Abstract
Two systems for the uptake of inorganic phosphate (Pi) in Escherichia coli, PitA and Pst, have been described. A revertant of a pitA pstS double mutant that could grow on Pi was isolated. We demonstrate that the expression of a new Pi transporter, PitB, is activated in this strain by a gene amplification event.
Transport of inorganic phosphate (Pi) across the cytoplasmic membrane of Escherichia coli is mediated by the PitA protein and Pst system. PitA, which transports metal phosphates (28) and is constitutively expressed (17), is driven by the proton motive force (PMF) (18, 28). The Pst system, which transports Pi at the expense of ATP (6, 9), is composed of a periplasmic Pi-binding protein (PstS), two integral membrane proteins (PstC and PstA), and an ATP-binding protein (PstB) (24). The genes encoding these four proteins constitute an operon (24), together with phoU, which encodes a protein not required for Pi transport (23). Under Pi limitation, the expression of the Pst system, which is produced at a basal level under Pi-replete conditions, is further induced. Like, for example, phoA, which encodes the periplasmic enzyme alkaline phosphatase (26), the pst-phoU operon is part of the pho regulon, which is under the control of a two-component regulatory system consisting of the proteins PhoB and PhoR (29). Furthermore, the Pst system appears to be involved in regulation, since mutations in the genes of the pst-phoU operon generally result in constitutive expression of the pho regulon (29–31). The exact mechanism by which the Pst system controls the expression of the pho regulon is not known. To study the role of PstS in regulation, we attempted to isolate mutants with mutations in the membrane components of the Pst system that can transport Pi in the absence of the PstS protein. In one of the mutants obtained, a new Pi transporter, PitB, appeared to be expressed.
Construction of a pstS pitA double mutant.
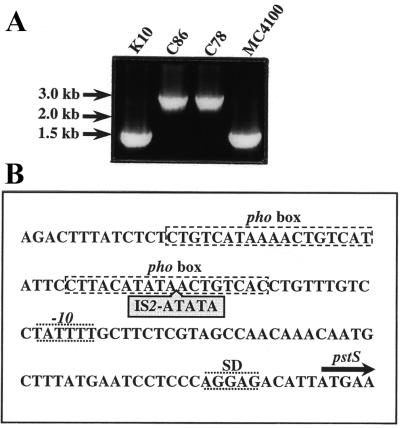
A pstS pitA double-mutant strain is expected to behave as an organic phosphate auxotroph (22), but previously described pstS pitA double mutants, such as strain C86, appear to take up Pi and to grow on Pi as the sole source of phosphate (reference 30 and data not shown). Western blotting revealed that the PstS protein was produced in this strain, although at much lower levels than in its parental strain, K10, grown under Pi limitation (data not shown). To characterize the pstS mutation in strain C86, a DNA fragment was amplified by PCR. The amplified fragment was considerably larger than the expected 1.4 kb, which was found when strains MC4100 and K10 were analyzed (Fig. 1A). An enlarged PCR fragment was also found in another pitA pstS strain, C78 (Fig. 1A). Sequencing of the PCR fragment from strain C86 revealed the presence of an IS2 element in the promoter region of pstS (Fig. 1B), whereas no other mutation was found in the pstS gene or the other genes of the pst-phoU operon. Hence, strain C86 and probably also strain C78 contain a Pst system, which is expressed at a lower level due to the insertion of an IS2 element in its promoter.
FIG. 1.
Characterization of the pstS mutation in C86. (A) Amplification of the pstS gene and its promoter region by PCR using chromosomal DNA of strains K10, C86, C78, and MC4100 (5) and the primers pst4 (5′-GAGTAATAAATGGATGCCC-3′) and pst5 (5′-CGGTGGGTTAAAAGCAGGC-3′). Strains K10 (pitA10), C86 (pstS21 derivative of K10), and C78 (pstS28 derivative of K10) were kindly provided by the E. coli Genetic Stock Center (Department of Biology, Yale University, New Haven, Conn.). The positions of the molecular size markers are indicated at the left. (B) Position of the IS2 element in the promoter region of the pstS gene in strain C86. The two pho boxes, the −10 region and the Shine-Dalgarno sequence (SD), are indicated. The start of the coding region of pstS is indicated by an arrow.
