Abstract
Understanding the neuroanatomical correlates of internalizing psychopathology during adolescence may shed light on to neurodevelopmental processes that make this a critical period for the trajectory of mental illness. However, few studies have simultaneously examined co-occurring and dissociable features of internalizing psychopathology during this formative developmental stage. In the current study we identify the neuroanatomical correlates of four dimensions of internalizing psychopathology symptoms in adolescents: a common internalizing dimension capturing covariance in symptoms across internalizing disorders, as well as low positive affect-, anxious arousal-, and anxious apprehension-specific residuals. Our results suggest that these dimensions are associated with neuroanatomy across much of the brain, including prefrontal and limbic regions implicated in case-control studies, but also regions supporting visual processing. Importantly, results differed between males and females in regions that are sexually dimorphic in adulthood and the direction of the effects were largely opposite to what has been observed in adults and children.
Introduction
Adolescence is a sensitive developmental period for brain systems relevant to internalizing psychopathology (i.e., anxiety and depression) (Romer & Walker, 2007). Symptoms of internalizing disorders often first manifest during adolescence (Hankin, 2006, 2009, 2015), and neurodevelopmental process during this period may have long lasting effect on mental health trajectories (Copeland et al., 2009; Kansky et al., 2016). As such, understanding the neural correlates of internalizing psychopathology during adolescence may have relevance to efforts to lessen the burden of mental illness across the lifespan. Despite this clinical relevance, the neuroanatomical correlates of co-occurring and dissociable symptoms of internalizing psychopathology during adolescence remains unclear. Such information may speak to the neural causes and consequences of mental illness and inform precision psychiatry (Fernandes et al., 2017), identifying specific neural systems that may drive heterogeneity in symptomology across individuals, potentially informing targeted treatment plans.
Research into the neuroanatomical correlates of internalizing psychopathology has been dominated by case-control studies in adults that compare properties of the brains of individuals who meet diagnostic criteria for some disorder (i.e., “cases”) to individuals who do not meet these criteria (i.e., “controls”). Such studies have confirmed models of psychopathology that posit central roles of the prefrontal cortex and limbic systems across most mental health disorders. For example, a meta-analysis of studies predominately involving adults showed that all major psychiatric disorders are associated with reduced gray matter volume in the rostral anterior cingulate and insula, whereas internalizing disorders are specifically associated with reduced volume in the hippocampus and amygdala (Goodkind et al., 2015). Functional MRI (fMRI) research into alterations of the brain with psychopathology largely aligns with these structural findings, demonstrating alterations in prefrontal and limbic regions. Specifically, accounts of psychopathology derived from fMRI studies suggest that brain systems supporting internally directed thought (e.g., default mode network [DMN]), emotional information processing (e.g., limbic and salience networks), and cognitive control (i.e., frontoparietal network [FPN]) may sit at the core of internalizing disorders, as well as psychopathology more broadly (Menon, 2011; Williams, 2016).
Taken together, it appears that the high rates of comorbidity (Kessler et al., 2005) and overlapping symptomologies (Zbozinek et al., 2012) between psychiatric disorders may emerge in part due to common neural substrates. While this insight is valuable, case-control designs rely on certain assumptions regarding the nature of psychopathology (Kotov et al., 2018). Specifically, such designs characterize psychopathology as being categorical, with individuals either meeting criteria for a disorder or not. However, psychopathology symptoms are distributed across the general population and individuals who do not meet criteria for a disorder often still experience impairment due to subclinical symptomology (Smith et al., 2018). Furthermore, case-control designs often fail to model comorbidity between disorders, drawing clear lines between disorders when, in fact, they may be characterized by common symptoms, and as such, may be influenced by common etiological factors. In addition, such classifications do not account for the considerable heterogeneity in symptomology that can occur within a single disorder (Kotov et al., 2018).
Considering these potential shortcomings, a more nuanced mapping of the neuroanatomical correlates of psychopathology can be gained through dimensional models (e.g., Caspi et al., 2014; Snyder et al., 2017). Such models decompose covariance in symptoms to differentiate between latent symptom dimensions and characterize these dimensions continuously (Kotov et al., 2018). This framework allows researchers to quantify comorbid aspects of psychopathology while simultaneously capturing heterogenous aspects that may be associated with certain disorders. A prominent dimensional model of psychopathology suggests that symptoms of all major psychiatric disorders can be differentiated into three latent factors: a p-factor capturing a general susceptibility to psychiatric disorders, as well as internalizing- and externalizing-specific factors (Caspi et al., 2014). Several recent studies have investigated the neural correlates of these dimensions, but results are inconsistent. For example, within a sample of children ages 6 -10, increased p-factor scores were associated with less volume in the prefrontal cortex, whereas increased internalizing-specific factor scores were associated with reduced volume in limbic/paralimbic regions (Snyder et al., 2017). However, in a sample of over 9,000 9-10 year old children, less global gray matter volume was associated with higher levels of a general psychopathology dimension, but no significant associations were observed with an internalizing-specific factor (Durham et al., 2021). Additionally, well powered whole brain analyses in adult found no associations between a p-factor and gray matter volume of prefrontal or limbic regions, instead finding that higher p-factor scores were associated with reduced volume in occipital (Romer et al., 2018, 2019) and cerebellar regions (Romer et al., 2018). In related work, p-factor, internalizing, and externalizing factor scores were all associated with overlapping patterns of reduced cortical thickness across much of the brain (Romer et al., 2020).
Inconsistencies in these results across studies may have occurred because the investigations were performed on individuals from different age groups. Recent evidence supports this possibility, as distinct patterns of gray matter alterations are observed in adolescent as compared to adults with major depression both in terms of the regions and morphometric properties that are altered (Schmaal et al., 2017). Considering the neurodevelopmental processes that unfold across adolescence, these age differences are not entirely surprising. Gray matter morphometry follows spatiotemporally trajectories, marked by brain growth and subsequent increases in cortical volume from birth to puberty. The onset of puberty and transition into adolescence marks a shift to widespread neuronal pruning and increased myelination of white matter (Gogtay & Thompson, 2010; Lenroot et al., 2007). These changes manifest as a reduction in cortical volume and thickness, observed first in lower-level sensorimotor regions and later in prefrontal regions (Gennatas et al., 2017; Gogtay et al., 2004). Given these considerable changes in neural organization coupled with the trend for symptoms of psychopathology to first emerge during adolescence, it is possible that the specific neuroanatomical correlates of psychopathology differ in adolescence as compared to other ages.
Previous studies investigating the neuroanatomical correlates of dimensions of psychopathology symptoms have also differed in the spatial resolution at which gray matter morphometry was measured, including low resolution ROIs (e.g., Durham et al., 2021; Snyder et al., 2017) to high resolution whole-brain analyses (e.g., Romer et al., 2018, 2019). These differences in resolution make it difficult to compare across studies and may affect the relative power to detect associations. Specifically, as one averages across larger areas of brain, the signal-to-noise ratio increases while the ability to precisely localize the effect is reduced (Zhao et al., 2013). This difference in power is further exacerbated by the need to account for a larger number of multiple comparisons in high resolution analyses, meaning comparably sized effects may be deemed significant in ROI analyses but not whole brain analyses. As such, research into the neural correlates of psychopathology may be well served to employ analyses that span multiple levels of spatially resolution, as is done in the current report.
Recent gray matter morphometry analyses employing dimensional models of psychopathology have begun to provide a degree of specificity that is lacking in case-control studies. However, these higher-order dimensions can be subdivided further into subfactors which may have unique neural bases. In now classic work, Watson and Clark proposed the tripartite model of internalizing psychopathology, which categorized symptoms of anxiety and depression as consisting of a common factor, termed negative affect, as well as two specific factors, anxious arousal and low positive affect (Clark & Watson, 1991). This original three-factor model has since been extended to include a fourth factor, anxious apprehension, an anxiety symptom dimension that is dissociable from anxious arousal (Nitschke et al., 2001) with distinct neural correlates (Sharp et al., 2015). Recent work from our research group supports this model further, demonstrating that it can be successfully parameterized as a bifactor model and that each dimensions shows unique associations with specific disorders (Snyder et al., submitted).
Importantly, previous research suggests that at least some of the symptom dimensions investigated in the current report show differential trajectories in adolescent males and females. Specifically, when compared to males, females showed higher levels of depressive symptoms in early adolescence, as well as a greater increase in the severity of both depressive symptoms and anxious arousal as they age towards late adolescence (Hankin, 2009). Interestingly, these and similar sex differences in symptomology are accompanied by the emergence of sexual dimorphisms in the brain, particularly in prefrontal and limbic brain systems linked to psychopathology (Lotze et al., 2019; Ruigork et al., 2014). While developmental models of sex differences in psychopathology often place a particular importance on differing neurodevelopmental trajectories, it remains unclear whether adolescent males and females show distinct neuroanatomical correlates of the four symptom dimensions .
In the current study, we employ a bifactor model of internalizing symptomology to identify brain regions associated with four internalizing dimensions: a common internalizing dimension capturing symptoms that are shared across depression and anxiety, as well as three specific symptom dimensions, including a low positive affect-specific dimension, an anxious arousal-specific dimension, and an anxious apprehension-specific dimension. While a handful of studies have investigated the neuroanatomical correlates of related dimensions (e.g., Cardinale et al., 2019; Castagna et al., 2018; Holmes et al., 2012; Lener et al. 2016), to our knowledge no study has employed bifactor modeling to parse common and unique variance between the four internalizing symptom dimensions. We utilize a bifactor model approach because it allows us to differentiate between common and specific symptom dimensions, something that alternative parameterizations are unable to do (Bornovalova et al., 2020). Considering large effects of sex on internalizing psychopathology and neuroanatomical trajectories during adolescence (Gennatas et al., 2017), we test for moderating effects of sex on the relations between internalizing dimensions and gray matter morphometry. To balance statistical power with anatomical specificity (Zhao et al., 2013), we carry out both ROI and exploratory whole brain analyses.
