Abstract
Useful Field of View (UFOV) computerized cognitive training improves older adults’ gait speed and balance and reduces dementia risk. We investigated a new form of UFOV training requiring physical movement, Training Under Cognitive Kinematics (TUCK). We hypothesized TUCK would be acceptable, feasible, and potentially efficacious to improve UFOV Test- and motor performance. Sixty-nine older adults were randomized to TUCK, computerized UFOV training, or an active control group. Cognitive- and motor function were assessed before and immediately after the intervention period. Participants rated TUCK as enjoyable, engaging and satisfying, indicating acceptability. Eighty-five percent of participants completed TUCK, demonstrating feasibility. Overall, effect sizes for TUCK did not indicate greater efficacy than computerized UFOV training relative to controls. UFOV training showed effect sizes indicating improved balance as measured by Turn 360 (d=0.37) and Optogait (d=0.51–0.69) from pre- to post- training relative to controls. Incorporating movement into UFOV cognitive training did not enhance cognitive or motor functional gains. Future investigations are needed to elucidate the underlying mechanisms of UFOV cognitive training to enhance motor function. Research should continue to investigate the association of cognitive and motor function and interventions to improve these outcomes among older adults. The trial and planned analyses were pre-registered: https://osf.io/7utgw.
Keywords: balance, cognitive training, speed of processing training, gait, cognitive intervention
Introduction
Dementia increases in prevalence with age and is the most expensive medical condition in the U.S. (Hurd et al. 2013). More than 5 million Americans have Alzheimer’s disease, the most common form of dementia (Alzheimer’s Association 2012). As the population of older adults continues to increase, so does the need for enhanced methods to reduce dementia risk. Efficacious interventions to delay or prevent the progression of cognitive decline to dementia are critically needed to promote health and well-being.
Declines in motor function (e.g., gait and balance) occur several years before a dementia diagnosis and may signal dementia risk (Albers et al. 2015). Older adults with cognitive decline demonstrate poor performance on motor function assessments (e.g., Maquet et al. 2010)). Furthermore, several cognitive processes are significantly associated with motor function including executive function (e.g., Desjardins-Crepeau et al. 2014; Martin et al. 2013; Soumaré et al. 2009; Donoghue et al. 2012), attention (e.g., Holtzer et al. 2006; Martin et al. 2013), and processing speed (e.g., Desjardins-Crepeau et al. 2014; Donoghue et al. 2012; Martin et al. 2013; Soumaré et al. 2009).
Given that age-related cognitive declines contribute to motor difficulties, research has recently examined whether cognitive interventions may improve motor function (e.g., Li and Lindenberger 2002; Smith-Ray et al. 2014a; Smith-Ray et al. 2014b; Verghese et al. 2010). Such studies indicate that psychomotor speed, postural-stability, balance, and gait speed may be enhanced by cognitive interventions.
UFOV cognitive training (a.k.a., cognitive speed of processing training) is one of the most well-studied cognitive interventions (Edwards et al. 2017a; Edwards et al. 2017b; F. Lin et al. 2016; Rebok et al. 2014; Smith-Ray et al. 2014a; Smith-Ray et al. 2014b). UFOV training is the only intervention to date, behavioral or pharmacological, shown to lower risk of dementia (Edwards et al. 2017a). Recent analyses from ACTIVE, a large, multi-site randomized trial, indicate that randomization to UFOV training reduced dementia risk by 29% across ten years (Edwards et al. 2017a). Interestingly, scientists have also shown that UFOV cognitive training enhances older adults’ motor function as evident by improved gait speed and balance (Smith-Ray et al. 2014a; Smith-Ray et al. 2014b). UFOV training may be a particularly efficacious approach because it specifically targets speed of processing, attention, and executive function, cognitive abilities strongly associated with motor function (e.g., Blankevoort et al. 2013; Clouston et al. 2013; Desjardins-Crepeau et al. 2014).
Three trials have examined the effects of UFOV training on balance and gait (Smith-Ray et al. 2014a; Smith-Ray et al. 2014b; Ross et al. 2016). Adults aged 70 and older with a history of falls and/or balance difficulties (N=51) were randomized to computerized UFOV training or falls education. Results indicated that those randomized to UFOV training showed medium effect sizes relative to controls for improved balance and gait speed. In a second study, researchers randomized 45 older minority adults with a history of falls or balance difficulties to UFOV training or a no-contact control group and found medium to large effect sizes for improved balance and gait speed (Smith-Ray et al. 2014b). Secondary analyses of the ACTIVE study were conducted to examine the longitudinal effects of UFOV training relative to no-contact controls on motor function among relatively healthy older adults. Across five years, higher doses of UFOV training were associated with better psychomotor speed and balance (Ross et al. 2016). Overall, these three studies demonstrate that computerized UFOV cognitive training enhances balance and gait speed.