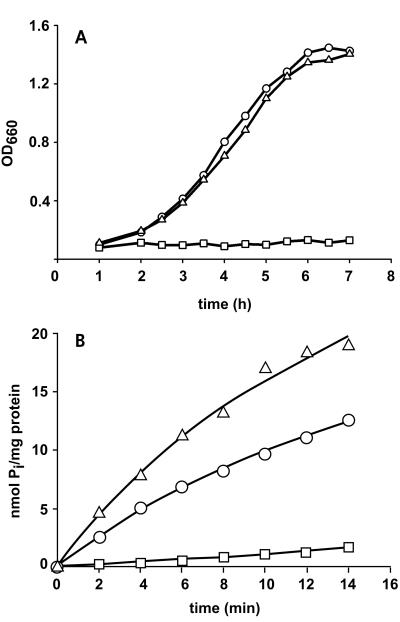
We decided to construct a new pitA pstS double mutant. Strain K10 carries an uncharacterized pitA mutation (30). Sequencing of a PCR fragment containing this pitA allele revealed a single point mutation with respect to the wild type (4), resulting in a Gly220Asp substitution in a putative membrane-spanning segment of PitA. A pstS::kan mutation was constructed by ligating a kanamycin resistance cassette from pUC18K (13) into the PvuI site of pSN5182 (15). The resulting plasmid, pSL15, was digested with NruI and BamHI, and the 5.8-kb DNA fragment carrying the pstS::kan allele was used to transform recBC sbcB strain AM1095 (8), to disrupt the chromosomal pstS gene. A pstS::kan derivative of strain K10, designated CE1485, was subsequently constructed by P1 transduction (14). Western blotting (Fig. 2A, lane 2) confirmed the absence of the PstS protein in this strain, which failed to grow on Pi as the sole source of phosphate (Fig. 3A), whereas growth on glycerol 3-phosphate (G3P) was not affected (results not shown). Furthermore, the efficiency of the cells in taking up 33Pi was drastically reduced (Fig. 3B). Whereas PhoU expression was induced in strain K10 under low-Pi (LPi) conditions, it was detected in strain CE1485 after growth in high-Pi (HPi) medium (Fig. 2), indicating that the pho regulon is constitutively expressed. Furthermore, this result shows that the kanamycin resistance cassette in pstS has no polar effect on the expression of the downstream genes in the pst operon. Alkaline phosphatase assays (25) confirmed the constitutive expression of the pho regulon in CE1485 (data not shown).
FIG. 2.
Expression of the PstS and the PhoU proteins in pitA mutant strain K10 (lane 1), pitA pstS mutant strain CE1485 (lane 2), and the pseudorevertant strain CE1487 (lane 3). Cells were grown in a peptone-based, phosphate-poor medium (11) supplemented with 0.5% glucose, 660 μM K2HPO4, and 1 mM G3P (HPi medium) (A) or with no K2HPO4 or G3P added (LPi medium) (B). The alternative phosphate source G3P was omitted from the LPi medium, since it may be degraded by alkaline phosphatase, thereby generating Pi. Although growth of CE1485 in the LPi medium was very poor, enough cells could be collected for this analysis. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12) and, for Western blotting (1), transferred to a nitrocellulose membrane (Schleicher & Schuell). Immunodetection was performed with polyclonal antisera directed against the Pi-binding protein (PstS) and PhoU. The positions of molecular size standard proteins are indicated on the left in kilodaltons.
FIG. 3.
