Abstract
Sodium-glucose co-transporter-2 (SGLT2) inhibitors offer significant outcome benefits beyond glucose lowering, including reduced risk of cardiovascular death, all-cause mortality, major adverse cardiovascular events, hospitalisations for heart failure and progression of renal disease. Considering these therapeutic effects, minimal incremental risk for hypoglycaemia and simplicity of administration, this drug class appears to be an attractive therapeutic option for older adults, and post hoc analysis of trial data provides support for the use of SGLT2 inhibitors in this population. Nevertheless, despite favourable clinical trial data, there has been some hesitance in clinical practice prescribing these drugs to older frail adults due to the limited therapeutic experience in this population and insufficient long-term safety data. In this review article, we evaluate the risk–benefit profile for the use of SGLT2 inhibitors in this population and suggest that rather than being a treatment to avoid, SGLT2 inhibitors should be considered a valid therapeutic option for older frail adults with or without diabetes.
Keywords: frail, sodium-glucose co-transporter-2, type 2 diabetes mellitus, older people
Key Points
SGLT2 inhibitors have demonstrated multiple favourable effects in older adults beyond glucose control, including reductions in the risk of cardiovascular death, all-cause mortality, major adverse cardiovascular events, hospitalisations for heart failure and progression of renal disease.
SGLT2 inhibitors are well tolerated in frail older adults with or without diabetes, with a low risk of serious adverse effects that should not overshadow the significant cardioprotective benefits.
Increased use of SGLT2 inhibitors in frail older adults with or without diabetes has the potential to provide early clinical benefits and improve symptoms and outcomes for this population.
Introduction
The prevalence of type 2 diabetes mellitus (T2DM) is increasing globally, with the highest increases anticipated to be in older populations. Indeed, it is projected that the number of people aged >65 years with T2DM will reach 195.2 million by 2030 and 276.2 million by 2045 [1]. Although the ageing population is in part responsible for this, the prevalence of T2DM in this population is higher than would be expected as a consequence of increasing life expectancy alone. In addition to metabolic dysfunction, ageing is also associated with vascular and renal changes that predispose older people to an increased risk of cardio-renal complications such as heart failure (HF) and chronic kidney disease (CKD) [2, 3]. Some of the symptoms of cardio-renal disease such as fatigue, confusion, depression and high blood pressure may be mistaken for normal ageing. Therefore, it is important that symptoms are properly assessed in this population and that undetected cardiorenal metabolic disease is diagnosed and appropriate treatment is prescribed. Identifying suitable treatment strategies for T2DM and its associated complications in older adults is an unmet medical need.
Older adults are a heterogeneous group, with high variability in the capacity for self-care, sarcopenia and overall dependency [4]. Collectively, these characteristics may be summarised under the term frailty, best defined as the presence of three or more of the following criteria: unintentional weight loss (more than 4.5 kg in 1 year), slow gait speed, weak grip strength and self-reported physical exhaustion or measured low physical activity [5]. Frailty is widely accepted as a better predictor of complications and mortality than chronological age. The prevalence of diabetes in the adult general population is 4%, whereas it is more than 10% in those aged >65 years [6] and up to 25% in frail older adults, such as those in care homes that require additional support and management of comorbidities [7]. It is uncertain whether diabetes contributes to the process of frailty or is a result of the polymorbidity process. Diabetes care in frail older adults is challenging, complicated by the multiple comorbidities, polypharmacy, and the increased detrimental impact of hypoglycaemia and other adverse events. Even though it is recognised that an individualised approach with frailty-specific higher glycated haemoglobin (HbA1c) targets of 64–70 mmol/mol are needed in this population to balance the risk of hypo- and hyperglycaemic events [8], there is limited specific guidance on the most appropriate treatment.
Sodium-glucose co-transporter-2 (SGLT2) inhibitors are a class of anti-hyperglycaemic drugs that lower blood glucose concentration by increasing urinary glucose excretion via inhibition of SGLT2 in the proximal renal tubules [9]. Previously, there have been concerns regarding the use of these agents in older adults, given the recognised side effects of polyuria and increased risk of candidiasis and hyperglycaemia. However, multiple clinical benefits beyond glucose lowering have been established with SGLT2 inhibitors including reduced risk of cardiovascular (CV) death, all-cause mortality, hospitalisations for heart failure (HHF), renal decline, hyperkalaemia, and improvements in weight and blood pressure [10–13]. Furthermore, the drugs are taken orally at any time of the day and have no known significant drug interactions. Considering their multiple favourable effects, simplicity of administration, good tolerability and negligible hypoglycaemia, they appear to be an attractive therapeutic option for older adults, and post hoc analysis of trial data demonstrates the clinical value of SGLT2 inhibitors in this population [14–16].
