Abstract
Acute heart failure (AHF), a global pandemic with high morbidity and mortality, exerts a considerable economic burden. AHF includes a broad spectrum of clinical presentations ranging from new-onset heart failure to cardiogenic shock. Key elements of the management rely on the clinical diagnosis confirmed on, both, increased natriuretic peptides and echocardiography, and on the prompt initiation of oxygen therapy, including non-invasive positive pressure ventilation, vasodilators, and diuretics. A care pathway is essential, specifically when an acute coronary syndrome is suspected or in the case of cardiogenic shock. Association or increasing doses of vasopressors despite an adequate volume status are markers of progression toward a refractory cardiogenic shock state. For the latter, mechanical circulatory support should be initiated early, optimally before the onset of renal or liver failure. Thus, a tertiary care center is recommended for the management of patients with AHF who require percutaneous coronary intervention or mechanical circulatory support. This narrative review provides multidisciplinary guidance for the management of AHF and cardiogenic shock from pre-hospital to intensive care unit/cardiac care unit, based on contemporary evidence and expert opinion.
Keywords: Heart failure, Emergency, Therapeutics, Cardiogenic shock
INTRODUCTION
Heart failure (HF) is a chronic condition resulting most often from left ventricular (LV) dysfunction of either systolic or diastolic origin. The natural history of HF is intercepted by acute life-threatening decompensation episodes requiring urgent hospital admission in the emergency room and subsequent transfer to an intensive care unit (ICU) or a cardiac care unit (CCU). These repeated unscheduled hospitalizations significantly deteriorate the patient's quality of life and short and/or long-term survival. Thus, acute bedside management of HF is crucial, and despite considerable research, no intravenous (IV) therapy has been implemented for patients with acute heart failure (AHF) over the past 30 years.1)
Specifically, the management of AHF is a complex process of care that requires, simultaneously, early identification of the HF clinical syndrome, confirmation by diagnostic tests (biological, echocardiography, and X-ray exams), assessment of severity, and adequate urgent treatment. The variety of clinical presentations ranging from moderate congestion, consisting of symptoms of breathless alone to a severe shock state syndrome, makes AHF extremely challenging to manage in an emergency setting. Thus, both, the cooperation of a multidisciplinary team (primary care physician, emergency physician, cardiologist, intensivist, and paramedic) and strict management protocols are cornerstones for the success of AHF treatment.
This narrative review provides guidance for the diagnosis and early management of AHF or cardiogenic shock (CS) from identification in the pre-hospital setting to the early phase after hospital admission.
DEFINITION
AHF can be defined as the rapid onset of or worsening of symptoms of HF secondary to cardiac dysfunction leading to low cardiac output, an elevation in ventricular filling pressure, and peripheral organ hypoperfusion. AHF could have two initial presentations: new onset of HF (“de novo”) or acute decompensation of chronic HF. The etiology of de novo AHF is most often acute coronary syndrome (ACS), while numerous causes can lead to the onset of acute decompensation of chronic HF: ischemic, infection, uncontrolled hypertension, arrhythmias, cardiac conduction disorders, dietary mistake (excessive salt intake), and medication non-compliance.1),2) The clinical phenotypes of AHF are heterogeneous and represent a broad range of different disease states: classical pulmonary edema decompensating a chronic HF, ACS-related HF, CS, and some specific patterns such as hypertensive HF and right ventricular (RV) failure. Pathophysiologically, AHF may occur on a reduced (<40%), mid-range (40–49%), or preserved (≥50%) left ventricular ejection fraction (LVEF). Roughly, half of the AHF occurs with a preserved LVEF that will often be hospitalized with a high level of congestion and a need for diuretics.3)
In summary, a correct assessment of the initial clinical phenotype is important as it guides immediate therapies, while measurement of LVEF will be important to guide long-term therapies.
CLASSIFICATION BASED ON THE INITIAL CLINICAL EXAM
In addition to correctly defining AHF clinical phenotypes, the American College of Cardiology Foundation/American Heart Association also stipulated stages of HF progression based on structural changes and symptoms. These clinical scenarios in AHF are based on very simple clinical signs such as systolic blood pressure (BP) and other symptoms at admission4):
1) Systolic BP >140 mmHg, predominantly diffuse pulmonary edema, and minimal systemic edema;
2) Systolic BP 100–140 mmHg, predominantly systemic edema, and minimal pulmonary edema;
3) Systolic BP <100 mmHg, predominantly signs of hypoperfusion, and minimal systemic and pulmonary edema;
4) AHF with ACS; and
5) AHF with isolated RV failure
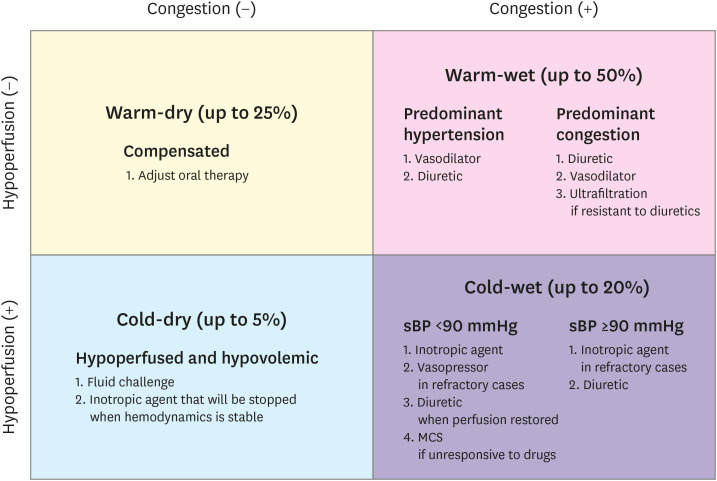
Clinical classification can also be based on a physical bedside evaluation of the symptoms of congestion (“wet” vs. “dry” if present vs. absent) and peripheral hypoperfusion (“cold” vs. “warm” if hypoperfused vs. normoperfused).5) The combination of these two familiar conditions allows the identification of four phenotypic groups, which will guide the therapy in the initial phase and will also have a prognostic value (Figure 1).5),6),7),8)
Figure 1. Clinical profiles of patients with acute HF based on the presence/absence of congestion and/or hypoperfusion. Adapted from the 2016 ESC guidelines on acute and chronic HF.2) This approach was proposed by the analysis of Nohria et al.5) Frequencies of the groups are given according to this analysis.
ESC = European Society of Cardiology; HF = heart failure; MCS = mechanical circulatory support; sBP = systolic blood pressure.
PRE-HOSPITAL AND EARLY MANAGEMENT OF ACUTE HEART FAILURE
The concept of early and aggressive management of AHF could be compared to the “golden hour” concept performed for patients with acute myocardial infarction (AMI).9) The early management of patients with AHF starts from the pre-hospital setting, including at home, and in the emergency department. Patients suspected of having AHF syndrome must continuously be monitored (pulse oximetry, BP, respiratory rate, and electrocardiogram) to detect very early signs of worsening.
Patients identified at an early stage with AHF should be transferred right away to the cardiology department or ICU/CCU according to the clinical assessment.10) On arrival at the hospital, immediate and concomitant diagnostic tests, physical assessment, and pharmacologic and nonpharmacologic treatments should be initiated or pursued in a dedicated HF care pathway. Concomitantly to hemodynamic and respiratory stabilization, diagnostic and treatment of the decompensating cause should be performed.
