Abstract
In a mouse model of systemic infection, the spv genes carried on the Salmonella enterica serovar Typhimurium virulence plasmid increase the replication rate of salmonellae in host cells of the reticuloendothelial system, most likely within macrophages. A nonpolar deletion in the spvB gene greatly decreased virulence but could not be complemented by spvB alone. However, a low-copy-number plasmid expressing spvBC from a constitutive lacUV5 promoter did complement the spvB deletion. By examining a series of spv mutations and cloned spv sequences, we deduced that spvB and spvC could be sufficient to confer plasmid-mediated virulence to S. enterica serovar Typhimurium. The spvBC-bearing plasmid was capable of replacing all of the spv genes, as well as the entire virulence plasmid, of serovar Typhimurium for causing systemic infection in BALB/c mice after subcutaneous, but not oral, inoculation. A point mutation in the spvBC plasmid preventing translation but not transcription of spvC eliminated the ability of the plasmid to confer virulence. Therefore, it appears that both spvB and spvC encode the principal effector factors for Spv- and plasmid-mediated virulence of serovar Typhimurium.
Salmonella enterica is best known as the cause of diarrhea produced by numerous serovars, including Salmonella enterica serovar Typhimurium. In fact, serovar Typhimurium is one of the leading causes of diarrhea in the United States, with more than 1 million cases estimated to occur each year (30). However, in compromised individuals, most notably those with immune deficiencies, the infection can spread beyond the intestines to cause enteric fever, resembling typhoid fever caused by S. enterica serovar Typhi (45). Of the thousands of diarrheagenic serovars of S. enterica, only a subset can cause systemic infection, and this subset has in common the presence of genetically related, high-molecular-weight virulence plasmids (14). Serovar Typhi does not possess a virulence plasmid (34).
The exact mechanism by which the virulence plasmid enables systemic infection is not known; however, the serovar Typhimurium virulence plasmid contributes to systemic disease in a mouse model of enteric fever by increasing the replication rate of the bacteria within host cells beyond the intestines (15). The primary plasmid-borne virulence attributes, including intracellular replication, are encoded by five spv genes (14). It is believed that the most relevant host cells for Spv-mediated intracellular replication are macrophages, based on cellular depletion experiments with mice infected with wild-type or Spv− serovar Typhimurium (17) and immunofluorescence analysis of tissues from mice infected with wild-type serovar Typhimurium (37; P. A. Gulig, S. A. Roberts, and T. J. Doyle, Abstr. 98th Gen. Meet. Am. Soc. Microbiol. abstr. B-212, 1998). Libby et al. (24) reproduced the Spv phenotype of increased growth rate within macrophages in cell culture. They subsequently determined that the spv genes were associated with the release of macrophages from cell culture substrates and rapid replication of salmonellae within these released macrophages (25). Most recently, SpvB was shown to be an ADP-ribosylating enzyme of actin, inhibiting its polymerization in host cells (23, 33, 42), and therefore could be involved with inhibition of phagosome-lysosome fusion. Others have shown that plasmid-cured S. enterica serovar Dublin was also less effective at lysing macrophages in culture than was the wild-type parent (9).
The relationship between the spv genes and virulence has been confirmed by both mutation of spv genes and placing the recombinant spvABCD genes into virulence plasmid-cured serovar Typhimurium or serovar Dublin (14). Virulence plasmid-cured salmonellae containing the cloned spv genes have virulence indistinguishable from that of the wild-type parent (10, 20, 47). The five spv genes, spvRABCD, have been sequenced; however, the only significant homology based on primary sequence offering a clue to their function was the identification of the spvR gene product as a positive regulatory protein in the LysR family (2, 4, 7, 27, 41). SpvR is essential for expression of the other spv genes, which form an operon (7, 19, 28). The ADP-ribosylating activity of SpvB, initially examined because of secondary amino acid structure (33), is discussed above.
There are reports that genes carried on the virulence plasmid other than the spv genes are involved in virulence. The virulence plasmid has been associated with resistance of salmonellae to complement, adherence to or invasion of host cells, and suppression of elicitation of γδ T cells; however, these results have not been repeated or have been contradicted (reviewed in reference 14). The virulence plasmid can affect growth of salmonellae in vitro under certain conditions (18, 21, 35). In addition to the spv genes, insertion mutations in other genetic loci have been shown to decrease virulence in animal models (5, 26, 31, 32, 36, 40, 46).
We wanted to determine the minimum complement of spv genes necessary to confer the plasmid-mediated virulence phenotype to serovar Typhimurium to enable a more focused genetic and functional analysis. It should be noted that the demonstration that genes are necessary for virulence by analysis of specific mutations and the demonstration that genes are sufficient to confer virulence by using cloned genes are different matters. It had been shown that the spvA gene was dispensable for virulence in orally inoculated mice (38, 49), and we reasoned that the spvR gene could be replaced by a suitable promoter for spvABCD provided on a recombinant plasmid. Through a combination of mutagenesis and cloning, we determined that the spvB and spvC genes could replace all of the spv genes and the entire virulence plasmid of serovar Typhimurium after subcutaneous (s.c.) inoculation of mice, in which spv genes are essential for full virulence.
Bacterial strains and plasmids.