Growth and 33Pi uptake. (A) Growth curve of pitA mutant strain K10 (○), pitA pstS double-mutant CE1485 (□), and the pseudorevertant CE1487 (▵). Cells were grown overnight in Luria broth supplemented with G3P, pelleted, and resuspended in HEPES-buffered synthetic medium (25) supplemented with 0.5% glucose and 660 μM K2HPO4. Growth was monitored for 7 h. (B) Uptake of 33Pi by cells of strains K10 (○), CE1485 (□), and CE1487 (▵). Cells were grown in Luria broth supplemented with 20 mM glucose and 1 mM G3P to an optical density at 660 nm (OD660) of approximately 0.9, washed, and resuspended in a solution of 20 mM potassium piperazine-N,N′-bis(2-ethanesulfonate) (PIPES) (pH 7.0)–10 mM MgSO4. These cells were stored on ice, and, within 2 h, transport assays were performed at 30°C with 50 μM 33P-labeled potassium phosphate as described previously (27). The experiments were repeated three times with essentially the same results, and data from a representative experiment are shown.
Isolation of a pseudorevertant of pitA pstS strain CE1485.
When CE1485 was plated on synthetic medium plates (25) with Pi as the sole source of phosphate, revertants appeared after overnight incubation. One of these revertants, designated CE1487, grew as efficiently as strain K10 in HPi medium (Fig. 3A), and 33Pi uptake was restored (Fig. 3B). Strain CE1487 was resistant to kanamycin, and the PstS protein could not be detected on Western blots (Fig. 2, lanes 3). Furthermore, sequencing of a PCR-amplified fragment revealed no other differences in the pitA gene besides the characterized single point mutation. Therefore, CE1487 is not a true revertant of strain CE1485 but classifies as a pseudorevertant.
The suppressor mutation in strain CE1487 was mapped by conjugation using a series of Hfr strains carrying Tn10 selection markers and by P1 transduction with an ordered set of transposon insertion mutants as donors (21). The wild-type allele was 37% cotransducible with the metC162::Tn10 marker and 32% cotransducible with the nupG511::Tn10 marker, which are located at min 67.9 and 66.9, respectively, on the chromosomal map (16). Inspection of the genome database (4) revealed a pitA homolog, designated pitB, in this region. PitA and PitB are 81% identical in their amino acid sequences. Thus, a mutation in pitB might be responsible for the restoration of growth of CE1485 on Pi.
To determine whether the pitB gene of the pseudorevertant CE1487 indeed encodes a functional Pi transporter, a 2.2-kb DNA fragment containing pitB and 360 bp of upstream DNA was amplified by PCR using primers pitB8 (5′-CGACCATAAACGGGAATCG) and pitB10 (5′-GCGGTGATGAATCACTGG-3′). The PCR product was ligated into HincII-digested pUC18 (33). Introduction of the resulting plasmid, pSL39, into the pitA pstS strain CE1485 restored growth on synthetic HPi plates. To inactivate pitB on the chromosome, a gentamicin resistance cassette from pBSL142 (2) was inserted into the MluI site in pitB on pSL39, yielding pSL37. AM1095 was transformed with EcoRI- and HindIII-digested pSL37, resulting in pitB::Gm mutant CE1490, and the mutation was transferred to pseudorevertant strain CE1487 by P1 transduction. The resulting strain, CE1491, failed to grow on synthetic HPi plates. Thus, the PitB protein is responsible for the growth of strain CE1487 on Pi as the sole source of phosphate.
Characterization of the pitB mutation in strain CE1487.
