Abstract
А clinical case of non-union in a cat after open fracture repair by intramedullary ostheosynthesis of the radius was described. The patient was presented with non-weight bearing lameness, fistulas with purulent discharge, swelling and severe pain. During the surgical revision, after bone sequestrum removal, the bone defect was filled with cancellous and cortical bone autografts. Osteosynthesis with a modified external bone fixator, made of Duracryl® Plus – a rapidly self-curing metacrylate polymer – and 6 Kirschner wires passing perpendicularly through both radial cortices was performed. The post-operative period was smooth, and after 23 weeks the external fixator was removed. Radiography showed very good bone healing, with excellent clinical result. The use of the ulna as a donor bone was very convenient because it allowed collecting a cortical graft of larger size. The extremely light model of external bone fixator provided adequate strength of fixation elements and proved to be an efficient and not expensive technique for osteosynthesis in cat with non-union fractures of the distal radius and ulna.
Keywords: cat, open radius and ulna fractures, external bone fixator, cancellous and cortical bone autograft
Resumo
Um caso clínico de correção de uma não união de uma fratura exposta em um gato após por osteossíntese intramedular do rádio foi descrito. O paciente apresentou claudicação, fístulas com secreção purulenta, edema e dor intensa. Durante a revisão cirúrgica, após a remoção do sequestro ósseo, o defeito ósseo foi preenchido com autoenxertos de osso esponjoso e cortical. Foi realizada osteossíntese com fixador ósseo externo modificado, confeccionado em Duracryl® Plus – polímero metacrilato de rápida autopolimerização – e 6 fios de Kirschner passando perpendicularmente por ambas as corticais radiais. O pós-operatório foi tranquilo e após 23 semanas o fixador externo foi removido. A radiografia mostrou boa consolidação óssea, com excelente resultado clínico. A utilização da ulna como osso doador foi muito conveniente, pois permitiu a coleta de um enxerto cortical de maior tamanho. O modelo extremamente leve de fixador ósseo externo proporcionou resistência adequada dos elementos de fixação e mostrou-se uma técnica eficiente e de baixo custo para osteossíntese em gatos com fraturas não consolidadas do rádio distal e ulna.
Palavras-chave: gato, pseudoartrose, fixação externa, autoenxertos ósseos
Introduction
In small dog breeds and cats, fractures of the radius and ulna are accompanied with highest non-union rates (Piras et al., 2011). Fractures with larger bone defects − “critical size defects” are a great challenge for veterinary orthopaedics. Self-healing of such bone defects is impossible, that is why they require additional bone healing stimulation. The principal technique to address such cases is application of various types of bone transplants (Kanczler & Oreffo, 2008) – autografts, allografts or xenografts. Bone grafts are also divided into cortical and cancellous. The former are applied for filling large bone defects, and the latter – for additional stimulation of osteogenesis. Most common sites for collection of bone autografts in animals are the proximal iliac wing, the tibia (Fitzpatrick et al., 2009), the ribs and iliac crest (Chung et al., 2021).
External bone fixation is a minimally invasive method of osteosynthesis commonly applied in open fractures with severe contamination, fragmented fractures, angular deformities of limbs, etc. (Bakici et al., 2019; Rovesti et al., 2007).
Only few clinical reports have been published regarding the use of fixation with autologous bone grafts in cats (Ferreira et al., 2018). The presented case describes the successful treatment of non-union in a cat with open infected fracture of the radius and ulna with a large defect using autogenous cancellous and cortical bone autografts.
Case report
A mixed-breed female intact cat, 4-year-old, weighing 3.4 kg was referred to the Small Animal Clinic to the Faculty of Veterinary Medicine, Stara Zagora, Bulgaria with the following history. Two months ago, the owners found out the cat with a contaminated lacerated wound with prominating bone ends of the right distal thoracic limb. The animal was operated in another clinic by intramedullary osteosynthesis of the radius with two Kirschner wires. The post operative period was characterized with complications (bone non-union and wound discharges).
