Abstract
Patients with multiple sclerosis acquire disability either through relapse-associated worsening (RAW) or progression independent of relapse activity (PIRA). This study addresses the relative contribution of relapses to disability worsening over the course of the disease, how early progression begins and the extent to which multiple sclerosis therapies delay disability accumulation.
Using the Novartis-Oxford multiple sclerosis (NO.MS) data pool spanning all multiple sclerosis phenotypes and paediatric multiple sclerosis, we evaluated ∼200 000 Expanded Disability Status Scale (EDSS) transitions from >27 000 patients with ≤15 years follow-up. We analysed three datasets: (i) A full analysis dataset containing all observational and randomized controlled clinical trials in which disability and relapses were assessed (n = 27 328); (ii) all phase 3 clinical trials (n = 8346); and (iii) all placebo-controlled phase 3 clinical trials (n = 4970). We determined the relative importance of RAW and PIRA, investigated the role of relapses on all-cause disability worsening using Andersen-Gill models and observed the impact of the mechanism of worsening and disease-modifying therapies on the time to reach milestone disability levels using time continuous Markov models.
PIRA started early in the disease process, occurred in all phenotypes and became the principal driver of disability accumulation in the progressive phase of the disease. Relapses significantly increased the hazard of all-cause disability worsening events; following a year in which relapses occurred (versus a year without relapses), the hazard increased by 31–48% (all P < 0.001). Pre-existing disability and older age were the principal risk factors for incomplete relapse recovery. For placebo-treated patients with minimal disability (EDSS 1), it took 8.95 years until increased limitation in walking ability (EDSS 4) and 18.48 years to require walking assistance (EDSS 6). Treating patients with disease-modifying therapies delayed these times significantly by 3.51 years (95% confidence limit: 3.19, 3.96) and 3.09 years (2.60, 3.72), respectively. In patients with relapsing-remitting multiple sclerosis, those who worsened exclusively due to RAW events took a similar length of time to reach milestone EDSS values compared with those with PIRA events; the fastest transitions were observed in patients with PIRA and superimposed relapses.
Our data confirm that relapses contribute to the accumulation of disability, primarily early in multiple sclerosis. PIRA begins in relapsing-remitting multiple sclerosis and becomes the dominant driver of disability accumulation as the disease evolves. Pre-existing disability and older age are the principal risk factors for further disability accumulation. The use of disease-modifying therapies delays disability accrual by years, with the potential to gain time being highest in the earliest stages of multiple sclerosis.
Keywords: multiple sclerosis, disability, disease progression, relapse, progression independent of relapse activity
Lublin et al. analyse the largest clinical trial dataset in multiple sclerosis—comprising ∼35 000 patients with ≤15 years of follow-up—to address questions including the relative contribution of relapses to disability worsening, how early progression begins, and the extent to which treatments delay disability accumulation.
Introduction
There are two main mechanisms by which patients with multiple sclerosis acquire disability: (i) step-wise accrual of impairment due to incomplete recovery from a relapse [i.e. relapse-associated worsening (RAW)]; and (ii) progression independent of relapse activity (PIRA). While the former is considered to be the main source of permanent disability in relapsing multiple sclerosis, the latter is thought to drive the insidious progression typical in primary and secondary progressive multiple sclerosis (PPMS and SPMS). The clinical distinction of relapsing and progressive forms of multiple sclerosis has recently been challenged: In a study by Kappos et al.1 including pooled data from two large phase 3 clinical trials in relapsing multiple sclerosis, most disability accumulation was not associated with overt relapses. The study used a composite end point of walking ability, hand coordination, and physical disability for the detection of PIRA events. The extent to which this finding reflects the pathophysiology of multiple sclerosis, rather than the particular definitions of relapse and progression used in the Kappos et al.1 study, warrants further exploration in a large independent dataset with more stringent definitions of PIRA.
While the role of clinical relapses in diagnosis is undisputed, and their impact on the patient’s quality of life is undeniable, the involvement of clinical relapse in long-term prognosis has been questioned.2,3 In natural history studies, patients with and without relapses progressed similarly and so a common mechanism of progression independent of relapses was suggested.4 However, more recent population-based studies have confirmed relapses play a role in the transition time from relapsing-remitting multiple sclerosis (RRMS) to SPMS (with poor recovery from relapses and high frequency of early relapses reducing the time to onset of progressive disease),5,6 in the accumulation of disability both in early and later RRMS,7–10 and even in progressive disease.11 The quantitative relevance of relapses and progression in driving the accumulation of disability in the different stages of multiple sclerosis therefore requires further study.
Relapses and focal inflammation are predominantly a feature of young patients with multiple sclerosis. Paediatric multiple sclerosis is almost exclusively of the relapsing subtype,12,13 with relapse rates being two to three times higher in paediatric-onset than adult-onset multiple sclerosis.14 In adult patients with RRMS, relapse frequency is highest in the youngest patients15 and decreases with age, even in placebo-treated patients.16 Current therapies for multiple sclerosis primarily target focal inflammation, and consequently their relative effect is strongest in patients where inflammation is most prominent, i.e. in young patients with multiple sclerosis.15,16 However, the extent to which disease-modifying therapies (DMTs) impact long-term outcomes and prolong the time to milestone disability levels is a question not fully answered.
The Novartis-Oxford multiple sclerosis (NO.MS) dataset is an ideal dataset in which to investigate the mechanisms of disability acquisition. It is currently the largest, most comprehensive clinical trial dataset in multiple sclerosis, spanning all phenotypes and containing data from ∼35 000 patients with up to 15 years of follow-up, with regular monitoring of patients’ neurological status by trained and certified raters and the inclusion of randomized placebo-controlled trials across all stages of multiple sclerosis.16 Here, using a large subset of NO.MS data with longitudinal evaluations of disability and relapses, we quantify all confirmed disability worsening (CDW) events, and we investigate the mechanisms of disability accrual, i.e. RAW versus PIRA, across all multiple sclerosis phenotypes. This study examines the role of clinical relapses in driving disease worsening and estimates the extent to which DMTs can alter the disease course and prolong the time to milestone disability levels.
Materials and methods
Data sources
All analyses were based on data from 23 Novartis multiple sclerosis clinical trials, plus their extensions, that collected longitudinal disability [measured by Kurtzke’s17 Expanded Disability Status Scale; EDSS) and relapse assessments, and formed part of the NO.MS dataset.16 An overview of the contributing studies can be found in Supplementary Table 1. Three sets of data were analysed, representing a gradient in size and data heterogeneity.
Full dataset (n = 27 328 patients). This was the main dataset and included all randomized controlled and observational trials plus all extension studies that evaluated disability and relapse outcomes. Patients could switch treatments and some patients were followed up to 15 years.
Phase 3 trials, plus all extensions (n = 8346 patients). This dataset included all NO.MS randomized placebo- or active-controlled phase 3 studies, with their extensions and follow-up times of up to 15 years. Data capture was highly standardized, and treatment switches were typically limited to switching from control treatment to open-label medication in the extensions.
Phase 3 double-blind, placebo-controlled trials (n = 4970 patients) were of shorter duration (typically 1–2 years) but highly standardized. The focus of analysis in this dataset was placebo-treated patients and the effects of DMTs.
In the double-blind phase 3 studies, disability was regularly assessed at 3-monthly visits by trained and certified independent EDSS raters who were not otherwise involved in the treatment of the patients and had no access to treatment or clinical information (EDSS-based disability criteria may be found in Supplementary Table 2A). In the extension studies, EDSS was assessed at 3- or 6-monthly intervals by trained and certified EDSS raters who could be the treating physician. In the full dataset, EDSS collection could be more heterogeneous and depended on the study protocols.
All trial protocols were approved by the appropriate institutional review boards or ethical committees and trials followed the principles of the Declaration of Helsinki and Good Clinical Practice. All patients provided written informed consent. All clinical and MRI scan data have been anonymized.18 The individual study results have been previously published elsewhere.
End point definitions
Multiple sclerosis relapses
Multiple sclerosis relapses were defined as the appearance of a new neurological abnormality or worsening of previously stable or improving pre-existing neurological abnormality (present for at least 24 h in the absence of fever or known infection), separated by at least 30 days from onset of a preceding clinical demyelinating event present for at least 24 h. Confirmed multiple sclerosis relapses were defined as those accompanied by a clinically relevant change in EDSS (e.g. an increase in EDSS score of at least 0.5 points) or functional scores (excluding bowel/bladder or cerebral scores) and confirmed programmatically and centrally in the NO.MS database. All studies collected investigator-reported relapses. Unless specifically noted, all investigator-reported relapses are included in the statistical analyses (irrespective of whether the relapse was EDSS-confirmed or not).
Confirmed and sustained disability worsening
CDW events were based on EDSS and defined by an increase in EDSS (either ≥1.5 points for patients with a baseline EDSS of zero, ≥1.0 point for patients with a baseline EDSS of 1–5 and by 0.5 points for patients with a baseline EDSS of ≥5.5; Supplementary Table 2A) confirmed by an EDSS assessment at least 3 or 6 months (3-month or 6-month CDW) apart from the onset of the worsening (and sustained in all intermediate EDSS assessments, if any).
Where applicable, we categorized CDW events as either RAW or PIRA. It is possible for patients to experience sequential RAW and PIRA events. Moreover, some CDW events did not fulfill either the criteria of RAW or PIRA and were left unclassified. The following definitions were used:
RAW was a 3- or 6-month CDW event with an onset within 90 days from the onset of an investigator-reported relapse (irrespective of the EDSS confirmation).