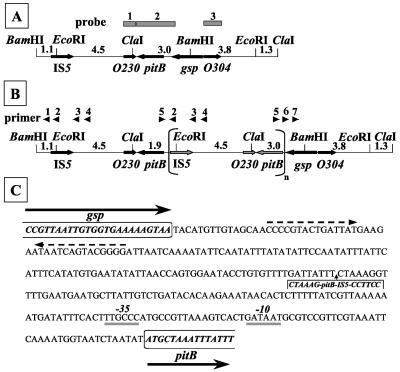
Sequencing the 2.2-kb DNA fragment containing pitB did not reveal any mutation. Therefore, a major chromosomal DNA rearrangement, possibly caused by the presence of an IS5 element near pitB (4) (Fig. 4A), could have affected gene expression. In Southern hybridizations with a pitB probe (probe 2, Fig. 4A), the expected 8.6-kb BamHI and 8.1-kb ClaI fragments were detected in the chromosomal DNA of strain CE1485 (Fig. 5A). In contrast, a much larger BamHI fragment and, besides the expected 8.1-kb fragment, an additional ClaI fragment of approximately 6.5 kb reacted with the probe in the DNA of CE1487 (Fig. 5A). These new hybridizing fragments gave much stronger signals than those obtained with the DNA of strain CE1485 (Fig. 5A), although equal amounts of DNA were used, suggesting that a DNA rearrangement took place in CE1487, resulting in the amplification of a DNA segment.
FIG. 4.
(A and B) Maps of the pitB chromosomal region of strains CE1485 (A) and CE1487 (B). Only the relevant BamHI, ClaI, and EcoRI sites are depicted. At the top of panel A, the probes used for Southern hybridization are indicated. At the top of panel B, the arrowheads indicate PCR primers with the following sequences: pr1, 5′-GGAAGATCGATGCGCTGG-3′; pr2, 5′-CCATTACCAGCCTTGGGG-3′; pr3, 5′-GGGGAAATTCTTCTCGGC-3′; pr4, 5′-GGATATCGTCAGCGGCGC-3′; pr5, 5′-CCTGTGTATATATCAAGGCC-3′; pr6, 5′-CAGGTAACGATGGTGCGG-3′; and pr7, 5′-CCTGCTCGGCACTCTCGG-3′. The numbers between the restriction sites indicate the lengths of the fragments in kilobases. (C) Nucleotide sequence of the pitB-gsp intercistronic region. Coding sequences for the glutathionylspermidine synthetase (Gsp), including the stop codon, and PitB proteins are indicated in bold italics and boxed. Dashed arrows indicate inverted repeats, which may function as the transcriptional terminator of the gsp gene. Putative −35 and −10 sequences of the pitB promoter are indicated. The insertion in strain CE1487 of the amplified DNA fragment containing IS5-pitB is indicated.
FIG. 5.
Southern blot analysis of chromosomal DNA of CE1485 and CE1487. Blots were hybridized with the pitB probe (A) and probe 3 (B) (see Fig. 4). DNA was digested with BamHI or ClaI, and fragments were separated by electrophoresis on a 0.8% agarose gel. The DNA was transferred from the gel to Hybond-N+ membranes (Amersham) with a vacuum blotter (Bio-Rad model 785). After transfer, the filter was washed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate [pH 7.0]) for 5 min, and DNA was cross-linked by UV irradiation for 2 min. Labeling of the probes, hybridization, and detection were done with digoxigenin labeling and detection kits (Boehringer Mannheim). Hybridization and stringency washes were carried out at 68°C. The positions of molecular size standard DNA fragments are indicated in kilobases.
To determine the extent of the DNA rearrangement in strain CE1487, additional hybridizations were performed with probes 1 and 3 (Fig. 4A), which were obtained after PCR amplification of the chromosomal segments. With probe 3, the expected 15.8-kb BamHI and 8.1-kb ClaI fragments were detected in the genomic DNA of both strains with equal intensities (Fig. 5B), indicating that gene O304 was not implicated in the DNA rearrangement. With probe 1, the large BamHI fragment and the 6.5-kb ClaI fragment, which were also detected with the pitB probe, gave strong hybridization signals with DNA from strain CE1487 but were not detected in CE1485 DNA (data not shown). Therefore, like pitB, the O230 gene appears to be amplified in strain CE1487.
