Summary
Over a quarter of the workforce in industrialized countries does shift work, which increases the risk for cardiometabolic disease. Yet shift workers are often excluded from lifestyle intervention studies to reduce this risk. In a randomized control trial with 137 firefighters who work 24-hour shifts completing (23-59 years old, 9% Female), 12 weeks of 10-h TRE was feasible, with TRE participants decreasing their eating window (baseline: mean 14.13 h, 95% CI 13.78 h to 14.47 h, intervention: 11.13 h, 95% CI 10.73 h to 11.54 h, p =3.29E-17) with no adverse effects, and improved quality of life assessed via SF-36 (ClinicalTrials.gov: NCT03533023). Compared to the standard of care (SOC) arm, TRE significantly decreased VLDL particle size. In participants that had elevated cardiometabolic risks at baseline, there were significant reductions in TRE compared to SOC in glycated Hemoglobin A1C and diastolic blood pressure. For individuals working a 24-hour shift schedule, TRE is feasible and can improve cardiometabolic health, especially for individuals with increased risk.
Keywords: time-restricted eating (TRE), shift work, randomized control trial, glucose homeostasis, cardiometabolic health, quality of life, feasibility
Graphical Abstract
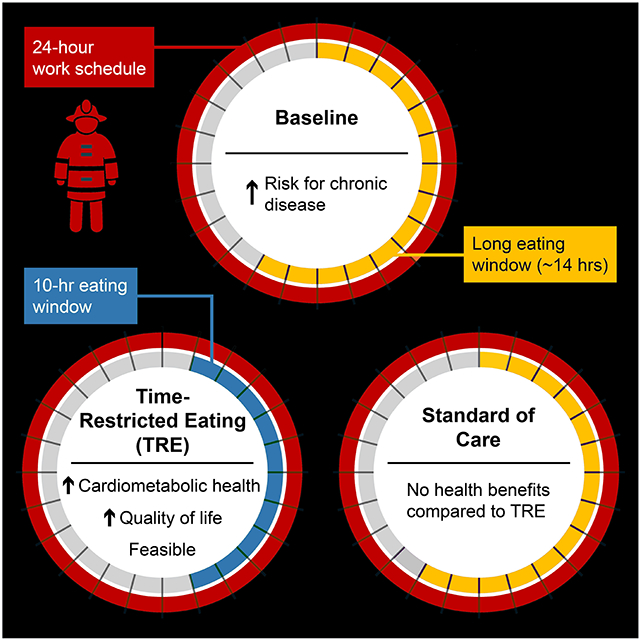
eTOC (In Brief)
Manoogian et al. assessed time-restricted eating (TRE) as a potential lifestyle intervention to improve the health of 24-hour shift workers. TRE was feasible and led to improvements in quality of life and cardiometabolic health, especially among participants that had abnormal cardiometabolic measures at baseline.
Introduction
Nearly 27% of the US workforce work outside the regular work hours and are considered shift workers (CDC and NIOHS, 2015). They include essential frontline workers such as physicians, nurses, police, pilots, and firefighters. These essential workers are the heroes of our society, hence the name The Healthy Heroes Study. Shift workers are frequently subject to erratic light-dark, sleep-activity, and eating-fasting patterns, leading to chronic circadian disruption (Boivin and Boudreau, 2014; Kosmadopoulos et al., 2020; Lunn et al., 2017; Yong et al., 2017). Shift work significantly increases the risk of cardiometabolic diseases (Kecklund and Axelsson, 2016; Kervezee et al., 2020; Sweeney et al., 2020). In addition to career shift workers, a large segment of the population also has a lifestyle that disrupts their circadian rhythms similar to a shift worker in which individuals have to stay awake for 2-3 h between 10 pm and 5 am for at least 50 days a year (IARC, 2010). Care responsibilities and social jet lag also contribute to circadian disruption (Vetter, 2018). Circadian disruption increases the risk for cardiometabolic disease as well as many other chronic diseases including cancer, infection, and autoimmune diseases (Hublin et al., 2010; Leclerc, 2010; Straif et al., 2007).
Despite the increased health risk of shift work, very little research has been done to identify lifestyle interventions to improve the health of this vulnerable population. Shift work or shift work-like lifestyle is frequently an exclusion criterion in lifestyle intervention studies because shift work can be a barrier to following the intervention, and/or be a potential confound to intervention outcomes due to circadian disruption. Due to the nature of the job, requiring wakefulness under light at night, lighting and sleeping opportunities are factors that are difficult to improve. Although access to a healthy diet is difficult, firefighter shift workers can improve diet quality and those who adopt a Mediterranean diet and lifestyle have reduced cardiometabolic disease risks (Romanidou et al., 2020), thus implying shift workers are receptive to pragmatic lifestyle interventions.
Ideal lifestyle intervention for shift workers should be (1) feasible to practice by people with a wide range of health conditions, (2) be compatible with their work schedule, (3) have minimal adverse effects which can impair their work, and (4) be both preventative and therapeutic for cardiometabolic risks. The timing of calorie intake (eating-fasting patterns) is a modifiable lifestyle factor that has the potential to lessen the burden of circadian disruption and support health in shift workers. Time-restricted eating (TRE) is a behavioral intervention that limits the time of daily caloric intake to a consistent window of 6-12 h, without overtly attempting to limit energy intake (Manoogian et al., 2019).
TRE imparts pleiotropic physiological benefits in both pre-clinical studies and pilot clinical trials on non-shift workers (Chaix et al., 2019b; Manoogian et al., 2021a; Rothschild et al., 2014). Clinical trials have demonstrated the cardiometabolic benefits of TRE in adults with elevated weight, prediabetes, and metabolic syndrome (Chow et al., 2020; Cienfuegos et al., 2020; Hutchison et al., 2019; Sutton et al., 2018; Wilkinson et al., 2020), even when already on medications (Wilkinson et al., 2020). These benefits include moderate weight loss, improved beta-cell function, decreased blood pressure, insulin resistance, oxidative stress, and appetite (Chow et al., 2020; Cienfuegos et al., 2020; Hutchison et al., 2019; Sutton et al., 2018; Wilkinson et al., 2020). Although a reduction in energy intake often accompanies TRE intervention in humans, the metabolic benefits are disproportionately larger than what is expected from the modest reduction in energy intake (Wilkinson et al., 2020) and improvements in glycemic regulation were seen without weight loss (Sutton et al., 2018). Furthermore, in contrast to intentional calorie restriction interventions which have a low rate of adherence due to adverse effects and difficulties following a reduced-calorie diet (Trepanowski et al., 2017), TRE participants often report an improved sense of energy, and improved mood (Gill and Panda, 2015). However, as with all behavioral interventions, assessment at baseline and participant engagement are needed. An 8-h TRE (12 pm-8 pm) randomized control trial (RCT) in 116 adults with elevated BMI saw significant reductions in weight within the TRE group, but not within the control group or between groups (Lowe et al., 2020). Yet, there was no assessment of eating time at baseline and 66 of the 116 participants were participating remotely with no contact with the research team. Although adherence was high (92.1% SOC, 83.5% TRE) when reported, it was only reported a fraction of days for both control (22.7%) and TRE arms (22.8%). On the other hand, daily participant engagement and coaching to sustain 25% calorie reduction with or without TRE found significant weight loss and health improvement in patients with obesity, but no difference between arms (Liu et al., 2022). While intentional CR for weight loss may not be an acceptable intervention for firefighters, these findings collectively suggest a need for an RCT with participant engagement to better understand the feasibility and impact of TRE on health outcomes among shift workers.
TRE can improve physical performance, which increases its acceptability for firefighters. Preclinical studies have reported improved endurance, motor coordination, and exercise capacity (Chaix et al., 2019a; Chaix et al., 2014; Hatori et al., 2012). Improved muscle performance and peak power/body weight results have been reported in clinical trials in healthy adults (Moro et al., 2020; Tinsley et al., 2017), whereas others reported weight loss and decreased energy intake with no change in performance (Brady et al., 2021; Tinsley et al., 2017; Tovar et al., 2021). Thus, TRE may potentially improve the health of shift workers without compromising their job performance. Yet, there is no RCT to assess TRE among shift workers.
In this RCT, we assess the feasibility and efficacy of 12 weeks of 10-h time-restricted eating (TRE) as a novel intervention to improve the health of firefighters who work 24-h shifts. We hypothesized that a self-selected 10-h TRE will be feasible among 24-h shift workers. This can be assessed by their ability to significantly reduce their habitual eating window to a level that is known to yield health benefits in published TRE studies on people with elevated cardiometabolic risks (Chow et al., 2020; Wilkinson et al., 2020). As the study cohort contains individuals with varying degrees of health status, we also hypothesized that TRE would result in a modest reduction in cardiometabolic risks between groups and a significant reduction among those with elevated risks.
Results
Time-restricted eating is feasible for firefighters on a 24-h shift
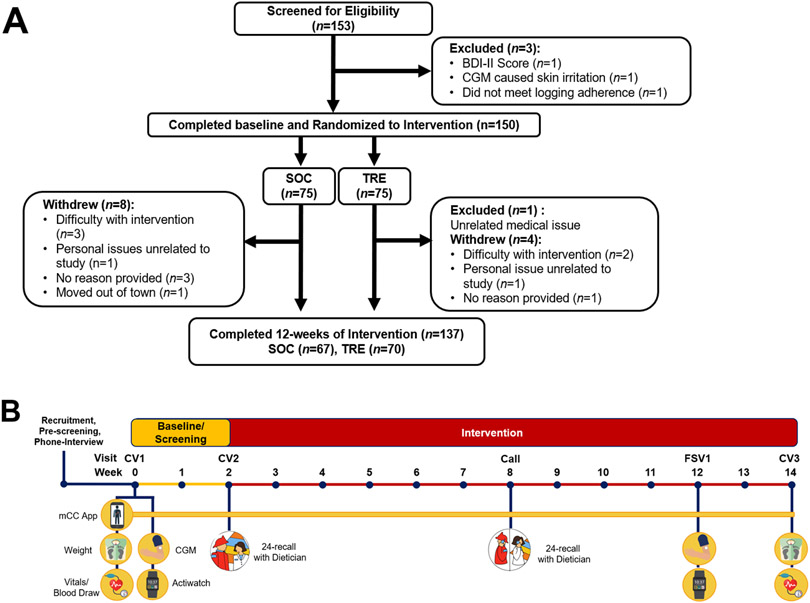
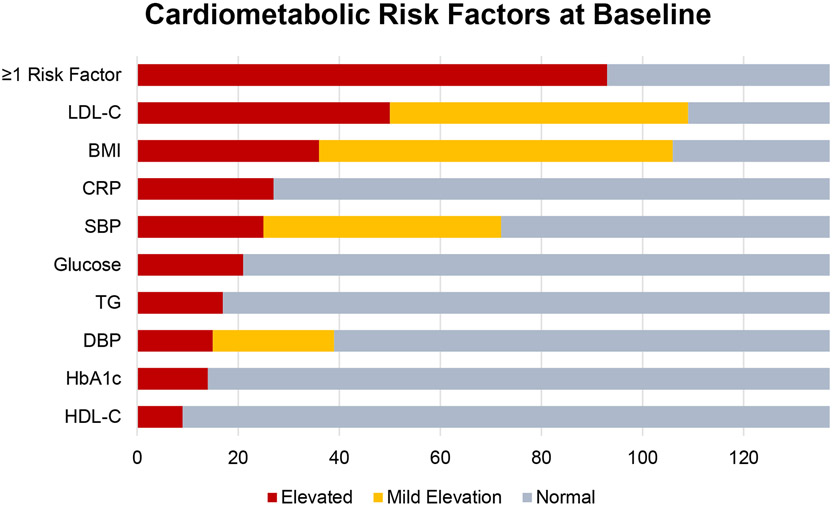
153 participants were screened from a volunteer sample of career first responders from the San Diego Fire-Rescue Department on 24-h shifts (4 excluded, Figure 1). 150 participants (136M/14F, age 21-59 y) were enrolled and randomized to either standard of care (SOC), or TRE (10-h TRE) (Table 1). Both arms were advised to follow a healthy Mediterranean diet, which is acceptable to firefighters (Yang et al., 2015). At baseline, the group averages for various measures of health risk were within a healthy range and were not significantly different between SOC and TRE groups. However, 71% (97/137) of participants had at least one cardiometabolic risk factor including elevated BMI (≥30 kg/m2, n=36), high blood pressure (SBP ≥130 mmHg, n=25 or DBP ≥85 mmHg, n=15), elevated low-density lipoprotein (LDL, ≥ 130 mg/dL, n=49), low high-density lipoprotein (HDL) cholesterol (<40 mg/dl males or <50 mg/dL females, n=9), hyperglycemia (fasting glucose ≥100mg/dL, n=21), elevated triglycerides (≥150 mg/dL, n=17), or elevated CRP (≥2.0 mg/dL, n=27) (Cannon, 2007; Karakas and Koenig, 2009; Ueda et al., 2018) (Figure 2, Table 1).
Figure 1. Consort Diagram and study timeline.
(A) Consort diagram and participants from screening through completion of the 12-week intervention. (B) Timeline of study events during baseline and 12-week intervention. SOC = Standard of Care, TRE = Time-restricted Eating, BDI-II = Beck Depression Inventory-II, CGM = Continuous Glucose Monitor.
Table 1.