There are currently four SGLT2 inhibitors licensed in the UK. Ertugliflozin and canagliflozin are licensed for the treatment of T2DM including individuals aged >65 years for which no dose adjustment is required, but renal function and risk of volume depletion need to be considered [17, 18]. Empagliflozin is licensed for managing T2DM and heart failure with reduced ejection fraction (HFrEF), with no dose adjustment necessary based on age [19]. In people ≥75 years of age, an increased risk for volume depletion should be taken into account. Initiation of empagliflozin is not recommended for individuals ≥85 years of age due to the limited therapeutic experience. Dapagliflozin is licensed for T2DM, HFrEF and CKD, and it can be prescribed to older adults aged ≥65 years, with no dose adjustment required based on age [20].
Despite favourable clinical trial data, and updated licensing and guidelines, there has been hesitance in clinical practice with regard to the prescription of SGLT2 inhibitors in older frail adults, who are at increased risk of both heart and renal failure. Indeed, real-world evidence suggests that prescriptions of this drug class in this population are generally lower than other T2DM treatments such as dipeptidyl peptidase-4 (DPP4) inhibitors, despite there being clear evidence of a lack of any additional benefit beyond glycaemic control for this class [21–24]. This may be due to the limited therapeutic experience of SGLT2 inhibitors in this population, particularly with regard to frailty, and insufficient long-term safety data.
Considering the recently updated NICE clinical guidelines for the management of T2DM in adults [25], it is anticipated that SGLT2 inhibitors will be more widely prescribed. Hence, in this review article we evaluate the benefit–risk profile for the use of SGLT2 inhibitors in the older population and aim to provide timely and important guidance to understanding the clinical role of SGLT2 inhibitors in older frail people with or without T2DM.
Benefit–risk profile of SGLT2 inhibitors in older frail adults
The benefit–risk profile of SGLT2 inhibitors in older frail adults (Figure 1) has yet to be fully explored, but pooled analyses have demonstrated good tolerability of SGLT2 inhibitors in adults aged ≥65 years [26, 27]. In addition, in a retrospective multicentre study, that analysed the efficacy and safety of SGLT2 inhibitors in people aged >70 years with T2DM in a real-life setting, with at least 1 year of follow-up, participants experienced a significant reduction in average HbA1c, from 7.7% (61 mmol/mol) at baseline to 7.4% (57 mmol/mol) at 1 year [28]. There was also a significant reduction in average fasting plasma glucose (FPG) levels over this period, from 157.7 to 140.6 mg/dL (8.75 to 7.8 mmol/L).
Figure 1.
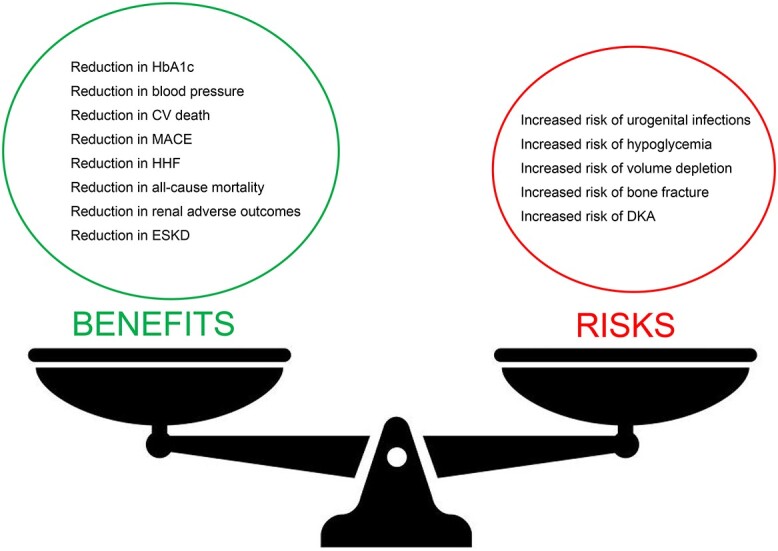
Benefit–risk profile of SGLT2 inhibitors in older frail adults. CV, cardiovascular; ESKD, end-stage kidney disease; UTI, urinary tract infection.
However, the real benefits of SGLT2 inhibitor use in the older population are unlikely to be due to a decrease in HbA1c or FPG, but as a consequence of a reduction in the risk of cardio-renal events and quality-of-life improvements (Table 1).
