Abstract
Background and Objectives
Persistent atrial fibrillation (PeAF) with heart failure (HF) arguably constitutes the sickest subset of atrial fibrillation (AF) patients.
Methods
A systematic search was made in PubMed, Embase, and Scopus databases. Network meta-analysis (NMA) of PeAF patients with systolic HF comparing all-cause mortality, change in HF-related quality of life (QoL) and hospitalization due to heart failure (HHF) were performed among catheter ablation (CA) of AF, rate-controlling drugs (RCDs), anti-arrhythmic drugs (AADs), and atrio-ventricular nodal ablation (AVNA) using Bayesian random effect model.
Results
Ablation strategies resulted significantly lower mortality than medical therapies (odds ratio [OR], 0.51; 95% confidence interval [CI], 0.35 to 0.76). CA of AF was associated with lower trend of mortality (OR, 0.78; 95% credible interval [CrI], 0.08 to 7.63) in comparison to AVNA in the Bayesian NMA. Rhythm control strategies resulted significantly higher improvement of QoL than rate control strategies (mean difference [MD], −12.78; 95% CI, −21.26 to −4.31). Bayesian NMA showed that CA of AF was better than AAD (MD, −7.98; 95% CrI, −27.68 to 8.27), however ranked AVNA to be lowest. Ablation strategies provided significantly lower HHF than medical therapies (OR, 0.42; 95% CI, 0.30 to 0.58). Bayesian NMA showed that CA of AF performed not only better than AAD (OR, 0.33; 95% CrI, 0.09 to 1.3) to reduce HHF, but also than AVNA (OR, 0.20; 95% CrI, 0.00 to 4.76). Of note, RCD ranked lowest with regard to mortality and HHF.
Conclusions
CA of AF remains the best strategy even for the sickest group of PeAF patients with systolic HF in regards to all-cause mortality, HF-related QoL and HHF.
Keywords: Persistent atrial fibrillation, Heart failure, Catheter ablation
INTRODUCTION
Use of catheter ablation (CA) in persistent atrial fibrillation (PeAF) requires extensive ablation strategy in addition to pulmonary vein isolation to get rid of non-PV triggers or complex atrial fractionated electrogram (CFAE), adoption of newer mapping technologies, such as focal impulse and rotor modulation and thoracoscopic convergence approaches.1),2),3),4),5),6) The CAMERA-MRI trial questioned the role of rate control strategy in atrial fibrillation (AF) patients with heart failure (HF)7) and the CASTLE-AF trial showed that CA for symptomatic paroxysmal or persistent AF patients with systolic heart failure was associated with a significantly lower rate of a composite end point of all-cause mortality or hospitalization due to heart failure (HHF) in comparison to medical therapy.8) Subsequently CA was generalized in AF with HF patients and that led to 2019 American Heart Association/American College of Cardiology (AHA/ACC) recommendation for CA as class IIb indication for CASTLE-AF-like patients.9),10) Analysis of the AF patients with HF (mostly HF with preserved ejection fraction) enrolled in the CABANA trial showed that, CA produced significant improvements in survival, freedom from AF-recurrence, and quality of life in comparison to drug therapy.11)
In real-world practice, in patients with PeAF with HF, a medical therapy (MT) with rate-controlling drugs (RCD) and/or anti-arrhythmic drugs (AADs) is the first-line of therapy, and if not benefitted or tolerated, CA is undertaken. In a selected group of patients, biventricular (BiV) pacing (or physiological pacing) may improve heart failure symptoms and atrio-ventricular nodal ablation (AVNA) could be the last resort if BiV pacing is not adequate due to AF despite RCDs. Meta-analysis including AF patients with HF demonstrated that CA was associated with better structural, functional and survival benefit than medical therapy.12),13) However, these studies did not distinguish between RCD or AADs and did not include pace and ablate strategy into consideration. ESC recommends CA of AF as class I recommendation in AF and HF patients to reduce mortality and HHF.14)
We have conducted a network analysis of all-cause mortality, improvement of HF-related quality of life (QoL), AF recurrence, and HHF in patients of PeAF with systolic heart failure comparing four treatment arms viz. RCDs, AADs, CA and AVNA.
METHODS
The meta-analysis consisted of PeAF patients with HF and compared rate control (RCDs and AVNA) versus rhythm control (AADs and CA) strategies and medical therapy (RCDs and AADs) versus CA in regards to all-cause mortality, change in QoL, AF recurrence and HHF. The network meta-analysis was aimed to conduct direct and indirect comparisons of all-cause mortality, change in QoL, AF recurrence, and HHF among four treatment arms (RCDs, AADs, CA of PeAF, and AVNA). The network meta-analysis has been described by the PRISMA guideline.15)
Search strategy
A systematic review was performed to search the existing literature published in the English language, as of October 2020. Three physician-reviewers (DK, AM, SD) queried PubMed, Embase, and Scopus databases for published literature; search terms were “atrial fibrillation,” “heart failure,” “rate control,” “rhythm control,” “anti-arrhythmic drugs,” “catheter ablation,” “radiofrequency ablation,” “pulmonary vein isolation,” “atrioventricular nodal ablation,” “pacing,” “death,” “cardiovascular death,” “heart failure hospitalization,” “quality of life,” “atrial fibrillation recurrence,” “implantable cardioverter-defibrillator (ICD),” “cardiac resynchronization therapy (CRT),” and combinations of these keywords. Additional literature was sought by searching the references of eligible articles. Any inter-reviewer discrepancies were resolved by a fourth reviewer (DP).
Study selection
Inclusion criteria
For the quantitative synthesis of the meta-analysis, we only selected randomized controlled trials (RCT) that directly compared two or more treatment modalities (RCDs, AADs, CA of PeAF, and AVNA) in the PeAF patients with systolic heart failure, i.e., left ventricular ejection fraction (LVEF) <50%, and provided all-cause mortality and change in QOL as outcomes.
Exclusion criteria
We excluded: (1) observational or non-randomized studies, single-arm studies, case reports, case series, review articles, and abstracts presented in the conferences, (2) studies with paroxysmal atrial fibrillation (PAF), (3) AF without HF, (4) diastolic HF. Studies that did not report outcomes for rate control (RCDs or AVNA) or rhythm control (AADs or CA) separately or did not specify medical therapy (whether RCDs or AADs) were excluded from the network meta-analysis (NMA).