PIRA was defined as a 3- or 6-month CDW event with either no prior relapse or an onset more than 90 days after the start date of the last investigator-reported relapse (irrespective of the EDSS confirmation). In addition, to qualify as a PIRA event, no relapse must occur within 30 days before or after the EDSS confirmation. If a relapse with incomplete recovery occurred, the baseline (i.e. the reference EDSS value) was reset >90 days after the relapse onset to identify the next PIRA event. In an individual patient, the baseline could be reset multiple times (i.e. after each relapse) until either a PIRA event was discovered, or until the individual EDSS profile ended.
Sustained PIRA was a 3- or 6-month PIRA event in which the EDSS-worsening was sustained in all following assessments, i.e. the patient never recovered in the available longitudinal data.
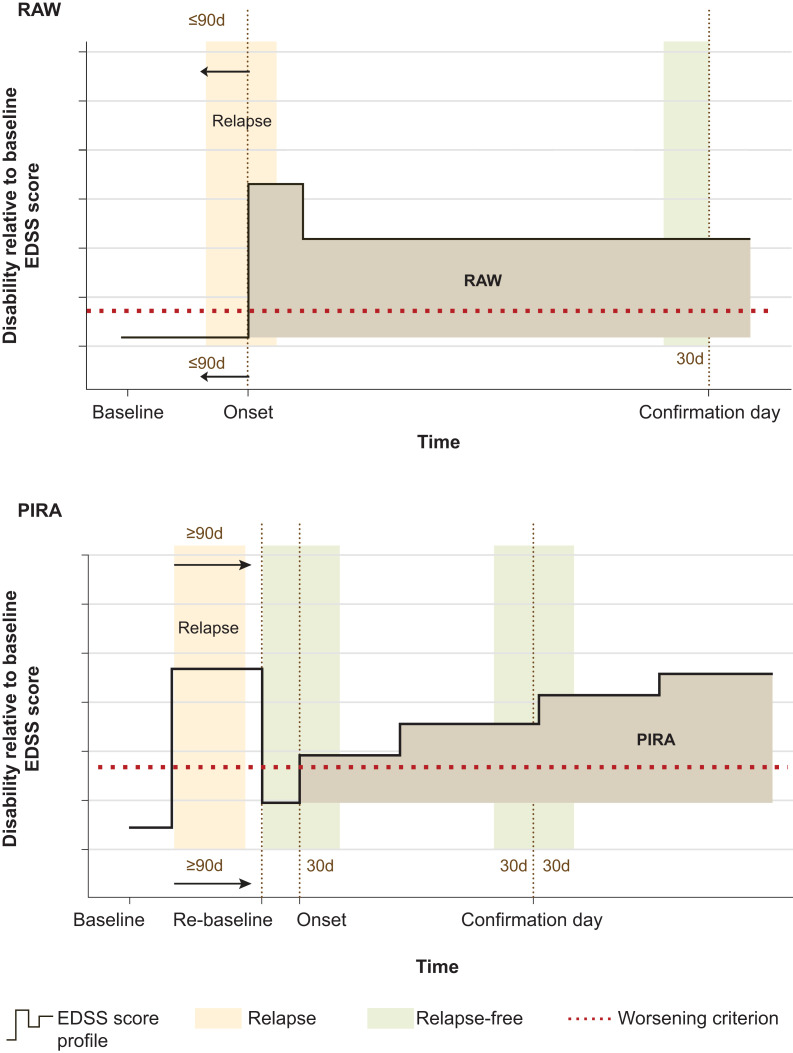
More detailed end point definitions may be found in Supplementary Table 2B, with Fig. 1 providing a schematic of the RAW and PIRA definitions.
Figure 1.
Schematic representations of RAW and PIRA event definitions. The EDSS-worsening threshold relative to baseline (or the ‘re-baseline’) is shown as a horizontal red dotted line; a disability event is defined by a clinically meaningful increase of the EDSS above the threshold, sustained until and confirmed by an EDSS assessment at least 3 or 6 months apart from the onset of the worsening. The bold black line represents the patient’s longitudinal disability trajectory (EDSS scores collected at visits). The onset and the confirmation are shown with vertical dotted brown lines. The vertical shaded area to the left in both schematics (peach) represents a relapse. The other vertical shaded areas (green) must be relapse free to fulfill the definition of RAW or PIRA, consistent with the definition in Kappos et al.1 In case a RAW or PIRA event could not be confirmed due to the occurrence of a relapse in the green interval, the confirmation was delayed to the next EDSS assessment. The onset of a RAW event has to occur ≤90 days since the start date of the most recent relapse. For PIRA events, the onset has to occur at >90 days from the onset of the most recent relapse. For PIRA events, if a relapse with incomplete recovery occurs, the baseline (i.e. the EDSS reference value) is reset >90 days after the relapse onset (‘re-baseline’; shown with vertical dotted line). The PIRA event is then relative to this new baseline. d = day.
Statistical analyses
Euler diagrams (adapted from Kappos et al.1) by multiple sclerosis phenotype (RRMS, SPMS or PPMS) for all three datasets and for paediatric patients (as a model of ‘pure’ RRMS, with a preponderance of focal inflammatory events) were used (i) to quantify the mechanisms of clinical disease worsening; and (ii) to determine the effect of DMTs on the mechanisms of clinical disease worsening.
We compared on-study MRI activity between patients with RAW and patients with PIRA events, based on the number (negative binomial model) and the percentage of patients free of gadolinium (Gd)-enhancing T1 lesions in each multiple sclerosis phenotype.
The prognostic value of recent relapses (within 1 year or the last 2 years) for subsequent all-cause 6-month CDW was analysed using Andersen-Gill models. The null hypothesis was tested that relapses have no prognostic value for future disability worsening. Results are reported as hazard ratios (HR) with 95% confidence intervals (CI) and the corresponding P-values. Details of the Andersen-Gill model are provided in the Supplementary material.
The effect of treatment on long-term disability outcomes (milestone EDSS scores) was assessed by estimating the transition times between predefined milestone EDSS scores in DMT-treated versus placebo-treated patients using Continuous Time Multistate Markov models (as described by Jackson et al.19; implemented in the ‘MSM’ package in R.)20 Analogous to Confavreux et al.,4 milestone EDSS values were defined as follows: (i) EDSS = 1 (no disability, minimal impairment in one functional system); (ii) EDSS = 4 (significant disability, but self-sufficient and up and about some 12 h a day; able to walk without aid or rest for 500 m); and (iii) EDSS = 6 [requires a single walking aid (e.g. cane, crutch) to walk about 100 m]. EDSS measures taken during a relapse (as judged by the treating physician) were omitted.21 The Markov Models included ‘age’ and ‘treatment’ as covariates because the distribution of EDSS scores and transition probabilities have a strong dependence on age and potentially on treatment. ‘Treatment’ was coded as (yes = any DMTs; no = no DMT). Treatments in the database included placebo, glatiramer acetate, interferon beta-1a, fingolimod, natalizumab, ofatumumab, siponimod, dimethyl fumarate and teriflunomide. Less frequently used DMTs in the NO.MS database were summarized under ‘other multiple sclerosis therapies’. Long-term data are primarily from fingolimod-treated patients (≈61 500 treatment-years). Patients with progressive multiple sclerosis (SPMS or PPMS) in the NO.MS database were predominantly treated with either placebo, fingolimod (PPMS) or siponimod (SPMS). DMTs and treatment-years of exposure to the specified medications have previously been reported.16 Of note, patients could switch from no treatment to treatment and vice versa, and the statistical model considered the treatment status of the patient at the time of the EDSS transition, i.e. it could handle treatment switches. Details about the calculation of transition times between milestone EDSS states from the Markov model are provided in the Supplementary material.
Data availability
The data from the NO.MS cohort are currently only available within the collaboration, due to data privacy requirements derived from the original signed informed consent forms and the risk-based anonymization, which takes IT security and access considerations into account.18 Anonymized clinical data from the individual studies are available on reasonable request provided that it is in line with current ethical and intellectual property requirements surrounding the use of data. Requests should be directed to ClinicalStudyDataRequest.com.
Results
Baseline characteristics
Baseline characteristics of patients in all three datasets were consistent with RRMS, SPMS and PPMS phenotypes in clinical trial populations and were reasonably comparable between datasets. Patients diagnosed with RRMS were younger, had a higher level of clinical and MRI disease activity and were less disabled at baseline compared with SPMS patients, while patients with a diagnosis of progressive multiple sclerosis (either SPMS or PPMS) were in their late 40s and showed lower levels of acute inflammation at baseline. Only 22% of RRMS and SPMS patients were treatment-naïve at baseline (Table 1).
Table 1.