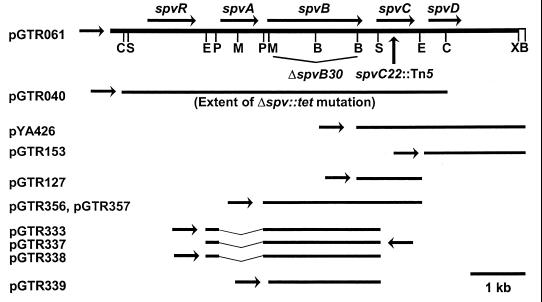
Bacterial strains and plasmids are listed and described in Table 1, and plasmid maps are depicted in Fig. 1. Unless noted otherwise, bacterial culture was at 37°C in L broth (LB) (22) or on LB containing 1.5% (wt/vol) agar. Cultures were stored frozen at −80°F in LB containing 35% (vol/vol) glycerol. For most applications, cultures were grown overnight as static cultures in LB. On the day of use, the cultures were diluted 1:20 into fresh, prewarmed LB and incubated with shaking at 37°C until the optical density at 600 nm reached approximately 0.4.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Relevant genotype | Comment(s) (reference[s]) |
|---|---|---|
| Serovar Typhimurium strains | ||
| χ3181 | pStSR100+ | Virulence plasmid positive (12) |
| χ3306 | gyrA1816 pStSR100+ | Virulence plasmid positive, Nalr (12) |
| χ3337 | gyrA1816 pStSR100− | Virulence plasmid-cured derivative of χ3306, Nalr (12) |
| χ3456 | pStSR100::Tnminitet | Tnminitet insertion in the virulence plasmid; does not affect virulence, Tetr (12) |
| UF009 | pStSR100::vir-44::aph | aph insertion in the virulence plasmid that does not affect virulence; used as Kanr wild-type strain (10) |
| UF012 | pStSR100spvB5::aph | aph insertion in the spvB gene of virulence plasmid that attenuates virulence (10) |
| UF051 | pStSR100ΔspvB30 | ΔspvB30, deleting amino acids 21 to 555 of spvB (49) |
| UF110 | pStSR100ΔspvRABCD′::tet | spvRABCD′ replaced with tet in χ3181; Tetr (16, 17) |
| Plasmids | ||
| pACYC184 | Cloning vector (3) | |
| pGTR040 | spvRABCD′ cloned into pYA2204 (10) | |
| pGTR061 | spvRABCD cloned into pYA2204 (10) | |
| pGTR100 | 4-kb EcoRI fragment of the virulence plasmid carrying spvABCD cloned into EcoRI site of pACYC184 (10) | |
| pGTR127 | spvC cloned into pACYC184 on a BamHI-EcoRI fragment so that spvC is expressed from the tet promoter (11) | |
| pGTR147 | pGTR061 spvC22::Tn5 (10) | |
| pGTR153 | spvD cloned into pACYC184 so that spvD is expressed from the tet promoter (11) | |
| pGTR175 | PstI deletion derivative of pGTR100, removing open reading frame of spvA; encodes spvBC downstream of spvA promoter (this work) | |
| pGTR309 | PstI-EcoRI fragment of pGTR061 encoding spvB and spvC cloned into the PstI-EcoRI site of pUC118 (this work) | |
| pGTR333 | 2.4-kb EcoRI-StuI fragment of pGTR175 cloned into the EcoRI site of pACYC184; carries spvB downstream of the spvA promoter and is codirectional with cat promoter of pACYC184 (this work) | |
| pGTR337 | 2.4-kb EcoRI fragment from pGTR333 cloned into the EcoRI site of pYA2204; carries spvB downstream of the spvA promoter; spvB is in the opposite orientation from lacZ′ (this work) | |
| pGTR338 | 2.4-kb EcoRI fragment from pGTR333 cloned into the EcoRI site of pYA2204; carries spvB downstream of the spvA promoter; spvB is in the same orientation as lacZ′ (this work) | |
| pGTR339 | 2.2-kb MscI-EcoRI fragment of pGTR333 cloned into pYA2204 digested with SmaI and EcoRI; carries the ribosome-binding site, start codon, and open reading frame of spvB, with spvB in the same orientation as lacZ′ (this work) | |
| pGTR356 | SphI-EcoRI fragment of pGTR309 cloned into the SphI-EcoRI site of pMW119 spvBC is codirectional with lacZ′ of pMW119 (this work) | |
| pGTR357 | pGTR356 with point mutation abolishing start codon of spvC (this work) | |
| pMW119 | Cloning vector derived from pSC101 (Nippon Gene Co., Tokyo, Japan) | |
| pUC118 | Cloning vector, Ampr (44) | |
| pYA426 | spvCD cloned into pACYC184 as a BamHI fragment so that spvCD are expressed by the tet promoter (11) | |
| pYA2204 | Low-copy-number cloning vector, Ampr (8) |
FIG. 1.
Physical and genetic maps of virulence plasmid sequences and recombinant plasmids used in this study. The top line depicts the insert of pGTR061, which carries spvRABCD, whose open reading frames are indicated by arrows with the direction of transcription shown. Restriction sites indicated below pGTR061 are as follows: B, BamHI; C, ClaI; E, EcoRI; M, MscI; P, PstI; and S, StuI. Note that pGTR061 extends to the XhoI site on the right. The rightmost BamHI site is indicated for reference to plasmids depicted below. The extent of the ΔspvB30 deletion and the location of the spvC22::Tn5 insertion are shown. The ClaI insert of pGTR040 corresponds to the Δspv::tet deletion of UF110. The arrows next to the insertion sequences of each clone indicate the direction of transcription of the promoters of the respective vectors. For pGTR333, pGTR337, and pGTR338, the broken line indicates that the EcoRI-PstI fragment carrying the spvA promoter has been juxtaposed to the spvB open reading frame by the PstI deletion. Details of plasmid construction are presented in Table 1.