We had several hypotheses regarding the brain systems associated with each dimension. Because the common internalizing factor captures symptoms that are shared across disorders, we predicted it would be associated with the anterior cingulate cortex and the insula, brain regions which show reduced gray matter across all major psychiatric disorders in adults (Goodkind et al., 2015), as well as associations with transdiagnostic dimensions in children (Snyder et al., 2017). Considering links between low positive affect and atypical reward processing (Nikolova et al., 2012), we predicted that the low positive affect-specific factor would be associated with gray matter morphometry in regions responsible for reward-related processes, including the basal ganglia and orbitofrontal cortex. We hypothesized that the anxious arousal-specific factor would be associated with gray matter morphometry of regions supporting threat processing, namely the amygdala (Ohman, 2005), and sensorimotor regions involved with the physiological responses to threat. Finally, we predicted that the anxious apprehension-specific factor would be associated with left-lateralized prefrontal regions associated with verbalizations of worry (Nitschke et al., 1999; Engels et al., 2007; Sharp et al., 2015), as well as the hippocampus due previous associations of anxious apprehension but not anxious arousal in children (Castagna et al., 2018).
Because adolescent females are thought to undergo neurodevelopmental processes earlier than males (Giedd et al., 2012; Lenroot et al., 2007), we predicted we would observe sex moderation effects in late-developing prefrontal regions while early-developing sensorimotor regions would demonstrate consistent effects across the sexes. Finally, whereas associations between psychopathology severity and gray matter morphometry have been consistently negative in adults (i.e., higher levels of symptom severity associated with less gray matter volume or thickness), findings in youth samples have shown more variability in terms of direction (e.g., Castagna et al., 2018) with some evidence suggesting that the direction of gray matter-psychopathology relations may shift during adolescence (Ducharne et al., 2014). As such, we hypothesize that, across different brain regions, we may observe either negative or positive associations (i.e., higher levels of symptom severity associated with greater gray matter volume or thickness) in the current adolescent sample.
Methods
Participants
Participants in the current report were drawn from 150 adolescent participants in the Colorado Cognitive Neuroimaging Family Emotion Research study (CoNiFER study), all of whom were originally recruited for studies in the Genes and Environment Mood (GEM) Lab (Benjamin Hankin, P.I.; for details of these studies, see Hankin et al., 2015). Initial recruitment was performed in the Denver metropolitan area via public schools and direct mail. Eligibility requirements included being free of a history of neurological insult and MRI contraindications. Participants were not screened for psychiatric disorders prior to data collection. Of the initial 150 participants, 128 successfully completed a T1 structural MRI scan with minimal head motion. Of these 128 participants, 10 did not complete the questionnaires needed to derive internalizing factor scores and were subsequently excluded from analyses, resulting in a final sample of 118 individuals. There were 16 pairs and one triad of siblings within the final sample. For full demographic information, see Table 1. Questionnaires used to produce the internalizing symptom dimension factor scores were acquired as part of a separate behavioral session with the two sessions completed 47 days apart on average. Minor participants assented with signed parental consent prior to participation, and participants 18 and older provided informed consent. Research protocols were approved by University of Colorado Boulder Institutional Review Board prior to data collection.
Table 1. Sample demographics and internalizing dimension subscale scores.
Demographic characteristics and scores on internalizing questionnaire subscales. MASQ= Mood and Anxiety Symptom Questionnaire; PSWQ= PennState Worry Questionnaire; SD= standard deviation.
| Characteristic | Mean (SD) |
|---|---|
| Age (years) | 17.0 (1.5) |
| MASQ | |
| Anxious arousal | 20.0 (5.5) |
| Low positive affect | 40.5 (11.7) |
| Loss of interest | 14.9 (4.2) |
| PSWQ | 43.5 (13.3) |
| Characteristic | # of participants (% of sample) |
| Sex | |
| Female | 59 (50%) |
| Male | 59 (50%) |
| Ethnicity | |
| Non-Hispanic | 95 (81%) |
| Hispanic | 23 (19%) |
| Race | |
| White | 82 (69.5%) |
| Black/African American | 7 (5.9%) |
| Asian | 2 (1.7%) |
| American Indian/Alaskan Native | 7 (5.9%) |
| Native Hawaiian/Pacific Islander | 1 (.8%) |
| Multiracial | 18 (15.2%) |
| Other | 1 (.8%) |
Internalizing psychopathology questionnaires
We used items from the Mood and Anxiety Symptom Questionnaire (MASQ) (Watson et al., 1995a,b) and the Penn State Worry Questionnaire (PSWQ) (Meyer et al., 1990) as manifest indicators for the four internalizing dimensions of interest. The current analyses used the 39 items capturing the anxious arousal (AA, e.g., “Hands were shaky), low positive affect (LPA, e.g., “Felt like I was having a lot of fun” – reverse coded), and loss of interest (LI, e.g., “felt really bored”) subscales within the MASQ. The MASQ has been previously shown to have good internal consistency, test-retest reliability, and convergent and discriminate validity for anxiety and depression disorders (Nitchke et al., 2001; Watson et al., 1995a,b). Within the current sample, all MASQ subscales had acceptable to high internal consistency (AA α = .83, LPA α = .95, LI α = .74). The PSWQ is a 16-item questionnaire assessing a tendency to worry (e.g., “My worries overwhelm me”) and has been previously shown to have good internal consistency, test-retest reliability, and convergent and discriminate validity for anxiety disorders (Brown et al., 1992; Molina & Borkovec, 1994). Within the current sample, the PSWQ had high internal consistency (α = .93).
Latent variable model
To derive factor scores of the latent internalizing dimensions, a bifactor model of was fit and factor scores for participants were saved for use as covariates in gray matter morphometry analyses. Work from our research group indicates that a similar bifactor model has a good fit in adult and that there is little evidence of a LI-specific residual (Banich et al., 2020; Snyder et al., submitted). Confirmatory factor analysis was conducted in Mplus (Muthén & Muthén, 2012) using full information maximum likelihood estimation. Good model fit was defined as, CFI>.95, RMSEA<.06 and SRMR<.08 (Hu & Bentler, 1999). We utilized parcels instead of using individual items as indicators because of the relatively small sample size and large number of items, with four parcels per factor (PSWQ: 4 item parcels; MASQ AA: 4-5 item parcels; MASQ LPA: 3-4 item parcels; MASQ LI: 2 item parcels). To create the parcels we used correlation parceling (Little et al., 2013), in which we first averaged the two items with highest correlations and then averaged together highly correlated 2-item parcels to create 4-item parcels. For scales with an odd number of items, we averaged the remaining single items with the parcel with which they were most highly correlated. In doing so, we were able to simultaneously reduce the number of indicators while preserving the construct structure. All parcels were specified to load onto the common internalizing factor that captures covariance across all indicators, and additionally onto their respective specific factors that represents the unique variance associated with each. Factors were constrained not to correlate because what is shared between factors is already captured by the common factor (e.g., Chen et al., 2012).
Structural MRI acquisition
Structural MRI data were acquired at the Intermountain Neuroimaging Consortium located at the University of Colorado Boulder using a Siemens 3-Tesla PRISMA MRI scanner for all but 18 participants for whom data were acquired on the pre-upgrade version of the same magnet (TIM TRIO). For all participants, a 32-channel head coil was used to collect a high-resolution T1-weighted Magnetization Prepared Gradient Echo (MPRAGE) sequence with the following parameters: number of slices= 224; repetition time (TR)= 2400 ms; echo time (TE)= 2.07 ms; flip angle= 8°; field of view (FoV)= 256 mm; and a voxel dimension= .8 x .8 x .8 mm.
Gray matter morphometry preprocessing
Analyses testing for relations between individual differences in internalizing dimension factor scores and surface-based morphometry were carried out employing the FreeSurfer analysis suite (http://surfer.nmr/mgh.harvard.edu/). T1-weighted structural images were brain extracted using a hybrid watershed/surface deformation procedure (Segonne et al., 2004), followed by a transformation into Talairach space, intensity normalization (Sled et al., 1998), tessellation of the gray/white matter boundary (Fischl et al., 2001), and surface deformation along intensity gradients to optimally differentiate gray matter, white matter, and cerebral spinal fluid (CSF) boundaries (Dale et al., 1999; Fischl and Dale, 2000). The resulting segmented surfaces were registered to a standard spherical inflated brain template (Fischl et al., 1999a,b), parcellated according to gyral and sulcal structure (Desikan et al., 2006; Fischl et al., 2004), and then used to compute a range of surface-based measurements, including cortical volume, surface area, and thickness.