Table 1.
Summary of SGLT2 inhibitor cardio-renal benefits
| SGLT2 inhibitor | CVOTs | Licensed indications | Use in older adult | Cardio-renal benefits |
|---|---|---|---|---|
| Dapagliflozin | DECLARE TIMI-58 [44] DAPA-HF [45] DAPA-CKD [36] |
T2DM HF CKD |
≥65 years | Reduction in CV mortality Reduction in HHF Improvement in HF-related health status Reduction in renal adverse events |
| Canagliflozin | CANVAS [46] CREDENCE [47] |
T2DM | ≥65 years Caution: Renal function and risk of volume depletion should be taken into account |
Prevention of MACE Reduction in HHF Reduction in systolic and diastolic blood pressure Reduction in renal adverse events |
| Empagliflozin | EMPA-REG OUTCOMES [48] EMPEROR- REDUCED [49] EMPEROR-PRESERVED [50] |
T2DM HF |
≥75 years Caution: risk of volume depletion should be taken into account ≥ 85 years: not recommended |
Reduction in CV mortality Reduction in HHF Reduction in renal adverse events |
| Ertugliflozin | VERTIS-CV [51] | T2DM | ≥65 years Caution: Renal function and risk of volume depletion should be taken into account |
Reduction in HHF |
Outcomes observed across age groups in the EMPA-REG OUTCOME and DECLARE-TIMI 58 cardiovascular outcomes trials (CVOTs) for empagliflozin and dapagliflozin, respectively, provide support for the efficacy and safety of SGLT2 inhibitors in older populations with T2DM (although it should be noted that frailty was not considered) [14, 15]. Post hoc analysis of EMPA-REG, in which 35% of participants were between 65 and < 75 years of age and 9% were ≥ 75 years of age , demonstrated that reductions in risks of CV mortality, HF and renal outcomes associated with empagliflozin were consistent across all age groups supporting its cardiorenal benefits in older individuals [15]. Furthermore, across all age categories, adverse events associated with empagliflozin reflected its known safety profile. A post hoc analysis of DECLARE, in which 54% of participants aged < 65 years, 40% ≥ 65 to <75 years, and 6% ≥ 75 years, demonstrated that the overall efficacy and safety of dapagliflozin was consistent regardless of age. For example, dapagliflozin reduced the composite of CV death or HHF consistently, with a hazard ratio (HR) of 0.88 (95% confidence interval [CI]: 0.72–1.07), 0.77 (95% CI: 0.63–0.94) and 0.94 (95% CI: 0.65–1.36) in age groups <65, ≥ 65 to <75, and ≥ 75 years, respectively (interaction P value 0.53). Although adverse events were generally more frequent in older versus younger individuals, in both treatment and control groups, there was no age-based treatment interaction for any of the safety outcomes assessed [14]. These findings were supported by post hoc analysis of DAPA-HF, in which 60% of participants aged > 65 years, and 24% > 75 years, with results demonstrating that dapagliflozin reduced worsening HF events and death, and improved symptoms, in people with HF, with and without T2DM, across all age groups [16]. Furthermore, as in the DECLARE trial, although adverse events increased with age, they were not associated with dapagliflozin in any age group [16].
A meta-analysis of EMPA-REG OUTCOMES, DECLARE TIMI 58 and CANVAS indicated that the effect of SGLT2 inhibitors on CV outcomes among people with T2DM was consistent across all age groups; the HR for major adverse cardiac events (MACE) was 0.83 (95% CI: 0.71–0.96) for people aged ≥65 years and 0.95 (95% CI: 0.86–1.05) in people aged <65 years, with no subgroup differences (P = 0.15) [29]. Similar results were observed in a meta-analysis of EMPA-REG OUTCOMES, DECLARE TIMI 58 and CREDENCE in which SGLT2 inhibitors were found to reduce MACE outcomes in older adults (> 65 years) by 17% (OR 0.83 (95% CI: 0.70–0.99)), numerically superior to the impact in younger individuals (OR 0.94 (95% CI: 0.79–1.11)) [30].