Construction of an spvC start codon mutation in pGTR356.
To address the possibility that spvC is required for virulence conferred by pGTR356, we constructed a site-directed mutation in the start codon of spvC using the Transformer system (Clontech, Palo Alto, Calif.). The sequence of the start codon and the preceding three nucleotides, CCCATG, was changed to CCCGGG, which not only destroyed the start codon but also created a new and unique SmaI site as a simple screen for the mutation. The mutagenic oligonucleotide sequence was CGCAAAGGAGATTTCCCGGGCCCATAAATAGGC (SmaI site underlined), and the presence of the mutation was confirmed by SmaI digestion coupled with the lack of BsaI digestion (as part of the mutagenesis procedure, a BsaI site within the bla gene of the vector pMW119 was destroyed while conserving the amino acid sequence of Bla). The SpvC− derivative plasmid was named pGTR357.
Infection of mice.
All mouse studies used BALB/c mice (Charles River, Wilmington, Mass.; University of Florida Department of Pathology, Immunology, and Laboratory Medicine Mouse Facility), which are sensitive to infection by serovar Typhimurium because of the Itys mutation (39). Mice were orally inoculated as described previously with approximately 108 CFU of serovar Typhimurium (11). Inoculation with 105 CFU of salmonellae was done s.c. either into both hind footpads, as described previously (15), or into the upper back near the shoulder blades. All experiments were repeated at least once, with results similar to those of the experiment shown.
Lack of the spvD gene can be complemented by increased expression of spvC.
It has been shown through mutational and complementation analysis that the spvD gene is essential for full virulence of serovar Typhimurium (10). However, the effects of mutating spvD were small compared with the effects of mutations in spvR or spvC. As part of our attempts to construct a recombinant plasmid which contained the minimal spv sequence needed to confer full virulence in plasmid-cured serovar Typhimurium, we constructed plasmid pGTR040, which contains the 6.3-kb ClaI fragment bearing spvRABCD′ subcloned into the low-copy-number vector pYA2204 (similarly to pGTR061; see Fig. 1) (10). Unlike pGTR061, pGTR040 could not restore virulence to plasmid-cured χ3337 (10). The lack of spvD in pGTR040 was complemented by placing into χ3337 (pGTR040) the plasmid pGTR153, which carries only the spvD gene (10) (Table 2). We also examined plasmid pYA426, which carries spvCD expressed from the tet promoter of pACYC184 (11, 13), for its ability to complement the lack of spvD in pGTR040. As expected, χ3337 (pGTR040, pYA426) was fully virulent in terms of splenic infection (data not shown). We then examined deletion derivatives of pYA426 produced from the 3′ end of spvD moving toward spvC. We expected that as soon as spvD experienced deletions, the resulting plasmids would not work with pGTR040 to confer full virulence. However, deletions of pYA426 extending completely through spvD still produced virulence in combination with pGTR040 (data not shown). The smallest deletion derivative conferring virulence was plasmid pGTR127, which carries only spvC (Table 2). Therefore, by providing spvC expressed from a recombinant plasmid, the necessity of providing spvD to spvRABC-carrying pGTR040 was abolished. It appeared that overexpressed spvC compensated for the lack of spvD.
TABLE 2.
Expression of recombinant spvC can complement lack of spvD to confer virulence to serovar Typhimurium in mice
| Plasmid used to transform χ3337(pGTR040) | CFU ofab:
|
Paired differencea | P valuec | |
|---|---|---|---|---|
| χ3337(pGTR040) | χ3456 | |||
| pGTR153 | 5.5 ± 1.2 | 5.1 ± 1.2 | −0.4 ± 1.1 | >0.35 |
| pGTR127 | 4.8 ± 0.8 | 5.0 ± 1.0 | 0.2 ± 0.6 | >0.35 |
| pACYC184 | 4.1 ± 1.4 | 5.2 ± 1.6 | 1.1 ± 0.5 | <0.005 |
Means ± standard deviations of log-transformed data are shown. n = 6 (for all groups).
Virulence plasmid-cured serovar Typhimurium χ3337(pGTR040), which carries spvRABCD′, was transformed with either pGTR153 (carrying only spvD), pGTR127 (carrying only spvC), or the vector pACYC184. These strains were mixed with wild-type serovar Typhimurium χ3456 and inoculated orally to mice at a dose of 108 CFU. Five days later, spleens were harvested and plated to enumerate CFU of each strain.
P value for the paired difference being different from 0.
If the mechanism of this spvC-mediated complementation of a lack of spvD was due to an effect of the spvD deletion on the cis-encoded spvC, then the spvD deletion of pGTR040 should not have been complemented by spvD alone. The data are consistent with SpvD either affecting expression of spvC in a trans-active manner or physically or functionally interacting with SpvC to facilitate its virulence function in a manner that is mimicked by overexpressing spvC in the absence of spvD.
A nonpolar deletion mutation of spvB attenuates virulence but is noncomplementable.