Critical appraisal
The randomized studies were appraised with the Risk of Bias 2.0 Scale16) which assessed individual studies in regards to patient selection, randomization, blinding, and outcome data reporting.
Data extraction
Baseline characteristics and outcomes were extracted from each of the selected studies. Baseline characteristics included AF treatment arms, sample size as per intention to treat analysis, study-design, follow-up duration, age, left atrial diameter, subjects receiving devices, LVEF, previous cardioversion, ischemic background. Complications of individual treatments, mortality, and changes in HF QoL were recorded when available. As most studies reported QoL as per Minnesota Heart Failure Questionnaire (MLHFQ), the quantitative meta-analysis and NMA excluded studies that reported QoL in other metrics.
Data analysis
Meta-analysis
Descriptive statistics are presented as means and standard deviations (SDs) for continuous variables and the number of cases or percentages for dichotomous and categorical variables. Statistical analysis was performed in line with recommendations from the Cochrane Collaboration using RStudio 1.4.17) Comparison of all-cause mortality and change in QoL between catheter ablation and medical therapy or rate vs. rhythm control have been conducted using the random-effects model of DerSimonian and Laird. Heterogeneity was described as I2 statistics; publication bias was assessed by funnel plot; covariate analysis was performed and depicted as a bubble plot.
NMA
Bayesian Network meta-analysis comparing four treatment arms (e.g., RCD, AAD, and AVNA) was performed. Network plot, league table, and rankogram were derived using MetInsight V3.1418) and using Markov chain Monte Carlo (MCMC) simulations.19) Gelman-Rubin convergence was assessed for all the studies.20) Estimates from Bayesian NMA were presented as odds ratio (OR) or mean difference (MD) with 95% credible interval (CrI), i.e., the value at 2.5% and 97.5% quantiles.21) The ranking table was derived, which showed the probability for each treatment to be ranked as the most effective treatment. The rankogram was based on the median ranking probabilities of each treatment arm.21) The median rank is the middle iteration rank when every simulated estimate of the class is ordered.
Inconsistency, deviance, and sensitivity analysis
The direct, indirect, and network estimates and a Bayesian p-value for the related test of inconsistency between the direct and indirect evidence for each treatment comparison were derived.22) Deviance analysis was conducted and contribution for each study arm to the residual deviance was also plotted and analyzed. The contour plot simultaneously looked at residual deviance and leverage.23) Leverage values outside the contour of 3 are considered poorly fitting.
RESULTS
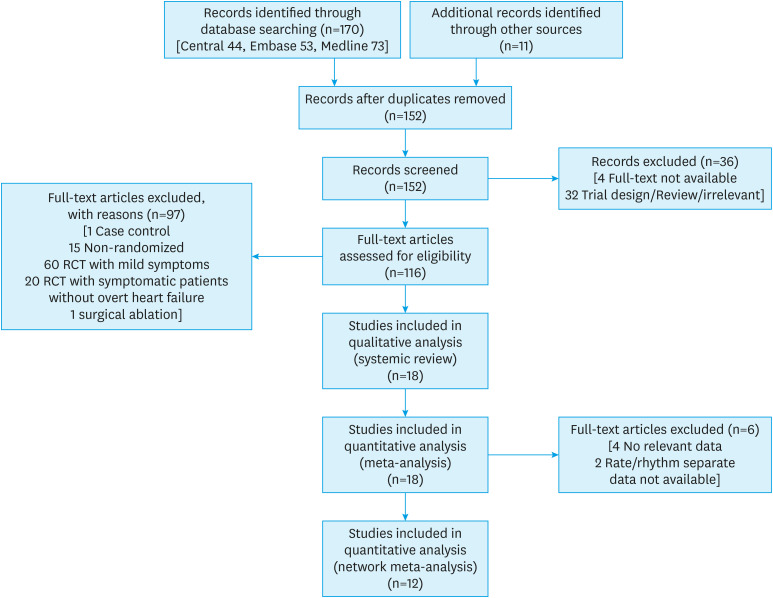
A total of 18 RCTs7),8),24),25),26),27),28),29),30),31),32),33),34),35),36),37),38),39) were included for the systematic review and qualitative meta-analysis and six studies were excluded from the quantitative meta-analysis (Figure 1).7),8),30),33),37),39) Most of the studies were not blinded and not all studies reported the required outcomes (Supplementary Figure 1). A total of 3,698 patients were included in the meta-analysis with a follow-up duration ranging from 6 to 60 months. Two landmark studies compared CA to MT but did not specify the subgroups like rate and rhythm control amongst those treated with MT.7),8) For comparison, RCD and AVNA were taken as ‘rate control’ strategy whereas AAD and CA were taken as ‘rhythm control’ strategy.
Figure 1. PRISMA flow diagram of study-selection.
Baseline parameters
Overall, the study populations were old; however, the eldest population groups were studied by Brignole et al for the pace/ ablate strategy.24),38) In most of the studies, left atria were severely dilated except two.29),37) All studies selected patients with severe LVSD, however, patients in pace/ablate studies by Brignole et al.24),38) has moderate LVSD. One study compared RCD to AAD among the patients who already underwent CRT and AVNA.35) In the study by Marrouche et al.8) and Di Biase et al.,36) all patients had CRT. We included the studies which assessed QoL by the MLHFQ excluded the study by Brignole et al.38) from the comparison since they used 6-item Specific Symptom Scale (SSS) score as a measure of QoL (Table 1).