Baseline characteristics of the datasets analysed, by multiple sclerosis phenotypes (means and standard deviations; counts and proportions)
| Full dataset (N = 27 328) | Phase 3 trials + extensions (n = 8346) | Phase 3 placebo-controlled trials (n = 4970) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RRMS n = 24 469 |
SPMS n = 1873 |
PPMS n = 986 |
RRMS n = 5623 |
SPMS n = 1753 |
PPMS n = 970 |
RRMS n = 2355 |
SPMS n = 1645 |
PPMS n = 970 |
|
| Agea (years) [min, max] |
n′ = 24 440 (99.9%) 39.4 ± 10.5 [10, 86] |
n′ = 1873 (100%) 47.6 ± 8.2 [21, 78] |
n′ = 986 (100%) 48.5 ± 8.5 [24, 67] |
n′ = 5623 (100%) 36.9 ± 9.8 [10, 59] |
n′ = 1753 (100%) 47.8 ± 8.0 [21, 63] |
n′ = 970 (100%) 48.4 ± 8.5 [24, 66] |
n′ = 2355 (100%) 38.7 ± 8.9 [16, 59] |
n′ = 1645 (100%) 48.0 ± 8.0 [21, 63] |
n′ = 970 (100%) 48.4 ± 8.5 [24, 66] |
| Females (%) |
n′ = 24 467 (99.99%) 17 490 (71.5%) |
n′ = 1873 (100%) 1123 (60.0%) |
n′ = 986 (100%) 477 (48.4%) |
n′ = 5623 (100%) 3938 (70.0%) |
n′ = 1753 (100%) 1050 (59.9%) |
n′ = 970 (100%) 469 (48.4%) |
n′ = 2355 (100%) 1733 (73.6%) |
n′ = 1645 (100%) 987 (60.0%) |
n′ = 970 (100%) 469 (48.4%) |
| Caucasian (%) |
n′ = 19 218 (78.5%) 16 400 (85.3%) |
n′ = 1860 (99.3%) 1728 (92.9%) |
n′ = 986 (100%) 945 (95.8%) |
n′ = 5607 (99.7%) 5132 (91.5%) |
n′ = 1742 (99.4%) 1661 (95.4%) |
n′ = 970 (100%) 933 (96.2%) |
n′ = 2355 (100%) 2175 (92.4%) |
n′ = 1635 (99.4%) 1559 (95.4%) |
n′ = 970 (100%) 933 (96.2%) |
| Duration of multiple sclerosis since first symptoms, years (%) | n′ = 21 943 (89.7%) | n′ = 1826 (97.5%) | n′ = 984 (99.8%) | n′ = 5622 (99.9%) | n′ = 1750 (99.8%) | n′ = 969 (99.9%) | n′ = 2355 (100%) | n′ = 1642 (99.8%) | n′ = 969 (99.9%) |
| Median categoryb | 5 to <10 | 10 to <30 | 5 to <10 | 5 to <10 | 10 to <30 | 5 to <10 | 5 to <10 | 10 to <30 | 5 to <10 |
| 0 to <2 years | 3213 (14.6%) | 5 (0.3%) | 7 (0.7%) | 1125 (20.0%) | 5 (0.3%) | 5 (0.5%) | 333 (14.1%) | 4 (0.2%) | 5 (0.5%) |
| 2 to <5 years | 4397 (20.0%) | 99 (5.4%) | 387 (39.3%) | 1235 (22.0%) | 94 (5.4%) | 386 (39.8%) | 457 (19.4%) | 90 (5.5%) | 386 (39.8%) |
| 5 to <10 years | 5867 (26.7%) | 334 (18.3%) | 554 (56.3%) | 1484 (26.4%) | 315 (18.0%) | 550 (56.8%) | 682 (29.0%) | 295 (18.0%) | 550 (56.8%) |
| 10 to <30 years | 8033 (36.6%) | 1265 (69.3%) | 36 (3.7%) | 1714 (30.5%) | 1214 (69.4%) | 28 (2.9%) | 843 (35.8%) | 1134 (69.1%) | 28 (2.9%) |
| ≥30 years | 433 (2.0%) | 123 (6.7%) | 0 (0.0%) | 64 (1.1%) | 122 (7.0%) | 0 (0.0%) | 40 (1.7%) | 119 (7.2%) | 0 (0.0%) |
| Previously treated (%) |
n′ = 15 935 (65.1%) 12 343 (77.5%) |
n′ = 1832 (97.8%) 1435 (78.3%) |
n′ = 977 (99.1%) 211 (21.6%) |
n′ = 5622 (99.9%) 3201 (56.9%) |
n′ = 1753 (100%) 1374 (78.4%) |
n′ = 969 (99.9%) 205 (21.2%) |
n′ = 2354 (99.9%) 1330 (56.5%) |
n′ = 1645 (100%) 1289 (78.4%) |
n′ = 969 (99.9%) 205 (21.2%) |
| Number of relapses in previous year |
n′ = 22 040 (90.1%) 1.2 ± 1.0 |
n′ = 1827 (97.5%) 0.3 ± 0.6 |
n′ = 983 (99.7%) 0.0 ± 0.1 |
n′ = 5623 (100%) 1.4 ± 0.8 |
n′ = 1751 (99.9%) 0.3 ± 0.6 |
n′ = 970 (100%) 0.0 ± 0.0 |
n′ = 2355 (100%) 1.5 ± 0.8 |
n′ = 1643 (99.9%) 0.3 ± 0.5 |
n′ = 970 (100%) 0.0 ± 0.0 |
| EDSS at baseline |
n′ = 20 535 (83.9%) 2.7 ± 1.6 |
n′ = 1852 (98.9%) 5.4 ± 1.1 |
n′ = 976 (99.0%) 4.7 ± 1.0 |
n′ = 5621 (99.9%) 2.5 ± 1.3 |
n′ = 1753 (100%) 5.4 ± 1.1 |
n′ = 970 (100%) 4.7 ± 1.0 |
n = 2355 (100%) 2.4 ± 1.3 |
n′ = 1645 (100%) 5.4 ± 1.1 |
n′ = 970 (100%) 4.7 ± 1.0 |
| Patients with Gd-enhancing lesions (%) |
n′ = 10 227 (41.8%) 3884 (38.0%) |
n′ = 1732 (92.5%) 391 (22.6%) |
n′ = 967 (98.1%) 124 (12.8%) |
n′ = 5578 (99.2%) 2122 (38.0%) |
n′ = 1701 (97.0%) 378 (22.2%) |
n′ = 967 (99.7%) 124 (12.8%) |
n′ = 2342 (99.4%) 863 (36.8%) |
n′ = 1593 (96.8%) 350 (22.0%) |
n′ = 967 (99.7%) 124 (12.8%) |
| T2 lesion volume (mm3) at baselinec |
n′ = 6178 (25.2%) 8375 ± 10 764 |
n′ = 1738 (92.8%) 15 717 ± 16 227 |
n′ = 967 (98.1%) 9764 ± 12 014 |
n′ = 5580 (99.2%) 8214 ± 10 545 |
n′ = 1707 (97.4%) 15 654 ± 16 238 |
n′ = 967 (99.7%) 9764 ± 12 014 |
n′ = 2342 (99.4%) 5890 ± 7752 |
n′ = 1599 (97.2%) 15 298 ± 16 010 |
n′ = 967 (99.7%) 9764 ± 12 014 |
n refers to the analysis set totals; n refers to the total number of subjects in the specific column; n′ (%) refers to the number and proportion of patients with the specific baseline feature evaluated; and the numbers in bold refer to the mean ± standard deviation or the number (proportion) of patients with the specific characteristic out of n′.
To preserve anonymization of the data, the age of patients has been randomly jittered, thus the age range may not be fully aligned with the inclusion criteria of individual studies. The phase 3 RRMS studies included adult RRMS patients per protocol inclusion criteria. Baseline characteristics of the paediatric phase 3 study (PARADIGMS) have previously been reported.27
To preserve anonymization of the data, exact duration of multiple sclerosis has been categorized.
MRI methods differ between different trials and MRI reading centers, which may introduce systematic biases for between-phenotype comparisons22; cross-phenotype comparisons should be done cautiously.
Mechanisms of disability worsening: RAW and PIRA
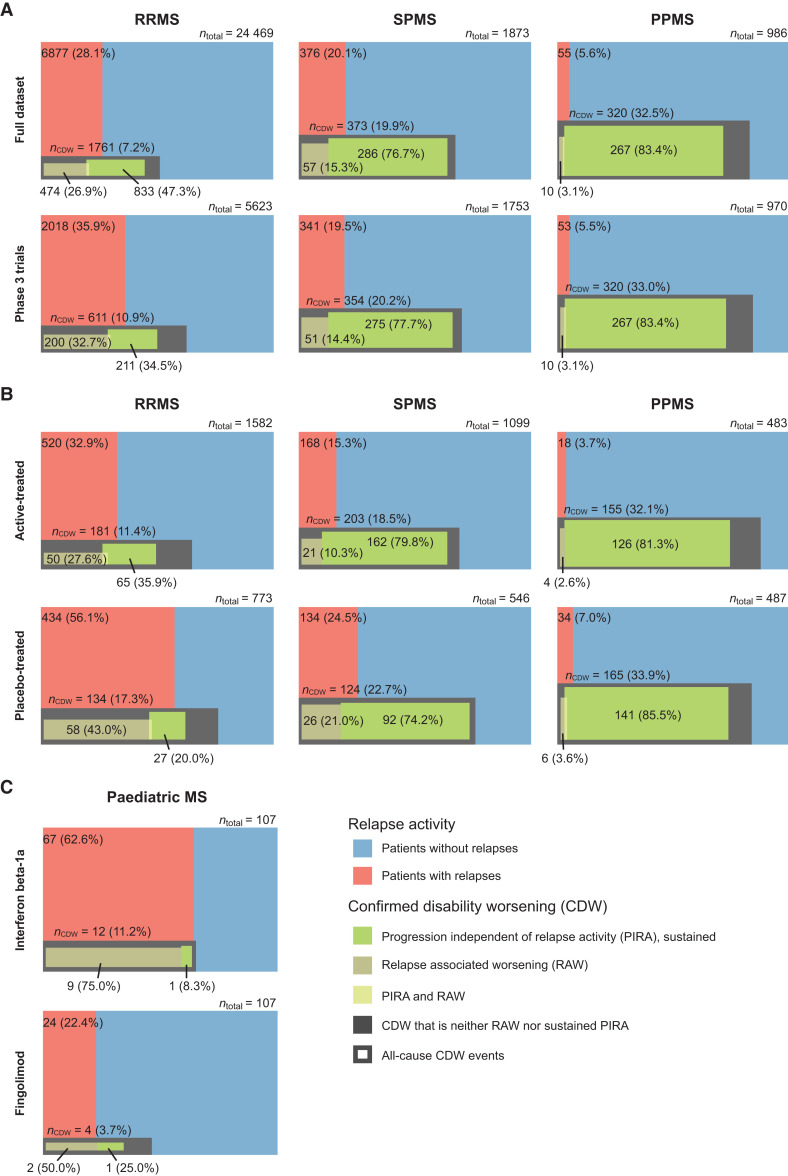
Relapses during the study were most common in RRMS, followed by SPMS, and least common (but still occurred) in PPMS patients in both the full dataset and in the phase 3 trials (Fig. 2A). The risk of an all-cause disability worsening (6-month CDW) event was higher in progressive than relapsing multiple sclerosis in both in the full dataset (PPMS: 320/986 = 32.5%; SPMS: 373/1873 = 19.9%; and RRMS: 1761/24 469 = 7.2%) and in the phase 3 trials (PPMS: 320/970 = 33.0%; SPMS: 354/1753 = 20.2%; and RRMS: 611/5623 = 10.9%).