To resolve the exact extent of the amplified DNA fragment, PCRs were performed with the primers shown in Fig. 4B. Only the primer combinations pr5-pr2, pr5-pr3, and pr5-pr4 yielded fragments when chromosomal DNA of strain CE1487 was used as the DNA template but not with DNA from strain CE1485. Sequencing of the PCR fragments revealed the fusion point to be located within the pitB-gsp intercistronic region (Fig. 4C). The amplified fragment begins with the sequence GGAAGGTCCGAACAAGTCCT from the IS5 element and ends again in the promoter region of pitB, making it 6.4 kb long (Fig. 4B). Both the increased copy number of pitB (19) and the presence of the IS5 element in the pitB promoter region after the DNA rearrangement could be responsible for the increased expression of pitB in the pseudorevertant strain. Such an IS5-mediated activation of gene expression has been described previously, for example in the cryptic bgl operon of E. coli K-12 (20).
Transport characteristics of PitB.
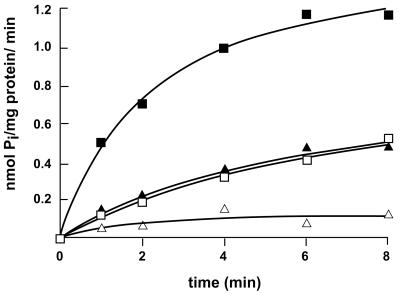
To compare the characteristics of PitA- and PitB-mediated Pi transport, pitB and pitA were PCR amplified with primer couples pitB17 (5′-CGGAATTCATGCTAAATTTATTTGTTG-3′) plus pitB18 (5′-CGCGGATCCTTAAATCAACTGCAATGC-3′) and pit1 (5′-CGGAATTCATGCTACATTTGTTTGC-3′) plus pit2 (5′-CGCGGATCCTTACAGGAACTGCAAGG-3′), respectively, and cloned in pJF118EH (7) under tac promoter control. Introduction of the resulting plasmids, pSL41 and pSL42, respectively, but not of vector pJF118EH enabled pitA pitB pstS strain CE1491, even without isopropyl-β-d-thiogalactopyranoside, to grow on Pi as the sole source of phosphate and to take up 33Pi (data not shown). To determine the PMF dependency of PitB-mediated Pi transport, Pi transport was studied in right-side-out membrane vesicles prepared from strain CE1491 carrying pSL41 or pSL42 as described previously (10). Like PitAmediated 33Pi uptake (data not shown), PitB-mediated 33Pi uptake was inhibited by valinomycin and nigericin, which selectively dissipate the transmembrane potential (Δψ) and transmembrane pH gradient (ΔpH), respectively (Fig. 6). Apparently, PitB activity is dependent on both components of the PMF. In addition, the initial velocities of 33Pi uptake were determined over the first 30 s of linear uptake at Pi concentrations between 4 and 320 μM and the Km value of PitB was determined by direct fitting of the data to the Michaelis-Menten equation (data not shown). The apparent Km for Pi found, 39 μM, is close to the reported value for PitA, 24 to 38 μM Pi (17, 32).
FIG. 6.
Uptake of 33Pi in right-side-out membrane vesicles of strain CE1491 expressing pitB from plasmid pSL41. Membrane vesicles were diluted to a final protein concentration of 0.1 to 0.5 mg of protein/ml in air-saturated 50 mM potassium PIPES (pH 7.0)–10 mM MgSO4. The membrane vesicles were preincubated for 3 min at 30°C with 2 μM pyrroloquinoline quinone in the absence (▵) or presence (■) of 20 mM glucose to generate the PMF and in the presence of glucose in combination with 2.5 μM valinomycin (□) or 2.5 μM nigericin (▴). Transport was initiated on addition of 50 μM 33P-labeled potassium phosphate and analyzed by rapid filtration (27). The experiments were repeated twice with essentially the same results, and data from a representative experiment are shown.