Table 1. Baseline parameters of the patients included in the selected randomized controlled trials for the meta-analysis.
| ID | Study (year) | Comparator arms | Follow up (months) | Age (years) | LAD (mm) | ICD/CRT | LVEF (%) | Previous cardioversion | Ischemic HF | On Digoxin |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Brignole et al. (1998)24) | Rate (n=34) | 12 | 72±9 | 49±6 | NA | 44±15 | NA | 18 (52.9) | 19 (55.9) |
| AVNA (n=32) | 72±9 | 52±10 | 43±12 | 14 (43.8) | 18 (56.3) | |||||
| 2 | Okçün et al. (2004)25) | Rate (n=84) | 36 | 58±12 | 47±17 | NA | 33±15 | NA | 0 (0) | 65 (77.4) |
| AAD (n=70) | 61±10 | 45±15 | 31±8 | 0 (0) | 3 (4.3) | |||||
| 3 | Khan et al. (2008)26) | CA (n=41) | 6 | 60±8 | 49±5 | NA | 27±8 | NA | 30 (73) | NA |
| [PABA-CHF] | AVNA (n=40) | 61±8 | 47±6 | 29±7 | 27 (68) | |||||
| 4 | Roy et al. (2008)27) | Rate (n=694) | 37 | 67±11 | 49±7 | NA | 27±6 | 7 (1) | 48 (6.9) | 450 (64.8) |
| AAD (n=682) | 66±11 | 49±7 | 27±6 | 7 (1) | 48 (7) | 437 (64.1) | ||||
| 5 | Shelton et al. (2009)28) | Rate (n=31) | 12 | 72.7±8.3 | 46±7 | NA | NA | 28 (90.3) | 17 (55) | 20 (64.5) |
| [CAFÉ-II] | AAD (n=30) | 72±5.4 | 48±6 | 16 (53.3) | 13 (44) | 17 (56.7) | ||||
| 6 | MacDonald et al. (2010)29) | Rate (n=19) | 6 | 64.4±8.3 | 32±6† | NA | 42.9±9.6 | 3 (15.8) | 10 (52.6) | 9 (47.4) |
| CA (n=22) | 62.3±6.7 | 33±8† | 36.1±11.9 | 6 (27.3) | 11 (50) | 12 (54.6) | ||||
| 7 | Talajic et al. (2010)30) | Rate (n=694) | 60 | NA | NA | NA | NA | NA | NA | NA |
| [AF-CHF] | AAD (n=682) | |||||||||
| 8 | Frasure-Smith et al. (2012)31) | Rate (n=463) | 39 | NA | NA | NA | NA | NA | NA | NA |
| AAD (n=470) | ||||||||||
| 9 | Jones et al. (2013)32) | Rate (n=26) | 12 | 62±9 | 46±7 | 4 (15.4) | 25±7‡ | 15 (57.7) | 7 (26.9) | 12 (46.2) |
| CA (n=26) | 64±10 | 50±6 | 10 (38.5) | 22±8‡ | 14 (53.8) | 10 (38.5) | 16 (61.5) | |||
| 10 | Suman-Horduna et al. (2013)33) | Rate (n=378) | 12 | 66±11 | 49.2±7 | 29 (7.7) | 26.4±6 | NA | 166 (43.9) | 238 (63) |
| [AF-CHF] | AAD (n=371) | 65±12 | 48.2±7.3 | 28 (7.6) | 26.5±6.5 | 166 (44.7) | 236 (63.6) | |||
| 11 | Hunter et al. (2014)34) | Rate (n=24) | 6 | 60±10 | 50±10 | NA | 33.7±12.1 | NA | 7 (29.2) | NA |
| [CAMTAF] | CA (n=26) | 55±12 | 52±11 | 31.8±7.7 | 6 (23.1) | |||||
| 12 | Schwartzman et al. (2015)35) | Rate (n=26) | 12 | 69±9 | 51±5 | 0 (0) | 28±9 | NA | 18 (69.2) | NA |
| AAD (n=26) | 71±7 | 53±7 | 0 (0) | 28±6 | 19 (73.1) | |||||
| 13 | Di Biase et al. (2016)36) | AAD (n=101) | 24 | 60±11 | 48±4.9 | 101 (100) | 30±8 | 52 (51.5) | 66 (65.3) | NA |
| [AATAC] | CA (n=102) | 62±10 | 47±4.2 | 102 (100) | 29±5 | 3 (2.9) | 63 (61.8) | |||
| 14 | Jones et al. (2016)37) | Rate (n=25) | 12 | 62±9 | 27±7† | 4 (16) | 25±7‡ | NA | 6 (24) | 12 (48) |
| CA (n=24) | 64±10 | 30±6† | 4 (16.7) | 22±8‡ | 10 (41.7) | 14 (58.3) | ||||
| 15 | Prabhu et al. (2017)7) | Rate (n=33) | 6 | 62±9.4 | 47±8.2 | NA | 35±9.3 | 31 (94) | 0 (0) | NA |
| [CAMERA-HF] | CA (n=33) | 59±11 | 48±5.5 | 35±9.8 | 32 (97) | 0 (0) | ||||
| 16 | Marrouche et al. (2018)8) | AAD (n=184) | 60 | 64* | 49* | 184 (100) | 31.5* | NA | 96 (52.2) | NA |
| [CASTLE-AF] | CA (n=179) | 64* | 48* | 179 (100) | 32.5* | 72 (40.2) | ||||
| 17 | Brignole et al. (2018)38) | Rate (n=52) | 12 | 72±9 | NA | 20 (38.5) | 40±12 | 21 (40.4) | 19 (36.5) | 13 (25) |
| [APAF-CRT] | AVNA (n=50) | 71±12 | 22 (44) | 41±12 | 18 (36) | 13 (26) | 30 (60) | |||
| 18 | Kuck et al. (2019)39) | CA (n=68) | 12 | 65±8 | 51±5 | 35 (51.5) | 24.8±8.8 | 54 (79.4) | 40 (58.8) | 21 (30.9) |
| [AMICA] | BMT (n=72) | 65±8 | 50±6 | 35 (48.6) | 27.8±9.5 | 45 (62.5) | 30 (41.7) | 20 (27.8) |
Values are presented as mean±standard deviation or number (%).
AAD = anti-arrhythmic drugs; AF = atrial fibrillation; AVNA = atrio-ventricular node ablation; BMT = best medical therapy; CA = catheter ablation; CRT-D = cardiac resynchronization therapy with defibrillator; HF = heart failure; ICD = implantable cardiac defibrillator; LAD = left atrial diameter; LVEF = left ventricular ejection fraction; NA = not available; RCD – Rate controlling drugs.
*Median; †Left atrial area; ‡LVEF by radio-nucleotide study.