Figure 2.
Confirmed disability worsening by mechanism across multiple sclerosis phenotypes. Euler diagrams are presented by multiple sclerosis phenotype for (A) the full dataset (n = 27 328) and in patients from randomized phase 3 trials plus extensions (n = 8346); (B) active-treated versus placebo-treated patients from phase 3 placebo-controlled trials (n = 4970); and (C) in paediatric patients treated with fingolimod or interferon beta-1a. In the full dataset and for the subset of patients from phase 3 clinical trials, progression and relapse onset was restricted to the first 2 years of the trials. Six-month CDW was used in adult patients. Three-month CDW was used in paediatric patients, as the mean study duration was 20 versus 17 months for patients treated with fingolimod or interferon beta-1a, respectively. PIRA events were 6-month or 3-month confirmed and sustained until the end of the follow-up time in adult and paediatric patients, respectively. Coloured areas are proportional to the represented groups. Each diagram is divided vertically into a red area (patients who relapsed) and a blue area (patients who did not relapse); this vertical division extends to the bottom of the figure. Superimposed is the proportion of patients who experienced a 6-month CDW or 3-month CDW (dark grey box); the overlap between the grey box and red area represents patients who relapsed and had all-cause disability worsening; the overlap between the grey box and the blue area represents patients who had all-cause disability worsening but no relapses. Patients with CDW events were further classified into RAW (bronze), PIRA sustained until the end of the follow-up period (green) or both (yellow). Due to the definition of RAW and PIRA events (Supplementary Table 2B), some patients experienced a disability worsening that could be classified neither as a RAW nor a sustained PIRA event (dark grey)—these unclassified events also include 6-month confirmed PIRA events that were not sustained in the longitudinal data. The baseline characteristics of patients who are diagnosed as RRMS but experienced PIRA events without having any relapses in the study are summarized in Supplementary Table 4 and an assessment of the MRI activity is provided in Supplementary Table 5.
In the full dataset and the phase 3 trials, as predicted, relapse-associated disability worsening (out of all 6-month CDW events) occurred more frequently in RRMS than SPMS and least frequently in PPMS (Fig. 2A). However, unexpectedly, PIRA had already begun in adult patients with RRMS: Sustained PIRA events occurred with similar or higher frequency to RAW events in adult patients with RRMS (in the full dataset RAW versus PIRA: 474 versus 833/1761; 26.9 versus 47.3%; and in the phase 3 dataset RAW versus PIRA: 200 versus 211/611; 32.7 versus 34.5%, respectively). The remaining 454 CDW events in the full dataset, and 200 CDW events in the phase 3 trials, could not be classified as either RAW or sustained PIRA according to our definitions (note that the unclassified events include 6-month confirmed PIRA events that were not sustained in the longitudinal follow-up data; these have not been counted as PIRA events, differing from the definition used by Kappos et al.1). The number of sustained and unstained PIRA events are tabulated in Supplementary Table 3: In RRMS, proportionally more 6-month confirmed PIRA events were not sustained (i.e. RRMS patients recovered at some stage), while in progressive multiple sclerosis the vast majority of 6-month confirmed PIRA events were sustained in the longitudinal data.
In the full dataset, sustained PIRA occurred across all multiple sclerosis phenotypes and meaningfully contributed to the overall clinical worsening of patients (sustained PIRA/6-month CDW events: RRMS, 833/1761 = 47.3%; SPMS, 286/373 = 76.7%; and PPMS, 267/320 = 83.4%). Similarly, in the phase 3 trials, sustained PIRA events occurred in all phenotypes (sustained PIRA/6-month CDW events: RRMS, 211/611 = 34.5%; SPMS, 275/354 = 77.7%; and PPMS, 267/320 = 83.4%). In the full dataset, the 6-month confirmed PIRA events were mostly sustained until the end of the follow-up data in all phenotypes, with the proportion of sustained PIRA events being highest in progressive multiple sclerosis (sustained PIRA/6-month PIRA events: PPMS, 267/310 = 86.1%; SPMS, 286/307 = 93.2%; and RRMS, 833/1175 = 70.9%; Supplementary Table 3). In the phase 3 dataset, corresponding numbers for sustained PIRA/6-month PIRA events were: PPMS, 267/310 = 86.1%; SPMS, 275/295 = 93.2%; and RRMS, 211/394 = 53.6%.
A minority of patients with RRMS experienced sustained PIRA events and thus fulfilled the definition of SPMS: in the full dataset, these were 833 out of 24 469 patients (3.4%), which is broadly consistent with the corresponding numbers from the clinical phase 3 studies (211/5623 = 3.8%; Fig. 2A). Baseline characteristics of adult patients diagnosed as RRMS who did not experience relapses but experienced sustained PIRA events were broadly similar to those with RAW events and to the total RRMS population (Supplementary Table 4).
For phase 3 double-blind, placebo-controlled trials, when MRI scans were available, we compared the MRI activity in adult RRMS patients who experienced either PIRA or RAW events. Placebo-treated RRMS patients with PIRA versus RAW events had less MRI disease activity (0.98 versus 1.35 Gd-enhancing T1 lesions/scan) and were more likely to be free of Gd-enhancing lesions (44 versus 28%; Supplementary Table 5).
RAW was the dominant driver of disability worsening only in patients with paediatric onset of RRMS (Fig. 2C). In interferon beta-1a treated paediatric patients, 9/12 (75%) of the 3-month CDW events were RAW, 2/12 (16.6%) did not fulfill the definition of RAW or PIRA, while only a single patient worsened in the absence of any reported relapse activity, fulfilling the definition of PIRA.
Treatment effect on mechanisms of disability worsening
We compared the impact of DMT treatment on the different types of disability worsening in the randomized placebo-controlled phase 3 trials (Fig. 2B). The data show that DMTs reduced the proportions of patients who relapsed and those who had all-cause disability worsening events, with the strongest effect in RRMS. In actively-treated patients with RRMS, 181/1582 = 11.4% experienced a 6-month CDW event compared with 134/773 = 17.3% of placebo-treated patients. In SPMS, 203/1099 = 18.5% of the DMT-treated patients experienced a 6-month CDW event compared with 124/546 = 22.7% of placebo-treated patients. In PPMS, corresponding numbers were 155/483 = 32.1% versus 165/487 = 33.9%.
In patients treated with any DMTs, compared with placebo, proportionally more of the 6-month CDW events were sustained PIRA events, most notably in RRMS: 65/181 6-month CDW (35.9%) in the active arms versus 27/134 (20.0%) in the placebo arms. Similarly, in patients with SPMS, the number of sustained PIRA events out of all 6-month CDW events was 162/203 (79.8%) in the active arms versus 92/124 (74.2%) in the placebo arms. In PPMS the vast majority of 6-month CDW events were sustained PIRA; corresponding numbers were 126/155 (81.3%) in DMT-treated versus 141/165 (85.5%) in placebo-treated patients.
A similar, but more pronounced pattern was seen in paediatric patients (Fig. 2C). In interferon beta-1a treated patients, all patients who had a CDW event also relapsed during the study; only a single patient had worsening that fulfilled the definition of PIRA. The more efficacious treatment (fingolimod compared with interferon beta-1a) reduced the proportion of patients who relapsed (fingolimod: 24/107 = 22.4% versus interferon beta-1a: 67/107 = 62.6%) and also reduced all-cause 3-month CDW events (fingolimod: 4/107 = 3.7% versus interferon beta-1a: 12/107 = 11.2%). However, in fingolimod-treated patients, only 2/4 (50%) of the CDW events were RAW, while 2/4 (50%) events occurred in the absence of any reported relapses; one of them was a sustained PIRA event (no relapses and no new lesions were detected in that patient), the second was a worsening that occurred in the absence of any reported relapse activity, but the patient recovered (i.e. an unclassifiable event).
The role of relapses in driving disease worsening and progression in multiple sclerosis
Relapses in the previous study year, at any time and across all phenotypes, increased the risk for a subsequent all-cause CDW event, with marked significance and consistently, by 31–48% across the three datasets (Table 2). The prognostic value of relapses 1 year prior (i.e. the annualized relapse rate-1 year) was similar or higher (based on a likelihood-ratio test) than that of relapses in the previous 2 years (i.e. the annualized relapse rate-2 years) in all three datasets.
Table 2.
The prognostic value of relapses for subsequent disability worsening
| Full dataset (n = 27 328) | Phase 3 trials (n = 8346) | Phase 3 placebo-controlled trials (n = 4970) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 6-month CDW | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
| ARR1 | 1.47 | 1.41, 1.55 | <0.001 | 1.48 | 1.40, 1.57 | <0.001 | 1.31 | 1.21, 1.41 | <0.001 |
| ARR2 | 1.38 | 1.29, 1.47 | <0.001 | 1.37 | 1.25, 1.49 | <0.001 | 1.17 | 1.03, 1.33 | <0.019 |
n refers to the analysis set totals. The prognostic value of relapses was analysed using Andersen-Gill models. The annualized relapse rate (ARR) 1 (ARR1) or 2 (ARR2) years prior to time ‘t’ were used in separate models as time-varying covariates. The prognostic value of relapses was summarized with hazard ratios (hazard ratio >1 corresponds to an increased risk) for a 6-month CDW event. The ARR1 is calculated as the cumulative number of relapses as reported by investigators in the year prior to time t and divided by 365.25 days if the patient has been observed for the full year. The ARR2 is calculated analogously but for 2 years, i.e. 2 × 365.25 days. Andersen-Gill models, adjusting for additional covariates, are further described in the Supplementary material.