Oxygenation and non-invasive ventilation
The first step will be to restore oxygenation if transcutaneous oxygen saturation (SpO2) <90% and non-invasive positive pressure ventilation (NIPPV) should be initiated early if the patient presents respiratory distress symptoms.11) The targeted SpO2 is not 100%, as this causes vasoconstriction and a reduction in cardiac output. Girardis et al.12) reported that among patients admitted to ICUs, conservative oxygen therapy (SpO2 target: 94–98%) compared with conventional oxygen therapy (SpO2 target: 97–100%) resulted in lower ICU mortality. The benefits of NIPPV are an increase in SpO2 and a decrease in breathing work due to a reduction in both pre- and afterload.13) A similar reduction in respiratory distress symptoms was observed with continuous positive airway pressure and bi-level positive pressure ventilation modalities.14) However, in the case of RV failure, the increase in intrathoracic pressure induced by NIPPV also translates into an increase in right afterload that will be detrimental to right heart function. In this specific setting, NIPPV should be avoided.13) Intubation should be considered if NIPPV fails or if NIPPV is contraindicated because of poor patient cooperation, apnea, vomiting, and suspicion of pneumothorax.15) However, NIPPV may be useful in cases of coma induced by hypercapnia, and such cases should be resolved in a timely manner after NIPPV initiation.
Intravenous diuretics
Together with non-invasive ventilation, administration of IV therapies is a crucial step in AHF management. As mentioned above, almost all AHF patients, including those with preserved LVEF, especially if the inferior vena cava (IVC) diameter is dilated, should receive IV diuretics, as early as possible after confirmation of AHF diagnosis by measurement of natriuretic peptides.
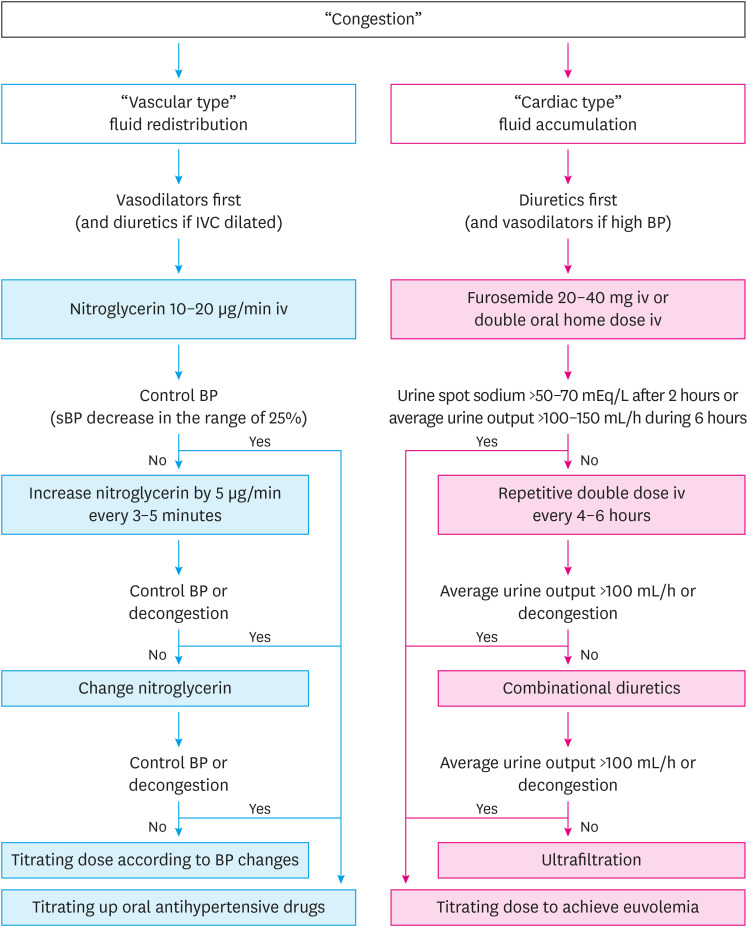
Loop diuretics inhibit the Na-K-2Cl symporter at the ascending loop of Henle, and have the most potent diuretic effect, promoting excretion of sodium and chloride.16) For congestion management, diuretics, such as furosemide, will enhance respiratory improvement and decrease the ventricular filling pressure. In addition, loop diuretics act as immediate venodilators and usually rapidly improve respiratory symptoms.15) In patients with AHF, the dose-response curve is decreased. Thus, the recommended initial dose should be at least 20–40 mg of furosemide (or equivalent, bumetanide) in new-onset patients, and at least the equivalent IV dose for patients chronically receiving diuretic therapy.17) The diuretic response may be evaluated using urinary volume output and post-diuretic spot urinary sodium content (the bladder should be empty before the administration of diuretics).18) If the spot urinary sodium content is <50–70 mEq/L after two hours, or if the hourly urine output is <100–150 mL during the first six hours, the bolus dose should be doubled. Nevertheless, if diuresis has not improved, repetitive bolus should be administered every 6 hours (Figure 2).18) Higher doses are associated with a transient worsening in renal function,4),19),20) although an increase in creatinine caused by decongestion has not been reported to lead to a worse outcome.21)
Figure 2. Flowchart for vasodilator and diuretic use in acute heart failure. Of note, the “vascular type” usually has high sBP and preserved LVEF while the “cardiac type” presents normal sBP and reduced LVEF.
IV = intravenous; IVC = inferior vena cava; LVEF = left ventricular ejection fraction; sBP = systolic blood pressure.
Loop diuretics may be beneficial in patients with volume overload; however, it has several limitations, such as diuretic resistance, neurohormonal activation, electrolyte disturbances, and worsening renal function. To solve these problems, combination therapies of loop diuretics with thiazide, mineralocorticoid receptor antagonist, and vasopressin antagonism may improve the diuretic effect.22),23),24) Tolvaptan, a selective vasopressin V2 receptor antagonist with electrolyte-free water diuretic properties, acts on the collecting tubules of the kidneys and is used for the treatment of volume overload in patients with HF in several Asian countries. Several clinical trials have demonstrated marked improvement in congestion and the prevention of renal function in AHF patients following tolvaptan treatment.25),26),27),28) In addition, recent trials have reported that sodium-glucose linked transporter type 2 (SGLT2) inhibitors could potentially reduce the risk of cardiovascular-related mortality in patients with diabetes.29),30) SGLT2 inhibitors, which inhibit proximal sodium absorption, have the potential to exert a diuretic effect, although more evidence is needed in AHF patients. Ultrafiltration may be considered in patients in whom decongestion goals are not met with a maximal dose of loop diuretics or a combination of diuretic therapy. However, ultrafiltration did not show a benefit compared to pharmacologic therapy in a randomized clinical trial of AHF patients with worsened renal function.31) The routine use of ultrafiltration is not recommended and should be confined to highly selected patients.