Meta-analysis
Medical therapy vs. catheter ablation
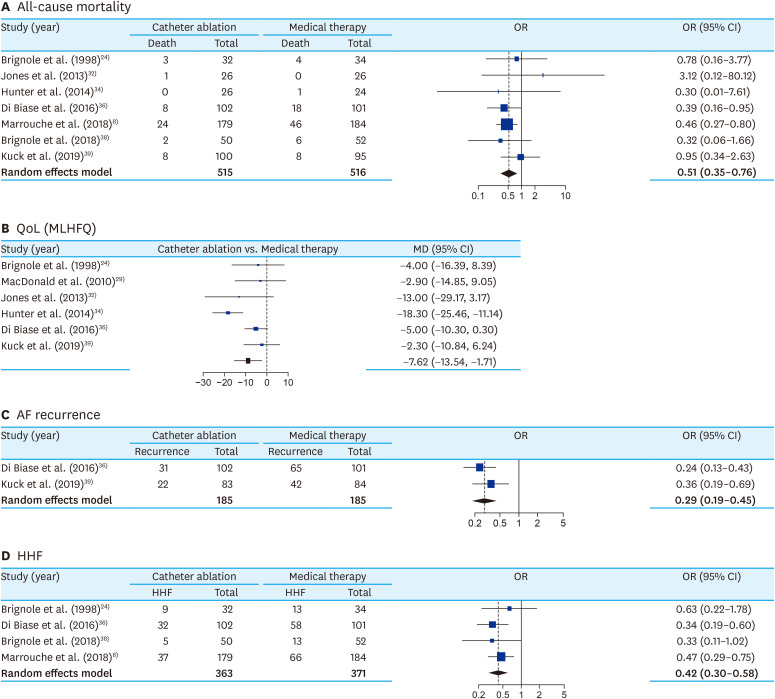
The meta-analysis directly comparing MT and CA, totaling 291 patients in MT and 423 patients in ablation therapy, showed significant mortality benefit (OR, 0.51; 95% confidence interval [CI], 0.35 to 0.76; I2, 0%) with the CA (Figure 2A). Subgroup analysis between AF ablation and AVNA showed no significant difference (p=0.98) (Supplementary Figure 2A). There was no asymmetry in the funnel plot (Supplementary Figure 2B). Meta-regression analysis with follow-up duration did not show any significant correlation with mortality (p=0.48). CA was also associated with significantly higher improvement in QoL than MT (MD, 12.78; 95% CI, 4.31 to 21.26; I2, 34%) (Figure 2B). AF-recurrence was also significantly lower with CA than MT (OR, 0.27; 95% CI, 0.19 to 0.45; I2, 0%) (Figure 2C) and there was no asymmetry in the funnel plot (Supplementary Figure 3). Similarly, HHF was also significantly lower with CA than MT (OR, 0.42; 95% CI, 0.30 to 0.58; I2, 0%) (Figure 2D). Subgroup analysis between AF ablation and AVNA showed no significant difference (p=0.75). There was no asymmetry in the funnel plot (Supplementary Figure 4B).
Figure 2. Forest plot comparing all-cause mortality (A), improvement in QoL (MLHFQ score) (B), AF recurrence (C) and HHF (D) between ablation therapy* and medical therapy for PeAF patients with HF.
AF = atrial fibrillation; CI = confidence interval; HF = heart failure; HHF= hospitalization due to heart failure; MD = mean difference; MLHFQ = Minnesota Heart Failure Questionnaire; PeAF = persistent atrial fibrillation; OR = odds ratio; QoL = quality of life.
*Includes both catheter ablation of AF and atrio-ventricular node ablation (vide online Supplementary Figure 5 for subgroup analysis).
Rate control vs. rhythm control
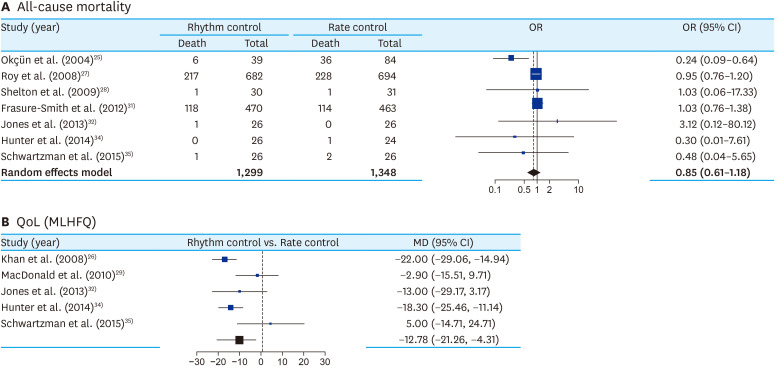
The meta-analysis directly comparing all-cause mortality between rate control (RCD group) and rhythm control (AAD and CA) found no significant difference (OR, 1.21; 95% CI, 0.83 to 1.74; I2, 34%) (Figure 3A). Subgroup analysis between AAD and AF ablation as rhythm control strategy, showed no significant difference (p=0.75) (Supplementary Figure 5A). There was no asymmetry in the funnel plot (Supplementary Figure 5B). Meta-regression analysis with follow-up duration did not show any significant correlation with mortality (p=0.68). However, rhythm control strategy was significantly associated with better QoL in comparison to rate control strategy (MD, 12.78; 95% CI, 4.31 to 21.26) (Figure 3B).
Figure 3. Forest plot comparing all-cause mortality (A) and improvement in QoL (MLHFQ score) (B) between rhythm control* and rate control strategy† for PeAF with HF.
AAD = anti-arrhythmic drug; AF = atrial fibrillation; CA = catheter ablation; CI = confidence interval; HF = heart failure; MD = mean difference; MLHFQ = Minnesota Heart Failure Questionnaire; OR = odds ratio; PeAF = persistent atrial fibrillation; QoL = quality of life; RCD = rate-controlling drug.
*Includes AAD and CA of AF; †Includes RCD and atrio-ventricular node ablation.
