Abstract
The Escherichia coli zitB gene encodes a Zn(II) transporter belonging to the cation diffusion facilitator family. ZitB is specifically induced by zinc. ZitB expression on a plasmid rendered zntA-disrupted E. coli cells more resistant to zinc, and the cells exhibited reduced accumulation of 65Zn, suggesting ZitB-mediated efflux of zinc.
Zinc is an essential component of many proteins and is required for life in all organisms. However, excess zinc is toxic, and as a result, cells require homeostatic mechanisms to control intracellular zinc levels. In Escherichia coli, zinc deficiency induces expression of a specific zinc uptake system, ZnuABC, which is an ABC transporter for zinc uptake (22). Under conditions of zinc sufficiency, expression of the pump is repressed by the Fur homologue Zur, which presumably binds to the bidirectional promoter region of znuA and znuBC. However, under toxic conditions Zn(II) enters the cells by an unknown pathway. The phosphate uptake system has been implicated in uptake of Zn(II), possibly as a metal phosphate (3). Growth of E. coli in high concentrations of Zn(II), Cd(II), or Pb(II) resulted in induction of ZntA, a Zn(II)-Cd(II)-Pb(II)-translocating P-type ATPase. ZntR, a MerR homologue, is a transcriptional activator of zntA (4, 18). Disruption of zntA resulted in sensitivity to Zn(II), Cd(II), and Pb(II) (2, 24, 25). However, in addition to zntA, there are two uncharacterized genes, ybgR and yiiP, encoding gene products belonging to the cation diffusion facilitator (CDF) family of proteins (17, 23). The CDF family has common structural characteristics, with six transmembrane domains and containing histidine-rich motifs predicted to extend into the cytosol (1, 6). In addition, overproduction of eukaryotic members of this family confers resistance to zinc in Saccharomyces cerevisiae (6, 15).
In this report we show that zitB (formerly ybgR) encodes an additional zinc transporter belonging to the CDF family of proteins. Double disruption of zitB and zntA rendered E. coli cells more zinc sensitive than a single disruption in zntA alone. Furthermore, overexpression of ZitB resulted in a significant increase in zinc resistance and reduced uptake of zinc. Expression of both zitB and yiiP was inducible by zinc in a concentration-dependent manner. However, in contrast to zitB, the overexpression of yiiP did not confer additional zinc resistance, and disruption of yiiP in different strains did not alter zinc resistance, so the function of its gene product remains unknown.
ZitB is an additional zinc transporter.
zitB deletions were introduced into E. coli W3110 and E. coli RW3110 (zntA::Km), producing E. coli strains GG51 (ΔzitB::Cm) and GG48 (ΔzitB::Cm zntA::Km). Chromosomal deletions were performed as described by Datsenko and Wanner (5), and the gene of interest was replaced by a chloramphenicol cassette (Cm). The ΔzitB::Cm cassette was transduced into E. coli W3110 and RW3110 (zntA::Km) by P1 transduction. Mutants with a single ΔzitB deletion did not exhibit significant differences in metal sensitivity compared to E. coli W3110 (data not shown). However, E. coli strain GG48 (ΔzitB::Cm zntA::Km) was more zinc sensitive than E. coli RW3110 (zntA::Km), indicating that zitB (formerly ybgR) might encode a zinc transporter (Fig. 1). There was no effect on the MICs of cobalt and cadmium when E. coli strains GG48 and RW3110 were compared (data not shown). Since zitB appears to be selective for zinc, ybgR was renamed zitB (for “zinc transporter”).
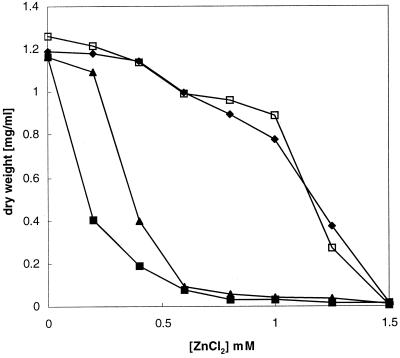
FIG. 1.
Effect of zinc on growth E. coli W3110 (□), RW3110 (zntA::Km) (▴), GG48 (ΔzitB::Cm zntA::Km) (■), and GG48 (ΔzitB::Cm zntA::Km)/pZITB (⧫). Growth curves with different ZnCl2 concentrations are shown. Overnight cultures were diluted 1:500 into fresh Luria-Bertani medium with the indicated concentrations of ZnCl2. Cell growth was monitored as the optical density at 600 nm after 15 h of incubation at 37°C with shaking and converted to dry weight. Experiments were performed in triplicate, values are averages.