Vasodilators
IV vasodilators should be systematically combined with diuretics to manage AHF patients when systolic BP is high. IV vasodilators are also indicated in AHF patients with normal systolic BP. Vasodilators have dual benefits by decreasing venous tone and arterial tone, reducing both preload and afterload.32) Nitroglycerin has been used as a vasodilator in AHF for many years.33) The hemodynamic effects of nitroglycerin are dose-dependent: venous dilatation occurs at low doses and arterial vasodilation occurs at higher doses. In a retrospective study,34) high doses of nitroglycerin decreased the rates of endotracheal intubation and ICU admission. Thus, patients should receive increased doses every 3–5 minutes to decrease BP in the range of 25% in the first few hours (Figure 2).2),20),35) In patients with heart failure with reduced ejection fraction (HFrEF), non-dihydropyridine calcium-channel blockers, such as diltiazem and verapamil, should be avoided because of their negative inotropic action.36) Vasodilators are not indicated in AHF with BP below 90 mmHg and should be used with caution, particularly in patients with low cardiac output, aortic stenosis, hypertrophic left ventricle with a small cavity, or predominant RV failure.15)
IN-HOSPITAL DIAGNOSTIC PROCESS OF ACUTE HEART FAILURE WITHOUT CARDIOGENIC SHOCK
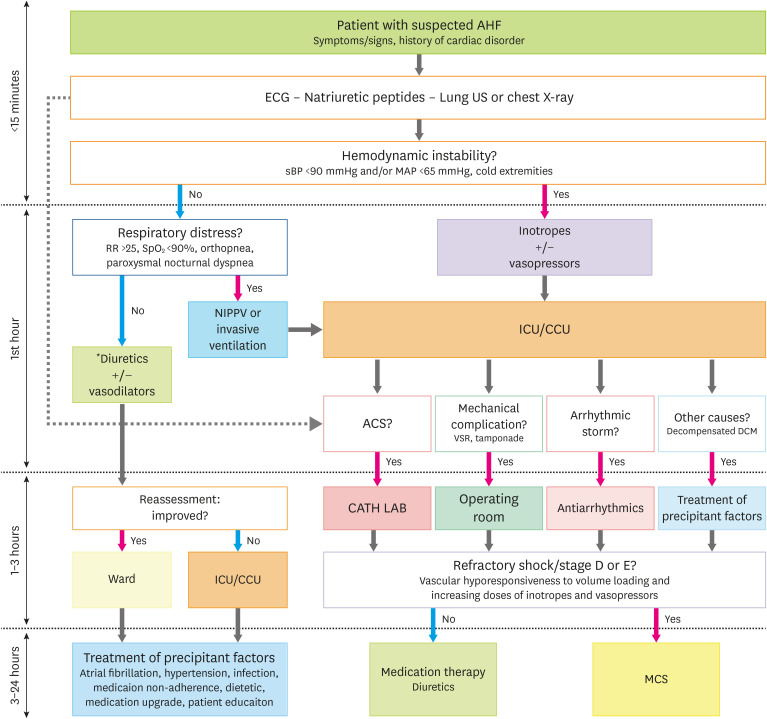
The hospital management of patients with suspected AHF is a timely step-by-step procedure (Figure 3). First, triage should be performed to assess initial severity. Second, AHF diagnosis should be confirmed based on clinical signs and measurement of plasma natriuretic peptides. Third, the causes of AHF should be identified (according to the CHAMP acronym—acute coronary syndrome, hypertension emergency, arrhythmia, acute mechanical cause, and pulmonary embolism).2) Fourth, the consequences of AHF on organ injuries should be assessed.15)
Figure 3. Hospital management of patients with suspected acute heart failure.
For more detail on the medications for congestion, refer to Figure 2.
ACS = acute coronary syndrome; AHF = acute heart failure; CATH LAB = cardiac catheterization laboratory; CCU = cardiac care unit; DCM = dilated cardiomyopathy; ECG = electrocardiogram; ICU = intensive care unit; MAP = mean arterial pressure; MCS = mechanical circulatory support; NIPPV = non-invasive positive pressure ventilation; RR = respiration rate; sBP = systolic blood pressure; SpO2 = oxygen saturation; US = ultrasound; VSR = ventricular septal rupture.
Complementing exams
Electrocardiography
A normal electrocardiogram (ECG) is rarely found in patients with AHF.37) Thus, an ECG, in patients with AHF, could play an important role in the determination of the decompensating cause. Supraventricular arrhythmia, such as atrial fibrillation, is most often found, followed by the presence of a new T wave or any other non-specific abnormalities. For the latter, a comparison with a previous ECG, if available, is strongly recommended.37)
Chest X-ray
Chest radiography is still recommended in patients with suspected AHF to exclude other causes of respiratory failure such as pneumothorax, pleural effusion, and tumoral syndrome.2) However, in up to 20% of patients with AHF, the chest X-ray is normal.38) Cephalization on chest radiography can be indicative of HFrEF. In fact, the diagnostic performance of chest X-rays is very low in terms of specificity and sensitivity to discriminate HFrEF from heart failure with preserved ejection fraction (HFpEF).39) Thus, chest radiography should not be a critical diagnostic tool in identifying specific causes of HF.
Echocardiography
Echocardiography, a non-irradiative exam, is a much better complementing exam to confirm the diagnosis of AHF and to investigate the underlying cause. It is increasingly used in the emergency room and in ICU/CCU to rule in or out pulmonary congestion. In AHF patients with hemodynamic instability, echocardiography is the complement cornerstone exam for the assessment of LV and RV systolic-diastolic functions as well as for the evaluation of pulmonary artery pressure. It could also promptly diagnose underlying causes such as tamponade, ventricular septal defect, valvular leakage, valvular stenosis, endocarditis, or intracardiac tumor (myxoma). A trained operator with at least a level III competence should perform and analyze echocardiography.40) The latter point restricts in an emergency setting the availability of echocardiography for the diagnostic of AHF. Indeed, in a recently published registry of 699 AHF patients admitted to 26 emergency departments, echocardiography was performed in only 15% of the cases.41) In summary, although echocardiography is not immediately needed in case of AHF with stable hemodynamics, it should be urgently performed in cases of hemodynamic instability.
Lung ultrasound
Lung ultrasound imaging is a non-invasive tool that allows both diagnostic and semi-quantification of congestive pulmonary edema. Briefly, hyperechoic artifacts arising from the pleural line and extending to the bottom of the screen without fading are known as B-lines. The latter is the ultrasound equivalent of the Kerley B lines found on a chest X-ray. Several protocols for lung ultrasound have been published. The simplified 4-Zone lung ultrasound method sums the number of B-lines in 4 pre-specified chest areas. Platz et al.42) recently demonstrated in an observational 2-site study that a number of B-lines >10 was associated with higher values of N-terminal pro-B type natriuretic peptide (NT-proBNP) and vascular congestion on chest X-ray. Moreover, lung ultrasound is a dynamic exam with rapid changes in response to therapies, making it a useful tool for follow-up.43)
Finally, unlike the skills required for performing a full echocardiography, lung ultrasound skills could be easily acquired, including by nurses with a short training program.44)
Pulmonary artery catheter
Although traditionally considered as a standard for the diagnosis of HF congestion, pulmonary artery catheter (PAC) is not routinely recommended.2) However, appropriate PAC use might be useful in patients with AHF who remain severely symptomatic despite appropriate therapies and for those with an unclear hemodynamic status.45)
Laboratory tests
BNP, NT-proBNP
Plasma natriuretic peptides play an important role in the early diagnosis of AHF. The natriuretic peptides, BNP and NT-proBNP, are mainly produced by the heart as a response to ventricular wall stretching by pressure overload or volume expansion.46) Plasma natriuretic peptides have been extensively studied in HF. This biomarker consistently demonstrated excellent negative but low predictive performance.47) Thus, both, BNP and NT-proBNP are recommended to exclude AHF in cases of acute respiratory distress.2) The specificity of these biomarkers is highly variable. Indeed, plasma natriuretic peptides are likely to be increased, for instance, in elderly patients or in cases of chronic renal failure and sepsis.2) Conversely, levels of natriuretic peptides are known to be lower in obese patients with AHF.48) Natriuretic peptides have prognostic value in AHF, in which higher concentrations mean more severe conditions and increased risk for readmission and mortality.49),50)
Cardiac troponin
Cardiac troponins are useful for the detection of ACS. In addition, the level of troponins, which suggests ongoing myocyte injury or necrosis, may be increased even in patients with AHF in the absence of an apparent myocardial ischemia or an acute coronary event.51) Elevated troponin levels are also associated with poorer outcomes in patients with AHF.52),53)
Other laboratory tests
Further laboratory examinations should be performed at the admission of a suspected AHF in order, first to detect and potentially correct triggering factors of AHF, and second, to assess the impact of AHF on organ dysfunction.
Thus, thyroid-stimulating hormone, hemoglobin, and whole blood counts are routinely performed as thyroid dysfunction, anemia, and infection are common triggers of AHF. Assessment of procalcitonin levels may also be considered to rule out acute infection or to guide the initiation of antibiotic therapy.54) Miró et al.55) reported that patients with AHF triggered by infection had a better outcome if antibiotics were administered early.