Besides Pi, arsenate is transported by PitA (3). Consequently, pitA mutants are resistant to arsenate. To investigate whether PitB can transport arsenate, various strains were streaked on plates containing synthetic medium (25) supplemented with 660 μM K2HPO4, 1 mM G3P, and 10 mM arsenate. Whereas the pitA mutant strains K10 and CE1485 were able to grow on this medium, growth of the PitB-expressing pseudorevertant strain CE1487 was greatly impaired (data not shown), indicating that PitB is able to transport arsenate.
In conclusion, we demonstrated that expression of a cryptic homolog of pitA, pitB, can be activated in vivo by a DNA rearrangement involving DNA amplification and insertion of IS5 in the promoter region and that this gene encodes a Pi transporter with similar characteristics to PitA. Interestingly, a screening of 34 completely sequenced genomes (http://www.ncbi.nlm.nih.gov/COG) revealed that several other bacteria, including, for example, Pseudomonas aeruginosa, contain more than one PitA homolog.
Acknowledgments
We thank A. Torriani for providing anti-PhoU serum and the Netherlands Culture Collection of Bacteria (NCCB) for providing several plasmids and strains.
This research was supported by the Life Sciences Foundation (A.L.W.), which is subsidized by the Netherlands Organization for Scientific Research (NWO). H.W.V.V. is a fellow of the Royal Netherlands Academy of Arts and Sciences (KNAW).
REFERENCES
- 1.Agterberg M, Fransen R, Tommassen J. Expression of Escherichia coli PhoE protein in avirulent Salmonella typhimurium aroA and galE strains. FEMS Microbiol Lett. 1988;50:295–299. [Google Scholar]
- 2.Alexeyev M F, Shokolenko I N, Croughan T P. Improved antibiotic-resistance gene cassettes and omega elements for Escherichia coli vector construction and in vitro deletion/insertion mutagenesis. Gene. 1995;160:63–67. doi: 10.1016/0378-1119(95)00108-i. [DOI] [PubMed] [Google Scholar]
- 3.Bennett R L, Malamy M H. Arsenate resistant mutants of Escherichia coli and phosphate transport. Biochem Biophys Res Commun. 1970;40:496–503. doi: 10.1016/0006-291x(70)91036-3. [DOI] [PubMed] [Google Scholar]
- 4.Blattner F R, Plunkett III G, Block C A, Perna N T, Burland V, Riley M, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 5.Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–544. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 6.Chan F Y, Torriani A. PstB protein of the phosphate-specific transport system of Escherichia coli is an ATPase. J Bacteriol. 1996;178:3974–3977. doi: 10.1128/jb.178.13.3974-3977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fürste J P, Pansegrau W, Frank R, Blöcker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 8.Hoekstra W P M, de Haan P G, Bergmans J E N, Zuidweg E M. Transformation in E. coli K12: relation of linkage to distance between markers. Mol Gen Genet. 1976;145:109–110. doi: 10.1007/BF00331565. [DOI] [PubMed] [Google Scholar]
- 9.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 10.Kaback H R. Bacterial membranes. Methods Enzymol. 1971;22:99–120. [Google Scholar]
- 11.Levinthal A, Signer E R, Fetherolf K. Reactivation and hybridisation of reduced alkaline phosphatase. Proc Natl Acad Sci USA. 1962;48:1230–1237. doi: 10.1073/pnas.48.7.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lugtenberg B, Meyers J, Peters R, van der Hoek P, van Alphen L. Electrophoretic resolution of the “major outer membrane protein” of Escherichia coli into four bands. FEBS Lett. 1975;58:254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- 13.Ménard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 15.Morita T, Amemura M, Makino K, Shinagawa H, Magota K, Otsuji N, Nakata A. Hyperproduction of phosphate-binding protein, PhoS, and pre-PhoS proteins in Escherichia coli carrying a cloned phoS gene. Eur J Biochem. 1983;130:427–435. doi: 10.1111/j.1432-1033.1983.tb07169.x. [DOI] [PubMed] [Google Scholar]