Prior to running surface-based analyses, data quality assurance was checked using FreeSurfer’s standard quality assurance tools (https://surfer.nmr.mgh.harvard.edu/fswiki/QATools), including checking for volume-based statistical outliers (+/− 3 standard deviations) of ROI segmentation data, signal-to-noise ratio, and mean and standard deviation of white matter intensity. Data quality for all 118 participants was deemed acceptable, with no outlying ROIs detected, SNR ranging between 15 and 25, and white matter intensity ranging between 103 and 108. Additionally, author HRS carried out visual inspection of the subcortical segmentation and whole brain cortical surfaces to ensure that the distinct subcortical structures appeared to be properly segmented and that the white and pial surfaces aligned with the distinction between cortical gray and white matter visible when viewing the T1 structural images. We carried out ROI analyses in fsaverage7 space (163,842 vertices per hemisphere) and whole brain analyses within fsaverage5 space (10,242 vertices per hemisphere). By doing so, we were provided a more spatially precise estimate of gray matter properties in our ROIs while reducing the number of multiple comparisons in whole brain analyses.
Gray matter morphometry analyses
Analyses were carried out in both a ROI and exploratory whole brain fashion. In the ROI approach, we first extracted total volume from prefrontal and limbic ROIs as defined by the Desikan-Killany atlas (Desikan et al., 2006) (see Supplementary Figure 1 for ROIs utilized in current study). Prefrontal ROIs included the frontal pole, superior frontal gyrus, middle frontal gyrus (combination of rostral and caudal middle frontal ROIs), inferior frontal gyrus (combination of pars triangularis, pars opercularis, and pars orbitalis ROIs), anterior cingulate (combination of rostral and caudal anterior cingulate ROIs), and orbitofrontal cortex (combination of medial and lateral orbitofrontal cortex ROIs). Limbic/paralimbic ROIs included the insula, amygdala, hippocampus, thalamus, and basal ganglia (combination of nucleus accumbens, caudate, putamen, and pallidum ROIs). For all ROIs we averaged across left and right hemisphere to create bilateral ROIs.
To test for relations between ROI gray matter morphometry and the internalizing dimension factor scores, we carried out mixed-effects models (‘lmer’ function in R), predicting gray matter volume for each ROI by factor scores for all four dimensions simultaneously, while controlling for sex, age, total intracranial volume, and MRI scanner operating system (i.e., PRISMA or TRIO TIM). To test for sex moderation effects, we ran additional analyses predicting ROI gray matter volume for each dimension separately, including a factor score-by-sex interaction term. For ROI analyses controlling for age and sex, as well as testing for sex interactions, a standard alpha of p <.05 was used to determine significant relations between gray matter and internalizing dimension factor scores. For all of effects that passed this alpha threshold, we carried out post hoc analyses to determine the degree to which results were driven by a particular subregion(s) that went into an given bilateral ROI, first by hemisphere (i.e., left versus right hemisphere), and then by regional subdivision (e.g., medial versus lateral orbitofrontal cortex).
Second, we carried out exploratory whole brain analyses using the general linear model (GLM) to test for associations of vertex-wise measurements of gray matter volume, surface area, and thickness with each internalizing factor score, separately. We carried out two sets of whole brain GLMs, including analyses in which we controlled for both age and sex, as well as analyses that tested for factor score-by-sex interactions. All whole brain analyses controlled for whole brain gray matter properties (i.e., total volume, total surface area, or mean thickness as appropriate).
To correct for multiple comparisons and non-independence between adolescent siblings in the whole brain analyses, we carried out non-parametric permutation testing as implemented by PALM (Winkler et al., 2014), utilizing exchangeability blocks (“-eb” option in PALM), in conjunction with sign flipping and exchangeable errors (“-ise” and “-ee” options in PALM, respectively). For all models, permutations were carried out across 10,000 iterations. We corrected for multiple comparisons using cluster-mass correction (Bullmore et al., 1999), with a cluster-forming threshold of p<.001. Resulting clusters were deemed significant at a cluster-wise family-wise error rate of p<.05.
Results
Internalizing dimensional model fit
In an initial model including all specific factors, loss of interest (LI) parcels had strong loadings only on the common internalizing factor and there was no significant variance associated with the LI-specific factor, indicating the common factor fully accounted for covariance among the LI indicators. Thus, the LI-specific factor was eliminated, and LI parcels were set to load only onto the common factor. This revised model had acceptable to good fit (CFI = .966, RMSEA= .056, SRMR = .072; χ2 (92) = 130.958, p <.005), and all parcels loaded significantly on their factor(s) (see Supplementary Figure 2 for final model factor loadings). Factor scores were then saved for further analyses. Factor determinacies (i.e., correlations between the true latent factor and the factor scores) were high for all factors, including common internalizing (.90), low positive affect-specific (.93), anxious arousal-specific (.87), and anxious apprehension-specific (.93) factors, supporting the use of factor scores in further analyses. Correlations between the factor scores for all four dimensions can be found in Supplementary Table 1.
Associations of gray matter morphometry with common internalizing factor scores
In ROI analyses controlling for sex and age (see Table 2 and Figure 1), higher levels of the common internalizing factor scores were significantly associated with greater volume of bilateral basal ganglia (β(SE)= .165(.072), p=.023). Post hoc analyses revealed that this association was observed in both the left (β(SE)= .155(.074), p=.037) and right (β(SE)= .169(.072), p=.020) basal ganglia, but were specific to the putamen (left: β(SE)= .185(.078), p=.019; right: β(SE)= .175(.074), p=.020) and, to a lesser degree, the caudate (left: β(SE)= .139(.077), p=.075; right: β(SE)= .139(.079), p=.087). There was significant sex moderation on the relation between level of an individual’s common internalizing factor score and bilateral superior frontal gyrus volume (β(SE)= −.252(.099), p=.043), with male youth showing a significant negative association and female youth showing a non-significant positive association (see Supplementary Figure 3 for scatter plots of sex moderation effects). Post hoc analyses revealed this sex moderation effect was largely driven by the right (β(SE)= .249(.110), p=.026) as compared to the left (β(SE)= .145(.102), p=.155) superior frontal gyrus.
Table 2. Results from region of interest (ROI) and whole brain analyses of associations between internalizing dimensions and gray matter volume.
ROI results include both main analyses investigating bilateral ROIs (bold), as well as post hoc subregion analyses. “Dimension” column indicates the internalizing dimension factor score. “Effect” column indicates if the result is when controlling for age and sex (“cntrl”) or sex moderation effects (“sex”).
| Region of Interest Analyses | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dimension | Effect | ROI | Direction | Std. β(SE) | p-value | ||||
| Common Internalizing | cntrl. | bi. BG | + | .165(.072) | .023 | ||||
| post hoc subregion | lh BG | + | .155(.074) | .037 | |||||
| rh BG | + | .169(.072) | .020 | ||||||
| lh putamen | + | .185(.078) | .019 | ||||||
| rh putamen | + | .175(.074) | .020 | ||||||
| lh pallidum | + | .020(.092) | .824 | ||||||
| rh pallidum | + | .099(.081) | .226 | ||||||
| lh accumbens | − | −.065(.086) | .453 | ||||||
| rh accumbens | + | .054(.089) | .545 | ||||||
| lh caudate | + | .139(.077) | .075 | ||||||
| rh caudate | + | .139(.079) | .081 | ||||||
| gender | bi. SFG | ♂=−* ♀= +ns |
.252(.099) | .043 | |||||
| post hoc subregion | lh SFG | ♂= −Λ ♀= −ns |
.145(402) | .155 | |||||
| rh SFG | ♂= −* ♀= +ns |
.249(.110) | .026 | ||||||
| Low Positive Affect-specific | cntrl. | bi. amygdala | − | −.138(.069) | .049 | ||||
| post hoc subregion | lh amygdala | − | −.214(.075) | .005 | |||||
| rh amygdala | − | −.050(.072) | .485 | ||||||
| cntrl. | bi. OFC | + | .177(.078) | .025 | |||||
| post hoc subregion | lh mOFC | + | .130(.090) | .148 | |||||
| rh mOFC | + | .158(.086) | .069 | ||||||
| lh lOFC | + | .132(.083) | .116 | ||||||
| rh lOFC | + | .152(.085) | .077 | ||||||
| gender | bi. FP | ♂= +* ♀= −ns |
−.203(.124) | .016 | |||||
| post hoc subregion | lh FP | ♂= +ns ♀= −ns |
−.053(434) | .694 | |||||
| rh FP | ♂= +* ♀= −* |
−.428(124) | <.001 | ||||||
| gender | bi. hippo. | ♂= +* ♀= −ns |
−.240(107) | .012 | |||||
| post hoc subregion | lh hippo. | ♂= +ns ♀= −ns |
−.217(.116) | .065 | |||||
| rh hippo. | ♂= +* ♀= −Λ |
−.298(097) | .003 | ||||||
| Anxious Apprehension-specific | cntrl. | bi. MFG | + | .143(.069) | .042 | ||||
| post hoc subregion | lh rMFG | + | .214(.075) | .005 | |||||
| rh rMFG | + | .142(.076) | .065 | ||||||
| lh cMFG | − | −.013(.082) | .869 | ||||||
| rh cMFG | + | .031(.081) | .700 | ||||||
| Whole Brain Analyses | |||||||||
| Dimension | Effect | Measure | Region | mm2 | X | Y | Z | Direc. | p-value |
| Common Internalizing | cntrl. | area | rh FG | 109 | 37 | −6 | −31 | + | <.05 |
| thickness | lh aIFG | 173 | −49 | 30 | −3 | + | <.05 | ||
| Low Positive Affect-specific | cntrl. | thickness | lh PC | 258 | −13 | −90 | 7 | − | <.05 |
| sex | area | rh SFS | 107 | 26 | 17 | 39 | ♂= −ns ♀= +ns |
<.05 | |
| Anxious Apprehension-specific | cntrl. | thickness | lh PCun | 124 | −7 | −69 | 43 | + | <.05 |
| area | rh FG | 109 | 37 | −6 | −31 | + | <.05 | ||
“Direction” or “Direc.” indicates the nature of the relationship, “mm2” indicates the size of cluster in. “X”, “Y”, and “Z” indicate coordinates of center of mass of cluster. + = increased internalizing dimension factor score is associated with increased gray matter morphometry; − = increased internalizing dimension factor score is associated with decreased gray matter; ♂ = relationship in males; ♀ = relationship in females; ns= relationship is non-significant in given sex;
= relationship is marginally significant in given sex;
= relationship is significant in a given sex. bi= bilateral; lh= left hemisphere; rh= right hemisphere; BG= basal ganglia; SFG= superior frontal gyms; amyg.= amygdala; OFC= orbitofrontal cortex; mOFC= medial orbitofrontal cortex; lOFC= lateral orbitofrontal cortex; FP= frontal pole; hippo.= hippocampus; MFG= middle frontal gyms; rMFG= rostral middle frontal gyms; cMFG= caudal middle frontal gyms; FG= fusiform gyms; aIFG= anterior inferior frontal gyurs; PC= pericalcarine cortex; SFS= superior frontal sulcus; PCun= precuneus
Figure 1. Results from region of interest analyses.