Other risk factors for 6-month CDW included pre-existing disability (i.e. the higher the level of disability, the more likely a further worsening), male sex and a diagnosis of progressive multiple sclerosis (either SPMS or PPMS). The impact of these additional risk factors is illustrated for each of the three datasets in Supplementary Fig. 1.
Relapses and relapse recovery
Risk factors for an incomplete recovery from relapse were investigated as contributors to the accumulation of disability. Overall, 15 921 relapses were reported in RRMS patients, 556 in SPMS and 115 in PPMS; this included all investigator-reported relapses, irrespective of an EDSS-based confirmation; the recovery from relapse was assessed as per the investigator’s judgement.
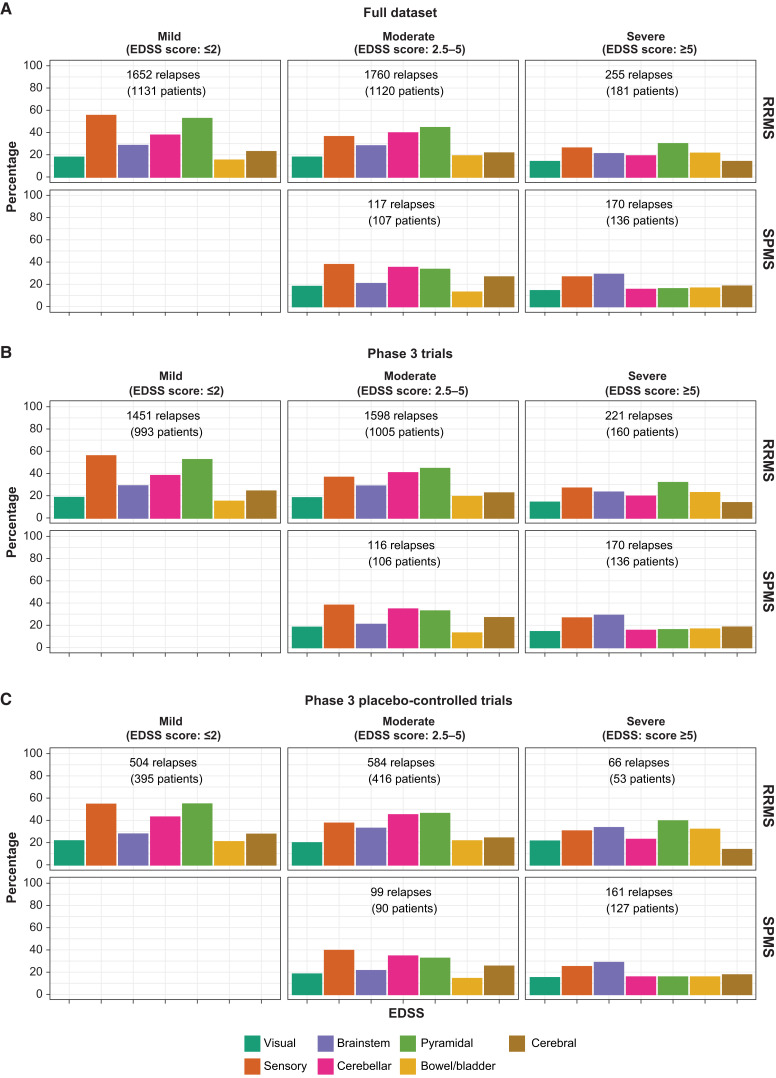
The functional systems affected by relapses in patients with RRMS and SPMS are summarized in Fig. 3. In patients with early RRMS and only mild disability (EDSS ≤ 2), sensory and pyramidal functional systems were most commonly affected by relapses. As the disease progressed, bowel and bladder involvement and other functional systems tended to gain in relative importance. Affected functional systems were not fundamentally different between RRMS and SPMS patients, when adjusting for the level of pre-existing disability. There were no striking differences in the functional systems involved in a relapse to explain why patients were judged to have fully or incompletely recovered from relapses (Supplementary Fig. 2). The investigator-judged level of relapse recovery was well in-line with the EDSS-based recovery from relapses (Supplementary Fig. 3).
Figure 3.
Relapse-affected functional systems in RRMS and SPMS patients by level of disability level (EDSS total score category) prior to the relapse (mild, moderate and severe). A–C describe the full dataset, randomized phase 3 trials plus extensions and phase 3 placebo-controlled trials, respectively. The number of patients corresponds to the number of patients with relapses with corresponding EDSS functional score assessments. Similar illustrations for complete and incomplete recovery are provided in Supplementary Fig. 2.
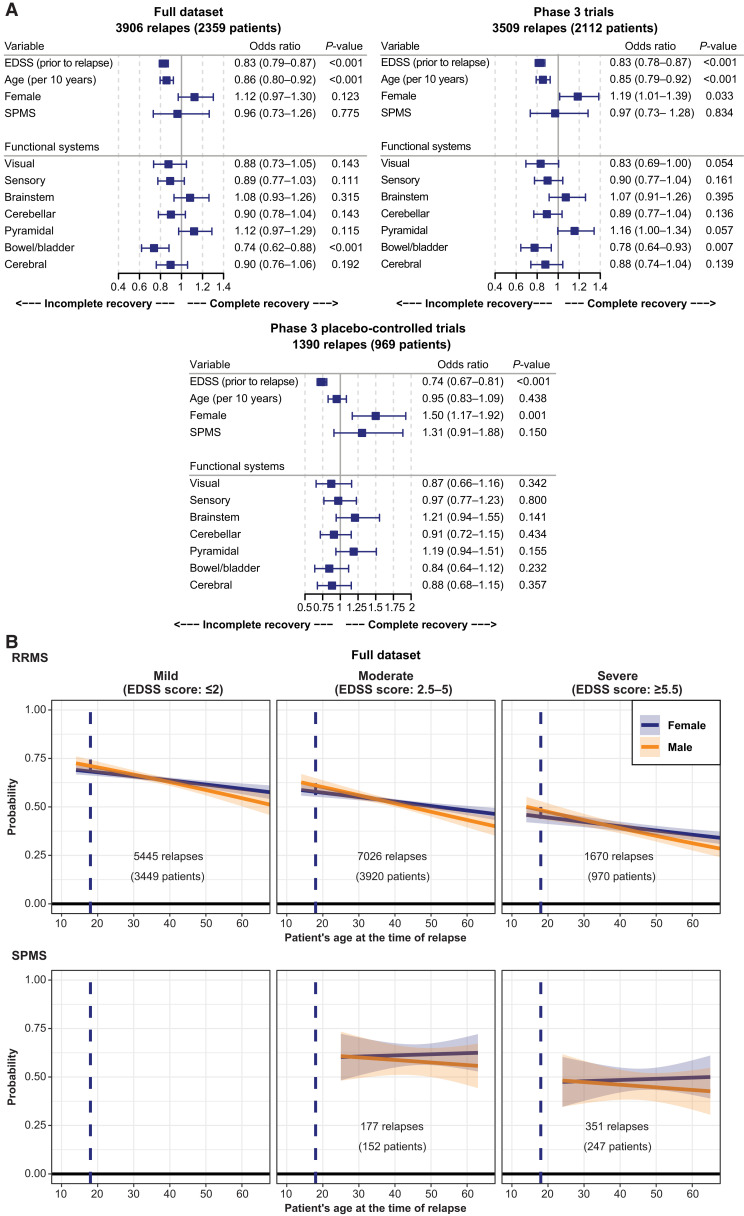
Pre-existing disability (as measured by the EDSS total score, prior to the relapse) and patient’s age at the time of the relapse were the most important and consistent risk factors for incomplete recovery from relapses in all datasets (Fig. 4A). The higher the level of pre-existing disability and the older the age, the lower the chances of a complete recovery. In addition, male sex was suggestive of a lower likelihood for a complete recovery from relapses. In the full and the phase 3 datasets, there was also a trend suggesting bowel and bladder involvement (which tends to occur at later stages in the disease) may be associated with risk of an incomplete recovery from relapses; however, the numerical differences were small and not consistent across all datasets. Other functional systems were not consistently prognostic after accounting for EDSS and age. Also, a diagnosis of RRMS or SPMS did not impact the recovery from relapse when accounting for age and pre-existing level of disability (Fig. 4A and B).
Figure 4.
Prognostic factors of a complete relapse recovery and probability of a complete relapse recovery (as judged by the Investigator; full dataset). (A) The number of patients represents the number of patients with relapses with corresponding EDSS functional score assessments and other covariates. Risk factors for an incomplete relapse recovery were analysed in a logistic regression model with adjustments for sex, age and EDSS score (prior to relapse), as well as for functional systems involved in the relapse. Odds ratios are displayed with 95% CIs; odds ratios significantly (<1) indicate risk factors for an incomplete recovery. (B) The number of patients noted in each panel corresponds to the number of patients with relapses and status of recovery available. The charts represent the mean probability of a complete relapse recovery by phenotype (top row RRMS, bottom row SPMS), sex, pre-existing level of disability (categorized) and as a function of the patient’s age at the time of the relapse. Relapse recovery was analysed in a logistic regression model with adjustments for sex, age and EDSS score (prior to relapse). Data for the phase 3 trials and the placebo-controlled phase 3 trials are presented in Supplementary Fig. 4 and are broadly consistent.