It has been reported that an aph insertion in spvB greatly attenuates splenic infection for serovar Typhimurium after oral inoculation (10). However, we were unable to complement the mutation with cloned sequences expressing spvB. In fact, no one has been able to complement an spvB mutation with only spvB to date. We considered the possibility that the aph insertion was polar on downstream genes or otherwise exerted pleiotropic effects. We therefore constructed strain UF051, in which amino acids 21 to 555 of the spvB gene present on the virulence plasmid were deleted using inverse PCR mutagenesis, resulting in the ΔspvB30 mutation (49). As expected, UF051 was greatly attenuated for splenic infection after oral inoculation of mice compared with wild-type χ3456 (paired difference in log splenic CFU, 3.8 ± 0.7 [mean ± standard deviation]; P < 0.002), similar to spvB5::aph strain UF012 compared with wild-type χ3456 (paired difference in log splenic CFU, 3.8 ± 1.2; P < 0.01).
To complete the molecular version of Koch's postulates and confirm that the ΔspvB30 mutation was responsible for the observed attenuation, we attempted to complement the mutation with a variety of recombinant plasmids expressing spvB. We constructed a series of plasmids, each carrying only spvB transcribed by either a vector-borne promoter and/or the spvABCD promoter (Table 1; Fig. 1). None of the plasmids were capable of complementing the ΔspvB30 mutation after oral inoculation of mice (data not shown). Whenever spvB was expressed from a vector-borne promoter, either cat or lacUV5, salmonellae containing these plasmids (pGTR333, pGTR338, and pGTR339) were poorly recovered in complementation experiments and were even attenuated for virulence when the plasmids were placed in wild-type serovar Typhimurium χ3181 (data not shown). pGTR337, which had spvB expressed from the spvABCD promoter alone, was not detrimental but did not complement. We verified that the spvB-containing plasmids expressed SpvB using in vitro transcription-translation and Western blot analyses (data not shown). This lack of complementation was not due to the ΔspvB30 mutation being trans dominant (e.g., by producing an SpvB product that interfered with other Spv or cellular virulence functions), because UF051 containing pGTR061 (bearing the entire spv region) was fully virulent for splenic infection after oral inoculation of mice (paired difference for log CFU per spleen in a mixed infection with wild-type χ3456 was −0.06 ± 1.3; P = 0.5). Similarly, the ΔspvB30 mutation was not polar on expression of spvC or spvD, because pGTR147, which is pGTR061 with an spvC22::Tn5 insertion (10), was able to complement the ΔspvB30 mutation (paired difference for log CFU per spleen in a mixed infection with wild-type χ3456 was −0.02 ± 1.3; P = 0.5).
A recombinant plasmid carrying spvBC complements ΔspvB30 and confers virulence to Spv− serovar Typhimurium by the s.c. route of inoculation.
Since previous results (38, 49) indicated that spvA was not essential for virulence and we demonstrated above that lack of spvD could be compensated for by providing excess spvC, we considered the possibility that the ΔspvB30 mutation could be complemented by a plasmid carrying spvB and spvC together. If the spvBC genes were expressed from an exogenous promoter, then spvR would be obviated. We therefore constructed plasmid pGTR356, which contains the spvBC expressed from the lacUV5 promoter genes in the low-copy-number vector pMW119 (Table 1; Fig. 1). In Lac− serovar Typhimurium, this promoter would be constitutive. UF051(pGTR356) was fully virulent when administered to mice by the oral route compared with wild-type χ3306 (mean log splenic CFU were 5.6 ± 0.76 and 6.0 ± 1.5, respectively; P > 0.6). UF051 containing the vector pMW119 yielded a log splenic CFU of 2.2 ± 1.0 [P < 0.005 compared with χ3306 or UF051(pGTR356)]. Therefore, the combined spvBC genes were able to complement the ΔspvB30 mutation. We still do not know why the spvB gene alone could not complement the ΔspvB30 mutation. However, with the breadth of spv clones attempted by us and others, it is unlikely that the reason was inadequate or insufficient expression.
Since pGTR356 complemented the ΔspvB30 mutation, and in light of the ancillary roles of spvA and spvD and the ability to compensate for SpvR by an exogenous promoter, we asked if pGTR356 could restore virulence to either Δspv::tet serovar Typhimurium UF110 (16, 17) or plasmid-cured χ3337 (12). In oral inoculations with mixed strains, UF110(pGTR356) sometimes approached wild-type UF009 for levels of splenic infection but most often failed to achieve wild-type levels (data not shown). UF110(pGTR356) was sometimes significantly higher than virulence plasmid-cured χ3337 for splenic infection but at other times was not significantly higher. Interestingly, UF110(pGTR356) was often recovered from Peyer's patches or feces in lower numbers, compared with UF009 or χ3337. Similarly, χ3337(pGTR356) failed to achieve wild-type levels of splenic infection after oral inoculation of mice (data not shown).