Baseline Characteristics (for 137 participants who completed the 12-week intervention)
| Metric | SOC (n=67) | TRE (n=70) | All (n=137) |
|---|---|---|---|
| Age, mean (SD), years | 39.61 (9.39) | 41.07 (8.71) | 40.36 (90.4) |
| Male, n, (%) | 65 (97%) | 60 (84%) | 125 (91%) |
| Female, n, (%) | 2 (3%) | 10 (15%) | 12 (9%) |
| Race, n, (%) | |||
| White | 42 (63%) | 40 (60%) | 82 (60%) |
| Black | 2 (3%) | 3 (4%) | 5 (4%) |
| Asian | 2 (3%) | 3 (4%) | 5 (4%) |
| Hispanic | 9 (13%) | 12 (17%) | 21 (15%) |
| Mixed Race | 2 (3%) | 0 (0%) | 2 (1%) |
| Unknown Race | 10 (15%) | 12 (17%) | 22 (16%) |
| Outcome Measures | |||
| 95% eating window, mean (SD), hours | 13.98 (1.79) | 14.19 (1.39) | 14.09 (1.60) |
| Fasting Glucose, mean (SD), mg/dL | 92.51 (7.45) | 92.31 (7.96) | 92.41 (7.69) |
| HbA1c, mean (SD), % | 5.28 (0.31) | 5.30 (0.40) | 5.29 (0.36) |
| HOMA-IR, mean (SD) | 1.09 (0.71) | 1.13 (0.78) | 1.11 (0.74) |
| Fasting Insulin, mean (SD), mIU/L | 4.70 (2.87) | 4.90 (3.13) | 4.80 (3.00) |
| Prevalence of Cardiometabolic Risk Factors | |||
| BMI ≥ 30 kg/m2, n (%) | 16 (24%) | 20 (29%) | 36 (26%) |
| Fasting Glucose ≥ 100 mg/dL, n (%) | 11 (16%) | 10 (14%) | 21 (15%) |
| HbA1c ≥ 5.7 %, n (%) | 7 (10%) | 7 (10%) | 14 (10%) |
| LDL Cholesterol ≥ 130 mg/dL, n (%) | 25 (37%) | 25 (36%) | 50 (36%) |
| Triglycerides ≥ 150 mg/dL, n (%) | 9 (13%) | 8 (11%) | 17 (12%) |
| HDL Cholesterol <40 mg/dL for males, <50 mg/dL for females, n (%) | 3 (4%) | 6 (9%) | 9 (7%) |
| Systolic Blood Pressure ≥ 135 mmHg, n (%) | 9 (13%) | 16 (23%) | 25 (18%) |
| Diastolic Blood Pressure ≥ 85 mmHg, n (%) | 6 (9%) | 9 (13%) | 15 (11%) |
| CRP ≥ 2.0 mg/dL, n (%) | 12 (18%) | 15 (21%) | 27 (20%) |
| Medications: Type (Drug Names) | |||
| Antihypertensive (Losartan, Olmesartan, Lisinopril, Lisinopril-Hydrochlorothiazide, Atenolol, Metoprolol, Atenolol, Timolol), n (%) | 5 (7%) | 6 (9%) | 11 (8%) |
| Statin, (Crestor, Atorvastatin), n (%) | 4 (6%) | 5 (7%) | 9 (7%) |
| Ezetimibe (Zetia), n (%) | 0 (0%) | 1 (1%) | 1 (1%) |
| Omegas (Omega-3, Fish Oil, Omega-6), n (%) | 14 (21%) | 18 (26%) | 32 (23%) |
| Antidiabetic (Metformin, Jardiance), n (%) | 0 | 2 (3%) | 2 (1%) |
| Melatonin, n (%) | 1 (1%) | 6 (9%) | 7 (5%) |
| Prescription Sleep aid (Ambien), n (%) | 1 (1%) | 0 | 1 (1%) |
Figure 2. Participants with cardiometabolic risk factors at baseline.
All values were taken in a fasted state. Elevated risk: Low-Density Lipoprotein Cholesterol (LDL-C) ≥ 130 mg/dL, BMI ≥ 30 mg/dL, C-Reactive Protein (CRP) ≥ 2.0 mg/dL, SBP ≥ 130 mmHg, Glucose ≥ 100 mg/dL, Triglycerides (TG) ≥ 150 mg/dL, Diastolic Blood Pressure (DBP) ≥ 85 mmHg, Glycated Hemoglobin (HbA1c) ≥ 5.7 %, High-Density Lipoprotein Cholesterol (HDL-C) <40 mg/dL for males, <50 mg/dL for females. Mild Risk: LDL ≥ 100 mg/dL, BMI ≥ 25 mg/dL, SBP ≥ 120 mmHg, DBP ≥ 80 mmHg. Risk factors included elevated risks as described, but not mild risks. Reported for n=137 who completed the study. Also, see Table 3.
All participants used the smartphone app myCircadianClock (Gill and Panda, 2015) to record all ingestion events during 2 weeks of baseline and 12 weeks of intervention. All participants also received study-related nudges and reminders through the app throughout the study. Participants were advised not to change any medication or supplements during the study. Medications are documented in Table 1. 137 participants completed the 12-week guided intervention (67 SOC: 65M/2F, 70 TRE: 59M/11F; 23-59 y) (Figure 1). Among 12 participants who dropped out, 8 were SOC. 2 participants in each arm dropped out due to the burden of the intervention. There was no significant difference in baseline factors in those who dropped out (Table S1).
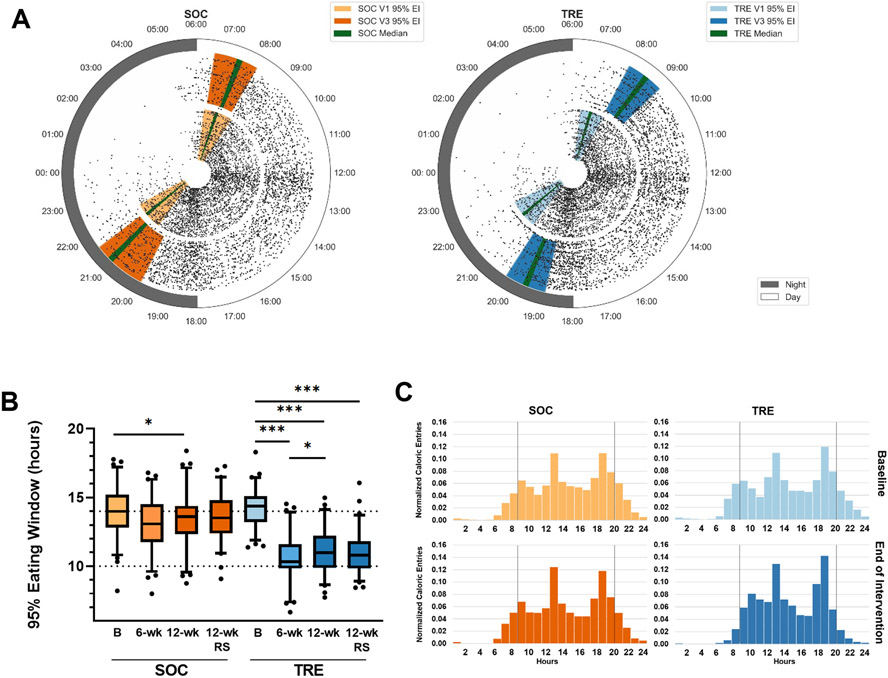
At baseline, both the SOC and TRE arms had a 95% eating window of approximately 14 h (mean: 13.98 h, 95% CI 13.52 h to 14.40 h, and 14.19 h, 95% CI 13.78 h to 14.47 h respectively). The 95% eating window is the 95% interval of time that all caloric items were logged during a given study period. The earliest and latest 2.5% of entries are removed. At baseline, two SOC participants had an eating window of ≤10 h (8.19 h and 9.65 h), and all TRE participants had an eating window of ≥11.37 h. Upon randomization, participants in the TRE arm were advised to select a 10-hour eating window that best suited their work and social life. Participants self-selected a window that began at 9 am (9.01 h, 95% CI 8.85 h to 9.18 h) and ended at 7 pm (18.99 h, 95% CI 18.82 h to 19.15 h). Thus, the TRE cohort chose to fast overnight, and maintain normal meal times, even though they were on 24-h shifts. None of the participants reported any adverse events.
By the end of the intervention, the SOC group maintained a long eating window of 13.35 h (95% CI 12.89 h to 13.82 h). Whereas the TRE group significantly decreased their eating window to 11.13 h, 95% CI 10.73 h to 11.54 h, p=3.29E-17) (Figure 3, Table 2). The eating windows for both SOC and TRE were consistent throughout the intervention with assessments at weeks 5-7 of intervention (6-wk) and randomly sampled throughout the intervention (12-wk-RS) (Figure 3, Table S2).
Figure 3. Changes in temporal eating pattern.
(A) 24-hour plots of all caloric events (15,732 entries) for SOC (left) and TRE (right) during baseline (baseline, inner ring, light orange, and light blue) and the last 2 weeks of the 12-week intervention (outer ring, dark orange, and dark blue). Each participant's data is represented for both baseline and end of intervention. Colored bars represent the 25-75% range of the 95% eating widow in each condition. The green line indicated the median 95% eating window in each condition.
(B) Box plots of the 95% eating window for SOC and TRE at baseline and intervention. TRE (blue) group had a significant decrease in eating window in all conditions compared to baseline. SOC (orange) showed a small decrease in the eating window between V1 and V3. There was no significant difference in the eating window between 6-wk and 12-wk. Baseline=14 consecutive days before the start of intervention), 6-wk = halfway through intervention – 1st day of week 5 through last day of week 6, 12-wk=14 consecutive days before the 12-week follow-up visit, 12-wk-RS = Random sample of 14 days throughout the 12-week intervention. * p<0.05, *** p<0.001. Box and Whisker– median, 25-75% (box) – whiskers 5-95%. Between-group differences were assessed by Mixed ANOVA (Also see Table S2).
(C) Histograms of caloric entries on the mCC app at baseline (Top) and the last 2 weeks of the 12-week intervention (bottom) for SOC (left, orange) and TRE (right, blue). Vertical black lines indicate 8 am to 8 pm for reference. TRE, but not SOC, participants decreased caloric events before 8 am and after 8 pm.
Table 2.
