infants and children with PHEU have been found to exhibit perturbed insulin sensitivity (107), dyslipidemia (108), high blood pressure (109, 110), and cardiac dysfunction (109), in comparison to HIV-unexposed individuals. Furthermore, in the oldest-aged cohort of adolescents and young adults with PHEU versus well-matched controls studied to date, in utero HIV exposure conferred an increased odds of obesity later in life (111). Notably, in this study, lower maternal CD4+ T cell count during pregnancy was associated with higher BMI among adolescents and young adults with PHEU, underscoring a potential biologic link between maternal HIV-associated immune dysregulation and the pathogenesis of metabolic disease in offspring (111). Intriguingly, reductions in T cell subsets including CD4+ T cells are a hallmark of COVID-19 (46), and thus careful monitoring of BMI trajectories and metabolic outcomes in offspring with in utero exposure to SARS-CoV-2 may be warranted.
In addition to prenatal HIV and influenza exposure, maternal obesity provides another instructive case study of MIA in pregnancy and offspring cardiometabolic risk. As the most common non-infectious cause of MIA, maternal obesity is characterized by a state of chronic low-grade immune activation that may predispose to adverse cardiometabolic health outcomes in offspring (112–114). In large epidemiologic studies, individuals born to mothers with obesity have been shown to be at higher risk of obesity (115), increased adiposity (116), nonalcoholic fatty liver disease (117), insulin resistance (118), high blood pressure (119), dyslipidemia (120), and cardiovascular disease (121). Animal models have further elucidated the biologic basis for these findings, demonstrating enhanced adipogenesis (122), impaired myogenesis (123), and reduced hepatic expression of insulin signaling proteins in exposed offspring (124). While obesity is characterized by alterations to the intrauterine milieu that extend beyond immune programming effects, correlations between C-reactive protein (CRP) levels in pregnant women with obesity and indices of adiposity among their infants and children suggest that the link between maternal BMI and adverse metabolic sequelae in offspring is at least partially mediated by MIA (125, 126).
While the molecular mechanisms by which MIA programs adverse cardiometabolic outcomes in offspring remain an area of active investigation, current evidence suggests that systemic maternal inflammation may modulate the immune response in the placenta and key fetal metabolic tissues, which in turn may impact cardiometabolic health of the child/adolescent. For example, placentas from mothers with obesity have been found to exhibit macrophage accumulation with enhanced expression of the pro-inflammatory cytokines TNF-α, IL-1, and IL-6 (127). Pregnancies complicated by obesity also have been shown to manifest lower placental efficiency (fetal-placental weight ratio) and fetal oxygen concentration, suggesting that placental inflammation may interfere with normal placental function and maternal-fetal exchange (128). Beyond influencing the function of the placenta, maternal and placental immune activation may lead to alterations in the fetal immune response that persist over the life course (129), which in turn may predispose to systemic insulin resistance and other cardiometabolic sequelae (130). Moreover, as shown in animal models, inflammation within key metabolic organs including skeletal muscle can occur following in utero exposure to MIA, which in turn may dysregulate metabolic processes including insulin signaling and adipogenesis (131). Other alterations to the intrauterine milieu in maternal obesity may also be relevant in the context of SARS-CoV-2, including altered free fatty acid trafficking between mother and fetus resulting in lipotoxicity of the placenta and downstream fetal organs, and perturbed maternal secretion of key hormones including insulin (113, 132, 133), which will require further study in this context.
Early and preliminary signals of adverse outcomes in children exposed to prenatal SARS-CoV-2
Based on compelling evidence that MIA contributes to multi-systemic morbidity in offspring, dedicated studies of neurologic and cardiometabolic outcomes among cohorts with in utero exposure to maternal COVID-19 are urgently needed. Although data remain limited, preliminary evidence suggests the possibility of adverse neurodevelopmental outcomes in infants and young children exposed to SARS-CoV-2 in utero, and/or born during the COVID-19 pandemic (134–138). Two studies available on pre-print servers that lack a non-infected comparator group identified developmental delay in 10% of infants exposed to maternal SARS-CoV-2 in utero by 12 months of age (138) and deficits in the social-emotional domain of neurodevelopmental testing at 3 months of age (137). A report of over 7,700 infants born during the COVID-19 pandemic to both SARS-CoV-2 infected and non-infected mothers identified an association between maternal SARS-CoV-2 exposure and offspring neurodevelopmental diagnosis at 12 months, including developmental disorders of motor and speech and language function (139). In addition, while the association between maternal SARS-CoV-2 exposure and child neurodevelopmental diagnosis by age 1 was enhanced by preterm delivery, modeling demonstrated that the increased risk for neurodevelopmental morbidity was not fully explained by prematurity, suggesting a more specific mechanism of effect than simply SARS-CoV-2 contributing to pregnancy complications. Not all studies have suggested definite associations between maternal SARS-CoV-2 infection and early indicators of adverse child neurodevelopment, however. A recently published cohort study of 255 infants did not find a significant association between maternal SARS-CoV-2 infection and differences on any Ages & Stages Questionnaire subdomain score at 6 months of age (140). Interestingly, the same study reported that both SARS-CoV-2 exposed and unexposed infants born during March-December 2020 had significantly lower gross motor, fine motor and personal-social subdomain scores compared to a historic cohort of infants born before the pandemic. Continuing to follow children exposed to SARS-CoV-2 in utero and their contemporaneous, unexposed counterparts will be critical in dissecting these complex exposures and differential and/or additive impacts of varied in utero exposures on offspring outcomes.
Longitudinal cohorts and cellular models to understand offspring risk
To our knowledge, no specific data are available regarding cardiometabolic health outcomes in babies born to mothers with prenatal COVID-19. Nonetheless, preterm birth and low birth weight – which are both known to occur at a higher frequency among babies with in utero exposure to COVID-19 (9, 10) – have been linked to heightened risk of chronic diseases in adulthood including obesity, type 2 diabetes mellitus, and cardiovascular disease (141–143). Given the potential magnitude of the impact, with as many as 20 million children exposed to SARS-CoV-2 in utero annually (84), developing large, diverse multi-center and multi-national cohorts of exposed children to follow their longer-term neurodevelopmental and cardiometabolic outcomes will be key to elucidating offspring risk.
While such cohorts will be critically important to our understanding, as we wait for offspring outcomes that may not become apparent for a decade or more, animal and cellular models may offer a more rapid assessment of potential offspring risk. Although animal models of SARS-CoV-2 in pregnancy have been used to characterize differences in the immune response between pregnant and non-pregnant dams (144), no studies to our knowledge have investigated offspring developmental outcomes using these models. While there are some data to suggest that hamster models of SARS-CoV-2 infection are most similar to humans (145), the impact of MIA on offspring brain and behavior has been most extensively characterized in rodents and non-human primates, which may be more favorable models for future work in this area.