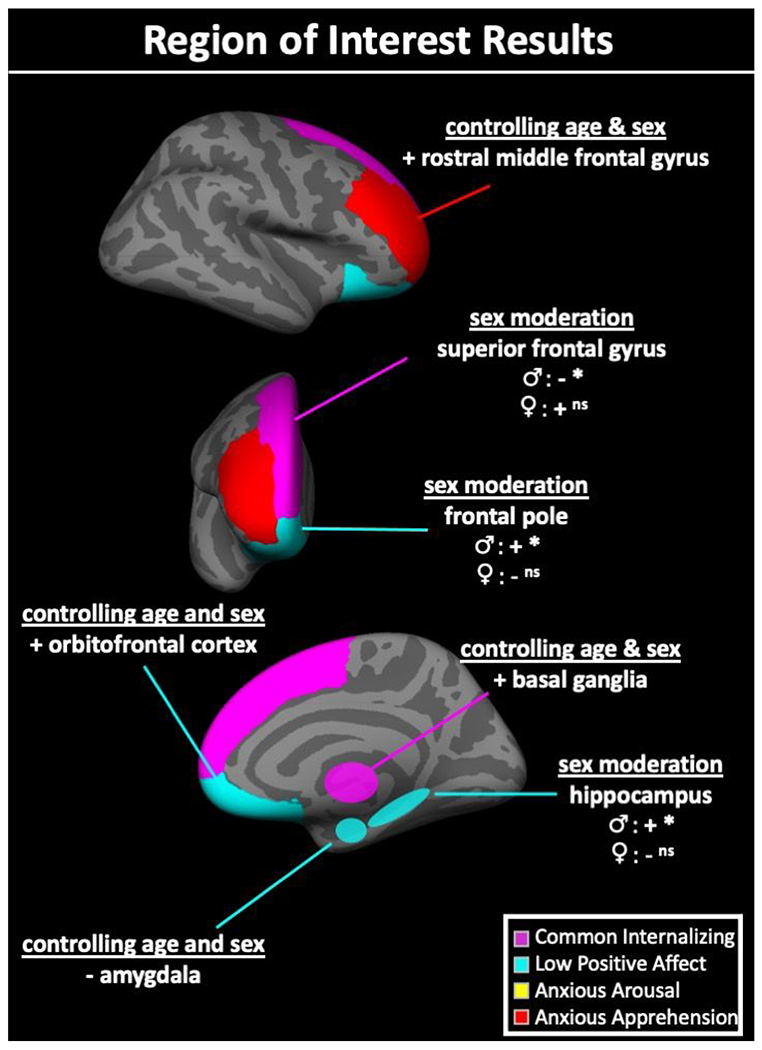
+ = higher internalizing dimension factor scores associated with higher gray matter volume; − = lower internalizing dimension factor scores associated with higher gray matter volume; ♂ = relationship in males; ♀ = relationship in females; ns= relationship is non-significant in given sex; Λ= relationship is marginally significant in given sex; *= relationship is significant in a given sex. bi= bilateral; lh= left hemisphere; rh= right hemisphere; BG= basal ganglia; SFG= superior frontal gyrus; amyg.= amygdala; OFC= orbitofrontal cortex; mOFC= medial orbitofrontal cortex; lOFC= lateral orbitofrontal cortex; FP= frontal pole; hippo.= hippocampus; MFG= middle frontal gyrus; rMFG= rostral middle frontal gyrus; cMFG= caudal middle frontal gyrus.
In whole brain analyses controlling for sex and age (see Table 2 and Figure 2), higher levels of common internalizing factor scores were associated with greater surface area and thickness in right anterior fusiform gyrus and left anterior inferior frontal gyrus (pars triangularis portion), respectively. No significant sex moderation effects were observed.
Figure 2. Results from whole brain analyses.
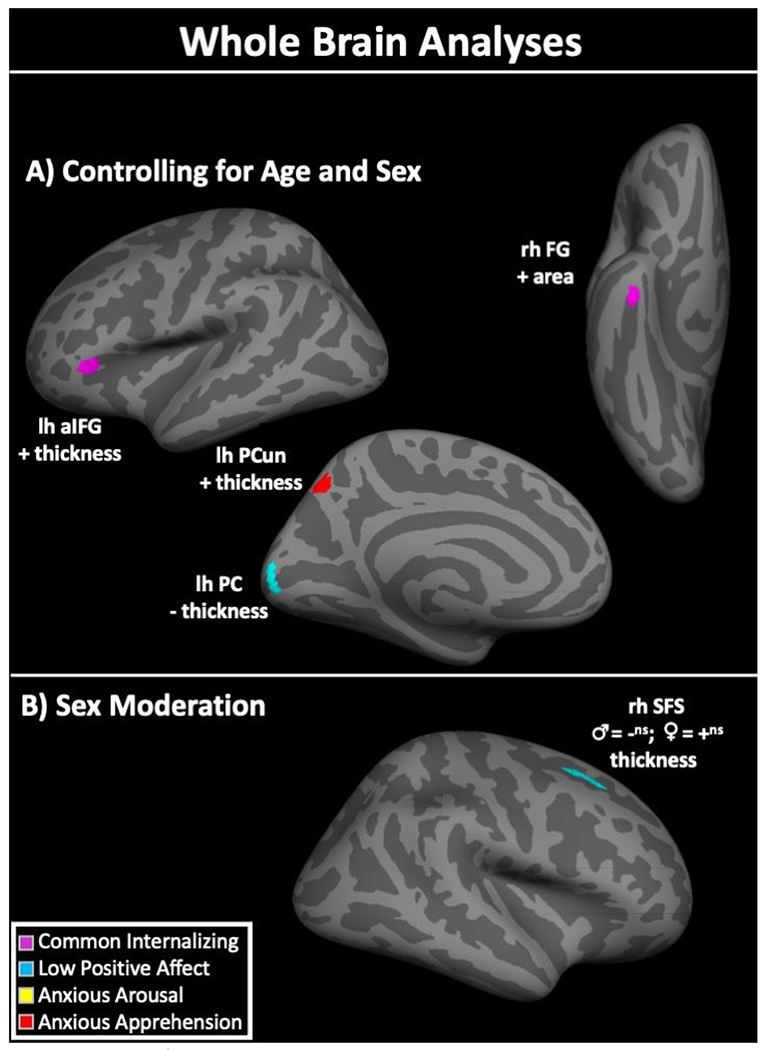
Panel A) shows results when controlling for age and sex. Panel B) shows significant sex moderation effects on the relationships between gray matter morphometry and internalizing dimension factor scores. += higher internalizing dimension factor scores associated with higher gray matter volume, surface area, or thickness; −= higher internalizing dimension factor scores associated with lower gray matter volume, surface area, or thickness; ♂= effect in males; ♀= effect in females; ns= non-significant effect; lh= left hemisphere; rh= right hemisphere; IFG= anterior inferior frontal gyrus; PCun= precuneus; PC= pericalcarine cortex; FG= fusiform gyrus; SFS= superior frontal sulcus.
Associations of gray matter morphometry with low positive affect-specific factor scores
In ROI analyses controlling for sex and age (see Table 2 and Figure 1), higher low positive affect-specific factor scores were associated with less volume of bilateral amygdala (β (SE)= −.138(.069), p=.049) and greater volume of bilateral orbitofrontal cortex (β(SE)= .177(.078), p=.025). Post hoc analyses revealed that the associations with the amygdala was largely driven by the left (β(SE)= −.214(.075), p=.005) but not right (β(SE)=−.050(.072), p=.485) homolog and the orbitofrontal effect was marginally significant in the two right subregions (medial: β(SE)= .130(.075), p=.069; lateral: β(SE)= .152(.085), p=.077) and non-significant in the left (medial: β(SE)= .130(.090), p=.148; lateral: β(SE)= .132(.083), p=.116).
There were significant sex moderation effects on the relations between low positive affect-specific factor scores and volume of bilateral frontal pole (β(SE)= −.203(.124), p=.016) and hippocampus (β(SE)= .165(.072), p=.023). For both regions, adolescent males showed a significant positive association between volume and low positive affect-specific factor scores whereas females showed a non-significant negative association (see Supplementary Figure 3 for scatter plots of sex moderation effects). Post hoc analyses revealed that the frontal pole effect was largely driven by the right (β(SE)= −.428(.124), p<.001) but not left (β(SE)= −.053(.134), p=.694) homolog, whereas the hippocampus effect was significant on the right (β(SE)= −.298(.097), p=.003) and marginally significant on the left (β(SE)= −.217(.116), p=.065).