Changes in Health Metrics for All Participants
| Outcome Measure |
SOC | TRE | Between Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Baseline | 12-wk | Change | P-value | N | Baseline | 12-wk | Change | P-value | Change (V3-V1) TRE-SOC |
P-value Time x Group |
|
| 95% eating window, hours | 66 | 13.96 (13.52 to 14.40) | 13.35 (12.89 to 13.82) | −0.60 (−1.08 to −0.13) | 0.013 * | 66 | 14.13 (13.78 to 14.47) | 11.13 (10.73 to 11.54) | −2.99 (−3.51 to −2.47) | 3.29E-17 *** | −2.39 (−3.08 to −1.69) | 3.84E-10 *** |
| Fasting Glucose, mg/dL | 66 | 92.47 (90.62 to 94.32) | 92.61 (90.83 to 94.38) | 0.14 (−1.56 to 1.83) | 0.873 | 70 | 92.31 (90.42 to 94.21) | 92.50 (90.95 to 94.05) | 0.19 (−1.47 to 1.84) | 0.824 | 0.05 (−2.30 to 2.40) | 0.967 |
| HbA1c, % | 66 | 5.28 (5.20 to 5.36) | 5.29 (5.20 to 5.36) | 0.01 (−0.05 to 0.07) | 0.802 | 70 | 5.30 (5.21 to 5.40) | 5.27 (5.20 to 5.33) | −0.04 (−0.1 to 0.03) | 0.290 | −0.04 (−0.13 to 0.05) | 0.340 |
| Fasting Insulin, mIU/L | 66 | 4.71 (4.00 to 5.42) | 4.85 (4.12 to 5.58) | 0.14 (−0.38 to 0.65) | 0.596 | 70 | 4.90 (4.15 to 5.65) | 4.84 (4.27 to 5.41) | −0.06 (−0.68 to 0.57) | 0.855 | −0.19 (−1.00 to 0.61) | 0.635 |
| HOMA-IR | 66 | 1.09 (0.92 to 1.27) | 1.13 (0.95 to 1.31) | 0.04 (−0.09 to 0.17) | 0.576 | 70 | 1.14 (0.95 to 1.32) | 1.12 (0.98 to 1.25) | −0.02 (−0.17 to 0.14) | 0.787 | −0.06 (−0.26 to 0.14) | 0.571 |
| Weight, kg | 66 | 89.29 (85.66 to 92.91) | 88.86 (85.27 to 92.45) | −0.43 (−0.94 to 0.08) | 0.098 | 70 | 87.25 (84.03 to 90.47) | 86.31 (83.08 to 89.54) | −0.94 (−1.46 to −0.42) | 0.0006 *** | −0.51 (−0.14 to 0.21) | 0.163 |
| BMI, kg/m2 | 67 | 27.65 (26.71 to 28.59) | 27.56 (26.64 to 28.48) | −0.09 (−0.28 to 0.11) | 0.371 | 70 | 27.77 (26.92 to 28.63) | 27.52 (26.66 to 28.38) | −0.26 (−0.45 to −0.07) | 0.009 ** | −0.17 (−0.44 to 0.10) | 0.219 |
| Systolic Blood Pressure, mmHg | 67 | 120.32 (118.12 to 122.52) | 119.72 (117.45 to 121.99) | −0.60 (−2.77 to 1.58) | 0.585 | 70 | 120.73 (118.12 to 123.35) | 118.86 (116.43 to 121.30) | −1.87 (−3.85 to 0.11) | 0.063 | −1.27 (−4.18 to 1.63) | 0.387 |
| Diastolic Blood Pressure, mmHg | 67 | 74.21 (72.10 to 76.33) | 72.78 (70.61 to 74.94) | −1.44 (−3.37 to 0.50) | 0.143 | 70 | 74.45 (72.22 to 76.68) | 72.45 (70.35 to 74.58) | −2.00 (−3.93 to −0.07) | 0.043 * | −0.56 (−3.27 to 2.15) | 0.682 |
| HDL Cholesterol, mg/dL | 66 | 55.48 (52.96 to 58.01) | 56.76 (53.83 to 59.68) | 1.27 (−0.70 to 3.25) | 0.203 | 70 | 59.53 (55.17 to 63.89) | 59.66 (55.66 to 63.67) | 0.13 (−1.51 to 1.76) | 0.876 | −1.14 (−3.67 to 1.38) | 0.373 |
| LDL Cholesterol, mg/dL | 66 | 121.47 (113.71 to 129.23) | 118.86 (110.92 to 126.81) | −2.61 (−7.32 to 2.11) | 0.274 | 69 | 122.36 (115.21 to 129.52) | 123.12 (116.15 to 130.08) | 0.75 (−3.54 to 5.05) | 0.727 | 3.36 (−2.95 to 9.67) | 0.294 |
| VLDL Cholesterol Particle Size, nm | 61 | 46.63 (45.91 to 47.35) | 46.39 (45.67 to 47.10) | −0.25 (−0.86 to 0.36) | 0.419 | 69 | 47.50 (46.66 to 48.33) | 46.15 (45.30 to 47.01) | −1.34 (−2.20 to −0.49) | 0.003 ** | −1.10 (−2.16 to −0.03) | 0.044 * |
| HDL particle number, umol/L | 61 | 35.16 (34.26 to 36.07) | 34.12 (33.09 to 35.16) | −1.04 (−1.82 to −0.26) | 0.010 * | 69 | 35.34 (34.47 to 36.22) | 35.34 (34.50 to 36.18) | −0.01 (−0.74 to 0.73) | 0.987 | 1.03 (−0.03 to 2.10) | 0.057 |
| Triglycerides, mg/dL | 66 | 86.67 (72.37 to 100.96) | 83.55 (71.58 to 95.51) | −3.12 (−12.15 to 5.91) | 0.493 | 70 | 94.24 (80.91 to 107.58) | 92.53 (77.04 to 108.02) | −1.96 (−10.89 to 7.47) | 0.711 | 1.41 (−11.37 to 14.19) | 0.828 |
| Body Fat, % | 67 | 23.73 (22.45 to 25.02) | 24.03 (22.86 to 25.21) | 0.30 (−0.19 to 0.80) | 0.229 | 70 | 25.72 (24.29 to 27.16) | 25.65 (24.25 to 27.04) | −0.08 (−0.42 to 0.27) | 0.657 | −0.38 (−0.97 to 0.21) | 0.209 |
| CRP, mg/L | 65 | 1.14 (0.78 to 1.50) | 1.08 (0.72 to 1.44) | −0.06 (−0.56 to 0.45) | 0.821 | 70 | 1.21 (0.89 to 1.53) | 1.13 (0.86 to 1.40) | −0.08 (−0.36 to 0.21) | 0.597 | −0.02 (−0.58 to 0.54) | 0.949 |
| Daily Calories, kcal | 66 | 2268.41 (2129.61 to 2407.21) | 1949.72 (1786.57 to 2112.87) | −318.69 (−531.05 to −106.33) | 0.003 ** | 69 | 2314.28 (2136.09 to 2490.48) | 1898.38 (1720.84 to 2075.92) | −415.91 (−600.87 to −230.94) | 1.27E-5 *** | −97.22 (−375.52 to 181.08) | 0.447 |
| Alcohol, g | 66 | 11.77 (3.87 to 19.67) | 9.50 (4.91 to 14.09) | −2.27 (−11.68 to 7.14) | 0.632 | 69 | 12.23 (6.83 to 17.62) | 6.34 (3.17 to 9.51) | −5.89 (−11.41 to −0.37) | 0.037 * | −3.62 (−14.31 to 7.07) | 0.504 |
| Sleep Duration, mins | 67 | 472.45 (461.61 to 483.29) | 471.65 (460.48 to 482.81) | −0.80 (−10.66 to 9.06) | 0.871 | 69 | 480.70 (469.44 to 491.95) | 478.08 (466.30 to 489.86) | −2.62 (−13.61 to 8.37) | 0.636 | −1.81 (−16.47 to 12.84) | 0.807 |
| Sleep efficiency, % | 67 | 89.78 (89.15 to 90.41) | 89.75 (89.08 to 90.43) | −0.03 (−0.39 to 0.34) | 0.897 | 69 | 90.34 (89.81 to 90.88) | 90.23 (89.68 to 90.82) | −0.07 (−0.49 to 0.29) | 0.616 | −0.04 (−0.06 to 0.05) | 0.782 |
| Daily Activity, A.U. | 67 | 296531.02 (278764.60 to 314297.44) | 286955.79 (269350.11 to 304561.48) | −9575.23 (−22097.98 to 2947.53) | 0.132 | 69 | 295900.53 (279582.45 to 312218.61) | 279797.29 (263712.34 to 295882.23) | −16103.24 (−23741.85 to −8464.63) | 7.76E-5 *** | −6528.02 (−20965.49 to 7909.46) | 0.373 |
Data presented as mean (95% Confidence Interval). Between-group differences were assessed by Mixed ANOVA. Within-group differences were assessed with paired t-tests. p<0.05 (*), p<0.01 (**), p<0.001 (***). Daily calories and alcohol were calculated from a 24-hour recall with a dietician at baseline and between weeks 5-7 of intervention, not the end of the intervention. A.U.= arbitrary units. See Table S4 for data on all lipoprofile measures.
To assess adherence to the self-selected 10-h eating window, a total of 27,982 food and beverage records and their time-stamps collected through the app (not including water or medication) were analyzed at baseline, 6-wk, 12-wk, and 12-wk-RS (Table S2). Participants in both groups logged 85% of days at baseline and 68-77% of days at all time points during the intervention (~5 days/week). Participants in the TRE group consumed a food or beverage item outside of the 10-hour eating window approximately 1-2 days/week throughout the intervention (Table S2).
Glucose regulation
For all participants, parameters reporting glucose homeostasis was in a normal range at baseline and there were no significant changes within or between groups (Table 2). However, multiple factors of glucose homeostasis were improved in participants that started with elevated fasting glucose (≥100 mg/dL) of HbA1c (≥5.7%) at baseline (Table 3 and Table S3). A post hoc analysis of patients with elevated fasting glucose, fasting glucose was significantly decreased within both the SOC (−4.64 mg/dL, 95% CI −8.45 mg/dL to −0.82 mg/dL, p=0.011) and TRE groups (−6.00 mg/dL, 95% CI −11.49 mg/dL to −0.51 mg/dL, p=0.002), but not between groups (Table 3). HbA1c was significantly decreased in the TRE group (n=10, −0.21 %, 95% CI −0.55 % to 0.13 %, p=0.033) and compared to SOC (n=11, 0.10 %, 95% CI −0.16 % to −0.36 %, p=0.170, between groups p=0.012). Fasting insulin was not significantly changed in either arm (Table 3). HOMA-IR (homeostasis model assessment-estimated insulin resistance) was significantly decreased within the TRE group (−0.49, 95% CI −1.12 to 0.15, p=0.013), but not within the SOC group (−0.12, 95% CI −0.48 to 0.25, p=0.300) or between groups (p=0.196) (Table 3).
Table 3.
Changes in health metrics in participants that had abnormal values at baseline
| Outcome Measure |
Value at baseline |
SOC | TRE | Time x Group x Elevated Value (SOC vs TRE) |
Time x Elevated Value (Combined groups, elevated vs normal at baseline) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Baseline | 12-wk | Change 12-wk - Baseline |
P-value Time x Elevated Value |
N | Baseline | 12-wk | Change 12-wk - Baseline |
P-value Time x Elevated Value |
Change (V3-V1) TRE- SOC |
P- value |
P-value | ||
| Fasting Glucose, mg/dL | ≥ 100 mg/dL | 11 | 104.82 (103.07 to 106.57) | 100.18 (95.87 to 104.49) | −4.64 (−8.45 to −0.82) | 0.011 * | 10 | 107.10 (101.75 to 112.45) | 101.10 (97.82 to 104.38) | −6.00 (−11.49 to −0.51) | 0.002 ** | −1.36 (−7.49 to 4.76) | 0.633 | 5.80E-5 *** |
| HbA1c, % | Fasting Glucose ≥ 100 mg/dL | 11 | 5.31 (5.06 to 5.55) | 5.41 (5.21 to 5.61) | 0.10 (−0.16 to 0.36) | 0.170 | 10 | 5.60 (5.02 to 6.18) | 5.39 (5.11 to 5.67) | −0.21 (−0.55 to 0.13) | 0.033 * | −0.31 (−0.70 to 0.08) | 0.012 * | 0.453 |
| Fasting Insulin mIU/L | Fasting Glucose ≥ 100 mg/dL | 11 | 5.91 (2.81 to 9.00) | 5.63 (3.05 to 8.23) | −0.27 (−1.62 to 1.07) | 0.480 | 10 | 6.10 (3.06 to 9.14) | 4.60 (2.87 to 6.33) | −1.50 (−3.77 to 0.77) | 0.059 | −1.23 (−3.63 to 1.17) | 0.287 | 0.053 |
| HOMA-IR | Fasting Glucose ≥ 100 mg/dL | 11 | 1.53 (0.74 to 2.33) | 1.41 (0.75 to 2.08) | −0.12 (−0.48 to 0.25) | 0.300 | 10 | 1.64 (0.79 to 2.49) | 1.15 (0.71 to 1.60) | −0.49 (−1.12 to 0.15) | 0.013 ** | −0.37 (−1.03 to 0.30) | 0.196 | 0.009 ** |
| Weight, kg | BMI ≥ 30 | 16 | 108.07 (100.80 to 115.35) | 107.90 (100.79 t 115.01) | −0.17 (−1.73 to 1.39) | 0.576 | 20 | 103.57 (99.71 to 107.43) | 102.41 (98.41 to 106.41) | −1.16 (−2.51 to 0.19) | 0.601 | −0.99 (−2.97 to 0.99) | 0.444 | 0.971 |
| Systolic Blood Pressure, mmHg | ≥ 130 mm Hg | 9 | 136.30 (132.00 to 140.60) | 127.92 (121.10 t 34.75) | −8.38 (−14.16 to −2.51) | 0.004** | 16 | 135.31 (132.69 to 137.94) | 127.64 (122.67 to 132.62) | −7.67 (−12.48 to −2.85) | 0.001 ** | 0.71 (−6.65 to 8.07) | 0.690 | 1.70E-5 *** |
| Diastolic Blood Pressure, mmHg | ≥ 85 mm Hg | 6 | 89.43 (83.84 to 95.03) | 85.67 (76.48 to 94.86) | −3.77 (−13.27 to 5.74) | 0.455 | 9 | 88.81 (86.15 to 91.48) | 76.67 (70.66 to 82.67) | −12.15 (−17.56 to −6.73) | 2.20E-5 *** | −8.38 (−17.35 to 0.59) | 0.033 * | 9.72E-4 *** |
| LDL Cholesterol, mg/dL | ≥ 130 mg/dL | 25 | 154.32 (146.48 to 162.16) | 148.52 (140.06 to 156.98) | −5.80 (−16.71 to 5.11) | 0.294 | 24 | 154.00 (144.23 to 163.77) | 149.08 (138.30 to 159.87) | −4.92 (−12.54 to 2.70) | 0.054 | 0.88 (−12.12 to 13.88) | 0.589 | 0.037 * |
| VLDL Particle Size, nm | LDL-C ≥ 130 mg/dL | 23 | 45.64 (44.27 to 47.02) | 46.12 (44.51 to 47.72) | 0.48 (−0.71 to 1.67) | 0.063 | 24 | 47.35 (45.49 to 49.22) | 45.59 (44.14 to 47.04) | −1.77 (−3.21 to −0.32) | 0.475 | −2.24 (−4.07 to 0.42) | 0.107 | 0.645 |
| CRP, mg/dL | ≥ 2 mg/dL | 11 | 3.57 (2.15 to 5.00) | 1.55 (0.47 to 2.64) | −2.02 (−4.07 to 0.03) | 2.50E-4 *** | 15 | 3.50 (2.90 to 4.10) | 2.07 (1.01 to 3.12) | −1.43 (−2.37 to −0.50) | 3.51E-8 *** | 0.59 (−1.34 to 2.51) | 0.323 | 2.48E-9 *** |
Post hoc sub-analysis. Data presented as mean (95% Confidence Interval). All p-values were determined via Mixed ANOVA. p<0.05 (*), p<0.01 (**), p<0.001 (***). See Table S3 for additional post hoc sub-analyses.