The liver and kidneys could be severely injured in the case of AHF. Acute kidney injury is a frequent adverse event in patients with AHF, which worsens the prognosis.56) Thus, blood urea nitrogen and creatinine levels should be repeatedly checked in patients with AHF. Acute liver dysfunction could reflect different profiles in AHF. An increase in liver transaminase (aspartate transaminase [AST], alanine transaminase [ALT]) levels mainly indicates liver cell ischemia/necrosis caused by low cardiac output,57) whereas an increase in cholestatic markers (alkaline phosphatase) may indicate right heart congestion.58) Consequently, repetitive tests of liver biomarkers are also needed in AHF patients.
Multiple novel biomarkers (circulating dipeptidyl peptidase 3, soluble suppression of tumorigenesis-2, galectin-3, growth differentiation factor-15, etc.) have been investigated in AHF. However, their role has not yet been defined, and they are not routinely used at bedside.2),59),60)
CARDIOGENIC SHOCK
Classification
CS is a heterogeneous syndrome in which the prognosis is deeply impacted by its origin, the patient's medical history, and the severity of the illness. Ranging from pre-shock syndrome to cardiac arrest, treatment and prognosis of CS vary widely according to the severity and the underlying cause. Thus, to improve the management of CS, a new stratification has been proposed.61) Briefly, 5 stages were defined from A to E and are presented in Table 1. The key points to keep in mind are that patients in stages A and B are at risk of acute degradation and should be carefully monitored. Patients reaching stage C require immediate treatment.
Table 1. Classification of cardiogenic shock.
| Stage | Description |
|---|---|
| Stage A | A patient who is not currently experiencing signs or symptoms of CS, but is at risk for its development. These patients may include those with large acute myocardial infarction or prior infarction acute and/or acute on chronic heart failure symptoms. |
| “At risk” | |
| Stage B | A patient who has clinical evidence of relative hypotension or tachycardia without hypoperfusion. |
| “Beginning” CS | |
| Stage C | A patient that manifests with hypoperfusion that requires intervention (inotrope, pressor or mechanical support, including ECMO) beyond volume resuscitation to restore perfusion. These patients typically present with relative hypotension. |
| “Classic” CS | |
| Stage D | A patient that is similar to category C but are getting worse. They fail to respond to initial interventions. |
| “Deteriorating/doom” | |
| Stage E | A patient that is experiencing cardiac arrest with ongoing CPR and/or ECMO, being supported by multiple interventions. |
| “Extremis” |
Adapted from the Society for Cardiovascular Angiography and Interventions clinical expert consensus statement on the classification of cardiogenic shock.61) Reproduced with permission of John Wiley and Sons from Baran et al., Catheter Cardiovasc Interv. 2019;94(1):29-37.
CS = cardiogenic shock; CPR = cardiopulmonary resuscitation; ECMO = extracorporeal membrane oxygenation.
Epidemiology and etiology
Patients presenting with CS should be systematically suspected of having ACS. Indeed, an ACS is diagnosed in approximately 70% of CS cases.62) However, only 4–8% of ACS cases evolve toward CS. Thus, in practice, at the bedside, an initial evaluation in patients with CS should include an ECG and repeated troponin dosages. In the first week following ACS, the onset of CS should always take into consideration mechanical complications such as papillary muscle rupture, ventricular septal defect, or free wall rupture.63) In such a situation, echocardiography is mandatory in order to exclude or confirm a mechanical complication. Congestive HF is the second most prevalent cause, accounting for more than 30% of CS.64) Other acute causes of CS, although rare, should be screened for and these include: myocarditis, septic cardiomyopathy, valvular dysfunction (native or prosthetic), arrhythmic storm, peripartum cardiomyopathy, stress-induced cardiomyopathy, pulmonary embolism, hypo- or hyperthyroidism, and cardiovascular agent poisoning.65),66),67),68),69),70),71),72) In most of these situations, clinical presentation, usual biological assessment, and echocardiography provide directions to caregivers at the bedside.
Initial assessment
There are 2 aspects of the initial assessment: severity and cause of the CS. Echocardiography will assess global cardiac function and estimate the cardiac index. Serum lactate levels reflect the severity of hypoxia-induced hypoperfusion. Renal (creatinine, glomerular filtration rate) and liver (prothrombin time, AST, and ALT) functions will reflect the consequences of both low cardiac index and venous congestion. The etiological investigation will depend on the clinical exam and the echocardiography results, but ECG and troponin are essential to exclude an ACS.
Etiological treatment
If ACS is suspected, a coronary angiogram should be performed irrespective of the delays of CS.2) Coronary revascularization should urgently be limited to the most probable culprit lesion given that complete revascularization is associated with increased 30-day risk of a composite outcome including death or severe renal failure in AMI-related CS patients.73),74) Fibrinolytic therapy should be considered only when percutaneous coronary intervention is not available. Other causes are rare and should be treated according to complementary test results.
Hemodynamic monitoring
A patient with CS should undergo continuous monitoring of organ perfusion and hemodynamic status. Echocardiography, which is a discontinuous and operator-dependent monitoring device, must be repeated throughout the day, specifically on the first day after admission, in which CS patients are at risk of evolving toward stage C or D.75) High-intensity monitoring should be initiated according to the severity of CS. Once a patient in CS reaches stage B, continuous monitoring has to be performed in the ICU or CCU with ECG, oximetry, and BP measurements.
Monitoring arterial BP is recommended through an arterial catheter ideally placed in the right radial artery to anticipate a possible CS stage D.76) In the case of CS stage D, most patients are under veno-arterial extracorporeal membrane oxygenation (VA-ECMO).
Repeated assessment of blood gas and lactate levels assess the effectiveness of treatments and will indicate an improvement or deterioration of organ perfusion.76),77) In CS, a central venous catheter must be placed to infuse catecholamine.77) Ideally, this catheter must be placed in the internal jugular vein. Thus, mixed venous oxygen saturation (SvO2) could be monitored continuously or discontinuously to estimate the global oxygen demand and supply; hence, to evaluate the variation of the cardiac index in response to therapy. Although there is no agreement on the use of PAC in assessing and treating CS, PAC may be considered to determine pulmonary artery pressures, right atrial pressure, stroke volume, and SvO2, and the effects of therapies, specifically in patients with RV dysfunction.76),78)
Vasopressor and inotrope treatments
Low cardiac output and congestion lead to neurohormonal and pro-inflammatory overwhelming responses that contribute to organ dysfunction or failure. Vasoplegia, a common feature in the most severe case of CS (stage C and over), is a surrogate marker of this inflammatory response. Moreover, generated inflammation will itself deteriorate cardiac function.79)
In CS stage B (classic wet and cold presentation), vasoplegia syndrome is less frequent and the main goal is to restore cardiac output. Thus, inotropes are most often sufficient.
In patients with CS, reaching stage C (from cold and dry to warm and wet presentations), vasoplegia syndrome stands in the foreground with low diastolic arterial pressure.80) Vasopressors, namely norepinephrine, should be initiated first to restore a mean arterial pressure of >65 mmHg. Then, after assessing the euvolemic status and the correction of the arterial pressure, inotrope should be started at low doses.