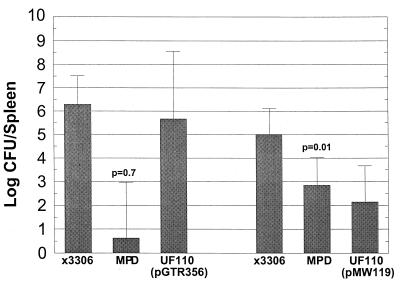
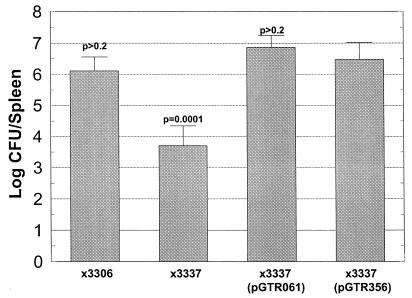
Since it appeared that pGTR356 could be detrimental to salmonellae in the gut, we injected pGTR356-containing strains s.c. into the hind footpads or backs of mice and examined splenic infection (Fig. 2 and 3). We had previously shown that the virulence plasmid and specifically the spv genes were essential for systemic infection of serovar Typhimurium inoculated s.c. into BALB/c mice (15, 17). pGTR356 was able to fully complement the Δspv::tet mutation of UF110 when examined in mixed infection with wild-type χ3306 (paired difference in log splenic CFU of 0.62 was not significantly different from 0; P > 0.6) (Fig. 2). Similarly, in the virulence plasmid-cured background of χ3337, pGTR356 fully restored splenic infection after s.c. inoculation, compared with wild-type χ3306 and χ3337 (pGTR061) (P > 0.2) (Fig. 3). Therefore, when the intestines, which are not involved with Spv-mediated pathogenesis in mice, are bypassed via s.c. inoculation, the spvBC genes are sufficient to replace the entire virulence plasmid for enabling splenic infection.
FIG. 2.
Recombinant spvBC complements Δspv::tet after s.c. inoculation of mice. Mice were inoculated s.c. with wild-type strain χ3306 and Δspv::tet strain UF110 containing either spvBC-bearing pGTR356 or the vector pMW119. Four days later, spleens were removed, homogenized, and plated to enumerate CFU. P values are for the mean paired difference (MPD) being greater than 0. Error bars represent standard deviations. n = 5 (for all groups).
FIG. 3.
Recombinant spvBC complements virulence plasmid-cured serovar Typhimurium after s.c. inoculation of mice. Mice were inoculated s.c. with wild-type strain χ3306 or virulence plasmid-cured strain χ3337 containing spvBC-bearing pGTR356, spvRABCD-bearing pGTR061, or no plasmid. Four days later, spleens were removed, homogenized, and plated to enumerate CFU. P values are for χ3337(pGTR356) being different from the other strains. Additionally, χ3456 and χ3337(pGTR061) were each significantly greater than χ3337 (P < 0.0001), and χ3337(pGTR061) was significantly greater than χ3456 (P < 0.02). Error bars represent standard deviations. n = 5 (for all groups).
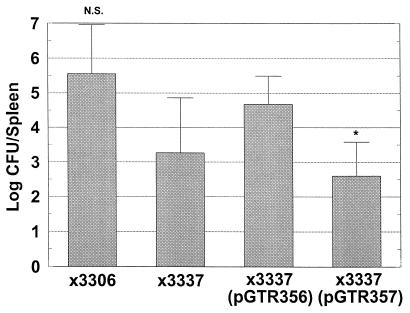
We considered the possibility that the presence of spvC on pGTR356 enabled virulence by affecting the stability of spvB mRNA in a cis-active manner by the presence of the spvC mRNA immediately downstream. Alternatively, the SpvC protein could interact with the SpvB protein to either aid in its function or prevent toxic effects to the salmonella cells. To confirm that the SpvC protein was essential for the virulence conferred by pGTR356 to plasmid-cured χ3337, we constructed a site-directed mutation in the start codon of spvC in pGTR356, yielding pGTR357 (Table 1). The mRNA structure of pGTR357 should have been intact, while translation of spvC should have been inhibited. When inoculated s.c. into the backs of BALB/c mice, pGTR357 was unable to confer virulence to χ3337 in terms of splenic CFU (Fig. 4), and splenic CFU were significantly lower than those attained by χ3306. Therefore, translation of SpvC protein is required for the virulence function encoded by pGTR356, and both spvB and spvC are necessary and sufficient to confer plasmid-mediated virulence to serovar Typhimurium after s.c. inoculation of mice.
FIG. 4.
spvC is required for virulence conferred by spvBC-bearing pGTR356. Mice were inoculated s.c. with 105 CFU of either wild-type χ3306, virulence plasmid-cured χ3337, χ3337(pGTR356), or χ3337(pGTR357). Six days later, spleens were removed, homogenized, and plated to enumerate CFU. The spvC mutation of pGTR357(∗) significantly decreased infection, compared with spvBC-bearing pGTR356 (P < 0.01). N.S., χ3306 was not significantly different from χ3337(pGTR356) (P > 0.3). Error bars represent standard deviations. n = 5 (for all groups).