Comparison among individual treatment arms: network meta-analysis
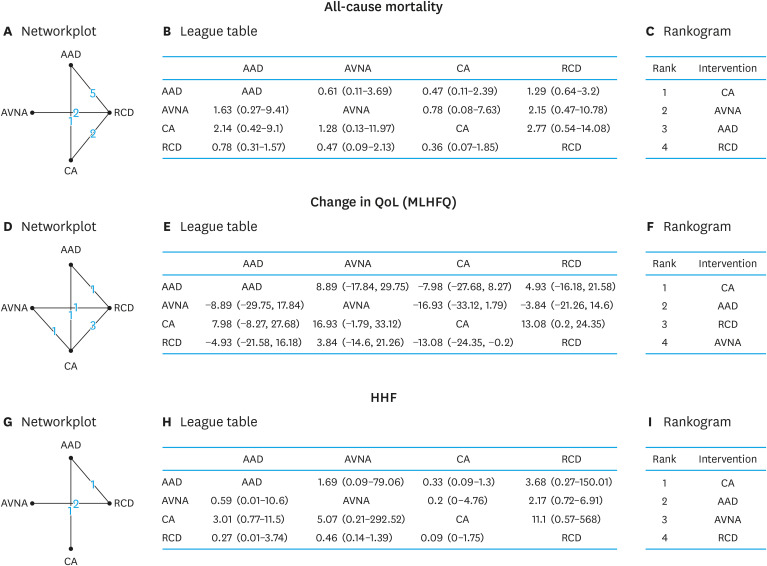
As rhythm control could be catheter ablation or AADs, likewise, rate control could be either RCDs or AVNA; we did NMA of the four different arms, based on available data. Studies that did not have specific data for individual strategy were excluded (Figure 4).7),8),30),33),37),39),40)
Figure 4. Network meta-analysis of all-cause mortality expressed in OR (95% CrI) (A-C), improvement of QoL (MLHFQ score), expressed in MD (95% CrI) (D-F), and HHF expressed in OR (95% CrI) among different treatment arms for PeAF with HF (G-I). Network-plot (A, D, G) each node on the plot represents an individual intervention (RCDs, AADs, CA, and AVNA), connecting lines between nodes indicating number of trials making each comparison; League table (B, E, H)) showing Bayesian comparison of all treatment pairs: the table displays the results for all treatment pairs in both the upper triangle and lower triangle, but with the comparison switched over. For both above and below the leading diagonal, the results are for the treatment at the top of the same column vs. treatment at the left-hand side of the same row; and Rankogram (C, F, I) showing median rank chart of intervention arms with all studies included in the network meta-analysis model. For treatment rankings, smaller outcome values were set as desirable.
AAD = anti-arrhythmic drug; AVNA = atrio-ventricular node ablation; CA = catheter ablation; CrI = credibility interval; HHF = hospitalization due to heart failure; MD = mean difference; MLHFQ = Minnesota Heart Failure Questionnaire; OR = odds ratio; QoL = quality of life; RCD = rate controlling drug.
All-cause mortality
In 10 randomized controlled studies with mortality data24),27),28),31),32),33),34),35),36),38) totaling 3,018 patients (Figure 4A), all-cause mortality was found to be lower with CA in the comparison to RCDs (OR, 0.36; 95% CrI, 0.07 to 1.85), AADs (OR, 0.48; 95% CrI, 0.11 to 2.31) and AVNA (OR, 0.76; 95% CrI, 0.09 to 7.37) (Figure 4B). The node split model did not show any significant incontinency in the comparisons of direct and indirect evidence between different treatment arms (Supplementary Figure 6A). Bayesian random effect modeling using MCMC simulations revealed the between-study SD (95% CrI) in the log-odds scale to be 0.62 (0.05 to 1.28). The highest probability of rankings (lower the better) in regards to all-cause mortality was with CA (56%), AVNA (35%) AAD (51%), and RCD (64%) as rank 1, rank 2, rank 3, and rank 4 respectively (Supplementary Figure 6B). When orders of median ranking probabilities were plotted (rankogram), CA and RCD were found to have the lowest and highest median probability of all-cause mortality (Figure 4C).
HF-related QoL (MLHFQ)
In 7 studies24),26),29),32),34),35),36) with mortality data totaling 485 patients, NMA showed that QoL was better with CA in the comparison to RCDs (MD, −13.0; 95% CI, −24.4 to −0.2), AADs (MD, −7.98; 95% CI, −27.7 to 8.27) and AVNA (MD, −16.9; 95% CI, −33.1 to 1.8) (Figure 4E). The node split model did not show any significant inconsistency in the comparisons of direct and indirect evidence between different treatment arms (Supplementary Figure 7A). Bayesian random effect modeling using MCMC simulations revealed between-study SD (95% CrI) in the log-odds scale was 8.78 (0.78 to 19.97). The highest probability of rankings (lower the better) in regards to change in QOL was with CA (84%), AAD (55%), RCD (57%), and AVNA (63%), as of rank 1, rank 2, rank 3, and rank 4, respectively (Supplementary Figure 7B). When orders of median ranking probabilities were plotted (rankogram), CA and AVNA were found to have the highest and lowest median probability of change in QoL (Figure 4C).
HHF
In 4 randomized controlled studies24),35),36),38) with data totaling 406 patients, NMA showed HHF was lower with CA in comparison to RCDs (OR, 0.09; 95% CrI, 0.00 to 1.75), AADs (OR, 0.33; 95% CrI, 0.09 to 1.3) and AVNA (OR, 0.2; 95% CrI, 0.00 to 4.76) (Figure 4H). The difference between direct and indirect comparisons are depicted in Supplementary Figure 8A. Bayesian random effect modeling using MCMC simulations revealed between-study SD (95% CrI) in the log-odds scale was 8.78 (0.78 to 19.97). The highest probability of rankings (lower the better) in regards to HHF was with CA (84%), AAD (55%), AVNA (57%), and RCD (63%) as rank 1, rank 2, rank 3, and rank 4, respectively (Supplementary Figure 8B). When orders of median ranking probabilities were plotted (rankogram), CA outscored all other treatment arms and RCD ranked last, in regards to improvement in HHF (Figure 4C).