The recommended association of catecholamines is norepinephrine and dobutamine in the case of CS reaching stage C. Indeed, dopamine is associated with harmful arrhythmic effects in a subset of patients with CS from the SOAP2 study81) and should not be recommended. Epinephrine compared to norepinephrine plus dobutamine in a small multicentric study of patients with CS post-AMI was also associated with more progression to CS stage D.80) A meta-analysis of 16 cohorts performed on individual data also confirmed that epinephrine administration was associated with increased mortality in patients with CS.82)
Dobutamine is the most often prescribed inotrope in patients with CS.2) However, levosimendan, a myofilament calcium sensitizer, and enoximone or milrinone phosphodiesterase 3 inhibitor are 2 non-adrenergic alternatives. Levosimendan failed to demonstrate any beneficial effect in many CS situations: prevention or treatment of post-cardiac surgery CS, in non-surgical stage B CS patients, and for the prevention of organ dysfunction in septic shock patients.83),84),85),86),87) However, levosimendan might be a valuable option in stage B CS due to beta-blocker poisoning or adrenergic-stress cardiomyopathy. Phosphodiesterase 3 inhibitors are controversial and awaiting future, correctly designed studies. Therefore, the prescription of such treatments for patients with cardiac dysfunction could not be recommended.88)
Repeated echocardiography should be performed to assess the evolution of cardiac function.76) Physicians must carefully monitor at least twice a day, hemodynamic status including the clinical presentation (diuresis, marbles, oxygenation), norepinephrine dose, cardiac function, and biological exams, including lactate, liver, and kidney function.
In summary, in the case of low diastolic arterial pressure in patients with CS, norepinephrine is the best vasopressor to be combined with dobutamine in case of low cardiac output. No data support the use of dopamine and epinephrine in CS.
Mechanical circulatory support
In patients with CS reaching the most severe stage C or stage D who cannot be stabilized with medical therapy, mechanical circulatory support (MCS) devices can be used to achieve adequate systemic tissue perfusion by increasing mean arterial pressure, while concurrently decreasing myocardial oxygen demand. The European Society of Cardiology (ESC) guidelines recommend that all patients with CS should rapidly be transferred to a tertiary care center, which has a 24/7 service of cardiac catheterization and a dedicated ICU/CCU with the availability of a short-term MCS.2)
Criteria for an MCS
The decision at the right time for the implantation of an MCS in a patient reaching stage D of CS remains puzzling. Briefly, the definition of a severe C/D at bedside could be interpreted in these terms: CS with sustained hypotension, end-organ hypoperfusion, and hyperlactatemia despite adequate intravascular volume and association of high-dose inotropes and vasopressors.61),80) An uncontrolled increase in the norepinephrine dose associated with major symptoms of hypoperfusion (cold extremities, extended marbles, cyanosis) is also a practical definition of severe C/D stage. The confirmation of myocardial failure is achieved by rapid echocardiography.
In such a situation, the implantation of an MCS should not be delayed. Indeed, if no relevant study has been published to predict the effective criteria of an MCS implantation, conversely, there is extensive literature on the criteria predicting the outcome after an MCS implantation. Briefly, whatever the studied score, liver and renal failure are consistently associated with a poor outcome.89),90),91) Thus, an MCS should be initiated before the onset of organ failure and specifically before liver and kidney failure.
Intra-aortic balloon pump
The intra-aortic balloon (IABP) pump, a counterpulsation pump placed percutaneously in the descending aorta, has been widely used in CS patients. However, the IABP-SHOCK II trial did not show a beneficial outcome with IABP treatment in patients with AMI and CS compared to medical therapy alone.92),93) The ESC guidelines do not recommend the routine use of IABP in CS (class III, level B).2) Nonetheless, IABP could be used in patients receiving VA-ECMO to reduce LV afterload. A meta-analysis reported that in patients with CS complicating AMI, the use of IABP with VA-ECMO was associated with lower mortality in comparison to patients on VA-ECMO alone.94)
LV assist devices and veno-arterial extracorporeal membrane oxygenation
The currently available left ventricular assist device (LVAD) options are Impella (Impella 2.5®, Impella CP®, or Impella 5.0®; Abiomed, Danvers, MA, USA), TandemHeart (Cardiac Assist, Inc., Pittsburgh, PA, USA), and iVAC 2L (PulseCath B.V., Amsterdam, The Netherlands). LVAD can be used to unload the failing ventricle and restore circulatory output in patients with preserved RV function and poor respiratory failure. However, the circulatory output is most often limited to 4 to 4.5 L/min, and these devices are not able to replace the respiratory function. Conversely, VA-ECMO is recommended in patients with left, right, or global cardiac failure with or without respiratory impairment. The key advantages of this device are the efficient delivery of a high circulatory output up to 6 L/min and to replace the respiratory function in both oxygenation and decarboxylation aspects.95) To date, there have been numerous large cohort descriptions on the use of VA-ECMO or LVAD. However, there is no randomized controlled study confirming that in stage D, an MCS decreases mortality.91),96)
The decision for an MCS implantation should be included in a more overall discussion on the patient's planned clinical management, i.e. anticipation of a bridge to recovery, an opportunity for a heart graft, or a bridge to a long-term mechanical circulatory device. Patients with no reasonable expectation or with intractable organ failure should not be implanted with an MCS.
OTHER CLINICAL SCENARIOS OF ACUTE HEART FAILURE SEEN IN THE INTENSIVE CARE UNIT
Acute coronary syndrome
Diagnosis and treatment should be managed according to the ESC guidelines on non-ST elevation ACS and ST-elevation myocardial infarction.97),98) ACS patients complicated by AHF should immediately undergo coronary angiography and percutaneous coronary intervention.99) The diagnosis of ACS is not straightforward, especially in cases of coexisting AHF, since AHF could mask signs of ACS, and since cardiac troponin levels could be slightly elevated in case of AHF. Repeated ECG and cardiac troponin measurements may improve the accuracy of ACS diagnosis.100)
Predominant right ventricular failure
RV failure can be caused by a wide range of factors including tricuspid regurgitation, pulmonary stenosis, RV myocardial infarction, myocarditis, arrhythmogenic RV cardiomyopathy, pulmonary hypertension, acute pulmonary embolism, and congenital heart diseases, such as atrial septal defect, Ebstein anomaly, and transposition of the great arteries. Early diagnosis is crucial because treatment depends on the underlying causes. Echocardiography assesses RV size, function and load, associated valvular diseases, and pulmonary pressures. PAC can also be used in RV failure to measure pulmonary artery wedge pressure, pulmonary artery pressure, right atrial pressure, stroke volume, and SvO2, and the effects of therapies. Volume expansion to increase stroke volume requires extreme caution in patients with pulmonary hypertension. RV over-distention by volume expansion could decrease LV diastolic compliance by a ventricular septal shift toward the left ventricle (ventricular interdependence).101) In addition, perfusion of the right ventricular free wall is determined by the difference between the RV free wall tension and the coronary artery pressure.101),102) Volume loading that results in RV over-distention and increases in RV free wall tension may decrease coronary perfusion of the RV without increasing systolic volume.101) In hypotensive patients with pulmonary hypertension, inotropes should be considered to increase right ventricular flow. Milrinone and levosimendan increase both RV contractility and RV systolic volume while reducing RV end-diastolic pressure.103) Nevertheless, in cases of acute refractory RV failure, an MCS should be considered.
Myocarditis
Myocarditis is an inflammatory disease of the myocardium that is caused by a variety of infectious and noninfectious conditions.104) The clinical manifestations of myocarditis vary from asymptomatic to fatigue, chest pain, HF, CS, arrhythmias, and sudden death.104) In the case of ACS-like presentations, such as acute chest pain, ST/T changes on the ECG, LV dysfunction on echocardiography, and elevated cardiac troponin, it is much more difficult to distinguish acute myocarditis from ACS. Therefore, in cases of suspected myocarditis, it is mandatory to exclude ACS. Endomyocardial biopsy remains the gold standard for the diagnosis of myocarditis and identifies the underlying etiology and the type of inflammation (lymphocytic, eosinophilic, polymorphic, giant cell myocarditis, and cardiac sarcoidosis). According to the underlying etiology, specific treatments are initiated.104),105) The ESC Working Group on Myocardial and Pericardial Diseases recommend that the core principles of treatment in myocarditis are optimal care of HF and etiology-targeted therapy.104) Steroid therapy is indicated in cardiac sarcoidosis in the presence of ventricular dysfunction or arrhythmia and in some forms of infection-negative eosinophilic or giant cell myocarditis with HF or ventricular arrhythmia.106) Interleukin (IL)-1 is considered pivotal in the pathogenesis of myocardial inflammation.107) The ongoing ARAMIS trial (NCT03018834) should assess whether IL-1 blockade with anakinra improves outcomes in acute myocarditis. In patients with fulminant myocarditis, an MCS may be needed as a bridge to recovery or heart transplantation. As fulminant myocarditis may heal completely, VA-ECMO therapy can efficiently restore circulatory output, awaiting myocardial recovery.