In whole brain analyses controlling for sex and age (see Table 2 and Figure 2), higher low positive affect-specific factor scores were associated with less thickness in a cluster in left pericalcarine cortex. Significant sex moderation effects were found with surface area of a cluster in right superior frontal sulcus, with male youth showing a non-significant negative association and female youth showing a non-significant positive association (see Supplementary Figure 3 for scatter plots of sex moderation effects).
Associations of gray matter morphometry with anxious arousal-specific factor scores
Across both ROI and whole brain analyses, no significant effects were observed, both when controlling for sex and age and when testing for sex moderation.
Associations of gray matter morphometry with anxious apprehension-specific factor scores
In ROI analyses controlling for sex and age (see Table 2 and Figure 1), higher anxious apprehension-specific factor scores were associated with greater volume of bilateral middle frontal gyrus (β(SE)= .143(.069), p=.042). Post hoc analyses revealed this effect was strongest in the left rostral middle frontal gyrus subregion (β(SE)= .214(.075), p=.005), marginally significant in the right rostral middle frontal gyrus (β(SE)= .142(.076), p= .065), and non-significant in the left (β(SE)= −.013(.082), p=.869) and right (β(SE)= .031(.081), p=.700) caudal middle frontal gyrus subregions.
In whole brain analyses controlling for sex and age (see Table 2 and Figure 2), higher anxious apprehension-specific factor scores were associated with less thickness in the left precuneus. No significant sex moderation effects were observed.
Discussion
Utilizing a bifactor model of internalizing psychopathology within a sample of adolescents, we found evidence that gray matter morphometry of brain regions implicated across anxiety and depression can be parsed into associations of certain subregions with specific symptom dimensions. These results demonstrate that symptoms that are common across internalizing disorders (i.e., common internalizing factor) are associated with individual differences in gray matter volume in prefrontal portions of the DMN, whereas the low positive affect-specific and anxious apprehension-specific factors are associated with portions of the limbic network and the FPN, respectively. Furthermore, some of these associations differed between the sexes in brain regions that are sexually dimorphic in adulthood, highlighting the importance of closely considering sex in studies into the neural correlates of psychopathology. In the following sections, we discuss results for each internalizing dimension in turn, with a focus on how the regional pattern of results align with well-established functional brain networks (e.g., Yeo et al., 2011). We then turn our focus to discussing moderating effects of sex on associations between gray matter morphometry and internalizing symptom dimensions.
Common internalizing factor associated with gray matter of the default mode network
Levels of the common internalizing factor were associated with gray matter morphometry of brain regions often altered across psychiatric conditions, including prefrontal components of the DMN and the dorsal striatum. The DMN supports a range of adaptative functions, including simulating future events, constructing personal meaning, and maintaining continuity of a sense of self (Andrews-Hanna et al., 2014). Atypical DMN function is considered a transdiagnostic risk factor for a range of psychopathologies (Barch, 2017; Whitfield-Gabreli & Ford, 2012). The current results support and extend this literature, demonstrating that gray matter of brain regions predominately within the DMN are associated with transdiagnostic symptoms of internalizing psychopathology, while also showing that these associations may be specific to prefrontal components of the DMN, namely the superior frontal and anterior inferior frontal gyri.
Functional and structural properties of these two prefrontal regions have been recently linked to bifactor symptom dimensions closely related to the common internalizing factor. For example, utilizing the same bifactor model as the current study but in adult women, higher levels of a common internalizing factor were associated with less deactivation of a cluster within the DMN subdivision of the superior frontal gyrus during an emotional word-emotional face Stroop task (Banich et al., 2020). Furthermore, two gray matter morphometry studies utilizing a more general bifactor model of psychopathology in children found that levels of a p-factor were associated with volume of both the superior frontal gyrus and pars orbitalis, the most anterior subdivision of the inferior frontal gyrus, amongst other regions (Durham et al., 2021; Snyder et al., 2017). While the current report focused on internalizing psychopathology only, any covariance across symptoms attributable to a more general p-factor is likely to be partitioned into our common internalizing factor. As such, it is possible that some of the associations observed between the common internalizing factor and gray matter morphometry in the current study may be driven by variance in this factor that is also shared more widely across psychopathology.
The common internalizing factor was also associated with volume of the basal ganglia, which are thought to contribute to a diverse set of psychiatric symptoms (Macpherson & Hikida, 2019). Importantly, this association was driven by bilateral aspects of the dorsal striatum subdivision frequently associated with cognitive control (i.e., caudate and putamen) (Provost et al., 2015), not the ventral striatum subdivision commonly associated with reward processing (Daniel & Pollmann, 2014). The concurrent associations of both prefrontal portions of the DMN and the dorsal striatum with the common internalizing factor may reflect a shared neurobiological mechanism that links these regions to each other as well as to psychopathology. Not only does recent evidence suggest that the basal ganglia largely coactivate with the DMN (Alves et al., 2019), but functional connectivity between the dorsal striatum and the DMN but not the FPN is associated with increased levels of general distress, a construct we believe is closely related to common internalizing (Hua et al., 2019). These findings jointly point to striatal and DMN circuitry as potential transdiagnostic mechanisms affecting comorbid features of internalizing psychopathology and begin to reconcile both network- and region-based approaches to characterizing the neural correlates of mental illness.
Low positive affect-specific factor associated with gray matter within the limbic network
Levels of the low positive affect-specific factor were associated with gray matter of a highly interconnected set of subcortical and cortical regions within the limbic network, including the amygdala, hippocampus, orbitofrontal cortex, and frontal pole. This constellation of regions supports reward-related processes often altered in depression, as well as other disorders (Holland & Gallagher, 2004), processes which are likely central to low positive affect. Though an oversimplification of the diverse range of functions these regions serve, each are thought to support distinct aspects of reward processing, including the signaling of rewarding experiences (i.e., amygdala), the mnemonic encoding and recall of these experiences (i.e., hippocampus), the representation and modulation of reward value (i.e., orbitofrontal cortex), and the instantiation of abstract reward-driven goal states (i.e., frontal pole).
The amygdala and hippocampus, located immediately adjacent to one another with rich bilateral connections, have been implicated in psychopathology. Together they support critical aspects of reward and punishment learning, as well as emotional memory formation and retrieval (Rutishauser et al., 2006; Yang & Wang, 2017). Meta-analytic evidence shows these medial temporal lobe structures may be altered specifically in internalizing disorders as compared to other classes of psychiatric conditions (Goodkind et al., 2015). The current results provide additional clarity as to these findings, suggesting that within internalizing disorders, the amygdala and hippocampus are specifically associated with low positive affect symptoms of depression as opposed to anxiety-specific or comorbid symptoms. Under this scenario, previous evidence of alterations in amygdala volume in anxiety disorders (Milham et al., 2005; Schienle et al., 2011; Suor et al., 2020) may not reflect specific aspects of anxious arousal or apprehension per se and may instead reflect symptoms of depression that often co-occur with anxiety.
The orbitofrontal cortex and frontal pole are contiguous areas of cortex within the limbic network and are broadly implicated in the representation of reward values (Gottfried et al., 2003; Sescousse et al., 2010) and abstract goals (Charron & Koechlin, 2010; Tsujimoto et al., 2011), respectively. While the orbitofrontal cortex is often discussed as contributing to psychopathology due to its role in reward/punishment-related processes (Kringelbach, 2005) and reciprocal connections with the amygdala (Rudebeck & Rich, 2018), associations between the frontal pole and psychopathology are less commonly noted. However, the position of the frontal pole atop the organizational hierarchy of the prefrontal cortex (Badre & D’Esposito, 2009) and coactivation with the limbic network (Yeo et al., 2011) make it well-positioned to play a prominent role in psychopathology symptomology. Additionally, its purported function in managing abstract goal representations align with features of low positive affect and depression, which can include alterations in goal-directed behavior (Johnson et al., 2010; Street, 2002). Specifically, depression has been associated with an inability to effectively disengage from failed goals (Street, 2002), something which may emerge in part through frontal polar mechanisms. Indeed, previous research suggests that gray matter morphometry of a cluster of anterior prefrontal cortex spanning into the frontal pole is associated with individual differences in common executive function, a dimension believed to capture goal-maintenance (Smolker et al., 2018). Taken together, we believe current and past results highlight the frontal pole as an area of particular interest for future research into impairments in goal-directed behavior that are characteristic of depression, as well as other mental illness (Gillan et al., 2016).
While the low positive affect-specific factor was associated with volumetric properties across many diverse portions of the limbic system, our results suggest that these association may not represent a single common underlying mechanism. Rather, effects were in different directions and of different magnitudes across these regions. As such, we speculate that the diversity of associations reflect heterogeneity in the neural bases of low positive affect, with individual differences in this symptom dimension potentially emerging at multiple, distinct subsystems within the limbic network. We speculate further that these could include blunted amygdala reactivity to rewarding stimuli in the environment, hippocampally-mediated biases towards self-punishing or away from rewarding memories, failures in the orbitofrontal cortex to properly represent reward value, or impairments in the frontal poles capacity to effectively manage abstract goal representations, particularly in the face of perceived goal failure. Indeed, anhedonia/low positive affect is thought to have several distinct subtypes (Cooper et al., 2018), suggesting not only a range of mechanisms but also highlighting the need for additional research into potential subfactors that may exist within the dimensions utilized in the current report and elsewhere.