Cellular models have historically been used to bridge a mechanistic gap between animal model studies and human epidemiological or cohort studies. Models that use primary human cells have the advantage of retaining epigenomic and other programming effects of the intrauterine environment. For example, microglia are resident brain macrophages whose function and programming may be critical in mediating many of the adverse neurodevelopmental outcomes noted in offspring after MIA (146). In a cross-disciplinary collaboration, our group has adapted protocols previously used to differentiate microglia-like cells from individual patient-derived peripheral blood mononuclear cells (147), to generate personalized microglial-like cellular models from umbilical cord blood mononuclear cells (148). These models can be used to characterize microglia descriptively (e.g. immunocytochemistry/marker expression, transcriptomics), to assess microglial-mediated functions such as synaptic pruning which may underlie many neurodevelopmental disorders, and to screen novel and repurposed therapeutic compounds if a phenotype is identified. Another promising candidate cell type for such models is Hofbauer cells, fetal placental macrophages which can be isolated from the placenta after delivery and differentiated (149). Hofbauer cells share a common yolk-sac origin with microglia, and thus their programs may reflect the exposures and developmental processes of fetal brain microglia (94). This is just one example of how personalized cellular models can be used to provide a direct and relatively rapid means of characterizing the impact of in utero exposures for a specific individual.
Conclusions
Direct placental infection is unlikely to be the leading driver of observable differences in health outcomes of children born to women infected with SARS-CoV-2 during pregnancy. Rather, the impact of SARS-CoV-2 on the next generation, if any, will likely be best understood by probing how maternal immune activation might alter cellular programs within the fetoplacental unit, and linking such programmatic alterations with long-term outcomes of offspring. Protective factors, or factors that might re-establish balance toward a non-infected phenotype, will be critical to identify, as will the potential for additive risk with multiple immune-activating maternal exposures, such as the common combination of maternal SARS-CoV-2 infection, obesity, and preeclampsia. Longitudinal cohorts will be essential to discovering the clinical impact, if any, of both dramatic and subtle immunological shifts that occur during fetal life, and this knowledge can be augmented with cellular models to help elucidate mechanism and generate data sooner, given the substantial time horizon required to characterize many outcomes of interest.
Funding:
NICHD: 1R01HD100022-01 and 3R01HD100022-02S2 (A.G.E.), 1K12HD103096 (L.L.S), and 5K23HD100266 (L.T.F). NIAID: 1U19AI167899-01 (A.G.E.). Simons Foundation SFARI award, grant #870754 (A.G.E.) The funders had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, nor in the preparation, review, or approval of the manuscript
References
- 1.CDC. Data on COVID-19 during Pregnancy: Severity of Maternal Illness.
- 2.Rader B, Gertz A, Iuliano AD, Gilmer M, Wronski L, Astley CM, Sewalk K, Varrelman TJ, Cohen J, Parikh R, Reese HE, Reed C, and Brownstein JS. 2022. Use of At-Home COVID-19 Tests - United States, August 23, 2021-March 12, 2022. MMWR Morb. Mortal. Wkly. Rep 71: 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke KEN, Jones JM, Deng Y, Nycz E, Lee A, Iachan R, Gundlapalli AV, Hall AJ, and MacNeil A. 2022. Seroprevalence of Infection-Induced SARS-CoV-2 Antibodies - United States, September 2021-February 2022. MMWR Morb. Mortal. Wkly. Rep. 71: 606–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, San JE, Cromer D, Scheepers C, Amoako DG, Karim F, Bernstein M, Lustig G, Archary D, Smith M, Ganga Y, Jule Z, Reedoy K, Hwa S-H, Giandhari J, Blackburn JM, Gosnell BI, Abdool Karim SS, Hanekom W, Ngs SA, Team C-K, von Gottberg A, Bhiman JN, Lessells RJ, Moosa M-YS, Davenport MP, de Oliveira T, Moore PL, and Sigal A. 2021. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Planas D, Saunders N, Maes P, Guivel-Benhassine F, Planchais C, Buchrieser J, Bolland W-H, Porrot F, Staropoli I, Lemoine F, Péré H, Veyer D, Puech J, Rodary J, Baele G, Dellicour S, Raymenants J, Gorissen S, Geenen C, Vanmechelen B, Wawina-Bokalanga T, Martí-Carreras J, Cuypers L, Sève A, Hocqueloux L, Prazuck T, Rey FA, Simon-Loriere E, Bruel T, Mouquet H, André E, and Schwartz O. 2021. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. [DOI] [PubMed] [Google Scholar]
- 6.Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, Nahabedian J, Anderson K, and Gilboa SM. 2020. Characteristics of Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep 69: 769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badr DA, Mattern J, Carlin A, Cordier AG, Maillart E, El Hachem L, El Kenz H, Andronikof M, De Bels D, Damoisel C, Preseau T, Vignes D, Cannie MM, Vauloup-Fellous C, Fils JF, Benachi A, Jani JC, and Vivanti AJ. 2020. Are clinical outcomes worse for pregnant women at >/=20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching. Am J Obstet Gynecol 223: 764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbloom JI, Raghuraman N, Carter EB, and Kelly JC. 2021. Coronavirus disease 2019 infection and hypertensive disorders of pregnancy. Am J Obstet Gynecol 224: 623–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piekos SN, Roper RT, Hwang YM, Sorensen T, Price ND, Hood L, and Hadlock JJ. 2022. The effect of maternal SARS-CoV-2 infection timing on birth outcomes: a retrospective multicentre cohort study. Lancet Digit Health 4: e95–e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuliani F, Oros D, Gunier RB, Deantoni S, Rauch S, Casale R, Nieto R, Bertino E, Rego A, Menis C, Gravett MG, Candiani M, Deruelle P, Garcia-May PK, Mhatre M, Ado Usman M, Abd-Elsalam S, Etuk S, Napolitano R, Liu B, Prefumo F, Savasi V, Silva M, Baafi E, Ariff S, Maiz N, Baffah Aminu M, Cardona-Perez JA, Craik R, Tavchioska G, Bako B, Benski C, Hassan-Hanga F, Savorani M, Sentilhes L, Carola Capelli M, Takahashi K, Vecchiarelli