SUMMARY AND KEY MESSAGES
• AHF patients benefit from a dedicated care pathway starting from the pre-hospital setting up to the cardiology department.
• AHF patients benefit from aggressive therapies, including NIPPV, diuretics, and vasodilators.
• Patients with AHF benefit from prompt identification and correction of the underlying cause.
• AHF patients evolving toward CS should be directed in a dedicated ICU/CCU with a 24/7 available service of mechanical assistance in case of stage D.
Footnotes
Conflict of Interest: Koji Takagi received speaker's honoraria from Otsuka, Sumitomo Dainippon, AstraZeneca, and Bayer and consultancy fees from Terumo. Antoine Kimmoun received a speaker's honoraria from Baxter, MSD, and Gilead. Naoki Sato has been a consultant for Otsuka, Terumo, Novartis, Toa-Eiyo, Tanabe-Mitsubishi, and BMS, and has received honoraria from Otsuka, Ono, Daiichi-Sankyo, Teijin, Boehringer Ingelheim, and Bayer. Alexandre Mebazaa reports personal fees from Novartis, Orion, Roche, Servier, Sanofi, Otsuka, Philips, grants and personal fees from Adrenomed, Abbott, grants from 4TEEN4.
- Supervision: Kimmoun A, Mebazaa A.
- Validation: Takagi K.
- Writing - original draft: Takagi K.
- Writing - review & editing: Kimmoun A, Sato N, Mebazaa A.
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–1488. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 3.Van Aelst LN, Arrigo M, Placido R, et al. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur J Heart Fail. 2018;20:738–747. doi: 10.1002/ejhf.1050. [DOI] [PubMed] [Google Scholar]
- 4.Mebazaa A, Gheorghiade M, Piña IL, et al. Practical recommendations for prehospital and early in-hospital management of patients presenting with acute heart failure syndromes. Crit Care Med. 2008;36:S129–39. doi: 10.1097/01.CCM.0000296274.51933.4C. [DOI] [PubMed] [Google Scholar]
- 5.Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41:1797–1804. doi: 10.1016/s0735-1097(03)00309-7. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson LW. Design of therapy for advanced heart failure. Eur J Heart Fail. 2005;7:323–331. doi: 10.1016/j.ejheart.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Nohria A, Lewis E, Stevenson LW. Medical management of advanced heart failure. JAMA. 2002;287:628–640. doi: 10.1001/jama.287.5.628. [DOI] [PubMed] [Google Scholar]
- 8.Javaloyes P, Miró Ò, Gil V, et al. Clinical phenotypes of acute heart failure based on signs and symptoms of perfusion and congestion at emergency department presentation and their relationship with patient management and outcomes. Eur J Heart Fail. 2019;21:1353–1365. doi: 10.1002/ejhf.1502. [DOI] [PubMed] [Google Scholar]
- 9.Peacock WF, Emerman C, Costanzo MR, Diercks DB, Lopatin M, Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail. 2009;15:256–264. doi: 10.1111/j.1751-7133.2009.00112.x. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi M, Kohsaka S, Miyata H, et al. Association between prehospital time interval and short-term outcome in acute heart failure patients. J Card Fail. 2011;17:742–747. doi: 10.1016/j.cardfail.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J 3CPO Trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359:142–151. doi: 10.1056/NEJMoa0707992. [DOI] [PubMed] [Google Scholar]
- 12.Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit the oxygen-ICU randomized clinical trial. JAMA. 2016;316:1583–1589. doi: 10.1001/jama.2016.11993. [DOI] [PubMed] [Google Scholar]
- 13.Masip J, Peacock WF, Price S, et al. Indications and practical approach to non-invasive ventilation in acute heart failure. Eur Heart J. 2018;39:17–25. doi: 10.1093/eurheartj/ehx580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray AJ, Goodacre S, Newby DE, et al. A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial. Health Technol Assess. 2009;13:1–106. doi: 10.3310/hta13330. [DOI] [PubMed] [Google Scholar]
- 15.Mebazaa A, Tolppanen H, Mueller C, et al. Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med. 2016;42:147–163. doi: 10.1007/s00134-015-4041-5. [DOI] [PubMed] [Google Scholar]
- 16.Brater DC. Pharmacokinetics of loop diuretics in congestive heart failure. Br Heart J. 1994;72:S40–3. doi: 10.1136/hrt.72.2_suppl.s40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mentz RJ, Kjeldsen K, Rossi GP, et al. Decongestion in acute heart failure. Eur J Heart Fail. 2014;16:471–482. doi: 10.1002/ejhf.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullens W, Damman K, Harjola VP, et al. The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:137–155. doi: 10.1002/ejhf.1369. [DOI] [PubMed] [Google Scholar]
- 19.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotter G, Metzkor E, Kaluski E, et al. Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary oedema. Lancet. 1998;351:389–393. doi: 10.1016/S0140-6736(97)08417-1. [DOI] [PubMed] [Google Scholar]
- 21.Brisco MA, Zile MR, Hanberg JS, et al. Relevance of changes in serum creatinine during a heart failure trial of decongestive strategies: insights from the DOSE trial. J Card Fail. 2016;22:753–760. doi: 10.1016/j.cardfail.2016.06.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaduganathan M, Mentz RJ, Greene SJ, et al. Combination decongestion therapy in hospitalized heart failure: loop diuretics, mineralocorticoid receptor antagonists and vasopressin antagonists. Expert Rev Cardiovasc Ther. 2015;13:799–809. doi: 10.1586/14779072.2015.1053872. [DOI] [PubMed] [Google Scholar]
- 23.ter Maaten JM, Valente MA, Damman K, Hillege HL, Navis G, Voors AA. Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nat Rev Cardiol. 2015;12:184–192. doi: 10.1038/nrcardio.2014.215. [DOI] [PubMed] [Google Scholar]
- 24.Channer KS, McLean KA, Lawson-Matthew P, Richardson M. Combination diuretic treatment in severe heart failure: a randomised controlled trial. Br Heart J. 1994;71:146–150. doi: 10.1136/hrt.71.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jujo K, Saito K, Ishida I, et al. Randomized pilot trial comparing tolvaptan with furosemide on renal and neurohumoral effects in acute heart failure. ESC Heart Fail. 2016;3:177–188. doi: 10.1002/ehf2.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang WL, Yang Y, Yang J, et al. Use of tolvaptan vs. furosemide in older patients with heart failure: meta-analysis of randomized controlled trials. Herz. 2018;43:338–345. doi: 10.1007/s00059-017-4563-4. [DOI] [PubMed] [Google Scholar]
- 27.Gheorghiade M, Konstam MA, Burnett JC, Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 28.Kinugawa K, Sato N, Inomata T, Shimakawa T, Iwatake N, Mizuguchi K. Efficacy and safety of tolvaptan in heart failure patients with volume overload. Circ J. 2014;78:844–852. [PubMed] [Google Scholar]
- 29.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 30.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 31.Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishihara S, Gayat E, Sato N, et al. Similar hemodynamic decongestion with vasodilators and inotropes: systematic review, meta-analysis, and meta-regression of 35 studies on acute heart failure. Clin Res Cardiol. 2016;105:971–980. doi: 10.1007/s00392-016-1009-6. [DOI] [PubMed] [Google Scholar]