Implementing interventions to prevent cognitive decline, improve motor abilities, maintain functional independence, and lower incidence of dementia is of paramount importance to helping older adults maintain their health and quality of life. Thus, we examined a new, dynamic application of UFOV cognitive training with the aim to enhance the cognitive, motor, and functional benefits of this training technique. This innovative version of UFOV training is referred to as Training Under Cognitive Kinematics (TUCK) and integrates motor responses with the established UFOV training exercises. Our objective was to examine the acceptability, feasibility, and potential efficacy of TUCK training. Based on the current body of knowledge regarding the relationship between cognitive and motor function and results that UFOV training improves gait speed and balance, we expected TUCK to show potential to improve cognitive and motor performance relative to controls. Furthermore, we expected the motor function gains from TUCK to be larger than those from traditional UFOV training.
Materials and Methods
Participants
All participants provided informed consent before participating in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the University of South Florida Institutional Review Board and Ethics Committee (Project identification code 00033916). Participants (N=107) were recruited through a registry of older adults interested in research. Advertisements were placed and mass-mailings were distributed to older adults residing in the greater Tampa Bay, Florida area.
Inclusion criteria were: at least 65 years of age, the ability to speak, read, and understand English, willingness and availability to complete the entire study, intact vision quantified as near visual acuity of 20/50 or better, Montreal Cognitive Assessment score (MoCA) of 21 or higher, adequate hearing as indicated by pure-tone thresholds ≤70 dB HL in the mid-frequency range in at least one ear, and adequate functional reach (detailed below). We included only those who were novice to cognitive training. Specifically, individuals who had less than 10 hours of previous cognitive training experience, which may be the minimum dose to induce cognitive/functional benefits (Kelly et al. 2014), were included. We recruited individuals with either a history of falls (1 or more in the past two years) or self-reported balance difficulties (Smith-Ray et al. 2014b; Smith-Ray et al. 2014a). Participants were required to self-report the ability to walk unassisted for two or more minutes. Exclusion criteria at baseline included a Geriatric Depression Scale (GDS) score of 5 or greater; limited step clearance (detailed below); plans to undergo general anesthesia, chemotherapy, or radiation treatment during the study period; or concurrent participation in another study. If at any time during study enrollment participants underwent anesthesia, chemotherapy or radiation treatment; participated in another research study; participated in a computerized cognitive training program outside of the study; or received diagnosis of a neurological impairment, dementia, stroke or serious brain injury, they were deemed ineligible.
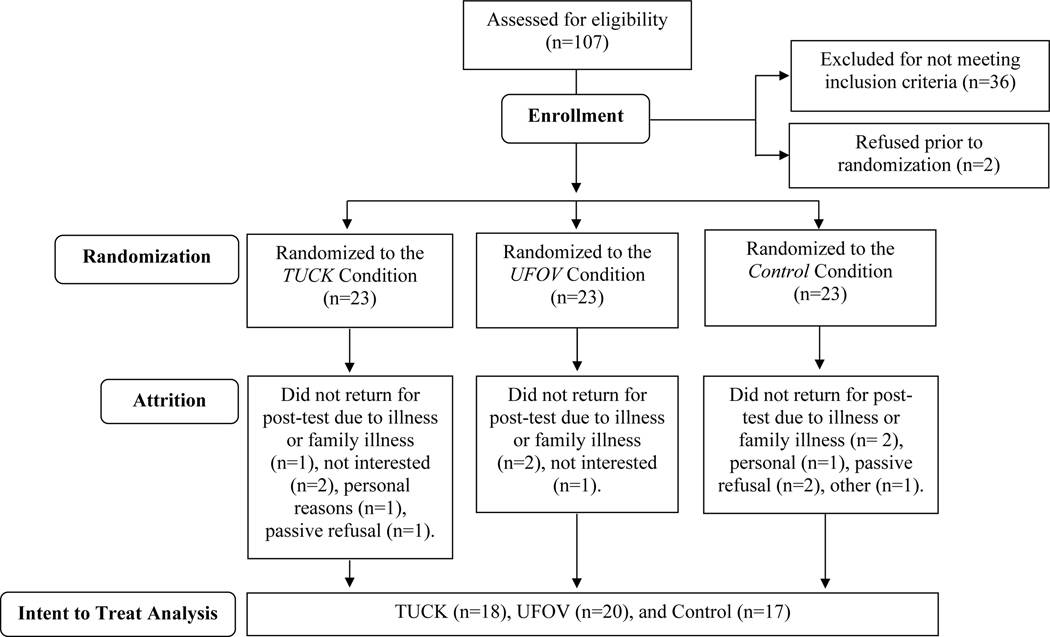
Thirty-eight participants were ineligible at baseline due to cognitive status (n=12), depressive symptoms (n=12), functional reach (n=4), step clearance (n=4), vision (n=1), neurological impairment (n=1), participation in another research study (n=1), planned general anesthesia (n=1), too busy (n=1), or not interested (n=1). See Figure 1. Sixty-nine participants were enrolled and randomized to one of three arms: TUCK (n=23), computerized cognitive training (n=23), or an active control condition of computer games (n=23). The randomized sample had an age range of 65 to 91 years and with an average age of 73 years, an average education of 15 years, and included 59% females. The majority of participants (91%) reported Caucasian race and 4% indicated Hispanic ethnicity.