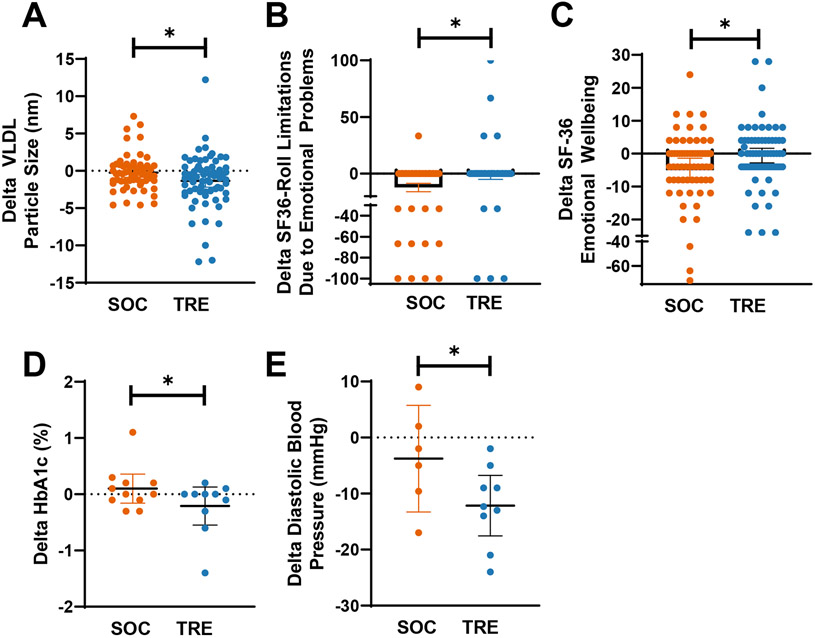
A post hoc sub-analysis based on elevated HbA1c (≥ 5.7%) at baseline, also found a significant decrease in HbA1c within the TRE group (−0.51%, 95% CI −0.97 % to −0.06 %, p=2.14E-7) and compared to the SOC group (−.010 %, 95% CI −0.19 % to −0.01 %, p=0.220; between groups, p=0.003) (Table S3, Figure 4).
Figure 4. Significant changes between TRE and SOC interventions.
Change in values (V3-V1) are shown as scatter plots with mean and 95% CI for SOC (orange) and TRE (blue). The significant difference between groups was assessed via Mixed ANOVA (* indicates p<0.05). (Also see Tables 2, 3, and Table S4 and S5).
A combined post hoc analysis of all SOC and TRE participants found that participants who started with elevated fasting glucose or HbA1c (SOC and TRE, n=21) had a significant decrease in fasting glucose, HbA1c, and HOMA-IR compared to those that had normal glucose levels at baseline (n= 115) (Table 3, Table S3).
Cardiometabolic disease risks
Lipids.
LDL cholesterol and triglycerides were within the respective normal range at baseline and showed no significant differences within or between SOC and TRE groups at the end of the 12-week intervention (Table 2). There were also no significant changes in LDL-particle number (LDL-P), small LDL-P, large HDL-P, large VLDL-P, LDL-P size, or HDL-P size (Table S4).
In all participants that completed the intervention, very-low-density lipoprotein (VLDL) particle size significantly decreased within the TRE group, and compared to SOC (TRE: n=69, −1.34 nm, 95% CI −2.20 nm to −0.49 nm, p=0.003, SOC: n=61, −0.25 nm, 95% CI −0.86 nm to 0.36 nm, p=0.419, between groups p=0.044) (Table 2, Figure 4, Table S4). HDL particle number (HDL-P) significantly decreased in the SOC group (−1.04 umol/L, 95% CI −1.82 umol/L to −.026 umol/L, p=0.010), but not in the TRE group (−0.01 umol/L, 95% CI −0.74 umol/L to 0.73 umol/L, p=0.987) or between groups (p=0.057) (Table 2, Table S4). However, the decrease in the SOC group was not clinically significant.
Blood pressure.
Both the SOC and TRE groups had normal systolic and diastolic blood pressure at baseline (Table 2). Despite being in a normal range before the intervention, diastolic blood pressure was significantly decreased within the TRE group (−2.00 mmHg, 95% CI −3.93 mmHg to −0.07 mmHg, p=0.043), but not within the SOC group or between groups (Table 2).
In a post hoc sub-analysis of participants that had elevated SBP (≥130 mmHg) at baseline, SBP significantly decreased within both the SOC (n=9, −8.38 mmHg, −14.16 mmHg to −2.51 mmHg, p=0.004) and TRE groups (n=16, −7.67 mmHg, −12.48 mmHg to −2.85 mmHg, p=0.001), but not between groups (p=0.690) (Table 3, Figure 4).
Among participants who had a baseline DBP of ≥85 mmHg, a post hoc analysis found that DBP was significantly decreased within the TRE group (n=9, −12.15 mmHg, −17.56 mmHg to −6.73 mmHg, p=2.20E-5) and compared to SOC (p=0.033), but not within SOC (n=6, −3.77 mmHg, −13.27 mmHg to 5.74 mmHg, p=0.455).
In a post hoc sub-analysis of participants with elevated DBP at baseline based on a lower threshold of ≥80 mmHg (SOC, n=17; TRE, n=22), DBP was still significantly decreased within TRE (−7.24 mmHg, 95% CI −11.25 mmHg to −3.24 mmHg, p=1.35E-4), but not within SOC or between groups (Table S3).
Combined, a post hoc analysis of SOC and TRE participants with elevated SBP (≥130 mmHg) or DBP (≥85 mmHg) at baseline displayed a significant decrease in blood pressure (SOC and TRE, SBP: n=25, −7.92 mmHg, 95% CI −11.37 mmHg to −4.47 mmHg; DBP, n=15, −8.80 mmHg, 95% CI −13.61 to −3.98 mmHg) compared to participants that had normal levels at baseline (SOC and TRE, SBP: n=112, +0.24 mmHg, 95% CI −1.24 mmHg to 1.73 mmHg; DBP: n=122, −0.86 mmHg, 95% CI −2.20 mmHg to 0.49 mmHg) (SBP: p=1.70E-5, DBP: p=9.72E-4) (Table 3).
Weight, BMI, and Body Composition.
TRE, but not SOC, had a significant decrease in body weight (TRE: −1.1%, −0.94 kg, −1.46 kg to −0.42 kg p=0.0006; SOC: −0.5%, −0.43kg, 95% CI −0.94 kg to 0.08 kg, p=0.098) (Table 2). Weight change was not significant between groups (p=0.163). BMI was also significantly decreased within the TRE group (−0.26 kg/m2, 95% CI −0.45 kg/m2 to −0.07 kg/m2, p=0.009), but not in the SOC group (−0.09 kg/m2, 95% CI −0.28 kg/m2 to 0.11 kg/m2, p=0.371) or between groups (p=0.219) (Table 2). There were no changes in percent body fat for TRE or SOC compared to baseline or between groups (Table 2).
C-Reactive Protein (CRP).
When all participants were included, CRP was within the normal range at baseline and did not change in either group (Table 2). In a post hoc analysis with participants that started with CRP ≥2.0 mg/dL at baseline, both SOC and TRE participants significantly decreased CRP within group (SOC, n=11, −2.02 mg/dL, 95% CI −4.07 mg/dL to 0.03 mg/dL, p=2.50E-4; TRE, n=15, −1.43 mg/dL, 95% CI −2.37 mg/dL to −0.50 mg/dL, p=3.51E-8) (Table 3). There was no significant change between groups (p=0.323) (Table 3). Combined, these SOC and TRE participants significantly decreased CRP compared to participants that started with normal levels (p=2.48E-9) (Table 3).
Energy intake and activity
24-hour dietary recall.
Daily energy intake was assessed by 24-hour dietary recall with a dietician at baseline and week six of the intervention. Both the SOC (−318.69 kcal, 95% CI −531.05 kcal to −106.33 kcal, p=0.003) and TRE (−415.91 kcal, 95% CI −600.87 kcal to −230.94 kcal, p=1.27E-5) groups significantly decreased their daily calories, but there was no significant difference between groups (p=0.447) (Table 2).
Mediterranean Diet.
From the 24 h dietary recall data for each arm, we identified food names and descriptors for major components of a Mediterranean diet – fruits, vegetables, fish, and olive oil. At baseline in both groups, 34% of food descriptors represented these Mediterranean diet components, which increased to 38% for SOC and 42% for TRE arms at 6 weeks (Table S6).
Alcohol.
The TRE group significantly reduced alcohol consumption at 6 weeks compared to baseline (−5.89 g, 95% CI −11.41 g to −0.37 g, p=0.037), but there was no significant change within SOC (−2.27 g, 95% CI −11.68 g to 7.14 g, p=0.632), or between groups (p=0.504) (Table 2). One alcoholic beverage is approximately 14 grams of alcohol. The TRE group decreased their alcohol intake by 48% from baseline, which was almost half of a drink for a given day. All participants who completed the dietary recall were included in the analysis, but a majority of participants did not have an alcoholic drink at either time point (39 of 66 SOC and 38 of 69 TRE).
Activity.
Daily activity, assessed via actiwatch, was significantly decreased at the end of the 3-month intervention within TRE participants (−16103.24 arbitrary units (A.U.), −23741.85 A.U. −8464.63 A.U., p=7.76E-5) but not within SOC (−9575.23 A.U., −22097.98 A.U. −2947.53 A.U., p=0.132) or between groups (p=0.373) (Table 2).
Sleep duration, efficiency, and self-reported sleep quality
Sleep.
There were no significant changes in daily sleep duration or sleep efficiency (assessed via actiwatch) within or between the SOC or TRE groups (Table 2).
Pittsburg Sleep Quality Index (PSQI).
There are seven categories to assess different aspects of sleep (duration, disturbance, latency, daytime dysfunction, efficiency, overall quality, need for medication, and a total score) with a lower score indicating better sleep (Buysse et al., 1989). The only significant change was a decrease in sleep disturbance at the end of intervention compared to baseline within the TRE group (−0.16, 95% CI −0.30 to −0.02, p=0.027), but not within the SOC group or between groups (Table S5).
Epworth Sleepiness Scale (ESS).
The ESS assesses sleepiness, with a lower score indicating less sleepiness. There were no significant changes in sleepiness, assessed via the ESS for all participants (Table S5). In a post hoc sub-analysis of participants with higher-than-normal daytime sleepiness at baseline (ESS score > 5; SOC: n=54, 10.43, 95% CI 9.49 to 11.36; TRE: n=52, 9.79 CI 8.94 to 10.63), both the SOC (−0.87, 95% CI −1.49 to −0.25, p=0.007) and TRE (−1.10, 95% CI −2.00 to −0.19, p=0.019) within group, and they were not significantly different between groups (p=0.442).
Quality of life
The SF-36 (36-Item Short Form Survey) is a quality-of-life questionnaire with subcategories for both physical and mental health scores (Busija et al., 2011; Ware and Sherbourne, 1992). A higher score indicates better health. The TRE group significantly improved the view of their health at the end of intervention compared to the baseline (+8.46 A.U., 95% CI 2.58 A.U. to 14.33 A.U., p=0.005), but there was no significant difference within SOC or between groups (Table S5). The SOC group had significantly worsened the measures of role limitations due to emotional problems (−12.50, 95% CI −19.84 to −5.16, p=0.001) and emotional well-being (−5.06, 95% CI −8.75 to −1.37, p=0.008) at the end of intervention compared to baseline and compared to TRE (role limitations due to emotional problems: −1.90, 95% CI −8.2 to 4.43, p=0.550; between groups p=0.030; emotional well-being: −0.60, 95% CI −2.80 to 1.60, p=0.588; between groups p=0.036) (Figure 4, Table S5). There were no other significant changes in the SF-36 within or between groups (Table S5).
Discussion
To our knowledge, this is the first RCT of TRE on individuals engaged in 24-h shift work. Shift workers, including firefighters, are at an elevated risk for cardiometabolic diseases (Donovan et al., 2009; Kales et al., 2009; Poston et al., 2011; Soteriades et al., 2011). Yet, shift work and the associated tasks are perceived barriers to the adoption of lifestyle interventions, such as calorie restrictions and sleep extension, that are known to reduce risks for cardiometabolic diseases (Fontana, 2018; Potter et al., 2016; Tobaldini et al., 2018). The primary outcomes of this RCT were to (1) assess the feasibility of a 10-hour TRE intervention in firefighters on a 24-hour shift schedule, and (2) evaluate change in glucose homeostasis in response to the intervention. Secondary outcomes assessed other cardiometabolic health measures and quality of life.
The design and implementation of this study involved the adoption of innovative strategies to overcome the known barriers to shift workers’ participation in intervention studies. An independent advisory committee headed by representatives of the National Fire Protection Association (NFPA) and composed of representatives of San Diego and other national fire departments, the National Institute for Occupational Safety & Health (NIOSH), National Volunteer Fire Council (NVFC), International Association of Firefighters (IAFF), and local firefighter union advised on strategies for recruitment and retention. The study protocol was reviewed and approved by the Compliance Assurance Program Office of the Department of Homeland Security. The study staff did at least one 24-h ride-along at the busiest fire station in San Diego to gain participants’ confidence and to understand their shift work lifestyle. The inclusion/exclusion criteria were developed in consultation with the firefighters to ensure participation was open to most firefighters, including those who may have no cardiometabolic health risk so that participation in the study would not be viewed as a sign of underlying disease condition which could be perceived as compromising their work. Mediterranean diet was advised for all participants (SOC and TRE) because this diet is associated with improved cardiovascular health among firefighters (Romanidou et al., 2020; Sotos-Prieto et al., 2020) and was favored by the independent advisory committee. Clinic visits were coordinated with their Kelly shift schedule and overtime so that the visits did not occur immediately after a 24-h shift to reduce any inadvertent acute effect of the 24-h shift on blood parameters. Instead of group- or individual-in-person sessions typically used to deliver lifestyle intervention, the mCC app was used to deliver health nudges to both SOC and TRE groups throughout the study. Both groups received the same number of messages.