C, Ikenoue S, Thiruvengadam R, Soto Conti CP, Cetin I, Nachinab VB, Ernawati E, Duro EA, Kholin A, Teji JS, Easter SR, Salomon LJ, Ayede AI, Cerbo RM, Agyeman-Duah J, Roggero P, Eskenazi B, Langer A, Bhutta ZA, Kennedy SH, Papageorghiou AT, and Villar J. 2022. Effects of prenatal exposure to maternal COVID-19 and perinatal care on neonatal outcome: results from the INTERCOVID Multinational Cohort Study. Am J Obstet Gynecol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeSisto CL, Wallace B, Simeone RM, Polen K, Ko JY, Meaney-Delman D, and Ellington SR. 2021. Risk for Stillbirth Among Women With and Without COVID-19 at Delivery Hospitalization - United States, March 2020-September 2021. MMWR Morb Mortal Wkly Rep 70: 1640–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arora N, Sadovsky Y, Dermody TS, and Coyne CB. 2017. Microbial Vertical Transmission during Human Pregnancy. Cell Host Microbe 21: 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodworth KR, Olsen EO, Neelam V, Lewis EL, Galang RR, Oduyebo T, Aveni K, Yazdy MM, Harvey E, Longcore ND, Barton J, Fussman C, Siebman S, Lush M, Patrick PH, Halai UA, Valencia-Prado M, Orkis L, Sowunmi S, Schlosser L, Khuwaja S, Read JS, Hall AJ, Meaney-Delman D, Ellington SR, Gilboa SM, Tong VT, Pregnancy CC-R, Infant Linked Outcomes T, Pregnancy C, and Infant Linked Outcomes T. 2020. Birth and Infant Outcomes Following Laboratory-Confirmed SARS-CoV-2 Infection in Pregnancy - SET-NET, 16 Jurisdictions, March 29-October 14, 2020. MMWR Morb. Mortal. Wkly. Rep 69: 1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaherman VJ, Afshar Y, Boscardin J, Keller RL, Mardy A, Prahl MK, Phillips C, Asiodu IV, Berghella WV, Chambers BD, Crear-Perry J, Jamieson DJ, Jacoby VL, and Gaw SL. 2020. Infant Outcomes Following Maternal Infection with SARS-CoV-2: First Report from the PRIORITY Study. Clin. Infect. Dis. 73: e2810–e2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dube R, and Kar SS. 2020. COVID-19 in pregnancy: the foetal perspective-a systematic review. BMJ Paediatr Open 4: e000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leyser M, Marques FJP, and Nascimento O. J. M. d.. 2021. POTENTIAL RISK OF BRAIN DAMAGE AND POOR DEVELOPMENTAL OUTCOMES IN CHILDREN PRENATALLY EXPOSED TO SARS-COV-2: A SYSTEMATIC REVIEW. Rev Paul Pediatr 40: e2020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz DA, and Levitan D. 2021. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infecting Pregnant Women and the Fetus, Intrauterine Transmission, and Placental Pathology During the Coronavirus Disease 2019 (COVID-19) Pandemic: It’s Complicated. Arch. Pathol. Lab. Med. 145: 925–928. [DOI] [PubMed] [Google Scholar]
- 18.Allotey J, Chatterjee S, Kew T, Gaetano A, Stallings E, Fernández-García S, Yap M, Sheikh J, Lawson H, Coomar D, Dixit A, Zhou D, Balaji R, Littmoden M, King Y, Debenham L, Llavall AC, Ansari K, Sandhu G, Banjoko A, Walker K, O’Donoghue K, van Wely M, van Leeuwen E, Kostova E, Kunst H, Khalil A, Brizuela V, Broutet N, Kara E, Kim CR, Thorson A, Oladapo OT, Zamora J, Bonet M, Mofenson L, Thangaratinam S, and Preg COVLSRC. 2022. SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission: living systematic review and meta-analysis. BMJ 376: e067696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirkle CM 2022. Transmission of SARS-CoV-2 from mother to baby is rare. BMJ 376: o593. [DOI] [PubMed] [Google Scholar]
- 20.Koliogiannis V 2021. SARS-CoV-2 infection during pregnancy: Does fetal MRI show signs of impaired fetal brain development? , Radiological Society of North America Annual Meeting. [Google Scholar]
- 21.Soto-Torres E, Hernandez-Andrade E, Huntley E, Mendez-Figueroa H, and Blackwell SC. 2021. Ultrasound and Doppler findings in pregnant women with SARS-CoV-2 infection. Ultrasound Obstet. Gynecol. 58: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz DA 2022. Stillbirth after COVID-19 in Unvaccinated Mothers Can Result from SARS-CoV-2 Placentitis, Placental Insufficiency, and Hypoxic Ischemic Fetal Demise, Not Direct Fetal Infection: Potential Role of Maternal Vaccination in Pregnancy. Viruses 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeSisto CL, Wallace B, Simeone RM, Polen K, Ko JY, Meaney-Delman D, and Ellington SR. 2021. Risk for Stillbirth Among Women With and Without COVID-19 at Delivery Hospitalization - United States, March 2020-September 2021. MMWR Morb. Mortal. Wkly. Rep. 70: 1640–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shook LL, Brigida S, Regan J, Flynn JP, Mohammadi A, Etemad B, Siegel MR, Clapp MA, Li JZ, Roberts DJ, and Edlow AG. 2022. SARS-CoV-2 Placentitis Associated With B.1.617.2 (Delta) Variant and Fetal Distress or Demise. J. Infect. Dis. 225: 754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanes ED, Miller ES, Otero S, Ebbott R, Aggarwal R, Willnow AS, Ozer EA, Mithal LB, and Goldstein JA. 2022. Placental Pathology After SARS-CoV-2 Infection in the Pre-Variant of Concern, Alpha / Gamma, Delta, or Omicron Eras. Int J Surg Pathol: 10668969221102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal P, Morriseau TS, Kereliuk SM, Doucette CA, Wicklow BA, and Dolinsky VW. 2018. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit Rev Clin Lab Sci 55: 71–101. [DOI] [PubMed] [Google Scholar]
- 27.Wadhwa PD, Buss C, Entringer S, and Swanson JM. 2009. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med 27: 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bronson SL, and Bale TL. 2016. The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology 41: 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald E, Hor K, and Drake AJ. 2020. Maternal influences on fetal brain development: The role of nutrition, infection and stress, and the potential for intergenerational consequences. Early human development 150: 105190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzgerald E, Parent C, Kee MZ, and Meaney MJ. 2021. Maternal distress and offspring neurodevelopment: Challenges and opportunities for pre-clinical research models. Frontiers in Human Neuroscience: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zoubovsky SP, Williams MT, Hoseus S, Tumukuntala S, Riesenberg A, Schulkin J, Vorhees CV, Campbell K, Lim H-W, and Muglia LJ. 2022. Neurobehavioral abnormalities following prenatal psychosocial stress are differentially modulated by maternal environment. Translational psychiatry 12: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spann MN, Monk C, Scheinost D, and Peterson BS. 2018. Maternal immune activation during the third trimester is associated with neonatal functional connectivity of the salience network and fetal to toddler behavior. Journal of Neuroscience 38: 2877–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Estes ML, and McAllister AK. 2016. Maternal immune activation: Implications for neuropsychiatric disorders. Science 353: 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain S, Baer RJ, McCulloch CE, Rogers E, Rand L, Jelliffe-Pawlowski L, and Piao X. 2021. Association of maternal immune activation during pregnancy and neurologic outcomes in offspring. The Journal of Pediatrics 238: 87-93. e83. [DOI] [PubMed] [Google Scholar]
- 35.Boulanger-Bertolus J, Pancaro C, and Mashour GA. 2018. Increasing role of maternal immune activation in neurodevelopmental disorders. Frontiers in behavioral neuroscience: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer U 2014. Prenatal poly (i: C) exposure and other developmental immune activation models in rodent systems. Biological psychiatry 75: 307–315. [DOI] [PubMed] [Google Scholar]
- 37.Rinaudo P, and Wang E. 2012. Fetal programming and metabolic syndrome. Annu Rev Physiol 74: 107–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ingvorsen C, Brix S, Ozanne SE, and Hellgren LI. 2015. The effect of maternal Inflammation on foetal programming of metabolic disease. Acta Physiol (Oxf) 214: 440–449. [DOI] [PubMed] [Google Scholar]
- 39.Han VX, Patel S, Jones HF, and Dale RC. 2021. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat Rev Neurol 17: 564–579. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y-M, and Shin E-C. 2021. Type I and III interferon responses in SARS-CoV-2 infection. Exp. Mol. Med. 53: 750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim M-H, Salloum S, Wang JY, Wong LP, Regan J, Lefteri K, Manickas-Hill Z, Gao C, Li JZ, Sadreyev RI, Yu XG, Chung RT, M. C.−. Collection, and T. Processing. 2021. Type I, II, and III Interferon Signatures Correspond to Coronavirus Disease 2019 Severity. J. Infect. Dis. 224: 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galani I-E, Rovina N, Lampropoulou V, Triantafyllia V, Manioudaki M, Pavlos E, Koukaki E, Fragkou PC, Panou V, Rapti V, Koltsida O, Mentis A, Koulouris N, Tsiodras S, Koutsoukou A, and Andreakos E. 2021. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 22: 32–40. [DOI] [PubMed] [Google Scholar]
- 43.Lowery SA, Sariol A, and Perlman S. 2021. Innate immune and inflammatory responses to SARS-CoV-2: Implications for COVID-19. Cell Host Microbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanacan A, Yazihan N, Erol SA, Anuk AT, Yucel Yetiskin FD, Biriken D, Ozgu-Erdinc AS, Keskin HL, Moraloglu Tekin O, and Sahin D. 2021. The impact of COVID-19 infection on the cytokine profile of pregnant women: A prospective case-control study. Cytokine 140: 155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherer ML, Lei J, Creisher PS, Jang M, Reddy R, Voegtline K, Olson S, Littlefield K, Park H-S, Ursin RL, Ganesan A, Boyer T, Elsayed N, Brown DM, Walch SN, Antar AAR, Manabe YC, Jones-Beatty K, Golden WC, Satin AJ, Sheffield JS, Pekosz A, Klein SL, and Burd I. 2021. Pregnancy alters interleukin-1 beta expression and antiviral antibody responses during severe acute respiratory syndrome coronavirus 2 infection. Am. J. Obstet. Gynecol. 225: 301.e301-301.e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Flores V, Romero R, Xu Y, Theis KR, Arenas-Hernandez M, Miller D, Peyvandipour A, Bhatti G, Galaz J, Gershater M, Levenson D, Pusod E, Tao L, Kracht D, Florova V, Leng Y, Motomura K, Para R, Faucett M, Hsu C-D, Zhang G, Tarca AL, Pique-Regi R, and Gomez-Lopez N. 2022. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat. Commun. 13: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taglauer ES, Dhole Y, Boateng J, Snyder-Cappione J, Parker SE, Clarke K, Juttukonda L, Devera J, Hunnewell J, Barnett E, Jia H, Yarrington C, Sabharwal V, and Wachman EM. 2022. Evaluation of maternal-infant dyad inflammatory cytokines in pregnancies affected by maternal SARS-CoV-2 infection in early and late gestation. J. Perinatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phetsouphanh C, Darley DR, Wilson DB, Howe A, Munier CML, Patel SK, Juno JA, Burrell LM, Kent SJ, Dore GJ, Kelleher AD, and Matthews GV. 2022. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol 23: 210–216. [DOI] [PubMed] [Google Scholar]
- 49.Gomez-Lopez N, Romero R, Tao L, Gershater M, Leng Y, Zou C, Farias-Jofre M, Galaz J, Miller D, Tarca AL, Arenas-Hernandez M, Bhatti G, Garcia-Flores V, Liu Z, Para R, Kanninen T, Hadaya O, Paredes C, and Xu Y. 2022. Distinct Cellular Immune Responses to SARS-CoV-2 in Pregnant Women. J. Immunol. 208: 1857–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo X, Semerci N, De Assis V, Kayisli UA, Schatz F, Steffensen TS, Guzeloglu-Kayisli O, and Lockwood CJ. 2022. Regulation of Proinflammatory Molecules and Tissue Factor by SARS-CoV-2 Spike Protein in Human Placental Cells: Implications for SARS-CoV-2 Pathogenesis in Pregnant Women. Front. Immunol. 13: 876555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bordt EA, Shook LL, Atyeo C, Pullen KM, De Guzman RM, Meinsohn M-C, Chauvin M, Fischinger S, Yockey LJ, James K, Lima R, Yonker LM, Fasano A, Brigida S, Bebell LM, Roberts DJ, Pépin D, Huh JR, Bilbo SD, Li JZ, Kaimal A, Schust DJ, Gray KJ, Lauffenburger D, Alter G, and Edlow AG. 2021. Maternal SARS-CoV-2 infection elicits sexually dimorphic placental immune responses. Sci. Transl. Med. 13: eabi7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ovies C, Semmes EC, and Coyne CB. 2021. Pregnancy influences immune responses to SARS-CoV-2. Sci Transl Med 13: eabm2070. [DOI] [PubMed] [Google Scholar]