Age does not fully account for differences in older adults with diabetes, and frailty assessments may be more important than chronological age alone [31]. A post hoc analysis of DAPA-HF examined the efficacy of dapagliflozin according to frailty status [32]. A 32-item frailty index was calculable for 4,742 patients and patients were stratified by three subgroups: 50.4% were in frailty index class 1 (non-frail patients); 33.9% in frailty index class 2 (more frail); and 15.7% in frailty index class 3 (most frail). In this analysis, dapagliflozin was found to reduce the risk of worsening HF or CV death in all frailty groups, with the largest absolute reductions in more frail patients. The reduction in the event rate per 100 years for dapagliflozin versus placebo was 3.5 for the not frail group, 3.6 for the more frail group and, notably, 7.9 for the most frail group. For other clinical events and health status, consistent benefits were observed, but the most frail patients generally had larger absolute reductions. Adverse events related to dapagliflozin treatment were not higher than for placebo, regardless of frailty status.
Post hoc analysis from DAPA-HF indicated that dapagliflozin significantly improves HF-related health status (symptoms, physical function and quality of life), as measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), with the benefits emerging early and being sustained long-term [33]. Treatment with dapagliflozin reduced the risk of clinical events to a similar extent across the range of KCCQ at baseline, indicating that the beneficial effects of dapagliflozin are independent of the health status impairment at baseline.
Additional data from DAPA-HF demonstrated that use of dapagliflozin was associated with a reduction in the risk of CV death and HHF, emerging as early as 28 days after randomisation (worsening HF: HR at 28 days 0.48 [95% CI: 0.23–0.94], CV death: HR at 28 days 0.87 [95% CI: 0.31–2.41]) [34]. These results are consistent with the findings from EMPEROR-Reduced, in which there was a sustained statistically significant benefit of empagliflozin on the primary efficacy end point at 34 days after randomisation [35]. These studies demonstrate that the outcome benefits can be realised quickly following SGLT2 inhibitor initiation and as such support the early addition of SGLT2 inhibitors to the treatment regimen of older adults for which early clinical benefits are important.
Renal impairment is common in older adults with diabetes. SGLT2 inhibitors can exert nephroprotection not only through improving glycaemic control but also through glucose-independent effects, such as blood pressure-lowering and direct renal effects including slowing the decline in glomerular filtration rate (GFR), reducing the onset of microalbuminuria and slowing or reversing the progression of proteinuria. The DAPA-CKD trial demonstrated that for individuals with CKD, regardless of the presence or absence of diabetes, the risk of a composite of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease or death from renal or CV causes was significantly lower with dapagliflozin than with placebo [36]. The event horizon for the outcome benefits was approximately 13 months. For older adults who are otherwise healthy, as well as those that are considered moderately frail, the benefits are likely to be realised within their anticipated life expectancy. However, for individuals with severe frailty the benefit is unlikely to be achieved within their anticipated life expectancy.
The effect of SGLT2 inhibitors on muscle mass in older adults prone to sarcopenia and frailty remains uncertain but is an important issue as loss of muscle is undesirable in this population. However, a clinical trial investigating empagliflozin in older Japanese people with T2DM is currently in progress (Empagliflozin in Elderly T2DM Patients (EMPA-ELDERLY)), to assess its effects on skeletal muscle mass, muscle strength and physical performance [37].
Concerns have been raised regarding the increased potential for adverse events with SGLT2 inhibitors in older adults who are generally more prone to hypoglycaemia, volume depletion, fractures and genital infections. However, a pooled analysis of phase II/III studies has recently demonstrated that dapagliflozin use over a 2-year treatment period was generally well tolerated in older people with T2DM, with comparable rates of hypoglycaemia, genital infections and urinary tract infections, low rates of volume reduction and no increased risk of bone fractures between older and younger populations [26]. Furthermore, as described above, across all age categories in the recently published CVOTs, the adverse event profile associated with SGLT2 inhibitors reflects the known safety profile.
Despite the outcomes benefits associated with SGLT2 inhibitors for older frail adults, there is still an element of confusion around prescription of these agents to these individuals as data regarding the efficacy and safety SGLT2 inhibitors are often lacking for this population, particularly in the very old (>75 years). In addition, comorbidities, frailty and polypharmacy may result in hesitancy from some healthcare professionals to prescribe these drugs. With these issues in mind, understanding the practical considerations around SGLT2 inhibitor use in older frail adults with or without T2DM is essential to optimise the benefit–risk profile of these agents in this population.
Practical considerations
Although current data provide assurance regarding the safety of SGLT2 inhibitors in older people, the general increased risk of some adverse events should be taken into consideration in this population. Older adults prescribed an SGLT2 inhibitor should be informed about the possibility of urogenital fungal infections and encouraged to maintain basic genital hygiene and seek medical attention if symptoms of candidiasis develop. In general, these infections present at a mild-to-moderate intensity, respond well to standard treatment and do not require treatment discontinuation, unless there is recurrent or persistent infection. It is important to be aware that, by the method of action, SGLT2 inhibitors cause acidification of the urine, and dysuria, particularly in the first few weeks, but is not necessarily an indicator of infection.