Such study design resulted in participation by firefighters with a wide spectrum of health status with none to multiple risks for cardiometabolic diseases. Thus, it offered a unique opportunity to assess the feasibility of adherence to behavioral lifestyle interventions among participants with a continuum of disease risks.
The health status of dropout participants was not significantly different from the participants who completed the 3 months intervention, thus suggesting the baseline health status did not affect study participation. The dropout rate in the TRE arm was less than that in the SOC group and the participants did not report any adverse effects of 10-h TRE. There were also no reports of TRE compromising participants’ readiness or ability to do their work.
We found that a 10-hour TRE intervention was feasible in firefighters on 24-hour shifts. The TRE group decreased their eating window by 3 hours to 11.13 hours (Table 2). Of days logged, TRE participants only logged entries outside their 10-hour designated eating window approximately 1-2 days a week. Such occasional eating outside the self-selected window was permitted to enable firefighters to eat a late dinner or snack when they attended extended evening- or night-calls. Previous studies found metabolic benefits of TRE in participants with excess body weight or cardiometabolic risk factors with similar adherence. A preclinical mouse model of TRF with restricted feeding limited to 5 days a week (2 days ad lib) (Chaix et al., 2014) and a pilot study in adults with metabolic syndrome with adherence to 10-h TRE 6 of 7 days (Wilkinson et al., 2020) demonstrated significant cardiometabolic benefits of TRE. Thus, we interpret that adherence to TRE for 5 days a week, as seen in our study, is likely sufficient to obtain the benefits of the intervention.
Both the SOC and TRE group received instruction to adhere to a Mediterranean diet which includes increased intake of fruits and vegetables, use of olive oil, and modest alcohol consumption. Analyses of dietary recall records of food descriptors revealed increased consumption of fruits, vegetables, fish, and olive oil. There was a significant decrease in alcohol intake (almost a half drink a day, −5.89 g, 1 drink=14 g) within the TRE group. This is important as decreased alcohol intake can be difficult to achieve and can have widespread health benefits (Haddock et al., 2015; Organization, 2018). Future studies are needed to determine if TRE could be used as a sustainable lifestyle to decrease alcohol consumption.
As seen in many dietary interventions, we also found remarkable changes in activity, sleep, and quality of life. There was a significant decrease in activity within the TRE group (−5%), but not within or compared to SOC (−3%). Similar decreases (−6%) in mean daily activity were seen in other TRE (Wilkinson et al., 2020) and intermittent fasting studies (Betts et al., 2014). However, the activity levels of this cohort of firefighters at both time points were >32% higher than the activity levels in a cohort of non-shift workers (Wilkinson et al., 2020) and there was no report of excessive fatigue or sense of “low-energy” underlying the observed decrease in activity.
There was a significant decrease in self-reported sleep disturbances in the TRE arm, but there were no significant changes to the quality of sleep assessed by the actiwatch. This discrepancy is likely because the actiwatch interprets wrist movement (or lack thereof) for sleep duration and quality, which may not be sensitive enough to assess changes in sleep disturbances or quality of sleep. Change in sleep duration did not change when assessed by self-report or actiwatch. This is not surprising as sleep opportunities are limited for shift workers. However, this demonstrates that health benefits can be achieved without significant increases in sleep duration.
We also found significant changes in quality-of-life scores assessed via the SF-36 questionnaire. The SOC group significantly worsened emotional well-being and resulting role limitations compared to TRE and baseline, whereas the TRE group did not change from baseline (Figure 4, Table S5). This is consistent with findings in a preclinical shift work model that demonstrated that TRF prevented depressive and anxiety-like behaviors (Guerrero-Vargas et al., 2021). Improvements in subjective views of sleep and health are important findings as they improve quality of life and were achieved without altering the work schedule.
Preclinical animal models have shown the negative metabolic impacts of shift-work are due to the mistiming of food intake (Guerrero-Vargas et al., 2018), which, in turn, disrupts circadian regulation of metabolism. Aligning food intake to the habitual wakeful or active period and maintaining a consistent eating window as in TRE can prevent or reverse excessive weight gain and cardiometabolic disease risks in animals (Chaix et al., 2019a). Although the TRE arm could maintain a shorter daytime eating window relative to baseline and SOC, we did not observe significant weight loss and improvement in glucose regulation or cardiometabolic risk factors between groups. The TRE group significantly decreased VLDL size compared to SOC, however, the overall effects on dyslipidemia will require further studies. There was a significant reduction in body weight, BMI, and DBP in the TRE but not in SOC. Such modest changes may be due to group averages of the health parameters at baseline being in the healthy range. This is consistent with preclinical studies in which animals with normal weight and metabolic health see modest benefits, while those with excessive weight or dysmetabolism benefit the most from TRF (Chaix et al., 2014). This preclinical observation prompted us to do subgroup analyses on participants with higher cardiometabolic disease risks. The finding that multiple measures of glucose regulation and cardiovascular health were improved in the subpopulation of participants that began with abnormal levels at baseline compared to those who started with normal levels suggests that TRE may have a greater immediate impact on individuals who are at risk for cardiometabolic disease.
Based on other lifestyle interventions, we predict that the results of the TRE intervention will translate well in the real world. These findings may not only apply to shift workers but would likely extend to a wider population of caretakers and other individuals who experience abnormal sleep-wake patterns. Participants that saw the greatest benefits of both TRE and SOC were older and had at least one risk factor for cardiometabolic disease. Thus, individuals with chronic disease, and likely older, are most likely to see these benefits. There are currently no systematic comparisons for the efficacy of circadian lifestyle interventions to improve health in the real world. The current leading lifestyle intervention to improve cardiometabolic health is the Diabetes Prevention Program (DPP). Weight loss achieved in real-world application of the DPP was about half to one-third of the weight loss reported in the original clinical trials (Dunkley et al., 2014). However, weight, diabetes progression rates, and other cardiometabolic risks were still significantly decreased (Dunkley et al., 2014). Furthermore, the use of mHealth technology (mCC app) in our study to deliver and monitor the intervention makes the TRE intervention both scalable and customizable for shift workers (this study) and non-shift workers (Chow et al., 2020; Hutchison et al., 2019; Phillips et al., 2021; Wilkinson et al., 2020).
Both SOC and TRE reduced their calorie intake by 14% and 18% respectively and improved their intake of fruits and vegetables, which may explain several health benefits relative to baseline shared between groups. Combined with a low dropout rate, the health improvements and lack of adverse events imply that a scalable lifestyle intervention program incorporating TRE and a Mediterranean diet for firefighters is feasible to improve cardiometabolic health and quality of life.
Strengths of Study
Unlike other TRE RCTs, this study used a real-world standard-of-care control (Mediterranean Diet) that is known to have health benefits (Sotos-Prieto et al., 2020). The participants were a representative sample (gender, ethnicity, age, and health status) of firefighters on a 24-hour shift in San Diego County who were generally healthy (BMI <30, normal blood pressure, and cardiometabolic biomarkers) at baseline. Even within this healthy population, cardiometabolic health benefits were observed. Additional benefits were seen in participants that have cardiometabolic abnormalities at baseline. Importantly, there were no adverse effects reported, regardless of health status at baseline. The study also demonstrated that 10-hour TRE is a feasible behavioral intervention for firefighters on a 24-hour schedule without modifying schedules. Another strength of this study is the combination of in-person visits and a mobile health app to implement the intervention and collect continuous assessment of food intake (in real-time).
Limitations of Study
One limitation of this study is that it is a short-term 12-week intervention. To determine if TRE can be both a preventative and treatment for cardiometabolic disease in 24-h shift workers, participants will need to be monitored over an extended period. Additionally, the study population was only 9% female. Although this is a good representation of firefighters (only 4% of 24-hour shift workers in SDFD are female), it is still a limitation of how the study findings can be translated to broader populations. As with many lifestyle interventions, the adherence measurements and some outcomes, such as sleep, were reliant on self-reporting. The participants’ health factors at baseline were heterogeneous with many participants starting with normal levels at baseline. This led to a post hoc sub-analysis of participants that had abnormal health factors at baseline which resulted in small n-values (exploratory outcomes). Similarly, due to the rarity of research on shift workers and firefighters, in particular, we assessed many exploratory outcomes and did not perform corrections for multiple testing as we deemed it more likely to result in false negatives than false positives. This study was also restricted to one shift work schedule in one county in the United States. As shift work schedules can vary greatly across jobs and even between fire departments, additional studies will need to be done to assess the feasibility of TRE for other shift work schedules. There was no control group as both groups were advised to follow a Mediterranean diet. Although this is a limitation, it also shows that the observed benefits of TRE were beyond that of a Mediterranean diet.
Conclusion:
Time-restricted eating is feasible for 24-hour shift workers and may serve as a novel intervention to treat and potentially prevent cardiometabolic disease.
Star Methods
RESOURCES AVAILABILITY
Lead contact.
Further information and requests for resources and data should be directed to and will be fulfilled by the lead contact, Satchidananda Panda (panda@salk.edu).
Materials Availability.
This study did not generate new unique reagents. Specific requests for data sharing will require a Material Transfer Agreement and/or Data Use Agreement and will be managed by the Salk Institute UC San Diego. Both Institutes abide by the Uniform Biological Material Transfer Agreement (UBMTA).
Data and Code Availability
Deidentified participants’ source data “Data S1 – Source Data” has been deposited at Mendeley and made publicly available. Data S1. Unprocessed data reported and underlying the display item in the manuscript, related to Figures 2, 3, and 4 and all health metrics reported. The DOI is listed in the key resources table. mCC app data will not be shared as it could indirectly identify individuals. This paper does not report an original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological Blood Samples | ||
| Human Blood Samples | This study | N/A |
| Critical Commercial Assays | ||
| Comprehensive Metabolic Panel | Center for Advanced Laboratory Medicine (CALM) (La Jolla, CA) | Cat#Cobas8000 (Roche; Indianapolis, IN) |
| Lipoprofile by NMR | ARUP (Salt Lake City, UT) | Cat#2013716, CobasC702 (Roche; Indianapolis, IN) |
| Insulin | ARUP (Salk Lad City, UT) | Cat#00770022, chemiluminescent Immunoassays on an ADVIA Centaur (Siemens Corporation; Washington, DC) |
| Glycated hemoglobin | UCSD Jacobs Medical Center (JMC) (La Jolla, CA) | Cat#Cobas8000 (Roche; Indianapolis, IN) |
| Complete Blood Count | JMC/CALM (La Jolla, CA) | Cat#XN-10 (Sysmex; Chuo-ku, Japan) |
| High sensitivity C-reactive protein | CALM (La Jolla, CA) | Cat#Cobas8000 (Roche; Indianapolis, IN) |
| Deposited Data | ||
| Data S1 - Source Data | This paper | Mendeley: DOI: 10.17632/b6h5kvn5pj.1 |
| Software and Algorithms | ||
| SPSS Statistics | IBM | https://www.ibm.com/products/spss-statistics |
| Prism 8.1.2 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| Analyzing Actiwatch Data: Actiware Software v.6.0.9 | Philips Respironics | Cat#1104775 |
| Other | ||
| Pittsburgh Sleep Quality Index | Buysse et al., 1989 | https://www.cmu.edu/ommon-cold-porject/measures-by-study/health-practices/sleep-habits/psqirev.pdf |
| Epworth Sleepiness Scale | Johns, 1991 | https://epworthsleepinessscale.com/about-the-ess/ |
| Beck Depression Inventory-II | Pearson Assessments | https://www.pearsonassessments.com/store/usassessments/en/Store/Professional-Assessments/Personality-%26-Biopsychosocial/Beck-Depression-Inventory/p/100000159.html |
| 36-Item Short Form Survey (SF-36) | Rand Corporation | https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html |
| Actiwatch Spectrum Plus | Philips Respironics | Cat#1101894 |
| DC-430U Dual Frequency Total Body Composition Analyzer | Tanita (Tokyo, Japan) | Cat#DC-430U |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human Subjects:
This study was conducted with approvals from the institutional review boards at the Salk Institute for Biological Studies (IRB# 18-0001) and the University of California, San Diego (IRB # 172083). The trial was registered at ClinicalTrials.gov (NCT03533023). Methods are also detailed in the study protocol paper (Manoogian et al., 2021b).
Participants and recruitment
All participants provided written informed consent. Participants were recruited from May 8, 2018, through December 18, 2019. To gain familiarity of the study staff with the work biography of 24-h firefighters for participant recruitment, better design study workflow, and improve study participation, the study staff did 24-h ride-along in busy fire stations before and during the recruitment phase. In consultation with the worker union and fire department, participation in this study was not restricted to those with hyperglycemia and/or elevated cardiometabolic risk factors. This was done to avoid any potential perceived stigma of being in a behavior intervention study to address any existing disease condition. As a result, participants included both healthy adults and those with aspects of cardiometabolic risk factors.
All members of the San Diego Fire and Rescue Department (SDFD) who were career first responders and worked 24-hour shifts were invited to participate in the study. See exclusion criteria below. 24 hours shifts started at 8 am (transition to next shift between 7 am – 8 am) for up to 4 days in a row (96 hours). Transitions between shifts start at 7 am.
153 participants were screened and enrolled in the study, and 150 completed the baseline/screening period and were randomized (136M/14F, age 21-59 yr). 137 participants completed the 3-month intervention (67 SOC: 65M/2F; 70 TRE: 59M/11F; 23-59yr) (Figure 1A). No medication adjustments were allowed during the study.