Figure 1.
Flow of Participants
Measures
Inclusion
MoCA was used to examine participants’ cognitive status (Nasreddine et al. 2005). This assessment has good internal consistency, Cronbach’s α=.83, and test-retest reliability, r=.92 (Nasreddine et al. 2005). The MoCA has 94% sensitivity to identify dementia and was used to briefly screen mental status to exclude those with probable dementia (Nasreddine et al. 2005).
Near visual acuity was assessed at 40 centimeters using standardized procedures with a Sloan letter chart (Good-Lite 2010).
Using standard procedures, air conduction hearing thresholds were assessed at 500, 1000, 2000, 4000, 6000 and 8000 Hz using insert earphones or over the ear headphones. Hearing was considered adequate for inclusion if pure-tone thresholds were less than or equal to 70 dB HL in the mid-frequency range (e.g., 1000, 2000 Hz) in at least one ear as determined by standard hearing evaluation. All audiometric equipment was calibrated, functioned properly, and located in an environment free of electrical interference as specified in American National Standards Institute Standards 3.6–2010 (Institute, 2010).
The GDS was used to screen depressive symptoms. The GDS has 92% sensitivity and 89% specificity to detect depression and has good test-retest reliability, r=.75-.81 (Friedman et al. 2005). Those with signs of moderate or worse depression (GDS score of 5 or greater) were excluded, as prior work indicates that depression attenuates UFOV training benefits (Wolinsky et al. 2009).
Participants were required to demonstrate step clearance capacity to lift each foot greater than 3 cm off the ground, a minimum functional reach of at least 0.1524 meters, and shoulder range motion of no less than 90 degrees flexion to indicate the physical capacity to complete the study safely, particularly the TUCK intervention.
Cognitive Function
Cognitive function was assessed with the Useful Field of View Test (UFOV). This reliable (r=.74-.81 test re-test reliability) and valid test measures speed of processing for visual attention tasks (Edwards et al. 2005a). The UFOV Test is the proximal outcome of UFOV cognitive training and determines the minimum display duration at which participants processed information for three increasingly difficult subtests. Display times were manipulated between 16 and 500 ms to determine the 75% correct threshold. In subtest one, a silhouette of either a car or a truck was presented inside a fixation box and identified by the participant. Subtest two required the participant to identify a car or a truck presented in a fixation box and localize a car presented in the periphery. Subtest three required the same two responses but included distractors that surrounded the peripheral car. Performance across the subtests was summed for use in analyses. Smaller scores indicate better performance.
Motor Function
Motor function was assessed by the Timed Up and Go Test (TUG), Functional Reach, Turn 360, and OptoGait analysis system. Gait speed, the primary motor outcome of interest, was measured by TUG (Shumway-Cook et al. 2000). During the test, the participant was asked to get up from a chair, walk a 3-meter course, turn around, walk back to the chair, and sit down. Participants completed two trials and the average time in seconds was used in analyses. Previous research indicates the TUG Test has good validity (M. R. Lin et al. 2004). Faster times indicate better performance.
Secondary motor outcomes included the Functional Reach and Turn 360 tests. The Functional Reach Test was used to examine postural control (Duncan et al. 1990). The Functional Reach Test has good construct validity and test-retest reliability, r=.89 (Duncan et al. 1990). For the Functional Reach test participants were positioned next to the wall with one arm raised 90 degrees. The distance in inches that a subject was able to reach forward from an initial upright posture to the maximal anterior leaning posture without moving or lifting the feet was measured. The distances of two trials were averaged as the total score, with a greater distance indicating better balance.
The well-established, reliable, and valid Turn 360 test was used to assess balance (Reuben and Siu 1990). Participants were asked to assume a standing starting position and make one complete 360-degree turn. The number of steps required to make the turn was recorded and the procedure was repeated for a second turn. Fewer steps represent better performance. The average number of steps across both turns was used for analyses. Smaller steps indicate better performance.
An exploratory motor function outcome was gait analysis from the OptoGait system while participants marched in place (Lienhard et al. 2013). The OptoGait system consists of a single transmitting bar and receiving bar that is 100×8 cm and contains 96 light diodes located 3 mm above floor level and approximately 1 cm apart, each working at 1000Hz. Participants stood in the middle of the transmitting and receiving bars and marched in place by lifting their knees and swinging their arms at a pace they felt comfortable. Participants performed this task for 30 seconds with their eyes open and again for 30 seconds with their eyes closed. The OptoGait software was used to obtain gait parameters of contact time and asymmetry to characterize dynamic balance control. Asymmetry refers to the difference in contact time between left and right strides. Several studies have demonstrated that OptoGait is reliable and a valid tool for gait analysis (Lienhard et al. 2013).