- 53.Argueta LB, Lacko LA, Bram Y, Tada T, Carrau L, Rendeiro AF, Zhang T, Uhl S, Lubor BC, Chandar V, Gil C, Zhang W, Dodson BJ, Bastiaans J, Prabhu M, Houghton S, Redmond D, Salvatore CM, Yang YJ, Elemento O, Baergen RN, tenOever BR, Landau NR, Chen S, Schwartz RE, and Stuhlmann H. 2022. Inflammatory responses in the placenta upon SARS-CoV-2 infection late in pregnancy. iScience 25: 104223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu-Culligan A, Chavan AR, Vijayakumar P, Irshaid L, Courchaine EM, Milano KM, Tang Z, Pope SD, Song E, Vogels CBF, Lu-Culligan WJ, Campbell KH, Casanovas-Massana A, Bermejo S, Toothaker JM, Lee HJ, Liu F, Schulz W, Fournier J, Muenker MC, Moore AJ, Yale IT, Konnikova L, Neugebauer KM, Ring A, Grubaugh ND, Ko AI, Morotti R, Guller S, Kliman HJ, Iwasaki A, and Farhadian SF. 2021. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med (N Y) 2: 591-610.e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hecht JL, Quade B, Deshpande V, Mino-Kenudson M, Ting DT, Desai N, Dygulska B, Heyman T, Salafia C, Shen D, Bates SV, and Roberts DJ. 2020. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod. Pathol 33: 2092–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharps MC, Garrod A, Aneni E, Jones CJP, Batra G, and Heazell AEP. 2022. Placental Macrophages Following Maternal SARS-CoV-2 Infection in Relation to Placental Pathology. Frontiers in Virology 2. [Google Scholar]
- 57.Shook LL, Atyeo CG, Yonker LM, Fasano A, Gray KJ, Alter G, and Edlow AG. 2022. Durability of Anti-Spike Antibodies in Infants After Maternal COVID-19 Vaccination or Natural Infection. JAMA 327: 1087–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halasa NB, Olson SM, Staat MA, Newhams MM, Price AM, Pannaraj PS, Boom JA, Sahni LC, Chiotos K, Cameron MA, Bline KE, Hobbs CV, Maddux AB, Coates BM, Michelson KN, Heidemann SM, Irby K, Nofziger RA, Mack EH, Smallcomb L, Schwartz SP, Walker TC, Gertz SJ, Schuster JE, Kamidani S, Tarquinio KM, Bhumbra SS, Maamari M, Hume JR, Crandall H, Levy ER, Zinter MS, Bradford TT, Flori HR, Cullimore ML, Kong M, Cvijanovich NZ, Gilboa SM, Polen KN, Campbell AP, Randolph AG, Patel MM, and Overcoming Covid I. 2022. Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants. N Engl J Med 387: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, and Swift M. 2021. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM 3: 100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gee S, Chandiramani M, Seow J, Pollock E, Modestini C, Das A, Tree T, Doores KJ, Tribe RM, and Gibbons DL. 2021. The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate. Nat. Immunol. 22: 1490–1502. [DOI] [PubMed] [Google Scholar]
- 61.Matute JD, Finander B, Pepin D, Ai X, Smith NP, Li JZ, Edlow AG, Villani A-C, Lerou PH, and Kalish BT. 2021. Single-cell immunophenotyping of the fetal immune response to maternal SARS-CoV-2 infection in late gestation. Pediatr. Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, and Huh JR. 2016. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351: 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, and Prinssen EP. 2014. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 10: 643–660. [DOI] [PubMed] [Google Scholar]
- 64.Kreitz S, Zambon A, Ronovsky M, Budinsky L, Helbich TH, Sideromenos S, Ivan C, Konerth L, Wank I, Berger A, Pollak A, Hess A, and Pollak DD. 2020. Maternal immune activation during pregnancy impacts on brain structure and function in the adult offspring. Brain Behav. Immun 83: 56–67. [DOI] [PubMed] [Google Scholar]
- 65.Baines KJ, Hillier DM, Haddad FL, Rajakumar N, Schmid S, and Renaud SJ. 2020. Maternal Immune Activation Alters Fetal Brain Development and Enhances Proliferation of Neural Precursor Cells in Rats. Front. Immunol. 11: 1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mednick SA, Machon RA, Huttunen MO, and Bonett D. 1988. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry 45: 189–192. [DOI] [PubMed] [Google Scholar]
- 67.Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, and Susser ES. 2004. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry 61: 774–780. [DOI] [PubMed] [Google Scholar]
- 68.Al-Haddad BJS, Jacobsson B, Chabra S, Modzelewska D, Olson EM, Bernier R, Enquobahrie DA, Hagberg H, Ostling S, Rajagopal L, Adams Waldorf KM, and Sengpiel V. 2019. Long-term Risk of Neuropsychiatric Disease After Exposure to Infection In Utero. JAMA Psychiatry 76: 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cordeiro CN, Tsimis M, and Burd I. 2015. Infections and Brain Development. Obstet Gynecol Surv 70: 644–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yockey LJ, Lucas C, and Iwasaki A. 2020. Contributions of maternal and fetal antiviral immunity in congenital disease. Science 368: 608–612. [DOI] [PubMed] [Google Scholar]
- 71.Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, and Croen LA. 2015. Maternal Infection During Pregnancy and Autism Spectrum Disorders. J Autism Dev Disord 45: 4015–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adams Waldorf KM, and McAdams RM. 2013. Influence of infection during pregnancy on fetal development. Reproduction 146: R151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Al-Haddad BJS, Oler E, Armistead B, Elsayed NA, Weinberger DR, Bernier R, Burd I, Kapur R, Jacobsson B, Wang C, Mysorekar I, Rajagopal L, and Adams Waldorf KM. 2019. The fetal origins of mental illness. Am J Obstet Gynecol 221: 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Massarali A, Adhya D, Srivastava DP, Baron-Cohen S, and Kotter MR. 2022. Virus-Induced Maternal Immune Activation as an Environmental Factor in the Etiology of Autism and Schizophrenia. Front Neurosci 16: 834058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He J, Liu ZW, Lu YP, Li TY, Liang XJ, Arck PC, Huang SM, Hocher B, and Chen YP. 2017. A Systematic Review and Meta-Analysis of Influenza A Virus Infection During Pregnancy Associated with an Increased Risk for Stillbirth and Low Birth Weight. Kidney Blood Press Res 42: 232–243. [DOI] [PubMed] [Google Scholar]
- 76.Song JY, Park KV, Han SW, Choi MJ, Noh JY, Cheong HJ, Kim WJ, Oh MJ, and Cho GJ. 2020. Paradoxical long-term impact of maternal influenza infection on neonates and infants. BMC Infect Dis 20: 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borren I, Tambs K, Gustavson K, Schjolberg S, Eriksen W, Haberg SE, Hungnes O, Mjaaland S, and Trogstad LIS. 2018. Early prenatal exposure to pandemic influenza A (H1N1) infection and child psychomotor development at 6months - A population-based cohort study. Early Hum Dev 122: 1–7. [DOI] [PubMed] [Google Scholar]