Inclusion Criteria
Firefighter or work a 24-hr shift schedule with San Diego Fire and Rescue
Age: 21-65 years
Own a smartphone (Apple or Android Operating System)
If participants are on cardiovascular medications (HMG-CoA reductase inhibitors (statins), other lipid-modifying drugs (including over-the-counter drugs such as red yeast rice and fish oil), anti-hypertensive, anti-diabetes drugs), no dose adjustments will be allowed during the study period
Exclusion Criteria
Insulin-dependent diabetes mellitus
Presence of acute chronic inflammatory or autoimmune disease (defined by acute symptoms or C-reactive protein >10 mg/L), malabsorption syndromes, liver disease, or kidney disease (stage 3 or greater)
Uncontrolled thyroid disease
Intake of drugs likely to interfere with study endpoints, including corticosteroids, anabolic steroids, anti-psychotics, antiretroviral drugs, and immunosuppressive drugs (within 3 months of starting the study)
Presence or recent history of anemia (hematocrit <33% within 3 months of starting the study)
History of bariatric surgery
Pregnant or breast-feeding women
Current or recent (within 12 months of starting the study) pregnancy or breastfeeding, or intention of becoming pregnant in the next 6 months
Any cancer other than non-melanoma skin cancer in the last 3 years
On a special or prescribed diet for other reasons (e.g., Celiac disease)
Depression is determined by the Beck Depression Inventory (BDI)
Planned international travel during the study period
Insufficient logging on the mCC app (does not log at least 2 entries a day for 10 of 14 days) during baseline will exclude from being randomized into the intervention period
METHOD DETAILS
Study Design
The Healthy Heroes study is a 1:1 RCT with 150 career firefighters on 24-hour shifts from San Diego Fire and Rescue. All participants completed a 2-week baseline/screening period during which they wore a continuous glucose monitor (CGM), and an actiwatch, and logged all food intake using the myCircadianClock (mCC) smartphone application. At the first visit, participants also completed a fasting blood draw, vitals, and questionnaires. At the end of the 2-week baseline/screening, participants were randomized to a 12-week intervention of either Standard of Care (SOC) or SOC and 10-h TRE (will be referred to as TRE). Randomization was done by the statistician, who did not interact with participants, via block randomization design. To avoid selection bias, two different block sizes of 4 and 8 were used at random to produce a randomization table prior to the start of the study (Efird, 2011). The SOC group received nutritional counseling and was instructed to follow a Mediterranean diet. The Mediterranean diet encourages eating meals with family or friends, uses olive oil as the main source of fat, and is comprised mainly of plant-based foods. Dairy, poultry, fish, and alcohol are allowed in moderation, and consumption of red meat and sweets are limited (Davis et al., 2015). The general goals for participants were 60% carbohydrate (whole grain, fruits, vegetables, beans, and legumes), 15% protein (Fist and seafood at least twice a week; yogurt, cheese, poultry, and eggs), and 25% fat (olive oil, canola oils, tree nuts, peanuts, avocado, and seeds). The TRE group received the same nutritional counseling and additionally were instructed to select a consistent 10-h eating window for all days of the week to consume all food and beverages (except water) for the duration of the 3-month intervention. Participants were allowed to consume caffeine (no cream, sugar, or artificial sweeteners) outside of their eating window as needed to stay alert and carry out their tasks at work. Participants were not prohibited from any emergency deployment within or outside the San Diego area but were instructed to notify the study staff if they were deployed outside San Diego during the study period as changing time zones could impact outcomes. All baseline measures were repeated at the end of the 12-week intervention.
Outcomes
There are two primary outcomes: (1) feasibility and adherence to TRE and (2) the impact of TRE on glucose homeostasis. Feasibility and adherence are measured as a percentage of days logged in which participants did not eat outside of their 10-h eating window. Changes in glucose homeostasis are assessed by fasting blood work. Abnormal glucose parameters such as elevated fasting glucose or HbA1c were not an inclusion criterion and thus, significant changes in glucose regulation are only expected in participants that have compromised glucose homeostasis at baseline. Glucose regulation was evaluated via fasting blood measures of glucose, HbA1c, Insulin, and HOMA-IR (Fasting Insulin*Fasting Glucose/405) (Rivas-Crespo, 2015). All participants were assessed for overall changes and any adverse events by speaking to the research team at each visit. Participants were also instructed to reach out to the research team if they experienced any adverse events. A post hoc sub-analysis was done among those who had compromised cardiometabolic health metrics at baseline.
Secondary outcomes include (1) changes in metabolic biomarkers in response to TRE including HDL cholesterol, LDL cholesterol, lipid sub particle numbers by NMR (Lipofit by NMR), Triglycerides, C-reactive Protein (CRP), (2) Systolic Blood Pressure, (3) Diastolic Blood Pressure, and (4) Body weight. Other outcomes include Body Mass Index (BMI), percent body fat (via Tanita Scale DC 430U; Tokyo, Japan), and questionnaires (quality of life, sleep, and health). For all outcomes, significant changes are only expected in participants that start with levels outside of the normal range at baseline.
In-Person Visits
Participants visited the Altman Clinic for Translational Research Institute (ACTRI) at the University of California San Diego Medical School for all clinic visits (CV1, 2, and 3). At CV1, participants had a fasting blood draw, completed questionnaires, and height, weight, blood pressure, waist, and hip circumference, and body composition (Tanita scale) were measured. They wore an actiwatch and CGM for two weeks and were trained to use the mCC app to log food. After 2 weeks, at CV2 participants were randomized to either SOC or TRE, completed questionnaires, and met with a dietician to complete a 24-hour dietary recall and receive nutritional counseling on the Mediterranean Diet. All participants were given a Mediterranean Diet cookbook. Participants in the TRE arm also self-selected a 10-h eating window with guidance from the research team. This initiated the 12-week monitored intervention. Six weeks into intervention (Study week 8). Participants had a phone call with the dietician to complete a second 24-hour dietary recall and received nutritional counseling. All food and beverage entries on the mCC app were provided to the dietician to aid in a 24-hour recall assessment. Two weeks prior to the end of the 12-week intervention (CV3), a member of the research team visited the fire station to provide an actiwatch and apply CGM to participants. Participants returned to the clinic at the end of the 12-week intervention to return the actiwatch and CGM and repeat all questionnaires and measures taken at CV1.
Due to the COVID-19 pandemic, a few of the clinic visits (3 out of 440) were moved from the ACTRI to the San Diego Fire Department Wellness center from April-July 2020. In place of fire station visits, participants were mailed the actiwatch and CGM, and zoom calls were used to help participants to apply the CGM themselves.
Blood Measurements
All blood samples were taken in a fasted state and performed by standard venipuncture. All participants were instructed to fast for at least 12 hours before blood draws. All blood samples were taken between 7 am – 11 am and taken at approximately the same time at baseline and the end of intervention for each participant. The following tests were performed at UCSD ACTRI: comprehensive metabolic panel, complete blood count, hemoglobin A1c (HbA1c), lipid panel, lipoprofile by nuclear magnetic resonance, insulin, and high-sensitivity C-reactive protein (hs-CRP or CRP).
Body Composition
Body fat percentage was obtained from the Tanita Scale DC 430U (Tokyo, Japan).
Eating Pattern Monitoring: The myCircadianClock (mCC) app
All participants used the mCC app (Gill and Panda, 2015) throughout the 14 weeks of baseline/screening and monitored intervention to log all food and beverages (except water). Food entries could be logged as a photo with a short annotation or as an annotation only. Participants in the TRE arm set their daily eating window on the app and had the option to set alerts for the beginning and end of their eating window.
The mCC app was customized with push notifications (nudges) for the SOC and TRE groups to provide reminders and educational materials to help guide firefighters throughout the study and improve adherence and retention (ex. “Tracking new habits is an effective way to help you maintain it.” Or “The Mediterranean Diet has been shown to decrease inflammation and promote a healthy digestive tract.”). Both groups received the same number of push notifications and most were identical. Approximately a quarter of the notifications also included information about circadian rhythms or eating time for the TRE group only (ex. “Keeping a regular eating window of about 10 h provides a healthy daily fasting interval and allows your body to prepare for digestion.”). The mCC app is available for both iPhones and Android and uses HIPAA-compliant Amazon Web Server (AWS) for server-side operations.
Logging and intervention adherence was monitored 2-3 times per week throughout the study. If participants had insufficient logging (logging less than 2 items over a 5-hour interval or more for 2 or more days), they were contacted via push notification through the mCC app, email, text, or telephone.
Wrist-worn Actigraphy (Actiwatches)
The Phillips accelerometer, Spectrum Plus™, was used to passively measure sleep and activity. Participants wore the actiwatch on their non-dominant arm for two weeks at a time during the study period (baseline and the last 2-weeks of the 12-week intervention). This data was used to measure sleep onset, sleep duration, sleep efficiency, and daily activity.
Questionnaires
Four questionnaires were used in this study. (1) Beck Depression Inventory-II (BDI-II), to screen for depression; (2) Short Form-36 Quality of Life (SF-36, developed at RAND as part of the Medical Outcomes Study (Busija et al., 2011; Ware and Sherbourne, 1992), to measure overall functional health score as well as separate physical and mental health dimension components; (3) Epworth Sleep Scale (ESS), to assess daytime sleepiness (Johns, 1991); and (4) Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989) to evaluate sleep in seven major areas – subjective sleep quality, sleep latency, sleep duration, sleep disturbances, sleep efficiency, use of sleeping aids, and daytime dysfunction. The BDI-II and SF-36 were given at CV1 and CV3 and the ESS and PSQI were given at CV2 and CV3.
Actigraphy Analysis
Philips Respironics Actiwatch Plus (van Wouwe et al., 2011) was used to assess sleep and activity during baseline and the last 2-weeks of intervention. Data were analyzed using Philips Actiware 6 software. Daily sleep duration and sleep efficiency were used to assess changes in sleep. Total activity counts were used to assess changes in activity.
Continuous Glucose Monitors
Participants wore an Abbott Freestyle LibrePro continuous glucose monitor (CGM) for 14 days during baseline and the last 14 days of the 12-week intervention (not reported in this manuscript). The CGM reading along with any self-report of lightheadedness or dizziness was intended to identify any potential hypoglycemia event.
QUANTIFICATION AND STATISTICAL ANALYSIS
Adherence Calculation
Adherence was assessed during baseline (B, 2 weeks leading up to CV2), weeks 5-7 of the intervention (6wk), the last 2 weeks of the 12-week intervention (12-wk), and randomly sampled 14 days throughout the 12-week intervention (12-wk-RS). For the TRE group, adherence to the self-selected 10-h eating window was assessed by the number of days in which a participant consumed a caloric item more than 15 mins outside of their self-selected 10-h eating window.
Eating Window Calculation
The eating window was calculated as the time window in which 95% of all calorie-containing food and beverage entries were logged on the mCC app during a designated interval (Gill and Panda, 2015): baseline, 6-wk, 12-wk, and 12-wk-RS. The earliest and latest 2.5% of entries were removed. Of the remaining entries, the earliest entry marked the beginning of the eating window and the latest entry as the end of the eating window.
Nutrition analysis
Calorie and alcohol intake were captured during a 24-hour recall with a dietician at the end of baseline and between weeks 5-7 of the intervention.
Statistical analysis
Data was collected in a pre-post design. Data from all participants who completed the 12-week assessments were included in the analysis. Participants that dropped out of the study and only had data from baseline were not included in the analysis. Baseline characteristics of participants that dropped out were assessed for randomness, and participants with elevated cardiometabolic risk factors at baseline, using a chi-square test for categorical data (sex and ethnicity) and independent t-tests for nominal data. For Independent T-tests, Levene’s Test for Equality of Variances was used to determine if equal variance should be assumed.
A general linear model for mixed design (Mixed ANOVA) was used to analyze differences between groups when there were two or more between-subject factors. All Mixed ANOVAs were run with one within-group factor of Time with 2 levels (Baseline and 12-weeks) for all analyses. For comparison between intervention groups (Table 2), there was one between-group factor of Intervention group (TRE or SOC). Differences were considered statistically significant provided a p-value of 0.05 or less was obtained. Post hoc sub-analyses were also performed with Mixed ANOVA to assess changes in participants who had health factors out of the normal range at baseline. For within intervention group analysis, there was one between-subjects factor of Elevated Value at Baseline (Elevated and Normal). For analysis between intervention groups, there were two between-subject factors, Elevated Value at Baseline and Intervention Group (SOC and TRE). For analysis of combined groups (all participants), there was one between groups factor of Elevated Value at Baseline (Table 3). Paired T-tests were used to compare within-group changes when there were no between-group factors. No corrections were made for multiple comparisons of primary or secondary outcomes. Data were analyzed using SPSS version 26 (IBM, USA).
Sample Size Estimation
Due to a lack of TRE RCT of similar length and because there has been no TRE RCT in shift workers, weight was used as an outcome for calculating sample size rather than glycemic measures. G*Power software was used to calculate the required sample size for the mixed model approach using the RMASS program provided by Hedeker (http://tigger.uic.edu/~hedeker/ml.html). The sample size of 150 provides a minimum power of 80% to detect a medium effect size for the primary hypotheses. For the mixed model, the medium effect size (based on previous studies) is defined as a between-group difference increasing linearly from 0 at baseline to .5 SD units at the last time point. The minimum power estimation is based on sample size calculation for 10% and 20% attrition, correlations of 0.2, 0.5, and 0.8 between the repeated measures, and for medium and large effect sizes.