Zinc resistance and transport by ZitB.
To determine whether ZitB transports zinc, the zitB gene was cloned into plasmid pASK-IBA3 (IBA Göttingen), leading to plasmid pZITB. Primer sequences are available on request. This plasmid was transferred into E. coli strain GG48 (ΔzitB::Cm zntA::Km). Induction of zitB on plasmid pZITB by addition of anhydrotetracycline (AHT) led to a significant increase in zinc resistance (Fig. 1). Induction by AHT was required to confer maximal zinc resistance. Expression of ZitB did not confer resistance to cobalt and cadmium (data not shown).
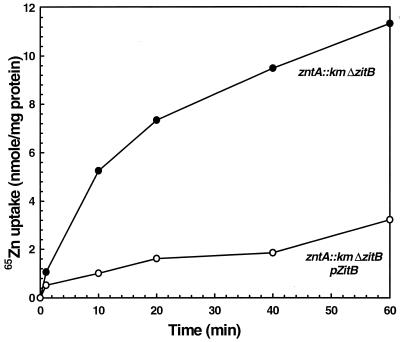
ZitB is homologous to members of the CDF family that have been implicated in transport of metal ions (6). Resistance mediated by a zinc transporter may be based on efflux, which decreases the intracellular concentration of metal ions. Uptake experiments were performed by filtration as described previously (16). When levels of cell-associated zinc ions in E. coli strain GG48 (ΔzitB::Cm zntA::Km) with and without expressed ZitB were compared, resistant cells accumulated significantly less zinc than the respective control cells (Fig. 2). Since it is a member of the CDF family, it is reasonable to propose that ZitB is located in the cytoplasmic membrane. Thus, reduced accumulation probably results from active transport of Zn(II) across the cytoplasmic membrane catalyzed by ZitB.
FIG. 2.
65Zn(II) uptake by cells of E. coli GG48 (ΔzitB::Cm zntA::Km)/pZITB expressing zitB. Cells were grown overnight in Luria-Bertani medium and diluted 100-fold into fresh prewarmed Luria-Bertani medium. The cells were grown to an optical density at 600 nm of 0.8 and induced with 200 μg of AHT per liter. After growth for 2.5 h, the cells were washed with buffer A (10 mM Tris-HCl [pH 7.0], 2 g of glucose per liter, 10 mM Na2HPO4) and concentrated fourfold in the same buffer. 65ZnSO4 was added to a final concentration of 5 μM. The cells were incubated at 37°C, and 0.1-ml aliquots were filtered through nitrocellulose membranes (0.45 μm) at various times and immediately washed with 10 ml of buffer B (10 mM Tris-HCl [pH 7.0], 10 mM MgCl2). The membranes were dried, and radioactivity was measured using a liquid scintillation counter. The protein concentration was determined using the bicinchoninic acid kit (Sigma), and the amount of Zn(II) per milligram of protein was calculated.
The yiiP gene product may also be involved in zinc homeostasis.
The yiiP gene encodes a putative gene product also belonging to the CDF family. Mutants with a ΔyiiP deletion were constructed from E. coli W3110, RW3110 (zntA::Km), and GG48 (ΔzitB zntA::Km), leading to strains GG180 (ΔyiiP::Cm), GG253 (ΔyiiP::Cm zntA::Km), and GG252 (ΔyiiP::Cm ΔzitB zntA::Km). Mutants with a ΔyiiP deletion did not show a decrease in zinc, cadmium, or cobalt resistance compared to the parental E. coli strains (data not shown). In contrast, strain GG253 (ΔyiiP::Cm zntA::Km) was slightly but significantly more zinc resistant than strain RW3110 (zntA::Km). However, overexpression of yiiP in plasmid pYIIP did not lead to an increase or decrease in zinc, cadmium, or cobalt tolerance (data not shown).
The zitB and yiiP genes are induced by zinc.