- 33.Metra M, Teerlink JR, Voors AA, et al. Vasodilators in the treatment of acute heart failure: what we know, what we don't. Heart Fail Rev. 2009;14:299–307. doi: 10.1007/s10741-008-9127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy P, Compton S, Welch R, et al. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Ann Emerg Med. 2007;50:144–152. doi: 10.1016/j.annemergmed.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein RE, Boccuzzi SJ, Cruess D, Nattel S. Diltiazem increases late-onset congestive heart failure in postinfarction patients with early reduction in ejection fraction. The Adverse Experience Committee; and the Multicenter Diltiazem Postinfarction Research Group. Circulation. 1991;83:52–60. doi: 10.1161/01.cir.83.1.52. [DOI] [PubMed] [Google Scholar]
- 37.Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944–1956. doi: 10.1001/jama.294.15.1944. [DOI] [PubMed] [Google Scholar]
- 38.Chakko S, Woska D, Martinez H, et al. Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: conflicting results may lead to inappropriate care. Am J Med. 1991;90:353–359. doi: 10.1016/0002-9343(91)80016-f. [DOI] [PubMed] [Google Scholar]
- 39.Thomas JT, Kelly RF, Thomas SJ, et al. Utility of history, physical examination, electrocardiogram, and chest radiograph for differentiating normal from decreased systolic function in patients with heart failure. Am J Med. 2002;112:437–445. doi: 10.1016/s0002-9343(02)01048-3. [DOI] [PubMed] [Google Scholar]
- 40.Popescu BA, Andrade MJ, Badano LP, et al. European Association of Echocardiography recommendations for training, competence, and quality improvement in echocardiography. Eur J Echocardiogr. 2009;10:893–905. doi: 10.1093/ejechocard/jep151. [DOI] [PubMed] [Google Scholar]
- 41.Chouihed T, Manzo-Silberman S, Peschanski N, et al. Management of suspected acute heart failure dyspnea in the emergency department: results from the French prospective multicenter DeFSSICA survey. Scand J Trauma Resusc Emerg Med. 2016;24:112. doi: 10.1186/s13049-016-0300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platz E, Campbell RT, Claggett B, et al. Lung ultrasound in acute heart failure: prevalence of pulmonary congestion and short- and long-term outcomes. JACC Heart Fail. 2019;7:849–858. doi: 10.1016/j.jchf.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Platz E, Merz AA, Jhund PS, Vazir A, Campbell R, McMurray JJ. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail. 2017;19:1154–1163. doi: 10.1002/ejhf.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitale J, Mumoli N, Giorgi-Pierfranceschi M, et al. Comparison of the accuracy of nurse-performed and physician-performed lung ultrasound in the diagnosis of cardiogenic dyspnea. Chest. 2016;150:470–471. doi: 10.1016/j.chest.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 45.Sotomi Y, Sato N, Kajimoto K, et al. Impact of pulmonary artery catheter on outcome in patients with acute heart failure syndromes with hypotension or receiving inotropes: from the ATTEND Registry. Int J Cardiol. 2014;172:165–172. doi: 10.1016/j.ijcard.2013.12.174. [DOI] [PubMed] [Google Scholar]
- 46.Vodovar N, Logeart D. Similar BNP and mortality association in patients with and without heart failure: any increase matters. J Am Coll Cardiol. 2018;71:2089–2091. doi: 10.1016/j.jacc.2018.03.454. [DOI] [PubMed] [Google Scholar]
- 47.Roberts E, Ludman AJ, Dworzynski K, et al. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ. 2015;350:h910. doi: 10.1136/bmj.h910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madamanchi C, Alhosaini H, Sumida A, Runge MS. Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol. 2014;176:611–617. doi: 10.1016/j.ijcard.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Kimmenade RR, Pinto YM, Bayes-Genis A, Lainchbury JG, Richards AM, Januzzi JL., Jr Usefulness of intermediate amino-terminal pro-brain natriuretic peptide concentrations for diagnosis and prognosis of acute heart failure. Am J Cardiol. 2006;98:386–390. doi: 10.1016/j.amjcard.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 50.Lee JH, Kim MS, Kim EJ, et al. KSHF guidelines for the management of acute heart failure: Part I. Definition, epidemiology and diagnosis of acute heart failure. Korean Circ J. 2019;49:1–21. doi: 10.4070/kcj.2018.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ilva T, Lassus J, Siirilä-Waris K, et al. Clinical significance of cardiac troponins I and T in acute heart failure. Eur J Heart Fail. 2008;10:772–779. doi: 10.1016/j.ejheart.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Thygesen K, Mair J, Giannitsis E, et al. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J. 2012;33:2252–2257. doi: 10.1093/eurheartj/ehs154. [DOI] [PubMed] [Google Scholar]
- 53.Peacock WF, 4th, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117–2126. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 54.Maisel A, Neath SX, Landsberg J, et al. Use of procalcitonin for the diagnosis of pneumonia in patients presenting with a chief complaint of dyspnoea: results from the BACH (Biomarkers in Acute Heart Failure) trial. Eur J Heart Fail. 2012;14:278–286. doi: 10.1093/eurjhf/hfr177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miró Ò, Takagi K, Gayat É, et al. Time-pattern of adverse outcomes after an infection-triggered acute heart failure decompensation and the influence of early antibiotic administration and hospitalisation: results of the PAPRICA-3 study. Clin Res Cardiol. 2020;109:34–45. doi: 10.1007/s00392-019-01481-3. [DOI] [PubMed] [Google Scholar]
- 56.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 57.van Deursen VM, Damman K, Hillege HL, van Beek AP, van Veldhuisen DJ, Voors AA. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail. 2010;16:84–90. doi: 10.1016/j.cardfail.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Nikolaou M, Parissis J, Yilmaz MB, et al. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur Heart J. 2013;34:742–749. doi: 10.1093/eurheartj/ehs332. [DOI] [PubMed] [Google Scholar]
- 59.Deniau B, Rehfeld L, Santos K, et al. Circulating dipeptidyl peptidase 3 is a myocardial depressant factor: dipeptidyl peptidase 3 inhibition rapidly and sustainably improves haemodynamics. Eur J Heart Fail. 2020;22:290–299. doi: 10.1002/ejhf.1601. [DOI] [PubMed] [Google Scholar]
- 60.Takagi K, Blet A, Levy B, et al. Circulating dipeptidyl peptidase 3 and alteration in haemodynamics in cardiogenic shock: results from the OptimaCC trial. Eur J Heart Fail. 2020;22:279–286. doi: 10.1002/ejhf.1600. [DOI] [PubMed] [Google Scholar]
- 61.Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94:29–37. doi: 10.1002/ccd.28329. [DOI] [PubMed] [Google Scholar]
- 62.Aissaoui N, Puymirat E, Tabone X, et al. Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: a report from the USIK 1995, USIC 2000, and FAST-MI French nationwide registries. Eur Heart J. 2012;33:2535–2543. doi: 10.1093/eurheartj/ehs264. [DOI] [PubMed] [Google Scholar]
- 63.Elbadawi A, Elgendy IY, Mahmoud K, et al. Temporal trends and outcomes of mechanical complications in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2019;12:1825–1836. doi: 10.1016/j.jcin.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 64.Fernando SM, Qureshi D, Tanuseputro P, et al. Mortality and costs following extracorporeal membrane oxygenation in critically ill adults: a population-based cohort study. Intensive Care Med. 2019;45:1580–1589. doi: 10.1007/s00134-019-05766-z. [DOI] [PubMed] [Google Scholar]
- 65.Graudins A, Lee HM, Druda D. Calcium channel antagonist and beta-blocker overdose: antidotes and adjunct therapies. Br J Clin Pharmacol. 2016;81:453–461. doi: 10.1111/bcp.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bourcier S, Coutrot M, Kimmoun A, et al. Thyroid storm in the ICU: a retrospective multicenter study. Crit Care Med. 2020;48:83–90. doi: 10.1097/CCM.0000000000004078. [DOI] [PubMed] [Google Scholar]