The probability of a complete relapse recovery (as judged by the investigator) was highest (∼75%) in the youngest, least disabled RRMS patients and decreased gradually with age and higher levels of disability (Fig. 4B, top row). For severely disabled patients above the age of 40 years, the probability of a full recovery from a relapse was not notably different between RRMS and SPMS patients and was approximately 50% or less (Fig. 4B). Similar findings were seen in the phase 3 studies (Supplementary Fig. 4).
Time between milestone disability levels and the effect of treatment
We investigated the time to milestone disability levels and the possible impact of current DMTs on these transition times. A total of 193 513 EDSS transitions from one visit to the next from n = 22 808 patients from the full analysis set informed the analysis. This included transitions to higher (worsening) or lower (improvement) EDSS values or no change (stable disease).
For illustration purposes, the Markov-model-estimated transition matrix for a patient with multiple sclerosis at the age of 40 years is presented in Supplementary Fig. 5. In our study, for each value of age and whether the patient was on or off a DMT at the time of the transition, a different transition matrix was estimated. For patients with no or only minimal disability (EDSS ≤ 2) the estimated probability to remain at the same stage for a year was approximately 40%; for patients with moderate to severe disability but who were ambulatory (EDSS = 3 to 5), the probability to remain at the same stage for a year was lower. Once patients needed a walking aid or were dependent on a wheelchair (EDSS = 6 to 7), the probability to remain at the same stage for a year was >60%. Thus, ‘stay times’ at a specific EDSS levels were initially long when patients were not (or only minimally) disabled; they were relatively short when patients were more impaired but able to walk, and they lengthened again when patients were dependent on a walking aid or a wheelchair. Supplementary Fig. 6 describes unequal ‘stay times’ at different EDSS scores based on the full dataset.23
The time to reach milestone disability levels and the impact of treatment, is summarized in Table 3. Placebo-treated patients were estimated to reach EDSS 4 on average within approximately 9.0 years, while patients who used DMTs took 12.5 years to reach the same level of disability; i.e. the time to reach EDSS 4 was significantly prolonged by treatment by approximately 3.5 years. Similarly, patients on placebo in the respective phase 3 trials needed a walking aid significantly earlier (after 18.5 years) compared with treated patients (21.6 years); the time gained by treatment was estimated at 3.1 years. At all tested stages of the disease, there was a significant time gain with treatment, but the time gain between EDSS 4 and 6 (1.4 years) was less than the time gain between EDSS 1 and 4 (3.5 years).
Table 3.
Transition time between milestone EDSS scores for placebo- versus any-DMT-treated patients by treatment effect and by mechanism of worsening
| Effect of treatment | By mechanism of worsening in RRMS patients who had a 6-month CDW event | |||||
|---|---|---|---|---|---|---|
| EDSS score | Placebo-treated (years) | DMT-treated (years) | Time gained due to treatment (years): Delta 95% CI | PIRA, with relapses (years, 95% CI) | PIRA, without relapses (years, 95% CI) | RAW (years, 95% CI) |
| 1 to 4 | 8.95 | 12.46 | 3.51 (3.19, 3.96) | 2.60 (2.30, 2.93) | 3.56 (3.31, 3.85) | 3.97 (3.61, 4.35) |
| 1 to 6 | 18.48 | 21.57 | 3.09 (2.60, 3.72) | 6.14 (5.51, 6.89) | 7.31 (6.84, 7.89) | 8.11 (7.36, 8.90) |
| 4 to 6 | 9.91 | 11.31 | 1.40 (0.86, 1.92) | 4.10 (3.49, 4.82) | 4.34 (3.98, 4.88) | 4.82 (4.41, 5.36) |
Mean transition times between milestone disability levels in the full dataset as measured using a continuous time Markov model. The time between milestone EDSS values considers all-cause disability worsening and improvement. The distribution of baseline disability stages (as measured by EDSS scores), as a function of the patient’s age, is presented in Supplementary Fig. 5. Based on inclusion and exclusion criteria for the clinical trials included in this study, the majority of the data points covered the range from EDSS 0 (normal neurological assessment) to EDSS 6 (requiring a walking aid), which is why we focused the analysis on this disability range. The gain in time between DMT- and placebo-treated patients (Delta) is statistically significant if the 95% CI does not include the value of zero. Transition times to milestone EDSS scores with corresponding 95% CI were also summarized by mechanism of worsening within the subgroup of RRMS patients who had a 6-month CDW event in the full dataset using continuous time Markov models; the 95% CI is to the time to milestone EDSS scores.
The time to milestone disability levels based on worsening attributed to PIRA (with or without relapses) or RAW in RRMS patients is reported in Table 3. The corresponding baseline characteristics are presented in Supplementary Table 4. The subgroup of RRMS patients who had all-cause CDW events reached milestone EDSS levels earlier (e.g. EDSS 1–4 in 2.60–3.97 years) than the overall group of placebo-treated RRMS patients, which also included a proportion of patients who did not worsen (i.e. 8.95 years for the same transition for the overall placebo group). RRMS patients who worsened due to PIRA reached milestone EDSS levels only slightly faster (CIs overlap) than those who worsened due to RAW events; the fastest worsening was observed in RRMS patients who experienced PIRA events in combination with superimposed relapses.
Discussion
In a large clinical trial dataset from the NO.MS database with >27 000 patients and ∼200 000 EDSS transitions, we investigated the mechanisms by which patients with multiple sclerosis accumulated disability. RAW was the main driver of worsening only in paediatric multiple sclerosis, while in adult multiple sclerosis PIRA was seen across all phenotypes, albeit at a lower frequency in RRMS than in progressive multiple sclerosis. Findings from two pooled phase 3 ocrelizumab studies by Kappos and colleagues1 were replicated, substantiated and expanded in the much larger and more heterogeneous NO.MS database: Our analysis specifically confirms that up to 50% of the disability accumulation in adult patients with RRMS is not associated with overt relapses. RAW and PIRA contributed relevantly to the overall accumulation of disability in adult RRMS while, in SPMS and PPMS, PIRA was the dominant driver of disease worsening. Baseline features of adult patients with RRMS who experienced either PIRA or RAW events were not substantially different, and were typical of RRMS, showing that progression plays a clinically relevant role also in RRMS. Patients with RAW events had on average higher on-study MRI activity compared with patients who experienced PIRA events, consistent with the expected underlying pathology.
In our analysis, we tested more stringent definitions of PIRA than those proposed by Kappos and colleagues1: To increase specificity, and to best answer our research question, we required disability worsening and progression events to be reflected in the EDSS score, while Kappos and colleagues1 used a composite of the EDSS score combined with hand-coordination (Nine-Hole Peg Test) and walking ability (Timed 25-Foot Walk Test), with a majority of progression events not being reflected in the EDSS. Furthermore, we required progression to be independent of all investigator-reported relapse activity (instead of only EDSS-confirmed or ‘protocol-defined’ relapses), thereby classifying fewer events as PIRA. Lastly, we required that PIRA events were not only 3- or 6-month confirmed but also sustained in all longitudinal follow-up data, i.e. we defined PIRA as an irreversible deterioration occurring in the absence of any reported relapse activity. Tightening of the PIRA definition in our study lowered the absolute numbers of PIRA events but produced a result that remained qualitatively unchanged in comparison to the findings by Kappos and colleagues1: PIRA, as detected by regular EDSS assessments, occurs and contributes to disability worsening in patients with RRMS.
Progression is underestimated as a contributing mechanism to the all-cause accumulation of disability in RRMS. This may be partly attributed to differences between clinical practice and clinical trials, such as the standardized visits and monitoring of EDSS scores in trials that is more conducive to detecting gradual worsening compared with clinical practice. In addition, in patients who experience a relapse, gradual worsening occurring prior to this event may be attributed incorrectly to the relapse, i.e. some progression may be masked by episodes of acute neurological symptoms. It may also be the case that the loss of function over time is so gradual in some patients as to be unnoticed by the patient or physician.9 However, in contrast to the results previously reported from the EPIC study,9 most (∼90%) of the 24 469 patients diagnosed with RRMS in our dataset did not experience a 6-month CDW, perhaps attributable to the use of DMTs, and the proportion of patients with RRMS who experienced PIRA events and thus fulfill the definition of SPMS was small (3.4%).
Our analysis of the NO.MS database confirmed evidence of progression in early RRMS, and that patients with progressive multiple sclerosis (SPMS and PPMS) can have focal lesions and relapses. In a previous study, the diagnostic uncertainty of the transition from RRMS to SPMS was estimated at 4.3 years.24 Our data suggest the extent and relative importance of focal inflammation and progression varies gradually with the patients’ age and pre-existing disability level, often without a sudden transition point to progressive disease.
Gradual clinical worsening may be more easily detectable when acute attacks are less frequent, either when relapse activity declines due to older age,16 or when acute inflammation is suppressed by treatment and underlying progression is revealed. In the phase 3 studies with ocrelizumab in patients with relapsing multiple sclerosis, ocrelizumab significantly reduced the frequency of relapses and all-cause disability worsening events versus interferon beta-1a25; however, out of the remaining all-cause disability worsening events, a higher proportion of PIRA events were seen in the ocrelizumab arm (∼90%) than in the interferon beta-1a arm (∼80%).1 Similarly, in the NO.MS database, DMT treatment reduced the frequency of relapses, all-cause disability worsening events, RAW and PIRA compared to placebo in the phase 3 trials, but resulted in a higher proportion of remaining disability worsening events occurring independently of relapse activity. This was not only observed in adult RRMS, but also in paediatric multiple sclerosis where progression is considered least likely to occur.