- 78.Short SJ, Lubach GR, Karasin AI, Olsen CW, Styner M, Knickmeyer RC, Gilmore JH, and Coe CL. 2010. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry 67: 965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conway F, and Brown AS. 2019. Maternal Immune Activation and Related Factors in the Risk of Offspring Psychiatric Disorders. Front. Psychiatry 10: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Missault S, Van den Eynde K, Vanden Berghe W, Fransen E, Weeren A, Timmermans JP, Kumar-Singh S, and Dedeurwaerdere S. 2014. The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain Behav Immun 42: 138–146. [DOI] [PubMed] [Google Scholar]
- 81.Careaga M, Murai T, and Bauman MD. 2017. Maternal Immune Activation and Autism Spectrum Disorder: From Rodents to Nonhuman and Human Primates. Biol Psychiatry 81: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mirabella F, Desiato G, Mancinelli S, Fossati G, Rasile M, Morini R, Markicevic M, Grimm C, Amegandjin C, Termanini A, Peano C, Kunderfranco P, di Cristo G, Zerbi V, Menna E, Lodato S, Matteoli M, and Pozzi D. 2021. Prenatal interleukin 6 elevation increases glutamatergic synapse density and disrupts hippocampal connectivity in offspring. Immunity 54: 2611-2631.e2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goeden N, Velasquez J, Arnold KA, Chan Y, Lund BT, Anderson GM, and Bonnin A. 2016. Maternal Inflammation Disrupts Fetal Neurodevelopment via Increased Placental Output of Serotonin to the Fetal Brain. J. Neurosci. 36: 6041–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shook LL, Sullivan EL, Lo JO, Perlis RH, and Edlow AG. 2022. COVID-19 in pregnancy: implications for fetal brain development. Trends Mol. Med. 28: 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rudolph MD, Graham AM, Feczko E, Miranda-Dominguez O, Rasmussen JM, Nardos R, Entringer S, Wadhwa PD, Buss C, and Fair DA. 2018. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci. 21: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spann MN, Monk C, Scheinost D, and Peterson BS. 2018. Maternal Immune Activation During the Third Trimester Is Associated with Neonatal Functional Connectivity of the Salience Network and Fetal to Toddler Behavior. J. Neurosci. 38: 2877–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith SE, Li J, Garbett K, Mirnics K, and Patterson PH. 2007. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 27: 10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rasmussen JM, Graham AM, Entringer S, Gilmore JH, Styner M, Fair DA, Wadhwa PD, and Buss C. 2019. Maternal Interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. Neuroimage 185: 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, Styner M, Potkin SG, Entringer S, Wadhwa PD, Fair DA, and Buss C. 2018. Maternal Systemic Interleukin-6 During Pregnancy Is Associated With Newborn Amygdala Phenotypes and Subsequent Behavior at 2 Years of Age. Biol. Psychiatry 83: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gilmore JH, Fredrik Jarskog L, Vadlamudi S, and Lauder JM. 2004. Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacology 29: 1221–1229. [DOI] [PubMed] [Google Scholar]
- 91.Wong H, and Hoeffer C. 2018. Maternal IL-17A in autism. Exp. Neurol. 299: 228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mass E, and Gentek R. 2021. Fetal-Derived Immune Cells at the Roots of Lifelong Pathophysiology. Front Cell Dev Biol 9: 648313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Edlow AG, Glass RM, Smith CJ, Tran PK, James K, and Bilbo S. 2018. Placental Macrophages: A Window Into Fetal Microglial Function in Maternal Obesity. Int. J. Dev. Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ceasrine AM, Batorsky R, Shook LL, Kislal S, Bordt EA, Devlin BA, Perlis RH, Slonim DK, Bilbo SD, and Edlow AG. 2021. Single cell profiling of Hofbauer cells and fetal brain microglia reveals shared programs and functions. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shook LL, Kislal S, and Edlow AG. 2020. Fetal brain and placental programming in maternal obesity: A review of human and animal model studies. Prenat. Diagn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nilsson C, Larsson BM, Jennische E, Eriksson E, Bjorntorp P, York DA, and Holmang A. 2001. Maternal endotoxemia results in obesity and insulin resistance in adult male offspring. Endocrinology 142: 2622–2630. [DOI] [PubMed] [Google Scholar]
- 97.Ni M, Zhang Q, Zhao J, Yao D, Wang T, Shen Q, Li W, Li B, Ding X, and Liu Z. 2022. Prenatal inflammation causes obesity and abnormal lipid metabolism via impaired energy expenditure in male offspring. Nutr Metab (Lond) 19: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cotechini T, Komisarenko M, Sperou A, Macdonald-Goodfellow S, Adams MA, and Graham CH. 2014. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med 211: 165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ascierto PA, Fu B, and Wei H. 2021. IL-6 modulation for COVID-19: the right patients at the right time? J Immunother Cancer 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Samuelsson AM, Ohrn I, Dahlgren J, Eriksson E, Angelin B, Folkow B, and Holmang A. 2004. Prenatal exposure to interleukin-6 results in hypertension and increased hypothalamic-pituitary-adrenal axis activity in adult rats. Endocrinology 145: 4897–4911. [DOI] [PubMed] [Google Scholar]
- 101.Mazumder B, Almond D, Park K, Crimmins EM, and Finch CE. 2010. Lingering prenatal effects of the 1918 influenza pandemic on cardiovascular disease. J Dev Orig Health Dis 1: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Illanes-Alvarez F, Marquez-Ruiz D, Marquez-Coello M, Cuesta-Sancho S, and Giron-Gonzalez JA. 2021. Similarities and differences between HIV and SARS-CoV-2. Int J Med Sci 18: 846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nesheim S, Taylor A, Lampe MA, Kilmarx PH, Fitz Harris L, Whitmore S, Griffith J, Thomas-Proctor M, Fenton K, and Mermin J. 2012. A framework for elimination of perinatal transmission of HIV in the United States. Pediatrics 130: 738–744. [DOI] [PubMed] [Google Scholar]