Rates of hypoglycaemia associated with the use of SGLT2 inhibitors in the CVOTs were not increased with age [14, 15, 26]. Across all age groups, however, they could potentiate the hypoglycaemic effects of insulin, sulfonylureas or tramadol. Further, due to impairment of counter-regulatory responses, these hypoglycaemia episodes have a greater chance of presenting with neuroglycopenic symptoms of hypoglycaemia (dizziness, weakness, delirium, confusion) compared with adrenergic symptoms (tremors, sweating). Thus, these symptoms may be missed or mistaken as primary neurological disease, leading to delayed recognition and treatment of hypoglycaemia [38]. In order to reduce the risk of hypoglycaemia, dose reductions of insulin or sulfonylureas may be required when initiating an SGLT2 inhibitor in people with HbA1c < 8.5% (< 69 mmol/mol).
Due to the diuretic action of SGLT2 inhibitors, they may be associated with volume loss, particularly in those with high circulating glucose. The impact of this may be more pronounced in older adults as a consequence of increased comorbidities, use of medications with hypotensive effects, impaired vascular homeostatic mechanisms, altered thirst response and changes in water and sodium balance that occur with ageing. Simple behavioural modification such as rising slowly from a supine position and maintaining good hydration is often all that is required to reduce these orthostatic symptoms. When measuring blood pressure, exploring for postural drop should be conducted, and if present, an individual should be treated to a blood pressure target based on standing blood pressure [39]. When considering de-intensification of therapies, priority for discontinuation should be given to diuretics. In addition to reducing the risk of postural hypotension, the downtitration of diuretics has additional potential benefit of reducing electrolyte abnormalities such as hyponatremia, hypokalaemia, hypomagnesemia and hypercalcemia which can exert a significant morbidity and cost burden in the older population, particularly with respect to hospitalisation and length of stay. Furthermore, diuretic downtitration offers additional benefits beyond reducing electrolyte abnormalities in terms of reducing dehydration and worsening renal function which also exert a significant healthcare burden in frail older adults.
Whereas the theoretical risk of accelerated osteoporosis or lower limb amputations have been raised in mechanistic studies, or post hoc analyses of single studies, neither of these have been borne out in larger clinical trials. For example, evaluation of the clinical trial program for dapagliflozin has not demonstrated any impact on markers of bone formation and resorption or bone mineral density at 1 year, nor in the risk of fragility fractures, independent of sex, frailty, age and insulin use [26, 40, 41]. With regard to the risk of amputation, while canagliflozin was associated with an increased risk in the CANVAS trial, a meta-analysis assessing the effect of SGLT2 inhibitors on peripheral artery disease and lower limb amputations in 27 randomised controlled trials reported that there is no evidence to suggest that empagliflozin and dapagliflozin increase the risk of either peripheral artery disease of lower limb amputations [42]. Canagliflozin may be associated with a specific risk and thus, this should be a consideration when prescribing SGLT2 inhibitors to older adults.
While the absolute risk is small, SGLT2 inhibitor therapy increases the risk of diabetic ketoacidosis (DKA), an acute metabolic complication of diabetes characterised by hyperglycaemia, hyperketonaemia and metabolic acidosis and which can lead to coma or even death. The incidence of DKA in clinical trials of SGLT2 inhibitors with T2DM was low and did not appear to increase according to age. A significant contributor this is relative insulinopenia, and thus it occurs predominantly at times of concomitant illness with associated restrictions in food intake, or dehydration. As older adults with T2DM are at increased risk of insulinopenia (osmotic symptoms, weight loss), compared to younger adults, due to the relatively longer duration of diabetes this puts them at higher risk of euglycemic ketoacidosis [43]. Pragmatically, this should not preclude their use; however, older adults prescribed an SGLT2 inhibitor should be advised to discontinue them in the presence of any acute dehydrating illness and seek medical assistance if they develop DKA symptoms in order that their ketone levels can be checked.
Effective integration of SGLT2 inhibitors into treatment pathways in older frail adults with and without T2DM will require multidisciplinary treatment pathways with an integrated approach from primary care, diabetologists, cardiovascular specialists, nephrologists, nursing staff and pharmacists to optimise the metabolic, CV and renal risk reduction in their shared patients. Furthermore, the treatment strategy in this population is likely to require greater individualisation to reflect the presence of comorbidities, disability and frailty and estimated life expectancy, as well as the individual’s preferences and goals of therapy.