- 16.Nichols B P, Shafiq O, Meiners V. Sequence analysis of Tn10 insertion sites in a collection of Escherichia coli strains used for genetic mapping and strain constructing. J Bacteriol. 1998;180:6408–6411. doi: 10.1128/jb.180.23.6408-6411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg H, Gerdes R G, Chegwidden K. Two systems for the uptake of phosphate in Escherichia coli. J Bacteriol. 1977;131:505–511. doi: 10.1128/jb.131.2.505-511.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg H, Gerdes R G, Harold F M. Energy coupling to the transport of inorganic phosphate in Escherichia coli K12. Biochem J. 1979;178:133–137. doi: 10.1042/bj1780133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roth J R, Benson N, Galitski T, Kaack K, Lawrence J G, Miesel L. Rearrangements of the bacterial chromosome: formation and applications. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: ASM Press; 1996. pp. 2256–2276. [Google Scholar]
- 20.Schnetz K, Rak B. IS5: a mobile enhancer of transcription in Escherichia coli. Proc Natl Acad Sci USA. 1992;89:1244–1248. doi: 10.1073/pnas.89.4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer M, Baker T A, Schnitzler S M, Deischel M, Goel W, Dove K J, et al. Collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989;53:1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprague G F, Bell R M, Cronan J E. A mutant of Escherichia coli auxotrophic for organic phosphates: evidence for two defects in inorganic phosphate transport. Mol Gen Genet. 1975;143:71–77. doi: 10.1007/BF00269422. [DOI] [PubMed] [Google Scholar]
- 23.Steed P M, Wanner B L. Use of the rep technique for allele replacement to construct mutants with deletions of the pstSCAB-phoU operon: evidence of a new role for the PhoU protein in the phosphate regulon. J Bacteriol. 1993;175:6797–6809. doi: 10.1128/jb.175.21.6797-6809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surin B P, Rosenberg H, Cox G B. Phosphate-specific transport system of Escherichia coli: nucleotide sequence and gene-polypeptide relationships. J Bacteriol. 1985;161:189–198. doi: 10.1128/jb.161.1.189-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tommassen J, Lugtenberg B. Outer membrane protein e of Escherichia coli is coregulated with alkaline phosphatase. J Bacteriol. 1980;143:151–157. doi: 10.1128/jb.143.1.151-157.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torriani A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960;38:460–470. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- 27.Van Veen H W, Abee T, Kortstee G J J, Konings W N, Zehnder A J B. Characterization of two phosphate transport systems in Acinetobacter johnsonii 210A. J Bacteriol. 1993;175:200–206. doi: 10.1128/jb.175.1.200-206.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Veen H W, Abee T, Kortstee G J J, Konings W N, Zehnder A J B. Translocation of metal phosphates via the phosphate inorganic transport system of Escherichia coli. Biochemistry. 1994;33:1766–1770. doi: 10.1021/bi00173a020. [DOI] [PubMed] [Google Scholar]
- 29.Wanner B L. Phosphorus assimilation and control of the phosphate regulon. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1357–1381. [Google Scholar]
- 30.Willsky G R, Bennett R L, Malamy M H. Inorganic phosphate transport in Escherichia coli: involvement of two genes which play a role in alkaline phosphatase regulation. J Bacteriol. 1973;113:529–539. doi: 10.1128/jb.113.2.529-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willsky G R, Malamy M H. Control of the synthesis of alkaline phosphatase and the phosphate-binding protein in Escherichia coli. J Bacteriol. 1976;127:595–609. doi: 10.1128/jb.127.1.595-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willsky G R, Malamy M H. Characterization of two genetically separable inorganic phosphate transport systems in Escherichia coli. J Bacteriol. 1980;144:356–365. doi: 10.1128/jb.144.1.356-365.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]