Why are constitutively expressed spvBC genes unable to confer plasmid-mediated virulence after oral inoculation of mice? The answer could lie in the detrimental nature of inappropriate expression of these genes. For example, it appears that salmonella genes that are involved with infection of macrophages (spv and salmonella pathogenicity island 2 [SPI2]) are regulated in a manner opposite that of those involved with infection of the intestines (salmonella pathogenicity island 1 [SPI1]), especially with regard to regulation by PhoP and PhoQ (spv and SPI2 are PhoP activated [6]; SPI1 is PhoP repressed [1]). In fact, we recently showed that the attenuating effects of constitutively expressed phoP in serovar Typhimurium are only apparent in an Spv+ background (29). Most recently it was shown that SpvB is an ADP-ribosylating toxin of actin in macrophages and inhibits polymerization of actin (23, 33, 42). If this activity contributes to inhibition of phagosome-lysosome fusion mediated by SPI2 (43), then it is possible that expression of these macrophage-specific genes might inhibit SPI1-mediated invasion of or transcytosis through the intestinal epithelium after oral inoculation. However, after s.c. inoculation, in which spv genes are essential for efficient systemic infection but SPI1 is dispensable (17), constitutive expression could be beneficial. We did not perform intraperitoneal infections because we have found that the spv genes are not nearly as important for virulence by this route as by oral and s.c. inoculation (12). This could be due to the fact that after intraperitoneal inoculation the salmonellae replicate extensively in extracellular fluid, where the spv genes are not required for virulence (12) and are not even induced for expression (48).
The mechanism by which the SpvB protein is secreted out of the salmonella cells and into the cytoplasm of macrophages to interact with actin is not known. A type III secretion-mediated process would be plausible, but there are no published data to support this hypothesis. It is possible that the SpvC and perhaps SpvD proteins participate in this process. The detrimental nature of spvB expressed by itself, either when cloned alone or when spvC is mutated in pGTR357, suggests that SpvB requires SpvC for appropriate, functional activity. This phenomenon is not restricted to virulence in mice, since some spvB-bearing recombinant plasmids are detrimental to serovar Typhimurium growing in vitro. Furthermore, our data suggest that SpvD interacts with SpvC for its function, since lack of SpvD could be complemented with overexpressed SpvC. In any case, it is clear that spvBC are sufficient to replace the entire virulence plasmid to enable systemic infection of mice under some circumstances. Coupled with the recent discovery of the molecular function of SpvB, our data should focus future investigations of Spv function on these two genes and their products.
(These results were presented in preliminary form at the 1994, 1995, and 1997 General Meetings of the American Society for Microbiology [C. M. Bacot, and P. A. Gulig, Abstr. 94th Gen. Meet. Am. Soc. Microbiol. 1994, abstr. B-322, 1994; J. A. Rogers, H. Matsui, and P. A. Gulig, Abstr. 95th Gen. Meet. Am. Soc. Microbiol. 1995, abstr. B-304, 1995; H. Matsui, K. Kawahara, A. Suzuki, K. Sekiya, H. Danbara, C. M. Bacot, and P. A. Gulig, Abstr. 97th Gen. Meet. Am. Soc. Microbiol., abstr. B-281, 1997].)
Acknowledgments
We thank Donna H. Duckworth for reviewing the manuscript.
This work was supported by NIH grant AI24821 and by American Heart Association—Florida Affiliate grants 89GIA81 and 92GIA868 to P.A.G., who was an American Heart Association Established Investigator with funds contributed in part by the American Heart Association—Florida Affiliate.
REFERENCES
- 1.Behlau I, Miller S I. A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J Bacteriol. 1993;175:4475–4484. doi: 10.1128/jb.175.14.4475-4484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caldwell A L, Gulig P A. The Salmonella typhimurium virulence plasmid encodes a positive regulator of a plasmid-encoded virulence gene. J Bacteriol. 1991;173:7176–7185. doi: 10.1128/jb.173.22.7176-7185.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coynault C, Robbe-Saule V, Popoff M Y, Norel F. Growth phase and SpvR regulation of transcription of Salmonella typhimurium spvABC virulence genes. Microb Pathog. 1992;13:133–143. doi: 10.1016/0882-4010(92)90073-w. [DOI] [PubMed] [Google Scholar]
- 5.Daifuku R, Chikami G K. Tn1725 transposon mutagenesis of 9–18Δ7, an EcoRI deletion derivative of Salmonella dublin Lane plasmid pSDL2. Infect Immun. 1991;59:4720–4723. doi: 10.1128/iai.59.12.4720-4723.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deiwick J, Nikolaus T, Erdogan S, Hensel M. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol Microbiol. 1999;31:1759–1773. doi: 10.1046/j.1365-2958.1999.01312.x. [DOI] [PubMed] [Google Scholar]