Cognitive Training Expectations
An established questionnaire was used to examine participants’ attitudes and expectations about the intervention (Rabipour and Davidson 2015). The questionnaire referred to cognitive function as abilities such as attention, memory, visual perception, information processing, and reasoning, and that cognitive training refers to activities that aim to improve cognitive functions by training within a specific timeframe (e.g., several weeks or months). Using this definition participants were asked to rate what effects they expected the intervention exercises to have including whether the exercises would result in improved general cognitive function, memory, concentration, distractibility, reasoning, multi-tasking, or everyday performance. Ratings were on a 7-point Likert scale ranging from completely unsuccessful (1), to no expectations (4), to completely successful (7). Participants also rated the degree to which the intervention was engaging, challenging, or enjoyable on a 7-point Likert scale ranging from very strongly disagree (1), to neither agree or disagree (4), to very strongly agree (7). Finally, participants rated whether they were satisfied with the program ranging from extremely dissatisfied (1), to neither satisfied or dissatisfied (4), to extremely satisfied (7).
Intervention and Control Conditions
UFOV Cognitive Training.
Participants randomized to the UFOV training condition completed traditional, computerized UFOV cognitive training. UFOV training, also referred to as speed of processing training, included repeated practice of visual exercises designed to improve information processing speed and attention. The exercises (i.e., Hawkeye, Double Decision, and Eye for Detail) were accessed using BrainHQ. The goal of the exercises was to improve UFOV Test performance by gradually increasing the amount of information one can quickly process. The exercises varied in difficulty ranging from basic visual processing speed (i.e., identifying central visual targets), to more advanced divided and selective attention tasks (i.e., identifying and localizing targets among distractors). Within each exercise, the stimuli (visual targets and distractors) became less discriminable and display duration decreases (i.e., briefer display times, making the exercises more difficult) as performance improved. Exercise difficulty is adapted automatically by the software based upon ongoing user performance. Within exercises, the stimuli were adapted in a graduated fashion moving from simple to complex visual stimuli.
TUCK Cognitive Training.
Participants randomized to the TUCK condition completed UFOV training exercises, which involved identifying central targets while simultaneously localizing peripheral targets at brief durations. Three BrainHQ training exercises were used: Hawkeye, Double Decision, and Eye for Detail. However, the stimuli were presented via light diodes rather than by computer. Training exercises were presented using a 7×5 LED matrix referred to as the TUCK system. The same adaptive algorithms used in the computer based UFOV exercises were programmed for use by the TUCK system. Thus, participants completed the same training tasks, but the responses required coordinated kinetics. As in traditional UFOV training, the software decreased the stimuli display duration as participants performed well. The trainer manipulated the light diodes to increase target eccentricity and add distractors as the participant’s performance improved. The TUCK system also differs from traditional UFOV training in that the stimuli are larger and involve fewer distractors.
Cognitive Stimulation Control.
Participants randomized to the active control condition of cognitive stimulation (i.e., computer games) completed three commercially available computer games. The control condition was designed to match TUCK and UFOV training with the expectation-based influence on cognitive performance, intensity, and overall engagement. Games providing face-valid cognitive stimulation that are rated E (for everyone) by the Entertainment Software Rating Board were used.
Procedure
The study protocol was approved by the University of South Florida Institutional Review Board (Pro00033916). The procedures performed in this study involving human participants were in accordance with the 1964 Helsinki declaration. All participants provided written consent prior to participating and all mandatory laboratory health and safety procedures were complied with during the course of conducting this work. The trial and planned analyses were pre-registered: https://osf.io/7utgw.
All participants were telephone screened to ascertain that they met the initial inclusion criteria. After obtaining informed consent, eligibility was further assessed at an in-person screening visit by measuring near visual acuity, hearing, cognitive status, depressive symptoms, and the physical capacity to complete the TUCK intervention safely. Participants completed pre- training measures of cognitive and motor functioning. Enrolled participants were randomized to one of three arms: TUCK, UFOV computerized cognitive training, or an active control condition. See Figure 1.
The training and control conditions were completed in groups of 2–5 participants, in 1-hour sessions, conducted two to three times per week over an approximate 12-week period. The first session of TUCK training was led by an Occupational Therapist (LG) to ensure participant safety. A 5-minute break was required mid-way through each training sessions, and participants were allowed to take additional breaks at any time during the training sessions. Participants completed the cognitive training expectations questionnaire at the final training visit or at the post-training visit. Participants were invited to complete post-test regardless of adherence and at this final visit they completed post- training measures of cognitive and motor functioning administered by blind assessors.
Analyses
Acceptability of the intervention was calculated using the median of participant ratings on the cognitive training expectations questionnaire (Rabipour and Davidson 2015). We assessed acceptability by participant ratings on the degree to which the intervention was enjoyable, challenging, engaging, and satisfying on a 7-point Likert scale (Rabipour and Davidson 2015). Our a priori criteria for acceptability were that if participants rate these four items with an overall median of greater than or equal to 5, we would consider TUCK to be an acceptable intervention.
Feasibility was measured by quantifying the proportion of participants randomized to TUCK who successfully completed the intervention protocol. Our a priori criteria were that if at least 75% of the study participants randomized to TUCK training successfully completed 15 or more hours of TUCK, the feasibility of the training would be confirmed.