The occurrence of clinical progression in the form of PIRA in early RRMS (despite effective anti-inflammatory treatment) suggests that a gradual pathological process (e.g. central or diffuse inflammation) and/or secondary degeneration (as a consequence of accumulating subclinical disease burden) plays a role in multiple sclerosis from the onset of the disease. Indeed, brain volume loss, instead of age-expected brain volume growth, was observed in paediatric patients treated with interferon beta-1a or fingolimod in the PARADIGMS study.26 Although fingolimod significantly reduced the frequency of relapses by 82% compared with interferon beta-1a27 and significantly lessened the amount of brain volume loss, a net loss of brain volume was observed in both active treatment arms. Brain volume and neuronal loss seem to be common features in multiple sclerosis, even in the earliest stages of the disease.16,28,29
Based on data from the TRANSFORMS30 and FREEDOMS31 studies (which are part of the NO.MS database), baseline T2 lesion volume was identified as the most important baseline risk factor associated with brain atrophy32 and neuronal loss33,34 in RRMS. Similar findings were reported in patients with progressive disease (PPMS) from the INFORMS trial.35 The prominent role of T2 lesion volume (which marks cumulative prior inflammation as a risk factor for further brain tissue and neuronal loss and clinical deterioration) is supportive of the topographical model of multiple sclerosis. In this model, clinical progression recapitulates a patient’s prior relapses and unmasks previously asymptomatic lesions.36 Early in the disease, the majority of lesions may go unnoticed by the patient,37 or patients may initially recover well from relapses. However, cumulative subclinical damage, measurable in the form of the total T2 lesion volume or the rate of brain atrophy, seems to play an important role in driving further brain tissue loss and the insidious disability of multiple sclerosis.9,38,39 Searching our NO.MS database for the youngest patients with a diagnosis of SPMS revealed that they had a mean T2 lesion volume >20 cm3 indicating a history of substantial inflammation, and many had a paediatric onset [observed in 67% (8/12) of patients diagnosed with SPMS at <25 years of age, and 40% (18/45) of those with a SPMS diagnosis at <30 years].16 This suggests that ongoing multiple sclerosis disease activity, either clinical or on MRI, is associated with irreversible damage to the CNS from disease onset, and that early prevention/treatment intervention could delay subsequent progression.
Our study confirmed that relapses are a significant and clinically meaningful contributor to all-cause disability worsening. This is in line with observations that relapses frequently lead to residual deficits,10,40–42 and that persistent focal inflammatory activity early in the disease is predictive of severe EDSS worsening.43–46 The authors of a natural history cohort of 1844 patients with multiple sclerosis from a single center came to a different conclusion, stating that relapses do not significantly influence the progression of irreversible disability.4 However, there were several limitations in the methodology of this study. Notably, the use of survival methods (time to event models) outside of a randomized controlled study, where baseline characteristics between the RRMS and PPMS cohorts included in the analysis likely differed in many aspects (including patients’ ages and baseline EDSS scores), thus limiting the validity of comparing EDSS transition times. In our study, we used Andersen-Gill models because they are statistically more suitable than survival methods as they take into account that patients may relapse and worsen repeatedly. They also can address the question of whether accumulation of disability is more likely following a time period (e.g. a year) with relapses than following a time period without relapses, based on a time-varying covariate analysis within the overall cohort (i.e. they do not require a separation of patients into different cohorts, which can lead to bias). We found that following a year with relapses (annualized relapse rate-1 year), the risk of an all-cause disability worsening significantly increased by approximately 30–50%, depending on the dataset.
We identified pre-existing disability and older age as the principal risk factors to further deterioration, in agreement with Chitnis et al.13 This suggests that patients developing residual disability after a relapse are more likely to do so on subsequent relapses, perhaps due to patients lacking adequate repair capacity or neuronal plasticity. Additional risk factors for an incomplete recovery from relapse, though of lower value, were male sex and bowel and bladder involvement. As a limitation, we could not study race or ethnicity as potential risk factors, due to the anonymization of the data.18
We analysed the time to reach milestone EDSS levels based on continuous time Markov models. Overall, our estimation of the disability trajectories from placebo-treated clinical trial patients closely resembled the long-term trajectories previously described in natural history cohorts, likely reflecting the disease as well as the scale properties of the EDSS scores.4,21,23 Based on our NO.MS database, we estimated that placebo-treated patients with RRMS take approximately 9.0 years from EDSS = 1 (minimal disability) to reach an increased limitation in walking ability (EDSS = 4) and 18.5 years to requiring walking assistance (EDSS = 6). Within the subset of patients with RRMS who experienced a 6-month CDW, we further analysed the time to milestone EDSS scores by clinical mechanism of worsening (the baseline characteristics of patients with RAW or PIRA events were roughly comparable only within the RRMS population). The fastest transition from EDSS 1–4 was estimated in the subgroup of patients with RRMS who experienced a combination of relapses and PIRA events (approximately 2.6 years). Finally, we analysed the impact of treatment in NO.MS, and found that DMTs can significantly delay the time to milestone disability by approximately 3.5 years between EDSS 1 and 4, and by approximately 1.4 years between EDSS 4 and 6, suggesting that the benefit of treatment is highest in the earliest stages of the disease.
In conclusion, relapses are significant drivers of the accumulation of disability primarily, but not exclusively, early in multiple sclerosis. Our study confirms that PIRA has been underestimated as a contributing factor in RRMS: PIRA plays a significant role in disease worsening in adult RRMS and gradually becomes the principle way by which patients with multiple sclerosis acquire disability in progressive disease. PIRA occurs early in the disease, as shown in our study in patients with successful suppression of inflammation with efficacious DMTs, which supports the existence of an ongoing treatment-resistant pathology from the start. Our study demonstrated that time to disability milestones can be delayed by several years with treatment, with the highest potential to gain time in the youngest, least disabled patients with multiple sclerosis.
Supplementary Material
Acknowledgements
The authors acknowledge the physicians and patients who participated in these studies for their valuable contribution towards the advancement of our knowledge in multiple sclerosis. The authors acknowledge the work of Ann-Marie Mallon, Luis Santos, Daniel Delbarre and Steve Gardiner in coordinating and conducting data wrangling work. They also thank Karen Stanford, Paul Coyle and Marie-Catherine Mousseau for medical writing assistance, which was funded by Novartis and included language checks, formatting, referencing, preparation of tables and figures as per journal guidelines and incorporating the authors’ revisions under the direction of the lead authors.
Abbreviations
- CDW =
confirmed disability worsening
- DMT =
disease-modifying therapy
- EDSS =
Expanded Disability Status Scale
- NO.MS =
Novartis-Oxford multiple sclerosis
- PIRA =
progression independent of relapse activity
- PPMS =
primary progressive multiple sclerosis
- RAW =
relapse-associated worsening
- RRMS =
relapsing-remitting multiple sclerosis
- SPMS =
secondary progressive multiple sclerosis
Contributor Information
Fred D Lublin, The Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Dieter A Häring, Novartis Pharma AG, Basel, Switzerland.
Habib Ganjgahi, Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Alex Ocampo, Novartis Pharma AG, Basel, Switzerland.
Farhad Hatami, Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Jelena Čuklina, Novartis Pharma AG, Basel, Switzerland.
Piet Aarden, Novartis Pharma AG, Basel, Switzerland.
Frank Dahlke, Novartis Pharma AG, Basel, Switzerland.
Douglas L Arnold, McConnell Brain Imaging Centre, Montreal Neurological Institute and Hospital, McGill University, Montréal, QC, Canada.
Heinz Wiendl, Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Münster, Germany.
Tanuja Chitnis, Department of Neurology, Brigham and Women’s Hospital, Boston, MA, USA.
Thomas E Nichols, Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Bernd C Kieseier, Novartis Pharma AG, Basel, Switzerland.
Robert A Bermel, Department of Neurology, Mellen Center for Multiple Sclerosis, Cleveland Clinic, Cleveland, OH, USA.
Funding
This study was supported by Novartis (Switzerland). Novartis employees contributed to study design, analysis of the data, and the decision to publish the results.
Competing interests
F.L. reports as sources of funding for research: Novartis, Actelion, Biogen, Sanofi, NMSS, NIH and Brainstorm Cell Therapeutics; consulting agreements/advisory boards/DSMB: Biogen, EMD Serono, Novartis, Teva, Actelion/Janssen, Sanofi/Genzyme, Acorda, Roche/Genentech, MedImmune/Viela Bio, Receptos/Celgene/BMS, TG Therapeutics, Medday, Atara Biotherapeutics, Mapi Pharma, Apitope, Orion Biotechnology, Brainstorm Cell Therapeutics, Jazz Pharmaceuticals, GW Pharma, Mylan, Immunic, Population Council, Avotres, Neurogene, Banner Life Sciences, Labcorp, Entelexo Biotherapeutics and NeuraLight; stock options: Avotres and NeuraLight; Speaker: Sanofi (non-promotional). D.A. reports consulting fees from Albert Charitable Trust, Alexion Pharma, Biogen, Celgene, Frequency Therapeutics, Genentech, Med-Ex Learning, Merck, Novartis, Population Council, Receptos, Roche and Sanofi-Aventis; grants from Biogen, Immunotec and Novartis and an equity interest in NeuroRx. H.W. receives honoraria for acting as a member of Scientific Advisory Boards for Biogen, Genzyme, Merck Serono, Novartis, Roche Pharma AG and Sanofi-Aventis and UCB; as well as speaker honoraria and travel support from Alexion, Biogen, Biologix, Cognomed, F. Hoffmann-La Roche Ltd., Gemeinnützige Hertie-Stiftung, Merck, Novartis, Roche Pharma AG, Genzyme, Teva and WebMD Global. H.W. is acting as a paid consultant for Actelion, Argenx, Biogen, Bristol Myers Squibb, EMD Serono, Idorsia, IGES, Immunic, Immunovant, Janssen, Johnson & Johnson, Novartis, Roche, Sanofi, the Swiss Multiple Sclerosis Society and UCB. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, Fresenius Foundation, the European Union, Hertie Foundation, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies (IZKF) Muenster and Biogen, GlaxoSmithKline, Roche Pharma AG and Sanofi-Genzyme. T.C. reports consulting fees from Biogen Idec, Novartis and Genentech; grants from Novartis, Octave Bioscience and Tiziana Life Sciences; all outside the submitted work. R.B. has served as a consultant for AstraZeneca, Biogen, EMD Serono, Genzyme, Genentech, Novartis and VielaBio. He receives research support from Biogen, Genentech and Novartis. H.G., F.H. and T.N. report no competing interests. F.D. was a salaried employee of Novartis during manuscript development, but is no longer employed by Novartis and reports no competing interests. D.H., A.O., J.Č., P.A. and B.K. are employees of Novartis.