Summary
The treatment paradigm of T2DM has shifted from a glucose-focused approach to an emphasis on reductions in morbidity and mortality. This is particularly true in the older population, whose life expectancy is shorter and for whom event rates are higher. Although SGLT2 inhibitor therapy has the potential to lead to volume depletion, that can be mitigated by adjusting diuretic therapy, the SGLT2 inhibitors generally lack some of the adverse effects associated with other therapies, such as renal dysfunction, hyperkalaemia and hypotension, that can make treatment challenging in older individuals. As such, SGLT2 inhibitors have proven to be a valuable therapeutic option for the treatment of T2DM in the older population due to their insulin independent mechanism, efficacious glycaemic control with low risk of hypoglycaemia, and their additional renal and CV benefits.
The benefits in a frail population have yet to be fully explored. However, in light of the recently updated NICE T2DM clinical guideline which recommends wider use of SGLT2 inhibitors, alongside metformin, in the first-line treatment of T2DM [25], additional data to support SGLT2 inhibitor use in the frail are anticipated. SGLT2 inhibitors have many benefits which are realised soon after initiation, and thus are likely to give benefit even within the shorter timescale of frail older adults. Consequently, age per se should not be a barrier to these agents and SGLT2 inhibitors should be considered a valid therapeutic option for older frail adults with T2DM, HF or CKD.
Contributor Information
Marc Evans, Diabetes Resource Centre, University Hospital Llandough, Cardiff, UK.
Angharad R Morgan, Health Economics and Outcomes Research Ltd., Cardiff, UK.
Sarah Davies, Woodlands Medical Centre, Cardiff, UK.
Hannah Beba, NHS Leeds Clinical Commissioning Group, Leeds, UK.
William David Strain, Diabetes and Vascular Research Centre, University of Exeter Medical School, Exeter, UK; The Academic Department of Healthcare for Older Adults, Royal Devon and Exeter Hospital, Exeter, UK.
Declaration of Conflicts of Interest
M.E. reports honoraria from AstraZeneca, NovoNordisk, Takeda and NAPP, and research support from NovoNordisk outside the submitted work. A.R.M. is an employee of Health Economics and Outcomes Research Ltd., Cardiff, UK who received fees from AstraZeneca in relation to this study. S.D. has received honorarium from AstraZeneca, Boehringer Ingelheim, Lilly, Novo Nordisk, Takeda, MSD, NAPP, Bayer and Roche for attending and participating in educational events and advisory boards, outside the submitted work. H.B. has received speaker honoraria and consultancy fees from Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Napp, Novo Nordisk, Sanofi and Takeda, outside the submitted work. W.D.S. holds research grants from Bayer, Novo Nordisk and Novartis and has received speaker honoraria from AstraZeneca, Bayer, Bristol-Myers Squibb, Merck, Napp, Novartis, Novo Nordisk and Takeda, outside the submitted work. W.D.S. is supported by the NIHR Exeter Clinical Research Facility and the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for the South West Peninsula.
Declaration of Sources of Funding
This work was supported by a grant from AstraZeneca in respect of medical writing and publication costs. AstraZeneca has not influenced the content of the publication or been involved in the writing of this publication. AstraZeneca has reviewed this document for factual accuracy only.
References
- 1. Sinclair A, Saeedi P, Kaundal A, Karuranga S, Malanda B, Williams R. Diabetes and global ageing among 65-99-year-old adults: findings from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 2020; 162: 108078. 10.1016/j.diabres.2020.108078. [DOI] [PubMed] [Google Scholar]
- 2. Jani B, Rajkumar C. Ageing and vascular ageing. Postgrad Med J 2006; 82: 357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. J Am Soc Nephrol 2017; 28: 407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mitnitski A, Howlett SE, Rockwood K. Heterogeneity of human aging and its assessment. J Gerontol A Biol Sci Med Sci 2017; 72: 877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fried LP, Tangen CM, Walston Jet al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–57. [DOI] [PubMed] [Google Scholar]
- 6. Diabetes UK. Diabetes in the UK 2010: Key Statistics on Diabetes. 2010. https://www.diabetes.org.uk/resources-s3/2017-11/diabetes_in_the_uk_2010.pdf(19 July 2022, date last accessed).