Model fit and deviation analyses
MCMC simulations for all the comparisons had DIC values more than 5 suggestive of Bayesian NMA to be more useful than frequentist NMA. Also, Gelman Rubin convergence plot for all the analyses converged around 1, suggesting good model fit. Residual deviances from the NMA model for all the studies were plotted in contour plots which showed leverage values of less than 3 (Supplementary Figures 6C, 7C, and 8C) and thus suggests that the amplitude of deviances from the NMA model were not significant.
DISCUSSION
Following CASTLE-AF study,8) CA has been advocated as class I recommendation as per ESC 2020 guideline in patients with AF with HF to reduce mortality and HHF when other rhythm control strategies fail.14) Important findings from our analysis are following:
(1) Ablation strategies were found to be associated with significantly lower mortality than medical therapies and CA of AF ranked better than pace and ablates strategy in the Bayesian NMA.
(2) Rhythm control strategies were found to be providing significantly higher improvement of HF-related QoL than rate control strategies and Bayesian NMA showed that CA of AF was better than AAD, however interestingly found pace/ ablate strategy to be lowest in the rank.
(3) Ablation strategies were associated with significantly lower HHF than medical therapies and Bayesian NMA found that CA of AF is not only better than AAD, but also it ranked higher than pace/ablate strategy.
(4) Of note, RCD ranked lowest in regards to mortality benefit as well as benefit from HHF.
In our analysis, CA showed a significant benefit in terms of mortality as compared to medical management in both the qualitative and quantitative analysis with no heterogeneity. This is in line with the earlier meta-analyses published, where there was a significant reduction in all-cause mortality, which was driven by patients with AF and HF with reduced ejection fraction.12),14) A large population-based study has evaluated the generalizability of the trial and found similar results as demonstrated in CASTLE-AF in the subjects who met the trial inclusion criteria.9) A recent subgroup analysis of the CABANA trial also supported the benefits of CA in this subset of patients.11) Pace/ablate strategy ranked just after CA of AF and possibly having ICD/CRT aided in reducing all-cause mortality, despite this particular strategy is usually utilized for sickest group of HF patients with poor response to CRT due to AF with first ventricular rate. AADs ranked third probably because of arrhythmic risks associated with them. Interestingly RCDs ranked last, despite their proven role in HF and their wide utilization in AF.
We have also shown that there was a significant improvement in QoL in the rhythm control strategies, both CA as well as AADs, as measured by the MLHFQ scale, on both the qualitative and quantitative analyses in the tone of HF sub-study of CABANA trial.11) AVNA performed worse than RCD, possible because pace/ ablate strategies were undertaken in the sickest group of patients.
AF itself causes increase HHF. Our findings are in line with earlier studies in which rhythm control strategies like CA and AADs caused lower HF hospitalizations.7),8),11),12),13) The reason may lie in the fact that cardiac output is improved in sinus rhythm due to preservation of atrial kick. Also, converting to sinus rhythm probably improves the underlying electro-anatomical substrate which improves outcomes.40) Pace and ablate strategy did better than RCDs in our NMA probably due to HF devices in patients undergoing AVNA and challenges the role of RCDs in HF patients with AF.
Catheter ablation was found to be significantly better than medical therapy in regards to AF-free survival in HF sub-study of CABANA trial11) similar to result in our meta-analysis. Studies with rate control strategies24),26),27),28),29),33),34),35),38) considered poor control of ventricular rates only but they were not considered for analysis in our study. NMA of the AF recurrence could not be performed due to poor set of available data.
This study has several limitations. Firstly, only a small number of trials could be included in the meta-analysis and further less in network meta-analysis. Secondly, covariate analysis could not be done due to the lack of individual patient data. Third, the studies were heterogeneous in terms of patient selection criteria (LVEF of the patients varied in all the trials), presence of devices (like ICD/CRT), and type of cardiomyopathy (ischemic or non-ischemic). Fourth, our analyses are limited to HF with reduced ejection fraction and should not be generalized to the HF with preserved ejection fraction group. Finally, included trials were conducted in high volume centres with experienced operators thus the applicability to wider real-world clinical settings remains uncertain.
PeAF with systolic HF is arguably the sickest subset of AF patients and CA is largely under-utilized despite upgradation of recommendation in recent guidelines.10),14),40) Although CASTLE-AF8) found CA of AF useful for reducing mortality and HHF, guideline recommends CA when medical therapy fails. Many of these patients receive ICD/CRT and go for AVNA subsequently. In our NMA on PeAF patients with systolic HF, CA of AF were found to be better than medical therapies (rate or rhythm control) and pace/ ablate strategy in regards to all-cause mortality, QoL, and HHF. Individualized treatment strategies are to be adopted for PeAF patients with systolic HF. Although randomized studies and cost-affectivity analysis are necessary to clarify, the evidence from our NMA poses the right question whether CA of AF should be utilized even before trial of AAD in AF patients with systolic HF.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Khanra D, Khan H, Kella D, Padmanabhan D.
- Data curation: Deshpande S, Mukherjee A, Mohan S.
- Formal analysis: Khanra D, Khan H.
- Investigation: Khanra D, Deshpande S, Mukherjee A, Khan H, Kella D.
- Methodology: Khanra D, Deshpande S, Khan H, Kella D, Padmanabhan D.
- Project administration: Deshpande S, Mohan S, Khan H, Kella D.
- Resources: Deshpande S, Mukherjee A, Mohan S, Khan H, Kella D.
- Software: Khanra D, Deshpande S, Khan H, Kella D, Padmanabhan D.
- Supervision: Khan H, Kella D, Kathuria S, Padmanabhan D.
- Validation: Khanra D, Mukherjee A, Mohan S, Khan H, Kella D, Padmanabhan D.
- Visualization: Khanra D, Khan H, Kella D, Padmanabhan D, Kathuria S.
- Writing - original draft: Mukherjee A.
- Writing - review & editing: Khanra D, Deshpande S, Mukherjee A, Mohan S, Kathuria S.
SUPPLEMENTARY MATERIALS
Critical appraisal of the selected studies in RoB 2.0 scale.
(A) Forrest plot comparing catheter therapy to medical therapy in regards to all-cause mortality with subgroup analysis with subgroup analysis between AVNA and CA of AF (B) Funnel plot for publication bias.
Funnel plot for publication bias in comparing catheter therapy to medical therapy in regards to AF recurrence.