- 7.Fang F C, Krause M, Roudier C, Fierer J, Guiney D G. Growth regulation of a Salmonella plasmid gene essential for virulence. J Bacteriol. 1991;173:6783–6789. doi: 10.1128/jb.173.21.6783-6789.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galan J E, Timoney J F, Curtiss R., III . Expression and localization of the Streptococcus equi M protein in Escherichia coli and Salmonella typhimurium. In: Powell D G, editor. Proceedings of the Fifth International Conference on Equine Infectious Diseases. Lexington: University Press of Kentucky; 1988. pp. 34–40. [Google Scholar]
- 9.Guilloteau L A, Wallis T S, Gautier A V, Maclntyre S, Platt D J, Lax A J. The Salmonella virulence plasmid enhances Salmonella-induced lysis of macrophages and influences inflammatory responses. Infect Immun. 1996;64:3385–3393. doi: 10.1128/iai.64.8.3385-3393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulig P A, Caldwell A L, Chiodo V A. Identification, genetic analysis, and DNA sequence of a 7.8-kilobase virulence region of the Salmonella typhimurium virulence plasmid. Mol Microbiol. 1992;6:1395–1411. doi: 10.1111/j.1365-2958.1992.tb00860.x. [DOI] [PubMed] [Google Scholar]
- 11.Gulig P A, Chiodo V A. Genetic and DNA sequence analysis of the Salmonella typhimurium virulence plasmid gene encoding the 28,000-molecular-weight protein. Infect Immun. 1990;58:2651–2658. doi: 10.1128/iai.58.8.2651-2658.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulig P A, Curtiss R., III Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987;55:2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulig P A, Curtiss R., III Cloning and transposon insertion mutagenesis of virulence genes of the 100-kilobase plasmid of Salmonella typhimurium. Infect Immun. 1988;56:3262–3271. doi: 10.1128/iai.56.12.3262-3271.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulig P A, Danbara H, Guiney D G, Lax A J, Norel F, Rhen M. Molecular analysis of virulence genes of the salmonella virulence plasmids. Mol Microbiol. 1993;7:825–830. doi: 10.1111/j.1365-2958.1993.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 15.Gulig P A, Doyle T J. The Salmonella typhimurium virulence plasmid increases the growth rate of salmonellae in mice. Infect Immun. 1993;61:504–511. doi: 10.1128/iai.61.2.504-511.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulig P A, Doyle T J, Clare-Salzler M J, Maiese R L, Matsui H. Systemic infection of mice by wild-type but not Spv−Salmonella typhimurium is enhanced by neutralization of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1997;65:5191–5197. doi: 10.1128/iai.65.12.5191-5197.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulig P A, Doyle T J, Hughes J A, Matsui H. Analysis of host cells associated with the Spv-mediated increased intracellular growth rate of Salmonella typhimurium in mice. Infect Immun. 1998;66:2471–2485. doi: 10.1128/iai.66.6.2471-2485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones G W, Rabert D K, Svinarich D M, Whitfield H J. Association of adhesive, invasive, and virulent phenotypes of Salmonella typhimurium with autonomous 60-megadalton plasmids. Infect Immun. 1982;38:476–486. doi: 10.1128/iai.38.2.476-486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krause M, Fang F C, Guiney D G. Regulation of plasmid virulence gene expression in Salmonella dublin involves an unusual operon structure. J Bacteriol. 1992;174:4482–4489. doi: 10.1128/jb.174.13.4482-4489.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krause M, Roudier C, Fierer J, Harwood J, Guiney D. Molecular analysis of the virulence locus of the Salmonella dublin plasmid pSDL2. Mol Microbiol. 1991;5:307–316. doi: 10.1111/j.1365-2958.1991.tb02111.x. [DOI] [PubMed] [Google Scholar]
- 21.Lax A J, Pullinger G D, Baird G D, Williamson C M. The virulence plasmid of Salmonella dublin: detailed restriction map and analysis by transposon mutagenesis. J Gen Microbiol. 1990;136:1117–1123. doi: 10.1099/00221287-136-6-1117. [DOI] [PubMed] [Google Scholar]
- 22.Lennox E S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955;1:190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- 23.Lesnick M L, Reiner N E, Fierer J, Guiney D G. The Salmonella spvB virulence gene encodes an enzyme that ADP-ribosylates actin and destabilizes the cytoskeleton of eukaryotic cells. Mol Microbiol. 2001;39:1464–1470. doi: 10.1046/j.1365-2958.2001.02360.x. [DOI] [PubMed] [Google Scholar]
- 24.Libby S J, Adams L G, Ficht T A, Allen C, Whitford H A, Buchmeier N A, Bossie S, Guiney D G. The spv genes on the Salmonella dublin virulence plasmid are required for severe enteritis and systemic infection in the natural host. Infect Immun. 1997;65:1786–1792. doi: 10.1128/iai.65.5.1786-1792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Libby S J, Lesnick M, Hasegawa P, Weidenhammer E, Guiney D G. The Salmonella virulence plasmid spv genes are required for cytopathology in human monocyte-derived macrophages. Cell Microbiol. 2000;2:49–58. doi: 10.1046/j.1462-5822.2000.00030.x. [DOI] [PubMed] [Google Scholar]
- 26.Manning E J, Baird G D, Jones P W. The role of plasmid genes in the pathogenicity of Salmonella dublin. J Med Microbiol. 1986;21:239–243. doi: 10.1099/00222615-21-3-239. [DOI] [PubMed] [Google Scholar]
- 27.Matsui H, Abe A, Kawahara K, Terakado N, Danbara H. Positive regulator for the expression of Mba protein of the virulence plasmid, pKDSC50, of Salmonella choleraesuis. Microb Pathog. 1991;10:459–464. doi: 10.1016/0882-4010(91)90111-m. [DOI] [PubMed] [Google Scholar]