Anxious arousal-specific factor shows limited relations with gray matter morphometry
Surprisingly, there was no evidence of significant associations between levels of the anxious arousal-specific factor and gray matter morphometry, both when controlling for sex and age as well as when testing for sex moderation effects. This lack of association contrasts with previous research in youth in which anxious arousal was positively associated with cortical thickness across several brain regions (Castagna et al., 2018). However, Castagna and colleagues had a younger youth sample than that in the current report and evidence suggests that anxiety symptomology may shift across development, with disorders characterized by more anxious arousal-like symptoms emerging earlier in life than disorders characterized by more anxious apprehension-like symptoms (de Lijster et al., 2007). Further research is needed to better understand the trajectory of anxious arousal across the lifespan and how this might influence the brain systems associated with this symptom dimension.
Anxious apprehension-specific factor associated with gray matter of the frontoparietal network
In support of models of anxious apprehension as being strongly linked to cognitive control mechanisms (Hallion et al., 2017; Madian et al., 2019), the anxious apprehension-specific factor was the only dimension in the current study to show associations with gray matter morphometry within the FPN, a brain network supporting cognitive control over working memory and attention (e.g., Marek & Dosenbach; Ptak, 2012). These results are particularly notable when contrasted to associations of the common internalizing factor scores with gray matter of prefrontal portions of the DMN. The DMN and FPN are consistently anti-correlated, with the DMN preferentially activating when participants are engaged in spontaneous internal mentation on the self and the FPN preferentially activating when individuals engage control over their internal mentation, including working memory and attention (Andrews-Hanna et al., 2014). Under this framework, the current results suggest that the common internalizing factor may capture symptoms related to the nature and content of internally directed mentation whereas the anxious apprehension-specific factor captures symptoms related to the ability to regulate internally directed mentation, including the contents of working memory and focus of attention.
Associations of anxious apprehension with gray matter morphometry of portions of the middle frontal gyrus have been observed in a youth previously (Castagna et al., 2018) but in that prior report it was unclear the degree to which these associations reflected variance in symptomology that is specific to anxious apprehension or shared with other aspects of psychopathology. In the current report, we find that associations between middle frontal gyrus gray matter may be specific to aspects of anxious apprehension that are dissociable from anxious arousal and common internalizing. In addition to the middle frontal gyrus, whole brain analyses revealed that anxious apprehension-specific factor scores were associated with volume of the posterior precuneus. fMRI research into the neural correlates of anxious apprehension have frequently implicated precuneus activity during worry-induction paradigms (Paulesu et al., 2010; Servaas et al., 2014) and functional connectivity work suggests that anxious apprehension may recruit a middle frontal gyrus to precuneus circuit, with levels of self-reported worry associated with increased connectivity of these two regions during a “Go/No-go” task (Forster et al., 2015). Our results build off these findings, demonstrating that trait levels of anxious apprehension-specific are not only associated with functional properties of the middle frontal gyrus and posterior precuneus, but gray matter morphometry as well.
Sex affects the neuroanatomical correlates of internalizing psychopathology
Sex moderation analyses found that the neuroanatomical correlates of adolescent internalizing psychopathology differ between adolescent males and females in both prefrontal and limbic brain regions. Looking across the four internalizing dimensions, sex moderation effects occurred in brain regions that are generally sexually dimorphic in adulthood (Lotze et al., 2019; Ruigork et al., 2014), including the superior frontal gyrus, frontal pole, and hippocampus. These effects were particularly notable for the associations between the low positive affect-specific factor and portions of the limbic network, namely the frontal pole and hippocampus. Post hoc analyses revealed that these effects were driven by the right homologs and were the only instances in which adolescent males and females showed significant or marginally significant associations that were in opposing directions (i.e., positive associations in males and negative associations in females). Interestingly, previous research suggesting that the neurodevelopmental trajectories of these regions differ between the sexes. For example, not only does the frontal pole show greater volume in adult women as compared to men (Lotze et al., 2019), but it is one of the few regions in which females show a significantly greater degree of reduction in volume than males across adolescence (Vijayakumar et al., 2016). Furthermore, sex differences in hippocampal development and function have been well documented in humans and rats (Siddiqui & Romeo, 2019; Persson et al., 2014; van Eijk et al., 2020; Yagi & Galea, 2019), with evidence suggesting that these sex differences are particularly relevant to disorders exacerbated by stress, as is the case for internalizing disorders (Hillerer et al., 2019). In fact, rats demonstrate opposite effects of stress between the sexes on hippocampal microstructure, with stress leading to an increase in neuronal spine density and dendrite length in males but a decrease in females (Weinstock, 2011). Though we are unable to speak to the effects of stress in the current report, the pattern of results coincides with these findings, with higher low positive affect-specific symptoms associating with greater hippocampal volume in males but less hippocampal volume in females. Future research will be well served to interrogate the mechanisms driving sexual dimorphisms in limbic neuroanatomy and their associations with psychopathology. For main effects of sex on gray matter morphometry in the current sample, see Supplementary Figure 4.
Direction of neuroanatomy-psychopathology associations change across the lifespan
Further insight into developmental effects on the neuroanatomical correlates of psychopathology can be gleaned by comparing results from the current adolescent sample to similar studies in adults and children. Most notably, whereas previous research in adults consistently show negative associations between overall severity of transdiagnostic psychopathology dimensions and gray matter morphometry, we and others observe the opposite in adolescents: higher levels of the internalizing factors are predominately associated with higher volume or thickness (e.g., Castagna et al., 2018; Gold et al., 2017). Though there are fewer gray matter morphometry studies that focus on psychopathology symptom severity and gray matter in child-only samples, a recent analysis across over 9,000 9–10-year-olds suggest that children, like adults, exclusively show negative associations between gray matter volume and general psychopathology dimensions (Durham et al., 2021). As such, the current results add to a growing body of literature suggesting that adolescence may mark a particularly unique period in the trajectory of the neuroanatomical correlates of psychopathology, presumably due to the dynamic neurodevelopmental processes that begin with puberty and unfold thereafter across adolescence.
We speculate that positive associations between gray matter morphometry and psychopathology during adolescence may reflect atypical, potentially pathological developmental processes, namely stunted synaptic pruning during adolescence. Typical adolescent neurodevelopment is characterized by extensive synaptic pruning, expansion of white matter, and a consolidation of neuronal cell bodies, all of which leads to a reduction in cortical thickness and volume, as well as more proficient neuronal communication, and mature psychological functioning. If these processes are somehow delayed or stunted, this would likely manifest as relatively higher levels of gray matter volume or thickness during adolescence. Indeed, neurodevelopmental models of other psychiatric conditions such as autism spectrum disorder and schizophrenia posit that altered pruning (e.g., Keshavan et al., 2020; Thomas et al., 2016) and neuronal proliferation (e.g., Reif et al., 2006) may be key etiological factors contributing to these disorders.
Limitations and future directions
The current study is not without limitations. First, the gray matter morphometry analyses reported in this manuscript are strictly correlational and do not imply causal relations. While the correlational nature of these results may limit our ability to make inferences regarding the etiology of internalizing psychopathology, our results provide a framework for delving deeper into the specific neural systems that may drive both comorbidity and heterogeneity in psychiatric symptoms. Second, it remains unclear the degree to which the neural systems associated with symptoms differ between general population and clinical samples. As such, it is unknown whether results in the current report reflect associations that are indeed relevant to current clinical methodologies. Third, while subject recruitment was targeted to maximize socioeconomic diversity, we did not explicitly test whether the current sample mirrors the national distribution of socioeconomic status. As such, the degree to which the current results are generalizable across socioeconomic statuses is unclear. Ongoing analyses are targeted at utilizing longitudinal approaches to characterize the developmental and psychopathology symptom trajectories of the current sample. In addition, an intriguing future direction is to evaluate potential indirect effects of prefrontal gray matter morphometry on associations between subcortical gray matter and psychiatric symptomology, as has been recently implemented in a large adult sample (Castagna et al., 2019).
Conclusions
To date, understanding the neurobiological underpinnings of psychiatric disorders has been challenging in part due to the comorbidity between disorders. The current report demonstrates the utility of bifactor modeling and individual differences approaches to address this issue, identifying neural systems that are preferentially associated with specific aspects of psychopathology. In addition to observing associations between gray matter morphometry and internalizing symptom dimensions across much of the brain, these associations differed between males and females in sexually dimorphic brain regions. These findings begin to parse the neuroanatomical correlates of mental illness into specific region-symptom associations, while identifying important regions of interest for further inquiry into the neurodevelopmental processes that make adolescents particularly vulnerable to psychopathology.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Kathy Pearson, David Caha, Rebecca Helmuth, and Kenny Carlson for invaluable contributions to this project.
Funding
This research was made possible through funding from grants R01MH105501 and T32MH016880.
Footnotes
Declaration of conflicting interests
The authors declared that they had no conflicts of interest related to this manuscript.