- 7. Sinclair AJ, Gadsby R, Penfold S, Croxson SC, Bayer AJ. Prevalence of diabetes in care home residents. Diabetes Care 2001; 24: 1066–8. [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes Association . 12. Older adults: standards of medical care in diabetes-2021. Diabetes Care 2021; 44: S168–s79. [DOI] [PubMed] [Google Scholar]
- 9. Abdul-Ghani MA, DeFronzo RA. Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr Pract 2008; 14: 782–90. [DOI] [PubMed] [Google Scholar]
- 10. McGuire DK, Shih WJ, Cosentino Fet al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021; 6: 148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neuen BL, Oshima M, Agarwal Ret al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized controlled trials. Circulation 2022; 145: 1460–70. [DOI] [PubMed] [Google Scholar]
- 12. Zelniker TA, Wiviott SD, Raz Iet al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019; 393: 31–9. [DOI] [PubMed] [Google Scholar]
- 13. Brown E, Wilding JPH, Alam U, Barber TM, Karalliedde J, Cuthbertson DJ. The expanding role of SGLT2 inhibitors beyond glucose-lowering to cardiorenal protection. Ann Med 2021; 53: 2072–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cahn A, Mosenzon O, Wiviott SDet al. Efficacy and safety of dapagliflozin in the elderly: analysis from the DECLARE-TIMI 58 study. Diabetes Care 2020; 43: 468–75. [DOI] [PubMed] [Google Scholar]
- 15. Monteiro P, Bergenstal RM, Toural Eet al. Efficacy and safety of empagliflozin in older patients in the EMPA-REG OUTCOME® trial. Age Ageing 2019; 48: 859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martinez FA, Serenelli M, Nicolau JCet al. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age: insights from DAPA-HF. Circulation 2020; 141: 100–11. [DOI] [PubMed] [Google Scholar]
- 17. Electronic Medicines Compendium (EMC) . SmPC: Steglatro 5 mg Film-Coated Tablets 2021. https://www.medicines.org.uk/emc/product/9803/smpc#gref(19 July 2022, date last accessed).
- 18. Electronic Medicines Compendium (EMC) . SmPC: Invokana 100 mg and 300 mg Film-Coated Tablets. 2021. https://www.medicines.org.uk/emc/medicine/28400#gref(19 July 2022, date last accessed).
- 19. Electronic Medicines Compendium (EMC) . SmPC: Jardiance 10 mg Film-Coated Tablets. 2021. https://www.medicines.org.uk/emc/product/5441/smpc#gref(19 July 2022, date last accessed).
- 20. Electronic Medicines Compendium (EMC) . SmPC: Forxiga 10 mg Film-Coated Tablets 2021. https://www.medicines.org.uk/emc/product/7607/smpc#gref(19 July 2022, date last accessed).
- 21. Ganz M, Ustyugova A, Sawalhi-Leckenby Net al. Utilization of glucose-lowering drugs in patients with T2DM and established CVD in US: a descriptive study using optum clinformatics data. J Am Coll Cardiol 2020; 75: 2017. [Google Scholar]
- 22. Knudsen JS, Baggesen LM, Lajer Met al. Changes in SGLT2i and GLP-1RA real-world initiator profiles following cardiovascular outcome trials: a Danish nationwide population-based study. PLoS One 2020; 15: 1–14. 10.1371/journal.pone.0229621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arnold SV, Inzucchi SE, Tang Fet al. Real-world use and modeled impact of glucose-lowering therapies evaluated in recent cardiovascular outcomes trials: an NCDR® Research to Practice project. Eur J Prev Cardiol 2017; 24: 1637–45. [DOI] [PubMed] [Google Scholar]
- 24. Schernthaner G, Shehadeh N, Ametov ASet al. Worldwide inertia to the use of cardiorenal protective glucose-lowering drugs (SGLT2i and GLP-1 RA) in high-risk patients with type 2 diabetes. Cardiovasc Diabetol 2020; 19: 185. 10.1186/s12933-020-01154-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. National Institute for Health and Care Excellence . NICE Guideline [NG28]: Type 2 Diabetes in Adults: Management. 2022. https://www.nice.org.uk/guidance/ng28(19 July 2022, date last accessed). [PubMed]
- 26. Fioretto P, Mansfield TA, Ptaszynska A, Yavin Y, Johnsson E, Parikh S. Long-term safety of dapagliflozin in older patients with type 2 diabetes mellitus: a pooled analysis of phase IIb/III studies. Drugs Aging 2016; 33: 511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sinclair AJ, Bode B, Harris Set al. Efficacy and safety of canagliflozin in individuals aged 75 and older with type 2 diabetes mellitus: a pooled analysis. J Am Geriatr Soc 2016; 64: 543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lunati M, Trevisan M, Gandolfi Aet al., eds. Safety and efficacy of SGLT2-inhibitors in over 70 years old type 2 diabetic patients: 1 year of follow up. In: Diabetologia. New York, NY, United States: Springer One New York Plaza, Suite 4600, 2021. [Google Scholar]