We measured the potential efficacy of TUCK by quantifying pre- to post- training d effect size improvements on cognitive and motor performance assessments relative to controls. We expected TUCK to show improved cognitive performance (measured by UFOV test) with an effect size of d=0.30 from pre- to post- test. We expected TUCK to improve motor function relative to controls as assessed by the TUG test with secondary indices being the Functional Reach Test and Turn 360 and Optogait as an exploratory outcome. We expected the motor function gains from TUCK to be larger than traditional UFOV training.
Results
Of the 69 eligible participants randomized, 55 participants completed post-test. Two participants withdrew after randomization, but before training participation and the remaining withdrew due to illness, disinterest, or personal reasons. See Figure 1 for details. With regard to missing data, two participants did not complete the UFOV Test post-training due to participant refusal (n=1) or computer software error (n=1) and 5 participants did not complete Optogait post-training.
The median ratings for the cognitive training expectations questionnaire items for each condition are reported in Table 1. Participants, on average, agreed that TUCK training was enjoyable, challenging, engaging, and satisfying, indicating acceptability.
Table 1.
Older Adults’ Post-Training Median Ratings on Cognitive Training Expectations Questionnaire by Training Condition.
| TUCK (n=20) |
UFOV Training (n=23) |
Control (n=21) |
|
|---|---|---|---|
| Challenging | 5 | 6 | 5 |
| Engaging | 5 | 5 | 5 |
| Enjoyable | 5 | 5 | 5 |
| Satisfying | 6 | 5 | 6 |
Note. TUCK=Training Under Cognitive Kinematics; UFOV=Useful Field of View. Scores reflect ratings on Likert scale ranging from 1 to 7.
For the TUCK condition, of the 23 randomized, 20 completed at least 15 or more sessions of TUCK (M=18 sessions completed, SD=4.94). Feasibility was thus demonstrated by 86.95% of participants randomized to TUCK completing the intervention protocol. There were no significant differences between number of sessions completed by training condition, F(2, 63)=.862, p=.427, ɳ2p=.027. On average participants completed 18 sessions of TUCK training, 17 sessions of UFOV training, or 16 sessions of the active control condition. Pre- and post-training means and standard deviations for the outcome measures by randomized condition are reported in Table 2.
Table 2.
Means and Standard Deviations for Outcomes Pre- and Post-Training by Randomized Condition
| TUCK | UFOV | Control | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Pre-Training (n=20) | Post-Training (n=18) | Pre-Training (n=23) | Post-Training (n=20) | Pre-Training (n=21) | Post-Training (n=17) | |
|
| ||||||
| M (SD) | M (SD) | M (SD) | ||||
| UFOV Test total score in ms+ | 322.69 (135.13) | 221.33 (118.06) | 373.87 (216.79) | 149.65 (67.11) | 423.91 (254.78) | 313.76 (177.49) |
| Functional Reach inches | 9.46 (3.21) | 9.93 (2.71) | 8.00 (2.50) | 9.36 (7.91) | 8.25 (3.07) | 9.66 (1.90) |
| Turn 360 # of steps+ | 6.17 (1.56) | 6.17 (2.20) | 6.61 (2.13) | 5.68 (1.91) | 5.87 (1.96) | 5.65 (2.04) |
| Timed Up and Go time in s+ | 12.17 (3.08) | 12.55 (4.04) | 12.91 (3.61) | 12.22 (2.84) | 13.16 (3.18) | 11.97 (1.78) |
| Optogait Asymmetry+ | 18.69 (60.14) | 6.26 (7.96) | 7.82 (8.26) | 3.27 (2.89) | 8.57 (11.15) | 6.21 (6.58) |
| Optogait Contact Time+ | 24.43 (33.16) | 22.16 (27.19) | 19.95 (19.98) | 7.98 (6.27) | 20.23 (25.59) | 20.02 (27.83) |
Note. TUCK=Training Under Cognitive Kinematics; UFOV=Useful Field of View.
lower is better
Effect sizes from pre- to post- training relative to controls were calculated after constraining outliers to +/− 2.5z and are reported in Table 3. The effect sizes for TUCK training indicated improved cognitive and motor performance relative to controls, with small effects on UFOV and assymetry, an index of dynamic balance control (i.e., Optogait). Relative to controls, UFOV training showed effect sizes indicating improved UFOV, Turn 360 performance, and both the asymmetry and contact time aspects of dynamic balance control.
Table 3.
Older Adults’ Pre- to Post- training Cognitive and Motor Performance Represented as d Effect Size Relative to Controls.
| UFOV Test | Functional Reach | Turn 360 | Timed Up and Go | Optogait Asymmetry | Optogait Contact Time | |
|---|---|---|---|---|---|---|
| TUCK Training | 0.13 | −0.29 | −0.05 | −0.42 | 0.17 | 0.03 |
| UFOV Training | 0.92 | −0.06 | 0.37 | −0.15 | 0.69 | 0.51 |
Note. Outliers were constrained to +/− 2.5 z prior to calculating effect sizes. Overall performance within the groups tended to improve from pre- to post-training. Positive d effects indicate pre-to post training changes were larger relative to controls- while negative d indicates pre- to post- test changes were smaller relative to controls. TUCK=Training Under Cognitive Kinematics; UFOV=Useful Field of View.