- 67.Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 68.Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;364:k5287. doi: 10.1136/bmj.k5287. [DOI] [PubMed] [Google Scholar]
- 69.Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bréchot N, Luyt CE, Schmidt M, et al. Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Crit Care Med. 2013;41:1616–1626. doi: 10.1097/CCM.0b013e31828a2370. [DOI] [PubMed] [Google Scholar]
- 71.Meneveau N, Guillon B, Planquette B, et al. Outcomes after extracorporeal membrane oxygenation for the treatment of high-risk pulmonary embolism: a multicentre series of 52 cases. Eur Heart J. 2018;39:4196–4204. doi: 10.1093/eurheartj/ehy464. [DOI] [PubMed] [Google Scholar]
- 72.Mirabel M, Luyt CE, Leprince P, et al. Outcomes, long-term quality of life, and psychologic assessment of fulminant myocarditis patients rescued by mechanical circulatory support. Crit Care Med. 2011;39:1029–1035. doi: 10.1097/CCM.0b013e31820ead45. [DOI] [PubMed] [Google Scholar]
- 73.Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419–2432. doi: 10.1056/NEJMoa1710261. [DOI] [PubMed] [Google Scholar]
- 74.Henriques JP, Claessen BE. Revascularization strategies in cardiogenic shock patients with MVD: for now, keep it simple. J Am Coll Cardiol. 2018;71:857–859. doi: 10.1016/j.jacc.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 75.De Backer D. Ultrasonic evaluation of the heart. Curr Opin Crit Care. 2014;20:309–314. doi: 10.1097/MCC.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 76.Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levy B, Bastien O, Karim B, et al. Experts' recommendations for the management of adult patients with cardiogenic shock. Ann Intensive Care. 2015;5:52. doi: 10.1186/s13613-015-0052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harjola VP, Lassus J, Sionis A, et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17:501–509. doi: 10.1002/ejhf.260. [DOI] [PubMed] [Google Scholar]
- 79.Harjola VP, Mullens W, Banaszewski M, et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur J Heart Fail. 2017;19:821–836. doi: 10.1002/ejhf.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Levy B, Clere-Jehl R, Legras A, et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018;72:173–182. doi: 10.1016/j.jacc.2018.04.051. [DOI] [PubMed] [Google Scholar]
- 81.De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 82.Léopold V, Gayat E, Pirracchio R, et al. Epinephrine and short-term survival in cardiogenic shock: an individual data meta-analysis of 2583 patients. Intensive Care Med. 2018;44:847–856. doi: 10.1007/s00134-018-5222-9. [DOI] [PubMed] [Google Scholar]
- 83.Gordon AC, Perkins GD, Singer M, et al. Levosimendan for the prevention of acute organ dysfunction in sepsis. N Engl J Med. 2016;375:1638–1648. doi: 10.1056/NEJMoa1609409. [DOI] [PubMed] [Google Scholar]
- 84.Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA. 2007;297:1883–1891. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 85.Cholley B, Caruba T, Grosjean S, et al. Effect of levosimendan on low cardiac output syndrome in patients with low ejection fraction undergoing coronary artery bypass grafting with cardiopulmonary bypass: the LICORN randomized clinical trial. JAMA. 2017;318:548–556. doi: 10.1001/jama.2017.9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mehta RH, Leimberger JD, van Diepen S, et al. Levosimendan in patients with left ventricular dysfunction undergoing cardiac surgery. N Engl J Med. 2017;376:2032–2042. doi: 10.1056/NEJMoa1616218. [DOI] [PubMed] [Google Scholar]
- 87.Landoni G, Lomivorotov VV, Alvaro G, et al. Levosimendan for hemodynamic support after cardiac surgery. N Engl J Med. 2017;376:2021–2031. doi: 10.1056/NEJMoa1616325. [DOI] [PubMed] [Google Scholar]
- 88.Koster G, Bekema HJ, Wetterslev J, Gluud C, Keus F, van der Horst IC. Milrinone for cardiac dysfunction in critically ill adult patients: a systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Intensive Care Med. 2016;42:1322–1335. doi: 10.1007/s00134-016-4449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J. 2015;36:2246–2256. doi: 10.1093/eurheartj/ehv194. [DOI] [PubMed] [Google Scholar]
- 90.Muller G, Flecher E, Lebreton G, et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med. 2016;42:370–378. doi: 10.1007/s00134-016-4223-9. [DOI] [PubMed] [Google Scholar]
- 91.Combes A, Brodie D, Chen YS, et al. The ICM research agenda on extracorporeal life support. Intensive Care Med. 2017;43:1306–1318. doi: 10.1007/s00134-017-4803-3. [DOI] [PubMed] [Google Scholar]
- 92.Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382:1638–1645. doi: 10.1016/S0140-6736(13)61783-3. [DOI] [PubMed] [Google Scholar]
- 93.Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 94.Vallabhajosyula S, O'Horo JC, Antharam P, et al. Concomitant intra-aortic balloon pump use in cardiogenic shock requiring veno-arterial extracorporeal membrane oxygenation. Circ Cardiovasc Interv. 2018;11:e006930. doi: 10.1161/CIRCINTERVENTIONS.118.006930. [DOI] [PubMed] [Google Scholar]
- 95.Thiele H, Ohman EM, Desch S, Eitel I, de Waha S. Management of cardiogenic shock. Eur Heart J. 2015;36:1223–1230. doi: 10.1093/eurheartj/ehv051. [DOI] [PubMed] [Google Scholar]
- 96.Thiele H, Jobs A, Ouweneel DM, et al. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J. 2017;38:3523–3531. doi: 10.1093/eurheartj/ehx363. [DOI] [PubMed] [Google Scholar]
- 97.Roffi M, Patrono C, Collet JP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 98.Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) Steg PG, James SK, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 99.Steg PG, Dabbous OH, Feldman LJ, et al. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE) Circulation. 2004;109:494–499. doi: 10.1161/01.CIR.0000109691.16944.DA. [DOI] [PubMed] [Google Scholar]
- 100.Januzzi JL, Jr, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J. 2012;33:2265–2271. doi: 10.1093/eurheartj/ehs191. [DOI] [PubMed] [Google Scholar]
- 101.Ventetuolo CE, Klinger JR. Management of acute right ventricular failure in the intensive care unit. Ann Am Thorac Soc. 2014;11:811–822. doi: 10.1513/AnnalsATS.201312-446FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Urabe Y, Tomoike H, Ohzono K, Koyanagi S, Nakamura M. Role of afterload in determining regional right ventricular performance during coronary underperfusion in dogs. Circ Res. 1985;57:96–104. doi: 10.1161/01.res.57.1.96. [DOI] [PubMed] [Google Scholar]
- 103.Arrigo M, Mebazaa A. Understanding the differences among inotropes. Intensive Care Med. 2015;41:912–915. doi: 10.1007/s00134-015-3659-7. [DOI] [PubMed] [Google Scholar]
- 104.Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 105.Leone O, Veinot JP, Angelini A, et al. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol. 2012;21:245–274. doi: 10.1016/j.carpath.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 106.Cooper LT, Jr, Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis--natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med. 1997;336:1860–1866. doi: 10.1056/NEJM199706263362603. [DOI] [PubMed] [Google Scholar]
- 107.De Luca G, Cavalli G, Campochiaro C, Tresoldi M, Dagna L. Myocarditis: an interleukin-1-mediated disease? Front Immunol. 2018;9:1335. doi: 10.3389/fimmu.2018.01335. [DOI] [PMC free article] [PubMed] [Google Scholar]