(A) Forrest plot comparing catheter ablation to medical therapy in regards to hospitalization due to heart failure with subgroup analysis between AVNA and CA of AF (B) Funnel plot for publication bias.
(A) Forrest plot comparing rhythm control to rate control in regards to all-cause mortality with subgroup analysis between AAD and CA of AF (B) Funnel plot for publication bias.
All-cause mortality (A) Node-split model for assessment of inconsistency between direct and indirect comparison in all studies: for each treatment comparison that has both direct and indirect estimates, the analysis provides the mean and credible intervals (the value at 2.5% and 97.5% quantiles) for the direct, indirect and network estimates together with a Bayesian p-value for the related test of inconsistency between the direct and indirect evidence for each treatment comparison. (B) Ranking probability table for all studies showing probability for each treatment at each rank expressed in percentage. (C) The contour plot looking at residual deviance and leverage (which is a statistical measure of influence of a data point on model estimation) simultaneously.
Quality of life change (A) Node-split model for assessment of inconsistency between direct and indirect comparison in all studies: for each treatment comparison that has both direct and indirect estimates, the analysis provides the mean and credible intervals (the value at 2.5% and 97.5% quantiles) for the direct, indirect and network estimates together with a Bayesian p-value for the related test of inconsistency between the direct and indirect evidence for each treatment comparison.(B) Ranking probability table for all studies showing probability for each treatment at each rank expressed in percentage. (C) The contour plot looking at residual deviance and leverage (which is a statistical measure of influence of a data point on model estimation) simultaneously.
Hospitalization due to heart failure (A)Node-split model for assessment of inconsistency between direct and indirect comparison in all studies: for each treatment comparison that has both direct and indirect estimates, the analysis provides the mean and credible intervals (the value at 2.5% and 97.5% quantiles) for the direct, indirect and network estimates together with a Bayesian p-value for the related test of inconsistency between the direct and indirect evidence for each treatment comparison. (B) Ranking probability table for all studies showing probability for each treatment at each rank expressed in percentage. (C) Thecontour plot looking at residual deviance and leverage (which is astatistical measure of influence of a data point on model estimation)simultaneously.
References
- 1.Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 2.Fink T, Schlüter M, Heeger CH, et al. Stand-alone pulmonary vein isolation versus pulmonary vein isolation with additional substrate modification as index ablation procedures in patients with persistent and long-standing persistent atrial fibrillation: the randomized Alster-Lost-AF Trial (ablation at St. Georg Hospital for Long-Standing Persistent Atrial Fibrillation) Circ Arrhythm Electrophysiol. 2017;10:e005114. doi: 10.1161/CIRCEP.117.005114. [DOI] [PubMed] [Google Scholar]
- 3.Lo LW, Lin YJ, Chang SL, Hu YF, Chung FP, Chen SA. Beyond pulmonary vein isolation: the role of additional sites in catheter ablation of atrial fibrillation. Curr Cardiol Rep. 2017;19:86. doi: 10.1007/s11886-017-0884-4. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg JS, Shah Y, Bhatt A, et al. Focal impulse and rotor modulation: acute procedural observations and extended clinical follow-up. Heart Rhythm. 2017;14:192–197. doi: 10.1016/j.hrthm.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Hong KL, Borges J, Glover B. Catheter ablation for the management of atrial fibrillation: current technical perspectives. Open Heart. 2020;7:e001207. doi: 10.1136/openhrt-2019-001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wats K, Kiser A, Makati K, et al. The convergent atrial fibrillation ablation procedure: evolution of a multidisciplinary approach to atrial fibrillation management. Arrhythm Electrophysiol Rev. 2020;9:88–96. doi: 10.15420/aer.2019.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prabhu S, Taylor AJ, Costello BT, et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI study. J Am Coll Cardiol. 2017;70:1949–1961. doi: 10.1016/j.jacc.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 8.Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 9.Noseworthy PA, Van Houten HK, Gersh BJ, et al. Generalizability of the CASTLE-AF trial: catheter ablation for patients with atrial fibrillation and heart failure in routine practice. Heart Rhythm. 2020;17:1057–1065. doi: 10.1016/j.hrthm.2020.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–51. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 11.Packer DL, Piccini JP, Monahan KH, et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation. 2021;143:1377–1390. doi: 10.1161/CIRCULATIONAHA.120.050991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew DS, Black-Maier E, Loring Z, et al. Diagnosis-to-ablation time and recurrence of atrial fibrillation following catheter ablation: a systematic review and meta-analysis of observational studies. Circ Arrhythm Electrophysiol. 2020;13:e008128. doi: 10.1161/CIRCEP.119.008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kheiri B, Osman M, Abdalla A, et al. Catheter ablation of atrial fibrillation with heart failure: An updated meta-analysis of randomized trials. Int J Cardiol. 2018;269:170–173. doi: 10.1016/j.ijcard.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 17.RStudio Team. RStudio: integrated development for R [Internet] Boston, MA: RStudio, PBC; 2020. [cited 2021 Mar 22]. Available from: http://www.rstudio.com/ [Google Scholar]
- 18.Owen RK, Bradbury N, Xin Y, Cooper N, Sutton A. MetaInsight: An interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Res Synth Methods. 2019;10:569–581. doi: 10.1002/jrsm.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Ravenzwaaij D, Cassey P, Brown SD. A simple introduction to Markov Chain Monte-Carlo sampling. Psychon Bull Rev. 2018;25:143–154. doi: 10.3758/s13423-016-1015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434–455. [Google Scholar]
- 21.Hu D, O'Connor AM, Wang C, Sargeant JM, Winder CB. How to conduct a Bayesian network meta-analysis. Front Vet Sci. 2020;7:271. doi: 10.3389/fvets.2020.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. 2013;33:641–656. doi: 10.1177/0272989X12455847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheeler DC, Hickson DA, Waller LA. Assessing local model adequacy in Bayesian hierarchical models using the partitioned deviance information criterion. Comput Stat Data Anal. 2010;54:1657–1671. doi: 10.1016/j.csda.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brignole M, Menozzi C, Gianfranchi L, et al. Assessment of atrioventricular junction ablation and VVIR pacemaker versus pharmacological treatment in patients with heart failure and chronic atrial fibrillation: a randomized, controlled study. Circulation. 1998;98:953–960. doi: 10.1161/01.cir.98.10.953. [DOI] [PubMed] [Google Scholar]