- 104.Xiao PL, Zhou YB, Chen Y, Yang MX, Song XX, Shi Y, and Jiang QW. 2015. Association between maternal HIV infection and low birth weight and prematurity: a meta-analysis of cohort studies. BMC Pregnancy Childbirth 15: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lohman-Payne B, Gabriel B, Park S, Wamalwa D, Maleche-Obimbo E, Farquhar C, Bosire RK, and John-Stewart G. 2018. HIV-exposed uninfected infants: elevated cord blood Interleukin 8 (IL-8) is significantly associated with maternal HIV infection and systemic IL-8 in a Kenyan cohort. Clin Transl Med 7: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dirajlal-Fargo S, Mussi-Pinhata MM, Weinberg A, Yu Q, Cohen R, Harris DR, Bowman E, Gabriel J, Kulkarni M, Funderburg N, Chakhtoura N, McComsey GA, and Protocol NL. 2019. HIV-exposed-uninfected infants have increased inflammation and monocyte activation. AIDS 33: 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jao J, Kirmse B, Yu C, Qiu Y, Powis K, Nshom E, Epie F, Tih PM, Sperling RS, Abrams EJ, Geffner ME, LeRoith D, and Kurland IJ. 2015. Lower Preprandial Insulin and Altered Fuel Use in HIV/Antiretroviral-Exposed Infants in Cameroon. J Clin Endocrinol Metab 100: 3260–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Claudio CC, Patin RV, Palchetti CZ, Machado DM, Succi RC, and Oliveira FL. 2013. Nutritional status and metabolic disorders in HIV-exposed uninfected prepubertal children. Nutrition 29: 1020–1023. [DOI] [PubMed] [Google Scholar]
- 109.Garcia-Otero L, Lopez M, Gonce A, Fortuny C, Salazar L, Valenzuela-Alcaraz B, Guirado L, Cesar S, Gratacos E, and Crispi F. 2021. Cardiac Remodeling and Hypertension in HIV-Uninfected Infants Exposed in utero to Antiretroviral Therapy. Clin Infect Dis 73: 586–593. [DOI] [PubMed] [Google Scholar]
- 110.Jao J, Jacobson DL, Yu W, Borkowsky W, Geffner ME, McFarland EJ, Patel K, Williams PL, Miller T, and Pediatric HIVACS. 2019. A Comparison of Metabolic Outcomes Between Obese HIV-Exposed Uninfected Youth From the PHACS SMARTT Study and HIV-Unexposed Youth From the NHANES Study in the United States. J Acquir Immune Defic Syndr 81: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fourman LT, Pan CS, Zheng I, Gerard ME, Sheehab A, Lee H, Stanley TL, and Grinspoon SK. 2020. Association of In Utero HIV Exposure With Obesity and Reactive Airway Disease in HIV-Negative Adolescents and Young Adults. J Acquir Immune Defic Syndr 83: 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pantham P, Aye IL, and Powell TL. 2015. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta 36: 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patel N, Pasupathy D, and Poston L. 2015. Determining the consequences of maternal obesity for offspring health. Exp Physiol 100: 1421–1428. [DOI] [PubMed] [Google Scholar]
- 114.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, and Sattar N. 2002. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab 87: 4231–4237. [DOI] [PubMed] [Google Scholar]
- 115.Yu Z, Han S, Zhu J, Sun X, Ji C, and Guo X. 2013. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One 8: e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gaillard R, Steegers EA, Duijts L, Felix JF, Hofman A, Franco OH, and Jaddoe VW. 2014. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension 63: 683–691. [DOI] [PubMed] [Google Scholar]
- 117.Ayonrinde OT, Oddy WH, Adams LA, Mori TA, Beilin LJ, de Klerk N, and Olynyk JK. 2017. Infant nutrition and maternal obesity influence the risk of non-alcoholic fatty liver disease in adolescents. J Hepatol 67: 568–576. [DOI] [PubMed] [Google Scholar]
- 118.Perng W, Gillman MW, Mantzoros CS, and Oken E. 2014. A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Ann Epidemiol 24: 793–800 e791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oostvogels AJ, Stronks K, Roseboom TJ, van der Post JA, van Eijsden M, and Vrijkotte TG. 2014. Maternal prepregnancy BMI, offspring’s early postnatal growth, and metabolic profile at age 5–6 years: the ABCD Study. J Clin Endocrinol Metab 99: 3845–3854. [DOI] [PubMed] [Google Scholar]
- 120.Hochner H, Friedlander Y, Calderon-Margalit R, Meiner V, Sagy Y, Avgil-Tsadok M, Burger A, Savitsky B, Siscovick DS, and Manor O. 2012. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation 125: 1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Razaz N, Villamor E, Muraca GM, Bonamy AE, and Cnattingius S. 2020. Maternal obesity and risk of cardiovascular diseases in offspring: a population-based cohort and sibling-controlled study. Lancet Diabetes Endocrinol 8: 572–581. [DOI] [PubMed] [Google Scholar]
- 122.Borengasser SJ, Zhong Y, Kang P, Lindsey F, Ronis MJ, Badger TM, Gomez-Acevedo H, and Shankar K. 2013. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology 154: 4113–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tong JF, Yan X, Zhu MJ, Ford SP, Nathanielsz PW, and Du M. 2009. Maternal obesity downregulates myogenesis and beta-catenin signaling in fetal skeletal muscle. Am J Physiol Endocrinol Metab 296: E917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martin-Gronert MS, Fernandez-Twinn DS, Poston L, and Ozanne SE. 2010. Altered hepatic insulin signalling in male offspring of obese mice. J Dev Orig Health Dis 1: 184–191. [DOI] [PubMed] [Google Scholar]
- 125.Gaillard R, Rifas-Shiman SL, Perng W, Oken E, and Gillman MW. 2016. Maternal inflammation during pregnancy and childhood adiposity. Obesity (Silver Spring) 24: 1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McCloskey K, Ponsonby AL, Collier F, Allen K, Tang MLK, Carlin JB, Saffery R, Skilton MR, Cheung M, Ranganathan S, Dwyer T, Burgner D, and Vuillermin P. 2018. The association between higher maternal pre-pregnancy body mass index and increased birth weight, adiposity and inflammation in the newborn. Pediatr Obes 13: 46–53. [DOI] [PubMed] [Google Scholar]
- 127.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, and Hauguel-de Mouzon S. 2008. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29: 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bianchi C, Taricco E, Cardellicchio M, Mando C, Massari M, Savasi V, and Cetin I. 2021. The role of obesity and gestational diabetes on placental size and fetal oxygenation. Placenta 103: 59–63. [DOI] [PubMed] [Google Scholar]