Discussion
This randomized, pilot trial examined a new, dynamic form of UFOV cognitive training, TUCK, to determine the acceptability, feasibility, and potential efficacy of the intervention. Participants rated the TUCK intervention as acceptable and it met our pre-specified feasibility criteria. The results suggest that a dynamic computerized cognitive training incorporating movement is a feasible and acceptable intervention among older adults. However, there were only small effect sizes of TUCK on the proximal outcome, UFOV performance, and one index of balance. In sum, we investigated if TUCK is a viable intervention for older adults. Although TUCK was acceptable and feasible, it did not show greater efficacy as compared to computerized UFOV training in this study.
We hypothesized that the TUCK intervention would result in improved cognitive and motor abilities, and that the motor function gains would be larger than those of the traditional UFOV training intervention. This hypothesis was not supported. This finding could be due to reduced complexity of the TUCK training. As previously mentioned, the TUCK system differed from the UFOV traditional training in that distractor stimuli were fewer. It is possible the reduction in peripheral distractors during TUCK training reduced the complexity of the training compared to UFOV, thereby reducing the efficacy of the training. However, in our early UFOV training investigations, we found that reducing the number of distractors by implementing training on a small- rather than a large- monitor, did not significantly alter efficacy (Ball et al. 1988; Edwards et al. 2005b). So, in our opinion, the number of distractors was not likely a major determinant of efficacy. The differential efficacy of TUCK could be due to increased cognitive load participants may experience from engaging in a dual task, monitoring visual targets, and initiating body movements accordingly. Perhaps a multimodal approach to UFOV cognitive training, or an active training matching complexity by number of distractors could show efficacy. In summary, our results indicate that incorporating movement into UFOV cognitive training exercises by presenting stimuli by light diodes may not be a superior approach to computerized UFOV cognitive training.
Consistent with previous findings (Smith-Ray et al. 2014a; Smith-Ray et al. 2014b), UFOV training resulted in improved balance as demonstrated by the Turn 360 test and Optogait parameters. Contrary to prior findings (Smith-Ray et al. 2014a; Smith-Ray et al. 2014b) gait speed, as measured by the TUG was not improved by UFOV training relative to controls in the current study. Both previous studies had relatively small sample sizes that were comparable in size to the current study, but these prior studies included more minority participants. Overall, these data and prior research are indicative that computerized, cognitive training in the UFOV paradigm improves balance among older adults.
This study is not without limitations. Our sample size was small and lacked diversity. The study population was predominantly Caucasian and relatively well-educated. Future studies would benefit from a larger, more heterogeneous sample in terms of race, ethnicity, and education. Prior works suggests that older adults with lower education levels, as included in previous studies (Smith-Ray et al. 2014a; Smith-Ray et al. 2014b), may particularly benefit from UFOV training (Clark et al. 2015). The nature of the TUCK intervention required more stringent inclusion criteria for safety than previous studies (Smith-Ray et al. 2014a; Smith-Ray et al. 2014b), which also may have affected results. UFOV training may be more likely to enhance gait speed in older adults with greater baseline motor function difficulties.
In summary, this pilot study demonstrates that traditional UFOV training may enhance balance, but TUCK, a dynamic version of UFOV training that incorporates movement does not show greater efficacy. Future research should examine what mechanisms of UFOV training result in motor function improvements. Future research should continue to further investigate the association between cognition and motor function as well as interventions to improve these outcomes among older adults.
Acknowledgments:
This research was funded by the Retirement Research Foundation, grant number 2017–179 to JE and JL. EH, JE, CH, and KB were additionally supported by National Institutes of Health National Institute on Aging (AG058324 and AG056428). We would like to thank our participants for their commitment to our ongoing research. We also thank Jade Sutfin, Raiza Carmenate-Nichols, Hannah Knott, Alice Le, Raquel Doblas-Shaw, and the USF Cognitive Aging Lab staff and volunteers for their roles recruiting participants and collecting study data.
Funding: This study was supported by the Retirement Research Foundation, grant number 2017-179 to JE and JL. EH, JE, CH, and KB were additionally supported by National Institutes of Health National Institute on Aging AG058324 and AG056428. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declarations
Conflicts of Interest/Competing Interests: Dr. Edwards worked between 1996 to 2005 as a consultant conducting related research studies for Visual Awareness, Inc., who owned the intellectual property surrounding the speed of processing training software. Posit Science now markets the UFOV training program. Over an approximate three-month period in 2008, Dr. Edwards worked as a limited consultant to Posit Science, Inc. to analyze data and prepare a publication. Dr. LaVere has consulted with and received equipment from Microgate, the developers of Optogait. The remaining authors declare no conflict of interest. The funders had no role in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Availability of Data and Material: The deidentified datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The trial and planned analyses were pre-registered: https://osf.io/7utgw.
Code availability: Not applicable.
Ethics approval: The study was approved by the USF Institutional Review Board prior to data collection. We certify that this study was performed in accordance with the ethical standards as ladi down in the 1964 Declaration of Helsinki.