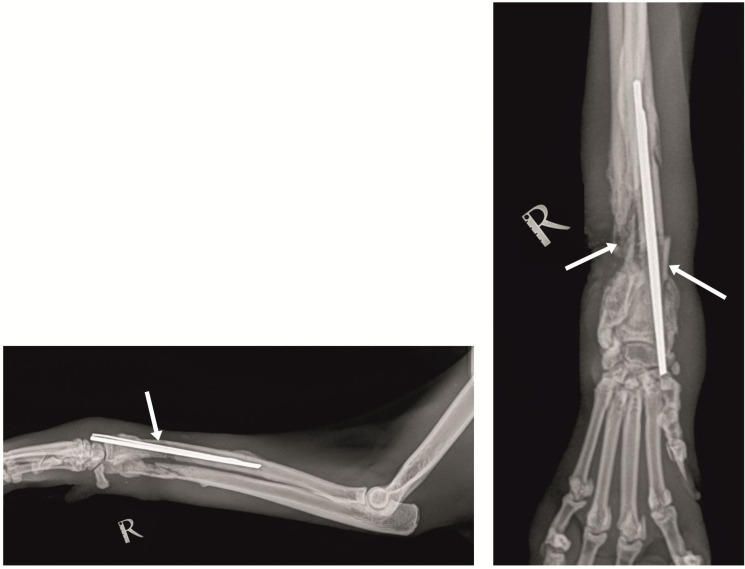
At referral, the initial orthopedic exam showed high-grade lameness with the right thoracic limb, swelling and fistulations, and severe pain upon palpation. Clinical and laboratory exams demonstrated fever (39.7°C), and moderate leukocytosis: 24.7 G/L (reference range 5.5-19.5 G/L; Weiser, 2012). Following deep sedation with medetomidine hydrochloride (Dorbene vet®, 1 mg/mL, Syva, Spain) at a dose of 0.075 mg/kg and ketamine hydrochloride (Anaket® , 100 mg/ml, Richter Pharma, Austria) at a dose of 7.5 mg/kg, applied intramuscularly in m. quadriceps femoris, mediolateral and craniocaudal radiographs were performed with a stationary X-ray unit (Philips, Bucky Diagnost CS4, Holland) and iQ-CR ACE digital station with iQ-VIEW/PRO v. 2.7 software. Radiographs showed osteolytic areas around fixation elements, a bone sequestrum and translucent zones due to pus collection, lack of bone callus and an obvious fracture line (Figure 1).
Figure 1. Radiograph of the patient at referral showing osteolytic areas surrounding fixation devices, bone sequestrum and translucent zones consequently to purulent exudate collection, lack of bone callus and obvious fracture line (arrows).
Anaesthesiological protocol included premedication with 0.2 mg/kg acepromazine maleate (Neurotranq®, 10 mg/ml, Alfasan International, Netherlands) and 0.01 mg/kg buprenorphine (Bupaq®, 0.3 mg/mL, Richter pharma, Austria; Vetergesic®, 0.3 mg/mL, Ceva, UK), applied i.m. in the same syringe in m. quadriceps femoris. Thirty minutes later, 5 mg/kg propofol (Propofol Fresenius®, Fresenius Kabi GmbH, Germany) was applied intravenously for induction. After endotracheal intubation, anaesthesia was maintained with isoflurane (Forane®, Abbott Laboratories Limited, United Kingdom% (Isoflurin®, 1000 mg/g, Vetpharma Animal Health, Barcelona – Spain) at concentration 1.5-2 vol%, mixed with 100% O2. Fluid therapy (Ringer lactate; Ringer Braun, B. Braun Melsungen AG, Germany) was applied at 10 mL/kg/h.
After routine limb preparation, a medial approach to the distal radius was used. The wound was cleansed, washed with physiological saline, and the bone sequestrum, bone fragments and Kirschner wires were removed. The proximal and distal edges of the radius were resected by means of oscillating saw.