Supplementary material
Supplementary material is available at Brain online.
References
- 1.Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol 2020;77(9):1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vukusic S, Confavreux C. Prognostic factors for progression of disability in the secondary progressive phase of multiple sclerosis. J Neurol Sci 2003;206(2):135–137. [DOI] [PubMed] [Google Scholar]
- 3.Casserly C, Ebers GC. Relapses do not matter in relation to long-term disability. Mult Scler. 2011;17(12):1412–1414. [DOI] [PubMed] [Google Scholar]
- 4.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343(20):1430–1438. [DOI] [PubMed] [Google Scholar]
- 5.Scalfari A, Neuhaus A, Daumer M, Muraro PA, Ebers GC. Onset of secondary progressive phase and long-term evolution of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014;85(1):67–75. [DOI] [PubMed] [Google Scholar]
- 6.Novotna M, Paz Soldan MM, Abou Zeid N, et al. Poor early relapse recovery affects onset of progressive disease course in multiple sclerosis. Neurology. 2015;85(8):722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott TF, Diehl D, Elmalik W, Gettings EJ, Hackett C, Schramke CJ. Multiple sclerosis relapses contribute to long-term disability. Acta Neurol Scand. 2019;140(5):336–341. [DOI] [PubMed] [Google Scholar]
- 8.Koch-Henriksen N, Thygesen LC, Sørensen PS, Magyari M. Worsening of disability caused by relapses in multiple sclerosis: A different approach. Mult Scler Relat Disord. 2019;32:1–8. [DOI] [PubMed] [Google Scholar]
- 9.Cree BAC, Hollenbach JA, Bove R, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85(5):653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology. 2003;61(11):1528–1532. [DOI] [PubMed] [Google Scholar]
- 11.Soldán MMP, Novotna M, Abou Zeid N, et al. Relapses and disability accumulation in progressive multiple sclerosis. Neurology. 2015;84(1):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banwell B, Ghezzi A, Bar-Or A, Mikaeloff Y, Tardieu M. Multiple sclerosis in children: clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurol. 2007;6(10):887–902. [DOI] [PubMed] [Google Scholar]
- 13.Chitnis T, Aaen G, Belman A, et al. Improved relapse recovery in paediatric compared to adult multiple sclerosis. Brain. 2020;143(9):2733–2741. [DOI] [PubMed] [Google Scholar]
- 14.Gorman MP, Healy BC, Polgar-Turcsanyi M, Chitnis T. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol. 2009;66(1):54–59. [DOI] [PubMed] [Google Scholar]
- 15.Gärtner J, Chitnis T, Ghezzi A, et al. Relapse rate and MRI activity in young adult patients with multiple sclerosis: A post hoc analysis of phase 3 fingolimod trials. Mult Scler J Exp Transl Clin. 2018;4(2):2055217318778610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahlke F, Arnold DL, Aarden P, et al. Characterisation of MS phenotypes across the age span using a novel data set integrating 34 clinical trials (NO.MS cohort): Age is a key contributor to presentation. Mult Scler. 2021;27(13):2062–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology. 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 18.Mallon A-M, Dahlke F, Aarden P, et al. Advancing data science in drug development through an innovative computational framework for datasharing and statistical analysis. BMC Med Res Methodol. 2021;21(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson CH, Sharples LD, Thompson SG, Duffy SW, Couto E. Multistate Markov models for disease progression with classification error. Statistician. 2003;52(Part 2):193–209. [Google Scholar]
- 20.Jackson CH. Multi-state modelling with R: the msm package. Version 1.6.8 16 December 2019. MRC Biostatistics Unit; 2007. [Google Scholar]
- 21.Palace J, Bregenzer T, Tremlett H, et al. UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model. BMJ Open. 2014;4(1):e004073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schach S, Scholz M, Wolinsky JS, Kappos L. Pooled historical MRI data as a basis for research in multiple sclerosis–a statistical evaluation. Mult Scler. 2007;13:509–516. [DOI] [PubMed] [Google Scholar]
- 23.Weinshenker BG, Rice GP, Noseworthy JH, Carriere W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study: 4. Applications to planning and interpretation of clinical therapeutic trials. Brain. 1991;114(Pt 2):1057–1067. [DOI] [PubMed] [Google Scholar]
- 24.Sand IK, Krieger S, Farrell C, Miller AE. Diagnostic uncertainty during the transition to secondary progressive multiple sclerosis. Mult Scler. 2014;20(12):1654–1657. [DOI] [PubMed] [Google Scholar]
- 25.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–234. [DOI] [PubMed] [Google Scholar]
- 26.Arnold DL, Banwell B, Bar-Or A, et al. Effect of fingolimod on MRI outcomes in patients with paediatric-onset multiple sclerosis: results from the phase 3 PARADIGMS study. J Neurol Neurosurg Psychiatry. 2020;91(5):483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chitnis T, Arnold DL, Banwell B, et al. Trial of fingolimod versus interferon beta-1a in pediatric multiple sclerosis. N Engl J Med. 2018;379(11):1017–1027. [DOI] [PubMed] [Google Scholar]
- 28.De Stefano N, Giorgio A, Battaglini M, et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology. 2010;74(23):1868–1876. [DOI] [PubMed] [Google Scholar]
- 29.Disanto G, Adiutori R, Dobson R, et al. Serum neurofilament light chain levels are increased in patients with a clinically isolated syndrome. J Neurol Neurosurg Psychiatry. 2016;87(2):126–129. [DOI] [PubMed] [Google Scholar]
- 30.Khatri B, Barkhof F, Comi G, et al. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol. 2011;10(6):520–529. [DOI] [PubMed] [Google Scholar]
- 31.Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401. [DOI] [PubMed] [Google Scholar]
- 32.Radue EW, Barkhof F, Kappos L, et al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology. 2015;84(8):784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain. 2018;141(8):2382–2391. [DOI] [PubMed] [Google Scholar]
- 34.Kuhle J, Kropshofer H, Häring DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92(10):e1007–e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387(10023):1075–1084. [DOI] [PubMed] [Google Scholar]
- 36.Krieger SC, Cook K, De Nino S, Fletcher M. The topographical model of multiple sclerosis: A dynamic visualization of disease course. Neurol Neuroimmunol Neuroinflamm. 2016;3(5):e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorpe JW, Kidd D, Moseley IF, et al. Serial gadolinium-enhanced MRI of the brain and spinal cord in early relapsing-remitting multiple sclerosis. Neurology. 1996;46(2):373–378. [DOI] [PubMed] [Google Scholar]
- 38.Miller DH, Lublin FD, Sormani MP, et al. Brain atrophy and disability worsening in primary progressive multiple sclerosis: insights from the INFORMS study. Ann Clin Transl Neurol. 2018;5(3):346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sormani MP, Kappos L, Radue EW, et al. Defining brain volume cutoffs to identify clinically relevant atrophy in RRMS. Multi Scler J. 2017;23(5):656–664. [DOI] [PubMed] [Google Scholar]
- 40.Vercellino M, Romagnolo A, Mattioda A, et al. Multiple sclerosis relapses: a multivariable analysis of residual disability determinants. Acta Neurol Scand. 2009;119(2):126–130. [DOI] [PubMed] [Google Scholar]
- 41.Bosca I, Coret F, Valero C, et al. Effect of relapses over early progression of disability in multiple sclerosis patients treated with beta-interferon. Mult Scler. 2008;14(5):636–639. [DOI] [PubMed] [Google Scholar]
- 42.Hirst C, Ingram G, Pearson O, Pickersgill T, Scolding N, Robertson N. Contribution of relapses to disability in multiple sclerosis. J Neurol. 2008;255(2):280–287. [DOI] [PubMed] [Google Scholar]
- 43.Bermel RA, You X, Foulds P, et al. Predictors of long-term outcome in multiple sclerosis patients treated with interferon beta. Ann Neurol. 2013;73(1):95–103. [DOI] [PubMed] [Google Scholar]
- 44.Jokubaitis VG, Spelman T, Kalincik T, et al. Predictors of long-term disability accrual in relapse-onset multiple sclerosis. Ann Neurol. 2016;80(1):89–100. [DOI] [PubMed] [Google Scholar]
- 45.Koch-Henriksen N, Sørensen PS, Magyari M. Relapses add to permanent disability in relapsing multiple sclerosis patients. Mult Scler Relat Disord. 2021;53:103029. [DOI] [PubMed] [Google Scholar]
- 46.Sotiropoulos MG, Lokhande H, Healy BC, et al. Relapse recovery in multiple sclerosis: Effect of treatment and contribution to long-term disability. Mult Scler J Exp Transl Clin. 2021;7(2):20552173211015503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from the NO.MS cohort are currently only available within the collaboration, due to data privacy requirements derived from the original signed informed consent forms and the risk-based anonymization, which takes IT security and access considerations into account.18 Anonymized clinical data from the individual studies are available on reasonable request provided that it is in line with current ethical and intellectual property requirements surrounding the use of data. Requests should be directed to ClinicalStudyDataRequest.com.