- 25.Okçün B, Yigit Z, Arat A, Küçükoglu SM. Comparison of rate and rhythm control in patients with atrial fibrillation and nonischemic heart failure. Jpn Heart J. 2004;45:591–601. doi: 10.1536/jhj.45.591. [DOI] [PubMed] [Google Scholar]
- 26.Khan MN, Jaïs P, Cummings J, et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359:1778–1785. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 27.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 28.Shelton RJ, Clark AL, Goode K, et al. A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure: (CAFE-II Study) Heart. 2009;95:924–930. doi: 10.1136/hrt.2008.158931. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald MR, Connelly DT, Hawkins NM, et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart. 2011;97:740–747. doi: 10.1136/hrt.2010.207340. [DOI] [PubMed] [Google Scholar]
- 30.Talajic M, Khairy P, Levesque S, et al. Maintenance of sinus rhythm and survival in patients with heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;55:1796–1802. doi: 10.1016/j.jacc.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Frasure-Smith N, Lespérance F, Talajic M, et al. Anxiety sensitivity moderates prognostic importance of rhythm-control versus rate-control strategies in patients with atrial fibrillation and congestive heart failure: insights from the Atrial Fibrillation and Congestive Heart Failure Trial. Circ Heart Fail. 2012;5:322–330. doi: 10.1161/CIRCHEARTFAILURE.111.964122. [DOI] [PubMed] [Google Scholar]
- 32.Jones DG, Haldar SK, Hussain W, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61:1894–1903. doi: 10.1016/j.jacc.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 33.Suman-Horduna I, Roy D, Frasure-Smith N, et al. Quality of life and functional capacity in patients with atrial fibrillation and congestive heart failure. J Am Coll Cardiol. 2013;61:455–460. doi: 10.1016/j.jacc.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 34.Hunter RJ, Berriman TJ, Diab I, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial) Circ Arrhythm Electrophysiol. 2014;7:31–38. doi: 10.1161/CIRCEP.113.000806. [DOI] [PubMed] [Google Scholar]
- 35.Schwartzman D, Housel D, Bazaz R, et al. A pilot study to assess benefit of atrial rhythm control after cardiac resynchronization therapy and atrioventricular node ablation. Pacing Clin Electrophysiol. 2015;38:275–281. doi: 10.1111/pace.12535. [DOI] [PubMed] [Google Scholar]
- 36.Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637–1644. doi: 10.1161/CIRCULATIONAHA.115.019406. [DOI] [PubMed] [Google Scholar]
- 37.Jones DG, Haldar SK, Donovan J, et al. Biomarkers in persistent AF and heart failure: impact of catheter ablation compared with rate control. Pacing Clin Electrophysiol. 2016;39:926–934. doi: 10.1111/pace.12919. [DOI] [PubMed] [Google Scholar]
- 38.Brignole M, Pokushalov E, Pentimalli F, et al. A randomized controlled trial of atrioventricular junction ablation and cardiac resynchronization therapy in patients with permanent atrial fibrillation and narrow QRS. Eur Heart J. 2018;39:3999–4008. doi: 10.1093/eurheartj/ehy555. [DOI] [PubMed] [Google Scholar]
- 39.Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA trial. Circ Arrhythm Electrophysiol. 2019;12:e007731. doi: 10.1161/CIRCEP.119.007731. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed A, Ullah W, Hussain I, et al. Atrial fibrillation: a leading cause of heart failure-related hospitalizations; a dual epidemic. Am J Cardiovasc Dis. 2019;9:109–115. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Critical appraisal of the selected studies in RoB 2.0 scale.
(A) Forrest plot comparing catheter therapy to medical therapy in regards to all-cause mortality with subgroup analysis with subgroup analysis between AVNA and CA of AF (B) Funnel plot for publication bias.
Funnel plot for publication bias in comparing catheter therapy to medical therapy in regards to AF recurrence.
(A) Forrest plot comparing catheter ablation to medical therapy in regards to hospitalization due to heart failure with subgroup analysis between AVNA and CA of AF (B) Funnel plot for publication bias.
(A) Forrest plot comparing rhythm control to rate control in regards to all-cause mortality with subgroup analysis between AAD and CA of AF (B) Funnel plot for publication bias.
All-cause mortality (A) Node-split model for assessment of inconsistency between direct and indirect comparison in all studies: for each treatment comparison that has both direct and indirect estimates, the analysis provides the mean and credible intervals (the value at 2.5% and 97.5% quantiles) for the direct, indirect and network estimates together with a Bayesian p-value for the related test of inconsistency between the direct and indirect evidence for each treatment comparison. (B) Ranking probability table for all studies showing probability for each treatment at each rank expressed in percentage. (C) The contour plot looking at residual deviance and leverage (which is a statistical measure of influence of a data point on model estimation) simultaneously.
Quality of life change (A) Node-split model for assessment of inconsistency between direct and indirect comparison in all studies: for each treatment comparison that has both direct and indirect estimates, the analysis provides the mean and credible intervals (the value at 2.5% and 97.5% quantiles) for the direct, indirect and network estimates together with a Bayesian p-value for the related test of inconsistency between the direct and indirect evidence for each treatment comparison.(B) Ranking probability table for all studies showing probability for each treatment at each rank expressed in percentage. (C) The contour plot looking at residual deviance and leverage (which is a statistical measure of influence of a data point on model estimation) simultaneously.
Hospitalization due to heart failure (A)Node-split model for assessment of inconsistency between direct and indirect comparison in all studies: for each treatment comparison that has both direct and indirect estimates, the analysis provides the mean and credible intervals (the value at 2.5% and 97.5% quantiles) for the direct, indirect and network estimates together with a Bayesian p-value for the related test of inconsistency between the direct and indirect evidence for each treatment comparison. (B) Ranking probability table for all studies showing probability for each treatment at each rank expressed in percentage. (C) Thecontour plot looking at residual deviance and leverage (which is astatistical measure of influence of a data point on model estimation)simultaneously.