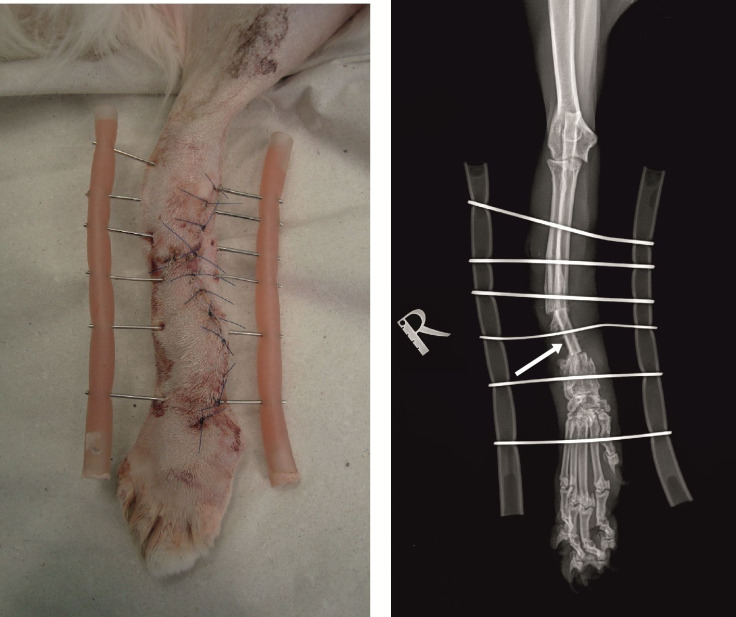
The bone defect was filled with autogenous cortical bone graft with length 2.7 сm, obtained from the ulnar diaphysis of the contralateral thoracic limb after caudal approach. At both edges of the cortical graft, a cancellous graft harvested from the proximal humeral metaphysis was applied for stimulation of the osteogenesis. After bone fragments adaptation, they were fixed with a modified external fixation device consisting of two bars made of silicone tubes filled with Duracryl® Plus (a rapidly self-curing metacrylate chemopolymer, Spofa Dental, Czech Republic) and 6 Kirschner wires. Fixation wires passed percutaneously and perpendicularly through both bone cortices (3 in the proximal radius with diameter 1.6 mm, one in cortical graft with diameter 1.2 mm, one 1.6-mm wire in the distal radius and another one, also with diameter 1.6 mm – in metacarpal bones) (Figure 2).
Figure 2. Моdified external fixator made of two silicone tube bars filled with Duracryl Plus (rapidly self-curing metacrylate polymer) and 6 Kirschner wires. The arrow points at the autograft.
During the post operative period, the external fixator was cleansed every day with 0.2% chlorhexidine followed by physiological saline for 14 days. The locomotion was restricted within a cage. For prevention of post operative infection i.v. cefriaxone (Tercef, Actavis, Bulgaria) at 30 mg/kg for 7 days were applied. Pain control was achieved with s.c. meloxicam (Meloxidolor, Produlab Pharma B.V., Netherlands) at 0.3 mg/kg for 3 days. By the 14th day, at dismissal, the use of the operated limb was satisfactory and no signs of infection were present.
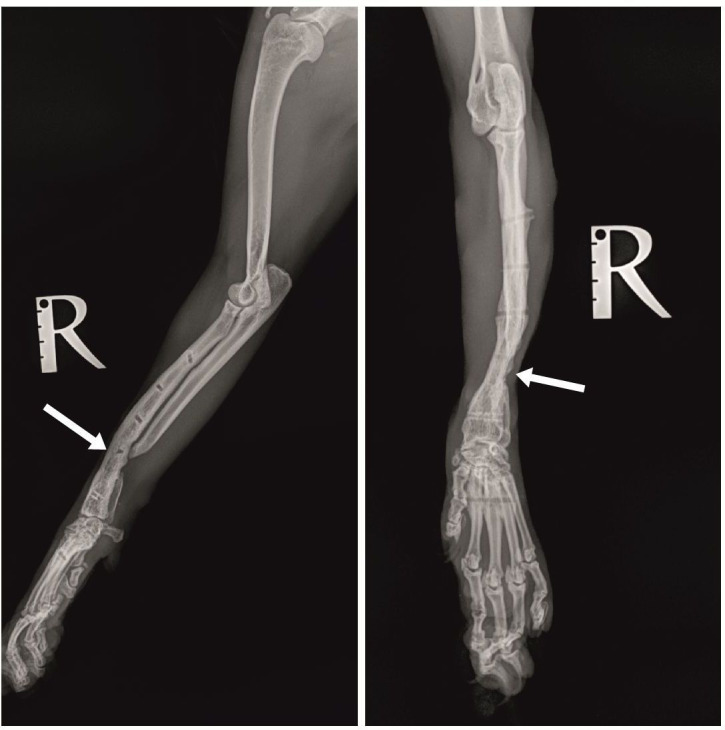
The first control examination by the 13th post-operative week showed lack of lameness, pain or swelling. Control radiography (Figure 3) demonstrated a very good bridging of the cortical graft without loosening of fixation elements. The second control exam by the 23rd post-operative week showed excellent use of the operated limb despite the partially impaired integrity of the lateral bar of the external fixator. Radiography showed excellent bridging of the cortical graft, no signs of migration or loosening of fixation elements. After removal of the external fixator, complete bone healing and bone callus remodelling proximally and distally to the cortical autograft were demonstrated (Figure 4). Regardless of the incomplete healing of the ulna and slight medial translation of the distal radius, pain, lameness and impaired gait were completely absent and the clinical results were excellent.