- 29. Giugliano D, Longo M, Maiorino MIet al. Efficacy of SGLT-2 inhibitors in older adults with diabetes: Systematic review with meta-analysis of cardiovascular outcome trials. Diabetes Res Clin Pract 2020; 162: 108114. 10.1016/j.diabres.2020.108114. [DOI] [PubMed] [Google Scholar]
- 30. Strain WD, Griffiths J. A systematic review and meta-analysis of the impact of GLP-1 receptor agonists and SGLT-2 inhibitors on cardiovascular outcomes in biologically healthy older adults. Br J Diabetes 2021; 21: 30–5. [Google Scholar]
- 31. Strain WD, Down S, Brown P, Puttanna A, Sinclair A. Diabetes and frailty: an expert consensus statement on the management of older adults with type 2 diabetes. Diabetes Therapy 2021; 12: 1227–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Butt JH, Dewan P, Merkely Bet al. Efficacy and safety of dapagliflozin according to frailty in heart failure with reduced ejection fraction: a post hoc analysis of the DAPA-HF trial. Ann Intern Med 2022; 175: 820–30. [DOI] [PubMed] [Google Scholar]
- 33. Kosiborod MN, Jhund PS, Docherty KFet al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation 2020; 141: 90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berg DD, Jhund PS, Docherty KFet al. Time to clinical benefit of dapagliflozin and significance of prior heart failure hospitalization in patients with heart failure with reduced ejection fraction. JAMA Cardiol 2021; 6: 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Packer M, Anker SD, Butler Jet al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Circulation 2021; 143: 326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heerspink HJL, Stefánsson BV, Correa-Rotter Ret al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436–46. [DOI] [PubMed] [Google Scholar]
- 37. Yabe D, Shiki K, Suzaki Ket al. Rationale and design of the EMPA-ELDERLY trial: a randomised, double-blind, placebo-controlled, 52-week clinical trial of the efficacy and safety of the sodium-glucose cotransporter-2 inhibitor empagliflozin in elderly Japanese patients with type 2 diabetes. BMJ Open 2021; 11: e045844. 10.1136/bmjopen-2020-045844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matyka K, Evans M, Lomas J, Cranston I, Macdonald I, Amiel SA. Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care 1997; 20: 135–41. [DOI] [PubMed] [Google Scholar]
- 39. National Institute for Health and Care Excellence . NICE Guideline [NG136]: Hypertension in adults: Diagnosis and Management. 2019. https://www.nice.org.uk/guidance/ng136(19 July 2022, date last accessed). [PubMed]
- 40. Ljunggren Ö, Bolinder J, Johansson Let al. Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes Obes Metab 2012; 14: 990–9. [DOI] [PubMed] [Google Scholar]
- 41. Zhuo M, Hawley CE, Paik JMet al. Association of sodium-glucose cotransporter-2 inhibitors with fracture risk in older adults with type 2 diabetes. JAMA Netw Open 2021; 4: e2130762. 10.1001/jamanetworkopen.2021.30762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dicembrini I, Tomberli B, Nreu Bet al. Peripheral artery disease and amputations with sodium-glucose co-transporter-2 (SGLT-2) inhibitors: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2019; 153: 138–44. [DOI] [PubMed] [Google Scholar]
- 43. Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care 2015; 38: 1638–42. [DOI] [PubMed] [Google Scholar]
- 44. Wiviott SD, Raz I, Bonaca MPet al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2018; 380: 347–57. [DOI] [PubMed] [Google Scholar]
- 45. McMurray JJV, Solomon SD, Inzucchi SEet al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 46. Neal B, Perkovic V, Mahaffey KWet al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–57. [DOI] [PubMed] [Google Scholar]
- 47. Perkovic V, Jardine MJ, Neal Bet al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–306. [DOI] [PubMed] [Google Scholar]
- 48. Zinman B, Wanner C, Lachin JMet al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–28. [DOI] [PubMed] [Google Scholar]
- 49. Packer M, Anker SD, Butler Jet al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–24. [DOI] [PubMed] [Google Scholar]
- 50. Anker SD, Butler J, Filippatos Get al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021; 385: 1451–61. [DOI] [PubMed] [Google Scholar]
- 51. Cannon CP, Pratley R, Dagogo-Jack Set al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020; 383: 1425–35. [DOI] [PubMed] [Google Scholar]