To analyze metal-dependent expression of zitB and yiiP, transcriptional fusions using lacZ as a reporter gene were constructed. To construct the chromosomal Φ(zitB-lacZ) transcriptional fusion in strain E. coli GG161 (W3110 ΔlacZYA::Km), the 400 bp upstream and downstream of the zitB stop codon were separately amplified by PCR from chromosomal DNA of E. coli W3110. These fragments were digested with BamHI, and both fragments were cloned into vector plasmid pGEM T-Easy (Promega, Madison, Wis.) in one step. As confirmed by control sequencing, this led to a plasmid harboring an 800-bp zitB fragment with a BamHI and an XbaI site located directly downstream of the stop codon of zitB, mutating the sequence CATTAATGGGACAGC (the TAA stop codon of zitB is in boldface) to CATTAAGGATCCGGGTCTAGAGGCCATTCACATCATCACCATTAA (underlining indicates restriction sites for BamHI and XbaI). A promoterless lacZ gene was inserted into the BamHI/XbaI site of this plasmid introduced by PCR, and the fragment containing zitB-lacZ was cloned as a NotI fragment into plasmid pKO3 (12). Finally, the pKO3 hybrid plasmid with Φ(zitB-lacZ) was used in a double-recombination event to insert the lacZ gene downstream of zitB on the chromosome of E. coli GG161 (W3110 ΔlacZYA::Km) as described previously (7). The correct insertion and orientation of lacZ in strain E. coli GG260 [W3110 ΔlacZYA::Km Φ(zitB-lacZ)] were verified by PCR. E. coli GG161 (W3110 ΔlacZYA::Km) was constructed by transfer of the lacZYA::Km replacement by generalized P1 transduction from strain E. coli BW25434 (5) into E. coli W3110. The β-galactosidase activity in permeabilized cells was determined as published previously (14). Likewise, a Φ(yiiP-lacZ) operon fusion was constructed, resulting in strain GG193 [W3110 ΔlacZYA::km Φ(yiip-lacZ)].
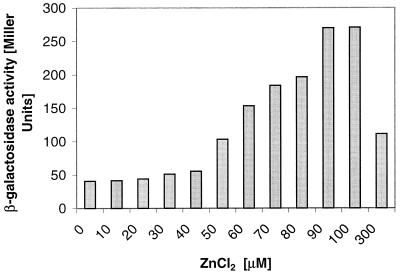
Expression of zitB was strongly induced by zinc and slightly induced by cadmium, while other metals did not significantly induce Φ(zitB-lacZ) (Table 1). The zinc concentration dependency of zitB expression was examined. Induction of zitB was observed with 50 μM ZnCl2 and reached a maximum at 100 μM in mineral salts medium. Higher concentrations of Zn(II) led to a decrease of zitB expression (Fig. 3). Northern blot analysis (8, 9) also showed an increase in zitB-specific transcript after addition of zinc (data not shown). Expression of yiiP was also maximally induced by zinc and also to a lesser degree by cadmium (Table 1).
TABLE 1.
Induction of zitB and yiiP by different metals
| Addition | Avg β-galactosidase activity (Miller units)a
|
|
|---|---|---|
| Φ(zitB-lacZ) | Φ(yiiP-lacZ) | |
| None | 21.7 | 36.9 |
| ZnCl2 | 223.5 | 212.5 |
| CdCl2 | 59.1 | 74.5 |
| CoCl2 | 24.5 | 30.7 |
| NiCl2 | 44.2 | 42.5 |
| EDTA | 25 | 35.9 |
| CuCl2 | 52.2 | 41.3 |
Cells of either E. coli GG260 Φ(zitB-lacZ) or GG193 Φ(yiiP-lacZ) were diluted 1:100 into fresh mineral salts medium with 0.2% glycerol and 0.1% yeast extract containing no added metal or were induced after 3 h of growth with different metals or EDTA, each at 0.1 mM. Incubation was continued with shaking for 3 h at 30°C, and the β-galactosidase activity was determined (14). The averages of three independent experiments are shown.
FIG. 3.
Induction of zitB. Induction of β-galactosidase activity in a zitB-lacZ transcriptional fusion strain. Overnight cultures of E. coli GG260 containing a Φ(zitB-lacZ) operon fusion on the bacterial chromosome were diluted 1:100 into fresh minimal medium with 0.2% glycerol and 0.1% yeast extract containing no added metal or were induced after 3 h of growth by increasing concentrations of ZnCl2. Incubation was continued with shaking for 3 h at 30°C, and the β-galactosidase activity was determined (14). Each experiment was performed in triplicate, and values are averages.
Conclusions.