Figure 3. Mediolateral (left) and craniocaudal (right) radiographs of operated limb by the 13th post operative week demonstrating cortical graft bridging (arrows).
Figure 4. Mediolateral (left) and craniocaudal (right) radiographs after removal of the external fixator. Arrows show bone callus remodelling proximally and distally to the cortical autograft.
Discussion
Fractures with large bone defects and bone non-union are a serious challenge for both human and veterinary orthopaedics (Minto et al., 2015). The main factors with adverse effect on fracture healing are the impaired blood supply in the fracture line area, presence of foreign bodies, necrotic tissues, bone sequestra, infection, maladaptation of bone fragments and inadequate osteosynthesis technique (Piermattei et al., 2006). In the presented clinical case, despite the well-adapted bone edges, bone union did not occur within 2 months after the surgery. In our opinion, the causes included the inappropriate osteosynthesis technique, presence of infection, reduced vascularisation of the distal radius and the presence of a large bone sequestrum. Intramedullary osteosynthesis does not provide the necessary stability of bone fragments in distal radial and ulnar fractures and at a later stage, leads to bone non-union. In fact, up to 80% of bone non-unions are attributed to improper surgical technique (Blaeser et al., 2003).
Dynamic compression plates are deemed the best technique of osteosynthesis in fractures accompanied with substantial bone defects and bone grafts (Alievi et al., 2007; Ferreira et al., 2018) as plates provide close contact between host bone and the bone graft. In our patient, due to fracture localisation and the lack of a large bone segment, the application of bone plate was impossible. Fractures with large bone defects and distal fractures of the radius are more specific and heal with difficulty, so the treatment should use minimally invasive methods of osteosynthesis, e.g. external bone fixator (Bakici et al., 2019). In this case a linear biplanar modified external fixator was used, whose bars were filled with a rapidly self-curing metacrylate polymer (Duracryl Plus), used in dentistry. Duracryl Plus is strong, cheap, available, easy for modelling, rapidly curing and extremely light. It provided considerable strength of fixation elements. Even when its integrity was partially compromised, no loosening of elements or other complications have occurred.
Although very effective methods of osteosynthesis, external fixators require daily care in the post-operative period comprising − cleansing and tightening of all screws of the external fixator and local application of antibiotic ointment on the skin. In our patient, necessary post operative care was reduced due to the modification of external fixator that did not require additional tightening. According to Beever et al. (2017) infections and exudation around the elements of the external fixation device were less frequent in cats than in dogs.