- 28.Matsui H, Abe A, Suzuki S, Kijima M, Tamura Y, Nakamura M, Kawahara K, Danbara H. Molecular mechanism of the regulation of expression of plasmid-encoded mouse bacteremia (mba) genes in Salmonella serovar choleraesuis. Mol Gen Genet. 1993;236:219–226. doi: 10.1007/BF00277116. [DOI] [PubMed] [Google Scholar]
- 29.Matsui H, Kawakami T, Ishikawa S, Danbara H, Gulig P A. Constitutively expressed phoP inhibits mouse-virulence of Salmonella typhimurium in an Spv-dependent manner. Microbiol Immunol. 2000;44:447–454. doi: 10.1111/j.1348-0421.2000.tb02519.x. [DOI] [PubMed] [Google Scholar]
- 30.Mead P S, Slutsker L, Dietz V, McCaig L F, Bresee J S, Shapiro C, Griffin P M, Tauxe R V. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michiels T, Popoff M Y, Durviaux S, Coynault C, Cornelis G. A new method for the physical and genetic mapping of large plasmids: application to the localisation of the virulence determinants on the 90 kb plasmid of Salmonella typhimurium. Microb Pathog. 1987;3:109–116. doi: 10.1016/0882-4010(87)90069-6. [DOI] [PubMed] [Google Scholar]
- 32.Norel F, Coynault C, Miras I, Hermant D, Popoff M Y. Cloning and expression of plasmid DNA sequences involved in Salmonella serotype typhimurium virulence. Mol Microbiol. 1989;3:733–743. doi: 10.1111/j.1365-2958.1989.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 33.Otto H, Tezcan-Merdol D, Girisch R, Haag F, Rhen M, Koch-Nolte F. The spvB gene-product of the Salmonella enterica virulence plasmid is a mono(ADP-ribosyl)transferase. Mol Microbiol. 2000;37:1106–1115. doi: 10.1046/j.1365-2958.2000.02064.x. [DOI] [PubMed] [Google Scholar]
- 34.Popoff M Y, Miras I, Coynault C, Lasselin C, Pardon P. Molecular relationships between virulence plasmids of Salmonella serotypes typhimurium and dublin and large plasmids of other Salmonella serotypes. Ann Microbiol (Paris) 1984;135:389–398. doi: 10.1016/s0769-2609(84)80080-0. [DOI] [PubMed] [Google Scholar]
- 35.Pullinger G D, Lax A J. A Salmonella dublin virulence-associated plasmid locus that affects bacterial growth under nutrient-limited conditions. Mol Microbiol. 1992;6:1631–1643. doi: 10.1111/j.1365-2958.1992.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 36.Rhen M, Virtanen M, Makela P H. Localization by insertion mutagenesis of a virulence-associated region on the Salmonella typhimurium 96 kilobase pair plasmid. Microb Pathog. 1989;6:153–158. doi: 10.1016/0882-4010(89)90018-1. [DOI] [PubMed] [Google Scholar]
- 37.Richter-Dahlfors A, Buchan A M J, Finlay B B. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roudier C, Fierer J, Guiney D G. Characterization of translation termination mutations in the spv operon of the Salmonella virulence plasmid pSDL2. J Bacteriol. 1992;174:6418–6423. doi: 10.1128/jb.174.20.6418-6423.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz L D, Sidman C L. Genetically determined murine models of immunodeficiency. Annu Rev Immunol. 1987;5:367–403. doi: 10.1146/annurev.iy.05.040187.002055. [DOI] [PubMed] [Google Scholar]
- 40.Sizemore D R, Fink P S, Ou J T, Baron L, Kopecko D J, Warren R L. Tn5 mutagenesis of the Salmonella typhimurium 100 kb plasmid: definition of new virulence regions. Microb Pathog. 1991;10:493–499. doi: 10.1016/0882-4010(91)90116-r. [DOI] [PubMed] [Google Scholar]
- 41.Taira S, Riikonen P, Saarilahti H, Sukupolvi S, Rhen M. The mkaC virulence gene of the Salmonella serovar Typhimurium 96 kb plasmid encodes a transcriptional activator. Mol Gen Genet. 1991;228:381–384. doi: 10.1007/BF00260630. [DOI] [PubMed] [Google Scholar]
- 42.Tezcan-Merdol D, Nyman T, Lindberg U, Haag F, Koch-Nolte F, Rhen M. Actin is ADP-ribosylated by the Salmonella enterica virulence-associated protein SpvB. Mol Microbiol. 2001;39:606–619. doi: 10.1046/j.1365-2958.2001.02258.x. [DOI] [PubMed] [Google Scholar]
- 43.Uchiya K, Barbieri M A, Funato K, Shah A H, Stahl P D, Groisman E A. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 1999;18:3924–3933. doi: 10.1093/emboj/18.14.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 45.Wilkins E G L, Roberts C. Extraintestinal salmonellosis. Epidemiol Infect. 1988;100:361–368. doi: 10.1017/s095026880006711x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williamson C M, Baird G D, Manning E J. A common virulence region on plasmids from eleven serotypes of Salmonella. J Gen Microbiol. 1988;134:975–982. doi: 10.1099/00221287-134-4-975. [DOI] [PubMed] [Google Scholar]
- 47.Williamson C M, Pullinger G D, Lax A J. Identification of an essential virulence region on Salmonella plasmids. Microb Pathog. 1988;5:469–473. doi: 10.1016/0882-4010(88)90008-3. [DOI] [PubMed] [Google Scholar]
- 48.Wilson J A, Doyle T J, Gulig P A. Exponential phase expression of spvA of the Salmonella typhimurium virulence plasmid: induction in intracellular salts medium and intracellularly in mice and cultured mammalian cells. Microbiology. 1997;143:3827–3839. doi: 10.1099/00221287-143-12-3827. [DOI] [PubMed] [Google Scholar]
- 49.Wilson J A, Gulig P A. Regulation of the spvR gene of the Salmonella typhimurium virulence plasmid during exponential phase growth in intracellular salts medium and at stationery phase in L broth. Microbiology. 1998;144:1823–1833. doi: 10.1099/00221287-144-7-1823. [DOI] [PubMed] [Google Scholar]