Consent to Participate: Informed consent was obtained from all individual participants included in the study.
Consent for publication: Participants signed informed consent regarding publishing their deidentified data.
Contributor Information
Elizabeth M. Hudak, Department of Psychiatry and Behavioral Neurosciences, University of South Florida, Tampa, FL, United States.
Karen L. Bell, Department of Communication Sciences & Disorders, University of South Florida, Tampa, FL, United States.
Cidnee Hall, Department of Psychiatry and Behavioral Neurosciences, University of South Florida, Tampa, FL, United States
Lori D. Grismore, Suncoast Gerontology Center, University of South Florida, Tampa, FL, United States; Neuroscience Institute, University of South Florida, Tampa, FL, United States.
Jake LaVere, LaVere Chiropractic & Performance Labs, Tarpon Springs, FL, United States.
Jerri D. Edwards, Department of Psychiatry and Behavioral Neurosciences, University of South Florida, Tampa, FL, United States.; Department of Communication Sciences & Disorders, University of South Florida, Tampa, FL, United States
References
- Albers MW, Gilmore GC, Kaye JA, Murphy C, Wingfield A, Bennett DA, et al. (2015). At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 11, 70–98, doi: 10.1016/j.jalz.2014.04.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association (2012). 2012 Alzheimer’s Disease Facts and Figures. http://www.alz.org/downloads/facts_figures_2012.pdf. Accessed March 27 2014.
- Ball KK, Beard BL, Roenker DL, Miller RL, & Griggs DS (1988). Age and visual search: Expanding the useful field of view. Journal of the Optical Society of America A. Optics and Image Science, 5(12), 2210–2219, doi: 10.1364/JOSAA.5.002210. [DOI] [PubMed] [Google Scholar]
- Blankevoort CG, Scherder EJ, Wieling MB, Hortobagyi T, Brouwer WH, Geuze RH, et al. (2013). Physical predictors of cognitive performance in healthy older adults: a cross-sectional analysis. PloS One, 8(7), e70799, doi: 10.1371/journal.pone.0070799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DO, Xu H, Unverzagt FW, & Hendrie H. (2015). Does targeted cognitive training reduce educational disparities in cognitive function among cognitively normal older adults? International Journal of Geriatric Psychiatry, 10.1002/gps.4395 [DOI] [PMC free article] [PubMed]
- Clouston SA, Brewster P, Kuh D, Richards M, Cooper R, Hardy R, et al. (2013). The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiologic Reviews, 35, 33–50, doi: 10.1093/epirev/mxs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins-Crepeau L, Berryman N, Vu TT, Villalpando JM, Kergoat MJ, Li KZ, et al. (2014). Physical functioning is associated with processing speed and executive functions in community-dwelling older adults. Journals of Gerontology. Series B: Psychological Sciences and Social Sciences, 69(6), 837–844, doi: 10.1093/geronb/gbu036. [DOI] [PubMed] [Google Scholar]
- Donoghue OA, Horgan NF, Savva GM, Cronin H, O’Regan C, & Kenny RA (2012). Association between timed up-and-go and memory, executive function, and processing speed. Journal of the American Geriatrics Society, 60(9), 1681–1686, doi: 10.1111/j.1532-5415.2012.04120.x. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Weiner DK, Chandler J, & Studenski S. (1990). Functional reach: A new clinical measure of balance. Journal of Gerontology, 45(6), M192–M197, doi: 10.1093/geronj/45.6.M192. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Clark D, Xu H, Guey LT, Ross LA, & Unverzagt FW (2017a). Speed of processing training results in lower risk of dementia. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 3, 603–611, doi: 10.1016/j.trci.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JD, Fausto BA, Tetlow AM, Corona RT, & Valdes EG (2017b). Systematic review and meta-analyses of useful field of view cognitive training. Neuroscience and Biobehavioral Reviews, doi: 10.1016/j.neubiorev.2017.11.004. [DOI] [PubMed]
- Edwards JD, Vance DE, Wadley VG, Cissell GM, Roenker DL, & Ball KK (2005a). Reliability and validity of useful field of view test scores as administered by personal computer. Journal of Clinical and Experimental Neuropsychology, 27(5), 529–543, doi: 10.1080/13803390490515432. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Wadley VG, Vance DE, Roenker DL, & Ball KK (2005b). The impact of speed of processing training on cognitive and everyday performance. Aging & Mental Health, 9, 262–271, doi: 10.1080/13607860412331336788. [DOI] [PubMed] [Google Scholar]
- Friedman B, Heisel MJ, & Delavan RL (2005). Psychometric properties of the 15-item geriatric depression scale in functionally impaired, cognitively intact, community-dwelling elderly primary care patients. Journal of the American Geriatrics Society, 53(9), 1570–1576, doi: 10.1111/j.1532-5415.2005.53461.x. [DOI] [PubMed] [Google Scholar]
- Good-Lite (2010). Sloan letter near vision card. www.good-lite.com.