Missing Data
Missing data were examined for randomness. The pattern of missing data was examined by comparing group differences in the primary outcomes of subjects with versus without missing data as recommended by Little and Rubin (Little and Rubin, 2019). This allows the inclusion of subjects with missing data or those who terminated the study early, without relying on data imputation procedures.
Excluded Participants and Data
Excluded Participants.
Four participants were excluded from the study (3 during baseline/screening and 1 after randomization).
Score on BDI-II.
Did not meet logging adherence during baseline/screening.
Skin irritation in response to CGM.
Unrelated medical issue (after randomization).
Excluded and Missing Data
| Category | Participant | Time Point | Data missing/excluded | Reason |
|---|---|---|---|---|
| Blood Test | HH-007 | V3 | Fasting blood work. | Did not complete a blood draw because not fasting at V3. |
| Blood Test | HH-096 | V1 | CRP | Excluded because the participant was recovering from flu at V1. Led to abnormally high CRP levels. |
| Blood Test | HH-006 | V1 and V3 | LDL cholesterol (V1 and V3), HDL particle number (V1), Lipoprofile (V3) | Lab error |
| Blood Test | HH-010 | V1 and V3 | Lipoprofile | Lab error |
| Blood Test | HH-023 | V3 | Lipoprofile | Lab error |
| Blood Test | HH-036 | V3 | Lipoprofile | Lab error |
| Blood Test | HH-037 | V1 and V3 | Small LDL particle number | interference |
| Blood Test | HH-041 | V1 | Large VLDL Particle Number | interference |
| Blood Test | HH-053 | V1 and V3 | Large HDL Particle Number | interference |
| Blood Test | HH-066 | V1 | Lipoprofile | interference |
| Blood Test | HH-067 | V1 | Lipoprofile | interference |
| Blood Test | HH-086 | V1 and V3 | Small LDL particle number | interference |
| Vitals | HH-002 | V3 | Waist circumference | Measure not taken at the visit. |
| Eating window | HH-052 | 6-wk | Eating Duration | Excluded: not enough days logged |
| Eating window | HH-140 | 6-wk | Eating Duration | Excluded: not enough days logged |
| Eating window | HH-024 | 6-wk | Eating Duration | Excluded: not enough days logged |
| Eating window | HH-129 | 6-wk and 12-wk | Eating Duration | Excluded: not enough days logged |
| Eating window | HH-078 | 12-wk | Eating Duration | Excluded: not enough days logged |
| Eating window | HH-125 | 12-wk | Eating Duration | Excluded: not enough days logged |
| Eating window | HH-110 | 12-wk | Eating Duration | Excluded: not enough days logged |
| Eating window | HH-034 | 12-wk | Eating Duration | Excluded: not enough days logged |
| Dietician Recall | HH-083 | Baseline | 24-hour Dietary Recall | Did not complete. |
| Dietician Recall | HH-112 | 6-wk | 24-hour Dietary Recall | Did not complete. |
| SF-36 | HH-037 | V1 | SF-36 Health change | did not answer the question |
| SF-36 | HH-079 | V1 | SF-36 Health change | did not answer the question |
| SF-36 | HH-140 | V1 | SF-36 Health change | did not answer the question |
| SF-36 | HH-130 | V3 | SF-36 | Excluded: broke their leg while surfing which led to limitations outside the experiment |
| SF-36 | HH-009 | V3 | SF-36 Health change | did not answer the question |
| SF-36 | HH-131 | V3 | SF-36 Health change | did not answer the question |
| SF-36 | HH-077 | V3 | SF-36 | did not complete |
| SF-36 | HH-017 | V3 | SF-36: role limitations due to emotional problems and emotional well-being | Exclude: family member recently passed away |
| PSQI | HH-032 | V1 | PSQI Sleep Quality | didn't answer the question |
| PSQI | HH-032 | V1 | PSQI total | could not calculate because of missing data |
| PSQI | HH-048 | V1 | PSQI Disruption | did not answer the question |
| PSQI | HH-048 | V1 | PSQI total | could not calculate because of missing data |
| PSQI | HH-072 | V1 | PSQI Disruption | did not answer the question |
| PSQI | HH-072 | V1 | PSQI total | could not calculate because of missing data |
| PSQI | HH-109 | V1 | PSQI Days Dys | didn't answer the question |
| PSQI | HH-109 | V1 | PSQI total | could not calculate because of missing data |
| PSQI | HH-130 | V1 | PSQI (all categories) | did not complete |
| PSQI | HH_105 | V1 | PSQI duration | did not answer the question |
| PSQI | HH_105 | V1 | PSQI HSE (sleep efficiency) | did not answer the question |
| PSQI | HH_105 | V1 | PSQI total | could not calculate because of missing data |
| PSQI | HH-018 | V1 | PSQI duration | did not answer the question |
| PSQI | HH-018 | V1 | PSQI HSE (sleep efficiency) | did not answer the question |
| PSQI | HH-018 | V1 | PSQI total | could not calculate because of missing data |
| PSQI | HH-024 | V1 | PSQI duration | did not answer the question |
| PSQI | HH-024 | V1 | PSQI HSE (sleep efficiency) | did not answer the question |
| PSQI | HH-024 | V1 | PSQI Latency | did not answer the question |
| PSQI | HH-024 | V1 | PSQI total | could not calculate because of missing data |
| PSQI | HH-063 | V1 | PSQI duration | did not answer the question |
| PSQI | HH-063 | V1 | PSQI HSE (sleep efficiency) | did not answer the question |
| PSQI | HH-063 | V1 | PSQI total | could not calculate because of missing data |
| PSQI | HH-131 | V1 | PSQI duration | did not answer the question |
| PSQI | HH-131 | V1 | PSQI HSE (sleep efficiency) | did not answer the question |
| PSQI | HH-131 | V1 | PSQI total | could not calculate because of missing data |
| PSQI | HH-032 | V3 | PSQI Sleep Quality | didn't answer the question |
| PSQI | HH-032 | V3 | PSQI total | could not calculate because of missing data |
| PSQI | HH-051 | V3 | PSQI Disruption | did not answer the question |
| PSQI | HH-051 | V3 | PSQI total | could not calculate because of missing data |
| PSQI | HH-077 | V3 | PSQI (all categories) | did not complete |
| PSQI | HH-094 | V3 | PSQI (all categories) | did not complete |
| PSQI | HH-124 | V3 | PSQI duration | did not answer the question |
| PSQI | HH-124 | V3 | PSQI HSE (sleep efficiency) | did not answer the question |
| PSQI | HH-124 | V3 | PSQI total | could not calculate because of missing data |
| PSQI | HH-133 | V3 | PSQI Disruption | did not answer the question |
| PSQI | HH-133 | V3 | PSQI total | could not calculate because of missing data |
| ESS | HH-048 | V1 | ESS | Lost |
| ESS | HH-130 | V1 | ESS | Lost |
Supplementary Material
Data S1. Unprocessed data reported and underlying the display item in the manuscript, related to Figures 2, 3, and 4 and all health metrics reported.
Highlights.
10-hour time-restricted eating is feasible for shift workers on a 24-hour schedule.
TRE improved very-low-density lipoprotein size and quality of life measures.
TRE decreased HbA1c and blood pressure for participants with cardiometabolic risk.
A consistent 10-hour eating window (TRE) had no adverse effects.
Acknowledgments
The authors would like to thank all of the participants and the San Diego Fire-Rescue Department for their support and aid throughout the study. We thank David Picone (Battalion Chief), Kyle O’Neill (Cancer and Health Coordinator), Brent Brainard (Captain/Wellness Officer), Kurtis Bennett (Health and Safety Director), Jeri Miuccio (Local 145), Chief Brian Fennessy (Former SDFD Fire Chief, when the study was initiated), and Chief Colin Stowell (SDFD Fire Chief) for their guidance and support for the study and ongoing assistance with coordinating the recruitment of study participants. The study was funded by FEMA-EMW-2016-FP-00788. E.N.C.M. was supported by the Larry L. Hillblom Foundation Postdoctoral Fellowship and Salk Women in Science Fellowship. P.R.T is supported by (DK118278), and S.P. is supported by (CA 258221 and CA236352), Robert Wood Johnson Foundation, Wu Tsai Human Performance Alliance and the Joe and Clara Tsai Foundation, William Doner Foundation, and Mack Foundation.
Inclusion and Diversity Statement
We worked to ensure gender balance in the recruitment of human subjects. We worked to ensure ethnic or other types of diversity in the recruitment of human subjects. We worked to ensure that the study questionnaires were prepared in an inclusive way. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
All authors have no disclosures concerning the manuscript. Affiliations are provided for all authors at the time the work was done. P.R.T is a consultant for Sanofi/Regeneron, Novo-Nordisk, Boehringer-Ingleheim, Janssen, Pfizer, Amarin, and Amgen. She is a stockholder of Cardero Therapeutics. S.P. is a consultant for Hooke London, the author of The Circadian Code and The Circadian Diabetes Code.
Additional Resources
Clinicaltrial.gov registration: NCT03533023, https://clinicaltrials.gov/ct2/show/study/NCT03533023?term=healthy+hero&draw=2&rank=1
Protocol Paper (Manoogian et al., 2021b): (doi: 10.1136/bmjopen-2020-045537): https://bmjopen.bmj.com/content/11/6/e045537
References.
- Betts JA, Richardson JD, Chowdhury EA, Holman GD, Tsintzas K, and Thompson D (2014). The causal role of breakfast in energy balance and health: a randomized controlled trial in lean adults. Am J Clin Nutr 100, 539–547. ajcn.114.083402 [pii] 083402 [pii] 10.3945/ajcn.114.083402 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin DB, and Boudreau P (2014). Impacts of shift work on sleep and circadian rhythms. Pathol Biol (Paris) 62, 292–301. 10.1016/j.patbio.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Brady AJ, Langton HM, Mulligan M, and Egan B (2021). Effects of 8 wk of 16:8 Time-restricted Eating in Male Middle- and Long-Distance Runners. Medicine and science in sports and exercise 53, 633–642. 10.1249/mss.0000000000002488. [DOI] [PubMed] [Google Scholar]
- Busija L, Pausenberger E, Haines TP, Haymes S, Buchbinder R, and Osborne RH (2011). Adult measures of general health and health-related quality of life: Medical Outcomes Study Short Form 36-Item (SF-36) and Short Form 12-Item (SF-12) Health Surveys, Nottingham Health Profile (NHP), Sickness Impact Profile (SIP), Medical Outcomes Study Short Form 6D (SF-6D), Health Utilities Index Mark 3 (HUI3), Quality of Well-Being Scale (QWB), and Assessment of Quality of Life (AQoL). Arthritis Care Res (Hoboken) 63 Suppl 11, S383–412. 10.1002/acr.20541. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, and Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Cannon CP (2007). Cardiovascular disease and modifiable cardiometabolic risk factors. Clin Cornerstone 8, 11–28. 10.1016/s1098-3597(07)80025-1. [DOI] [PubMed] [Google Scholar]
- CDC, and NIOHS (2015). Adjusted Prevalence of Shift Work (Any Alternative Shift) among Currently Employed Workers by Industry. https://wwwn.cdc.gov/Niosh-whc/chart/ohs-workorg?T=I&OU=WORKSCHDRCD&V=R2&chkcodes=False.