- 129.Apostol AC, Jensen KDC, and Beaudin AE. 2020. Training the Fetal Immune System Through Maternal Inflammation-A Layered Hygiene Hypothesis. Front Immunol 11: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shoelson SE, Lee J, and Goldfine AB. 2006. Inflammation and insulin resistance. J Clin Invest 116: 1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yan X, Zhu MJ, Xu W, Tong JF, Ford SP, Nathanielsz PW, and Du M. 2010. Up-regulation of Toll-like receptor 4/nuclear factor-kappaB signaling is associated with enhanced adipogenesis and insulin resistance in fetal skeletal muscle of obese sheep at late gestation. Endocrinology 151: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shook LL, Kislal S, and Edlow AG. 2020. Fetal brain and placental programming in maternal obesity: A review of human and animal model studies. Prenat Diagn 40: 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Parisi F, Milazzo R, Savasi VM, and Cetin I. 2021. Maternal Low-Grade Chronic Inflammation and Intrauterine Programming of Health and Disease. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang P, Zhou F, Guo Y, Yuan S, Lin S, Lu J, Tu S, Lu M, Shen S, Guedeney A, Xia H, and Qiu X. 2021. Association Between the COVID-19 Pandemic and Infant Neurodevelopment: A Comparison Before and During COVID-19. Front Pediatr 9: 662165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Deoni SC, Beauchemin J, Volpe A, Da Sa V, and R. Consortium. 2021. Impact of the COVID-19 Pandemic on Early Child Cognitive Development: Initial Findings in a Longitudinal Observational Study of Child Health. medRxiv. [Google Scholar]
- 136.Shuffrey LC, Firestein MR, Kyle M. Fields, Alcantara A, Amso C, Austin D, Bain J, Barbosa JM, Bence J, Bianco M, Fernandez C, Goldman C, Gyamfi-Bannerman S, Hott C, Hu V, Hussain Y, Factor-Litvak M, Lucchini P, Marsh M, McBrian R, Mourad D, Muhle M, Noble R, Penn K, Rodriguez A, Sania C, Silver A, O’Reilly WG, Stockwell KC, Tottenham M, Welch N, Zork MG, Fifer N, Monk WP, Dumitriu C, and D. 2021. Birth during the COVID-19 pandemic, but not maternal SARS-CoV-2 infection during pregnancy, is associated with lower neurodevelopmental scores at 6-months. medRxiv 10.1101/2021.07.12.21260365, accessed 10.28.21. [DOI]
- 137.Wang Y, Chen L, Wu T, Shi H, Li Q, Jiang H, Zheng D, Wang X, Wei Y, Zhao Y, and Qiao J. 2020. Impact of Covid-19 in pregnancy on mother’s psychological status and infant’s neurobehavioral development: a longitudinal cohort study in China. BMC Med. 18: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ayed M, Embaireeg A, Kartam M, More K, Alqallaf M, AlNafisi A, Alsaffar Z, Bahzad Z, Buhamad Y, Alsayegh H, Al-Fouzan W, and Alkandari H. 2021. Neurodevelopmental outcomes of infants secondary to in utero exposure to maternal SARS-CoV-2 infection: A national prospective study in Kuwait. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Edlow AG, Castro VM, Shook LL, Kaimal AJ, and Perlis RH. 2022. Neurodevelopmental Outcomes at 1 Year in Infants of Mothers Who Tested Positive for SARS-CoV-2 During Pregnancy. JAMA Netw Open 5: e2215787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shuffrey LC, Firestein MR, Kyle MH, Fields A, Alcantara C, Amso D, Austin J, Bain JM, Barbosa J, Bence M, Bianco C, Fernandez CR, Goldman S, Gyamfi-Bannerman C, Hott V, Hu Y, Hussain M, Factor-Litvak P, Lucchini M, Mandel A, Marsh R, McBrian D, Mourad M, Muhle R, Noble KG, Penn AA, Rodriguez C, Sania A, Silver WG, O’Reilly KC, Stockwell M, Tottenham N, Welch MG, Zork N, Fifer WP, Monk C, and Dumitriu D. 2022. Association of Birth During the COVID-19 Pandemic With Neurodevelopmental Status at 6 Months in Infants With and Without In Utero Exposure to Maternal SARS-CoV-2 Infection. JAMA Pediatr: e215563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liao L, Deng Y, and Zhao D. 2020. Association of Low Birth Weight and Premature Birth With the Risk of Metabolic Syndrome: A Meta-Analysis. Front Pediatr 8: 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Markopoulou P, Papanikolaou E, Analytis A, Zoumakis E, and Siahanidou T. 2019. Preterm Birth as a Risk Factor for Metabolic Syndrome and Cardiovascular Disease in Adult Life: A Systematic Review and Meta-Analysis. J Pediatr 210: 69-80 e65. [DOI] [PubMed] [Google Scholar]
- 143.Nordman H, Jaaskelainen J, and Voutilainen R. 2020. Birth Size as a Determinant of Cardiometabolic Risk Factors in Children. Horm Res Paediatr 93: 144–153. [DOI] [PubMed] [Google Scholar]
- 144.Zhu G, Du S, Wang Y, Huang X, Hu G, Lu X, Li D, Zhu Y, Qu D, Cai Q, Liu L, and Du M. 2021. Delayed Antiviral Immune Responses in Severe Acute Respiratory Syndrome Coronavirus Infected Pregnant Mice. Front Microbiol 12: 806902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Munoz-Fontela C, Widerspick L, Albrecht RA, Beer M, Carroll MW, de Wit E, Diamond MS, Dowling WE, Funnell SGP, Garcia-Sastre A, Gerhards NM, de Jong R, Munster VJ, Neyts J, Perlman S, Reed DS, Richt JA, Riveros-Balta X, Roy CJ, Salguero FJ, Schotsaert M, Schwartz LM, Seder RA, Segales J, Vasan SS, Henao-Restrepo AM, and Barouch DH. 2022. Advances and gaps in SARS-CoV-2 infection models. PLoS Pathog 18: e1010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Smolders S, Notter T, Smolders SMT, Rigo JM, and Brone B. 2018. Controversies and prospects about microglia in maternal immune activation models for neurodevelopmental disorders. Brain Behav Immun 73: 51–65. [DOI] [PubMed] [Google Scholar]
- 147.Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM, Whittredge PB, Fu T, Worringer K, Brown HE, Wang J, Kaykas A, Karmacharya R, Goold CP, Sheridan SD, and Perlis RH. 2019. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci 22: 374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sheridan SD, Thanos JM, De Guzman RM, McCrea LT, Horng JE, Fu T, Sellgren CM, Perlis RH, and Edlow AG. 2021. Umbilical cord blood-derived microglia-like cells to model COVID-19 exposure. Transl Psychiatry 11: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Megli C, and Coyne CB. 2021. Gatekeepers of the fetus: Characterization of placental macrophages. J. Exp. Med. 218. [DOI] [PMC free article] [PubMed] [Google Scholar]