References
- Alves PN, Foulon C, Karolis V, Bzdok D, Margulies DS, Volle E, & de Schotten MT (2019). An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings. Communications biology, 2(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316(1), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, & D’esposito M (2009). Is the rostro-caudal axis of the frontal lobe hierarchical?. Nature Reviews Neuroscience, 10(9), 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Smith LL, Smolker HR, Hankin BL, Silton RL, Heller W, & Snyder HR (2020). Common and specific dimensions of internalizing disorders are characterized by unique patterns of brain activity on a task of emotional cognitive control. International Journal of Psychophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM (2017). The neural correlates of transdiagnostic dimensions of psychopathology. American Journal of Psychiatry, 174, 613–615. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Choate AM, Fatimah H, Petersen KJ, & Wiernik BM (2020). Appropriate Use of Bifactor Analysis in Psychopathology Research: Appreciating Benefits and Limitations. Biological Psychiatry, 88(1), 18–27. 10.1016/j.biopsych.2020.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos MG, Peters S, van de Kamp FC, Crone EA, & Tamnes CK (2018). Emerging depression in adolescence coincides with accelerated frontal cortical thinning. Journal of Child Psychology and Psychiatry, 59(9), 994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Antony MM, & Barlow DH (1992). Psychometric properties of the Penn State Worry Questionnaire in a clinical anxiety disorders sample. Behaviour Research and Therapy, 30(1), 33–37. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, & Brammer MJ (1999). Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE transactions on medical imaging, 18(1), 32–42. [DOI] [PubMed] [Google Scholar]
- Cardinale EM, Kircanski K, Brooks J, Gold AL, Towbin KE, Pine DS, … & Brotman MA (2019). Parsing neurodevelopmental features of irritability and anxiety: Replication and validation of a latent variable approach. Development and psychopathology, 31(3), 917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, … & Moffitt TE (2014). The p factor: one general psychopathology factor in the structure of psychiatric disorders?. Clinical Psychological Science, 2(2), 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna PJ (2019). Structure related to function: prefrontal surface area has an indirect effect on the relationship between amygdala volume and trait neuroticism. Brain Structure and Function, 224(9), 3309–3320. [DOI] [PubMed] [Google Scholar]
- Castagna PJ, Roye S, Calamia M, Owens-French J, Davis TE, & Greening SG (2018). Parsing the neural correlates of anxious apprehension and anxious arousal in the grey-matter of healthy youth. Brain imaging and behavior, 12(4), 1084–1098. [DOI] [PubMed] [Google Scholar]
- Charron S, & Koechlin E (2010). Divided representation of concurrent goals in the human frontal lobes. Science, 325(5976), 360–363. [DOI] [PubMed] [Google Scholar]
- Chen FF, Hayes A, Carver CS, Laurenceau JP, & Zhang Z (2012). Modeling general and specific variance in multifaceted constructs: A comparison of the bifactor model to other approaches. Journal of personality, 80(1), 219–251. [DOI] [PubMed] [Google Scholar]
- Clark LA, & Watson D., (1991) Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology, 100 (3), 316–336. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Arulpragasam AR, & Treadway MT (2018). Anhedonia in depression: biological mechanisms and computational models. Current opinion in behavioral sciences, 22, 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Costello EJ, & Angold A (2009). Childhood and adolescent psychiatric disorders as predictors of young adult disorders. Archives of general psychiatry, 66(7), 764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179–194. [DOI] [PubMed] [Google Scholar]
- Daniel R, & Pollmann S (2014). A universal role of the ventral striatum in reward-based learning: evidence from human studies. Neurobiology of learning and memory, 114, 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lijster JM, Dierckx B, Utens EM, Verhulst FC, Zieldorff C, Dieleman GC, & Legerstee JS (2017). The age of onset of anxiety disorders: a meta-analysis. Canadian journal of psychiatry. Revue canadienne de psychiatrie, 62(4), 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … & Albert MS (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980. [DOI] [PubMed] [Google Scholar]
- Ducharme S, Albaugh MD, Hudziak JJ, Botteron KN, Nguyen TV, Truong C, … & O’Neill J (2014). Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cerebral cortex, 24(11), 2941–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham EL, Jeong HJ, Moore TM, Dupont RM, Cardenas-Iniguez C, Cui Z, … & Kaczkurkin AN (2021). Association of gray matter volumes with general and specific dimensions of psychopathology in children. Neuropsychopharmacology, 46(7), 1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels AS, Heller W, Mohanty A, Herrington JD, Banich MT, Webb AG, et al. (2007). Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology, 44, 352–363. [DOI] [PubMed] [Google Scholar]
- Fernandes BS, Williams LM, Steiner J, Leboyer M, Carvalho AF, & Berk M (2017). The new field of ‘precision psychiatry’. BMC medicine, 15(1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences, 97(20), 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, & Dale AM (2001). Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE transactions on medical imaging, 20(1), 70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, & Dale AM (1999a). Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage, 9(2), 195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, & Dale AM (1999b). High-resolution intersubject averaging and a coordinate system for the cortical surface. Human brain mapping, 8(4), 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, … & Caviness V (2004). Automatically parcellating the human cerebral cortex. Cerebral cortex, 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Forster S, Nunez Elizalde AO, Castle E, & Bishop SJ (2015). Unraveling the anxious mind: anxiety, worry, and frontal engagement in sustained attention versus off-task processing. Cerebral Cortex, 25(3), 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennatas ED, Avants BB, Wolf DH, Satterthwaite TD, Ruparel K, Ciric R, … & Gur RC (2017). Age-related effects and sex differences in gray matter density, volume, mass, and cortical thickness from childhood to young adulthood. Journal of Neuroscience, 37(20), 5065–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Mills KL, & Lenroot RK (2012). Magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biology of sex differences, 3(1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan CM, Kosinski M, Whelan R, Phelps EA, & Daw ND (2016). Characterizing a psychiatric symptom dimension related to deficits in goal-directed control. Elife, 5, e11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, … & Thompson PM (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101(21), 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, & Thompson PM (2010). Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain and cognition, 72(1), 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Steuber ER, White LK, Pacheco J, Sachs JF, Pagliaccio D, … & Pine DS (2017). Cortical thickness and subcortical gray matter volume in pediatric anxiety disorders. Neuropsychopharmacology, 42(12), 2423–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, … & Grieve SM (2015). Identification of a common neurobiological substrate for mental illness. JAMA psychiatry, 72(4), 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, & Dolan RJ (2003). Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science, 301(5636), 1104–1107. [DOI] [PubMed] [Google Scholar]
- Hallion LS, Tolin DF, Assaf M, Goethe J, & Diefenbach GJ (2017). Cognitive control in generalized anxiety disorder: relation of inhibition impairments to worry and anxiety severity. Cognitive Therapy and Research, 41(4), 610–618. [Google Scholar]
- Hankin BL (2006). Adolescent depression: Description, causes, and interventions. Epilepsy & behavior, 8(1), 102–114. [DOI] [PubMed] [Google Scholar]
- Hankin BL (2009). Development of sex differences in depressive and co-occurring anxious symptoms during adolescence: Descriptive trajectories and potential explanations in a multiwave prospective study. Journal of Clinical Child & Adolescent Psychology, 38(4), 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL (2015). Depression from childhood through adolescence: risk mechanisms across multiple systems and levels of analysis. Current opinion in psychology, 4, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Wetter E, & Cheely C (2008). Sex differences in child and adolescent depression: A developmental psychopathological approach. [Google Scholar]
- Hankin BL, Young JF, Abela JR, Smolen A, Jenness JL, Gulley LD, … & Oppenheimer CW (2015). Depression from childhood into late adolescence: Influence of gender, development, genetic susceptibility, and peer stress. Journal of abnormal psychology, 124(4), 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillerer KM, Slattery DA, & Pletzer B (2019). Neurobiological mechanisms underlying sex-related differences in stress-related disorders: Effects of neuroactive steroids on the hippocampus. Frontiers in neuroendocrinology, 55, 100796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, & Buckner RL (2012). Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. Journal of Neuroscience, 32(50), 18087–18100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural equation modeling: a multidisciplinary journal, 6(1), 1–55. [Google Scholar]
- Hua JP, Karcher NR, Merrill AM, O’Brien KJ, Straub KT, Trull TJ, & Kerns JG (2019). Psychosis risk is associated with decreased resting-state functional connectivity between the striatum and the default mode network. Cognitive, Affective, & Behavioral Neuroscience, 19(4), 998–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Carver CS, & Fulford D (2010). Goal dysregulation in the affective disorders. [Google Scholar]
- Kansky J, Allen JP, & Diener E (2016). Early adolescent affect predicts later life outcomes. Applied Psychology: Health and Well-Being, 8(2), 192–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan M, Lizano P, & Prasad K (2020). The synaptic pruning hypothesis of schizophrenia: promises and challenges. World Psychiatry, 19(1), 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, & Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry, 62(6), 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, & Watson D (2018). A paradigm shift in psychiatric classification: the Hierarchical Taxonomy Of Psychopathology (HiTOP). World Psychiatry, 17(1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML (2005). The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience, 6(9), 691. [DOI] [PubMed] [Google Scholar]
- Lener MS, Kundu P, Wong E, Dewilde KE, Tang CY, Balchandani P, & Murrough JW (2016). Cortical abnormalities and association with symptom dimensions across the depressive spectrum. Journal of affective disorders, 190, 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, … & Thompson PM (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage, 36(4), 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Domin M, Gerlach FH, Gaser C, Lueders E, Schmidt CO, & Neumann N (2019). Novel findings from 2,838 adult brains on sex differences in gray matter brain volume. Scientific reports, 9(1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson T, & Hikida T (2019). Role of basal ganglia neurocircuitry in the pathology of psychiatric disorders. Psychiatry and clinical neurosciences, 73(6), 289–301. [DOI] [PubMed] [Google Scholar]