- Chaix A, Lin T, Le HD, Chang MW, and Panda S (2019a). Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab 29, 303–319 e304. 10.1016/j.cmet.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Manoogian ENC, Melkani GC, and Panda S (2019b). Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu Rev Nutr 39, 291–315. 10.1146/annurev-nutr-082018-124320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, and Panda S (2014). Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20, 991–1005. 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow LS, Manoogian ENC, Alvear A, Fleischer JG, Thor H, Dietsche K, Wang Q, Hodges JS, Esch N, Malaeb S, et al. (2020). Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans who are Overweight: A Feasibility Study. Obesity (Silver Spring) 28, 860–869. 10.1002/oby.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, Lin S, Oliveira ML, and Varady KA (2020). Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab 32, 366–378 e363. 10.1016/j.cmet.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Bryan J, Hodgson J, and Murphy K (2015). Definition of the Mediterranean Diet; a Literature Review. Nutrients 7, 9139–9153. nu7115459 [pii] nutrients-07-05459 [pii] 10.3390/nu7115459 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan R, Nelson T, Peel J, Lipsey T, Voyles W, and Israel RG (2009). Cardiorespiratory fitness and the metabolic syndrome in firefighters. Occup Med (Lond) 59, 487–492. 10.1093/occmed/kqp095. [DOI] [PubMed] [Google Scholar]
- Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, and Khunti K (2014). Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care 37, 922–933. 37/4/922 [pii] 10.2337/dc13-2195 [doi]. [DOI] [PubMed] [Google Scholar]
- Efird J (2011). Blocked randomization with randomly selected block sizes. Int J Environ Res Public Health 8, 15–20. ijerph8010015 [pii] ijerph-08-00015 [pii] 10.3390/ijerph8010015 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L (2018). Interventions to promote cardiometabolic health and slow cardiovascular ageing. Nat Rev Cardiol 15, 566–577. 10.1038/s41569-018-0026-8. [DOI] [PubMed] [Google Scholar]
- Gill S, and Panda S (2015). A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 22, 789–798. 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Vargas NN, Espitia-Bautista E, Buijs RM, and Escobar C (2018). Shift-work: is time of eating determining metabolic health? Evidence from animal models. Proc Nutr Soc 77, 199–215. S0029665117004128 [pii] 10.1017/S0029665117004128 [doi]. [DOI] [PubMed] [Google Scholar]
- Guerrero-Vargas NN, Zárate-Mozo C, Guzmán-Ruiz MA, Cárdenas-Rivera A, and Escobar C (2021). Time-restricted feeding prevents depressive-like and anxiety-like behaviors in male rats exposed to an experimental model of shift-work. Journal of neuroscience research 99, 604–620. 10.1002/jnr.24741. [DOI] [PubMed] [Google Scholar]
- Haddock CK, Day RS, Poston WS, Jahnke SA, and Jitnarin N (2015). Alcohol use and caloric intake from alcohol in a national cohort of U.S. career firefighters. J Stud Alcohol Drugs 76, 360–366. 10.15288/jsad.2015.76.360. [DOI] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, et al. (2012). Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15, 848–860. 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hublin C, Partinen M, Koskenvuo K, Silventoinen K, Koskenvuo M, and Kaprio J (2010). Shift-work and cardiovascular disease: a population-based 22-year follow-up study. European journal of epidemiology 25, 315–323. 10.1007/s10654-010-9439-3. [DOI] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. (2010). Shiftwork: Definition and Occurrence of Exposure (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Painting, Firefighting, and Shiftwork. Lyon (FR): ). [PMC free article] [PubMed] [Google Scholar]
- Hutchison AT, Regmi P, Manoogian ENC, Fleischer JG, Wittert GA, Panda S, and Heilbronn LK (2019). Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity (Silver Spring) 27, 724–732. 10.1002/oby.22449. [DOI] [PubMed] [Google Scholar]
- Johns MW (1991). A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14, 540–545. 10.1093/sleep/14.6.540 [doi]. [DOI] [PubMed] [Google Scholar]
- Kales SN, Tsismenakis AJ, Zhang C, and Soteriades ES (2009). Blood pressure in firefighters, police officers, and other emergency responders. American journal of hypertension 22, 11–20. 10.1038/ajh.2008.296. [DOI] [PubMed] [Google Scholar]
- Karakas M, and Koenig W (2009). CRP in cardiovascular disease. Herz 34, 607–613. 10.1007/s00059-009-3305-7. [DOI] [PubMed] [Google Scholar]
- Kecklund G, and Axelsson J (2016). Health consequences of shift work and insufficient sleep. BMJ 355, i5210. 10.1136/bmj.i5210. [DOI] [PubMed] [Google Scholar]
- Kervezee L, Kosmadopoulos A, and Boivin DB (2020). Metabolic and cardiovascular consequences of shift work: The role of circadian disruption and sleep disturbances. Eur J Neurosci 51, 396–412. 10.1111/ejn.14216. [DOI] [PubMed] [Google Scholar]
- Kosmadopoulos A, Kervezee L, Boudreau P, Gonzales-Aste F, Vujovic N, Scheer F, and Boivin DB (2020). Effects of Shift Work on the Eating Behavior of Police Officers on Patrol. Nutrients 12. 10.3390/nu12040999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc A (2010). Shift-work and cardiovascular disease. European journal of epidemiology 25, 285–286. 10.1007/s10654-010-9456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA, and Rubin DB (2019). Statistical analysis with missing data, 3rd edition Edition (John Willey & Sons Inc; ). [Google Scholar]
- Liu D, Huang Y, Huang C, Yang S, Wei X, Zhang P, Guo D, Lin J, Xu B, Li C, et al. (2022). Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N Engl J Med 386, 1495–1504. 10.1056/NEJMoa2114833 [doi]. [DOI] [PubMed] [Google Scholar]
- Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, Philip E, Vittinghoff E, Heymsfield SB, Olgin JE, et al. (2020). Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern Med. 10.1001/jamainternmed.2020.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, Hansen J, Nelson RJ, Panda S, Smolensky MH, et al. (2017). Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program's workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ 607-608, 1073–1084. 10.1016/j.scitotenv.2017.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoogian EN, Chow LS, Taub PR, Laferrere B, and Panda S (2021a). Time-restricted eating for the prevention and management of metabolic diseases. Endocr Rev. 10.1210/endrev/bnab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoogian ENC, Chaix A, and Panda S (2019). When to Eat: The Importance of Eating Patterns in Health and Disease. J Biol Rhythms 34, 579–581. 10.1177/0748730419892105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoogian ENC, Zadourian A, Lo HC, Gutierrez NR, Shoghi A, Rosander A, Pazargadi A, Wang X, Fleischer JG, Golshan S, et al. (2021b). Protocol for a randomised controlled trial on the feasibility and effects of 10-hour time-restricted eating on cardiometabolic disease risk among career firefighters doing 24-hour shift work: the Healthy Heroes Study. BMJ open 11, e045537. 10.1136/bmjopen-2020-045537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro T, Tinsley G, Longo G, Grigoletto D, Bianco A, Ferraris C, Guglielmetti M, Veneto A, Tagliabue A, Marcolin G, and Paoli A (2020). Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: a randomized controlled trial. J Int Soc Sports Nutr 17, 65. 10.1186/s12970-020-00396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W.H. (2018). Global Status Report on Alcohol and Health 2018 (World Health Organization, Geneva: ). [Google Scholar]
- Phillips NE, Mareschal J, Schwab N, Manoogian ENC, Borloz S, Ostinelli G, Gauthier-Jaques A, Umwali S, Rodriguez EG, Aeberli D, et al. (2021). The Effects of Time-Restricted Eating versus Standard Dietary Advice on Weight, Metabolic Health and the Consumption of Processed Food: A Pragmatic Randomised Controlled Trial in Community-Based Adults. Nutrients 13. 10.3390/nu13031042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston WS, Haddock CK, Jahnke SA, Jitnarin N, Tuley BC, and Kales SN (2011). The prevalence of overweight, obesity, and substandard fitness in a population-based firefighter cohort. Journal of occupational and environmental medicine 53, 266–273. 10.1097/JOM.0b013e31820af362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter GD, Skene DJ, Arendt J, Cade JE, Grant PJ, and Hardie LJ (2016). Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences and Countermeasures. Endocr Rev, er20161083. 10.1210/er.2016-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Crespo MF (2015). Comment on Boyko and Jensen. Do We Know What Homeostatis Model Assessment Measures? If Not, Does It Matter? Diabetes Care 2007;30:2725–2728. Diabetes Care 38, e213. 38/12/e213 [pii] 10.2337/dc15-1172 [doi]. [DOI] [PubMed] [Google Scholar]
- Romanidou M, Tripsianis G, Hershey MS, Sotos-Prieto M, Christophi C, Moffatt S, Constantinidis TC, and Kales SN (2020). Association of the Modified Mediterranean Diet Score (mMDS) with Anthropometric and Biochemical Indices in US Career Firefighters. Nutrients 12. 10.3390/nu12123693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild J, Hoddy KK, Jambazian P, and Varady KA (2014). Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutrition reviews 72, 308–318. 10.1111/nure.12104. [DOI] [PubMed] [Google Scholar]
- Soteriades ES, Smith DL, Tsismenakis AJ, Baur DM, and Kales SN (2011). Cardiovascular disease in US firefighters: a systematic review. Cardiology in review 19, 202–215. 10.1097/CRD.0b013e318215c105. [DOI] [PubMed] [Google Scholar]
- Sotos-Prieto M, Ruiz-Canela M, Song Y, Christophi C, Mofatt S, Rodriguez-Artalejo F, and Kales SN (2020). The Effects of a Mediterranean Diet Intervention on Targeted Plasma Metabolic Biomarkers among US Firefighters: A Pilot Cluster-Randomized Trial. Nutrients 12. 10.3390/nu12123610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Benbrahim-Tallaa L, and Cogliano V (2007). Carcinogenicity of shift-work, painting, and fire-fighting. The Lancet. Oncology 8, 1065–1066. [DOI] [PubMed] [Google Scholar]
- Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, and Peterson CM (2018). Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab 27, 1212–1221 e1213. 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney E, Yu ZM, Dummer TJB, Cui Y, DeClercq V, Forbes C, Grandy SA, Keats M, Parker L, and Adisesh A (2020). The relationship between anthropometric measures and cardiometabolic health in shift work: findings from the Atlantic PATH Cohort Study. Int Arch Occup Environ Health 93, 67–76. 10.1007/s00420-019-01459-8. [DOI] [PubMed] [Google Scholar]
- Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, Morgan GB, and Grandjean PW (2017). Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur J Sport Sci 17, 200–207. 10.1080/17461391.2016.1223173. [DOI] [PubMed] [Google Scholar]
- Tobaldini E, Fiorelli EM, Solbiati M, Costantino G, Nobili L, and Montano N (2018). Short sleep duration and cardiometabolic risk: from pathophysiology to clinical evidence. Nat Rev Cardiol. 10.1038/s41569-018-0109-6. [DOI] [PubMed] [Google Scholar]
- Tovar AP, Richardson CE, Keim NL, Van Loan MD, Davis BA, and Casazza GA (2021). Four Weeks of 16/8 Time Restrictive Feeding in Endurance Trained Male Runners Decreases Fat Mass, without Affecting Exercise Performance. Nutrients 13. nu13092941 [pii] nutrients-13-02941 [pii] 10.3390/nu13092941 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, Gabel K, Freels S, Rigdon J, Rood J, et al. (2017). Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern Med 177, 930–938. 10.1001/jamainternmed.2017.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda P, Gulayin P, and Danaei G (2018). Long-term moderately elevated LDL-cholesterol and blood pressure and risk of coronary heart disease. PLoS One 13, e0200017. 10.1371/journal.pone.0200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wouwe NC, Valk PJ, and Veenstra BJ (2011). Sleep monitoring: a comparison between three wearable instruments. Mil Med 176, 811–816. [DOI] [PubMed] [Google Scholar]
- Vetter C (2018). Circadian disruption: What do we actually mean? Eur J Neurosci. 10.1111/ejn.14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE Jr., and Sherbourne CD (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30, 473–483. [PubMed] [Google Scholar]
- Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S, and Taub PR (2020). Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab 31, 91–104. 10.1016/j.cmet.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Farioli A, Korre M, and Kales SN (2015). Dietary Preferences and Nutritional Information Needs Among Career Firefighters in the United States. Glob Adv Health Med 4, 16–23. gahmj.2015.050 [pii] 10.7453/gahmj.2015.050 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong LC, Li J, and Calvert GM (2017). Sleep-related problems in the US working population: prevalence and association with shiftwork status. Occupational and environmental medicine 74, 93–104. 10.1136/oemed-2016-103638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Unprocessed data reported and underlying the display item in the manuscript, related to Figures 2, 3, and 4 and all health metrics reported.
Data Availability Statement
Deidentified participants’ source data “Data S1 – Source Data” has been deposited at Mendeley and made publicly available. Data S1. Unprocessed data reported and underlying the display item in the manuscript, related to Figures 2, 3, and 4 and all health metrics reported. The DOI is listed in the key resources table. mCC app data will not be shared as it could indirectly identify individuals. This paper does not report an original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological Blood Samples | ||
| Human Blood Samples | This study | N/A |
| Critical Commercial Assays | ||
| Comprehensive Metabolic Panel | Center for Advanced Laboratory Medicine (CALM) (La Jolla, CA) | Cat#Cobas8000 (Roche; Indianapolis, IN) |
| Lipoprofile by NMR | ARUP (Salt Lake City, UT) | Cat#2013716, CobasC702 (Roche; Indianapolis, IN) |
| Insulin | ARUP (Salk Lad City, UT) | Cat#00770022, chemiluminescent Immunoassays on an ADVIA Centaur (Siemens Corporation; Washington, DC) |
| Glycated hemoglobin | UCSD Jacobs Medical Center (JMC) (La Jolla, CA) | Cat#Cobas8000 (Roche; Indianapolis, IN) |
| Complete Blood Count | JMC/CALM (La Jolla, CA) | Cat#XN-10 (Sysmex; Chuo-ku, Japan) |
| High sensitivity C-reactive protein | CALM (La Jolla, CA) | Cat#Cobas8000 (Roche; Indianapolis, IN) |
| Deposited Data | ||
| Data S1 - Source Data | This paper | Mendeley: DOI: 10.17632/b6h5kvn5pj.1 |
| Software and Algorithms | ||
| SPSS Statistics | IBM | https://www.ibm.com/products/spss-statistics |
| Prism 8.1.2 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| Analyzing Actiwatch Data: Actiware Software v.6.0.9 | Philips Respironics | Cat#1104775 |
| Other | ||
| Pittsburgh Sleep Quality Index | Buysse et al., 1989 | https://www.cmu.edu/ommon-cold-porject/measures-by-study/health-practices/sleep-habits/psqirev.pdf |
| Epworth Sleepiness Scale | Johns, 1991 | https://epworthsleepinessscale.com/about-the-ess/ |
| Beck Depression Inventory-II | Pearson Assessments | https://www.pearsonassessments.com/store/usassessments/en/Store/Professional-Assessments/Personality-%26-Biopsychosocial/Beck-Depression-Inventory/p/100000159.html |
| 36-Item Short Form Survey (SF-36) | Rand Corporation | https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html |
| Actiwatch Spectrum Plus | Philips Respironics | Cat#1101894 |
| DC-430U Dual Frequency Total Body Composition Analyzer | Tanita (Tokyo, Japan) | Cat#DC-430U |