In fractures with large bone defects, bone healing is not possible without application of bone graft (autogenous, allogeneic or xenogeneic) serving to restore the anatomical integrity of bone and to enhance bone healing (Bisgard et al., 2011). The application of autogenous bone grafts is reported to be associated with best remedial effect and least complications. Disadvantages related to collection of autogenous grafts are pain, bleeding, fracture and prolonged anaesthesia period. Also, in small dog breeds and cats, the size of bone autografts is limited, especially in fractures with large bone defects. In our patient, the ulna of the contralateral thoracic limb was used as donor bone. The ulna was judged to be a better alternative for collection of cortical bone autograft than the tibia and the iliac wing as the shape, size and diameter of obtained ulnar cortical autograft are identical to the anatomical shape of the radius. Also, it is easily accessible and not associated with considerably prolonged anesthesia period. The ulna is not a weight-bearing bone and poses no limitation regarding graft size unlike the tibia and proximal iliac wing.
According to Nather et al. (2010), cortical bone grafts are stronger than cancellous ones, yet osteogenesis and osteoconduction events are less pronounced. That is why, proximally and distally to the cortical autograft, a cancellous autograft was applied to enhance bone healing. In this patient, complete bone healing occurred by the 13th week.
The commonest complications after bone grafting within the bone defect are impaired integrity, infections, repulsion graft rejection or necrosis (Thompson Junior et al., 2000). These complications are more frequently observed with allogeneic bone grafts than with autografts (Coronado Junior et al., 2000). In our patient, the post-operative period was smooth, without complications, possibly due to the utilization of a combination of cortical and cancellous bone autografts, external fixation using a light modified device, restriction of locomotor activity within the cage and antimicrobial therapy.
Healing of fractures treated with external skeletal fixators occurs mainly between the 3rd and the 12th week. It is affirmed that fractures treated with external skeletal fixators heal earlier than those with internal bone fixation techniques (Johnson et al., 1989). In our patient, the fracture healed completely 13 weeks after the surgery.
Conclusion
The ulna is exceptionally appropriate as a donor bone, as it is easily accessible, not weight-bearing, and the cortical graft size could be larger than that obtained from other donor bone sites. The extremely light model of external bone fixator, made of two silicone tubes filled with a rapidly self curing dental metacrylate polymer (Duracryl® Plus) provided considerable strength of fixation elements and proved to be an efficient and not expensive technique for osteosynthesis in cat with non-union fractures of the distal radius and ulna. Despite the numerous unfavorable circumstances from the medical history of the patient, the application of autogenous cortical and cancellous bone grafts resulted in excellent clinical outcome.
Footnotes
How to cite: Garnoeva, R., Roydev, R., & Halil, M. (2022). Successful outcome of distal radius non-union after open fracture osteomyelitis treated by external fixation and bone grafting in a cat: case report. Brazilian Journal of Veterinary Medicine, 44, e003322. https://doi.org/10.29374/2527-2179.bjvm003322
Ethics statement: The case description was consented by the animal owner.
Financial support: RG, RR and MH – None.
Availability of complementary results: Not available.
The study was carried out at the University Veterinary Hospital, Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria
References
- Alievi M. M., Schossler J. E. W., Guimarães L. D., Oliviera A. N. C., Traeslel C. K., Ferreiria P. A. Implante ósseo cortical alógeno conservado em mel na reconstrução de falha óssea diafisária em fêmur de cães: Avaliação clínica e radiográfica. Ciência Rural. 2007;37(2):450–457. doi: 10.1590/S0103-84782007000200024. [DOI] [Google Scholar]
- Bakici M., Birkan K., Cebeci M. T. External skeletal fixation. International Journal of Veterinary and Animal Research. 2019;2(3):69–73. [Google Scholar]