In this report we describe the identification of two genes, ybgR (zitB) and yiiP, on the E. coli chromosome that encode putative CDF proteins. Most CDF transporters analyzed thus far are responsible for zinc transport from the cytosol across different membranes. Four mammalian CDF transporters have been characterized: ZnT-1, ZnT-2, ZnT-3, and ZnT-4. ZnT-1 is responsible for zinc transport across the plasma membrane (19). ZnT-2 is responsible for zinc transport into lysosomes, and ZnT-3 is responsible for zinc transport into synaptic vesicles (20, 21). ZnT-4 is also thought to function in zinc efflux (10). Prokaryotic members of the CDF family include CzcD from Ralstonia metallidurans CH34 and CzrB (also named ZntA) from Staphylococcus aureus (1, 11, 26). In addition to zinc, these transporters were also shown to transport cobalt and cadmium (1). CzcD appears to have an additional regulatory function in repressing the CzC system by exporting inducing cations (1). However, in all CDF transporters characterized so far, neither the transport mechanism nor the actual substrate of the pump is known. It might therefore be premature to speculate about their physiological function.
In this study we examined the physiological role of the yiiP and zitB gene products in E. coli. No clear phenotype of a yiiP-disrupted strain was observed, so the physiological role of YiiP remains obscure. On the other hand, there was a clear relationship between expression of the zitB gene product and zinc tolerance in E. coli. Disruption of both zitB and zntA, which encodes a Zn(II)-translocating P-type ATPase (24), resulted in hypersensitivity to zinc. A strain disrupted only in zitB did not exhibit a decreased zinc tolerance, perhaps because ZntA could pump out zinc efficiently at high zinc concentrations. However, expression of zitB on a plasmid led to a significant increase in zinc resistance. It is possible that ZitB contributes to zinc homeostasis at low concentrations of zinc, while ZntA is required for growth at higher and more toxic concentrations. Additionally, zinc induction of a Φ(zitB-lacZ) transcriptional fusion showed a steady increase of transcription up to approximately 0.1 mM. Higher medium concentrations of zinc did not lead to a further increase in zitB transcription. This may reflect the fact that ZntA maintains the intracellular zinc concentration lower than the medium concentration. These studies indicate that zinc resistance is not due to a single transport system or any one factor but rather is due to many systems interacting in an as-yet-undefined way. The residual zinc resistance in a strain disrupted in both zntA and zitB suggests that there are additional factors or systems involved in zinc resistance.
Acknowledgments
This work was supported by hatch project 136713 to C.R., U. S. Public Health Service grant GM 55425 to B.P.R., and Ni262/3-3 of the Deutsche Forschungsgemeinschaft and Fonds der Chemischen Industrie to D.H.N.
REFERENCES
- 1.Anton A, Große C, Reissmann J, Pribyl T, Nies D H. CzcD is a heavy metal ion transporter involved in regulation of heavy metal resistance in Ralstonia sp. strain CH34. J Bacteriol. 1999;181:6876–6881. doi: 10.1128/jb.181.22.6876-6881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beard S J, Hashim R, Membrillo-Hernandez J, Hughes M N, Poole R K. Zinc(II) tolerance in Escherichia coli K12; evidence that the zntA gene (o732) encodes a cation transport ATPase. Mol Microbiol. 1997;25:883–891. doi: 10.1111/j.1365-2958.1997.mmi518.x. [DOI] [PubMed] [Google Scholar]
- 3.Beard S J, Hashim R, Wu G, Binet M R B, Hughes M N, Poole R K. Evidence for the transport of zinc(II) ions via the Pit inorganic phosphate transport system in Escherichia coli. FEMS Microbiol Lett. 2000;184:231–235. doi: 10.1111/j.1574-6968.2000.tb09019.x. [DOI] [PubMed] [Google Scholar]
- 4.Brocklehurst K R, Hobman J L, Lawley B, Blank L, Marshall S J, Brown N L, Morby A P. ZntR is a Zn(II)-responsive MerR-like transcriptional regulator of zntA in Escherichia coli. Mol Microbiol. 1999;31:893–902. doi: 10.1046/j.1365-2958.1999.01229.x. [DOI] [PubMed] [Google Scholar]