- Holtzer R, Verghese J, Xue X, & Lipton RB (2006). Cognitive processes related to gait velocity: Results from the Einstein aging study. Neuropsychology, 20(2), 215–223, doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, & Langa KM (2013). Monetary costs of dementia in the United States. New England Journal of Medicine, 368(14), 1326–1334, doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute ANS (2010). Specification for audiometers (ANSI S3.6–2010).
- Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, & Brennan S. (2014). The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: A systematic review and meta-analysis. Ageing Research Reviews, 15, 28–43, doi: 10.1016/j.arr.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Li KZ, & Lindenberger U. (2002). Relations between aging sensory/sensorimotor and cognitive functions. Neuroscience and Biobehavioral Reviews, 26(7), 777–783. [DOI] [PubMed] [Google Scholar]
- Lienhard K, Schneider D, & Maffiuletti NA (2013). Validity of the Optogait photoelectric system for the assessment of spatiotemporal gait parameters. Medical Engineering and Physics, 35(4), 500–504, doi: 10.1016/j.medengphy.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Lin F, Heffner KL, Ren P, Tivarus ME, Brasch J, Chen DG, et al. (2016). Cognitive and neural effects of vision-based speed-of-processing training in older adults with amnestic mild cognitive impairment: A pilot study. Journal of the American Geriatrics Society, 64(6), 1293–1298, doi: 10.1111/jgs.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MR, Hwang HF, Hu MH, Wu HD, Wang YW, & Huang FC (2004). Psychometric comparisons of the Timed Up and Go, One-Leg Stand, Functional Reach, and Tinetti Balance Measures in community-dwelling older people. Journal of the American Geriatrics Society, 52(8), 1343–1348, doi: 10.1111/j.1532-5415.2004.52366.x. [DOI] [PubMed] [Google Scholar]
- Maquet D, Lekeu F, Warzee E, Gillain S, Wojtasik V, Salmon E, et al. (2010). Gait analysis in elderly adult patients with mild cognitive impairment and patients with mild Alzheimer’s disease: simple versus dual task: a preliminary report. Clinical Physiology and Functional Imaging, 30(1), 51–56, doi: 10.1111/j.1475-097X.2009.00903.x. [DOI] [PubMed] [Google Scholar]
- Martin KL, Blizzard L, Wood AG, Srikanth V, Thomson R, Sanders LM, et al. (2013). Cognitive function, gait, and gait variability in older people: a population-based study. Journals of Gerontology: Series A, Biological Sciences and Medical Sciences, 68(6), 726–732, doi: 10.1093/gerona/gls224. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–699. [DOI] [PubMed] [Google Scholar]
- Rabipour S, & Davidson PS (2015). Do you believe in brain training? A questionnaire about expectations of computerized cognitive training. Behavioural Brain Research, 10.1016/j.bbr.2015.01.002. [DOI] [PubMed]
- Rebok GW, Ball K, Guey LT, Jones RN, Kim HY, King JW, et al. (2014). Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society, 62(1), 16–24, doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben DB, & Siu AL (1990). An objective measure of physical function of elderly outpatients. The Physical Performance Test. Journal of the American Geriatrics Society, 38(10), 1105–1112. [DOI] [PubMed] [Google Scholar]
- Ross LA, Sprague BN, & Phillips C. (2016). The impact of three cognitive training interventions on older adults’ physical functioning across 5 year. Journal of Aging and Health, 30, 475–498, doi: 10.1177/0898264316682916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway-Cook A, Brauer S, & Woollacott M. (2000). Predicting the probability for falls in community-dwelling older adults using the Timed Up and Go Test. Physical Therapy, 80, 896–903. [PubMed] [Google Scholar]
- Smith-Ray RL, Hughes SL, Prohaska TR, Little DM, Jurivich DA, & Hedeker D. (2014a). Impact of cognitive training on balance and gait in older adults. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 70, 357–366, doi: 10.1093/geronb/gbt097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Ray RL, Makowski-Woidan B, & Hughes SL (2014b). A randomized trial to measure the impact of a community-based cognitive training intervention on balance and gait in cognitively intact black older adults. Health Education and Behavior, 41(1S), 62S–69S, doi: 10.1177/1090198114537068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumaré A, Tavernier B, Alpérovitch A, Tzourio C, & Elbaz A. (2009). A cross-sectional and longitudinal study on the relationship between walking speed and cognitive function in community-dwelling elderly people. Journals of Gerontology: Series A, Biological Sciences and Medical Sciences, 64(10), 1058–1065, doi: 10.1093/gerona/glp077. [DOI] [PubMed] [Google Scholar]
- Verghese J, Mahoney J, Ambrose AF, Wang C, & Holtzer R. (2010). Effect of cognitive remediation on gait in sedentary seniors. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 65(12), 1338–1343, doi: 10.1093/gerona/glq127. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Mahncke HW, Vander Weg MW, Martin R, Unverzagt FW, Ball KK, et al. (2009). The ACTIVE cognitive training interventions and the onset of and recovery from suspected clinical depression. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 64B, 577–585, doi: 10.1093/geronb/gbp061 [DOI] [PMC free article] [PubMed] [Google Scholar]