- Madian N, Bredemeier K, Heller W, Miller GA, & Warren SL (2019). Repetitive negative thought and executive dysfunction: An interactive pathway to emotional distress. Cognitive Therapy and Research, 43(2), 464–480. [Google Scholar]
- Marek S, & Dosenbach NU (2018). The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues in clinical neuroscience, 20(2), 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Goodkind MS, & Etkin A (2016). Transdiagnostic impairment of cognitive control in mental illness. Journal of psychiatric research, 83, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends in cognitive sciences, 15(10), 483–506. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, & Borkovec TD (1990). Development and validation of the Penn State worry questionnaire. Behaviour research and therapy, 28(6), 487–495. [DOI] [PubMed] [Google Scholar]
- Milham MP, Nugent AC, Drevets WC, Dickstein DS, Leibenluft E, Ernst M, … & Pine DS (2005). Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biological psychiatry, 57(9), 961–966. [DOI] [PubMed] [Google Scholar]
- Molina S, & Borkovec TD (1994). The Penn State Worry Questionnaire: Psychometric properties and associated characteristics. In Davey GCL & Tallis F (Eds.), Wiley series in clinical psychology. Worrying: Perspectives on theory, assessment and treatment (pp. 265–283). Oxford, England: John Wiley & Sons [Google Scholar]
- Muthén LK, & Muthén BO (2012). MPlus: statistical analysis with latent variables--User’s guide. [Google Scholar]
- Nikolova YS, Bogdan R, Brigidi BD, & Hariri AR (2012). Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biological psychiatry, 72(2), 157–163. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Imig JC, McDonald RP, & Miller GA (2001). Distinguishing dimensions of anxiety and depression. Cognitive Therapy and Research, 25(1), 1–22. [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, & Miller GA (1999). Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology, 36(5), 628–637. [PubMed] [Google Scholar]
- Öhman A (2005). The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology, 30(10), 953–958. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Sambugaro E, Torti T, Danelli L, Ferri F, Scialfa G, … & Sassaroli S (2010). Neural correlates of worry in generalized anxiety disorder and in normal controls: a functional MRI study. Psychological medicine, 40(1), 117. [DOI] [PubMed] [Google Scholar]
- Persson J, Spreng RN, Turner G, Herlitz A, Morell A, Stening E, … & Söderlund H (2014). Sex differences in volume and structural covariance of the anterior and posterior hippocampus. Neuroimage, 99, 215–225. [DOI] [PubMed] [Google Scholar]
- Provost JS, Hanganu A, & Monchi O (2015). Neuroimaging studies of the striatum in cognition Part I: healthy individuals. Frontiers in systems neuroscience, 9, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R (2012). The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. The Neuroscientist, 18(5), 502–515. [DOI] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, & Lesch KP (2006). Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Molecular psychiatry, 11(5), 514–522. [DOI] [PubMed] [Google Scholar]
- Romer AL, Elliott ML, Knodt AR, Sison ML, Ireland D, Houts R, … & Moffitt TE (2020). Pervasively thinner neocortex as a transdiagnostic feature of general psychopathology. American Journal of Psychiatry, appi-ajp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer AL, Knodt AR, Houts R, Brigidi BD, Moffitt TE, Caspi A, & Hariri AR (2018). Structural alterations within cerebellar circuitry are associated with general liability for common mental disorders. Molecular psychiatry, 23(4), 1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer AL, Knodt AR, Sison ML, Ireland D, Houts R, Ramrakha S, … & Caspi A (2019). Replicability of structural brain alterations associated with general psychopathology: evidence from a population-representative birth cohort. Molecular Psychiatry, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, & Walker EF (Eds.). (2007). Adolescent psychopathology and the developing brain: Integrating brain and prevention science. Oxford University Press. [Google Scholar]
- Rudebeck PH, & Rich EL (2018). Orbitofrontal cortex. Current Biology, 28(18), R1083–R1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, & Suckling J (2014). A meta-analysis of sex differences in human brain structure. Neuroscience & Biobehavioral Reviews, 39, 34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U, Mamelak AN, & Schuman EM (2006). Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron, 49(6), 805–813. [DOI] [PubMed] [Google Scholar]
- Schienle A, Ebner F, & Schäfer A (2011). Localized gray matter volume abnormalities in generalized anxiety disorder. European archives of psychiatry and clinical neuroscience, 261(4), 303–307. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, … & Vernooij MW (2017). Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Molecular psychiatry, 22(6), 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ségonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, & Fischl B (2004). A hybrid approach to the skull stripping problem in MRI. Neuroimage, 22(3), 1060–1075. [DOI] [PubMed] [Google Scholar]
- Servaas MN, Riese H, Ormel J, & Aleman A (2014). The neural correlates of worry in association with individual differences in neuroticism. Human brain mapping, 35(9), 4303–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Redouté J, & Dreher JC (2010). The architecture of reward value coding in the human orbitofrontal cortex. Journal of neuroscience, 30(39), 13095–13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PB, Miller GA, & Heller W (2015). Transdiagnostic dimensions of anxiety: neural mechanisms, executive functions, and new directions. International Journal of Psychophysiology, 98(2), 365–377. [DOI] [PubMed] [Google Scholar]
- Siddiqui A, & Romeo RD (2019). Sex differences and similarities in hippocampal cellular proliferation and the number of immature neurons during adolescence in rats. Developmental neuroscience, 41(1-2), 132–138. [DOI] [PubMed] [Google Scholar]
- Smith L, Reichenberg A, Rabinowitz J, Levine SZ, & Velthorst E (2018). Psychiatric symptoms and related dysfunction in a general population sample. Schizophrenia Research: Cognition, 14, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolker HR, Friedman NP, Hewitt JK, & Banich MT (2018). Neuroanatomical correlates of the unity and diversity model of executive function in young adults. Frontiers in human neuroscience, 12, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Silton RL, Hankin BL, Smolker HR, Kaiser RH, Banich MT, Miller GA, & Heller W (submitted). The dimensional structure of internalizing psychopathology: Relation to diagnostic categories. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Hankin BL, Sandman CA, Head K, & Davis EP (2017). Distinct patterns of reduced prefrontal and limbic gray matter volume in childhood general and internalizing psychopathology. Clinical Psychological Science, 5(6), 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street H (2002). Exploring relationships between goal setting, goal pursuit and depression: A review. Australian Psychologist, 37(2), 95–103. [Google Scholar]
- Suor JH, Jimmy J, Monk CS, Phan KL, & Burkhouse KL (2020). Parsing differences in amygdala volume among individuals with and without social and generalized anxiety disorders across the lifespan. Journal of Psychiatric Research, 128, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MS, Davis R, Karmiloff-Smith A, Knowland VC, & Charman T (2016). The over-pruning hypothesis of autism. Developmental Science, 19(2), 284–305. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Genovesio A, & Wise SP (2011). Frontal pole cortex: encoding ends at the end of the endbrain. Trends in cognitive sciences, 15(4), 169–176. [DOI] [PubMed] [Google Scholar]
- van Eijk L, Hansell NK, Strike LT, Couvy-Duchesne B, de Zubicaray GI, Thompson PM, … & Wright MJ (2020). Region-specific sex differences in the hippocampus. NeuroImage, 215, 116781. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N, Allen NB, Youssef G, Dennison M, Yücel M, Simmons JG, & Whittle S (2016). Brain development during adolescence: A mixed-longitudinal investigation of cortical thickness, surface area, and volume. Human brain mapping, 37(6), 2027–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, … & Weiss SR (2018). The conception of the ABCD study: From substance use to a broad NIH collaboration. Developmental cognitive neuroscience, 32, 4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, & McCormick RA (1995a). Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of abnormal Psychology, 104(1), 15. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, & McCormick RA (1995b). Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of abnormal psychology, 104(1), 3. [DOI] [PubMed] [Google Scholar]
- Weinstock M (2011). Sex-dependent changes induced by prenatal stress in cortical and hippocampal morphology and behaviour in rats: an update. Stress, 14(6), 604–613. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Ford JM (2012). Default mode network activity and connectivity in psychopathology. Annual review of clinical psychology, 8, 49–76. [DOI] [PubMed] [Google Scholar]
- Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, … & Allen NB (2014). Structural brain development and depression onset during adolescence: a prospective longitudinal study. American Journal of Psychiatry, 171(5), 564–571. [DOI] [PubMed] [Google Scholar]
- Williams LM (2016). Precision psychiatry: a neural circuit taxonomy for depression and anxiety. The Lancet Psychiatry, 3(5), 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, & Nichols TE (2014). Permutation inference for the general linear model. Neuroimage, 92, 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi S, & Galea LA (2019). Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology, 44(1), 200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Peng Z, Ma X, Meng Y, Li M, Zhang J, … & Ma X (2017). Sex differences in the clinical characteristics and brain gray matter volume alterations in unmedicated patients with major depressive disorder. Scientific reports, 7(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, & Wang JZ (2017). From structure to behavior in basolateral amygdala-hippocampus circuits. Frontiers in neural circuits, 11, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … & Fischl B (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbozinek TD, Rose RD, Wolitzky-Taylor KB, Sherbourne C, Sullivan G, Stein MB, … & Craske MG (2012). Diagnostic overlap of generalized anxiety disorder and major depressive disorder in a primary care sample. Depression and anxiety, 29(12), 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Boucher M, Rosa-Neto P, & Evans AC (2013). Impact of scale space search on age-and gender-related changes in MRI-based cortical morphometry. Human brain mapping, 34(9), 2113–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisook S, Lesser I, Stewart JW, Wisniewski SR, Balasubramani GK, Fava M, … & Rush AJ (2007). Effect of age at onset on the course of major depressive disorder. American Journal of Psychiatry, 164(10), 1539–1546 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