- 5.Datsenko K A, Wanner B L. One-step inactivation of chromosomal genes in Escherichia coli K12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eide D J. The molecular biology of metal ion transport in Saccharomyces cerevisiae. Annu Rev Nutr. 1998;18:441–469. doi: 10.1146/annurev.nutr.18.1.441. [DOI] [PubMed] [Google Scholar]
- 7.Franke S, Grass G, Nies D H. The product of the ybdE gene of the Escherichia coli chromosome is involved in detoxification of silver ions. Microbiology. 2000;147:965–972. doi: 10.1099/00221287-147-4-965. [DOI] [PubMed] [Google Scholar]
- 8.Grass G, Große C, Nies D H. Regulation of the cnr cobalt and nickel resistance determinant from Ralstonia sp. strain CH34. J Bacteriol. 2001;182:1390–1398. doi: 10.1128/jb.182.5.1390-1398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Große C, Grass G, Anton A, Franke S, Navarrete Santos A, Lawley B, Brown N L, Nies D H. Transcriptional organization of the czc heavy metal homeostasis determinant from Alcaligenes eutrophus. J Bacteriol. 1999;181:2385–2393. doi: 10.1128/jb.181.8.2385-2393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L, Gitschier J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat Genet. 1997;17:292–297. doi: 10.1038/ng1197-292. [DOI] [PubMed] [Google Scholar]
- 11.Kuroda M, Hayashi H, Ohta T. Chromosome-determined zinc-responsible operon czr in Staphylococcus aureus strain 912. Microbiol Immunol. 1999;43:115–125. doi: 10.1111/j.1348-0421.1999.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 12.Link A J, Phillips D, Church G M. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol. 1997;179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mergeay M, Nies D, Schlegel H G, Gerits J, Charles P, van Gijsegem F. Alcaligenes eutrophus is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J Bacteriol. 1985;162:328–334. doi: 10.1128/jb.162.1.328-334.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Laboratory Press; 1992. [Google Scholar]
- 15.Miyabe S, Izawa S, Inoue Y. Expression of ZRC1 coding for suppressor of zinc toxicity is induced by zinc-starvation stress in Zap1-dependent fashion in Saccharomyces cerevisiae. Biochem Biophys Res Comm. 2000;276:879–884. doi: 10.1006/bbrc.2000.3580. [DOI] [PubMed] [Google Scholar]
- 16.Mobley H L T, Rosen B P. Energetics of plasmid-mediated arsenate resistance in Escherichia coli. Proc Natl Acad Sci USA. 1982;79:6119–6122. doi: 10.1073/pnas.79.20.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nies D H, Silver S. Ion efflux systems involved in bacterial metal resistances. J Ind Microbiol. 1995;14:186–199. doi: 10.1007/BF01569902. [DOI] [PubMed] [Google Scholar]
- 18.Outten C E, Outten F W, O'Halloran T V. DNA distortion mechanism for transcriptional activation by ZntR, a Zn(II)-responsive MerR homologue in Escherichia coli. J Biol Chem. 1999;274:37517–37524. doi: 10.1074/jbc.274.53.37517. [DOI] [PubMed] [Google Scholar]
- 19.Palmiter R D, Findley S D. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995;14:639–649. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmiter R D, Cole T B, Findley S D. ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J. 1996;15:1784–1791. [PMC free article] [PubMed] [Google Scholar]
- 21.Palmiter R D, Cole T B, Quaife C J, Findley S D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci USA. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patzer S I, Hantke K. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol Microbiol. 1998;28:1199–1210. doi: 10.1046/j.1365-2958.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- 23.Paulsen I T, Saier M J. A novel family of ubiquitous heavy metal ion transport proteins. J Membr Biol. 1997;156:99–103. doi: 10.1007/s002329900192. [DOI] [PubMed] [Google Scholar]
- 24.Rensing C, Mitra B, Rosen B P. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc Natl Acad Sci USA. 1997;94:14326–14331. doi: 10.1073/pnas.94.26.14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rensing C, Sun Y, Mitra B, Rosen B P. Pb(II)-translocating P-type ATPases. J Biol Chem. 1998;273:32614–32617. doi: 10.1074/jbc.273.49.32614. [DOI] [PubMed] [Google Scholar]
- 26.Xiong A, Jayaswal R K. Molecular characterization of a chromosomal determinant conferring resistance to zinc and cobalt ions in Staphylococcus aureus. J Bacteriol. 1998;180:4024–4029. doi: 10.1128/jb.180.16.4024-4029.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]