- Beever L., Giles K., Meeson R. Postoperative complications associated with external skeletal fixators in cats. Journal of Feline Medicine and Surgery. 2017;19(7):727–736. doi: 10.1177/1098612X17699466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgard S. K., Barnhart M. D., Shiroma J. T., Kennedy S. C., Schertel E. R. The effect of cancellous autograft and novel plate design on radiographic healing and postoperative complications in tibial tuberosity advancement for cranial cruciate-deficient canine stifles. Veterinary Surgery. 2011;40(4):402–407. doi: 10.1111/j.1532-950X.2011.00829.x. [DOI] [PubMed] [Google Scholar]
- Blaeser L. L., Gallagher J. G., Boudrieau R. J. Treatment of biologically inactive nonunions by a limited en bloc ostectomy and compression plate fixation: A review of 17 cases. Veterinary Surgery. 2003;32(1):91–100. doi: 10.1053/jvet.2003.50014. [DOI] [PubMed] [Google Scholar]
- Chung C. S., Lin L. S., Teo Y. M. Case report: Treatment of femoral non-union with rib and iliac crest autografts and rhBMP-2 in a cat. Frontiers in Veterinary Science. 2021;8:756167. doi: 10.3389/fvets.2021.756167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado G. S., Junior, Swenson C. L., Martinez S. A., Burkhardt K. S., Arnoczky S. P. Effects of a 98% solution of glycerol or sterilization with ethylene oxide on FeLV in bone allografts and effects on bone incorporation of allografts in cats. American Journal of Veterinary Research. 2000;61(6):665–671. doi: 10.2460/ajvr.2000.61.665. [DOI] [PubMed] [Google Scholar]
- Ferreira M. P., Alievi M. M., Dal-Bó I. S., Gonzalez P. C. S., Nóbrega F. S., Gouvêa A. S., Beck C. A. C. Surgical management of long bone fractures in cats using cortical bone allografts preserved in honey. The Canadian Veterinary Journal. La Revue Veterinaire Canadienne. 2018;59(4):393–396. [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick N., Smith T. J., O’Riordan J., Yeadon R. Treatment of incomplete ossification of the humeral condyle with autogenous bone grafting techniques. Veterinary Surgery. 2009;38(2):173–184. doi: 10.1111/j.1532-950X.2008.00485.x. [DOI] [PubMed] [Google Scholar]
- Johnson A. L., Kneller S. K., Weigel R. M. Radial and tibial fracture repair with external skeletal fixation, effects of fracture type, reduction and complications on healing. Veterinary Surgery. 1989;18(5):367–372. doi: 10.1111/j.1532-950X.1989.tb01102.x. [DOI] [PubMed] [Google Scholar]
- Kanczler J. M., Oreffo R. O. Osteogenesis and angiogenesis: The potential for engineering bone. European Cells & Materials. 2008;15:100–114. doi: 10.22203/eCM.v015a08. [DOI] [PubMed] [Google Scholar]
- Minto B. W., Prada T. C., Marinho P. V. T., Zani C. C., Menezes M. P. D. Successful use of autogenous bone graft for the treatment of a radius-ulna nonunion in an amputee dog. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 2015;67(4):979–983. doi: 10.1590/1678-4162-8054. [DOI] [Google Scholar]
- Nather A., David V., Teng J. W., Lee C. W., Pereira B. P. Effect of autologous mesenchymal stem cells on biological healing of allografts in critical-sized tibial defects simulated in adult rabbits. Annals of the Academy of Medicine, Singapore. 2010;39(8):599–606. [PubMed] [Google Scholar]
- Piermattei D. L., Flo G. L., DeCamp C. E. Handbook of Small Animal Orthopedics and Fracture Repair. Saunders Elsevier; 2006. [Google Scholar]
- Piras L., Cappellari F., Peirone B., Ferretti A. Treatment of fractures of distal radius and ulna in toy breed dogs with circular external skeletal fixation: A retrospective study. Veterinary and Comparative Orthopaedics and Traumatology. 2011;24(3):228–235. doi: 10.3415/VCOT-10-06-0089. [DOI] [PubMed] [Google Scholar]
- Rovesti G. L., Bosio A., Marcellin-Little D. J. Management of 49 antebrachial and crural fractures in dogs using circular external fixators. The Journal of Small Animal Practice. 2007;48(4):194–200. doi: 10.1111/j.1748-5827.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Junior, Garg A., Clohisy D. R., Cheng E. Y. Fractures in large segment allografts. Clinical Orthopaedics and Related Research. 2000;370:227–235. doi: 10.1097/00003086-200001000-00023. [DOI] [PubMed] [Google Scholar]
- Weiser G. In: Veterinary Haematology and Clinical Chemistry. Thrall M. A., Weiser G., Allison R. W., Campbell T. W., editors. Wiley-Blackwell; 2012. Introduction to leukocytes and the leukogram. p. 122. [Google Scholar]